Engineered Phage Endolysin Eliminates Gardnerella Biofilm without Damaging Beneficial Bacteria in Bacterial Vaginosis Ex Vivo
Abstract
:1. Introduction
2. Results
2.1. Gardnerella Genomes Contain Prophage Genes That Encode Active Endolysins
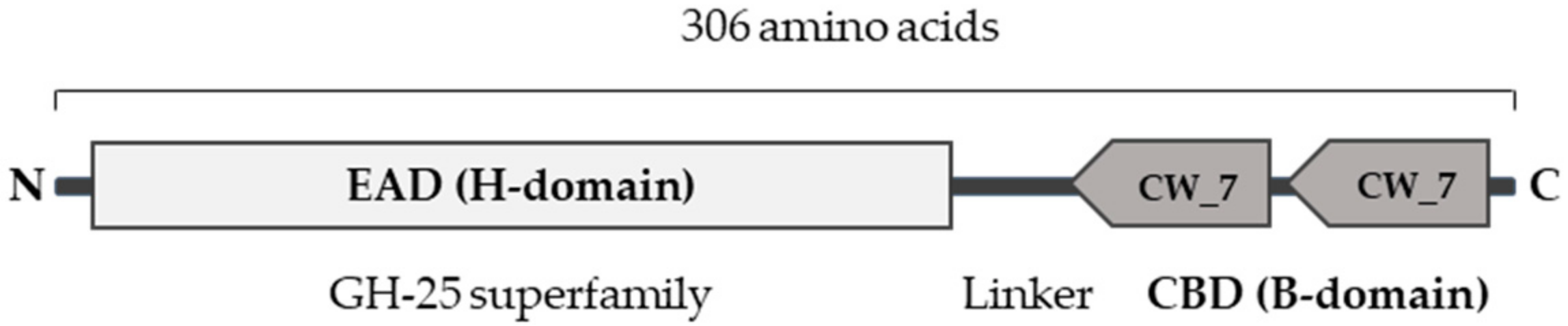
2.2. Engineering of a Gardnerella Endolysin with Enhanced Bactericidal Activity
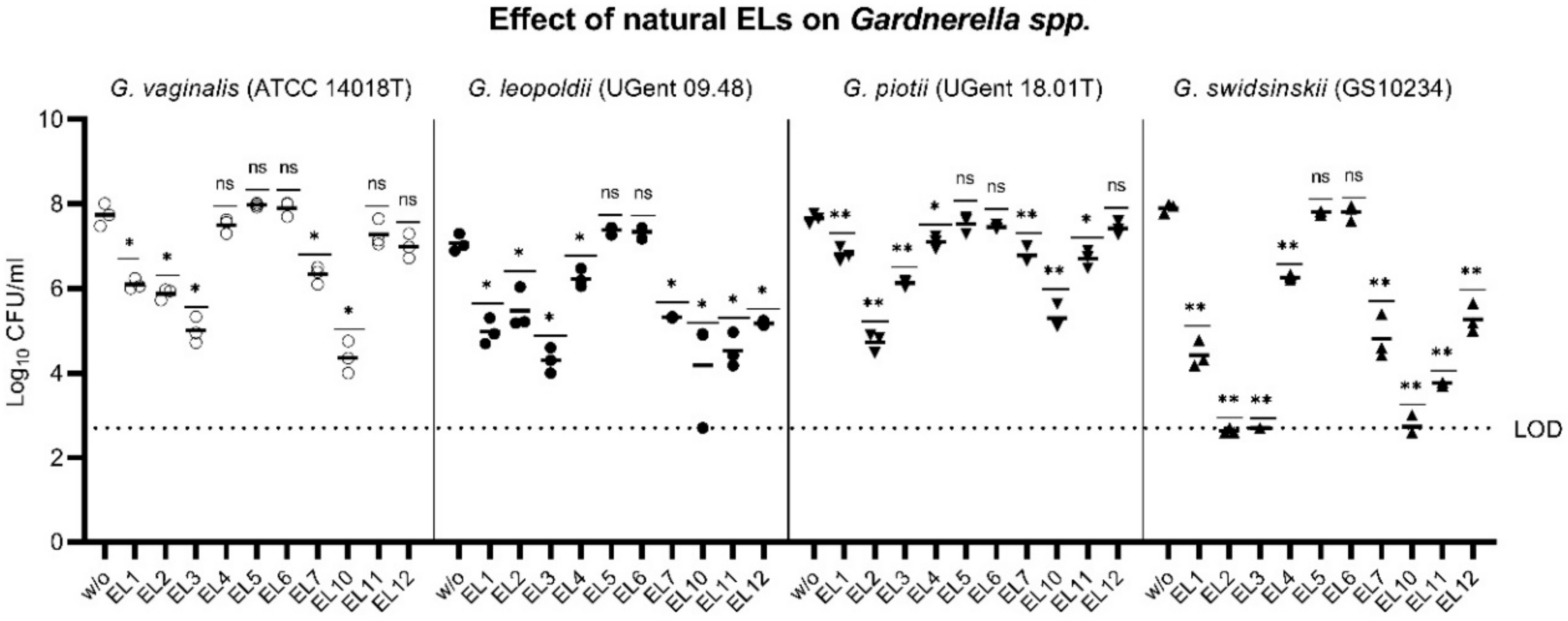
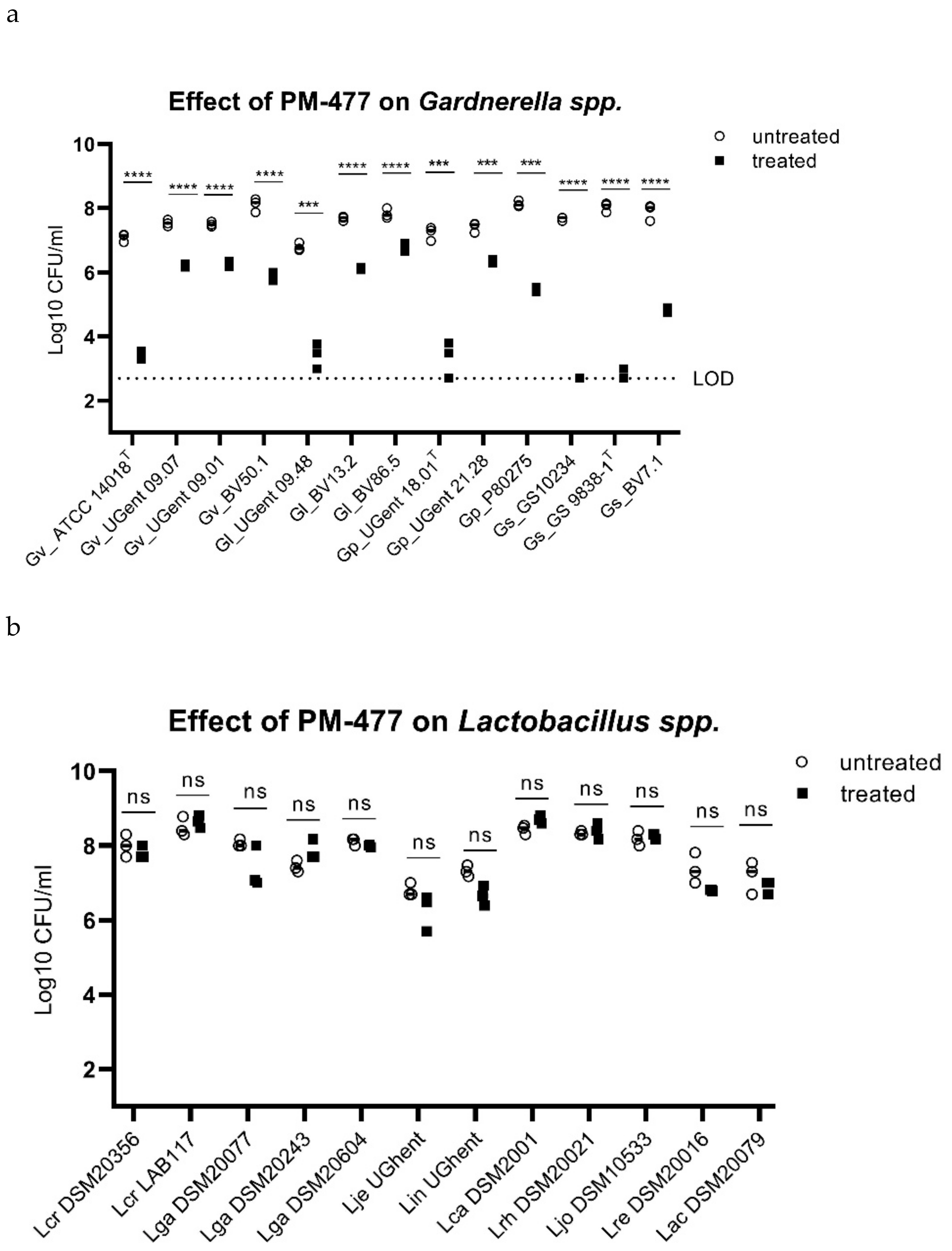
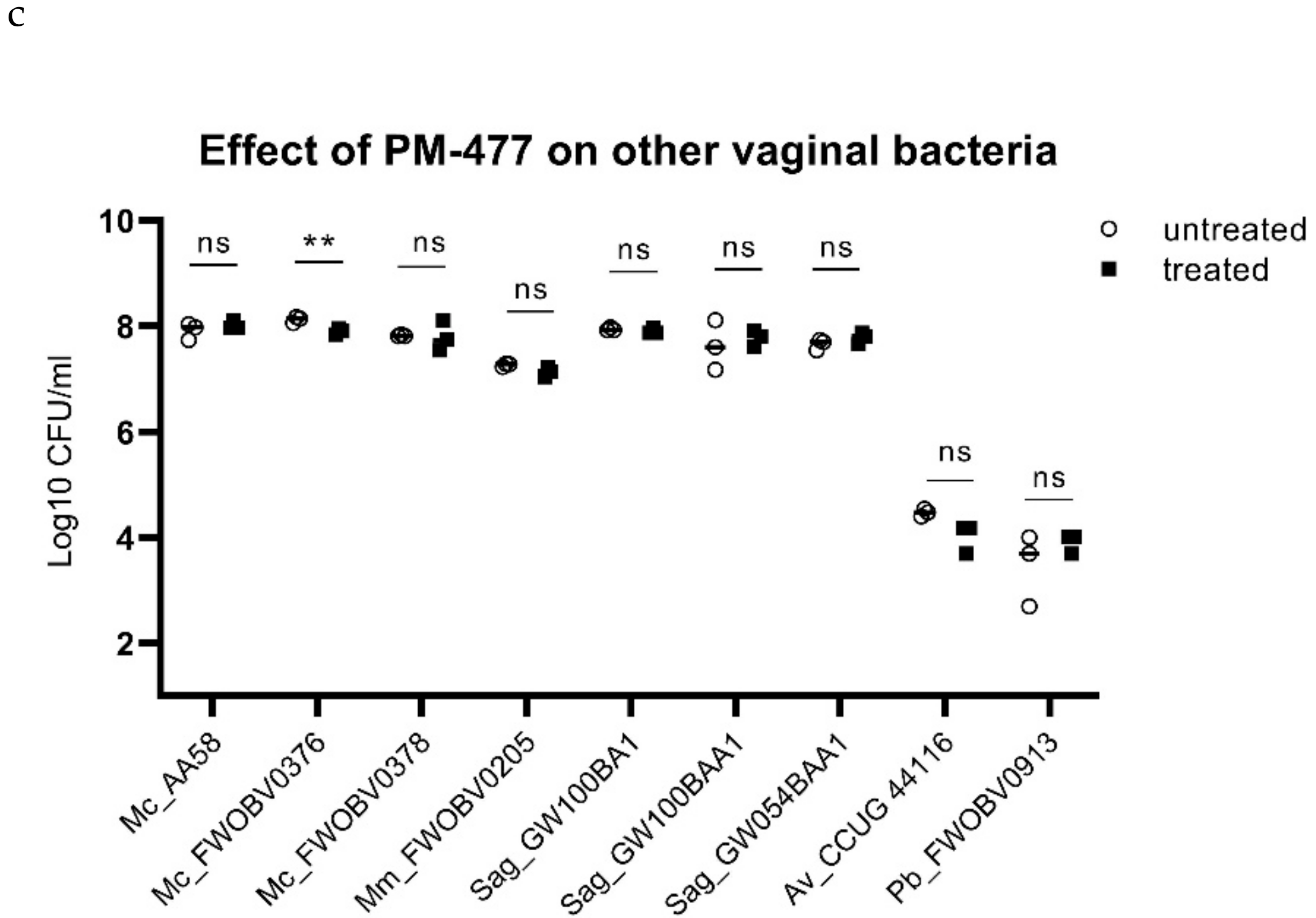
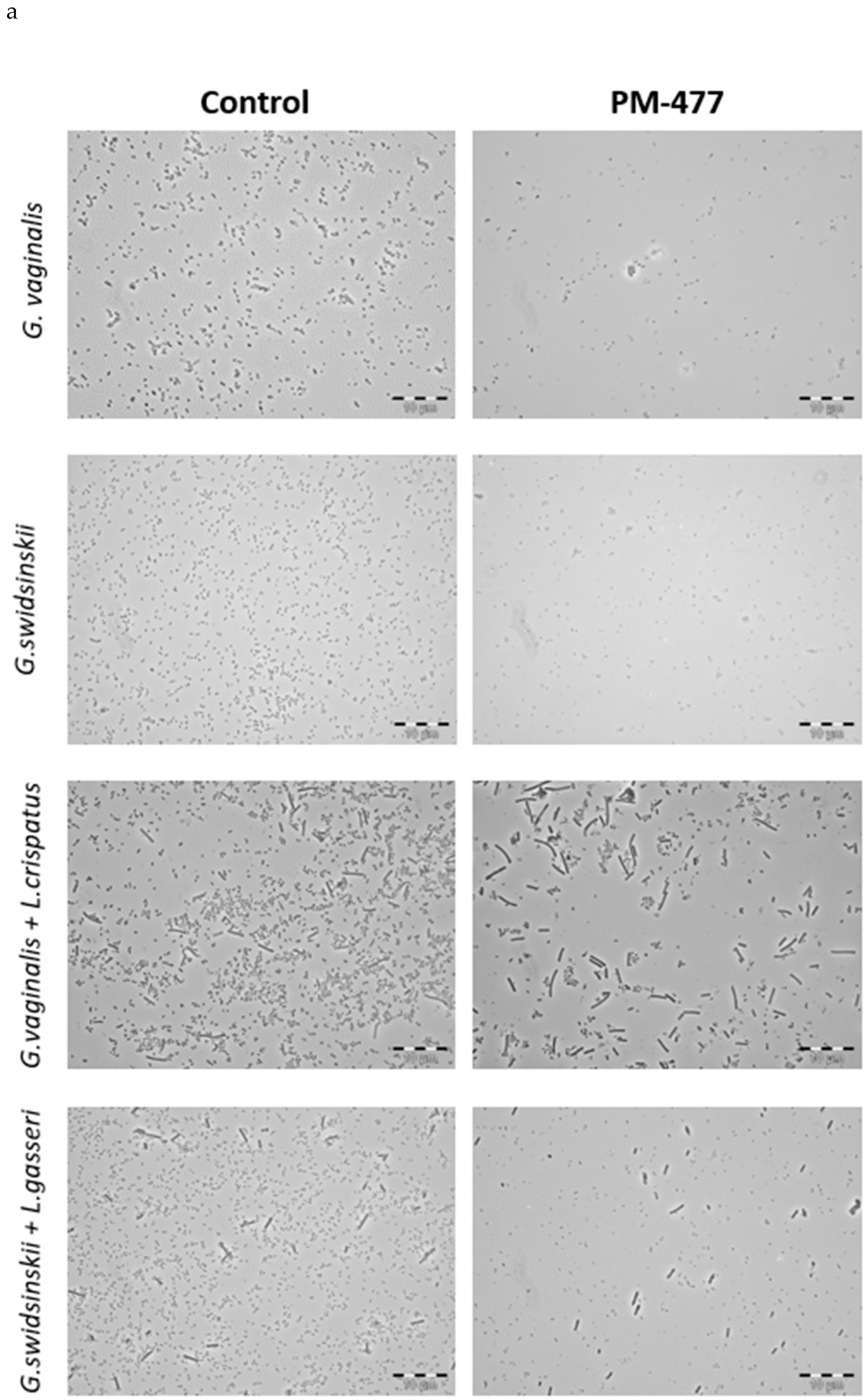
2.3. Specificity of PM-477 for the Genus Gardnerella
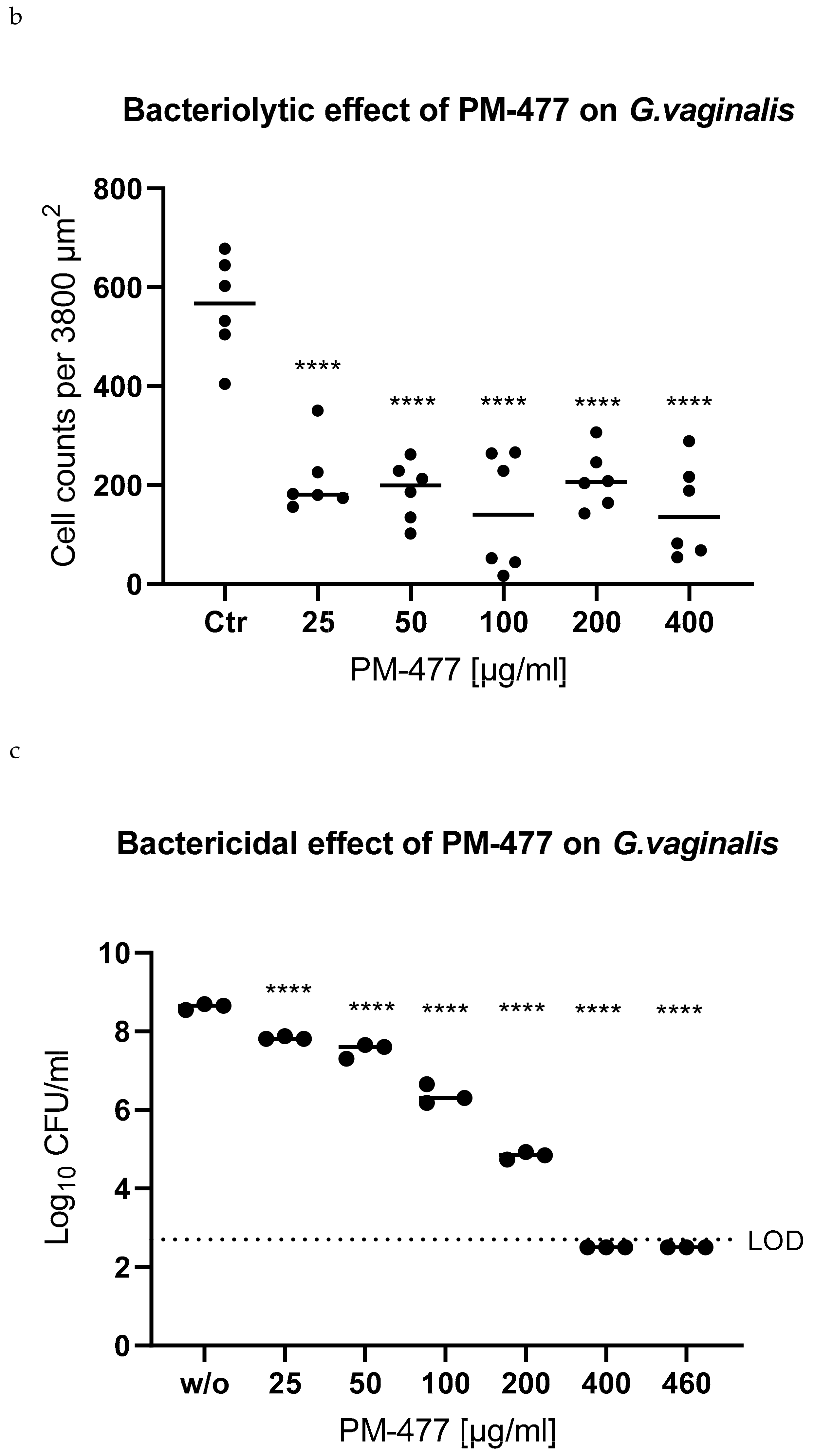
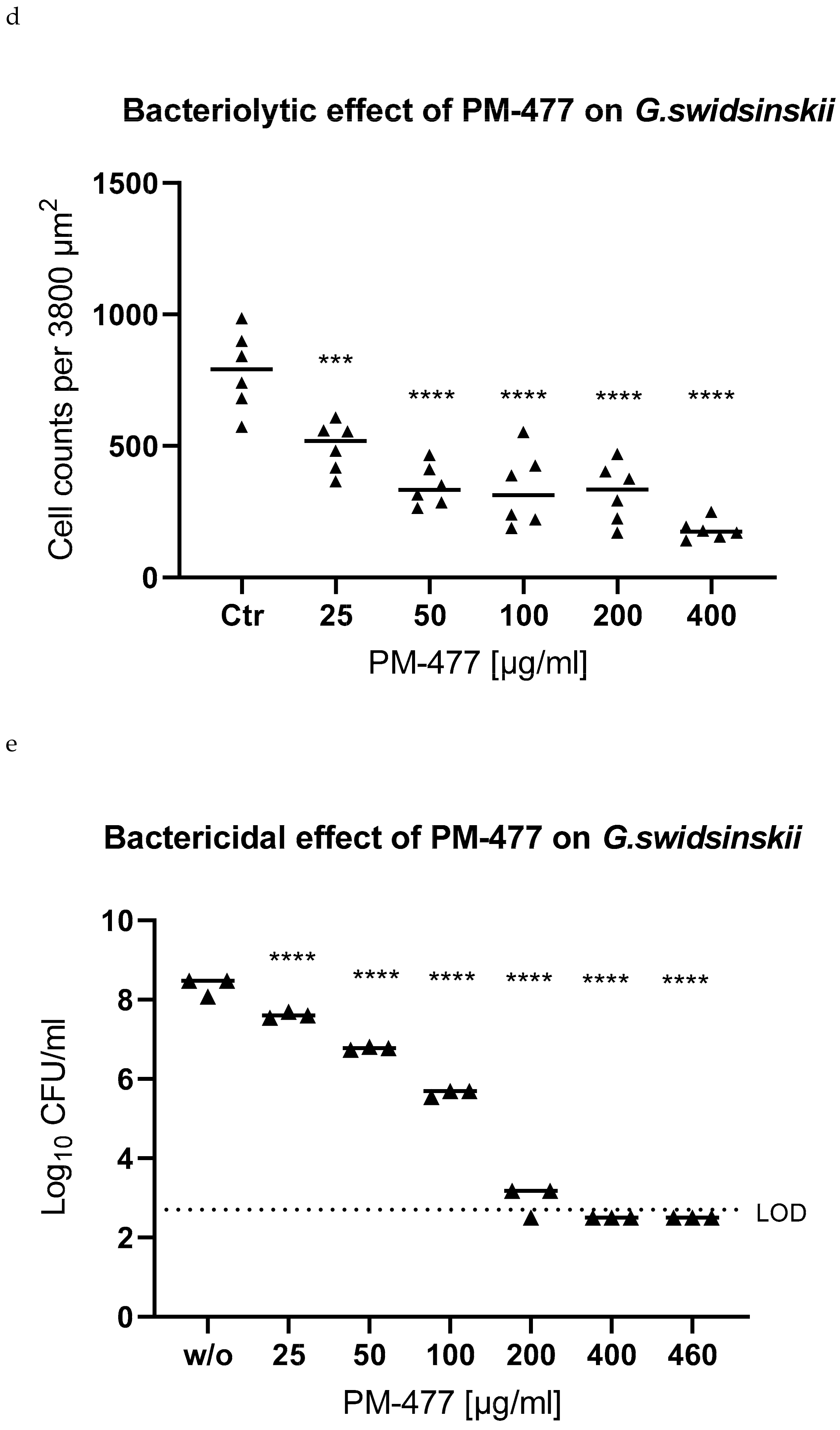
2.4. Efficacy of PM 477 Compared to Standard Antimicrobials
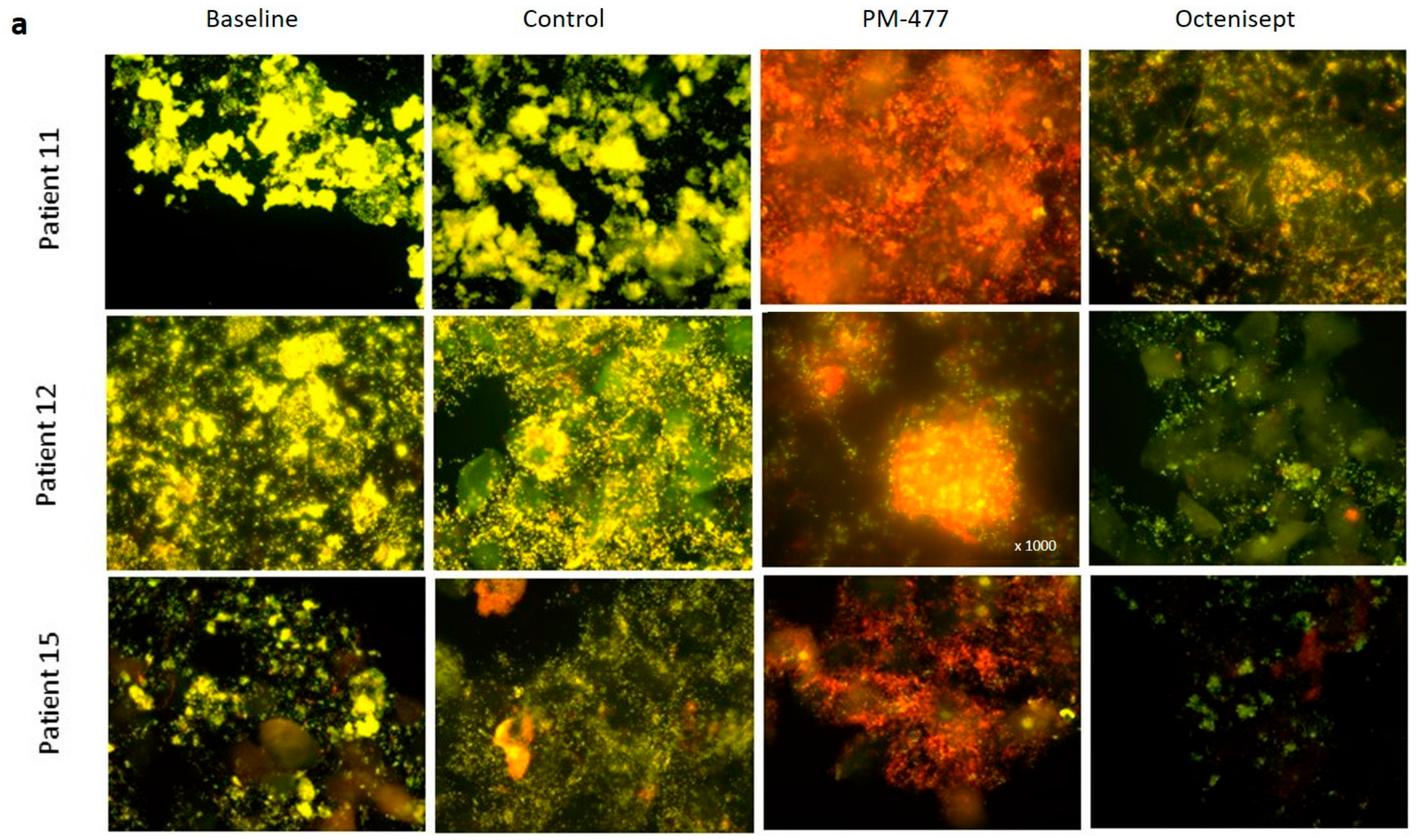
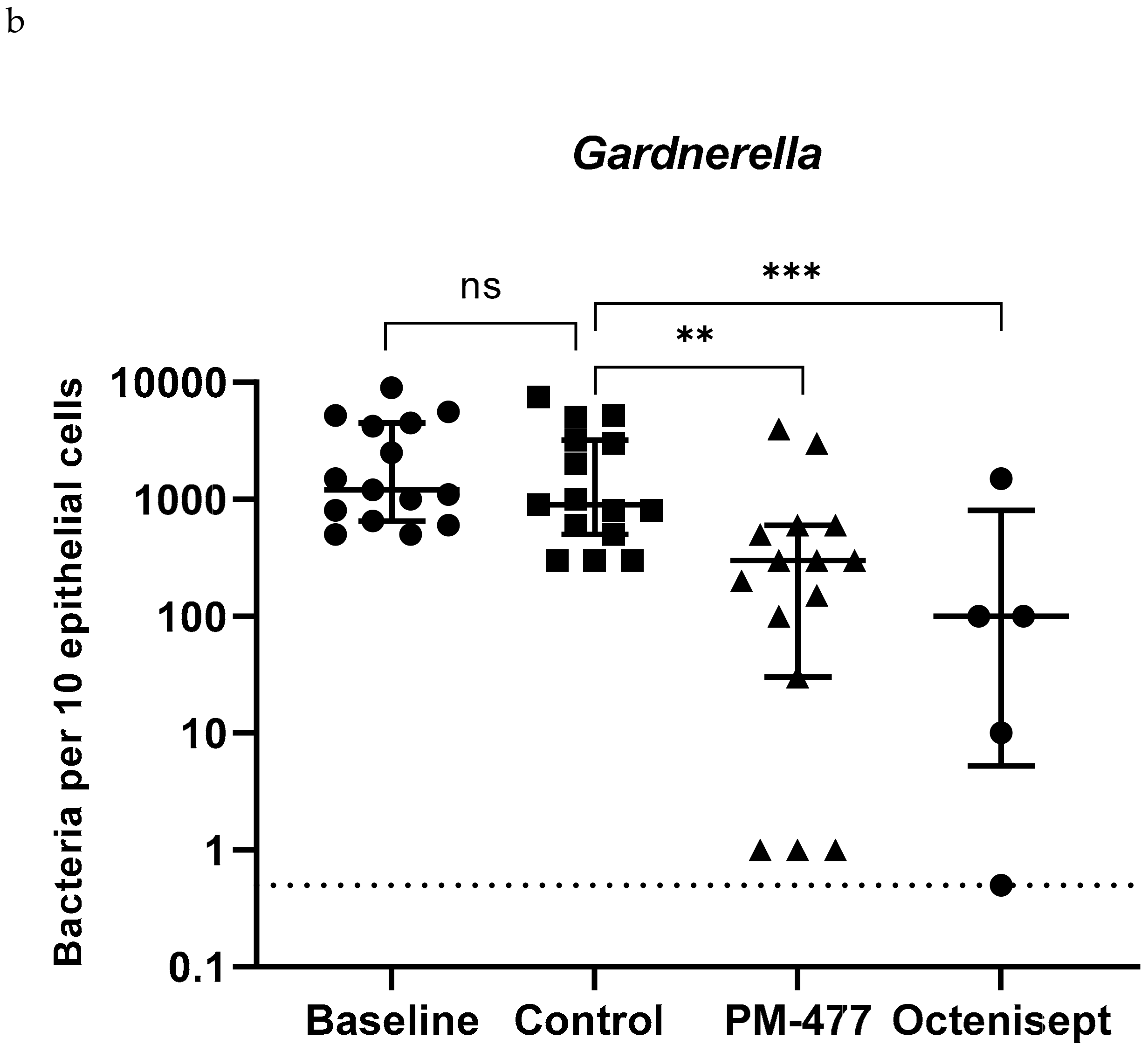
2.5. Efficacy of PM-477 against Biofilms of Gardnerella in Human BV Samples
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. Identification of Gardnerella Genes Encoding Endolysin within Prophage Regions
4.3. Gene Cloning and Overexpression of Phage Endolysins
4.4. Culture-Based Assessment of Bactericidal Activity
4.5. MIC and MBC Assessment
4.6. Phase Contrast Microscopy
4.7. Lytic Effects of PM-477 and Octenisept® on Ex Vivo BV Patient Samples as Detected by Fluorescence in situ Hybridization (FISH) Microscopy
4.8. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Gardnerella spp. | MBC99.5 of Antimicrobials [µg/mL] | |||||
|---|---|---|---|---|---|---|
| Species | Identification | Clade | PM-477 | CLI | MDZ | TDZ |
| G. vaginalis | ATCC 14018T | I | 2 | 0.5 | 16 | >128 |
| G. vaginalis | UGent 09.07 | I | 16 | 0.25 | >128 | >128 |
| G. vaginalis | UGent 09.01 | I | 8 | 0.25 | 32 | 8 |
| G. vaginalis | UGent 25.49 | I | 0.5 | 0.25 | 32 | 32 |
| G. vaginalis | BV50.1 | n.d. | 8 | 0.5 | >128 | >128 |
| G. vaginalis | BV111.5.1 | n.d. | 8 | 2 | 64 | 128 |
| G. vaginalis | FB049-01 | n.d. | 8 | 1 | 64 | 64 |
| G. vaginalis | FB061-03 | n.d. | >53 | 1 | 32 | 32 |
| G. leopoldii | UGent 09.48 | IV | 16 | 1 | >128 | >128 |
| G. leopoldii | BV13.2 | n.d. | n.d. | 1 | >128 | 128 |
| G. leopoldii | BV86.5 | n.d. | n.d. | 32 | >128 | n.d. |
| G. piotii | UGent 18.01T | II | 4 | 1 | 64 | >128 |
| G. piotii | UGent 21.28 | II | 4 | 0.5 | >128 | >128 |
| G. piotii | P80275 | n.d. | 2 | 1 | 64 | 128 |
| G. piotii | FB041 | n.d. | 8 | 2 | 64 | 128 |
| G. piotii | VMF1800 SVT21 | n.d. | >53 | 2 | >128 | >128 |
| G. swidsinskii | GS 10234 | IV | 1 | 0.25 | >128 | >128 |
| G. swidsinskii | GS 9838-1T | IV | 1 | 0.5 | >128 | >128 |
| G. swidsinskii | BV7.1 | n.d. | 2 | 1 | >128 | >128 |
| G. swidsinskii | BV112.4 | n.d. | n.d. | >128 | >128 | >128 |
| L. crispatus | DSM 20584 | >128 | 4 | >128 | >128 | |
| L. gasseri | DSM 20077 | >128 | 64 | >128 | >128 | |
| L. gasseri | DSM 20243 | >128 | >128 | >128 | >128 | |
| L. jensenii | PB2003-073-T2-2 | >128 | 4 | >128 | >128 | |
| Patient Samples | Gardnerella | L. iners | L. crispatus | A. vaginae | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Control | PM-477 | Octenisept | Baseline | Control | PM-477 | Octenisept | Baseline | Control | PM-477 | Octenisept | Baseline | Control | PM-477 | Octenisept | |
