Abstract
Cracks and other defects in concrete will affect its durability, thermal insulation effect, and energy consumption. ASR is one of the common causes of concrete cracks. In this study, mortar specimens modified with fly ash were prepared using the mortar bar rapid method, and the inhibition effect of ASR in two alkali environments and the building energy efficiency characteristics were comparatively analyzed. SEM, EDS, and XRD were used to analyze the microstructure, elemental distribution, and products in the specimens’ interfacial transition zone comparatively. The results show that a replacement amount of 20–30% fly ash can restrain ASR expansion and maintain high mechanical strength. In both alkaline environments, K and Al are enriched in the interface transition zone and combine with SiO2 and Al2O3 to form a stable framework aluminosilicate mineral. In addition to the inhibition effect of fly ash on ASR, the external wall heat dissipation flux decreased from 132.6 W/m2 to 117.6 W/m2, a decrease of 11.3%, and the overall envelope heat dissipation flux decreased by 9.2%, significantly reducing building energy consumption. This study provides a new perspective for the development of building energy-saving materials and helps green buildings and sustainable development.
1. Introduction
The construction industry consumes a substantial amount of energy, accounting for 40% of global total energy consumption [1,2]. A significant portion of this energy loss originates from building envelope systems, making enhancing concrete insulation performance crucial for energy conservation [3,4]. However, alkali–silica reaction (ASR) is one of the main causes of reduced durability in concrete structures. The formation of ASR gel increases the volumetric expansion within the concrete and leads to surface cracking [5]. If concrete is affected by ASR, it can result in cracking, a decline in mechanical properties, and increased heat transfer, which poses a serious threat to energy efficiency and the structural safety of buildings [6,7,8]. It has been shown that the thermal conductivity after cracking the material increases with the increase in water content, and the value of the thermal conductivity of the cracked material may be higher than that of the uncracked material [9]. The occurrence of ASR is complex [10], and the failure phenomenon still occurs occasionally. The cracking of ASR can be effectively inhibited by adding the appropriate amount of fly ash [11,12]. Fly ash is a kind of industrial waste. Adding fly ash into concrete not only realizes the reuse of resources, but also makes the concrete structure more compact, effectively hinders the circulation of alkali, and improves the durability of concrete [13]. The addition of fly ash can form a gel with low Ca/Si, which can adsorb a large amount of alkali [14,15] and reduce the alkalinity of pore solution [16] to inhibit ASR expansion. In addition, the aluminum in mineral admixtures containing more Al2O3 can react to form C-A-S-H gel with a stable structure, which can also reduce the risk of ASR [17,18]. However, the occurrence of ASR is affected by many factors, especially the types, characteristics, and products of alkali [19,20]. It has been found that ASR expansion is closely related to Na/Si and K/Si values [21], and the ASR expansion caused by Na is much larger than that of K [22]. In addition, ASR products generated by different lye at high-temperature vapor pressures are also different [23]. Shuaicheng Guo et al. found that different types of alkali (K+, Na+), alkali–silica ratio, and calcium content cause great differences in the gel’s swelling [24]. Most research on the inhibition of ASR by admixtures focuses on the Na environment, but the K environment is more common in nature [19], while there are few studies on the inhibition of ASR by admixtures in different alkali environments.
This study aims to deeply understand the inhibitory effect of admixtures on ASR in different alkali environments and explore the impact of ASR on building energy efficiency. Concrete specimens were prepared with fly ash as the admixture and soaked in a NaOH/KOH environment. The expansion rate and mechanical properties were measured. The microstructure, element distribution, and products of the sample interface transition zone were analyzed with a scanning electron microscope (SEM), Energy Dispersive Spectroscopy (EDS), and X-ray diffraction (XRD). The simulation software was used to explore fly ash’s influence on the building envelope’s heat dissipation. The research results will provide the basis for the selection and proportion of mineral admixtures in the future and will have important significance for improving concrete safety, durability, and energy saving.
2. Materials and Method
2.1. Materials
This study uses P.II 42.5R Portland cement, the relevant physical and mechanical properties and chemical composition of which are shown in Table 1 and Table 2, respectively. The main physical properties of cement are determined according to the GB175-2007 [25] Standard for General Portland Cement, and the specific surface area is 360 m2/Kg (which meets the requirements of standard > 300 m2/kg).

Table 1.
Main physical properties of cement.

Table 2.
Chemical component content of cement, fly ash.
The fly ash is grade II, which is obtained from the industrial waste residue of a power plant. According to the standard fly ash used for cement and concrete (GB/T1596-2017) [26], the sieve residue of 45 µm sieve was 18.7%, the loss on ignition was 3.19%, and the activity index of 28 days was 73%. The chemical composition is shown in Table 2.
