Rifabutin for the Treatment of Helicobacter pylori Infection: A Review
Abstract
1. Introduction
2. Bibliographic Search and Statistical Analysis
3. Rifabutin General Antimicrobial Activity and Mechanism of Action
4. Pharmacokinetics and Pharmacodinamics of Rifabutin
5. Resistance of H. pylori to Rifabutin
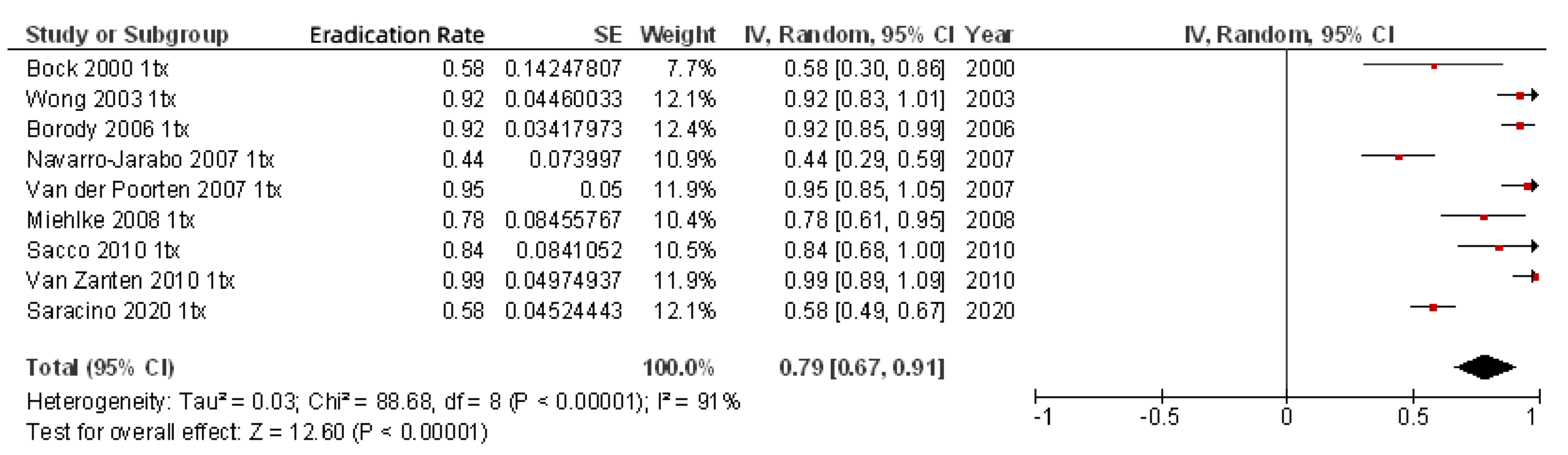
6. Efficacy of Rifabutin for H. pylori Eradication
7. How to Optimize Rifabutin-Based Treatments for H. pylori
7.1. Rifabutin Dose and Frequency
7.2. Duration of Treatment
7.3. Type and Dose of Antisecretory Drug
7.4. Addition of Bismuth
7.5. All-in-One Single Capsule Including Omeprazole, Amoxicillin, and Rifabutin
8. Tolerability of Rifabutin
9. Limitations of Rifabutin Treatment
10. Conclusions
Funding
Conflicts of Interest
Abbreviations
| Helicobacter pylori | H. pylori |
| proton pump inhibitor | PPI |
References and Notes
- Crowe, S.E. Helicobacter pylori Infection. N. Engl. J. Med. 2019, 380, 1158–1165. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Calvet, X. Review article: The effectiveness of standard triple therapy for Helicobacter pylori has not changed over the last decade, but it is not good enough. Aliment. Pharmacol. Ther. 2011, 34, 1255–1268. [Google Scholar] [CrossRef]
- Megraud, F.; Coenen, S.; Versporten, A.; Kist, M.; Lopez-Brea, M.; Hirschl, A.M.; Andersen, L.P.; Goossens, H.; Glupczynski, Y. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 2013, 62, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Pajares, J.M. Review article: Helicobacter pylori “rescue” regimen when proton pump inhibitor-based triple therapies fail. Aliment Pharmacol. Ther. 2002, 16, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P. “Rescue” regimens after Helicobacter pylori treatment failure. World J. Gastroenterol. 2008, 14, 5385–5402. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Molina-Infante, J.; Amador, J.; Bermejo, F.; Bujanda, L.; Calvet, X. IV Spanish Consensus Conference on Helicobacter pylori infection treatment. Gastroenterol. Hepatol. 2016, 39, 697–721. [Google Scholar] [CrossRef]
- Fallone, C.A.; Chiba, N.; van Zanten, S.V.; Fischbach, L.; Gisbert, J.P.; Hunt, R.H.; Jones, N.L.; Render, C.; Leontiadis, G.I.; Moayyedi, P.; et al. The Toronto Consensus for the Treatment of Helicobacter pylori Infection in Adults. Gastroenterology 2016, 151, 51–69. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 2017, 112, 212–239. [Google Scholar] [CrossRef]
- Nyssen, O.P.; Bordin, D.; Tepes, B.; Perez-Aisa, A.; Vaira, D.; Caldas, M.; Bujanda, L.; Castro-Fernandez, M.; Lerang, F.; Leja, M.; et al. European Registry on Helicobacter pylori management (Hp-EuReg): Patterns and trends in first-line empirical eradication prescription and outcomes of 5 years and 21 533 patients. Gut 2020, 70, 40–54. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Morena, F. Systematic review and meta-analysis: Levofloxacin-based rescue regimens after Helicobacter pylori treatment failure. Aliment. Pharmacol. Ther. 2006, 23, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Bermejo, F.; Castro-Fernandez, M.; Perez-Aisa, A.; Fernandez-Bermejo, M.; Tomas, A.; Barrio, J.; Bory, F.; Almela, P.; Sanchez-Pobre, P.; et al. Second-line rescue therapy with levofloxacin after H. pylori treatment failure: A Spanish multicenter study of 300 patients. Am. J. Gastroenterol. 2008, 103, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Castro-Fernandez, M.; Bermejo, F.; Perez-Aisa, A.; Ducons, J.; Fernandez-Bermejo, M.; Bory, F.; Cosme, A.; Benito, L.M.; Lopez-Rivas, L.; et al. Third-line rescue therapy with levofloxacin after two H. pylori treatment failures. Am. J. Gastroenterol. 2006, 101, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P. Empirical or susceptibility-guided treatment for Helicobacter pylori infection? A comprehensive review. Therap. Adv. Gastroenterol. 2020, 13, 1–16. [Google Scholar] [CrossRef]
- Megraud, F.; Lamouliatte, H. The treatment of refractory Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2003, 17, 1333–1343, Review article. [Google Scholar] [CrossRef]
- Megraud, F. Basis for the management of drug-resistant Helicobacter pylori infection. Drugs 2004, 64, 1893–1904. [Google Scholar] [CrossRef]
- Maddix, D.S.; Tallian, K.B.; Mead, P.S. Rifabutin: A review with emphasis on its role in the prevention of disseminated Mycobacterium avium complex infection. Ann. Pharmacother. 1994, 28, 1250–1254. [Google Scholar] [CrossRef]
- Rothstein, D.M. Rifamycins, Alone and in Combination. Cold Spring Harb. Perspect. Med. 2016, 6. [Google Scholar] [CrossRef]
- Kuo, C.J.; Lin, C.Y.; Le, P.H.; Chang, P.Y.; Lai, C.H.; Lin, W.R.; Chang, M.L.; Hsu, J.T.; Cheng, H.T.; Tseng, C.N.; et al. Rescue therapy with rifabutin regimen for refractory Helicobacter pylori infection with dual drug-resistant strains. BMC Gastroenterol. 2020, 20, 218. [Google Scholar] [CrossRef]
- Fiorini, G.; Zullo, A.; Vakil, N.; Saracino, I.M.; Ricci, C.; Castelli, V.; Gatta, L.; Vaira, D. Rifabutin Triple Therapy is Effective in Patients With Multidrug-resistant Strains of Helicobacter pylori. J. Clin. Gastroenterol. 2018, 52, 137–140. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Calvet, X. Review article: Rifabutin in the treatment of refractory Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2012, 35, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Kunin, C.M. Antimicrobial activity of rifabutin. Clin. Infect. Dis 1996, 22 (Suppl. 1), S3–S13. [Google Scholar] [CrossRef]
- Brogden, R.N.; Fitton, A. Rifabutin. A review of its antimicrobial activity, pharmacokinetic properties and therapeutic efficacy. Drugs 1994, 47, 983–1009. [Google Scholar] [CrossRef] [PubMed]
- Heep, M.; Beck, D.; Bayerdorffer, E.; Lehn, N. Rifampin and rifabutin resistance mechanism in Helicobacter pylori. Antimicrob. Agents Chemother. 1999, 43, 1497–1499. [Google Scholar] [CrossRef] [PubMed]
- Hays, C.; Burucoa, C.; Lehours, P.; Tran, C.T.; Leleu, A.; Raymond, J. Molecular characterization of Helicobacter pylori resistance to rifamycins. Helicobacter 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Heep, M.; Rieger, U.; Beck, D.; Lehn, N. Mutations in the beginning of the rpoB gene can induce resistance to rifamycins in both Helicobacter pylori and Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2000, 44, 1075–1077. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, T.; Suzuki, H.; Matsuzaki, J.; Muraoka, H.; Tsugawa, H.; Hirata, K.; Hibi, T. Helicobacter pylori resistance to rifabutin in the last 7 years. Antimicrob. Agents Chemother. 2011, 55, 5374–5375. [Google Scholar] [CrossRef]