| 1 | 4200 | 3200 | 150 | 150 | 80 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| 2 | 500 | 500 | 300 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| 3 | 5600 | 5000 | 3000 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | ||||
| 4 | 1500 | 1000 | 100 | 200 | 200 | 20 | 0 | 0 | 0 | 50 | 20 | 500 | ||||
| 5 | 1000 | 300 | 300 | 0 | 0 | 0 | 0 | 0 | 0 | 200 | 0 | 500 | ||||
| 6 | 2500 | 3000 | 200 | 500 | 800 | 400 | 20 | 100 | 100 | 200 | 200 | 200 | ||||
| 7 | 9000 | 7500 | 4000 | 60 | 60 | 20 | 0 | 0 | 0 | 1000 | 1000 | 500 | ||||
| 8 | 1100 | 900 | 600 | 0 | 0 | 0 | 0 | 0 | 10 | 50 | 0 | 100 | ||||
| 9 | 800 | 800 | 1 | 5 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 1 | ||||
| 10 | 500 | 300 | 500 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| 11 | 5200 | 5200 | 30 | 100 | 150 | 100 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 12 | 1200 | 600 | 300 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 30 | 30 | 3 |
| 13 | 4500 | 2000 | 600 | 1500 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 400 | 400 | 600 | 0 |
| 14 | 650 | 300 | 1 | 0.5 | 60 | 50 | 50 | 0 | 0 | 0 | 0 | 0 | 40 | 20 | 40 | 0 |
| 15 | 600 | 800 | 1 | 10 | 120 | 60 | 20 | 4 | 0 | 0 | 0 | 0 | 100 | 120 | 80 | 0 |
| Median | 1200 | 900 | 300 | 100 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 50 | 0 | 40 | 0 |
| Treatment | Baseline | Puffer | 2 µg/mL PM-477 | 20 µg/mL PM-477 | 200 µg/mL PM-477 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incubation time | 0 h | 2 h | 6 h | 24 h | 2 h | 6 h | 24 h | 2 h | 6 h | 24 h | 2 h | 6 h | 24 h |
| No. of Gardnerella cells | 4200 | 4000 | 3900 | 3200 | 4300 | 4200 | 4200 | 4200 | 3500 | 2300 | 4400 | 2000 | 150 |
References
- Bautista, C.T.; Wurapa, E.; Sateren, W.B.; Morris, S.; Hollingsworth, B.; Sanchez, J.L. Bacterial vaginosis: A synthesis of the literature on etiology, prevalence, risk factors, and relationship with chlamydia and gonorrhea infections. Mil. Med. Res. 2016, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Kenyon, C.; Colebunders, R.; Crucitti, T. The global epidemiology of bacterial vaginosis: A systematic review. Am. J. Obstet. Gynecol. 2013, 209, 505–523. [Google Scholar] [CrossRef]
- Tabatabaei, N.; Eren, A.; Barreiro, L.; Yotova, V.; Dumaine, A.; Allard, C.; Fraser, W. Vaginal microbiome in early pregnancy and subsequent risk of spontaneous preterm birth: A case-control study. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Leitich, H.; Kiss, H. Asymptomatic bacterial vaginosis and intermediate flora as risk factors for adverse pregnancy outcome. Best Pract. Res. Clin. Obstet. Gynaecol. 2007, 21, 375–390. [Google Scholar] [CrossRef]
- Haahr, T.; Zacho, J.; Bräuner, M.; Shathmigha, K.; Skov Jensen, J.; Humaidan, P. Reproductive outcome of patients undergoing in vitro fertilisation treatment and diagnosed with bacterial vaginosis or abnormal vaginal microbiota: A systematic PRISMA review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Mania-Pramanik, J.; Kerkar, S.C.; Salvi, V.S. Bacterial vaginosis: A cause of infertility? Int. J. STD AIDS 2009, 20, 778–781. [Google Scholar] [CrossRef]
