Abstract
This paper introduces the author’s practice-based research and the developed pioneering methods of application of printing inclusions and metal inclusions in glass. It also describes and examines the problems occurring during the application of printing and metal inclusions in glass and presents methods for the selection of suitable materials and techniques.
1. Introduction
The possibility of the application of metal inclusions in glass initially arose in response to the requirements to be able to materialize the author’s artistic ideas. The research reported in this paper is based on (Bialek 2017) that was informed by, and built on, earlier experiments of using metals (Figure 1) and the immersion of prints in glass (Figure 2 and Figure 3).

Figure 1.
Goshka Bialek; What’s Left? 2004; cast bronze and glass on marble base; photographed by Tim Adams, used by permission.

Figure 2.
Goshka Bialek; Newspaper, 2002; inclusion of screen-printing images on transfer paper in glass; 180 cm × 50 cm × 30 cm; photographed by Tim Adams, used by permission.
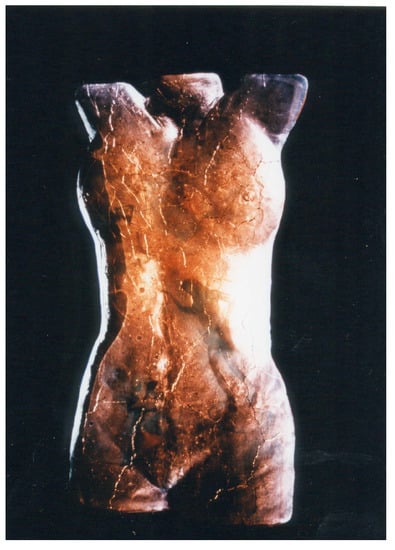
Figure 3.
Goshka Bialek; Imagination I, 2001; cast uranium glass with an immersion of printings; 180 cm × 50 cm × 30 cm; photographed by Tim Adams, used by permission.
The intention of the research was, using an earlier experience (Bialek 2000, 2003), firstly to develop techniques for the expansion of sculptors’ creative potential of combining glass in their studio practice. Secondly, the aim was to equip glass artists with a wider “palette” of inclusions for utilization of the “positive space” (internal space) in sculptures. Positive space is the space taken by an object, but the management of the internal space is possible only if the materials employed are transparent or semi-transparent, for example, glass (Figure 2, Figure 3 and Figure 4).

Figure 4.
Goshka Bialek; Self-portrait I & II, 2017; inclusion of nickel, lead, and tin alloy in Cristalica glass; hot pouring into the mold; created by author.
Hence, the idea was to consider an additional space to apply inclusions and the illusion of perspective. This led to the idea of utilizing the additional space in sculpture (particularly internal space) created by using transparent and semi-transparent materials.
This paper firstly surveys technological problems of applying metal inclusions in glass. Then, based on the literature survey and consultation with artists and technologists, it presents methods for the selection of suitable metals and techniques.
2. Drivers for Research
The most important reason for the research was to achieve a theoretical understanding of the application of inclusions, as this always brings some technical problems. The process of intentional inclusion always involves seeking to achieve a positive balance between materials if a combination of two or more different materials are involved, and especially if one of them is glass. Glass is a very complex material to work with and requires a great deal of knowledge and experience. Even experts cannot answer some questions pertaining to the technological problems of how to control the process of sticking glass to other materials during casting in high temperatures (Zasadzinski 2012, 2013, 2014, 2015; Hand 2014, 2015; Greiner-Wrona 2013, 2014, 2015). The investigation concentrated on a choice of the most suitable materials and adapting them, and the techniques of applying them to achieve the research goals. The main problem was to develop a technology which would deliver the desired results despite the problems encountered during the experiments. These problems were associated with the quality of the glass and materials available to artists on the market; the lack of information about the chemical and physical parameters of these materials; the compatibility, strength, and thickness of the inclusion materials; oxidation; and the fusion of glass, mold, and inclusion surfaces due to the high wetting parameters of materials at high temperatures. Most of these problems were solved during the investigation and through collaboration with metal and glass scientists. In the final research, two types of metals—nickel and lead tin alloys—were chosen because their chemical and physical properties are so different from each other. The most important difference is their melting points; nickel’s melting point is 1453 °C (Engineering ToolBox 2005), which is above the melting point of glass (600 °C), whereas lead’s melting point is 327.5 °C (Engineering ToolBox 2005), which is below that of glass. On the other hand, they have common features, which are also very important for this research. They are both sheen silver and corrosion resistant. Thanks to these properties and, at the same time, their diversity, it was hoped to realize some of their potential in their application in art works, and to further an understanding of the problems which the application of metal inclusions can involve.
