Electromigration of Chloride Ions in Cementitious Material: Extension of Nernst–Planck Theory
Abstract
1. Introduction
2. Experiment
2.1. Materials and Specimen Preparation
2.2. Experimental Procedure
2.2.1. Electrical Field Conduction
2.2.2. Measurement of Chloride Concentration
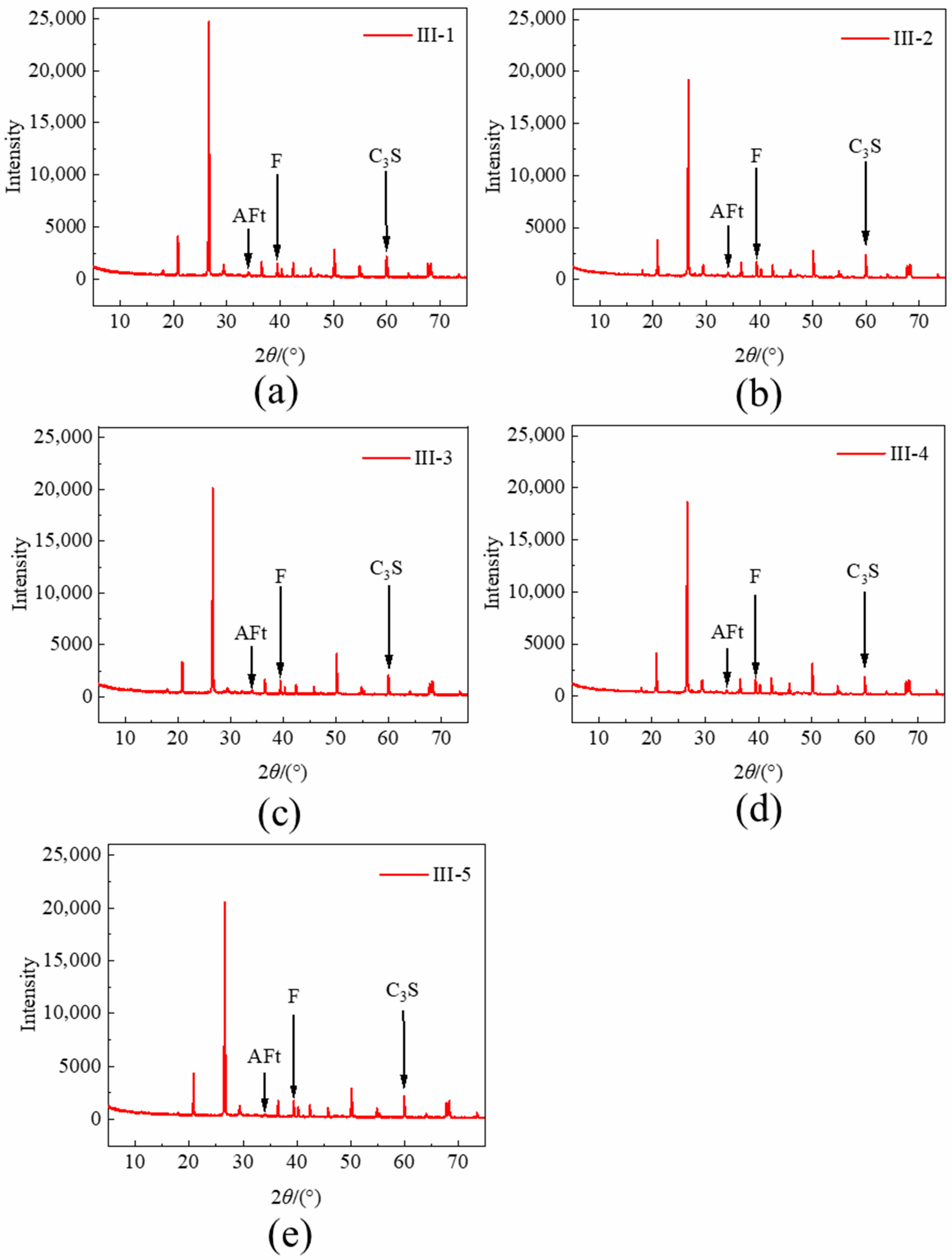
2.2.3. XRD and MIP Test
3. Results and Discussion
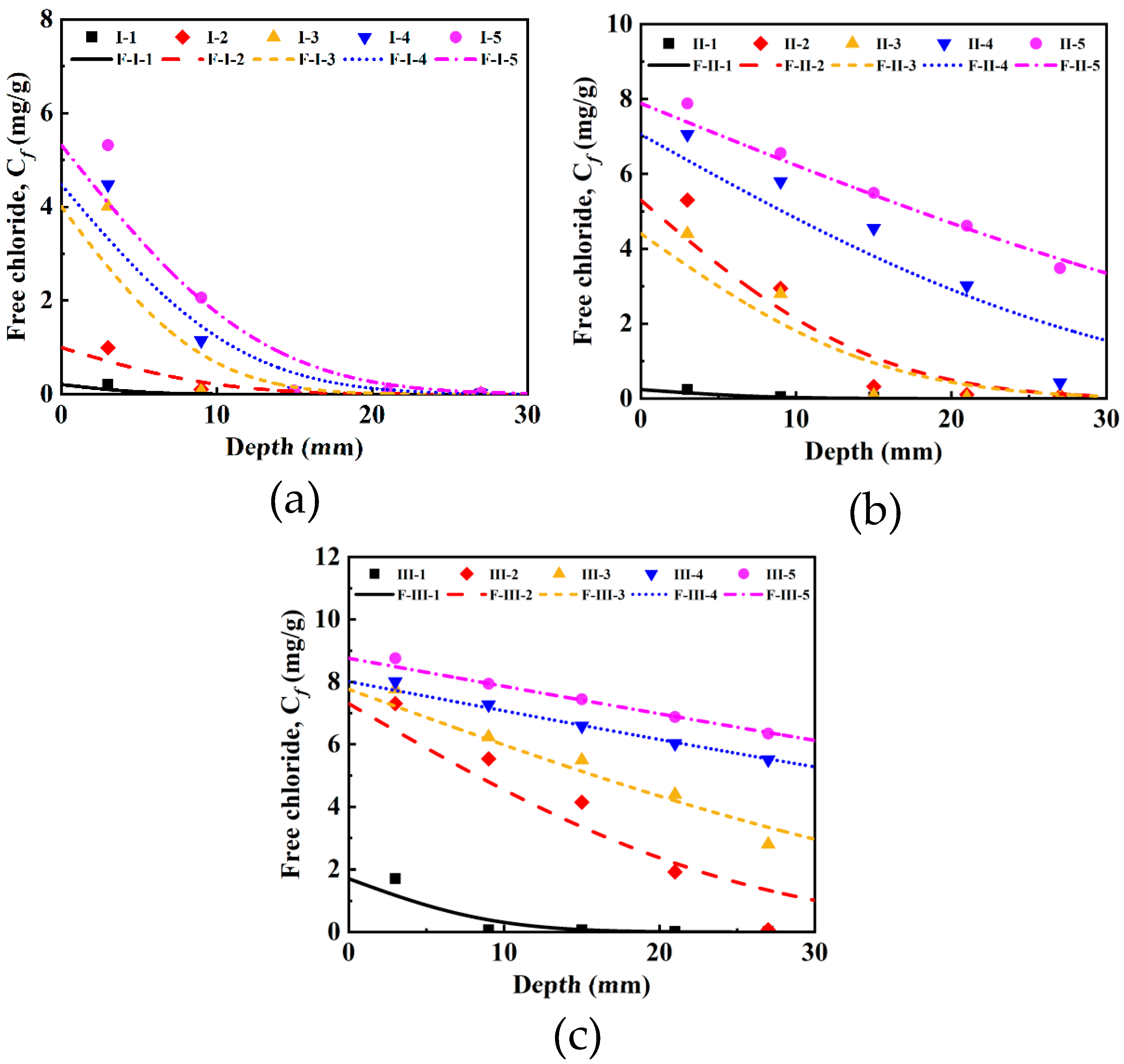
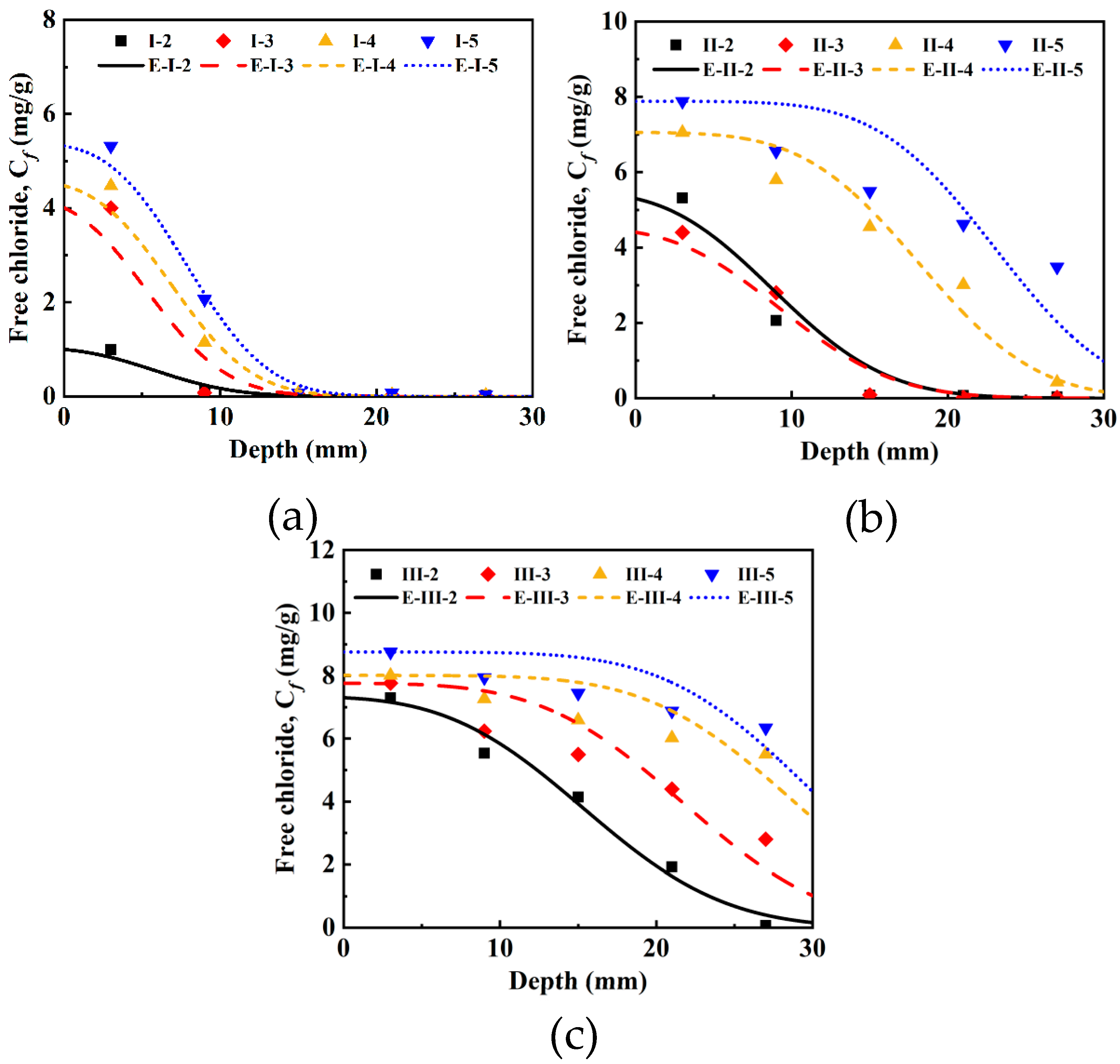
3.1. Investigation of Free Chlorides Under Current Densities
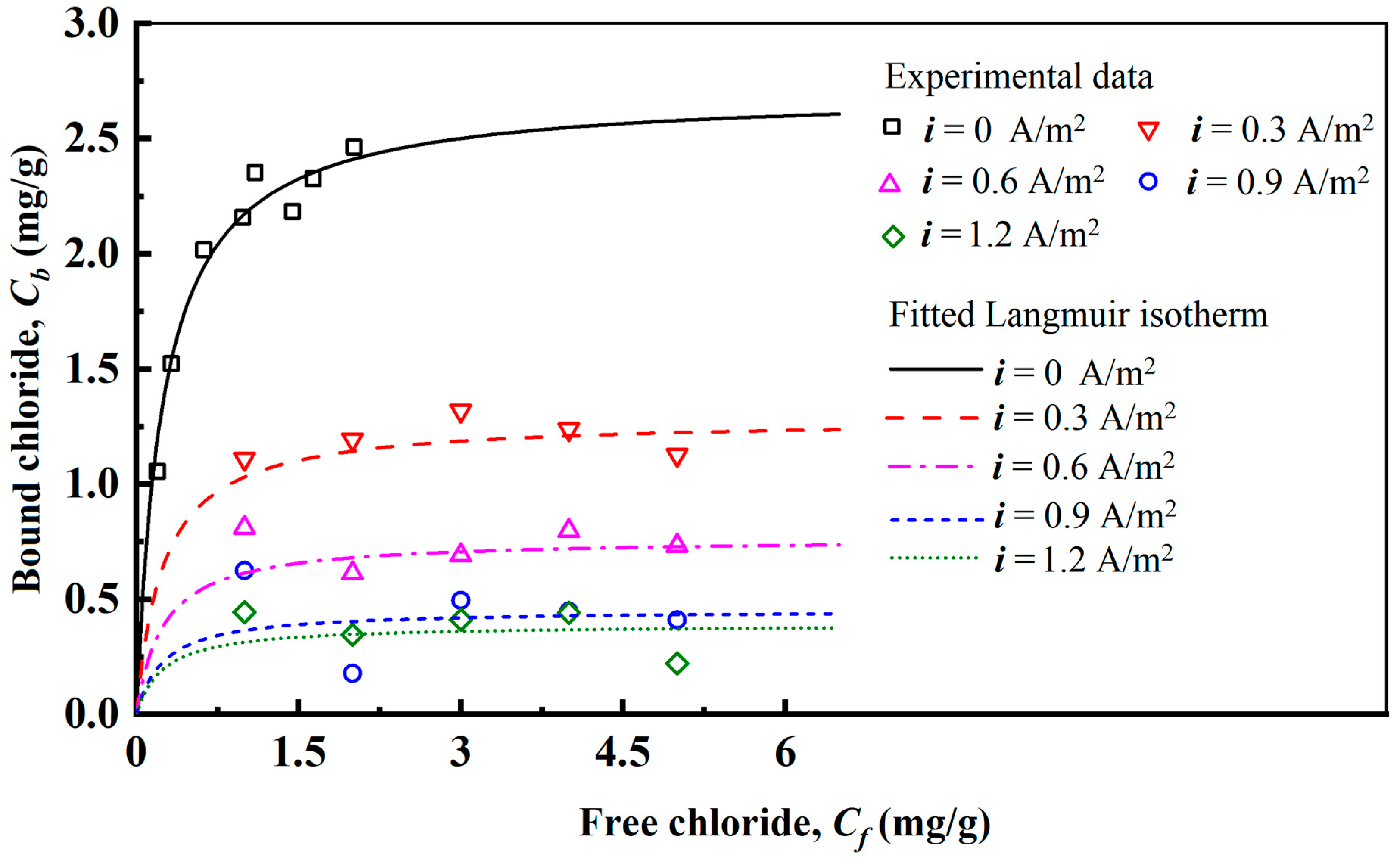
3.2. Chloride Binding Isotherms Under Current Densities
3.3. Discussion of Chloride Comprehensive Migration Coefficient
3.4. Determination of Electromigration Coefficient of Chloride Ions
3.4.1. One-Dimensional Analytical Solution of Nernst–Planck Equation Containing Independent Electromigration Coefficient
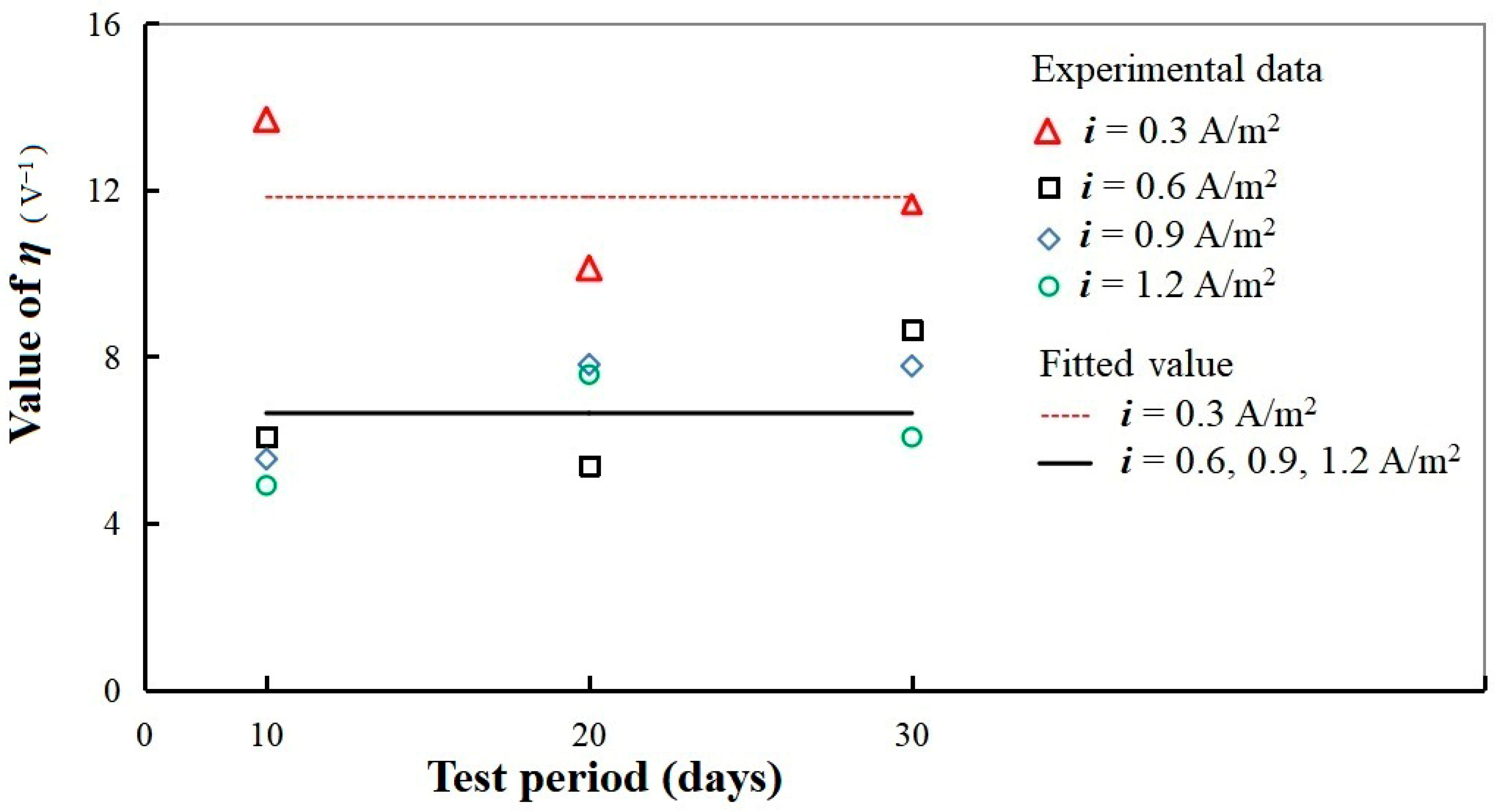
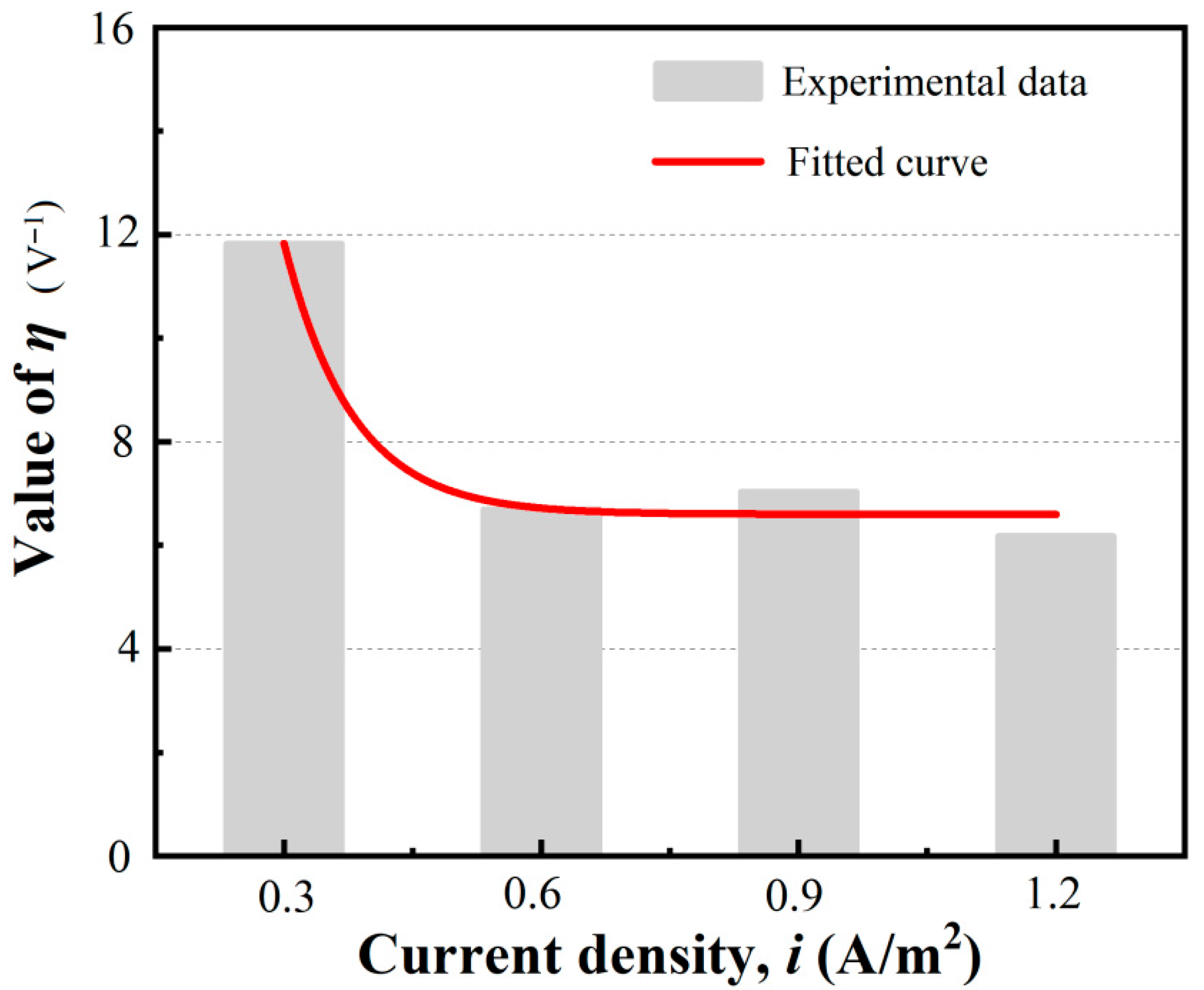
3.4.2. Electromigration Coefficient Fitting Based on Experimental Data Corresponding to Different Current Densities
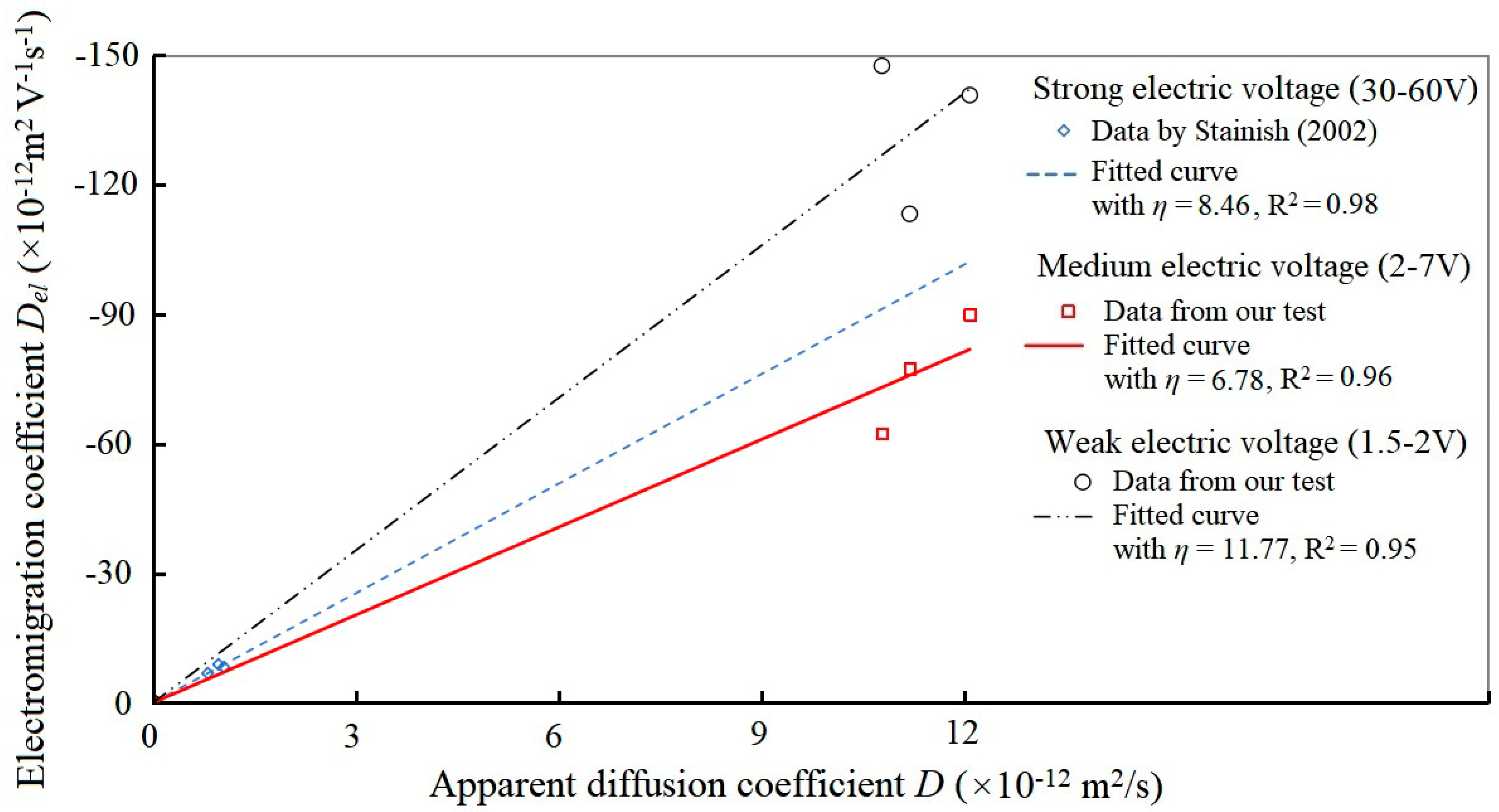
3.4.3. Relationship Between Electromigration Coefficient and Diffusion Coefficient
3.5. Microscopic Investigation and Pore Distribution
3.5.1. Microscopic Investigation of Bound Chloride Content
3.5.2. Investigation of Pore Distribution of Specimen
4. Conclusions
- (1)
- An analytical solution of the Nernst–Planck equation with an independent electromigration coefficient was derived, and its value was quantitatively measured corresponding to various current densities. At weak current densities, the electromigration coefficient is significantly higher, and at medium or high current densities, the electromigration coefficient remains at a constant value. For current densities of 0.3, 0.6, 0.9, and 1.2 (A/m2), the average electromigration coefficients of the specimens used in our test were determined to be 133 × 10−12, 76.6 × 10−12, 80.3 × 10−12, and 70.3 × 10−12 , respectively.
- (2)
- The chloride binding capacity of cementitious material rapidly decreases with the increase in current density. But, at weak or moderate current densities, its binding ability is to some extent preserved. For current densities of 0.3, 0.6, 0.9, and 1.2 (A/m2), the decay ratio of the binding ability was measured as 52.6%, 71.8%, 83.2%, and 85.6%, respectively.