- Suzuki, S.; Suzuki, H.; Nishizawa, T.; Kaneko, F.; Ootani, S.; Muraoka, H.; Saito, Y.; Kobayashi, I.; Hibi, T. Past rifampicin dosing determines rifabutin resistance of Helicobacter pylori. Digestion 2009, 79, 1–4. [Google Scholar] [CrossRef]
- Akada, J.K.; Shirai, M.; Fujii, K.; Okita, K.; Nakazawa, T. In vitro anti-Helicobacter pylori activities of new rifamycin derivatives, KRM-1648 and KRM-1657. Antimicrob. Agents Chemother. 1999, 43, 1072–1076. [Google Scholar] [CrossRef][Green Version]
- Rossi, G. An update on the antibiotic therapy of tuberculosis. Recenti. Prog. Med. 1999, 90, 241–243. [Google Scholar]
- Koudriakova, T.; Iatsimirskaia, E.; Tulebaev, S.; Spetie, D.; Utkin, I.; Mullet, D.; Thompson, T.; Vouros, P.; Gerber, N. In vivo disposition and metabolism by liver and enterocyte microsomes of the antitubercular drug rifabutin in rats. J. Pharmacol. Exp. Ther. 1996, 279, 1300–1309. [Google Scholar] [PubMed]
- Blaschke, T.F.; Skinner, M.H. The clinical pharmacokinetics of rifabutin. Clin. Infect. Dis 1996, 22, S15–S21. [Google Scholar] [CrossRef] [PubMed]
- Iatsimirskaia, E.; Tulebaev, S.; Storozhuk, E.; Utkin, I.; Smith, D.; Gerber, N.; Koudriakova, T. Metabolism of rifabutin in human enterocyte and liver microsomes: Kinetic parameters, identification of enzyme systems, and drug interactions with macrolides and antifungal agents. Clin. Pharmacol. Ther. 1997, 61, 554–562. [Google Scholar] [CrossRef]
- Piccolomini, R.; Di Bonaventura, G.; Picciani, C.; Laterza, F.; Vecchiet, J.; Neri, M. In vitro activity of clarithromycin against intracellular Helicobacter pylori. Antimicrob. Agents Chemother. 2001, 45, 1568–1571. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Jabes, D.; Della Bruna, C. In vitro Activity of Rifabutin, a Potential Antibiotic in the Therapy of Helicobacter Pylori, the 6th International Congress of Infectious Diseases (ICID). Prague, Czech Republic, 1994; Volume 69. [Google Scholar]
- Perri, F.; Festa, V.; Clemente, R.; Villani, M.R.; Quitadamo, M.; Caruso, N.; Bergoli, M.L.; Andriulli, A. Randomized study of two “rescue” therapies for Helicobacter pylori-infected patients after failure of standard triple therapies. Am. J. Gastroenterol. 2001, 96, 58–62. [Google Scholar] [PubMed]
- Heep, M.; Lehn, N.; Brandstatter, B.; Rieger, U.; Senzenberger, S.; Wehrl, W. Detection of rifabutin resistance and association of rpoB mutations with resistance to four rifamycin derivatives in Helicobacter pylori. Eur. J. Clin. Microbiol. Infect. Dis. 2002, 21, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Glocker, E.; Bogdan, C.; Kist, M. Characterization of rifampicin-resistant clinical Helicobacter pylori isolates from Germany. J. Antimicrob. Chemother. 2007, 59, 874–879. [Google Scholar] [CrossRef]
- Van der Poorten, D.; Katelaris, P.H. The effectiveness of rifabutin triple therapy for patients with difficult-to-eradicate Helicobacter pylori in clinical practice. Aliment. Pharmacol. Ther. 2007, 26, 1537–1542. [Google Scholar] [CrossRef]
- Graham, D.Y. Antibiotic resistance in Helicobacter pylori: Implications for therapy. Gastroenterology 1998, 115, 1272–1277. [Google Scholar] [CrossRef]
- Toracchio, S.; Capodicasa, S.; Soraja, D.B.; Cellini, L.; Marzio, L. Rifabutin based triple therapy for eradication of H. pylori primary and secondary resistant to tinidazole and clarithromycin. Dig. Liver Dis. 2005, 37, 33–38. [Google Scholar] [CrossRef]
- Matsuoka, M.; Yoshida, Y.; Hayakawa, K.; Fukuchi, S.; Sugano, K. Simultaneous colonisation of Helicobacter pylori with and without mutations in the 23S rRNA gene in patients with no history of clarithromycin exposure. Gut 1999, 45, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Kim, J.G.; Kwon, D.H. Mixed-infection of antibiotic susceptible and resistant Helicobacter pylori isolates in a single patient and underestimation of antimicrobial susceptibility testing. Helicobacter 2003, 8, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Cellini, L.; Grande, R.; Di Campli, E.; Di Bartolomeo, S.; Capodicasa, S.; Marzio, L. Analysis of genetic variability, antimicrobial susceptibility and virulence markers in Helicobacter pylori identified in Central Italy. Scand. J. Gastroenterol. 2006, 41, 280–287. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, V.; Giorgio, F.; Hassan, C.; Manes, G.; Vannella, L.; Panella, C.; Ierardi, E.; Zullo, A. Worldwide H. pylori antibiotic resistance: A systematic review. J. Gastrointestin. Liver Dis. 2010, 19, 409–414. [Google Scholar]
- Ghotaslou, R.; Leylabadlo, H.E.; Asl, Y.M. Prevalence of antibiotic resistance in Helicobacter pylori: A recent literature review. World J. Methodol. 2015, 5, 164–174. [Google Scholar] [CrossRef]
- Miehlke, S.; Schneider-Brachert, W.; Kirsch, C.; Morgner, A.; Madisch, A.; Kuhlisch, E.; Haferland, C.; Bastlein, E.; Jebens, C.; Zekorn, C.; et al. One-week once-daily triple therapy with esomeprazole, moxifloxacin, and rifabutin for eradication of persistent Helicobacter pylori resistant to both metronidazole and clarithromycin. Helicobacter 2008, 13, 69–74. [Google Scholar] [CrossRef]
- Bock, H.; Koop, H.; Lehn, N.; Heep, M. Rifabutin-based triple therapy after failure of Helicobacter pylori eradication treatment: Preliminary experience. J. Clin. Gastroenterol. 2000, 31, 222–225. [Google Scholar] [CrossRef]
- Pilotto, A.; Franceschi, M.; Rassu, M.; Furlan, F.; Scagnelli, M. In vitro activity of rifabutin against strains of Helicobacter pylori resistant to metronidazole and clarithromycin. Am. J. Gastroenterol. 2000, 95, 833–834. [Google Scholar] [CrossRef]
- Marzio, L.; Coraggio, D.; Capodicasa, S.; Grossi, L.; Cappello, G. Role of the preliminary susceptibility testing for initial and after failed therapy of Helicobacter pylori infection with levofloxacin, amoxicillin, and esomeprazole. Helicobacter 2006, 11, 237–242. [Google Scholar] [CrossRef]
- Miehlke, S.; Hansky, K.; Schneider-Brachert, W.; Kirsch, C.; Morgner, A.; Madisch, A.; Kuhlisch, E.; Bastlein, E.; Jacobs, E.; Bayerdorffer, E.; et al. Randomized trial of rifabutin-based triple therapy and high-dose dual therapy for rescue treatment of Helicobacter pylori resistant to both metronidazole and clarithromycin. Aliment. Pharmacol. Ther. 2006, 24, 395–403. [Google Scholar] [CrossRef]
