Effects of Ion-Regulated Mechanisms on Calcite Precipitation in the Enzyme-Induced Carbonate Precipitation Treatment of Loess
Abstract
1. Introduction
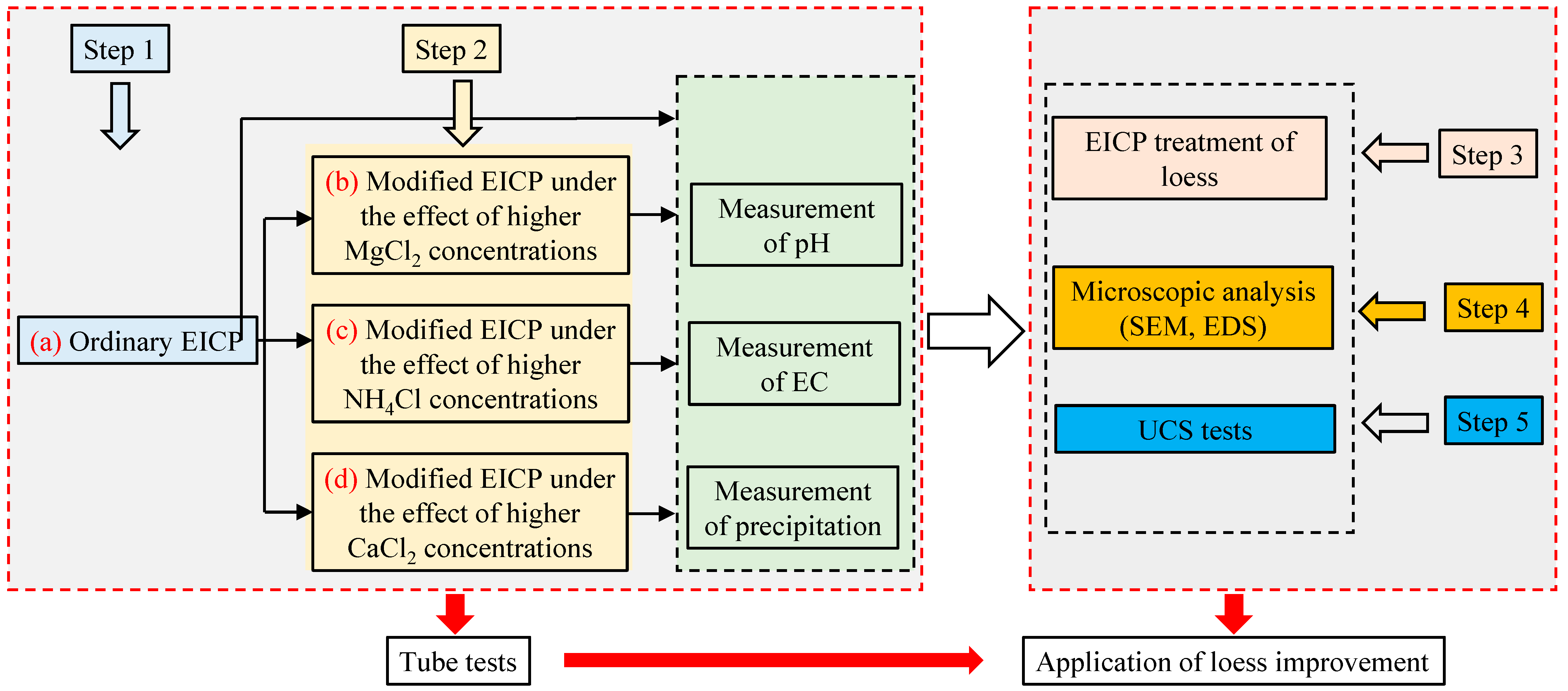
2. Materials and Methods
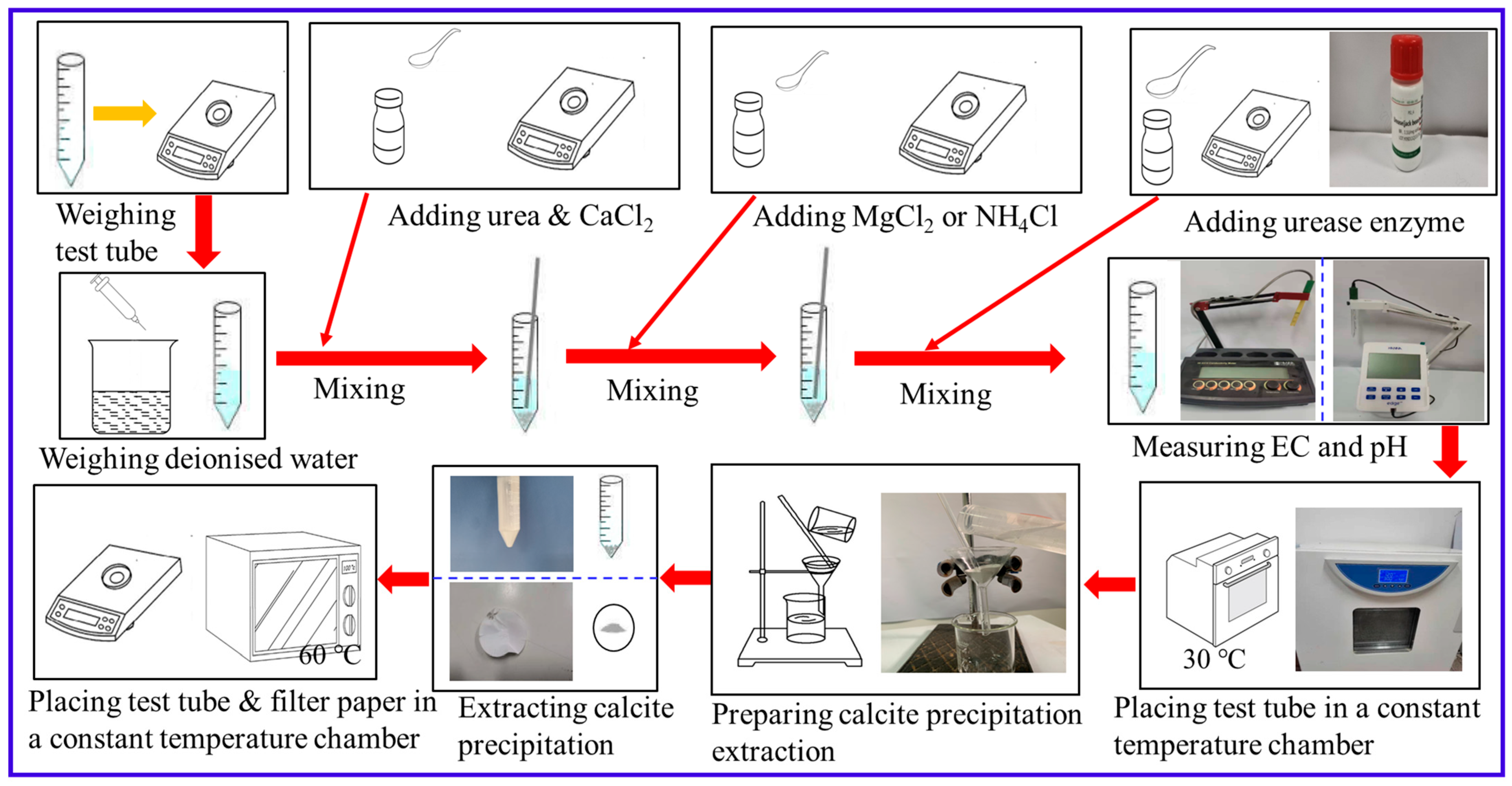
2.1. Test Tube Experiments