- (3)
- There is no clear linear relationship between the electromigration coefficient and the apparent diffusion coefficient for chloride ions, especially at weak current densities. This indicated that the traditional Nernst–Planck equation has significant errors in predicting particle transport in non-ideal porous media such as cementitious material. However, the electromigration coefficient shows a high degree of linear fit with the pure effective diffusion coefficient at all electric field strengths, and the ratio of the two was measured as −2.97 V−1.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, S.W.; Yao, Y.; Andrade, C.; Li, Z. Recent durability studies on concrete structure. Cem. Concr. Res. 2015, 78, 143–154. [Google Scholar] [CrossRef]
- Van Damme, H. Concrete material science: Past, present, and future innovations. Cem. Concr. Res. 2018, 112, 5–24. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Yan, B.; Yuan, C.; Wang, D.; Guo, W. Experimental study of chloride transport law in concrete considering the coupling effects of dry-wet ratio and freeze–thaw damage. Constr. Build. Mater. 2022, 351, 128940. [Google Scholar] [CrossRef]
- Tong, L.-y.; Xiong, Q.X.; Zhang, M.; Meng, Z.; Meftah, F.; Liu, Q.-f. Multi-scale modelling and statistical analysis of heterogeneous characteristics effect on chloride transport properties in concrete. Constr. Build. Mater. 2023, 367, 130096. [Google Scholar] [CrossRef]
- Angst, U.; Elsener, B.; Larsen, C.K.; Vennesland, Ø. Critical chloride content in reinforced concrete—A review. Cem. Concr. Res. 2009, 39, 1122–1138. [Google Scholar] [CrossRef]
- Jakobsen, U.H.; De Weerdt, K.; Geiker, M.R. Elemental zonation in marine concrete. Cem. Concr. Res. 2016, 85, 12–27. [Google Scholar] [CrossRef]
- Kenny, A.; Katz, A. Steel-concrete interface influence on chloride threshold for corrosion—Empirical reinforcement to theory. Constr. Build. Mater. 2020, 244, 10.1016. [Google Scholar] [CrossRef]
- Chalhoub, C.; François, R.; Carcasses, M. Critical chloride threshold values as a function of cement type and steel surface condition. Cem. Concr. Res. 2020, 134, 106086. [Google Scholar] [CrossRef]