- Van Oostrum, N.; De Sutter, P.; Meys, J.; Verstraelen, H. Risks associated with bacterial vaginosis in infertility patients: A systematic review and meta-analysis. Hum. Reprod. 2013, 28, 1809–1815. [Google Scholar] [CrossRef] [Green Version]
- Unemo, M.; Bradshaw, C.S.; Hocking, J.S.; de Vries, H.J.C.; Francis, S.C.; Mabey, D.; Marrazzo, J.M.; Sonder, G.J.B.; Schwebke, J.R.; Hoornenborg, E.; et al. Sexually transmitted infections: Challenges ahead. Lancet Infect. Dis. 2017, 17, e235–e279. [Google Scholar] [CrossRef]
- Hoang, T.; Toler, E.; DeLong, K.; Mafunda, N.A.; Bloom, S.M.; Zierden, H.C.; Moench, T.R.; Coleman, J.S.; Hanes, J.; Kwon, D.S.; et al. The cervicovaginal mucus barrier to HIV-1 is diminished in bacterial vaginosis. PLoS Pathog. 2020, 16, e1008236. [Google Scholar] [CrossRef] [Green Version]
- Vaneechoutte, M. The human vaginal microbial community. Res. Microbiol. 2017, 168, 811–825. [Google Scholar] [CrossRef]
- Ceccarani, C.; Foschi, C.; Parolin, C.; D’Antuono, A.; Gaspari, V.; Consolandi, C.; Laghi, L.; Camboni, T.; Vitali, B.; Severgnini, M.; et al. Diversity of vaginal microbiome and metabolome during genital infections. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Petrova, M.I.; Reid, G.; Vaneechoutte, M.; Lebeer, S. Lactobacillus iners: Friend or Foe? Trends Microbiol. 2017, 25, 182–191. [Google Scholar] [CrossRef]
- Swidsinski, A.; Mendling, W.; Loening-Baucke, V.; Ladhoff, A.; Swidsinski, S.; Hale, L.P.; Lochs, H. Adherent biofilms in bacterial vaginosis. Obstet. Gynecol. 2005, 106, 1013–1023. [Google Scholar] [CrossRef] [Green Version]
- Machado, A.; Cerca, N. Influence of Biofilm Formation by Gardnerella vaginalis and Other Anaerobes on Bacterial Vaginosis. J. Infect. Dis. 2015, 212, 1856–1861. [Google Scholar] [CrossRef] [Green Version]
- Muzny, C.A.; Taylor, C.M.; Swords, W.E.; Tamhane, A.; Chattopadhyay, D.; Cerca, N.; Schwebke, J.R. An Updated Conceptual Model on the Pathogenesis of Bacterial Vaginosis. J. Infect. Dis. 2019, 220, 1399–1405. [Google Scholar] [CrossRef]
- Swidsinski, A.; Loening-Baucke, V.; Mendling, W.; Dörffel, Y.; Schilling, J.; Halwani, Z.; Jiang, X.F.; Verstraelen, H.; Swidsinski, S. Infection through structured polymicrobial Gardnerella biofilms (StPM-GB). Histol. Histopathol. 2014, 29, 567–587. [Google Scholar] [CrossRef]
- Castro, J.; Alves, P.; Sousa, C.; Cereija, T.; França, Â.; Jefferson, K.K.; Cerca, N. Using an in-vitro biofilm model to assess the virulence potential of bacterial vaginosis or non-bacterial vaginosis Gardnerella vaginalis isolates. Sci. Rep. 2015, 5, 11640. [Google Scholar] [CrossRef] [Green Version]
- Castro, J.; Jefferson, K.K.; Cerca, N.; Biomédicas, E.; Biomédicas, B.; Salazar, A. Innate immune components affect growth and virulence traits of bacterial-vaginosis-associated and non-bacterial-vaginosis-associated Gardnerella vaginalis strains similarly. Pathog. Dis. 2019, 76, 89. [Google Scholar] [CrossRef] [Green Version]
- Murphy, K.; Mitchell, C.M. The Interplay of Host Immunity, Environment and the Risk of Bacterial Vaginosis and Associated Reproductive Health Outcomes. J. Infect. Dis. 2016, 214, S29–S35. [Google Scholar] [CrossRef] [Green Version]
