Theoretical Calculation Modeling of Thermal Conductivity of Geopolymer Foam Concrete in Building Structures Based on Image Recognition
Abstract
1. Introduction
2. Calculation Model of Thermal Conductivity of GFC
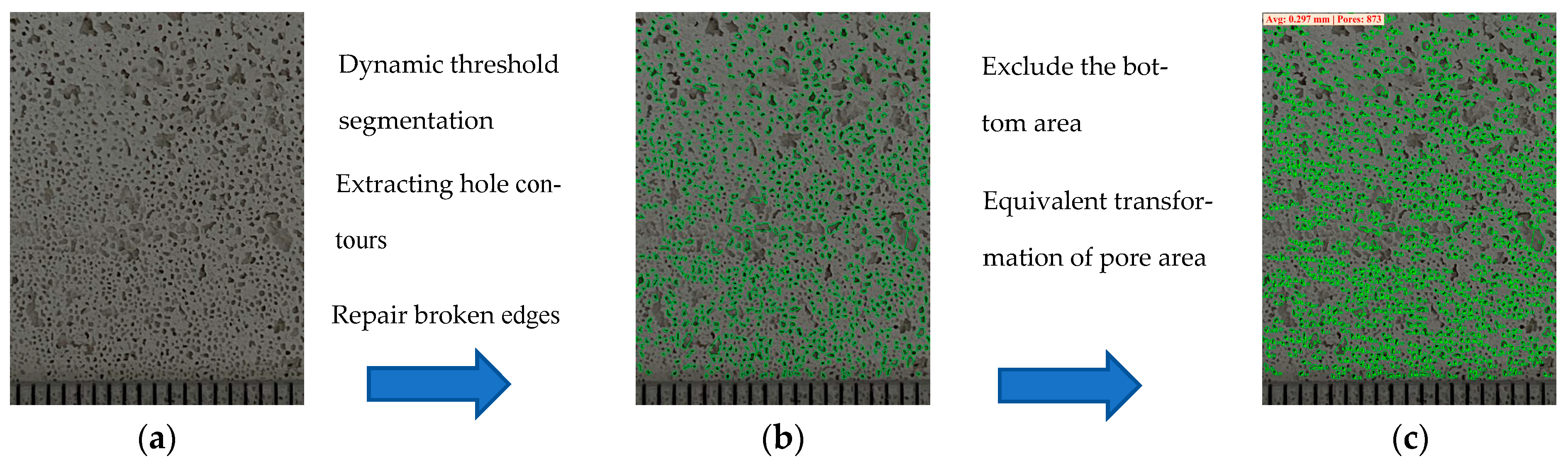
3. Pore Size Statistical Method Based on Image Recognition
4. Test Verification
4.1. Materials and Methods
4.2. Test Methods
4.2.1. Thermal Conductivity
4.2.2. Dry Density
4.2.3. Porosity
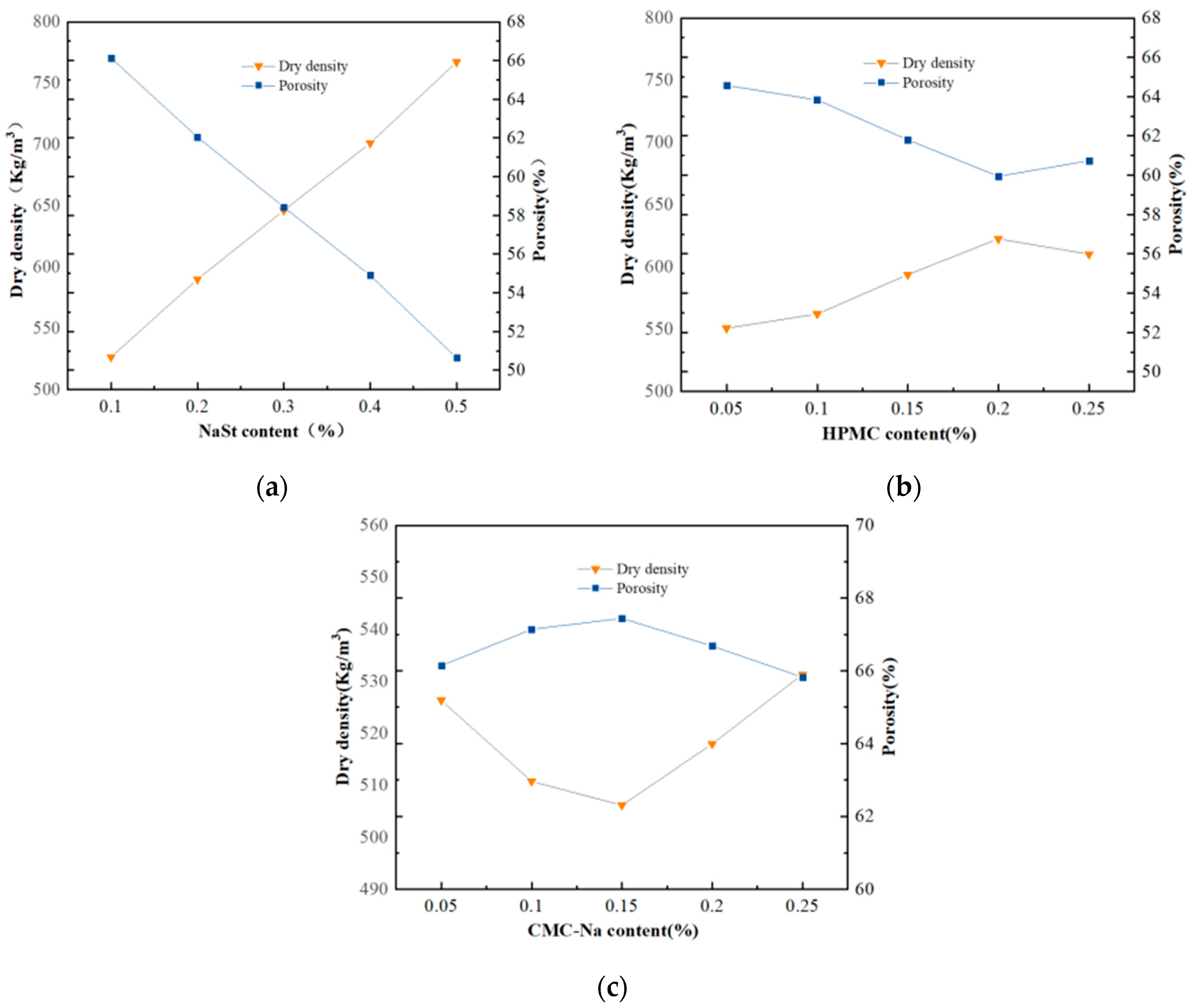
4.3. Dry Density and Porosity