- Borody, T.J.; Pang, G.; Wettstein, A.R.; Clancy, R.; Herdman, K.; Surace, R.; Llorente, R.; Ng, C. Efficacy and safety of rifabutin-containing ‘rescue therapy’ for resistant Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2006, 23, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, S.A.; Owen, R.J. Frequency and molecular characteristics of ciprofloxacin- and rifampicin-resistant Helicobacter pylori from gastric infections in the UK. J. Med. Microbiol. 2009, 58, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.L.; Navaratnam, P. High Helicobacter pylori resistance to metronidazole but zero or low resistance to clarithromycin, levofloxacin, and other antibiotics in Malaysia. Helicobacter 2011, 16, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Marzio, L.; Cellini, L.; Amitrano, M.; Grande, R.; Serio, M.; Cappello, G.; Grossi, L. Helicobacter pylori isolates from proximal and distal stomach of patients never treated and already treated show genetic variability and discordant antibiotic resistance. Eur. J. Gastroenterol. Hepatol. 2011, 23, 467–472. [Google Scholar] [CrossRef] [PubMed]
- McNulty, C.A.; Lasseter, G.; Shaw, I.; Nichols, T.; D’Arcy, S.; Lawson, A.J.; Glocker, E. Is Helicobacter pylori antibiotic resistance surveillance needed and how can it be delivered? Aliment. Pharmacol. Ther. 2012, 35, 1221–1230. [Google Scholar] [CrossRef]
- Tay, C.Y.; Windsor, H.M.; Thirriot, F.; Lu, W.; Conway, C.; Perkins, T.T.; Marshall, B.J. Helicobacter pylori eradication in Western Australia using novel quadruple therapy combinations. Aliment. Pharmacol. Ther. 2012, 36, 1076–1083. [Google Scholar] [CrossRef]
- O’Connor, A.; Taneike, I.; Nami, A.; Fitzgerald, N.; Ryan, B.; Breslin, N.; O’Connor, H.; McNamara, D.; Murphy, P.; O’Morain, C. Helicobacter pylori resistance rates for levofloxacin, tetracycline and rifabutin among Irish isolates at a reference centre. Ir. J. Med. Sci. 2013, 182, 693–695. [Google Scholar] [CrossRef]
- Larsen, A.L.; Ragnhildstveit, E.; Moayeri, B.; Eliassen, L.; Melby, K.K. Resistance rates of metronidazole and other antibacterials in Helicobacter pylori from previously untreated patients in Norway. APMIS 2013, 121, 353–358. [Google Scholar] [CrossRef]
- Selgrad, M.; Meissle, J.; Bornschein, J.; Kandulski, A.; Langner, C.; Varbanova, M.; Wex, T.; Tammer, I.; Schluter, D.; Malfertheiner, P. Antibiotic susceptibility of Helicobacter pylori in central Germany and its relationship with the number of eradication therapies. Eur. J. Gastroenterol. Hepatol. 2013, 25, 1257–1260. [Google Scholar] [CrossRef]
- Seo, J.H.; Jun, J.S.; Yeom, J.S.; Park, J.S.; Youn, H.S.; Ko, G.H.; Baik, S.C.; Lee, W.K.; Cho, M.J.; Rhee, K.H. Changing pattern of antibiotic resistance of Helicobacter pylori in children during 20 years in Jinju, South Korea. Pediatr. Int. 2013, 55, 332–336. [Google Scholar] [CrossRef]
- Gosciniak, G.; Biernat, M.; Grabinska, J.; Binkowska, A.; Poniewierka, E.; Iwanczak, B. The antimicrobial susceptibility of Helicobacter pylori strains isolated from children and adults with primary infection in the Lower Silesia Region, Poland. Pol. J. Microbiol. 2014, 63, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Biernat, M.M.; Poniewierka, E.; Blaszczuk, J.; Czapla, L.; Kempinski, R.; Ksiadzyna, D.; Grabinska, J.; Binkowska, A.; Megraud, F.; Gosciniak, G. Antimicrobial susceptibility of Helicobacter pylori isolates from Lower Silesia, Poland. Arch. Med. Sci. 2014, 10, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Selgrad, M.; Tammer, I.; Langner, C.; Bornschein, J.; Meissle, J.; Kandulski, A.; Varbanova, M.; Wex, T.; Schluter, D.; Malfertheiner, P. Different antibiotic susceptibility between antrum and corpus of the stomach, a possible reason for treatment failure of Helicobacter pylori infection. World J. Gastroenterol. 2014, 20, 16245–16251. [Google Scholar] [CrossRef] [PubMed]
- John, A.; Al Kaabi, S.; Doiphode, S.; Chandra, P.; Sharma, M.; Babu, R.; Yacoub, R.; Derbala, M. Does emerging Clarithromycin resistance signal an obituary to empirical standard triple therapy for Helicobacter pylori infection? Indian J. Gastroenterol. 2015, 34, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, M.; Di Campli, E.; Di Bartolomeo, S.; Cataldi, V.; Marzio, L.; Grossi, L.; Ciccaglione, A.F.; Nostro, A.; Cellini, L. In vitro antimicrobial susceptibility of Helicobacter pylori to nine antibiotics currently used in Central Italy. Scand. J. Gastroenterol. 2016, 51, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Suzuki, H.; Matsuzaki, J.; Tsugawa, H.; Fukuhara, S.; Miyoshi, S.; Hirata, K.; Seino, T.; Matsushita, M.; Nishizawa, T.; et al. Rifabutin-based 10-day and 14-day triple therapy as a third-line and fourth-line regimen for Helicobacter pylori eradication: A pilot study. United Eur. Gastroenterol. J. 2016, 4, 380–387. [Google Scholar] [CrossRef]
- Ciccaglione, A.F.; Tavani, R.; Grossi, L.; Cellini, L.; Manzoli, L.; Marzio, L. Rifabutin Containing Triple Therapy and Rifabutin with Bismuth Containing Quadruple Therapy for Third-Line Treatment of Helicobacter pylori Infection: Two Pilot Studies. Helicobacter 2016, 21, 375–381. [Google Scholar] [CrossRef]
- Sung, J.; Kim, N.; Park, Y.H.; Hwang, Y.J.; Kwon, S.; Na, G.; Choi, J.Y.; Kang, J.B.; Kim, H.R.; Kim, J.W.; et al. Rifabutin-based Fourth and Fifth-line Rescue Therapy in Patients with for Helicobacter pylori Eradication Failure. Korean J. Gastroenterol. 2017, 69, 109–118. [Google Scholar] [CrossRef][Green Version]
- Siavoshi, F.; Saniee, P.; Malekzadeh, R. Effective antimicrobial activity of rifabutin against multidrug-resistant Helicobacter pylori. Helicobacter 2018, 23, e12531. [Google Scholar] [CrossRef]
- Choi, Y.I.; Jeong, S.H.; Chung, J.W.; Park, D.K.; Kim, K.O.; Kwon, K.A.; Kim, Y.J.; So, S.; Lee, J.H.; Jeong, J.Y.; et al. Rifabutin and Furazolidone Could Be the Candidates of the Rescue Regimen for Antibiotic-Resistant H. pylori in Korea. Can. J. Infect. Dis. Med. Microbiol. 2019, 2019, 9351801. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, N.; Nam, R.H.; In Choi, S.; Lee, J.W.; Lee, D.H. Primary and secondary antibiotic resistance of Helicobacter pylori in Korea from 2003 to 2018. Helicobacter 2019, 24, e12660. [Google Scholar] [CrossRef] [PubMed]