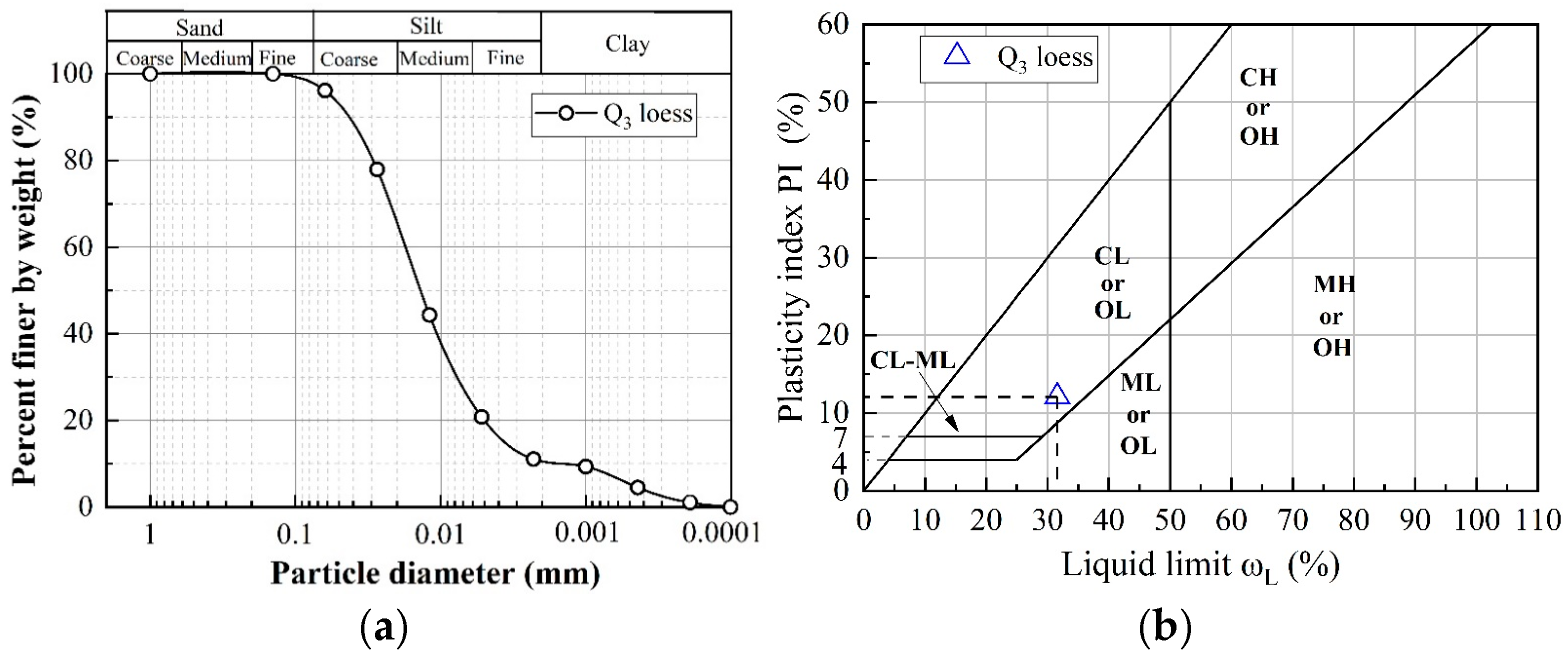
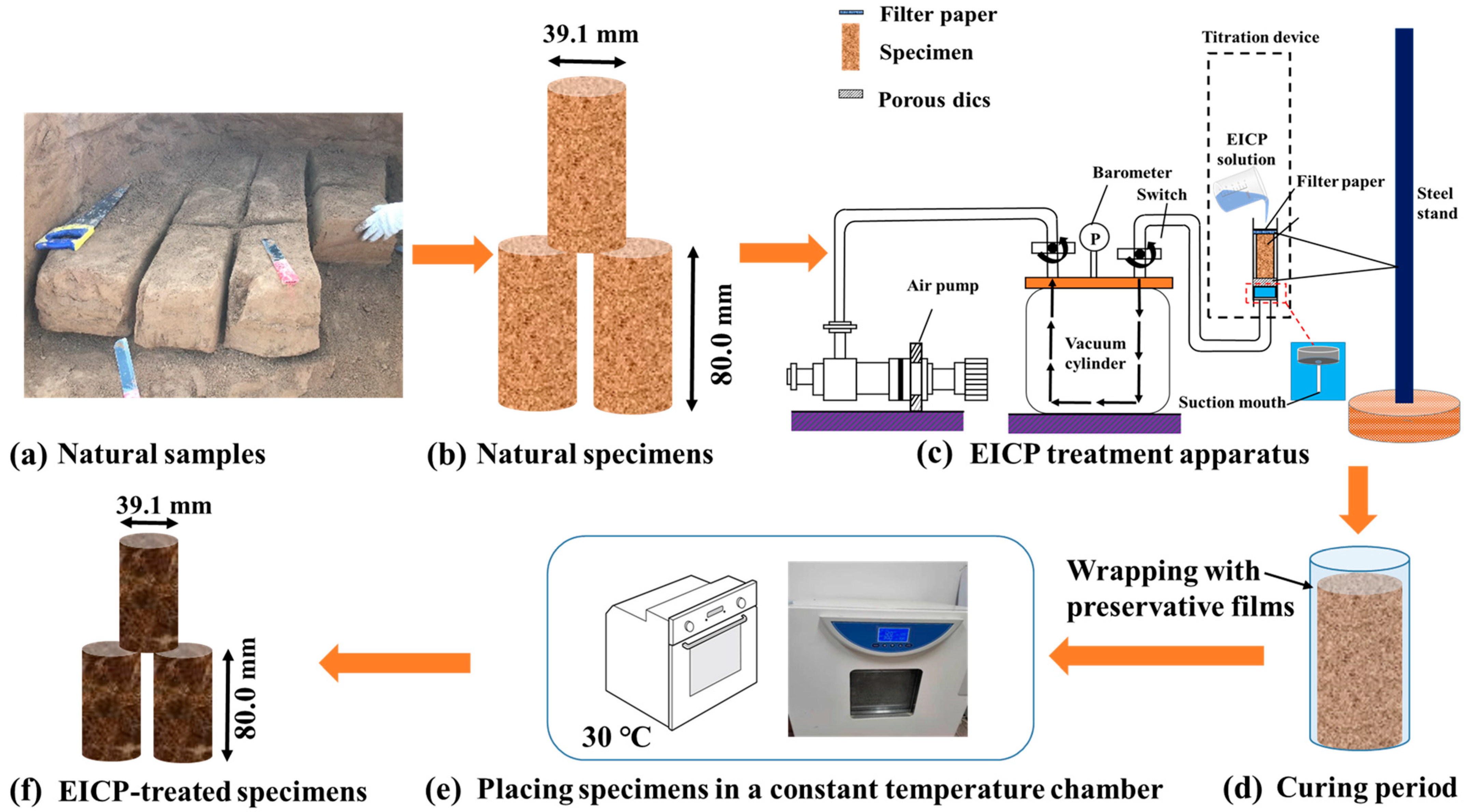
2.2. Soil Specimen Preparation
2.3. Experimental Procedure