The crushed stone of a tunnel, meta-feldspathic quartz sandstone, is taken as the aggregate of this test. The surface impurities of large aggregates are cleaned and then crushed with a crusher to a range of 0.15–4.75 mm. The expansion rates of pure cement samples in KOH and NaOH alkali environments for 14 days determined in this test are 0.27% and 0.35%, respectively. The expansion rate of the sample meets the requirements of the JGJ 52-2006 [27] standard for technical requirements and the test method of sand and crushed stone (or gravel) for ordinary concrete. The standard stipulates that the 14 d expansion rate of the specimen must be greater than 0.2% when the alkali activity test method (rapid method) of gravel or pebble is used, indicating that the aggregates in this test are active aggregates with potentially hazardous reactions.
2.2. Experimental Methods
The test piece is made according to the alkali activity test of gravel or pebble (rapid method), derived from JGJ 52-2006, the standard for technical requirements, and the sand and crushed stone (or gravel) test method for ordinary concrete. The equivalent alkali of cement is calculated as Na2O + 0.658 K2O, and the Na2Oeq value is 0.5%, which is adjusted to 1.0% by adding analytical pure NaOH/KOH. Two test pieces are made as follows: the reference test piece (pure cement + active aggregate) and the admixture test piece (20%, 30%, and 40% fly ash instead of Portland cement + active aggregate). The specific proportions of the test specimens are shown in Table 3.

Table 3.
The ratio of mortar specimens.
One group of specimens with a size of 25 mm × 25 mm × 280 mm (3 pieces in each group) is used to measure the expansion rate, as shown in Figure 1a, and four groups of specimens with a size of 40 mm × 40 mm × 160 mm (3 pieces in each group) are made to measure the strength, as shown in Figure 1b. The test piece is demolded and numbered after standard curing for 24 ± 2 h, then cured in water at 80 ± 2 °C for 24 h, and the reference length (L0) is measured. WN1/WK1 is immersed in tap water, while the admixture test piece and WN2/WK2 are immersed in 1 mol/L NaOH/KOH alkali solution. On the 3rd, 7th, 14th, 28th, 58th, and 60th days of specimen maintenance, its expansion rate is measured, and its mechanical properties at the 7th, 14th, 28th, and 60th days are measured.

Figure 1.
Two sizes of mortar bar specimens. (a) 25 mm × 25 mm × 280 mm; (b) 40 mm × 40 mm × 160 mm.
Where W refers to the pure cement reference specimen, N refers to NaOH, K refers to KOH, and WN1/WK1 refers to using the NaOH/KOH analytical reagent to make the equivalent alkali of cement reach 1% and soaking in tap water at 80 °C. WN2/WK2 are pure cement specimens soaked in an 80 °C, 1 mol/L NaOH/KOH environment. F refers to the fly ash test piece. Here, 352/387 represents a cement consumption of 25 mm × 25 mm × 280 mm/40 mm × 40 mm × 160 mm specimens, respectively, and the consumption of other materials is similar.
The samples soaked for 60 days are taken out, and the microstructure and element distribution of the interface transition zone between the aggregate and the cementitious material is observed using SEM and EDS. XRD is used to investigate the products of ASR.
3. Results
3.1. ASR Expansion of Specimens in Different Alkali Environments
On 3 d, 7 d, 14 d, 28 d, 58 d, and 60 d, the specimens WK1, WN1, WK2, WN2, FK2, FK3, FK4, FN2, FN3, and FN4 were taken out from the solution to determine their expansion rates.
It can be seen from Figure 2 that the WK1 and WN1 specimens did not expand significantly within 60 days, due to a short erosion time with a low erosion degree. However, the 14 d expansion rate of WK2 and WN2 specimens far exceeded the ASR threshold specified in the JGJ 52-2006 standard, and ASR occurred obviously. The expansion rate of the WK2 specimen is smaller than that of the WN2 specimen because the hydration radius of Na+ is larger, and the expansion of Na-rich alkali silica gel is more significant [23]. As shown in Figure 2, under different alkali environments, the expansion rate of some cement specimens replaced by fly ash is lower than that of the reference specimens (WK2 and WN2), indicating that the addition of fly ash can effectively inhibit ASR, and the higher the content, the lower the expansion rate. When the replacement amount is low, the inhibition effect of fly ash on ASR in the NaOH environment is better. With the increase in the substitution amount, its inhibition effect in the KOH environment is more significant. Based on the 60-day expansion rate, the expansion rate of each specimen is ranked as follows: FK4 < FN4 < FK3 < FN3 < FN2 < FK2.