- Bolzoni, F.; Brenna, A.; Ormellese, M. Recent advances in the use of inhibitors to prevent chloride-induced corrosion in reinforced concrete. Cem. Concr. Res. 2022, 154, 106719. [Google Scholar] [CrossRef]
- Xu, L.; Wu, P.; Zhu, X.; Zhao, G.; Ren, X.; Wei, Q.; Xie, L. Structural characteristics and chloride intrusion mechanism of passive film. Corros. Sci. 2022, 207, 110563. [Google Scholar] [CrossRef]
- Collepardi, M.; Marcialis, A.; Turriziani, R. Penetration of chloride ions into cement pastes and concretes. J. Am. Ceram. Soc. 1972, 55, 534–535. [Google Scholar] [CrossRef]
- Mangat, P.; Molloy, B. Prediction of long term chloride concentration in concrete. Mater. Struct. 1994, 27, 338–346. [Google Scholar] [CrossRef]
- Nilsson, L.; Massat, M.; Tang, L. Effect of non-linear chloride binding on the prediction of chloride penetration into concrete structures. In Proceedings of the ACI International Conference on Durability of Concrete, Gothenburg, Sweden, 1 May 1994; pp. 469–486. [Google Scholar]
- Thomas, M.D.; Bamforth, P.B. Modelling chloride diffusion in concrete: Effect of fly ash and slag. Cem. Concr. Res. 1999, 29, 487–495. [Google Scholar]
- Sun, W. Durability Evaluation and Service Life Prediction of Modern Concrete; China Architecture & Building Press: Beijing, China, 2015. [Google Scholar]
- Amey, S.L.; Johnson, D.A.; Miltenberger, M.A.; Farzam, H. Predicting the service life of concrete marine structures: An environmental methodology. Struct. J. 1998, 95, 205–214. [Google Scholar]
- Kassir, M.K.; Ghosn, M. Chloride-induced corrosion of reinforced concrete bridge decks. Cem. Concr. Res. 2002, 32, 139–143. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, M.; Pickering, H. On electric field induced breakdown of passive films and the mechanism of pitting corrosion. J. Electrochem. Soc. 1993, 140, 3448. [Google Scholar] [CrossRef]
- Bertolini, L.; Carsana, M.; Pedeferri, P. Corrosion behaviour of steel in concrete in the presence of stray current. Corros. Sci. 2007, 49, 1056–1068. [Google Scholar] [CrossRef]
- Hernández, F.R.; Plascencia, G.; Koch, K. Rail base corrosion problem for North American transit systems. Eng. Fail. Anal. 2009, 16, 281–294. [Google Scholar] [CrossRef]
- Wang, J.; Niu, D.; Wang, Y.; He, H.; Liang, X. Chloride diffusion of shotcrete lining structure subjected to nitric acid, salt–frost degradation, and bending stress in marine environment. Cem. Concr. Compos. 2019, 104, 103396. [Google Scholar] [CrossRef]