- Kouitcheu Mabeku, L.B.; Eyoum Bille, B.; Tepap Zemnou, C.; Tali Nguefack, L.D.; Leundji, H. Broad spectrum resistance in Helicobacter pylori isolated from gastric biopsies of patients with dyspepsia in Cameroon and efflux-mediated multiresistance detection in MDR isolates. BMC Infect. Dis. 2019, 19, 880. [Google Scholar] [CrossRef] [PubMed]
- Miftahussurur, M.; Cruz, M.; Doohan, D.; Subsomwong, P.; Abreu, J.A.J.; Hosking, C.; Waskito, L.A.; Yamaoka, Y. Five alternative Helicobacter pylori antibiotics to counter high levofloxacin and metronidazole resistance in the Dominican Republic. PLoS ONE 2019, 14, e0213868. [Google Scholar] [CrossRef] [PubMed]
- Miftahussurur, M.; Waskito, L.A.; Syam, A.F.; Nusi, I.A.; Siregar, G.; Richardo, M.; Bakry, A.F.; Rezkitha, Y.A.A.; Wibawa, I.D.N.; Yamaoka, Y. Alternative eradication regimens for Helicobacter pylori infection in Indonesian regions with high metronidazole and levofloxacin resistance. Infect. Drug Resist. 2019, 12, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Miftahussurur, M.; Aftab, H.; Shrestha, P.K.; Sharma, R.P.; Subsomwong, P.; Waskito, L.A.; Doohan, D.; Fauzia, K.A.; Yamaoka, Y. Effective therapeutic regimens in two South Asian countries with high resistance to major Helicobacter pylori antibiotics. Antimicrob Resist. Infect. Control. 2019, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.Y.; Canaan, Y.; Maher, J.; Wiener, G.; Hulten, K.G.; Kalfus, I.N. Rifabutin-Based Triple Therapy (RHB-105) for Helicobacter pylori Eradication: A Double-Blind, Randomized, Controlled Trial. Ann. Intern. Med. 2020, 172, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Yamada, A.; Niikura, R.; Shichijo, S.; Hayakawa, Y.; Koike, K. Efficacy and safety of a new rifabutin-based triple therapy with vonoprazan for refractory Helicobacter pylori infection: A prospective single-arm study. Helicobacter 2020, 25, e12719. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; Lv, Z.; Wang, Y.; Wang, B.; Xie, Y.; Zhou, X.; Lv, N. Rescue Therapy with a Proton Pump Inhibitor Plus Amoxicillin and Rifabutin for Helicobacter pylori Infection: A Systematic Review and Meta-Analysis. Gastroenterol. Res. Pract. 2015, 2015, 415648. [Google Scholar] [CrossRef]
- Gingold-Belfer, R.; Niv, Y.; Levi, Z.; Boltin, D. Rifabutin triple therapy for first-line and rescue treatment of Helicobacter pylori infection: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef]
- Perri, F.; Festa, V.; Andriulli, A. Treatment of antibiotic-resistant Helicobacter pylori. N. Engl. J. Med. 1998, 339, 53. [Google Scholar] [CrossRef]
- Perri, F.; Festa, V.; Clemente, R.; Quitadamo, M.; Andriulli, A. Rifabutin-based ‘rescue therapy’ for Helicobacter pylori infected patients after failure of standard regimens. Aliment. Pharmacol. Ther. 2000, 14, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Beales, I.L. Efficacy of Helicobacter pylori eradication therapies: A single centre observational study. BMC Gastroenterol. 2001, 1, 7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Canducci, F.; Ojetti, V.; Pola, P.; Gasbarrini, G.; Gasbarrini, A. Rifabutin-based Helicobacter pylori eradication ‘rescue therapy’. Aliment. Pharmacol. Ther. 2001, 15, 143. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Calvet, X.; Bujanda, L.; Marcos, S.; Gisbert, J.L.; Pajares, J.M. ‘Rescue’ therapy with rifabutin after multiple Helicobacter pylori treatment failures. Helicobacter 2003, 8, 90–94. [Google Scholar] [CrossRef]
- Wong, W.M.; Gu, Q.; Lam, S.K.; Fung, F.M.; Lai, K.C.; Hu, W.H.; Yee, Y.K.; Chan, C.K.; Xia, H.H.; Yuen, M.F.; et al. Randomized controlled study of rabeprazole, levofloxacin and rifabutin triple therapy vs. quadruple therapy as second-line treatment for Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2003, 17, 553–560. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Gisbert, J.L.; Marcos, S.; Pajares, J.M. Empirical Helicobacter pylori “rescue” therapy after failure of two eradication treatments. Dig. Liver Dis. 2004, 36, 7–12. [Google Scholar] [CrossRef]
- Qasim, A.; Sebastian, S.; Thornton, O.; Dobson, M.; McLoughlin, R.; Buckley, M.; O′Connor, H.; O′Morain, C. Rifabutin- and furazolidone-based Helicobacter pylori eradication therapies after failure of standard first- and second-line eradication attempts in dyspepsia patients. Aliment. Pharmacol. Ther. 2005, 21, 91–96. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Gisbert, J.L.; Marcos, S.; Moreno-Otero, R.; Pajares, J.M. Third-line rescue therapy with levofloxacin is more effective than rifabutin rescue regimen after two Helicobacter pylori treatment failures. Aliment. Pharmacol. Ther. 2006, 24, 1469–1474. [Google Scholar] [CrossRef]
- Gonzalez Carro, P.; Perez Roldan, F.; De Pedro Esteban, A.; Legaz Huidobro, M.L.; Soto Fernandez, S.; Roncero Garcia Escribano, O.; Esteban Lopez-Jamar, J.M.; Pedraza Martin, C.; Ruiz Carrillo, F. Efficacy of rifabutin-based triple therapy in Helicobacter pylori infected patients after two standard treatments. J. Gastroenterol. Hepatol. 2007, 22, 60–63. [Google Scholar] [CrossRef]
- Navarro-Jarabo, J.M.; Fernandez, N.; Sousa, F.L.; Cabrera, E.; Castro, M.; Ramirez, L.M.; Rivera, R.; Ubina, E.; Vera, F.; Mendez, I.; et al. Efficacy of rifabutin-based triple therapy as second-line treatment to eradicate helicobacter pylori infection. BMC Gastroenterol. 2007, 7, 31. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Gisbert, J.L.; Marcos, S.; Jimenez-Alonso, I.; Moreno-Otero, R.; Pajares, J.M. Empirical rescue therapy after Helicobacter pylori treatment failure: A 10-year single-centre study of 500 patients. Aliment. Pharmacol. Ther. 2008, 27, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Sacco, F.; Spezzaferro, M.; Amitrano, M.; Grossi, L.; Manzoli, L.; Marzio, L. Efficacy of four different moxifloxacin-based triple therapies for first-line H. pylori treatment. Dig. Liver Dis. 2010, 42, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Van Zanten, S.V.; Desai, S.; Best, L.; Cooper-Lesins, G.; Malatjalian, D.; Haldane, D.; Peltekian, K. Rescue therapy using a rifabutin-based regimen is effective for cure of Helicobacter pylori infection. Can. J. Gastroenterol. 2010, 24, 303–306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zullo, A.; De Francesco, V.; Manes, G.; Scaccianoce, G.; Cristofari, F.; Hassan, C. Second-line and rescue therapies for Helicobacter pylori eradication in clinical practice. J. Gastrointestin. Liver Dis. 2010, 19, 131–134. [Google Scholar]