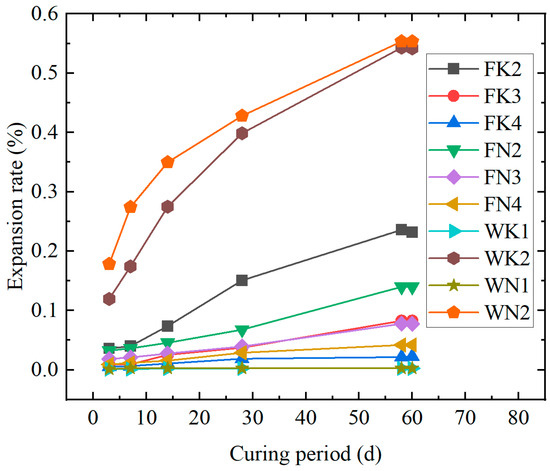
Figure 2.
Comparison of the expansion rate of fly ash mixed specimens under different alkali environments.
3.2. Comparative Analysis of Mechanical Properties
As shown in Figure 3, the compressive strength of the fly ash specimens (FK2, FN2, FK3, and FN3) was 7–12% higher than that of the pure cement specimens (WK2 and WN2) after 28 days. However, only FN2 remained dominant at 28 days. The strength of the fly ash specimens is higher than that of the pure cement specimens (WK2 and WN2) because the fineness of the admixtures is better than that of cement, which can more effectively fill the pores, thus improving the early strength. However, after the cement was partially replaced, the amount of cement was relatively reduced, the hydration products were reduced, and the secondary hydration reaction was delayed, resulting in a slowdown in the strength growth at 28 days. The strength of the WK2 and WN2 specimens decreased significantly due to ASR after 28 days, while the specimens mixed with fly ash showed higher strength. When the replacement amount of fly ash reached 40% (FK4 and FN4), due to the reduction in cement hydration products, the strength-enhancing gel generated by the secondary hydration reaction also reduced accordingly, and the admixture itself could not play the role of hydration setting, resulting in low strength. At the same time, the strength of the specimens mixed with fly ash decreased with the increase in replacement amount, and the strength is negatively correlated with the expansion rate. The greater the expansion rate, the lower the strength. Comprehensive analysis shows that the amount of fly ash replacing cement should be between 20% and 30%, which can not only effectively inhibit ASR expansion, but also ensure the strength of specimens.
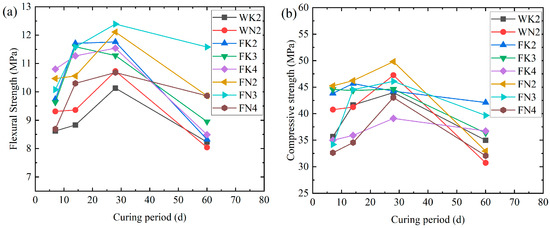
Figure 3.
Relationship between (a) flexural strength, (b) compressive strength, and specimen age.
3.3. SEM/EDS Analysis of the Interface Transition Zone
ASR mainly occurs in the interface transition zone between aggregates and cementitious materials. This study focuses on the elements and products in this region. The samples cured for 60 d were scanned using SEM and EDS to obtain the distribution of Si, Ca, Na, K, and Al elements in the interface transition zone.
It can be seen from Figure 4 that the interface structure of fly ash replacing part of the cement sample is denser than that of the pure cement reference sample. It can be seen from Figure 5 that, after replacing cement with fly ash, the content of the Si element increases compared with that of WK2 and WN2, increases with the increase in replacement amount, and is evenly distributed. The content of Ca decreases with the increase in fly ash content, which is more uniform than that of WK2 and WN2. In the KOH environment, the Na element is not enriched. Still, in a NaOH environment, WN2 is enriched with Na; in addition, the others gradually decrease and tend to be uniform with the admixture increase. It is worth noting that the K and Al elements are enriched in the interface transition zone in both alkali environments, indicating a mutual attraction between Al and K elements.
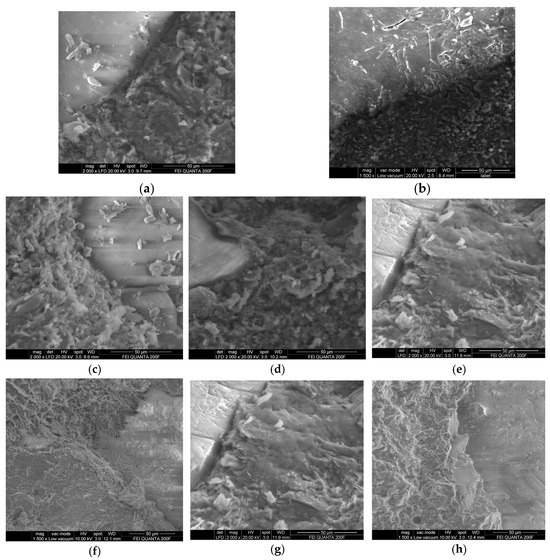
Figure 4.