- Wu, P.; Zhu, X.; Xu, L.; Peng, W.; Zhao, G. Effect of stray current coupled with chloride concentration and temperature on the corrosion resistance of a steel passivation film. Electrochem. Commun. 2020, 118, 106793. [Google Scholar] [CrossRef]
- de Almeida Souza, L.R.; de Medeiros, M.H.F.; Pereira, E.; Capraro, A.P.B. Electrochemical chloride extraction: Efficiency and impact on concrete containing 1% of NaCl. Constr. Build. Mater. 2017, 145, 435–444. [Google Scholar] [CrossRef]
- Zhu, Z.; Chu, H.; Guo, M.-z.; Shen, M.; Jiang, L.; Yu, L. Effect of silica fume and fly ash on the stability of bound chlorides in cement mortar during electrochemical chloride extraction. Constr. Build. Mater. 2020, 256, 119481. [Google Scholar] [CrossRef]
- Xia, J.; Cheng, X.; Liu, Q.F.; Xie, H.B.; Zhong, X.P.; Jin, S.J.; Mao, J.H.; Jin, W.L. Effect of the stirrup on the transport of chloride ions during electrochemical chloride removal in concrete structures. Constr. Build. Mater. 2020, 250, 118898. [Google Scholar] [CrossRef]
- Li, L.; Dong, S.; Wang, W.; Hu, R.; Du, R.; Lin, C.; Zhuo, X.; Wang, J. Study on interaction between macrocell and microcell in the early corrosion process of reinforcing steel in concrete. Sci. Sin. Technol. 2010, 40, 1104–1108. [Google Scholar] [CrossRef]
- Kranc, S.; Sagüés, A.A. Detailed modeling of corrosion macrocells on steel reinforcing in concrete. Corros. Sci. 2001, 43, 1355–1372. [Google Scholar] [CrossRef]
- Yu, Y.; Gao, W.; Castel, A.; Chen, X.; Liu, A. An integrated framework for modelling time-dependent corrosion propagation in offshore concrete structures. Eng. Struct. 2021, 228, 111482. [Google Scholar] [CrossRef]
- Feng, G.; Jin, Z.; Zhu, D.; Xiong, C.; Li, Z.; Wang, X. Corrosion propagation of steel reinforcement in pre-cracked mortar attacked by seawater using wire beam electrode. Corros. Sci. 2022, 208, 110655. [Google Scholar] [CrossRef]
- Zhu, X.; Zi, G. A 2D mechano-chemical model for the simulation of reinforcement corrosion and concrete damage. Constr. Build. Mater. 2017, 137, 330–344. [Google Scholar] [CrossRef]
- Zhu, X.; Meng, Z.; Liu, L.; Xu, L. An equivalent smeared layer method for simulating the non-uniform corrosion-induced damage of concrete. Eng. Fract. Mech. 2020, 224, 106791. [Google Scholar] [CrossRef]