- Gisbert, J.P.; Castro-Fernandez, M.; Perez-Aisa, A.; Cosme, A.; Molina-Infante, J.; Rodrigo, L.; Modolell, I.; Cabriada, J.L.; Gisbert, J.L.; Lamas, E.; et al. Fourth-line rescue therapy with rifabutin in patients with three Helicobacter pylori eradication failures. Aliment. Pharmacol. Ther. 2012, 35, 941–947. [Google Scholar] [CrossRef]
- Jeong, M.H.; Chung, J.W.; Lee, S.J.; Ha, M.; Jeong, S.H.; Na, S.; Na, B.S.; Park, S.K.; Kim, Y.J.; Kwon, K.A.; et al. Comparison of rifabutin- and levofloxacin-based third-line rescue therapies for Helicobacter pylori. Korean J. Gastroenterol. 2012, 59, 401–406. [Google Scholar] [CrossRef][Green Version]
- Lim, H.C.; Lee, Y.J.; An, B.; Lee, S.W.; Lee, Y.C.; Moon, B.S. Rifabutin-based high-dose proton-pump inhibitor and amoxicillin triple regimen as the rescue treatment for Helicobacter pylori. Helicobacter 2014, 19, 455–461. [Google Scholar] [CrossRef][Green Version]
- Fiorini, G.; Vakil, N.; Zullo, A.; Saracino, I.M.; Castelli, V.; Ricci, C.; Zaccaro, C.; Gatta, L.; Vaira, D. Culture-based selection therapy for patients who did not respond to previous treatment for Helicobacter pylori infection. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2013, 11, 507–510. [Google Scholar] [CrossRef]
- Ierardi, E.; Giangaspero, A.; Losurdo, G.; Giorgio, F.; Amoruso, A.; De Francesco, V.; Di Leo, A.; Principi, M. Quadruple rescue therapy after first and second line failure for Helicobacter pylori treatment: Comparison between two tetracycline-based regimens. J. Gastrointestin. Liver Dis. 2014, 23, 367–370. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Barrio, J.; Modolell, I.; Molina-Infante, J.; Aisa, A.P.; Castro-Fernandez, M.; Rodrigo, L.; Cosme, A.; Gisbert, J.L.; Fernandez-Bermejo, M.; et al. Helicobacter pylori first-line and rescue treatments in the presence of penicillin allergy. Dig. Dis. Sci. 2015, 60, 458–464. [Google Scholar] [CrossRef]
- Cosme, A.; Montes, M.; Ibarra, B.; Tamayo, E.; Alonso, H.; Mendarte, U.; Lizasoan, J.; Herreros-Villanueva, M.; Bujanda, L. Antimicrobial susceptibility testing before first-line treatment for Helicobacter pylori infection in patients with dual or triple antibiotic resistance. World J. Gastroenterol. 2017, 23, 3367–3373. [Google Scholar] [CrossRef] [PubMed]
- Papastergiou, V.; Mathou, N.; Licousi, S.; Evgenidi, A.; Paraskeva, K.D.; Giannakopoulos, A.; Stavrou, P.Z.; Platsouka, E.; Karagiannis, J.A. Seven-day genotypic resistance-guided triple Helicobacter pylori eradication therapy can be highly effective. Ann. Gastroenterol. 2018, 31, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Cosme, A.; Torrente Iranzo, S.; Montes Ros, M.; Fernandez-Reyes Silvestre, M.; Alonso Galan, H.; Lizasoain, J.; Bujanda, L. Helicobacter pylori antimicrobial resistance during a 5-year period (2013-2017) in northern Spain and its relationship with the eradication therapies. Helicobacter 2019, 24, e12557. [Google Scholar] [CrossRef] [PubMed]
- Ribaldone, D.G.; Fagoonee, S.; Astegiano, M.; Durazzo, M.; Morgando, A.; Sprujevnik, T.; Giordanino, C.; Baronio, M.; De Angelis, C.; Saracco, G.M.; et al. Rifabutin-Based Rescue Therapy for Helicobacter pylori Eradication: A Long-Term Prospective Study in a Large Cohort of Difficult-to-Treat Patients. J. Clin. Med. 2019, 8, 199. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Santamaría, D.; McNicholl, A.; Gisbert, J.P. Empirical Helicobacter pylori rescue therapy: An 18-year singlecentre study of 1200 patients. GastroHep 2019, 1, 311–324. [Google Scholar] [CrossRef]
- Kalfus, I.N.; Graham, D.Y.; Riff, D.S.; Panas, R.M. Rifabutin-Containing Triple Therapy (RHB-105) for Eradication of Helicobacter pylori: Randomized ERADICATE Hp Trial. Antibiotics 2020, 9. [Google Scholar] [CrossRef]
- Saracino, I.M.; Pavoni, M.; Zullo, A.; Fiorini, G.; Saccomanno, L.; Lazzarotto, T.; Antonelli, G.; Cavallo, R.; Borghi, C.; Vaira, D. Rifabutin-Based Triple Therapy Or Bismuth-Based Quadruple Regimen As Rescue Therapies For Helicobacter pylori Infection. Eur. J. Intern. Med. 2020. [Google Scholar] [CrossRef]
- Song, M.; Ang, T.L. Second and third line treatment options for Helicobacter pylori eradication. World J. Gastroenterol. 2014, 20, 1517–1528. [Google Scholar] [CrossRef]
- Boyanova, L.; Markovska, R.; Hadzhiyski, P.; Kandilarov, N.; Mitov, I. Rifamycin use for treatment of Helicobacter pylori infection: A review of recent data. Future Microbiol. 2020, 15, 1185–1196. [Google Scholar] [CrossRef]
- Fallone, C.A.; Moss, S.F.; Malfertheiner, P. Reconciliation of Recent Helicobacter pylori Treatment Guidelines in a Time of Increasing Resistance to Antibiotics. Gastroenterology 2019, 157, 44–53. [Google Scholar] [CrossRef]
- Gasbarrini, A.; Ojetti, V.; Armuzzi, A.; Branca, G.; Canducci, F.; Torre, E.S.; Candelli, M.; Pastorelli, A.; Anti, M.; Fedeli, G.; et al. Efficacy of a multistep strategy for Helicobacter pylori eradication. Aliment. Pharmacol. Ther. 2000, 14, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Seppala, K.; Kosunen, T.U.; Nuutinen, H.; Sipponen, P.; Rautelin, H.; Sarna, S.; Hyvarinen, H.; Farkkila, M.; Miettinen, T.A. Cure of Helicobacter pylori infection after failed primary treatment: One-center results from 120 patients. Scand. J. Gastroenterol. 2000, 35, 929–934. [Google Scholar] [PubMed]
- Chan, F.K.; Sung, J.J.; Suen, R.; Wu, J.C.; Ling, T.K.; Chung, S.C. Salvage therapies after failure of Helicobacter pylori eradication with ranitidine bismuth citrate-based therapies. Aliment. Pharmacol. Ther. 2000, 14, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Zullo, A.; Hassan, C.; Campo, S.M.; Lorenzetti, R.; Febbraro, I.; De Matthaeis, M.; Porto, D.; Morini, S. A triple therapy regimen after failed Helicobacter pylori treatments. Aliment. Pharmacol. Ther. 2001, 15, 1193–1197. [Google Scholar] [CrossRef] [PubMed]
- Gomollon, F.; Sicilia, B.; Ducons, J.A.; Sierra, E.; Revillo, M.J.; Ferrero, M. Third line treatment for Helicobacter pylori: A prospective, culture-guided study in peptic ulcer patients. Aliment. Pharmacol. Ther. 2000, 14, 1335–1338. [Google Scholar] [CrossRef] [PubMed]