for Loess Treatment Using the EICP Method
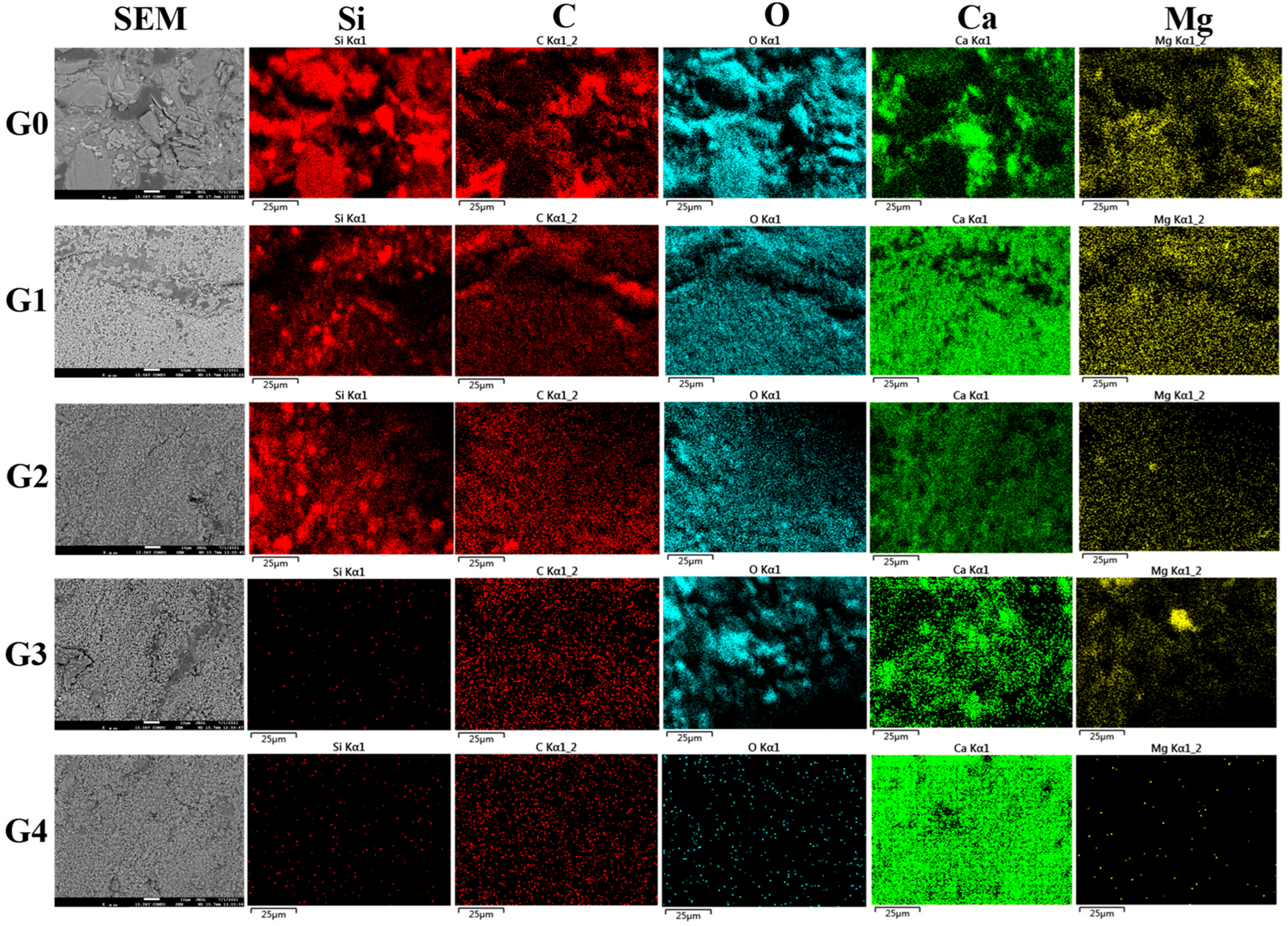
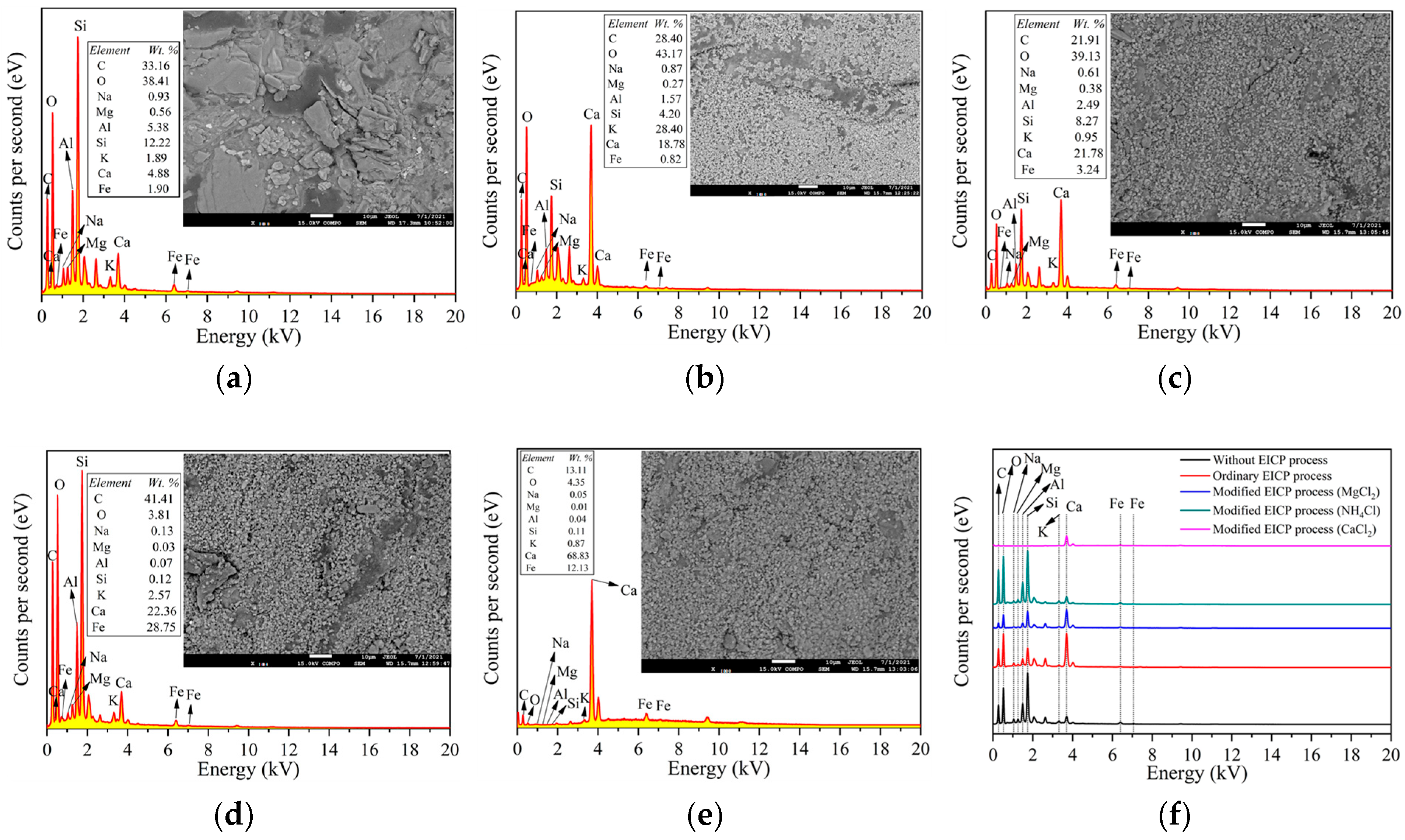
2.4. SEM and EDS Analysis