- Castro, J.; Machado, D.; Cerca, N. Unveiling the role of Gardnerella vaginalis in polymicrobial Bacterial Vaginosis bio fi lms: The impact of other vaginal pathogens living as neighbors. ISME J. 2019, 13, 1306–1317. [Google Scholar] [CrossRef]
- Machado, A.; Jefferson, K.K.A.; Cerca, N. Interactions between Lactobacillus crispatus and bacterial vaginosis (BV)-associated bacterial species in initial attachment and biofilm formation. Int. J. Mol. Sci. 2013, 14, 12004–12012. [Google Scholar] [CrossRef] [Green Version]
- Schwebke, J.R.; Muzny, C.A.; Josey, W.E. Role of Gardnerella vaginalis in the pathogenesis of bacterial vaginosis: A conceptual model. J. Infect. Dis. 2014, 210, 338–343. [Google Scholar] [CrossRef]
- Vaneechoutte, M.; Guschin, A.; Van Simaey, L.; Gansemans, Y.; Van Nieuwerburgh, F.; Cools, P. Emended description of Gardnerella vaginalis and description of Gardnerella leopoldii sp. nov., Gardnerella piotii sp. nov. and Gardnerella swidsinskii sp. nov., with delineation of 13 genomic species within the genus Gardnerella. Int. J. Syst. Evol. Microbiol. 2019, 69, 679–687. [Google Scholar] [CrossRef]
- Hill, J.E.; Albert, A.Y.K. Resolution and cooccurrence patterns of Gardnerella leopoldii, G. Swidsinskii, G. Piotii, and G. Vaginalis within the vaginal microbiome. Infect. Immun. 2019, 87. [Google Scholar] [CrossRef]
- Shipitsyna, E.; Krysanova, A.; Khayrullina, G.; Shalepo, K.; Savicheva, A.; Guschin, A.; Unemo, M. Quantitation of all Four Gardnerella vaginalis Clades Detects Abnormal Vaginal Microbiota Characteristic of Bacterial Vaginosis More Accurately than Putative G. vaginalis Sialidase A Gene Count. Mol. Diagnosis Ther. 2019, 23, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Bradshaw, C.S.; Sobel, J.D. Current Treatment of Bacterial Vaginosis-Limitations and Need for Innovation. J. Infect. Dis. 2016, 214, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Swidsinski, A.; Mendling, W.; Loening-Baucke, V.; Swidsinski, S.; Dörffel, Y.; Scholze, J.; Lochs, H.; Verstraelen, H. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am. J. Obstet. Gynecol. 2008, 198, 97.e1–97.e6. [Google Scholar] [CrossRef]
- Sobel, J.D. Vulvovaginal candidosis. Lancet 2007, 369, 1961–1971. [Google Scholar] [CrossRef]
- Buffie, C.G.; Jarchum, I.; Equinda, M.; Lipuma, L.; Gobourne, A.; Viale, A.; Ubeda, C.; Xavier, J.; Pamer, E.G. Profound alterations of intestinal microbiota following a single dose of clindamycin results in sustained susceptibility to Clostridium difficile-induced colitis. Infect. Immun. 2012, 80, 62–73. [Google Scholar] [CrossRef] [Green Version]
- Vardakas, K.Z.; Trigkidis, K.K.; Boukouvala, E.; Falagas, M.E. Clostridium difficile infection following systemic antibiotic administration in randomised controlled trials: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2016, 48, 1–10. [Google Scholar] [CrossRef]
- Petrina, M.A.B.; Cosentino, L.A.; Rabe, L.K.; Hillier, S.L. Susceptibility of bacterial vaginosis (BV)-associated bacteria to secnidazole compared to metronidazole, tinidazole and clindamycin HHS Public Access. Anaerobe 2017, 47, 115–119. [Google Scholar] [CrossRef]