- Rokkas, T.; Sechopoulos, P.; Robotis, I.; Margantinis, G.; Pistiolas, D.; Cumulative, H. pylori eradication rates in clinical practice by adopting first and second-line regimens proposed by the Maastricht III consensus and a third-line empirical regimen. Am. J. Gastroenterol. 2009, 104, 21–25. [Google Scholar] [CrossRef]
- De Boer, W.A.; Tytgat, G.N. Regular review: Treatment of Helicobacter pylori infection. BMJ 2000, 320, 31–34. [Google Scholar] [CrossRef]
- Malfertheiner, P. Infection: Bismuth improves PPI-based triple therapy for H. pylori eradication. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 538–539. [Google Scholar] [CrossRef]
- Liao, J.; Zheng, Q.; Liang, X.; Zhang, W.; Sun, Q.; Liu, W.; Xiao, S.; Graham, D.Y.; Lu, H. Effect of fluoroquinolone resistance on 14-day levofloxacin triple and triple plus bismuth quadruple therapy. Helicobacter 2013, 18, 373–377. [Google Scholar] [CrossRef]
- Goodwin, C.S.; Marshall, B.J.; Blincow, E.D.; Wilson, D.H.; Blackbourn, S.; Phillips, M. Prevention of nitroimidazole resistance in Campylobacter pylori by coadministration of colloidal bismuth subcitrate: Clinical and in vitro studies. J. Clin. Pathol. 1988, 41, 207–210. [Google Scholar] [CrossRef]
- Megraud, F. The challenge of Helicobacter pylori resistance to antibiotics: The comeback of bismuth-based quadruple therapy. Therap. Adv. Gastroenterol. 2012, 5, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, A.J.; Benfield, P.; Monk, J.P. Colloidal bismuth subcitrate. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic use in peptic ulcer disease. Drugs 1988, 36, 132–157. [Google Scholar] [CrossRef] [PubMed]
- McNicholl, A.G.; O’Morain, C.A.; Megraud, F.; Gisbert, J.P.; As Scientific Committee of the Hp-Eureg on Behalf of the National Coordinators. Protocol of the European Registry on the management of Helicobacter pylori infection (Hp-EuReg). Helicobacter 2019, 24, e12630. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Santamaría, D.; Nyssen, O.P.; Vaira, D.; Niv, Y.; Tepes, B.; Fiorini, G.; Perez-Aisa, A.; Rodrigo-Sáez, L.; Castro-Fernández, M.; Pellicano, R.; et al. European registry on H. pylori management (Hp-EuReg): Analysis of 1,782 empirical rescue therapies on third and subsequent lines. Helicobacter 2020, 25 (Suppl. 1), 41. [Google Scholar]
- Gisbert, J.P.; McNicholl, A.G. Optimization strategies aimed to increase the efficacy of H. pylori eradication therapies. Helicobacter 2017, 22, e12392. [Google Scholar] [CrossRef]
- Brughera, M.; Scampini, G.; Newman, A.J.; Castellino, S.; Sammartini, U.; Mazue, G. Overview of toxicological data on rifabutin. Exp. Toxicol. Pathol. 1995, 47, 1–9. [Google Scholar] [CrossRef]
- Dautzenberg, B. The use of rifabutin in Europe for the treatment of mycobacterial infection in AIDS patients. Infection 1997, 25, 63–66. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Calvet, X. Review article: Non-bismuth quadruple (concomitant) therapy for eradication of Helicobater pylori. Aliment. Pharmacol. Ther. 2011, 34, 604–617. [Google Scholar] [CrossRef]
- Griffith, D.E.; Brown, B.A.; Girard, W.M.; Wallace, R.J., Jr. Adverse events associated with high-dose rifabutin in macrolide-containing regimens for the treatment of Mycobacterium avium complex lung disease. Clin. Infect. Dis. 1995, 21, 594–598. [Google Scholar] [CrossRef]
- Apseloff, G.; Fluids, G.; LaBoy-Goral, L.; Kraut, E.; Vincent, J. Severe neutropenia caused by recommended prophylactic doses of rifabutin. Lancet 1996, 348, 685. [Google Scholar] [CrossRef]
- Burman, W.; Benator, D.; Vernon, A.; Khan, A.; Jones, B.; Silva, C.; Lahart, C.; Weis, S.; King, B.; Mangura, B.; et al. Acquired rifamycin resistance with twice-weekly treatment of HIV-related tuberculosis. Am. J. Respir. Crit. Care Med. 2006, 173, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Munsiff, S.S.; Driver, C.R.; Sackoff, J. Relapse and acquired rifampin resistance in HIV-infected patients with tuberculosis treated with rifampin- or rifabutin-based regimens in New York City, 1997–2000. Clin. Infect. Dis. 2005, 41, 83–91. [Google Scholar] [CrossRef] [PubMed]






| Author | Year | Country | Pre-Treatment (Naïve) or Post-Treatment Setting | Number of Patients | Resistance Rate (%) |
|---|---|---|---|---|---|
| Heep [24] | 1999 | Germany | Post (mostly) | 81 | 0 |
| Bock [48] | 2000 | Germany | Post (R) | 2 | 0 |
| Pilotto [49] | 2000 | Italy | Pre | 87 | 0 |
| Toracchio [41] | 2005 | Italy | Pre | 420 | 0 |
| Toracchio [41] | 2005 | Italy | Post (C and M; no R) | 104 | 1 |
| Marzio [50] | 2006 | Italy | Pre | 41 | 2.4 |
| Marzio [50] | 2006 | Italy | Post | 51 | 4 |
| Miehlke [51] | 2006 | Germany | Pre | 145 | 0.7 |
| Miehlke [51] | 2006 | Germany | Post (C and M; no R) | 25 | 0 |
| Borody [52] | 2006 | Australia | Post | 114 | 0 |
| Suzuki [28] | 2009 | Japan | Pre | 48 | 0 |
| Suzuki [28] | 2009 | Japan | Post | 46 | 17 |
| Chisholm [53] | 2007 | UK | Post (70%) | 255 | 0.8 |
| Glocker [38] | 2007 | Germany | Post (R in some) | 1585 | 1.4 |
| Miehlke [47] | 2008 | Germany | Post (R) | 16 | 31 |
| Goh [54] | 2011 | Malaysia | Pre | 90 | 2.2 |
| Marzio [55] | 2011 | Italy | Pre | 46 | 2.2 |
| Marzio [55] | 2011 | Italy | Post | 34 | 5.9 |
| Nishizawa [27] | 2011 | Japan | Pre and post | 414 | 0.2 |
| McNulty [56] | 2012 | UK | Pre and post | 169 | 0 |
| Tay [57] | 2012 | Australia | Post | 306 | 2 |
| Megraud(adults) [3] | 2013 | Europe | Pre | 1893 | 1.2 |
| Megraud(children) [3] | 2013 | Europe | Pre | 311 | 0.3 |
| O’Connor [58] | 2013 | Ireland | Pre | 85 | 0 |
| Larsen [59] | 2013 | Norway | Pre | 102 | 0 |
| Selgrad [60] | 2013 | Germany | Pre | 122 | 0.8 |
| Selgrad [60] | 2013 | Germany | Post | 262 | 5 |
| Seo [61] | 2013 | Korea | - | 91 | 7.7 |
| Gosciniak [62] | 2014 | Poland | Pre | 165 | 0 |
| Biernat [63] | 2014 | Poland | Pre | 50 | 0 |
| Selgrad [64] | 2014 | Germany | Pre | 29 | 0 |
| Selgrad [64] | 2014 | Germany | Post | 37 | 8 |
| John [65] | 2015 | Qatar | Pre | 105 | 4.8 |
| Di Giulio [66] | 2016 | Italy | Pre | 83 | 1.2 |
| Mori [67] | 2016 | Japan | Post | 25 | 3.4 |
| Ciccaglione [68] | 2016 | Italy | Post | 56 | 0 |
| Sung [69] | 2017 | Korea | Post | 6 | 0 |
| Hays [25] | 2018 | France | Pre and post | 1015 | 1 |
| Siavoshi [70] | 2018 | Iran | - | 104 | 7.7 |
| Choi [71] | 2019 | Korea | Pre and post | 31 | 0 |