SEM images of different specimens. (a) WK2; (b) WN2; (c) FK2; (d) FK3; (e) FK4; (f) FN2; (g) FN3; (h) FN4.
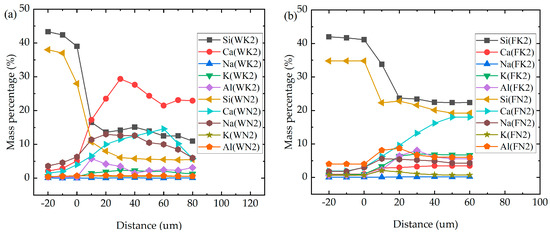
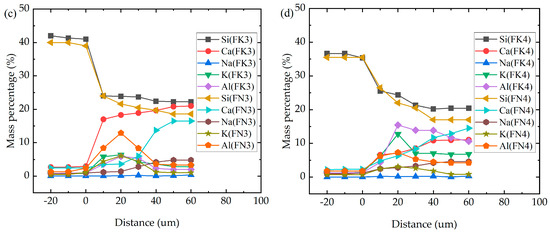
Figure 5.
Elements in the interface area of pure cement and fly ash mixed specimen. (a) pure cement specime; (b) 20% fly ash instead of Portland cement; (c) 30% fly ash instead of Portland cement; (d) 40% fly ash instead of Portland cement.
Al is adsorbed by SiO2 in the aggregate in the pore solution to form aluminosilicate and then reacts with Ca(OH)2 to form C-A-S-H gel [17,18]. In this gel, Al3+ will replace part of Si4+ to form a negatively charged aluminum oxide tetrahedron. Because the valence state of Al3⁺ (aluminum ion) is one unit lower than that of Si4⁺ (silicon ion), it just attracts alkali ions with positive valence (such as K+ and Na+). It enhances the gel’s alkali fixation ability. In addition, K+ and Na+ are more straightforward to combine with Al2O3 than Ca2+ to form alkali aluminosilicate gel [28]. Because the radius of the K+ ion is more significant than that of Na+, K+ is more straightforward to combine with Al3+, and K+ is attracted by the active ingredients in aggregates and cementitious materials, enriching K and Al in the interface region. Although there is K enrichment in the interface region, the expansion rate of the sample mixed with fly ash is very low, indicating that K and Al may form substances that do not readily absorb water. In the KOH environment, when a small amount of fly ash is added, the Al content is low, and the K content is high, more Al reacts in the far aggregate area, while the K enrichment in the interface transition zone occurs, resulting in ASR. In the NaOH environment, the high silica content of fly ash prevents the migration of Na+ to the interface transition zone and effectively prevents ASR, which may be the reason why the expansion rate of FK2 is slightly higher than that of FN2. When the content of fly ash is sufficient, Al2O3 increases, K+ reacts with active SiO2 and Al2O3 to form alkali aluminosilicate gel with a stable volume, and it finally crystallizes into a stable mineral [29]. The influence of K+ on ASR is lower than that of Na+, so the expansion rate of the FK series decreases with the increase in dosage, which is better than that of the FN series.
3.4. Element Ratio of Products in the Interface Area
The properties of ASR gel are not only related to Si, Ca, and other elements, but also depend on the atomic ratio of these elements [21]. As shown in Figure 6, compared with WK2 and WN2, the element ratio of the interface transition zone of the sample mixed with fly ash is lower than that of the reference sample in Ca/Si, Na/Si, and Ca/Al, while K/Si and Al/Si are higher and have a sudden increase. The higher the replacement amount, the more obvious the change. It has been found that the higher the Na/Si and K/Si values, the greater the swelling rate and water absorption of the gel, which is more likely to cause ASR [21]. However, after the admixture partially replaces the cement, Na/Si decreases, K/Si increases, and the expansion rate is lower than that of WK2 and WN2 specimens. It can be seen that it is not comprehensive to judge whether ASR occurs only by Na/Si and K/Si values. In addition, studies have shown that the C-S-H gel with low Ca/Si generated by the secondary hydration reaction has a high specific surface area and a strong ability to adsorb cations [30]. This experiment found that Ca/Al also has a low concave phenomenon in the interface region. In some experiments, ASR gels with different Al/Si were prepared. It was found that an increase in aluminum can reduce swelling and silicate leaching, create a compact structure, and reduce water absorption [31]. Schumacher believes mineral admixtures with high Al2O3 content can more effectively inhibit ASR [17]. SiO2 first reacts with OH to form acidic Si-OH [32]. The introduction of Al can also increase the acidity and concentration of Si-OH and enhance the adsorption capacity of alkali. Adding mineral admixtures can reduce the gel’s Ca/Si and Ca/Al, enhancing its adsorption and fixation ability to alkali. Therefore, it is one-sided to attribute the mechanism of mineral admixture inhibiting ASR solely to the low CaO content, and adding Al2O3 is also key.