3. Overview of Technological Problems
An early example of the experiments using inclusions in glass (Figure 2 and Figure 3) incorporated the immersion of screen-printing images in glass. The initial research (Bialek 2003) was concentrated, in general, on the use of inclusions (such as fabrics, metals, organic objects, clay, and images) in cast glass (including sand cast technique, cast glass in the mold, hot cast glass, and fusing). Following these experiments, the investigation began focusing on inclusions, such as prints, photographs, and holograms, and on the capabilities of controlling their shapes during the casting process. As a result of these studies, a novel technique of image transfer to glass was developed in 2000–2003, and results were published in Kevin Petrie’s (2006) book. This technique was predominantly used on the surfaces of cast and blown glass. The innovation was to fuse and/or cast multiple printed surfaces to create graphic image layers within the glass (Figure 2 and Figure 3). Applying the printings directly to flat window glass and controlling a shape of the inclusions during the fusing method was quite a straightforward process. However, slumping or casting into a mold required assembling more complicated objects than glass boxes. Additionally, the application of silkscreen printed images directly on a surface of the casting glass created problems, as the surface of the glass is never perfectly flat.
It was difficult to control the shape of the inclusions in cast glass in a mold; thus, it was necessary to find a material that would better meet the objective of retaining the inclusion shape. Therefore, the research concentrated on the search for a suitable material which would satisfy these objectives. Upon reviewing the literature and conducting initial experiments (Bialek 2017, pp. 114–30), metal was selected as the most appropriate medium for adding inclusions into glass. Initial experiments showed that some of metals, e.g., nickel, kept their shape even during a slumping or a cast glass into a mold or hot cast glass process. The experiments also showed some technological disadvantages of the material as an inclusion medium. It was difficult to understand the compatibility rules (Table 1) of metals with glass. Also, shortages of a technical description of the material and difficulties for artists to understand complicated processes during the application of metal with glass could constitute a barrier to using the medium in artistic practice.

Table 1.
Comparison of the linear thermal expansion coefficient of most common metals and glass used by artists.
Research also showed that the palette of metals used by artists is restricted, and the present technologies available for artists, which are widely drawn from the industrial sector, have many limitations. Therefore, it was crucial to extend the range of materials and techniques for creative practice. In addition, the research investigated the adaptation of existing technologies, particularly metal casting into molds, for immersing different kinds of metal inclusions into glass sculptures in order to utilize the space occupied by the investigated object.
Metal as an inclusion medium was chosen for a number of other reasons. Firstly, a similar technology is involved in casting both metal and glass (Figure 1). Both materials can be turned into liquid and back to a solid state many times. Also, some solid brittle metals, for example, gallium, fracture conchoidally like glass, which means that these materials fracture as waves rather than following any natural planes of separation. These similarities can be helpful, because most sculptors are familiar with the metal medium. This should help them understand the technology of the glass medium more easily and give them confidence to adopt glass as a medium in their artistic practice.
Secondly, some metals can be turned to amorphous material, also known as glassy metal, by extremely rapid cooling, physical vapor deposition, solid-state reaction, ion irradiation, or mechanical alloying (Ojovan and Lee 2010). The third reason was to introduce new metals (other than those usually used by glass artists in the past) for utilization in inclusions in glass.