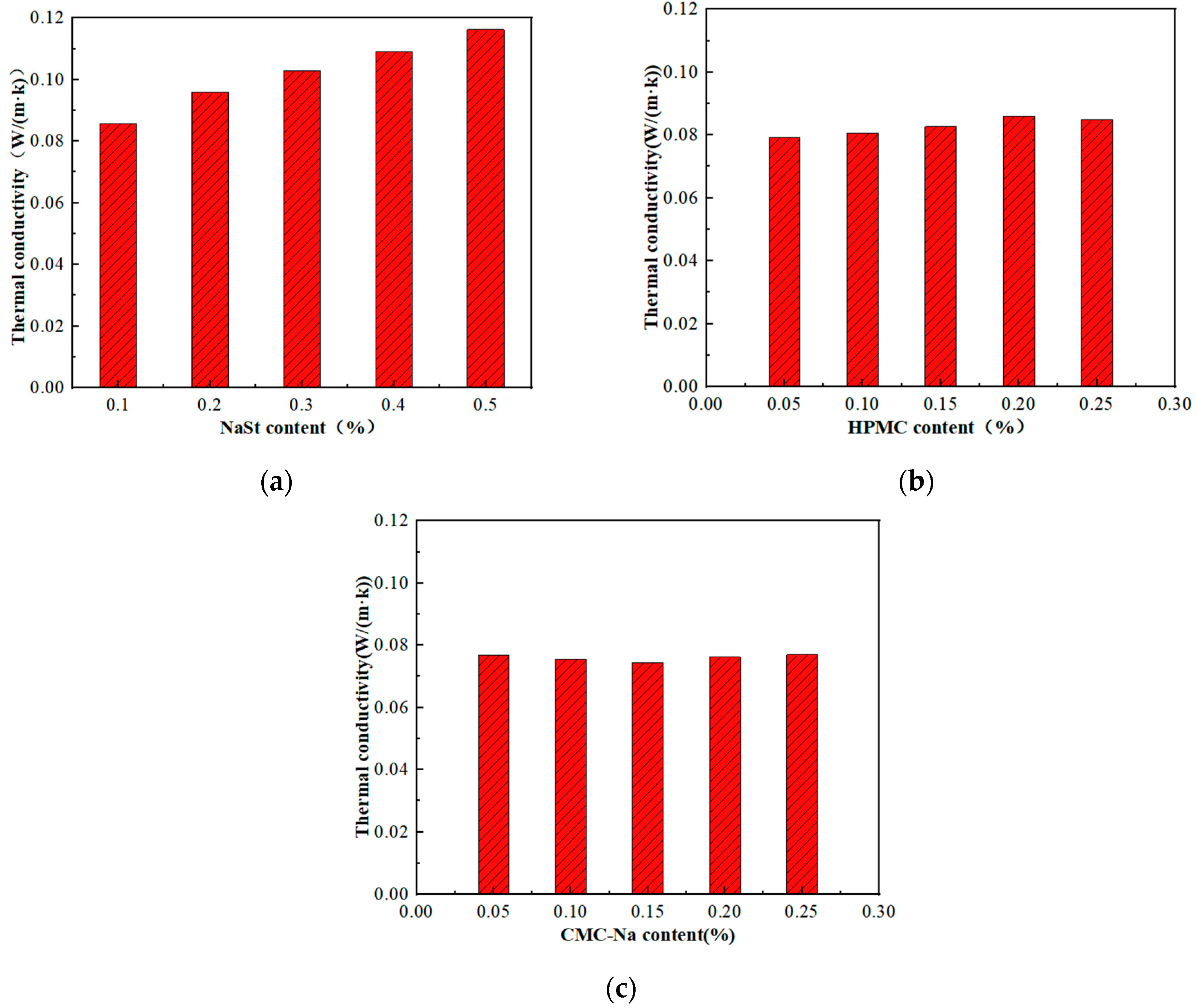
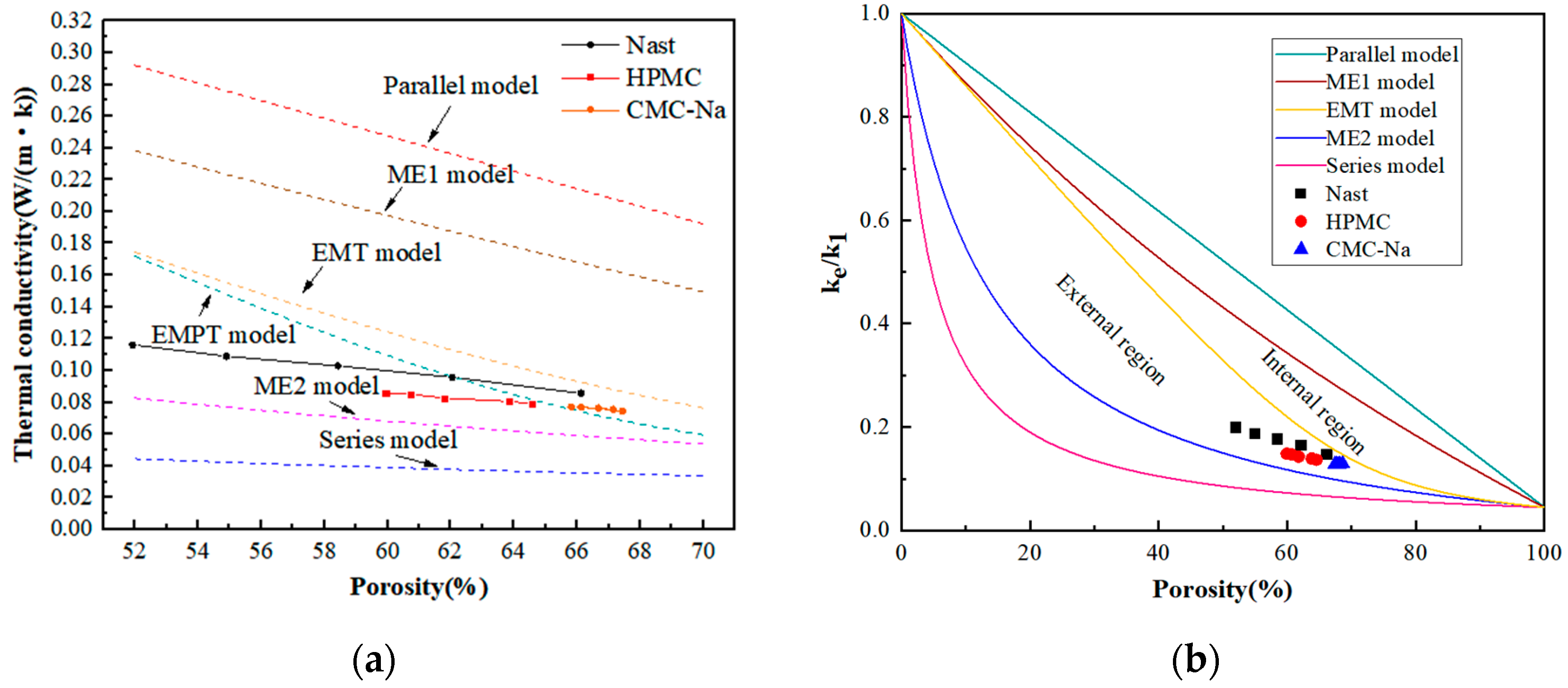
4.4. Thermal Conductivity
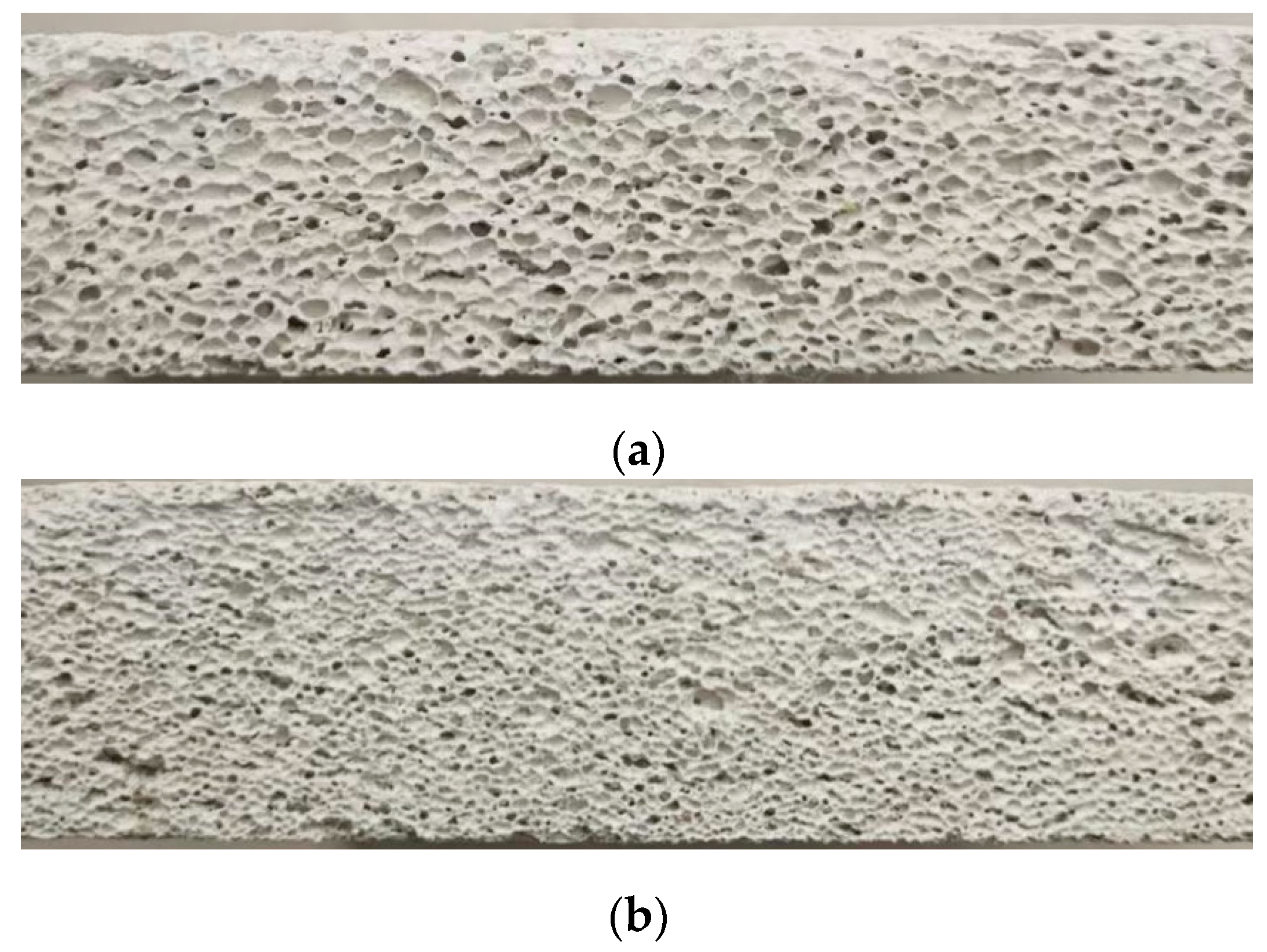
4.5. Material Bubble Pore Size
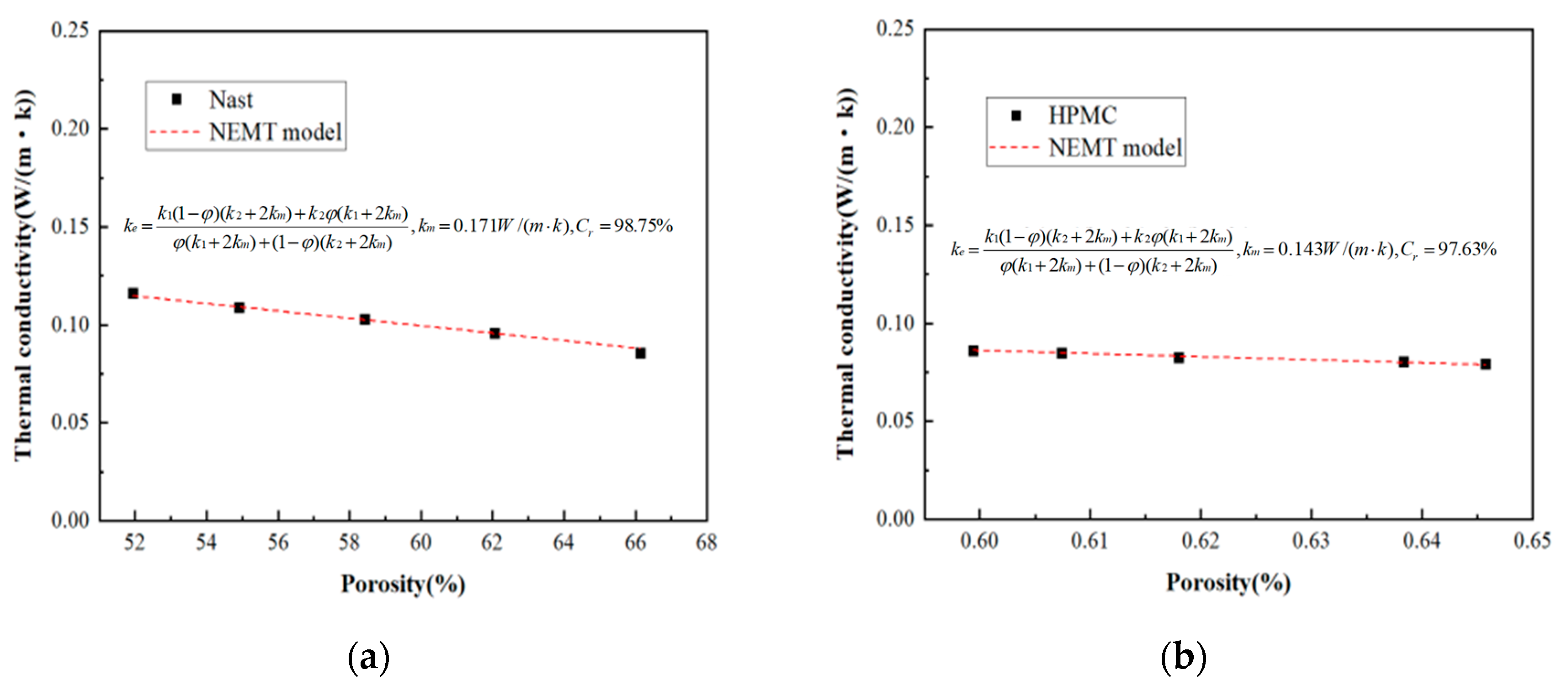
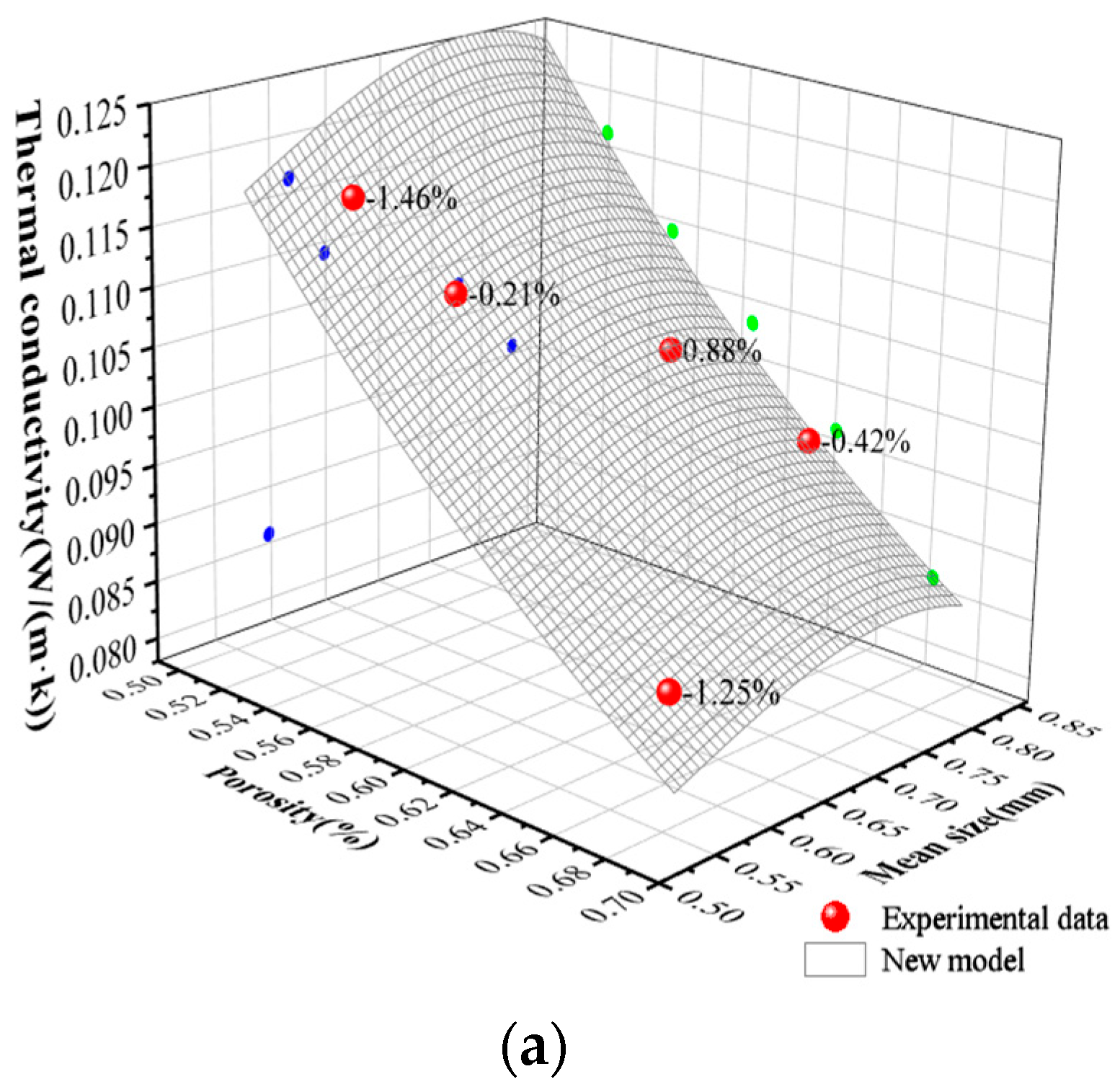
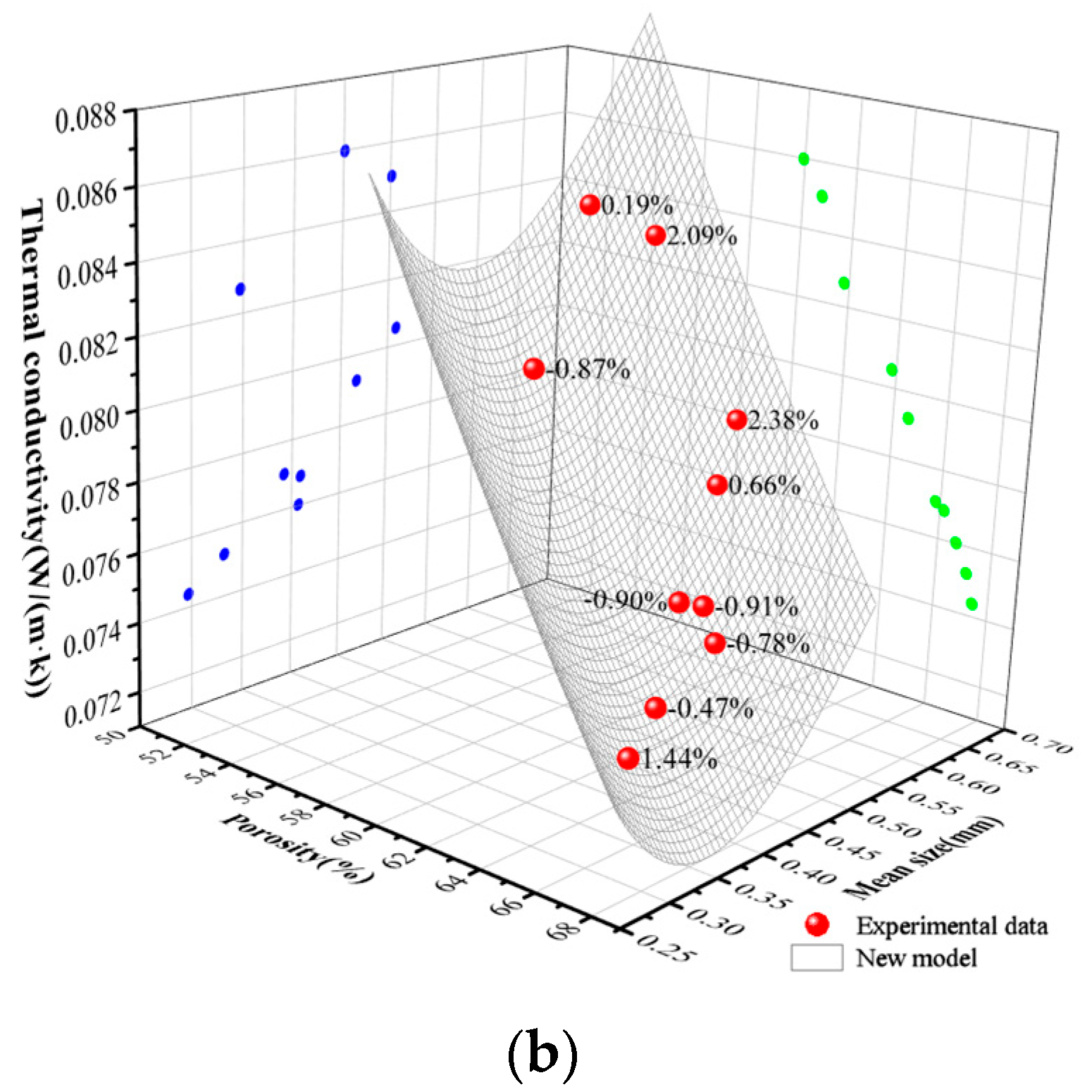
5. Results and Analysis
6. Conclusions
- (1)
- The thermal conductivity of GFC reaches the lowest value of 0.07438 W/(m·k) when CMC-Na with a dosage of 0.15% is used as the foam stabilizer. The viscosity increasing