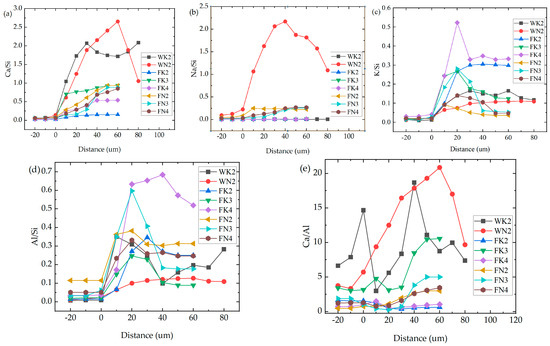
Figure 6.
Element ratio of pure cement and fly ash samples. (a) Ca/Si; (b) Na/Si; (c) K/Si; (d) Al/Si; (e) Ca/Al.
3.5. XRD Analysis
To further verify the mechanism of mineral admixtures inhibiting ASR, the samples of WK2, WN2, FK4, and FN4 soaked for 60 days were analyzed using XRD (Figure 7). The results show that KAlSi2O6 (leucite) and KAl3Si3O11 (nepheline) are formed in FK4 under the KOH environment, and KAlSi2O4 also exists in FN4, which are all framework aluminosilicate minerals, further indicating that the enrichment of K and Al in the interface transition zone is related to the inhibition of ASR. In such minerals, aluminum atoms replace some silicon atoms to form aluminum oxygen tetrahedrons, which are negatively charged and attract positive alkali ions for compensation due to the charge imbalance caused by the lack of one valence of aluminum [31]. This structure allows cations and water molecules to pass freely, keeps the crystal stable and not easy to expand, and enhances the cation adsorption capacity due to the large specific surface area.
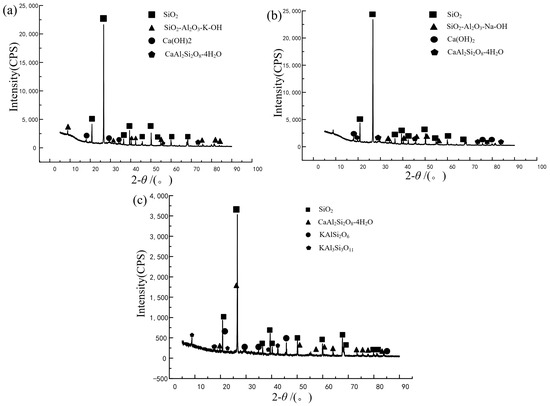
Figure 7.
XRD pattern. (a) WK2;, (b) WN2; (c) FK4.
To sum up, the internal mechanism of mineral admixtures’ inhibition of ASR mainly lies in the fact that they are rich in active SiO2 and Al2O3, are especially high in Al2O3 content, and produce C-A-S-H gel with low Ca/Si and Ca/Al through a secondary hydration reaction, which has a strong alkali fixation ability. Because of its larger ion radius, K is more straightforward to combine with Al2O3, forming a stable alkali Al-Si ternary system gel in the interface transition zone and finally crystallizing into a frame like an aluminosilicate mineral that cannot quickly expand. Meanwhile, Na+ reacts with the excess silicon in the admixture, which is far away from the aggregate area and retained.
3.6. The Impact of Building Energy Conservation
The research shows that fly ash affects the thermal conductivity of concrete [33], and the thermal conductivity of ordinary concrete is 1.82 W/(m∙K) [9]. Under the same curing procedure of concrete specimens, the thermal conductivity values of F2 and F4 concrete specimens with 20% and 40% fly ash are 1.54 W/(m∙K) and 1.47 W/(m∙K) [33], respectivley, and the density values of F2 and F4 specimens are 2185 kg/m3 and 2086.67 kg/m3, respectively.
To explore the inhibition of ASR and its impact on building energy conservation, we established a model of a three-dimensional shear wall room with a shear wall thickness of 250 mm, an internal wall thickness of 250 mm, and a floor thickness of 150 mm, as shown in Figure 8. The code for design of heating ventilation and air conditioning of civil buildings (GB50736-2012) [34] takes the winter working conditions in Nanjing as an example. The design temperature of the outdoor air conditioning is −4.1 °C, the average wind speed is 2.6 m/s, the heating room temperature is 26 °C, and the unheated temperature of three adjacent rooms is 10 °C. The influence of different types of concrete on building heat dissipation is analyzed. The calculation formula for concrete heat dissipation flux is as follows [35]:
where q is the heat dissipation flux, W/m2; λ is the thermal conductivity, W/(m·K); to and ti are the outdoor and indoor temperatures, °C, respectively; δ is the thickness of the wall, m; A is the area of the wall, m2; and ho and hi are the outdoor and indoor convective heat transfer coefficients W(m·K), respectivley.