Also, metals were chosen for their symbolic significance. Metals were used in the past as a base for inscriptions. For example, Romans used metal tablets for inscriptions as carriers of information. This not only helps us understand the evolution of the art and the history of the Roman Empire, but also helps us learn more about mundane affairs between people living at that time. Most of the Roman metal inscriptions were on bronze, lead, gold, or silver (Gordon 1968). Metals and quartz, which is a component of glass consisting of silica in an amorphous (non-crystalline) structure, are mediums which contemporary scientists still use to store data for future civilizations using high-tech technologies (Laursen 2013, p. 12).
The practical knowledge of forming metals was developed over thousands of years, but the understanding of the physical phenomena associated with deformation only developed within the last seventy years. By the 19th century, the number of known metals was 23; by the beginning of the 20th century, the number rose to 65; in the second part of the century, it rose to 70; currently, 81 metallic elements are known to science. Modern metallurgy, similarly to glass technology, has its roots in the ancient crafts of smelting, shaping, and treatment of metal. In the contemporary world, the combination of metal and glass is widely applied. Artists, designers, craftsmen, and architects create objects in varying styles, both traditional and ultra-modern; they use different types of metal and metallic patinas, a variety of glass, and diverse techniques to apply this combination of materials. However, the application of these two materials, especially by employing hot glass techniques (most suitable in this research), creates many problems and, as a consequence, often forces artists to acquire a significant scientific and empirical knowledge necessary for working with these materials.
In glassmaking terms, the word “metal” refers to the molten glass in a furnace, as per the definition from Bray’s (2001) glass dictionary. It is misleading because glass is not a metallic substance, and the use of that term in the context of glass is discouraged (Whitehouse 1993). Physically, any of the elementary class substances, such as gold, silver, or copper, are crystalline when solid. Metal is a medium that is typically hard, opaque, shiny, and has good electrical and thermal conductivity. It is an incredibly versatile material, used in applications ranging from high-tech lightweight aluminum to air-purifying titanium dioxide, and from crude and raw Corten steel used in marine transportation to gold leaf. These metals, as other chemicals, are arranged in order of atomic weight in Mendeleev’s periodic table.
4. Methods for the Selection of Suitable Metals and Application Techniques in Glass
In this section, the complexity of developing application techniques of metal inclusions in glass is discussed, as used to create a body of the author’s artwork.
In the initial stages, the research undertaken was more focused and concentrated on the recognition of limitations involved in the use of different kinds of metal inclusions in glass, and it concentrated on investigating the compatibility of glass with metallic materials, mainly non-iron or non-ferrous metals such as lead, tin, nickel, titanium, cobalt, chromium, aluminum, gallium, and a variety of alloys of these metals (Bialek 2017, pp. 130–53), because non-ferrous metals are more malleable, and mostly non-magnetic with a higher resistance to rust and corrosion. Several of the metals which are difficult to produce in pure form, or can be more conveniently used when alloyed, are available as ferroalloys. These are used as additions to steel, for example, molybdenum, vanadium, tungsten, and titanium. However, mostly pure metals were examined because it was easier to predict their behavior and they usually are softer than ferroalloys. The most important property of solid materials is the “thermal expansion” or expansion-with-temperature relationship. This is the property that complicates sealing different materials together, as per Schuler and Schuler’s definition (Schuler and Schuler 1971, p. 36). Consequently, the two materials will usually have their respective expansion–temperature relationships differ during the heating and cooling process. If this problem occurs using two different metals bonded with each other, the metals would bend. However, if we use glass and metal, because glass is brittle, it may fracture from stress if the length difference is excessive. In the course of research, it was found that, to reduce the effects associated with this phenomenon, it is important to carry out an appropriate annealing for both materials. Glass artists and glass technicians focus only on annealed glass, and they forget that they should also take into account relaxing metal at the same time in their calculations. Heat expands all metals, which again contract during cooling, and no danger of fracture is caused even by rapid cooling (Pellatt 1949, p. 64), but it is a different matter if the metal is combined with glass. Annealing of metals is a form of heat treatment and is made use of when the metal is required for use in a soft but tough state. Hence, most metals and their alloys selected in this study were annealed. Annealing may proceed in three separate stages depending on the extent of the required treatment. On the basis of consultation with scientists such as Professor Hand (2014, 2015), Professor Zasadzinski (2012, 2013, 2014, 2015), and Dr. Greiner-Wrona (2013, 2014, 2015), as well as available literature on metallurgy and annealing of materials (Skrzypek and Przybylowicz 2012; Young 2008; Jacop 1997), firing programs of cast glass with metal inclusions taking into account annealing for both materials were established (Bialek 2017, pp. 172–82).