3. Results
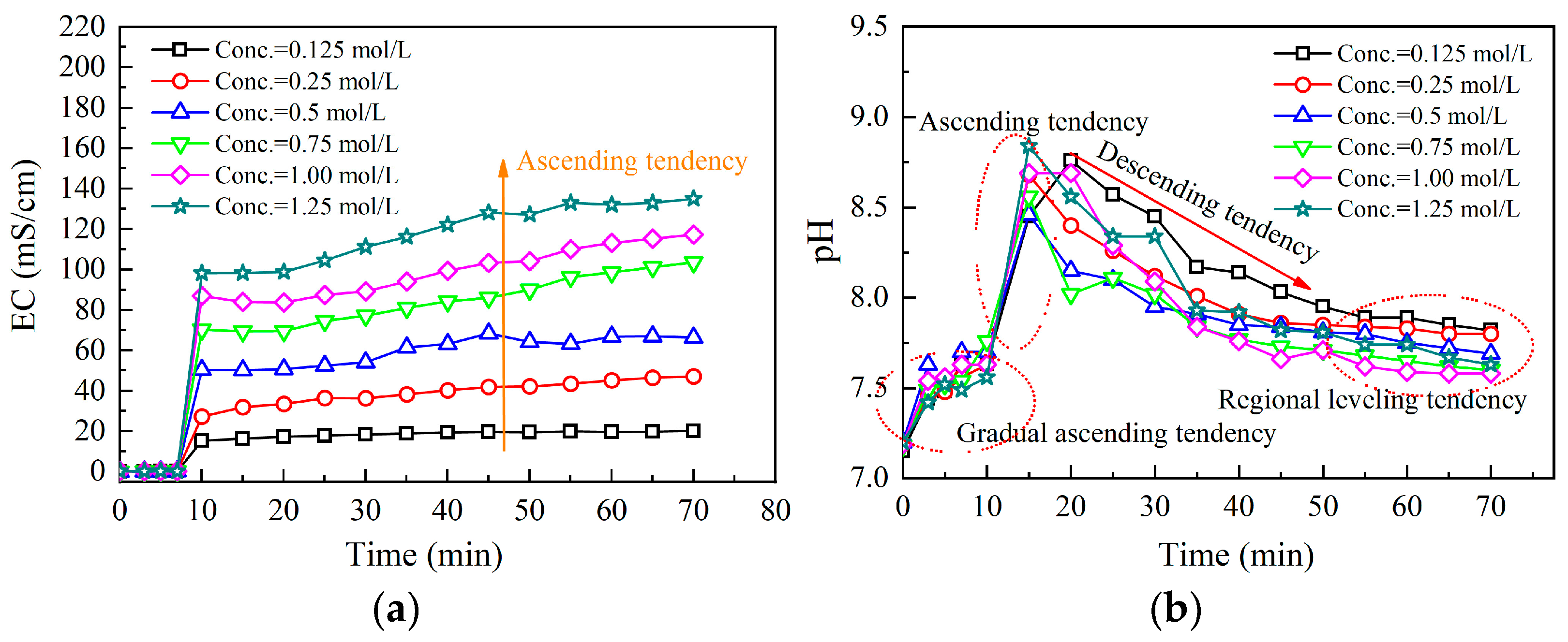
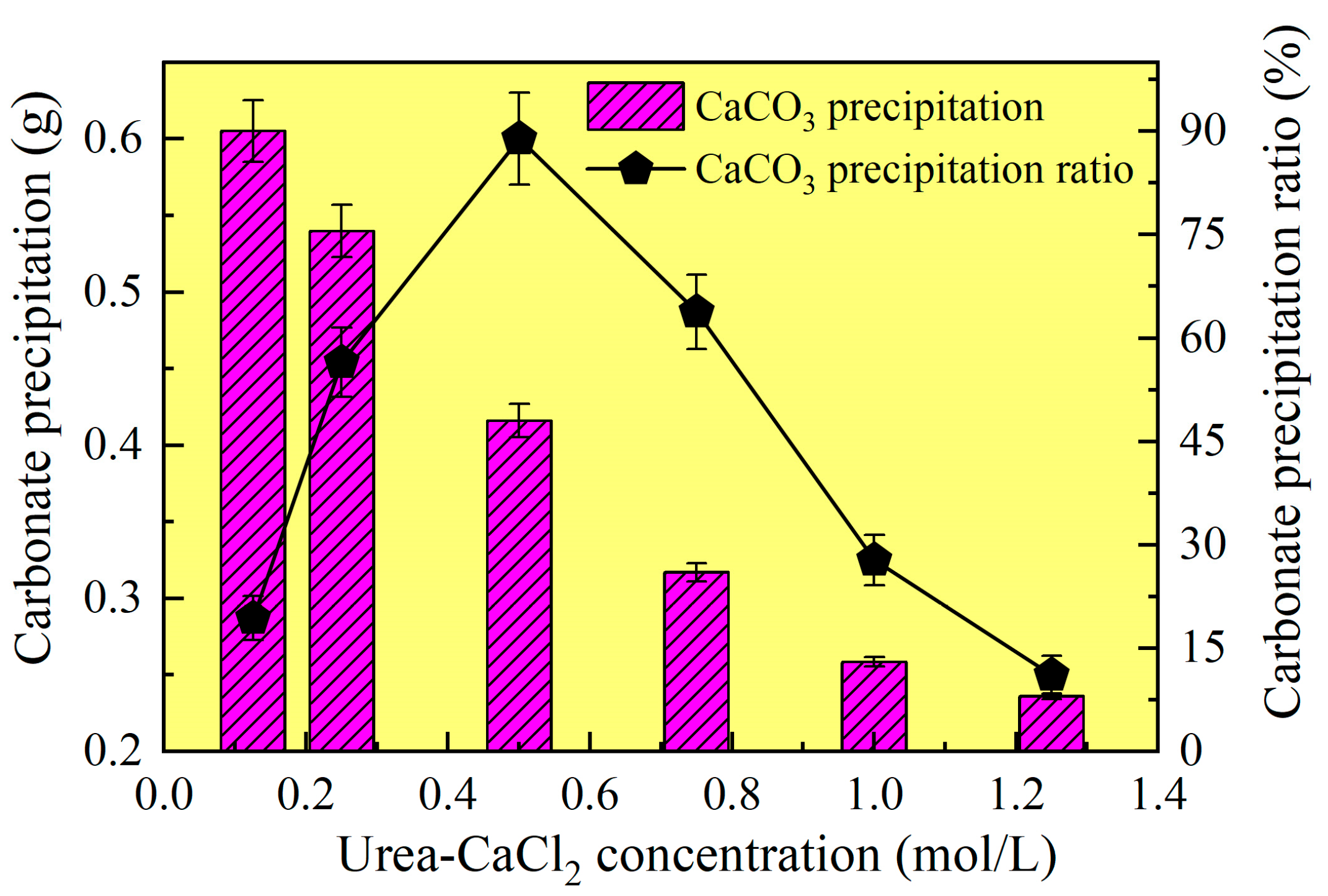
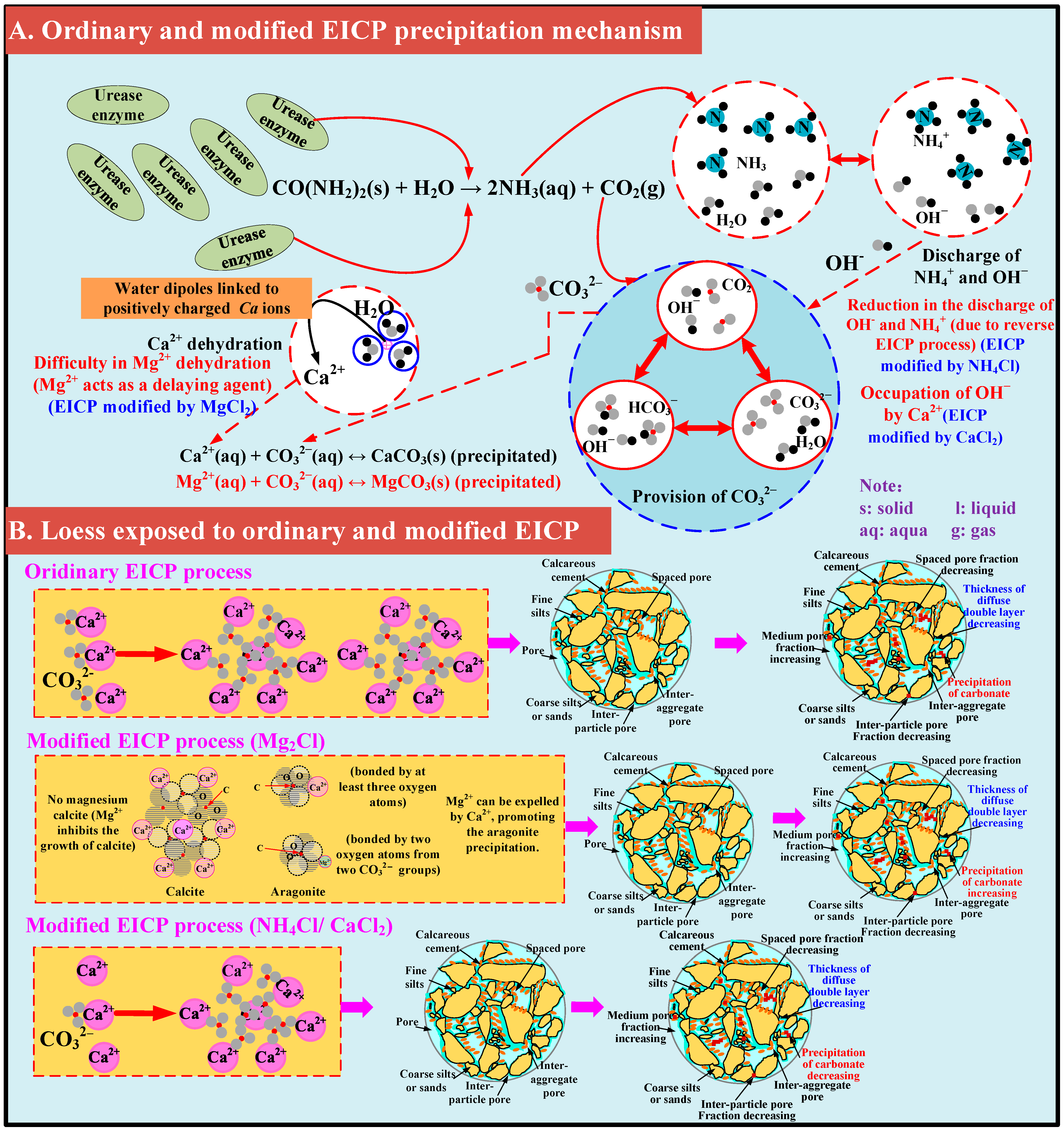