stabilizing agents such as CMC-Na and HPMC have a better effect than the synergistic stabilizing agent NaSt in improving the thermal conductivity, and the change in viscosity increasing stabilizing agent mixing amount has less of an effect on the thermal conductivity.
- (2)
- In this paper, when using the image recognition technology to measure the pore size of GFC bubbles, the range of macroporous pore sizes of GFC made by using CMC-Na, HPMC, and NaSt is 0.297~0.409 mm, 0.347~0.509 mm, and 0.598~0.831 mm, respectively; GFC made by viscosity stabilizing agent has a smaller pore size.
- (3)
- A new theory is proposed to predict the thermal conductivity of GFC. The experimental data of GFC in this paper and the experimental data of previous literature show that the thermal conductivity predicted by the model is basically consistent with the experimental results. Meanwhile, the pore distribution parameter t in the new model is categorized by the experimental data in this paper and the previous literature. Our long-term objective is to conduct extensive and systematic experiments to build a comprehensive database encompassing different material systems and processes. This database will then be used to train a machine learning algorithm, ultimately creating a big data model capable of recommending or predicting the values of parameters a and t directly based on various input conditions such as material formulation and foaming agent type. This approach is expected to significantly enhance the model’s universality and predictive power.
- (4)
- In the present study, due to the high cost and relatively low efficiency of 3D-CT scanning, an image recognition technique was employed for pore size measurement based on 2D images. With the increasing maturity of AI-3D reconstruction technology, which can potentially achieve reconstruction from multiple photographs and address the cost and efficiency limitations of 3D-CT scanning, the image recognition method adopted in this study can serve as a valuable reference for subsequent researchers.