A suitable metal to combine with glass should be soft and, therefore, weak enough to allow glass to ‘brief’ during a cooling process, even if it has a bond to the surface of the metal due to the wetting action of glass. Metals at high temperature will all oxidize to a certain extent, with some exceptions, for example, gold.
Based on a review of the literature and interviews with artists (Bialek 2017, pp. 58–89), it was deduced that most common metals used by artists in the past were gold, silver, platinum, copper, brass, and bronze; in the last few decades, we can add to this list aluminum and zinc. Artists justify their choice of metals because, in their opinion, these metals are most compatible with glass.
A comparison of the coefficients of expansion (COEs) of these metals (Table 1) reveals that the metal COEs are not well correlated with the COE of casting glass; however, in practice, they may be compatible. Take, for example, Kovar, which is an iron-based alloy with nickel and cobalt and which was specially designed to be used with borosilicate glass (Figure 5). Kovar has a COE of 1.5, while borosilicate glass has a COE of 5.5; yet, they are perfectly compatible despite their COEs being quite different.

Figure 5.
Kovar inclusion applied in borosilicate glass by lampworking; created by author.
Experiments conducted in this research showed that some metals produce a coat of gas bubbles during the casting process (Figure 5). It was noted that this phenomenon helps prevent bonding glass with metal at high temperatures and to overtake the compatibility problems, even if the COEs of both materials differ.
However, in some cases, bonding between metal and glass does not have any impact on the entire process, even if the COEs of both materials differ. Conducted experiments (Figure 6) proved that gallium, which turns into a liquid at temperatures greater than 29.76 °C, is such a metal. Gallium wetted the glass surface and built a very strong bond; however, because it stays in a liquid state during the entire heating and cooling process, it has an elastic bond with glass and, hence, it may be used as a compatible inclusion in glass.

Figure 6.
Gallium inclusion applied in borosilicate glass by lampworking; created by author.
A review of the literature and questionnaires sent out to artists (Bialek 2017, pp. 58–89) also showed that the thickness of a metal inclusion has an effect during annealing, with the belief that any metal thicker than a foil of 2–5 mil (=0.0508–0.127 mm) would cause the glass to crack (Walker 2010). However, experiments with metals of greater thickness than foil, up to 3 mm = 118.11 mil (nickel 99.9, aluminum, gallium, or lead alloys) showed that thicker inclusions could be regularly produced effectively (Bialek 2017, pp. 130–53).
The conclusion is that COEs of metals can be different from glass if there is not a strong bond between the metal inclusion and glass. However, if a chemical bond between the metal and the glass is formed, probably through oxidation of the metal surface, then, as the metal attempts to shrink away from the glass, stresses emerge. Although these stresses could simply break the metal/glass bond, they might lead to other fracture problems, especially if the surface roughness leads to some interlocking of the metal and the glass (Figure 7).

Figure 7.
The metal/glass bond caused stress during the cooling process; created by author.
As for the thickness of the inclusion, there are many variables in play. What the research showed is that, for softer metals, the thickness of the inclusion can be greater than that for harder metals, which are more likely to cause glass to crack; however, definitive results are difficult to predict, as the annealing process and any lubricant or separator will also have an effect on final outcomes. The study showed that, to avoid glass wetting at high temperatures, it is necessary to use a separator to coat metal surfaces or to find a metal which can produce for itself such a coat during the process of heating (Bialek 2017, pp. 164–71).