- Xua, F.; Han, J.; Wang, S.; Liu, W. Progress in Theoretical Models of Chloride Ion Transport in Cement-based Materials. Mater. Rev. 2014, 28, 172–178. [Google Scholar]
- Liu, Q.-f.; Feng, G.-l.; Xia, J.; Yang, J.; Li, L.-y. Ionic transport features in concrete composites containing various shaped aggregates: A numerical study. Compos. Struct. 2018, 183, 371–380. [Google Scholar] [CrossRef]
- Spiesz, P.; Brouwers, H.J.H. The apparent and effective chloride migration coefficients obtained in migration tests. Cem. Concr. Res. 2013, 48, 116–127. [Google Scholar] [CrossRef]
- NT Build. Concrete, Mortar and Cement-Based Repair Materials; NORDTEST: Espoo, Finland, 1999. [Google Scholar]
- Tang, L.; Lars-Olof, N. Rapid determination of the chloride diffusivity in concrete by applying an electric field. ACI Mater. J. 1993, 89, 49–53. [Google Scholar] [CrossRef] [PubMed]
- McGrath, P.F.; Hooton, R.D. Influence of voltage on chloride diffusion coefficients from chloride migration tests. Cem. Concr. Res. 1996, 26, 1239–1244. [Google Scholar] [CrossRef]
- Chen, M.-Y.; Chen, R.-P.; Chen, Y.-Q.; Ma, X.-Y.; Kang, X. Stray current induced chloride ion transport and corrosion characteristics of cracked ultra-high performance concrete. Constr. Build. Mater. 2023, 398, 132536. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Jin, L.; Zhang, R. Experimental and numerical study on chloride transmission in cracked concrete. Constr. Build. Mater. 2016, 127, 425–435. [Google Scholar] [CrossRef]
- Wang, K.; Zha, X.; Zhang, X.; Zhai, X. Mesoscale numerical simulation of chloride ion penetration in geopolymer concrete under the externally applied electric field. Constr. Build. Mater. 2023, 394, 131380. [Google Scholar] [CrossRef]
- Xu, W.; Li, Y.; Li, H.; Wang, K.; Zhang, C.; Jiang, Y.; Qiang, S. Corrosion mechanism and damage characteristic of steel fiber concrete under the effect of stray current and salt solution. Constr. Build. Mater. 2022, 314, 125618. [Google Scholar] [CrossRef]
- Elsener, B.; Molina, M.; Böhni, H. The electrochemical removal of chlorides from reinforced concrete. Corros. Sci. 1993, 35, 1563–1570. [Google Scholar] [CrossRef]