- (5)
- Additionally, pore structural parameters such as the proportion of micropores and connectivity significantly impact thermal conductivity. However, these parameters exhibit complex interdependencies—for instance, in GFC, high porosity drastically increases pore connectivity. This interplay makes it challenging to isolate the influence of individual parameters on thermal modeling. Given the inherent complexity of pore structures, incorporating all potential factors is impractical. Therefore, this study focuses on key pore structural parameters to balance model complexity with accuracy. Future research will expand predictive capabilities by integrating additional influential variables.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tuyan, M.; Andiç-Çakir, Ö.; Ramyar, K. Effect of alkali activator concentration and curing condition on strength and microstructure of waste clay brick powder-based geopolymer. Compos. Part B Eng. 2018, 135, 242–252. [Google Scholar] [CrossRef]
- Pasupathy, K.; Ramakrishnan, S.; Sanjayan, J. Formulating eco-friendly geopolymer foam concrete by alkali-activation of ground brick waste. J. Clean. Prod. 2021, 325, 129180. [Google Scholar] [CrossRef]
- Dang, J.; Hao, L.; Xiao, J.; Ding, T. Utilization of excavated soil and sewage sludge for green lightweight aggregate and evaluation of its influence on concrete properties. J. Clean. Prod. 2023, 390, 136061. [Google Scholar] [CrossRef]
- Dang, J.; Zhao, S.; Chen, G.; Cao, X.; Yang, J. Effect of polyethylene powder and heating treatment on the microstructure and hardened properties of foam concrete. J. Build. Eng. 2022, 50, 104143. [Google Scholar] [CrossRef]
- Dhasindrakrishna, K.; Pasupathy, K.; Ramakrishnan, S.; Sanjayan, J. Effect of yield stress development on the foam-stability of aerated geopolymer concrete. Cem. Concr. Res. 2020, 138, 106233. [Google Scholar] [CrossRef]
- She, W.; Du, Y.; Miao, C.; Liu, J.; Zhao, G.; Jiang, J.; Zhang, Y. Application of organic- and nanoparticle-modified foams in foamed concrete: Reinforcement and stabilization mechanisms. Cem. Concr. Res. 2018, 106, 12–22. [Google Scholar] [CrossRef]
- Huo, W.; Zhu, Z.; Sun, H.; Yang, L.; Zhang, C. Estimating the relationships between initial constituent molar ratios and physical–mechanical properties of RCFP-GBFS based geopolymers. Constr. Build. Mater. 2023, 406, 133409. [Google Scholar] [CrossRef]
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S. Geopolymer concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Liew, Y.; Heah, C.; Mustafa, A.; Kamarudin, H. Structure and properties of clay-based geopolymer cements: A review. Prog. Mater. Sci. 2016, 83, 595–629. [Google Scholar] [CrossRef]
- Nie, Q.; Hu, W.; Huang, B.; Shu, X.; Qiang, H. Synergistic utilization of red mud for flue-gas desulfurization and fly ash-based geopolymer preparation. J. Hazard. Mater. 2019, 369, 503–511. [Google Scholar] [CrossRef]
- Hu, W.; Nie, Q.; Huang, B.; Shu, X. Investigation of the strength development of cast-in-place geopolymer piles with heating systems. J. Clean. Prod. 2019, 215, 1481–1489. [Google Scholar] [CrossRef]
- Mclellan, B.; Williams, R.; Lay, J.; Riessen, A.; Corderet, G. Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef]
- Feng, W.; Jin, Y.; Zheng, D.; Li, Z.; Cui, H. Synergic effect of compositions and processing method on the performance of high strength alkali activated slag foam. Constr. Build. Mater. 2022, 352, 128991. [Google Scholar] [CrossRef]
- Kamseu, E.; Ngouloure, Z.; Benoît Nait Ali Zekeng, S.; Melo, U.; Rossignol, S.; Leonelli, C. Cumulative pore volume, pore size distribution and phases percolation in porous inorganic polymer composites: Relation microstructure and effective thermal conductivity. Energy Build. 2015, 88, 45–56. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, D.; Zhao, J.; Li, D.; Serina, N.; Rui, Y. Effect of calcium stearate based foam stabilizer on pore characteristics and thermal conductivity of geopolymer foam material. J. Build. Eng. 2018, 20, 21–29. [Google Scholar] [CrossRef]
- Gong, L.; Wang, Y.; Cheng, X.; Zhang, R.; Zhang, H. A novel effective medium theory for modelling the thermal conductivity of porous materials. Int. J. Heat Mass Transf. 2014, 68, 295–298. [Google Scholar] [CrossRef]
- Chen, G.; Li, F.; Geng, J.; Jing, P.; Si, Z. Identification, generation of autoclaved aerated concrete pore structure and simulation of its influence on thermal conductivity. Constr. Build. Mater. 2021, 294, 123572. [Google Scholar] [CrossRef]
- Gao, R.; Zhou, Z.; Chen, K.; Hu, X.; Jia, H.; Zhang, H.; Han, L. Mechanisms of the effect of surfactant types on the pore structure feature and properties of geopolymer foams. Constr. Build. Mater. 2025, 458, 139696. [Google Scholar] [CrossRef]
- Othuman, M.; Wang, Y. Elevated-temperature thermal properties of lightweight foamed concrete. Constr. Build. Mater. 2011, 25, 705–716. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Mao, J.; Lin, J.; Xiao, F.; Chen, S. Modeling the thermal conductivity of the ground granulated blast furnace slag-based foam geopolymer based on its multi-scale pore structure. J. Build. Eng. 2025, 105, 112487. [Google Scholar] [CrossRef]