Further studies were focused on adjusting both materials (glass and metal) used in this research to minimalize differences of physical characteristics between them. To increase the COE of glass, sodium carbonate is frequently added, but this should be done during the melting of the raw materials to manufacture glass (Holland 1966). In Svensson’s (Vice Director/Managing Director of Glasma AB) opinion, it is too late to add this ingredient to the melted glass nuggets in the furnaces as it would cause blisters in glass (Svensson 2013). Consequently, this study focused on the choice of the most suitable metals on the basis of the physical and chemical properties of these metals and preliminary experiments with these metals. This resulted in the following conditions:
- The thermal expansion of both metal and glass should be similar. This requirement was selected first, because it was mentioned as a central condition in the selection of materials for casting glass projects in most publications for glass artists. However, then again, as mentioned earlier, the COE parameter is not fully correlated with the compatibility of metal with glass. Professor Zasadziński (Krakow Academy of Technology) pointed out that the COE parameter is usually measured between room temperature and 300 °C, while the process of the application of metal inclusions is in a range of 25 °C to 1000 °C. The thermal expansion of materials in this temperature bracket is often not linear and does not change uniformly as the temperature increases; thus, it should be taken into account too.
- If possible, the curves of thermal expansions of the two materials (metal and glass) should be analogous during the process of cooling. Two problems appear here. Firstly, it is very difficult to find those parameters in literature (some of them are published by distributors of materials, but it is only about popular materials on the market) and, secondly, it is very rare that the two materials have matching curves of thermal expansions. To get around these problems, it was sometimes required to use empirical experience and intuition.
- Metal melting point should be higher than the highest casting point used for glass. This condition was determined on the basis of information gathered from metals which were used by artists as inclusions in the past.
- Metal boiling point (the boiling point is the temperature at which the liquid changes into a gas) should be much higher than the highest casting point used for glass. This condition was placed because of safe working with metals at high temperatures due to emitted gases during the boiling process.
- Low probability of oxidation. When metals are exposed to high temperatures, deposits often accumulate on the metal surfaces and initiate the oxidation processes. This process in literature is called high-temperature hot corrosion (Pettit 2011). For any metal, there exists a value of oxygen potential, which is called the “free energy of formation” of the oxide (Crouse-Hinds Series 2016). Above this value, the metal will be oxidized; however, below this value, no oxidation will take place. This value is very much dependent upon temperature and usually increases with increasing temperature.
- The selected metal should preferably not react with glass in the process of casting; this means that glass and metal should not stain each other.
The last condition was placed due to one of the aims of the research, whereby metal will not adversely react with glass. It would be very difficult to find a metal which satisfies all six conditions. Hence, in some cases, to increase the list of metal inclusions, a choice has to be made based on experience. Preliminary tests were conducted with different forms of metal, such as metal oxides or metals deposited via the thermal evaporation method, as they should be easier to apply as inclusions; nonetheless, these attempts did not yield the expected results (Bialek 2017, pp. 130–53). However, even if this part of the research was not entirely successful, it still brought about a better understanding of the metals and their application with glass.
Initially, experiments applying metal inclusions into glass were carried out using hot pouring, cast glass technique, and fusing technique. The results and conclusions are outlined in Table 2. During the heating process, when the glass is still solid, the metal inclusions are exposed to air at a high temperature. In many cases, this causes deformation and discoloration of the metal surface. As a result, studies were also directed to find better techniques to overcome these problems.

Table 2.
Comparison of hot kiln techniques used in the research.
The research showed that hot pouring into a mold was more efficient than other techniques (Table 2); however, it required further development of the issues discussed below.
4.1. Exploration to Prevent Metal Oxidation during the Heating Process
The best way to avoid the oxidation of metal inclusions during heating is to protect the metal from air. This can be achieved by tight-fit inclusions in glass. If inclusions are applied by fusing or cast kiln techniques, this can be achieved using waterjet.
To prevent oxidation of metals, the following methods can be used: overglaze, silver flakes (Figure 8), or coatings of metal with separators or lubricants, such as Borax, Flexi-Glass medium, jewelry enamels, graphites (Figure 9), clear varnish, transfers (Bialek 2017, pp. 164–71), or pouring hot glass onto metal (Figure 10).