| Lee [72] | 2019 | Korea | Pre | 202 | 1.5 |
| Kouitcheu M. [73] | 2019 | Cameroon | Pre | 140 | 0 |
| Miftahussurur [74] | 2019 | Indonesia | Pre | 63 | 0 |
| Miftahussurur [75] | 2019 | Indonesia | Pre | 105 | 0 |
| Miftahussurur [76] | 2019 | Nepal & Bangladesh | Pre | 98 | 0 |
| Graham [77] | 2020 | USA | Pre | 345 | 0 |
| Hirata [78] | 2020 | Japan | Post | 18 | 0 |
| Graham [77] | 2020 | USA | Post | 99 | 0 |
| Author | Year | Country | Drugs and Doses | Duration of Treatment (Days) | Number of Patients | Number of Previous Failed Treatments | Type of Previous Treatments | Eradication Rate ¶ (%) | Adverse Events (%) |
|---|---|---|---|---|---|---|---|---|---|
| Perri [81] | 1998 | Italy | Rifabutin 300 mg/24 h Amoxicillin 1 g/12 h Pantoprazole 40 mg/12 h | 7 | 28 | ≥2 | PPI-containing tx | 79 | 7.1 |
| Bock [48] | 2000 | Germany | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Lansoprazole 30 mg/12 h | 7 | 12 | 1 | PPI+A- and C-containing tx | 58 | 0 |
| Bock [48] | 2000 | Germany | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Lansoprazole 30 mg/12 h | 7 | 5 | 2 | PPI+A- and C-containing tx | 80 | 0 |
| Bock [48] | 2000 | Germany | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Lansoprazole 30 mg/12 h | 7 | 2 | 3 | PPI+A- and C-containing tx | 100 | 0 |
| Bock [48] | 2000 | Germany | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Lansoprazole 30 mg/12 h | 7 | 6 | ≥1 | PPI+A- and C-containing tx | 83 | 0 |
| Perri [82] | 2000 | Italy | Rifabutin 300 mg/24 h Amoxicillin 1 g/12 h Pantoprazole 40 mg/12 h | 7 | 25 | 2 | (1) PPI+C+A (2) PPI+C+M, PPI+C+T | 80 | 3 |
| Perri [82] | 2000 | Italy | Rifabutin 300 mg/24 h Amoxicillin 1 g/12 h Pantoprazole 40 mg/12 h | 7 | 16 | ≥3 | (1) PPI+C+A (2) PPI+C+A, PPI+C+M, PPI+C+T 3/(4) PPI+A, PPI+C+M, PPI+A+T, PPI+C+T, RBC+A+T, RBC+C, Q | 56 | 3 |
| Beales [83] § | 2001 | UK | Rifabutin 300 mg/24 h Amoxicillin 1 g/12 h Omeprazole 20 mg/12 h | 14 | 10 | 2 | C- and M-containing tx | 60 | - |
| Canducci [84] | 2001 | Italy | Rifabutin 300 mg/24 h Amoxicillin 1 g/12 h Omeprazole 20 mg/12 h | 10 | 10 | ≥3 | - | 70 | 10 |
| Perri [36] | 2001 | Italy | Rifabutin 150 mg/24 h Amoxicillin 1 g/12 h Pantoprazole 40 mg/12 h | 10 | 45 | ≥1 | PPI+C+A, PPI+C+M, RBC+C | 67 | 9 |
| Perri [36] | 2001 | Italy | Rifabutin 300 mg/24 h Amoxicillin 1 g/12 h Pantoprazole 40 mg/12 h | 10 | 45 | ≥1 | PPI+C+A, PPI+C+M, RBC+C | 87 | 11 |
| Gisbert [85] | 2003 | Spain | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Omeprazole 20 mg/12 h | 14 | 14 | 2 | (1) PPI+C+A (2) Q, RBC+T+M | 79 | 36 |
| Wong [86] | 2003 | China | Rifabutin 300 mg/24 h Levofloxacin 500 mg/24 h Rabeprazole 20 mg/12 h | 7 | 37 | 1 | PPI+C+A, PPI+C+M, PPI+A+M | 92 | 34 |
| Wong [86] | 2003 | China | Rifabutin 300 mg/24 h Levofloxacin 500 mg/24 h Rabeprazole 20 mg/12 h | 7 | 19 | ≥2 | C-containing tx (100%), M-containing tx (76%) | 89 | 34 |
| Gisbert [87] | 2004 | Spain | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Omeprazole 20 mg/12 h | 14 | 12 | 2 | (1) PPI+C+A (2) RBC+T+M | 67 | - |
| Gisbert [87] | 2004 | Spain | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Omeprazole 20 mg/12 h | 14 | 2 | 2 | (1) RBC+C+A (2) RBC+T+M | 100 | - |
| Qasim [88] | 2005 | Ireland | Rifabutin 300 mg/24 h Amoxicillin 1 g/12 h PPI/12 h | 10 | 34 | 2 | (1) PPI+C+A, PPI+C+M (2) PPI+C+A, PPI+C+M, Q | 38 | 2.9 |
| Toracchio [41] ¥ | 2005 | Italy | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Pantoprazole 40 mg/12 h | 10 | 65 | ≥1 | - | 78 | 1.5 |
| Borody [52] | 2006 | Australia | Rifabutin 150 mg/24 h Amoxicillin 1–1.5 g/8 h Pantoprazole 80 mg/8 h | 12 | 63 | 1 | C-containing tx | 92 | 40 |
| Borody [52] | 2006 | Australia | Rifabutin 150 mg/24 h Amoxicillin 1–1.5 g/8 h Pantoprazole 80 mg/8 h | 12 | 67 | ≥2 | C-containing tx | 90 | 40 |
| Gisbert [89] | 2006 | Spain | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Omeprazole 20 mg/12 h | 10 | 20 | 2 | (1) PPI+C+A (2) Q, RBC+T+M | 45 | 60 |
| Miehlke [51] § | 2006 | Germany | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Esomeprazole 20 mg/12 h | 7 | 73 | ≥1 (24% 1 tx, 52% 2 tx, 24% 3 tx) | PPI+C+A, PPI+C+M, PPI+A+M, Q, PPI+A+L, others | 74 | 35 |
| Gonzalez Carro [90] | 2007 | Spain | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Pantoprazole 40 mg/12 h | 10 | 92 | 2 | (1) PPI+C+A (2) Q | 61 | 2 |
| Navarro-Jarabo [91] | 2007 | Spain | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Omeprazole 20 mg/12 h | 7 | 45 | 1 | PPI+C+A, PPI+C+M | 44 | 44 |
| Van der Poorten [39] | 2007 | Australia | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h PPI/12 h | 10 | 19 | 1 | C-containing tx | 95 | 10 |
| Van der Poorten [39] | 2007 | Australia | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h PPI/12 h | 10 | 16 | 2 | (1) C-containing tx (2) Q (42% of the cases) | 64 | 10 |
| Van der Poorten [39] | 2007 | Australia | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h PPI/12 h | 10 | 31 | ≥3 | (1) C-containing tx (2) Q (42% of the cases) (3) Other | 62 | 10 |
| Gisbert [92] | 2008 | Spain | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Omeprazole 20 mg/12 h | 10-14 | 51 | 2 | (1) PPI+C+A (2) Q, RBC+T+M, PPI+A+L | 55 | 47 |
| Gisbert [92] | 2008 | Spain | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Omeprazole 20 mg/12 h | 10-14 | 7 | 3 | (1) PPI+C+A (2) Q, RBC+T+M (3) PPI+A+L | 71 | 86 |
| Miehlke [47] ¥ | 2008 | Germany | Rifabutin 300 mg/24 h Moxifloxacin 400 mg/24 h Esomeprazole 40 mg/24 h | 7 | 24 | 1 | PPI+A, PPI+C, PPI+C+A, PPI+C+M, Q, PPI+A+L | 78 | 30 |
| Miehlke [47] ¥ | 2008 | Germany | Rifabutin 300 mg/24 h Moxifloxacin 400 mg/24 h Esomeprazole 40 mg/24 h | 7 | 52 | 2 | PPI+A, PPI+C, PPI+C+A, PPI+C+M, Q, PPI+A+L | 90 | 30 |
| Miehlke [47] ¥ | 2008 | Germany | Rifabutin 300 mg/24 h Moxifloxacin 400 mg/24 h Esomeprazole 40 mg/24 h | 7 | 17 | 3 | PPI+A, PPI+C, PPI+C+A, PPI+C+M, Q, PPI+A+L | 69 | 30 |
| Miehlke [47] ¥ | 2008 | Germany | Rifabutin 300 mg/24 h Moxifloxacin 400 mg/24 h Esomeprazole 40 mg/24 h | 7 | 10 | ≥4 | PPI+A, PPI+C, PPI+C+A, PPI+C+M, Q, PPI+A+L | 89 | 30 |
| Sacco [93] | 2009 | Italy | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Esomeprazole 20 mg/12 h | 10 | 19 | 1 | PPI+A+Mx | 84 | 0 |
| Van Zanten [94] | 2010 | Canada | Rifabutin 300 mg/24 h Amoxicillin 1 g/12 h PPI/12 h | 7 | 4 | 1 | - | 100 | 0 |