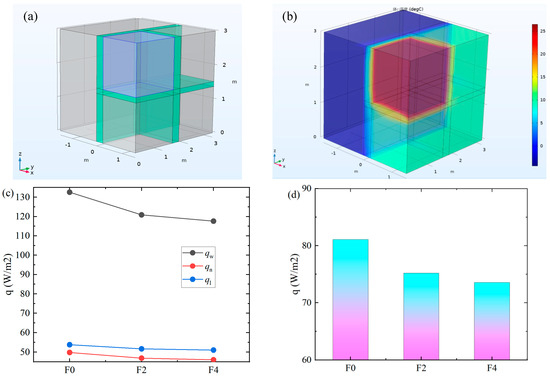
Figure 8.
Influence of fly ash on building heat dissipation. (a) 3D room model; (b) Thermal diagram of the room (“体: 温度”: Body surface: temperature); (c) heat dissipation flux of concrete; (d) average heat dissipation flux.
Figure 8a shows the distribution of shear wall rooms through external walls, internal walls, floors, and three adjacent rooms. It can be seen from Figure 8b that the temperature of the heated room is significantly higher than that of the outdoor and unheated rooms, so the thermal insulation performance of the concrete plays a vital role in the heat dissipation of the room. Figure 8c shows the heat dissipation flux of ordinary concrete F0, concrete F2 with 20% fly ash content, and concrete F4 with 40% fly ash content through the external wall qw, internal wall qn, and floor ql. It can be seen from the figure that, with the increase in fly ash content, the heat dissipation flux through the maintenance structure is smaller, and the heat dissipation flux of the external wall is reduced from 132.6 W/m2 to 117.6 W/m2. qw, qn, and ql are reduced by 11.3%, 7.6%, and 5.2%, respectively. It can be seen that adding fly ash most obviously improves the heat dissipation of the external wall. The heat dissipation flux of the external wall is the largest, followed by that of the floor, and the heat dissipation flux of the internal wall is the smallest. The heat dissipation flux of the external wall is 1.66 times that of the internal wall. This is mainly because the temperature difference in the external wall is large and the thickness of the internal wall is greater than that of the floor. The inhibition effect of ASR reduces the cracking and thermal conductivity of concrete. The average heat dissipation flux of the enclosure structure is reduced from 81.0 W/m2 to 73.6 W/m2, which is a reduction of 9.2%, and the building energy consumption is reduced, as shown in Figure 8d.
4. Conclusions
- (1)
- Fly ash can effectively inhibit the ASR expansion in NaOH and KOH environments. The expansion rate gradually decreases with the increase in fly ash content, and the inhibition effect is more evident in the KOH environment. A replacement amount of 20–30% fly ash can restrain ASR expansion and maintain high mechanical strength.
- (2)
- The internal mechanism of mineral admixture inhibition of ASR mainly lies in the fact that they are rich in active SiO2 and Al2O3, are especially high in Al2O3 content, and produce C-A-S-H gel with low Ca/Si and Ca/Al through the secondary hydration reaction, which has a strong solid alkali ability. The results show that both alkali environments enrich K and Al elements in the interface transition zone. K+ combines with a high content of SiO2 and Al2O3 in mineral admixtures and aggregates and crystallizes into non-expansive frames like aluminosilicate minerals in the interface transition zone, enhancing concrete’s durability and crack resistance. Na+ reacts with the excess silicon in the admixture, which is far away from the aggregate area and is retained.
- (3)
- The addition of fly ash also significantly improves the thermal insulation performance of concrete. With the addition of fly ash, the heat dissipation flux of the external wall is reduced by 11.3%, and the heat dissipation flux of the overall enclosure structure is reduced by 9.2%, effectively reducing the building energy consumption.
Author Contributions
Conceptualization, L.C. and L.W.; Investigation, J.Z. (Jiang Zheng); Data curation, L.C., L.W. and J.Z. (Jiang Zheng); Writing—original draft, L.C.; Writing—review & editing, J.Z. (Junming Zhou); Project administration, J.Z. (Junming Zhou). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Open Project Program of the Engineering Research Center of Building Energy Efficiency Control and Evaluation, Ministry of Education, grant number [AHJZNX-2022].