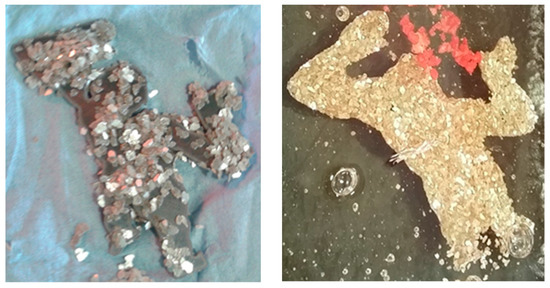
Figure 8.
Silver flakes used as a lubricant to prevent oxidation of metal inclusions; created by author.
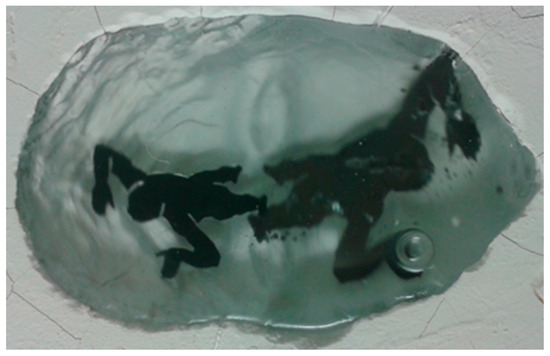
Figure 9.
Inclusion of nickel 825 alloy coated with black graphite; created by author.

Figure 10.
Method of pouring hot glass into plaster molds. The high temperature resistant moulds, up to 900 °C; created by author.
4.2. Development of Separators and Lubricants
Experiments showed that the best separators between glass and the main body of the plaster mold are a layer of shelf wash and/or china clay with the addition of boron nitride (white graphite) on the top surface of the mold. These molds are high temperature resistant moulds, up to 900 °C (Figure 11).

Figure 11.
Three types of separators: boron nitride, a slab of cast glass from furnace, and only 1% kiln wash, and/or China clay in the mold mixture; created by author.
These separators are quite effective in keeping the cast object clean from mold components. However, it was noted that the pouring of hot glass into plaster molds caused numerous gas bubbles (Figure 12). This is a known phenomenon among glassmakers, which causes them to avoid that technique.

Figure 12.
Gas bubbles in the glass as a result of pouring hot glass into the plaster mold; created by author.
It was noticed that a glass layer fired in the mold before pouring hot glass into the mold could act as an effective separator to reduce gas bubbles in the cast glass (Figure 10 and Figure 11).
To increase the possibility of using metal inclusions in glass, it is necessary to introduce appropriate lubricants or separators. A metal lubricant has to be applied to stop the process of wetting metal by glass in the hot stage, and a separator has to be applied to prevent oxidation of the metals and keeping the cast object clean from mold components. Experiments (Figure 8, Figure 9, Figure 10, Figure 11 and Figure 12) showed that many materials can fulfil these roles. Furthermore, even iron rust flakes or Gallium can also act as a separator or lubricant to prevent problems of incompatibility between some metals and glass (Bialek 2017, pp. 164–71).
4.3. Development of Tools
As previously mentioned, the application of metal inclusions causes many problems such as bonding materials to each other, a difficulty to control the shape of inclusions, and oxidation and rusting of the metal surface during the heating process. To solve these problems, it was necessary to develop new methods for applications available in a studio and to develop new tools. One of the newly designed tools involved stamps in the shape and size of metal inclusions (both parts were cut with waterjet) to make an indentation into the hot glass that the inclusion fits exactly into (Figure 13).

Figure 13.
Stamps to make indentations in hot glass and application of lead and tin alloy inclusions: (a) Three sizes of nickel alloy stamps and lead and tin alloy inclusions exactly in the shape of the stamps; (b,c) indentation made by a stamp in the GB shape; (d) indentation made by a stamp in a man silhouette shape; (e) application of lead and tin alloy inclusion; (f) lead and tin alloy inclusion covered by second layer of hot glass; created by author.