| Van Zanten [94] | 2010 | Canada | Rifabutin 300 mg/24 h Amoxicillin 1 g/12 h PPI/12 h | 7 | 10 | 2 | (1) PPI+C+A, PPI+C+M, RBC+C (2) PPI+C+A, PPI+C+M, Q | 50 | 0 |
| Van Zanten [94] | 2010 | Canada | Rifabutin 300 mg/24 h Amoxicillin 1 g/12 h PPI/12 h | 7 | 2 | 3 | (1) PPI+C+A, Q (2) PPI+A, PPI+C+M (3) PPI+C+A | 50 | 0 |
| Zullo [95] | 2010 | Italy | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Omeprazole 20 mg/12 h | 10 | 13 | 2 | (1) PPI+C+A, PPI+C+M, PPI+A+M (2) PPI+A+L | 85 | 15 |
| Tay [57] | 2012 | Australia | Rifabutin 150 mg/12 h Amoxicillin 1 g/8 h Ciprofloxacin 500 mg/12 h Rabeprazole 20 mg/8 h | 5-7 | 210 | ≥1 | (1) PPI+C+A +/- others (mostly PPI+C+M) | 95 | - |
| Tay [57] | 2012 | Australia | Rifabutin 150 mg/12 h Bismuth 240 mg/6 h Ciprofloxacin 500 mg/12 h Rabeprazole 20 mg/8 h | 7 | 69 | ≥1 | PPI+C+A +/- others (mostly PPI+C+M) | 94 | - |
| Gisbert [96] | 2012 | Spain | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h PPI/12 h | 10 | 100 | 3 | (1) PPI+C+A (2) Q (3) PPI+A+L | 50 | 30 |
| Jeong [97] | 2012 | Korea | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h PPI/12 h | 10 | 7 | 2 | (1) PPI+C+A (2) Q | 71 | - |
| Fiorini [99] | 2013 | Italy | Rifabutin 150 mg/24 h Amoxicillin 1 g/8 h Esomeprazole 40 mg/12 h | 12 | 105 | ≥1 | - | 89 | 15 |
| Ierardi [100] | 2014 | Italy | Rifabutin 150 mg/12 h Minocycline 100 mg/12 h Bismuth 120 mg/6 h Rabeprazole 20 mg/12 h | 10 | 21 | 2 | (1) PPI+C+A, sequential (2) PPI+A+L | 78 | 7.4 |
| Lim [98] | 2014 | Korea | Rifabutin 150 mg/12 h Amoxicillin 1 g/8 h Lansoprazole 60 mg/12 h | 7 | 27 | 2 | (1) PPI+C+A (2) Q | 96 | 11 |
| Lim [98] | 2014 | Korea | Rifabutin 150 mg/12 h Amoxicillin 1 g/8 h Lansoprazole 30 mg/12 h | 7 | 32 | 2 | (1) PPI+C+A (2) Q | 78 | 31 |
| Gisbert [101] # | 2015 | Spain | Rifabutin 150 mg/12 h Clarithromycin 500 mg/12 h Omeprazole 20 mg/12 h | 10 | 7 | 2 | (1) PPI+C+M (2) Q | 14 | 71 |
| Gisbert [101] # | 2015 | Spain | Rifabutin 150 mg/12 h Clarithromycin 500 mg/12 h Omeprazole 20 mg/12 h | 10 | 2 | 3 | (1) PPI+C+M (2) Q (3) PPI+C+L | 50 | 100 |
| Ciccaglione [68] | 2016 | Italy | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Pantoprazole 20 mg/12 h | 10 | 27 | 2 | (1) PPI+C+A (2) PPI+A+M/L/Mx | 67 | 0 |
| Ciccaglione [68] | 2016 | Italy | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Pantoprazole 20 mg/12 h Bismuth 240 mg/12 h | 10 | 29 | 2 | (1) PPI+C+A (2) PPI+A+M/L/Mx | 97 | 0 |
| Mori [67] | 2016 | Japan | Rifabutin 300 mg/24 h Amoxicillin 500 mg/8 h Esomeprazole 20 mg/8 h | 10 | 12 | 2 | (1) PPI+C+A (2) PPI+A+M | 83 | 75 |
| Mori [67] | 2016 | Japan | Rifabutin 300 mg/24 h Amoxicillin 500 mg/8 h Esomeprazole 20 mg/8 h | 10 | 17 | 2 | (1) PPI+C+A (2) PPI+A+M | 94 | 94 |
| Sung [69] | 2017 | Korea | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h PPI 20 mg/12 h | 7-14 | 12 | ≥3 | - | 50 | 18 |
| Cosme [102] | 2017 | Spain | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Omeprazole 20 mg/12 h | 10 | 12 | ≥1 | - | 58 | 17 |
| Fiorini [20] | 2018 | Italy | Rifabutin 150 mg/24 h Amoxicillin 1 g/12 h Esomeprazole 40 mg/12 h | 12 | 257 | ≥1 | - | 83 | 18 |
| Papastergiou [103] | 2018 | Greece | Rifabutin 150 mg/12 h Amoxicillin 1 g/8 h Esomeprazole 40 mg/12 h | 7 | 2 | 0 (naïve) | - | 50 | 25 |
| Papastergiou [103] | 2018 | Greece | Rifabutin 150 mg/12 h Amoxicillin 1 g/8 h Esomeprazole 40 mg/12 h | 7 | 2 | ≥1 | - | 50 | 25 |
| Ribaldone [105] | 2019 | Italy | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h PPI/12 h | 14 | 302 | 4 | (1) PPI+C+A (2) PPI+C+A+M (3) Q (4) PPI+A+L | 72 | 7.3 |
| Burgos-Santamaría [106] | 2019 | Spain | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Omeprazole 20 mg/12 h | 10-14 | 64 | 2 | (1) PPI+C+A (2) Q | 56 | 45 |
| Burgos-Santamaría [106] | 2019 | Spain | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Omeprazole 20 mg/12 h | 10-14 | 60 | 3 | (1) PPI+C+A (2) Q (3) PPI+A+L (or vice versa) | 55 | 45 |
| Burgos-Santamaría [106] | 2019 | Spain | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Omeprazole 20 mg/12 h | 10-14 | 4 | 4 | (1) PPI+C+A (2) Q (3) PPI+A+L (or vice versa) (4) Other | 50 | 45 |
| Burgos-Santamaría [106] # | 2019 | Spain | Rifabutin 150 mg/12 h Clarithromycin 500 mg/12 h Omeprazole 20 mg/12 h | 10-14 | 13 | 2 | (1) PPI+C+M (2) Q (or vice versa) | 23 | 75 |
| Burgos-Santamaría [106] # | 2019 | Spain | Rifabutin 150 mg/12 h Clarithromycin 500 mg/12 h Omeprazole 20 mg/12 h | 10-14 | 3 | 3 | (1) PPI+C+M (2) Q (or vice versa) (3) PPI+C+L | 67 | 75 |
| Saracino [108] | 2020 | Italy | Rifabutin 150 mg/24 h Amoxicillin 1 g/12 h Esomeprazole 40 mg/12 h | 12 | 119 | 1 | - | 58 | 46 |
| Saracino [108] | 2020 | Italy | Rifabutin 150 mg/24 h Amoxicillin 1 g/12 h Esomeprazole 40 mg/12 h | 12 | 66 | 2 | - | 56 | 46 |
| Saracino [108] | 2020 | Italy | Rifabutin 150 mg/24 h Amoxicillin 1 g/12 h Esomeprazole 40 mg/12 h | 12 | 44 | 3 | - | 54 | 46 |
| Saracino [108] | 2020 | Italy | Rifabutin 150 mg/24 h Amoxicillin 1 g/12 h Esomeprazole 40 mg/12 h | 12 | 41 | ≥4 | - | 24 | 46 |
| Graham [77] | 2020 | USA | Rifabutin 50 mg/8 h Amoxicillin 1 g/8 h Omeprazole 40 mg/12 h | 14 | 228 | 0 (naïve) | - | 84 | 36 |
| Hirata [78] | 2020 | Japan | Rifabutin 150 mg/12 h Amoxicillin 750 mg/12 h Vonoprazan 20 mg/12 h | 10 | 19 | 3 | (1) C-containing tx (2) M-containing tx (3) S-containing tx | 100 | 42 |
| Kalfus [107] | 2020 | USA | Rifabutin 150 mg/8 h Amoxicillin 1 g/8 h Omeprazole 40 mg/8 h (Talicia® single capsule) | 14 | 77 | 0 (naïve) | - | 77 | 51 |
| Kuo [19] | 2020 | China | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Esomeprazole 40 mg/12 h | 10 | 40 | ≥2 | - | 78 | 23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gisbert, J.P. Rifabutin for the Treatment of Helicobacter pylori Infection: A Review. Pathogens 2021, 10, 15. https://doi.org/10.3390/pathogens10010015
Gisbert JP. Rifabutin for the Treatment of Helicobacter pylori Infection: A Review. Pathogens. 2021; 10(1):15. https://doi.org/10.3390/pathogens10010015
Chicago/Turabian StyleGisbert, Javier P. 2021. "Rifabutin for the Treatment of Helicobacter pylori Infection: A Review" Pathogens 10, no. 1: 15. https://doi.org/10.3390/pathogens10010015
APA StyleGisbert, J. P. (2021). Rifabutin for the Treatment of Helicobacter pylori Infection: A Review. Pathogens, 10(1), 15. https://doi.org/10.3390/pathogens10010015