- Swidsinski, A.; Loening-Baucke, V.; Swidsinski, S.; Verstraelen, H. Polymicrobial Gardnerella biofilm resists repeated intravaginal antiseptic treatment in a subset of women with bacterial vaginosis: A preliminary report. Arch. Gynecol. Obstet. 2015, 291, 605–609. [Google Scholar] [CrossRef]
- Lev-Sagie, A.; Goldman-Wohl, D.; Cohen, Y.; Dori-Bachash, M.; Leshem, A.; Mor, U.; Strahilevitz, J.; Moses, A.E.; Shapiro, H.; Yagel, S.; et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat. Med. 2019, 25, 1500–1504. [Google Scholar] [CrossRef]
- Schmelcher, M.; Donovan, D.M.; Loessner, M.J. Bacteriophage endolysins as novel antimicrobials. Future Microbiol. 2012, 7, 1147–1171. [Google Scholar] [CrossRef] [Green Version]
- Fischetti, V.A. Development of phage lysins as novel therapeutics: A historical perspective. Viruses 2018, 10, 310. [Google Scholar] [CrossRef] [Green Version]
- Fischetti, V.A. Bacteriophage endolysins: A novel anti-infective to control Gram-positive pathogens. Int. J. Med. Microbiol. 2010, 300, 357–362. [Google Scholar] [CrossRef] [Green Version]
- Schuch, R.; Lee, H.M.; Schneider, B.C.; Sauve, K.L.; Law, C.; Khan, B.K.; Rotolo, J.A.; Horiuchi, Y.; Couto, D.E.; Raz, A.; et al. Combination therapy with lysin CF-301 and antibiotic is superior to antibiotic alone for treating methicillin-resistant staphylococcus aureus-induced murine bacteremia. J. Infect. Dis. 2014, 209, 1469–1478. [Google Scholar] [CrossRef]
- Oliveira, H.; Melo, L.D.R.; Santos, S.B.; Nobrega, F.L.; Ferreira, E.C.; Cerca, N.; Azeredo, J.; Kluskens, L.D. Molecular Aspects and Comparative Genomics of Bacteriophage Endolysins. J. Virol. 2013, 87, 4558–4570. [Google Scholar] [CrossRef] [Green Version]
- Sadhu, K.; Domingue, P.A.G.; Chow, A.W.; Nelligan, J.; Cheng, N.; Costerton, J.W. Gardnerella vaginalis has a gram-positive cell-wall ultrastructure and lacks classical cell-wall lipopolysaccharide. J. Med. Microbiol. 1989, 29, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Malki, K.; Shapiro, J.W.; Price, T.K.; Hilt, E.E.; Thomas-White, K.; Sircar, T.; Rosenfeld, A.B.; Kuffel, G.; Zilliox, M.J.; Wolfe, A.J.; et al. Genomes of Gardnerella Strains Reveal an Abundance of Prophages within the Bladder Microbiome. PLoS ONE 2016, 11, e0166757. [Google Scholar] [CrossRef]
- Bohr, L.L.; Mortimer, T.D.; Pepperell, C.S. Lateral Gene Transfer Shapes Diversity of Gardnerella spp. Front. Cell. Infect. Microbiol. 2020, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Schmelcher, M.; Tchang, V.S.; Loessner, M.J. Domain shuffling and module engineering of Listeria phage endolysins for enhanced lytic activity and binding affinity. Microb. Biotechnol. 2011, 4, 651–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, D.C.; Schmelcher, M.; Rodriguez-Rubio, L.; Klumpp, J.; Pritchard, D.G.; Dong, S.; Donovan, D.M. Endolysins as Antimicrobials. In Advances in Virus Research; Academic Press Inc.: Cambridge, MA, USA, 2012; Volume 83, pp. 299–365. [Google Scholar]
- Carpenter, D.E.; Anderson, K.; Citron, D.M.; Dzink-Fox, J.L.; Hackel, M.; Jenkins, S.G.; Knapp, C.; Koeth, L.; Schuetz, A.N.; Wexler, H. Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria, 9th ed.; Clinical and Laboratory Standards Institute: Annapolis Junction, MD, USA, 2018; Chapter 2. [Google Scholar]