- Fajardo, G.; Escadeillas, G.; Arliguie, G. Electrochemical chloride extraction (ECE) from steel-reinforced concrete specimens contaminated by “artificial” sea-water. Corros. Sci. 2006, 48, 110–125. [Google Scholar] [CrossRef]
- Orellan, J.; Escadeillas, G.; Arliguie, G. Electrochemical chloride extraction: Efficiency and side effects. Cem. Concr. Res. 2004, 34, 227–234. [Google Scholar] [CrossRef]
- Tritthart, J.; Pettersson, K.; Sorensen, B. Electrochemical removal of chloride from hardened cement paste. Cem. Concr. Res. 1993, 23, 1095–1104. [Google Scholar] [CrossRef]
- Chu, H.; Zhang, B.; Zhao, S.; Guo, M.; Liang, Y.; Jiang, L.; Song, Z. Effect of electric current on the stability of bound chloride. Cem. Concr. Compos. 2019, 103, 71–79. [Google Scholar] [CrossRef]
- Zhu, X.; Peng, W.; Xiao, C.; Ma, L.; Hou, J. Effect of electric field intensity on chloride binding capacity of concrete. J. Mater. Res. Technol. 2023, 23, 4466–4478. [Google Scholar] [CrossRef]
- Zhu, X.; Zi, G.; Cao, Z.; Cheng, X. Combined effect of carbonation and chloride ingress in concrete. Constr. Build. Mater. 2016, 110, 369–380. [Google Scholar] [CrossRef]
- Zhu, X.; Zi, G.; Lee, W.; Kim, S.; Kong, J. Probabilistic analysis of reinforcement corrosion due to the combined action of carbonation and chloride ingress in concrete. Constr. Build. Mater. 2016, 124, 667–680. [Google Scholar] [CrossRef]
- GB 175-2007; Common Portland Cement. State Administration for Market Regulation and Standardization Administration of China: Beijing, China, 2007.
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). State Administration for Market Regulation and Standardization Administration of China: Beijing, China, 2021.
- Sui, S.; Wilson, W.; Georget, F.; Maraghechi, H.; Kazemi-Kamyab, H.; Sun, W.; Scrivener, K. Quantification methods for chloride binding in Portland cement and limestone systems. Cem. Concr. Res. 2019, 125, 105864. [Google Scholar] [CrossRef]
- Noushini, A.; Castel, A.; Aldred, J.; Rawal, A. Chloride diffusion resistance and chloride binding capacity of fly ash-based geopolymer concrete. Cem. Concr. Compos. 2020, 105, 103290. [Google Scholar] [CrossRef]
- Ishida, T.; Iqbal, P.O.N.; Anh, H.T.L. Modeling of chloride diffusivity coupled with non-linear binding capacity in sound and cracked concrete. Cem. Concr. Res. 2009, 39, 913–923. [Google Scholar] [CrossRef]