3.1. Ordinary EICP Process
3.2. Modified EICP Process
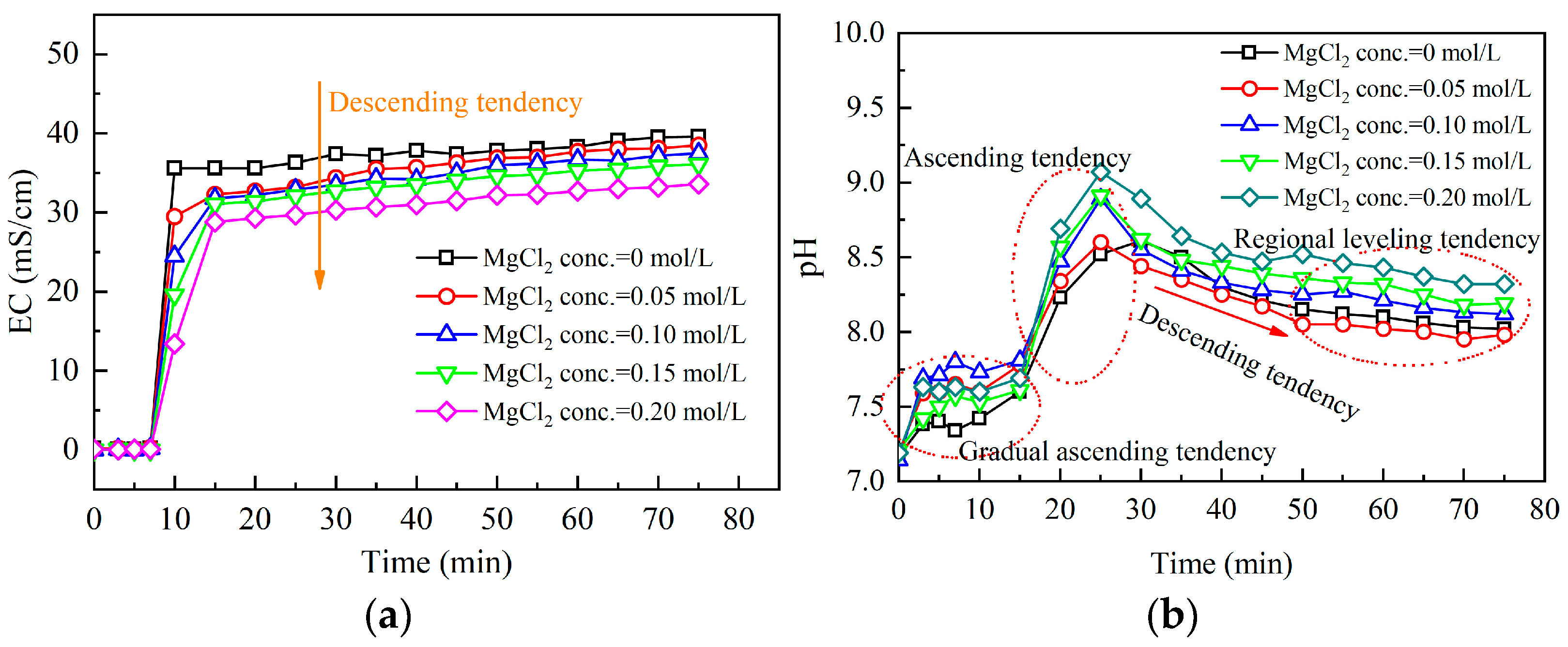
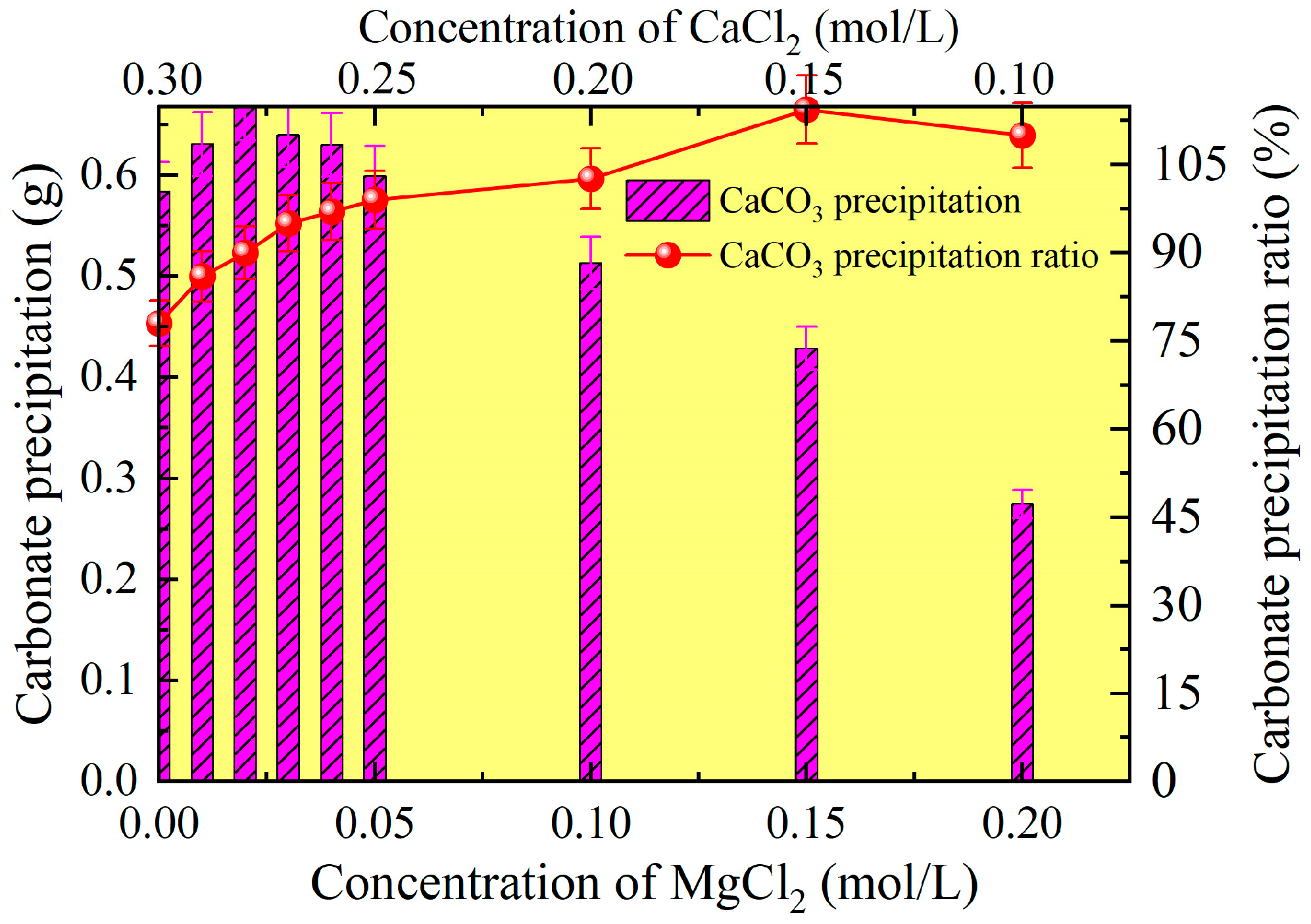