- Wang, Q.; Euler, C.W.; Delaune, A.; Fischetti, V.A. Using a novel lysin to help control Clostridium difficile infections. Antimicrob. Agents Chemother. 2015, 59, 7447–7457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuyler, J.A.; Mordechai, E.; Adelson, M.E.; Sobel, J.D.; Gygax, S.E.; Hilbert, D.W. Identification of intrinsically metronidazole-resistant clades of Gardnerella vaginalis. Diagn. Microbiol. Infect. Dis. 2016, 84, 1–3. [Google Scholar] [CrossRef]
- Morrill, S.; Gilbert, N.M.; Lewis, A.L. Gardnerella vaginalis as a Cause of Bacterial Vaginosis: Appraisal of the Evidence From in vivo Models. Front. Cell. Infect. Microbiol. 2020, 10, 168. [Google Scholar] [CrossRef]
- Muytjens, C.M.J.; Vasiliou, S.K.; Oikonomopoulou, K.; Prassas, I.; Diamandis, E.P. Putative functions of tissue kallikrein-related peptidases in vaginal fluid. Nat. Rev. Urol. 2016, 13, 596–607. [Google Scholar] [CrossRef]
- Swidsinski, A.; Doerffel, Y.; Loening-Baucke, V.; Swidsinski, S.; Verstraelen, H.; Vaneechoutte, M.; Lemm, V.; Schilling, J.; Mendling, W. Gardnerella biofilm involves females and males and is transmitted sexually. Gynecol. Obstet. Investig. 2010, 70, 256–263. [Google Scholar] [CrossRef]
- Alves, P.; Castro, J.; Sousa, C.; Cereija, T.B.; Cerca, N. Gardnerella vaginalis outcompetes 29 other bacterial species isolated from patients with bacterial vaginosis, using in an in vitro biofilm formation model. J. Infect. Dis. 2014, 210, 593–596. [Google Scholar] [CrossRef] [Green Version]
- Pybus, V.; Onderdonk, A.B. Evidence for a commensal, symbiotic relationship between Gardnerella vaginalis and Prevotella bivia involving ammonia: Potential significance for bacterial vaginosis. J. Infect. Dis. 1997, 175, 406–413. [Google Scholar] [CrossRef]
- Chen, K.C.S.; Forsyth, P.S.; Buchanan, T.M.; Holmes, K.K. Amine content of vaginal fluid from untreated and treated patients with nonspecific vaginitis. J. Clin. Investig. 1979, 63, 828–835. [Google Scholar] [CrossRef]
- Briselden, A.M.; Moncla, B.J.; Stevens, C.E.; Hillier, S.L. Sialidases (neuraminidases) in bacterial vaginosis and bacterial vaginosis-associated microflora. J. Clin. Microbiol. 1992, 30, 663–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amsel, R.; Totten, P.A.; Spiegel, C.A.; Chen, K.C.S.; Eschenbach, D.; Holmes, K.K. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am. J. Med. 1983, 74, 14–22. [Google Scholar] [CrossRef]
- Swidsinski, A.; Loening-Baucke, V. Evaluation of Polymicrobial Involvement Using Fluorescence In Situ Hybridization (FISH) in Clinical Practice. In Fluorescence In Situ Hybridization, 2nd ed.; Liehr, T., Ed.; Springer: New York, NY, USA, 2017; pp. 531–543. [Google Scholar] [CrossRef]
- Swidsinski, A.; Guschin, A.; Tang, Q.; Dörffel, Y.; Verstraelen, H.; Tertychnyy, A.; Khayrullina, G.; Luo, X.; Sobel, J.D.; Jiang, X. Vulvovaginal candidiasis: Histologic lesions are primarily polymicrobial and invasive and do not contain biofilms. Am. J. Obstet. Gynecol. 2019, 220, 91.e1–91.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amann, R.I.; Binder, B.J.; Olson, R.J.; Chisholm, S.W.; Devereux, R.; Stahl, D.A. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 1990, 56, 1919–1925. [Google Scholar] [CrossRef] [Green Version]