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Salihi, M.; El Mastouri, M.; El Fiti, M.; Harmen, Y.; Chebak, A.; Jama, C.; Chhiti, Y. Calcium Carbonate-Shelled Microencapsulated Phase Change Materials in Cement Mortar: A Pathway to Enhancing Energy Efficiency in Building Envelopes. J. Energy Storage 2025, 110, 115306. [Google Scholar] [CrossRef]
- Dominguez, C.; Kakkos, E.; Gross, D.; Hischier, R.; Orehounig, K. Renovated or Replaced? Finding the Optimal Solution for an Existing Building Considering Cumulative CO2 Emissions, Energy Consumption and Costs—A Case Study. Energy Build. 2024, 303, 113767. [Google Scholar] [CrossRef]
- Biswas, K.; Shrestha, S.; Hun, D.; Atchley, J. Thermally Anisotropic Composites for Improving the Energy Efficiency of Building Envelopes. Energies 2019, 12, 3783. [Google Scholar] [CrossRef]
- Salihi, M.; Chhiti, Y.; El Fiti, M.; Harmen, Y.; Chebak, A.; Jama, C. Enhancement of Buildings Energy Efficiency Using Passive PCM Coupled with Natural Ventilation in the Moroccan Climate Zones. Energy Build. 2024, 315, 114322. [Google Scholar] [CrossRef]
- Olajide, O.D.; Nokken, M.R.; Sanchez, L.F.M. Evaluation of the Induced Mechanical Deterioration of ASR-Affected Concrete Under Varied Moisture and Temperature Conditions. Cem. Concr. Compos. 2025, 157, 105942. [Google Scholar] [CrossRef]
- Doğruyol, M. Determination of ASR in Concrete Using Characterization Methods. Buildings 2024, 14, 657. [Google Scholar] [CrossRef]
- Tapas, M.J.; Thomas, P.; Vessalas, K.; Nsiah-Baafi, E.; Martin, L.; Sirivivatnanon, V. Comparative Study of the Efficacy of Fly Ash and Reactive Aggregate Powders in Mitigating Alkali-Silica Reaction. J. Build. Eng. 2023, 63, 105571. [Google Scholar] [CrossRef]
- Rajabipour, F.; Giannini, E.; Dunant, C.; Ideker, J.H.; Thomas, M.D.A. Alkali–Silica Reaction: Current Understanding of the Reaction Mechanisms and the Knowledge Gaps. Cem. Concr. Res. 2015, 76, 130–146. [Google Scholar] [CrossRef]
- Vejmelková, E.; Padevět, P.; Černý, R. Effect of Cracks on Hygric and Thermal Characteristics of Concrete. Bauphysik 2008, 30, 438–444. [Google Scholar] [CrossRef]
- Fanijo, E.O.; Kolawole, J.T.; Almakrab, A. Alkali-Silica Reaction (ASR) in Concrete Structures: Mechanisms, Effects and Evaluation Test Methods Adopted in the United States. Case Stud. Constr. Mater. 2021, 15, e00563. [Google Scholar] [CrossRef]
- Latifee, E.R. State-of-the-Art Report on Alkali Silica Reactivity Mitigation Effectiveness Using Different Types of Fly Ashes. J. Mater. 2016, 2016, 7871206. [Google Scholar] [CrossRef]
- Kandasamy, S.; Shehata, M.H. The Capacity of Ternary Blends Containing Slag and High-Calcium Fly Ash to Mitigate Alkali Silica Reaction. Cem. Concr. Compos. 2014, 49, 92–99. [Google Scholar] [CrossRef]
- Kawabata, Y.; Yamada, K. Evaluation of Alkalinity of Pore Solution Based on the Phase Composition of Cement Hydrates with Supplementary Cementitious Materials and Its Relation to Suppressing ASR Expansion. ACT 2015, 13, 538–553. [Google Scholar] [CrossRef]
- Kawabata, Y.; Yamada, K. The Mechanism of Limited Inhibition by Fly Ash on Expansion Due to Alkali–Silica Reaction at the Pessimum Proportion. Cem. Concr. Res. 2017, 92, 1–15. [Google Scholar] [CrossRef]
- Shayan, A.; Diggins, R.; Ivanusec, I. Effectiveness of Fly Ash in Preventing Deleterious Expansion Due to Alkali-Aggregate Reaction in Normal and Steam-Cured Concrete. Cem. Concr. Res. 1996, 26, 153–164. [Google Scholar] [CrossRef]
- Stade, H. On the Reaction of C-S-H(Di, Poly) with Alkali Hydroxides. Cem. Concr. Res. 1989, 19, 802–810. [Google Scholar] [CrossRef]
- Schumacher, K.A.; Ideker, J.H. New Considerations in Predicting Mitigation of Alkali-Silica Reaction Based on Fly Ash Chemistry. J. Mater. Civ. Eng. 2015, 27, 04014144. [Google Scholar] [CrossRef]