3.2.1. Effect of Magnesium Ion Addition
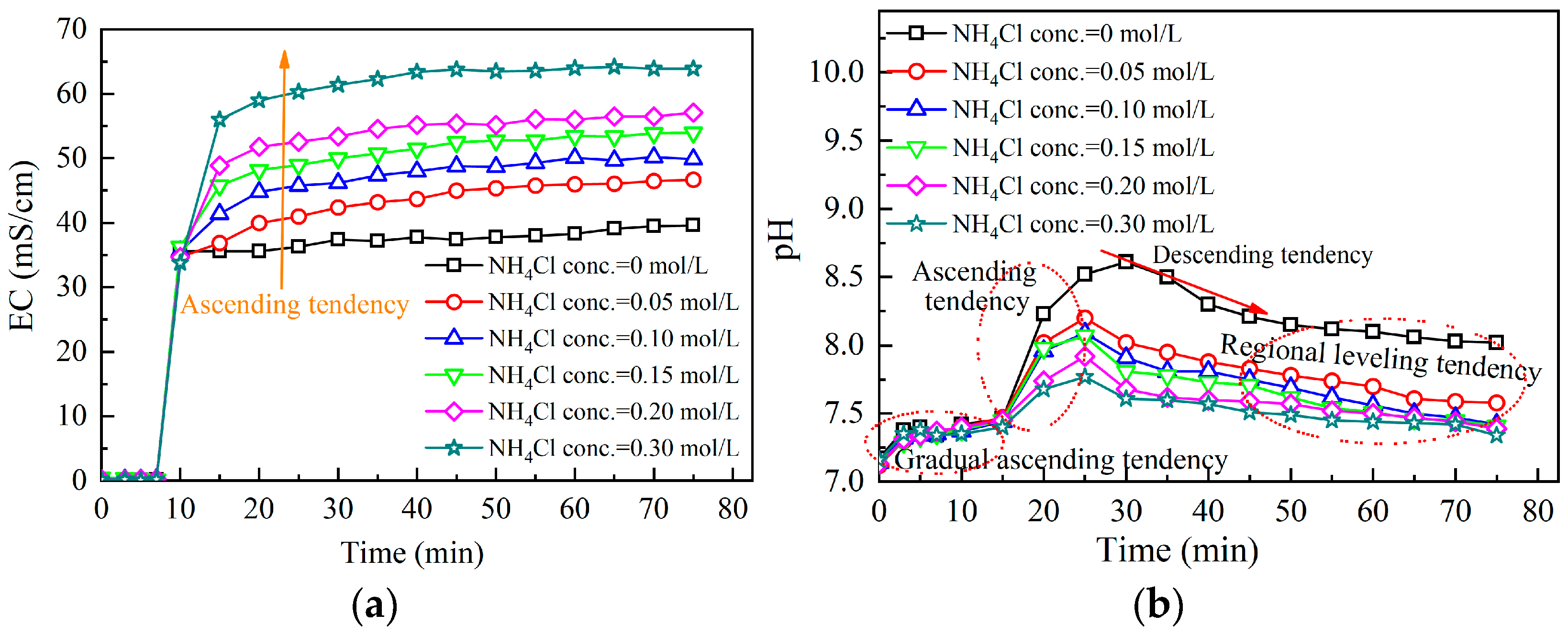
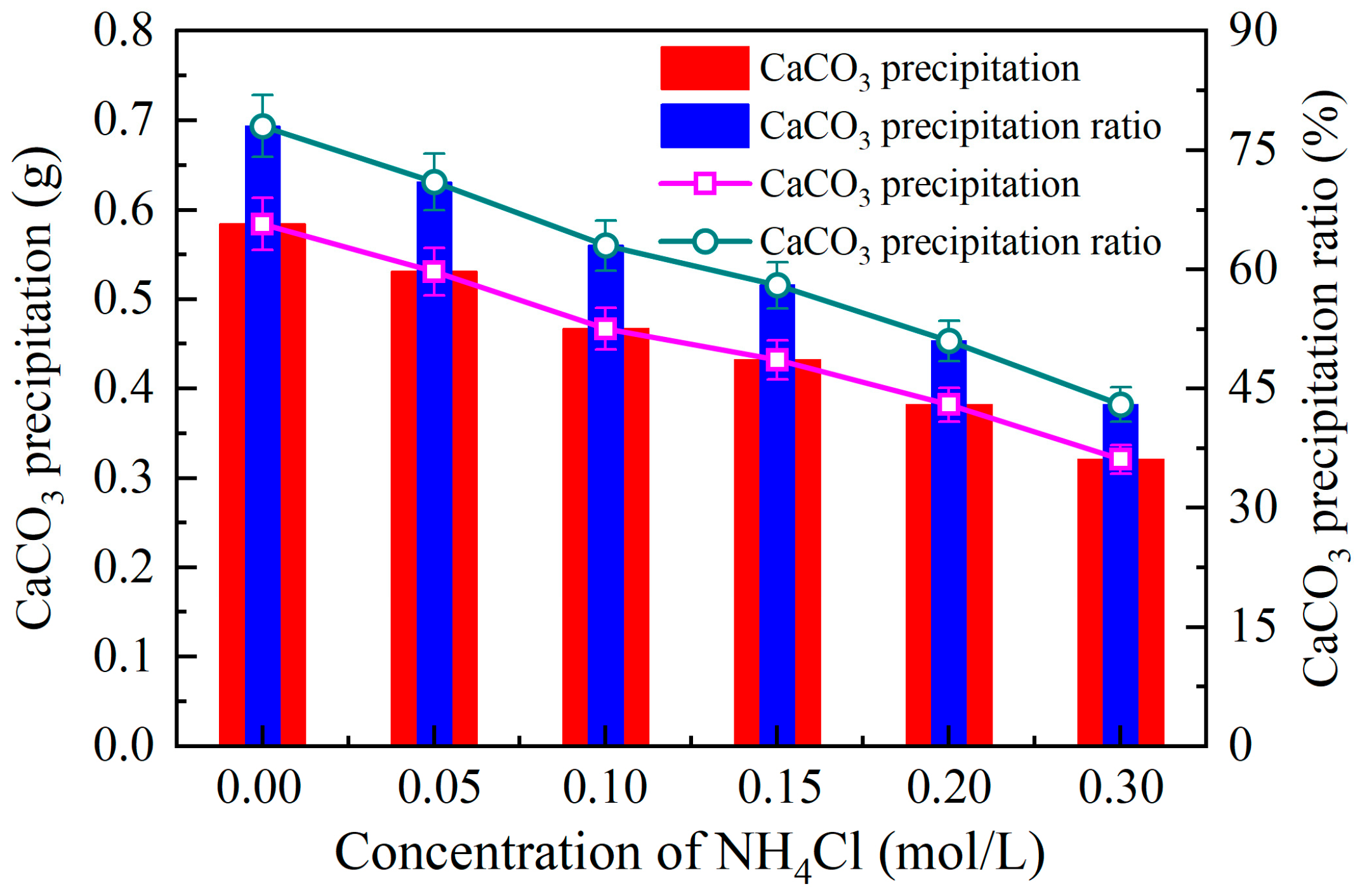
3.2.2. Effect of Ammonium Ion Addition