- Ishida, T.; Miyahara, S.; Maruya, T. Chloride Binding Capacity of Mortars Made with Various Portland Cements and Mineral Admixtures. J. Adv. Concr. Technol. 2008, 6, 287–301. [Google Scholar] [CrossRef]
- Azad, A.K. Chloride diffusion in concrete and its impact on corrosion of reinforcement. In Proceedings of the Symposium on Performance of Concrete Structures in the Arabian Gulf Environment, Dhahran, Saudi Arabia, 15–17 November 1998; pp. 262–273. [Google Scholar]
- Tang, L. Chloride Transport in Concrete-Measurement and Prediction. Ph.D. Thesis, Chalmers University of Technology, Gothenburg, Sweden, 1996. [Google Scholar]
- Spiesz, P.; Brouwers, H.J.H. Influence of the applied voltage on the Rapid Chloride Migration (RCM) test. Cem. Concr. Res. 2012, 42, 1072–1082. [Google Scholar] [CrossRef]
- Kribes, Z.E.; Cherif, R.; Aït-Mokhtar, A. Modelling of Chloride Transport in the Standard Migration Test including Electrode Processes. Materials 2023, 16, 6200. [Google Scholar] [CrossRef]
- Yang, P.; Sant, G.; Neithalath, N. A refined, self-consistent Poisson-Nernst-Planck (PNP) model for electrically induced transport of multiple ionic species through concrete. Cem. Concr. Compos. 2017, 82, 80–94. [Google Scholar] [CrossRef]
- Rios, R.T.; Rigaud, T.; Kopp, T.; Lolli, F.; Kurtis, K.E. Impact of curing solution on concrete surface resistivity and formation factor. Constr. Build. Mater. 2024, 426, 136070. [Google Scholar] [CrossRef]
- Stanish, K.D. The Migration of Chloride Ions in Concrete; University of Toronto: Toronto, ON, Canada, 2002. [Google Scholar]
- Yuan, Q. Fundamental Studies on Test Methods for the Transport of Chloride Ions in Cementitious Materials. Ph.D. Thesis, Ghent University, Gent, Belgium, 2009. [Google Scholar]
- Zibara, H. Binding of External Chlorides by Cement Pastes. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 2001. [Google Scholar]
- Birnin-Yauri, U.; Glasser, F. Friedel’s salt, Ca2Al(OH)6(Cl,OH)·2H2O: Its solid solutions and their role in chloride binding. Cem. Concr. Res. 1998, 28, 1713–1723. [Google Scholar] [CrossRef]
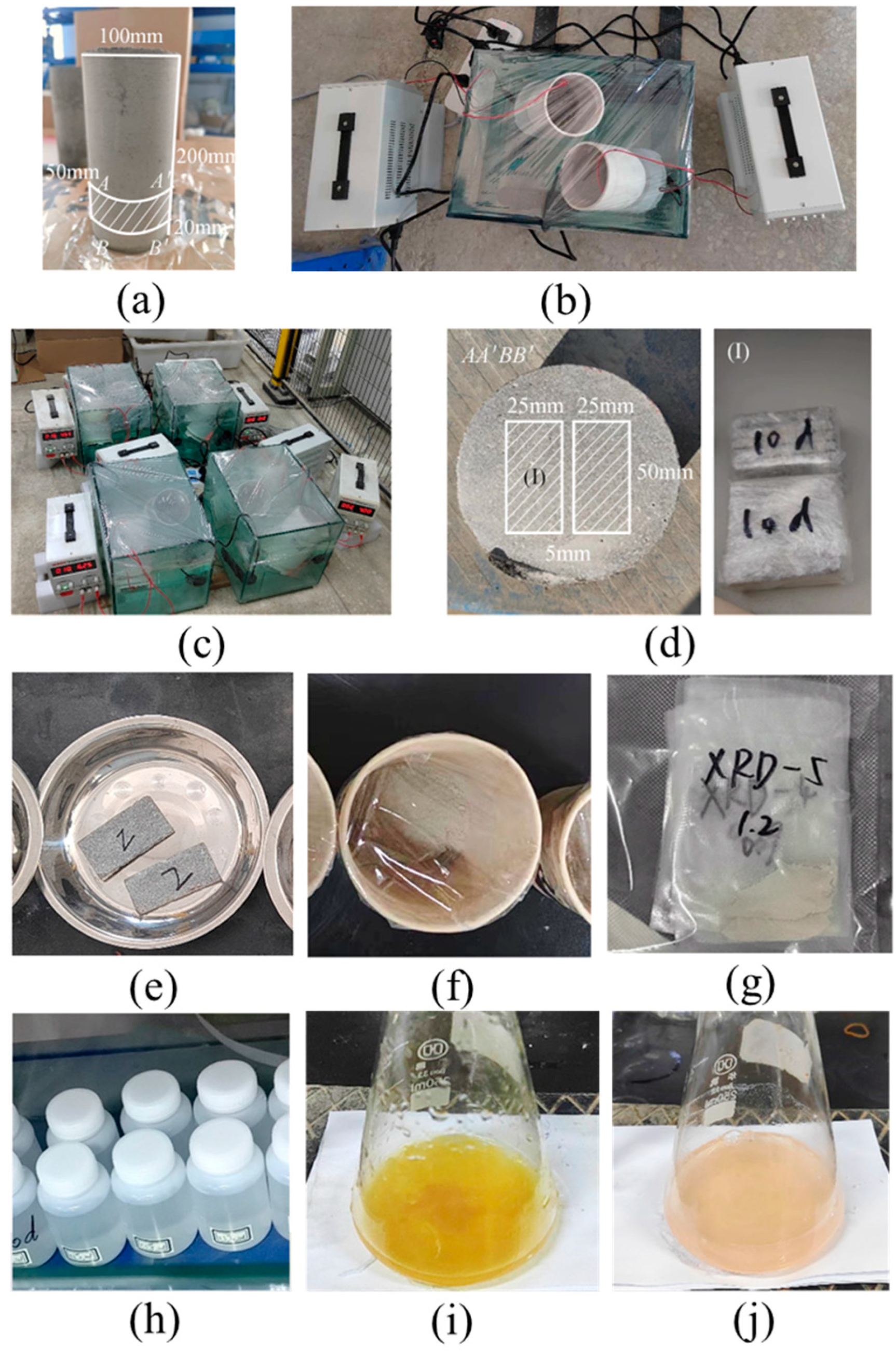





| Component | Content (%) |
|---|---|
| 55.5 | |
| 7.2 | |
| 24.0 | |
| 3.9 | |
| 3.2 | |
| 2.9 | |
| LOI | 1.9 |
| Specific surface area | 356 (/g) |