- Hay, R.; Ostertag, C.P. On Utilization and Mechanisms of Waste Aluminium in Mitigating Alkali-Silica Reaction (ASR) in Concrete. J. Clean. Prod. 2019, 212, 864–879. [Google Scholar] [CrossRef]
- Shi, Z.; Geng, G.; Leemann, A.; Lothenbach, B. Synthesis, Characterization, and Water Uptake Property of Alkali-Silica Reaction Products. Cem. Concr. Res. 2019, 121, 58–71. [Google Scholar] [CrossRef]
- Leemann, A.; Lothenbach, B. The Influence of Potassium–Sodium Ratio in Cement on Concrete Expansion Due to Alkali-Aggregate Reaction. Cem. Concr. Res. 2008, 38, 1162–1168. [Google Scholar] [CrossRef]
- Gholizadeh-Vayghan, A.; Rajabipour, F. The Influence of Alkali–Silica Reaction (ASR) Gel Composition on Its Hydrophilic Properties and Free Swelling in Contact with Water Vapor. Cem. Concr. Res. 2017, 94, 49–58. [Google Scholar] [CrossRef]
- Ma, P.; Li, J.; Bai, J.; Zhuo, Y.; Chi, L.; Zhu, Y.; Shi, Z.; Ma, H.; Chen, G. Effect of Type and Quantity of Inherent Alkali Cations on Alkali-Silica Reaction. Cem. Concr. Res. 2023, 173, 107293. [Google Scholar] [CrossRef]
- Lu, D.; Mei, L.; Xu, Z.; Tang, M.; Mo, X.; Fournier, B. Alteration of Alkali Reactive Aggregates Autoclaved in Different Alkali Solutions and Application to Alkali-Aggregate Reaction in Concrete (II) Expansion and Microstructure of Concrete Microbar. Cem. Concr. Res. 2006, 36, 1191–1200. [Google Scholar] [CrossRef]
- Guo, S.; Dai, Q.; Si, R. Effect of Calcium and Lithium on Alkali-Silica Reaction Kinetics and Phase Development. Cem. Concr. Res. 2019, 115, 220–229. [Google Scholar] [CrossRef]
- GB175-2007; Common Portland Cement. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2007.
- GB/T1596-2017; Fly Ash Used for Cement and Concrete. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2007.
- JGJ 52-2006; Standard for Technical Requirements and Test Method of Sand and Crushed Stone (or Gravel) for Ordinary Concrete. Ministry of Construction of the People’s Republic of China: Beijing, China, 2006.
- Saha, A.K.; Khan, M.N.N.; Sarker, P.K.; Shaikh, F.A.; Pramanik, A. The ASR Mechanism of Reactive Aggregates in Concrete and Its Mitigation by Fly Ash: A Critical Review. Constr. Build. Mater. 2018, 171, 743–758. [Google Scholar] [CrossRef]
- Park, S.-M.; Jang, J.-G.; Chae, S.-A.; Lee, H.-K. An NMR Spectroscopic Investigation of Aluminosilicate Gel in Alkali-Activated Fly Ash in a CO2-Rich Environment. Materials 2016, 9, 308. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Miura, M. Modified Model of Alkali-Silica Reaction. Cem. Concr. Res. 2007, 37, 1291–1297. [Google Scholar] [CrossRef]
- Krüger, M.E.; Hilbig, H.; Stelzner, L.; Machner, A. Effect of the Chemical Composition of Synthetic Alkali-Silica Gels on Their Structure, Swelling Behavior and Water Uptake. Cem. Concr. Res. 2024, 184, 107596. [Google Scholar] [CrossRef]
- Thomas, M. The Effect of Supplementary Cementing Materials on Alkali-Silica Reaction: A Review. Cem. Concr. Res. 2011, 41, 1224–1231. [Google Scholar] [CrossRef]
- Yang, I.-H.; Park, J.; Kim, K.-C.; Yoo, S.-W. A Comparative Study on the Thermal Conductivity of Concrete with Coal Bottom Ash under Different Drying Conditions. Adv. Civ. Eng. 2021, 2021, 7449298. [Google Scholar] [CrossRef]
- GB50736-2012; Design Code for Heating Ventilation and Air Conditioning of Civil Buildings. Ministry of Housing and Urban Rural Development of the People’s Republic of China: Beijing, China, 2012.
- Lin, W.; Liu, X.; Li, S.; Zhang, T. Investigation on Thermal Environment and Heat Transfer Characteristics in Ice Rinks with Different Envelopes. Build. Environ. 2022, 219, 109250. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).