| B1 | B2 | B3 | B4 | B5 | B7 | B10 | B11 | B12 | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gv_ATCC 14018T | Gl_UGent 09.48 | Gp_UGent 18.01T | Gs_GS10234 | Gv_ATCC 14018T | Gl_UGent 09.48 | Gp_UGent 18.01T | Gs_GS10234 | Gv_ATCC 14018T | Gl_UGent 09.48 | Gp_UGent 18.01T | Gs_GS10234 | Gv_ATCC 14018T | Gl_UGent 09.48 | Gp_UGent 18.01T | Gs_GS10234 | Gv_ATCC 14018T | Gl_UGent 09.48 | Gp_UGent 18.01T | Gs_GS10234 | Gv_ATCC 14018T | Gl_UGent 09.48 | Gp_UGent 18.01T | Gs_GS10234 | Gv_ATCC 14018T | Gl_UGent 09.48 | Gp_UGent 18.01T | Gs_GS10234 | Gv_ATCC 14018T | Gl_UGent 09.48 | Gp_UGent 18.01T | Gs_GS10234 | Gv_ATCC 14018T | Gl_UGent 09.48 | Gp_UGent 18.01T | Gs_GS10234 | |
| H1 | −1.9 | −1.3 | −0.9 | −3.5 | −0.4 | −0.3 | −0.2 | −1.4 | −0.7 | −0.7 | −0.3 | −1.9 | −0.8 | −0.7 | −0.7 | −2.3 | −1.8 | −0.8 | −0.8 | −3.3 | −1.6 | −1.2 | −0.9 | −3.3 | −2.5 | −2.3 | −1.5 | −4.9 | −1.5 | −1.6 | −0.8 | −3.8 | −0.7 | −0.6 | −0.4 | −2.3 |
| H2 | −1.6 | −1.2 | −1.0 | −3.5 | −1.2 | −1.1 | −2.5 | −5.4 | −2.9 | −2.1 | −1.6 | −5.4 | −2.3 | −1.8 | −1.6 | −4.5 | −1.1 | −1.5 | −2.7 | −4.7 | −1.0 | −1.3 | −2.6 | −6.7 | −3.6 | −3.3 | −3.8 | −6.7 | −3.3 | −3.0 | −3.7 | −6.7 | −2.8 | −3.3 | −3.6 | −6.7 |
| H3 | −1.1 | −1.3 | −2.9 | −4.3 | −1.6 | −1.4 | −3.1 | −4.1 | −3.0 | −2.0 | −1.6 | −5.4 | −1.9 | −1.4 | −1.2 | −4.0 | −1.4 | −1.2 | −0.6 | −3.5 | −0.9 | −1.1 | −0.3 | −2.8 | −1.5 | −2.2 | −0.6 | −3.8 | −1.6 | −2.0 | −0.8 | −4.2 | −1.3 | −1.9 | −0.5 | −3.7 |
| H4 | 0.1 | 0.7 | 0.4 | 0.0 | 0.1 | 0.5 | 0.4 | 0.0 | 0.0 | 0.4 | 0.2 | −0.3 | −0.4 | −0.5 | −0.4 | −2.0 | 0.1 | 0.3 | 0.5 | 0.1 | 0.1 | 0.1 | −0.4 | −1.3 | −3.0 | −2.9 | −1.9 | −4.8 | −2.9 | −3.3 | −1.8 | −4.8 | −0.3 | −1.1 | −0.3 | −3.9 |
| H5 | −1.3 | −1.5 | −1.1 | −3.8 | −1.2 | −1.3 | −0.9 | −3.3 | −2.8 | −3.0 | −1.8 | −4.8 | −1.8 | −3.0 | −2.2 | −4.5 | 0.2 | 0.3 | −0.1 | −0.1 | −1.6 | −2.2 | −1.2 | −4.2 | −2.5 | −3.6 | −1.1 | −4.7 | −2.4 | −1.6 | −1.6 | −4.4 | −2.3 | −1.9 | −1.6 | −4.3 |
| H6 | −0.3 | −0.1 | −0.4 | −1.8 | −0.8 | −0.4 | −0.7 | −2.4 | −0.3 | −0.5 | −0.3 | −1.6 | −0.4 | −0.7 | −0.3 | −1.6 | −0.4 | −0.5 | −0.4 | −1.7 | −0.4 | −0.8 | −0.6 | −2.0 | −0.5 | −0.7 | −0.6 | −1.8 | −1.0 | −1.5 | −0.7 | −2.7 | −0.4 | −0.6 | −0.3 | −1.3 |
| H7 | −0.9 | −1.4 | −0.9 | −3.1 | −2.0 | −2.2 | −1.3 | −4.2 | −3.2 | −3.3 | −2.0 | −4.8 | −2.5 | −3.1 | −1.7 | −4.0 | −1.6 | −2.1 | −1.2 | −3.2 | −1.6 | −2.1 | −1.2 | −3.2 | −3.4 | −3.0 | −1.8 | −5.1 | −3.5 | −3.3 | −1.9 | −3.7 | −2.6 | −3.3 | −1.7 | −4.1 |
| H10 | −1.7 | −1.1 | −1.4 | −3.2 | −1.6 | −1.2 | −1.2 | −3.9 | −2.7 | −1.9 | −2.1 | −4.9 | −2.3 | −1.5 | −1.7 | −4.2 | −1.2 | −0.8 | −1.2 | −3.5 | −1.4 | −0.8 | −1.2 | −3.5 | −3.5 | −1.6 | −2.4 | −5.5 | −3.2 | −1.7 | −1.8 | −4.6 | −3.6 | −2.8 | −1.6 | −5.0 |
| H11 | −0.8 | −1.3 | −0.8 | −2.3 | −0.2 | −0.4 | −0.4 | −0.3 | −2.5 | −2.2 | −1.7 | −4.2 | −1.7 | −1.7 | −1.1 | −2.8 | −1.5 | −1.7 | −0.9 | −3.2 | −0.1 | −0.9 | −0.7 | −1.9 | −2.8 | −2.2 | −1.4 | −4.1 | 0.1 | −1.6 | −1.0 | −3.5 | −3.0 | −2.3 | −1.3 | −4.1 |