| Density | 3.15 (g/) |
| Cement () | Sand () | Water () | Superplasticizer () | w/c |
|---|---|---|---|---|
| 636 | 1273.2 | 235.6 | 3.18 | 0.37 |
| I-1 | I-2 | I-3 | I-4 | I-5 | |
| II-1 | II-2 | II-3 | II-4 | II-5 | |
| III-1 | III-2 | III-3 | III-4 | III-5 |
| ) | |||
|---|---|---|---|
| I-1 | |||
| I-2 | |||
| I-3 | |||
| I-4 | |||
| I-5 |
| ) | |||
|---|---|---|---|
| I-2 | |||
| I-3 | |||
| I-4 | |||
| I-5 |
| ) | F | AFt | C3S | |
|---|---|---|---|---|
| III-1 | 0 | 1518 | 708 | 2185 |
| III-2 | 0.3 | 1721 | 702 | 2395 |
| III-3 | 0.6 | 1564 | 607 | 2115 |
| III-4 | 0.9 | 1615 | 569 | 1910 |
| III-5 | 1.2 | 1746 | 466 | 2211 |
| Porosity (%) | More Harmful Holes (>200 nm) (%) | Harmful Holes (50–200 nm) (%) | Less Harmful Holes (20–50 nm) (%) | Harmless Holes (<20 nm) (%) | Total Specific Pore Volume (mL/g) | |
|---|---|---|---|---|---|---|
| III-1 | 13.821 | 28.464 | 20.375 | 22.826 | 28.333 | 0.065 |
| III-2 | 15.854 | 30.587 | 16.828 | 24.511 | 28.072 | 0.076 |
| III-4 | 14.718 | 30.818 | 13.261 | 26.559 | 29.361 | 0.070 |
| ) | |||
|---|---|---|---|
| II-1 | |||
| II-2 | |||
| II-3 | |||
| II-4 | |||
| II-5 |
| ) | |||
|---|---|---|---|
| III-1 | |||
| III-2 | |||
| III-3 | |||
| III-4 | |||
| III-5 |
| ) | |||
|---|---|---|---|
| II-2 | |||
| II-3 | |||
| II-4 | |||
| II-5 |
| ) | |||
|---|---|---|---|
| III-2 | |||
| III-3 | |||
| III-4 | |||
| III-5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Hao, Y.; Wang, J.; Xiao, C. Electromigration of Chloride Ions in Cementitious Material: Extension of Nernst–Planck Theory. Buildings 2025, 15, 3429. https://doi.org/10.3390/buildings15183429
Zhu X, Hao Y, Wang J, Xiao C. Electromigration of Chloride Ions in Cementitious Material: Extension of Nernst–Planck Theory. Buildings. 2025; 15(18):3429. https://doi.org/10.3390/buildings15183429
Chicago/Turabian StyleZhu, Xingji, Yujie Hao, Jie Wang, and Changrong Xiao. 2025. "Electromigration of Chloride Ions in Cementitious Material: Extension of Nernst–Planck Theory" Buildings 15, no. 18: 3429. https://doi.org/10.3390/buildings15183429
APA StyleZhu, X., Hao, Y., Wang, J., & Xiao, C. (2025). Electromigration of Chloride Ions in Cementitious Material: Extension of Nernst–Planck Theory. Buildings, 15(18), 3429. https://doi.org/10.3390/buildings15183429