These stamps, when frequently used at high temperatures, should maintain an unchanged shape and surface. Nickel alloy (Cronifer 1925) in the form of a 3-mm-thick sheet met these conditions (Bialek 2017, pp. 151–52) and, therefore, was selected for the production of stamps.
4.4. Development of Firing Programs; Consideration of Annealing of Glass and Metal Inclusions Together
Annealing is a heat process, whereby a metal is heated to a specific temperature and then allowed to cool slowly. The question becomes how to define the annealing temperature for both materials in the same process, if they have different stress point temperatures. That is why annealing as a part of the firing process needs special consideration. Each object has to be treated separately due to the process used, combinations of types of glass and metal, and the size of object (Bialek 2017, pp. 172–81). However, the basis to constitute a general program was developed by the masters, Brychtová and Libensky, for glass annealing (Bialek 2017, p. 177).
By addressing these challenges, with the help of experts and experimentation, it was possible to introduce industrial processes to creative practice and, thus, extend the options available to artists for working with metal and glass in a workshop.
5. Conclusions
This paper introduced the author’s practice-based research. Firstly, it presented the developed novel printing methodologies (in 2000–2003), exposing new methods to embed imagery into glass. The method was introduced in Kevin Petrie’s (2006) book. This technique of image transfer to glass was predominantly used on the surfaces of cast and blown glass. The innovation was to fuse and/or cast multiple printed surfaces to create graphic image layers within the glass. Secondly, the paper defined and examined problems occurring during the application of metal inclusions in glass and presented methods for the selection of suitable materials and techniques.
In more detail, this paper described developments made around the process of inserting metal into glass with the aim of making it more efficient and practical. Some of the findings made a unique contribution to the artistic practice, for example, the development of a technique for pouring hot glass into a plaster mold without producing glass-distorting bubbles (Figure 12), which was previously thought impossible.
This paper also described experiments that incorporated the heating and annealing processes of both glass and metal to produce bespoke casting processes for various combinations of metals and kinds of glass. These processes can then be used with highly temperature-resistant molds, separators/lubricants, and metal stamps to eliminate sticking of the glass to the molds in a studio setting. Through the application of different lubricants and separators, the experiments also addressed the major problems of oxidation and corrosion of metals.
The research described in this paper discussed how the application of metal inclusions can be achieved by understanding reactions of metals in different working conditions and during the cooling and annealing process, and by the development of appropriate, controlled processes to reduce the difficulties faced during the inclusion application in glass. This was possible to achieve through a review of specialist literature and through collaboration with materials engineers who specialize in metal and/or glass. On the basis of these experiences, it was concluded that, to reduce the problems of applications of metal inclusions in glass in creative use, it would be very helpful to widen the cooperation between scientists and artists, and to encourage the publication of literature based on scientific knowledge expressed in a way that would be understandable by artists.
It is hoped that the subject of this research will provide a stimulus to increase the cooperation between scientists and artists, as well as promote an understanding of inclusions through a different approach to this subject.
Funding
Research support from Academy of Technology and Academy of Fine Art in Krakow, Poland; Wroclaw Fine Art Academy; Society of Glass Technology; Hempel Metals Ltd.; Durham University; Sunderland University.
Conflicts of Interest
The author declares no conflict of interest.
References
- Bialek, Goshka. 2000. Application of Inclusions in Glass by Hot Glass Techniques. Bachelor thesis, University of Sunderland, Sunderland, UK. [Google Scholar]
- Bialek, Goshka. 2003. Application of Inclusions in Glass; Particularly Printed Images. Master thesis, University of Sunderland, Sunderland, UK. [Google Scholar]
- Bialek, Goshka. 2017. Inner Space in Sculpture: The Use of Metal Inclusions in Glass. Ph.D. thesis, University of Sunderland, Sunderland, UK. Available online: http://sure.sunderland.ac.uk/8738/ (accessed on 29 November 2018).