| H12 | 0.0 | −0.8 | −0.2 | −1.5 | −0.2 | −0.9 | −0.3 | −2.1 | −0.2 | −1.2 | −0.6 | −2.5 | 0.0 | −0.7 | −0.3 | −2.3 | −0.2 | −1.1 | −0.4 | −2.1 | −0.8 | −0.6 | −0.4 | −2.6 | −1.3 | −0.3 | −0.4 | −2.9 | −2.4 | −1.0 | −1.0 | −3.9 | −0.2 | −1.0 | −0.3 | −1.9 |
| MIC of Antimicrobials [µg/mL] | ||||||
|---|---|---|---|---|---|---|
| Species | Identification | Clade | PM-477 | CLI | MDZ | TDZ |
| G. vaginalis | ATCC 14018T | I | 1 | 0.25 | 8 | 128 |
| G. vaginalis | UGent 09.07 | I | 2 | 0.25 | >128 | >128 |
| G. vaginalis | UGent 09.01 | I | 0.25 | 0.13 | 8 | 4 |
| G. vaginalis | UGent 25.49 | I | 0.13 | <0.06 | 8 | 4 |
| G. vaginalis | BV50.1 | n.d. | 8 | 0.25 | 32 | 64 |
| G. vaginalis | BV111.5.1 | n.d. | 2 | 0.125 | 8 | 4 |
| G. vaginalis | FB049-01 | n.d. | 4 | 0.5 | 16 | 8 |
| G. vaginalis | FB061-03 | n.d. | 8 | 0.25 | 8 | 4 |
| G. leopoldii | UGent 09.48 | IV | 4 | 0.5 | 128 | 128 |
| G. leopoldii | BV13.2 | n.d. | 4 | 0.5 | >128 | >128 |
| G. leopoldii | BV86.5 | n.d. | 1 | 0.25 | >128 | >128 |
| G. piotii | UGent 18.01T | II | 8 | 0.5 | 32 | 64 |
| G. piotii | UGent 21.28 | II | 2 | 0.25 | 64 | >128 |
| G. piotii | P80275 | n.d. | 1 | 0.5 | 16 | 32 |
| G. piotii | FB041 | n.d. | 4 | 1 | 32 | 64 |
| G. piotii | VMF1800 SVT21 | n.d. | 8 | 1 | 32 | 64 |
| G. swidsinskii | GS 10234 | IV | 0.5 | 0.25 | 64 | >128 |
| G. swidsinskii | GS 9838-1T | IV | 0.25 | <0.06 | >128 | >128 |
| G. swidsinskii | BV7.1 | n.d. | 1 | 0.5 | >128 | 128 |
| G. swidsinskii | BV112.4 | n.d. | 1 | 0.06 | 64 | 64 |
| L. crispatus | DSM 20584 | >128 | 4 | >128 | >128 | |
| L. gasseri | DSM 20077 | >128 | 64 | >128 | >128 | |
| L. gasseri | DSM 20243 | >128 | 32 | >128 | >128 | |
| L. jensenii | PB2003-073-T2-2 | >128 | 0.25 | >128 | >128 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landlinger, C.; Tisakova, L.; Oberbauer, V.; Schwebs, T.; Muhammad, A.; Latka, A.; Van Simaey, L.; Vaneechoutte, M.; Guschin, A.; Resch, G.; et al. Engineered Phage Endolysin Eliminates Gardnerella Biofilm without Damaging Beneficial Bacteria in Bacterial Vaginosis Ex Vivo. Pathogens 2021, 10, 54. https://doi.org/10.3390/pathogens10010054
Landlinger C, Tisakova L, Oberbauer V, Schwebs T, Muhammad A, Latka A, Van Simaey L, Vaneechoutte M, Guschin A, Resch G, et al. Engineered Phage Endolysin Eliminates Gardnerella Biofilm without Damaging Beneficial Bacteria in Bacterial Vaginosis Ex Vivo. Pathogens. 2021; 10(1):54. https://doi.org/10.3390/pathogens10010054
Chicago/Turabian StyleLandlinger, Christine, Lenka Tisakova, Vera Oberbauer, Timo Schwebs, Abbas Muhammad, Agnieszka Latka, Leen Van Simaey, Mario Vaneechoutte, Alexander Guschin, Gregory Resch, and et al. 2021. "Engineered Phage Endolysin Eliminates Gardnerella Biofilm without Damaging Beneficial Bacteria in Bacterial Vaginosis Ex Vivo" Pathogens 10, no. 1: 54. https://doi.org/10.3390/pathogens10010054
APA StyleLandlinger, C., Tisakova, L., Oberbauer, V., Schwebs, T., Muhammad, A., Latka, A., Van Simaey, L., Vaneechoutte, M., Guschin, A., Resch, G., Swidsinski, S., Swidsinski, A., & Corsini, L. (2021). Engineered Phage Endolysin Eliminates Gardnerella Biofilm without Damaging Beneficial Bacteria in Bacterial Vaginosis Ex Vivo. Pathogens, 10(1), 54. https://doi.org/10.3390/pathogens10010054