- Gadkar, A.; Subramaniam, K. Tailoring porosity and pore structure of cellular geopolymers for strength and thermal conductivity. Constr. Build. Mater. 2023, 393, 132150. [Google Scholar] [CrossRef]
- Li, X.; Zhao, X.; Guo, X.; Shao, Z.; Ai, M. New theoretical equation for effective thermal conductivity of two-phase composite materials. Mater. Sci. Technol. 2012, 28, 620–626. [Google Scholar] [CrossRef]
- Bi, C.; Tang, G.; Tao, W. Prediction of the gaseous thermal conductivity in aerogels with non-uniform pore-size distribution. J. Non-Cryst. Solids 2012, 358, 3124–3128. [Google Scholar] [CrossRef]
- Ordonez-Miranda, J.; Alvarado-Gil, J. Effect of the pore shape on the thermal conductivity of porous media. J. Mater. Sci. 2012, 47, 6733–6740. [Google Scholar] [CrossRef]
- Pimenov, D.; da Silva, L.; Ercetin, A.; Der, O.; Mikolajczyk, T.; Giasin, K. State-of-the-art review of applications of image processing techniques for tool condition monitoring on conventional machining processes. Int. J. Adv. Manuf. Technol. 2024, 130, 57–85. [Google Scholar] [CrossRef]
- Vishavkarma, A.; Venkatanarayanan, H. Assessment of pore structure of foam concrete containing slag for improved durability performance in reinforced concrete applications. J. Build. Eng. 2024, 86, 108939. [Google Scholar] [CrossRef]
- Maglad, A.; Othuman Mydin, M.; Datta, S.; Tayeh, B. Assessing the mechanical, durability, thermal and microstructural properties of sea shell ash based lightweight foamed concrete. Constr. Build. Mater. 2023, 402, 133018. [Google Scholar] [CrossRef]
- Maglad, A.; Mydin, M.; Kaze, R.; Abbood, I.; Tayeh, B. Synergistic effect of waste gypsum plasterboard and fly ash as partial cement replacement on fresh-state, microstructural, mechanical and transport properties of foamed concrete. Constr. Build. Mater. 2025, 463, 140079. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, Y.; Hu, H.; Arachchi, E.; Cen, X.; Li, W.; Wang, Q. Role of red mud as a foam stabilizer in geopolymer foam concrete: Enhancing stability, rheology, and mechanical performance. J. Build. Eng. 2025, 105, 112446. [Google Scholar] [CrossRef]
- Carson, J.; Lovatt, S.; Tanner, D.; Cleland, A. Thermal conductivity bounds for isotropic, porous materials. Int. J. Heat Mass Transf. 2005, 48, 2150–2158. [Google Scholar] [CrossRef]
- Wei, S.; Chen, Y.; Zhang, Y.; Jones, M. Characterization and simulation of microstructure and thermal properties of foamed concrete. Constr. Build. Mater. 2013, 47, 1278–1291. [Google Scholar] [CrossRef]
- Vaou, V.; Panias, D. Thermal insulating foamy geopolymers from perlite. Miner. Eng. 2010, 23, 1146–1151. [Google Scholar] [CrossRef]



| Components | CaO | SiO2 | Al2O3 | MgO | Fe2O3 | SO3 | Loss on Ignition |
|---|---|---|---|---|---|---|---|
| Content (%) | 34.00 | 34.50 | 17.70 | 6.01 | 1.03 | 1.64 | 0.84 |
| Foam Stabilizer Percent (%) | Slag Powder /g | H2O2 /g | Water Glass /g | NaOH /g | Water /g |
|---|---|---|---|---|---|
| NaSt (0.1–0.5%) | 9000 | 495 | 1895.36 | 283.54 | 3653.10 |
| HPMC (0.05–0.25%) | 9000 | 495 | 1895.36 | 283.54 | 3653.10 |
| CMC-Na (0.05–0.25%) | 9000 | 495 | 1895.36 | 283.54 | 3653.10 |
| (a) | |||||
| Foam Stabilizer Dosage (%) | 0.05 | 0.10 | 0.15 | 0.20 | 0.25 |
| Porosity (%) | 66.14 | 67.14 | 67.44 | 66.68 | 65.82 |
| Average pore size of large holes (mm) | 0.409 | 0.332 | 0.297 | 0.407 | 0.392 |
| Thermal conductivity (W/(m·k)) | 0.07686 | 0.07522 | 0.07438 | 0.07602 | 0.07705 |
| (b) | |||||
| Foam Stabilizer Dosage (%) | 0.05 | 0.10 | 0.15 | 0.20 | 0.25 |
| Porosity (%) | 64.57 | 63.83 | 61.80 | 59.94 | 60.74 |
| Average pore size of large holes (mm) | 0.467 | 0.509 | 0.347 | 0.451 | 0.501 |
| Thermal conductivity (W/(m·k)) | 0.07924 | 0.08052 | 0.08268 | 0.08598 | 0.08502 |
| (c) | |||||
| Foam Stabilizer Dosage (%) | 0.05 | 0.10 | 0.15 | 0.20 | 0.25 |
| Porosity (%) | 66.13 | 62.05 | 58.43 | 54.90 | 51.93 |
| Average pore size of large holes (mm) | 0.598 | 0.831 | 0.776 | 0.649 | 0.617 |
| Thermal conductivity (W/(m·k)) | 0.08578 | 0.09577 | 0.10292 | 0.10903 | 0.11620 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Chen, W.; Li, J.; Xie, Q.; Lin, M.; Fang, H.; Du, Z.; Jiang, L. Theoretical Calculation Modeling of Thermal Conductivity of Geopolymer Foam Concrete in Building Structures Based on Image Recognition. Buildings 2025, 15, 3494. https://doi.org/10.3390/buildings15193494
Xu Y, Chen W, Li J, Xie Q, Lin M, Fang H, Du Z, Jiang L. Theoretical Calculation Modeling of Thermal Conductivity of Geopolymer Foam Concrete in Building Structures Based on Image Recognition. Buildings. 2025; 15(19):3494. https://doi.org/10.3390/buildings15193494
Chicago/Turabian StyleXu, Yanqing, Wenwen Chen, Jie Li, Qun Xie, Mingqiang Lin, Haibo Fang, Zhihao Du, and Liqiang Jiang. 2025. "Theoretical Calculation Modeling of Thermal Conductivity of Geopolymer Foam Concrete in Building Structures Based on Image Recognition" Buildings 15, no. 19: 3494. https://doi.org/10.3390/buildings15193494
APA StyleXu, Y., Chen, W., Li, J., Xie, Q., Lin, M., Fang, H., Du, Z., & Jiang, L. (2025). Theoretical Calculation Modeling of Thermal Conductivity of Geopolymer Foam Concrete in Building Structures Based on Image Recognition. Buildings, 15(19), 3494. https://doi.org/10.3390/buildings15193494