- Bray, Charles. 2001. Dictionary of Glass; Materials and Techniques, 2nd ed.London: A & C Black. [Google Scholar]
- Crouse-Hinds Series. 2016. Oxygen Potential Measurements in Metal Heat Treatment Processes, Technical Note MTL Gas Analysers & Systems. TN05 520-1004 Rev3. Luton: Eaton Electric Limited. [Google Scholar]
- Engineering ToolBox. 2005. Metals-Melting Temperatures. Available online: https://www.engineeringtoolbox.com/melting-temperature-metals-d_860.html (accessed on 28 November 2018).
- Gordon, Cyrus H. 1968. Forgotten Scripts. New York: Basic Books. [Google Scholar]
- Greiner-Wrona, E. 2013. Interviews with Goshka Bialek by telephone, internet and meetings. Krakow and Durham. [Google Scholar]
- Greiner-Wrona, E. 2014. Interviews with Goshka Bialek by telephone, internet and meetings. Krakow and Durham. [Google Scholar]
- Greiner-Wrona, E. 2015. Interviews with Goshka Bialek by telephone, internet and meetings. Krakow and Durham. [Google Scholar]
- Hand, R. 2014. Interviews with Goshka Bialek by telephone and meetings. Durham, Cambridge and Sheffield. [Google Scholar]
- Hand, R. 2015. Interviews with Goshka Bialek by telephone and meetings. Durham, Cambridge and Sheffield. [Google Scholar]
- Holland, L. 1966. The Properties of Glass Surfaces. London: Chapman and Hall. [Google Scholar]
- Jacop, Leon. 1997. Factors that influence Spontaneous Failure in Thermally Treated Glass—Nickel Sulphide. Paper presented at Glass Processing Days, Tampere, Finland, 13–15 September. [Google Scholar]
- Laursen, Lucas. 2013. Data for the 31st Century. IEEE Spectrum 50: 14. [Google Scholar] [CrossRef]
- Ojovan, Michael I., and William Bill E. Lee. 2010. Connectivity and glass transition in disordered oxide systems. Journal of Non-Crystalline Solids 356: 2534–2540. [Google Scholar] [CrossRef]
- Pellatt, Apsley. 1949. Curiosities of Glass Making. London: David Bogue, p. 64. [Google Scholar]
- Petrie, Kevin. 2006. Glass and Print. London: A&C Black/American Ceramic Society. [Google Scholar]
- Pettit, Fred. 2011. Hot Corrosion of metals and Alloys. Oxidation of Metals 76: 1–21. [Google Scholar] [CrossRef]
- Schuler, Frederic, and Lilli Schuler. 1971. Glassforming, Glassmaking for the Craftsman. London: Pitman Publishing. [Google Scholar]
- Skrzypek, Stanisław J., and Karol Przybylowicz. 2012. Inzynieria metali i ich stopow. Krakow: Wydawnistwa AGH. [Google Scholar]
- Svensson, K. 2013. Telephone conversations with Goshka Bialek in Durham. 6 June 2013. [Google Scholar]
- The Engineering ToolBox. 2014. Coefficients of Linear Thermal Expansion. Resources, Tools and Basic Information for Engineering and Design of Technical Applications. Available online: http://www.engineeringtoolbox.com/linear-expansion-coefficients-d95.html (accessed on 24 November 2016).
- Walker, Brad. 2010. Contemporary Fused Glass. Clemmons: Four Corners International, Inc. [Google Scholar]
- Whitehouse, David. 1993. Glass: A Pocket Dictionary of Terms Commonly Used to Describe Glass and Glassmaking. Corning: The Corning Museum of Glass. [Google Scholar]
- Young, David John. 2008. High Temperature Oxidation and Corrosion of Metals. Oxford: Elsevier. [Google Scholar]
- Zasadzinski, J. 2012. Interview with Goshka Bialek by telephone, internet and meetings. Krakow. [Google Scholar]
- Zasadzinski, J. 2013. Interview with Goshka Bialek by telephone, internet and meetings. Krakow. [Google Scholar]
- Zasadzinski, J. 2014. Interview with Goshka Bialek by telephone, internet and meetings. Krakow. [Google Scholar]
- Zasadzinski, J. 2015. Interview with Goshka Bialek by telephone, internet and meetings. Krakow. [Google Scholar]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).