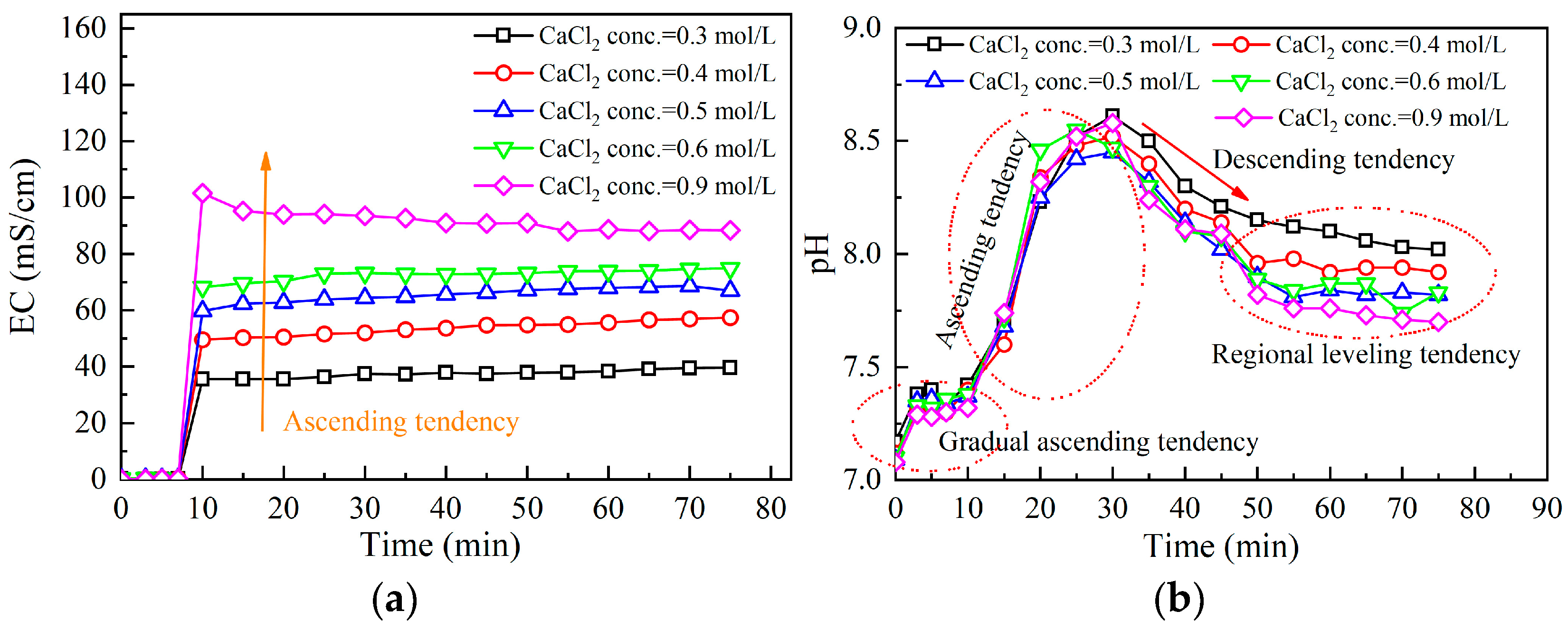
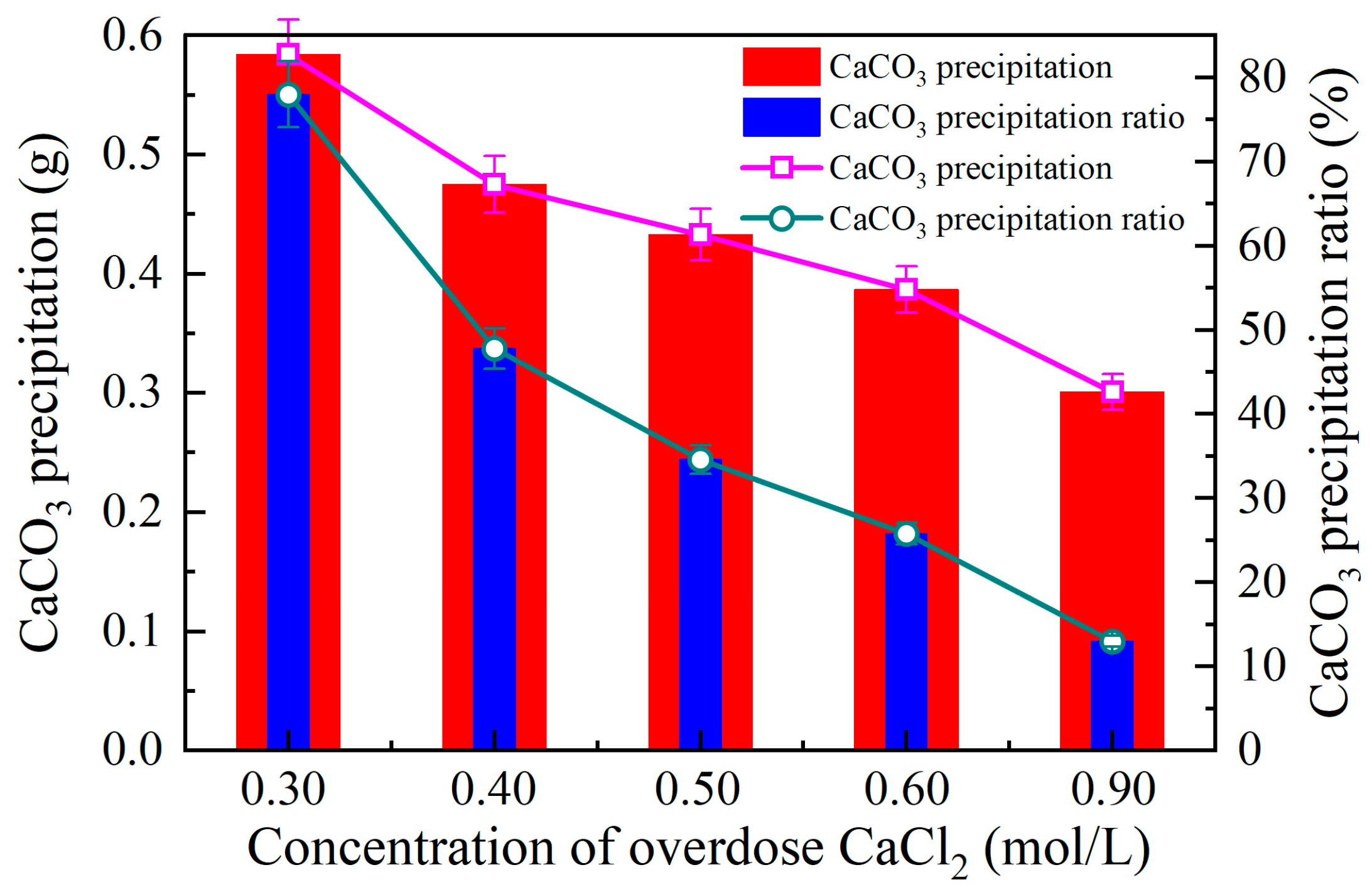
3.2.3. Effect of Calcium Chloride Addition
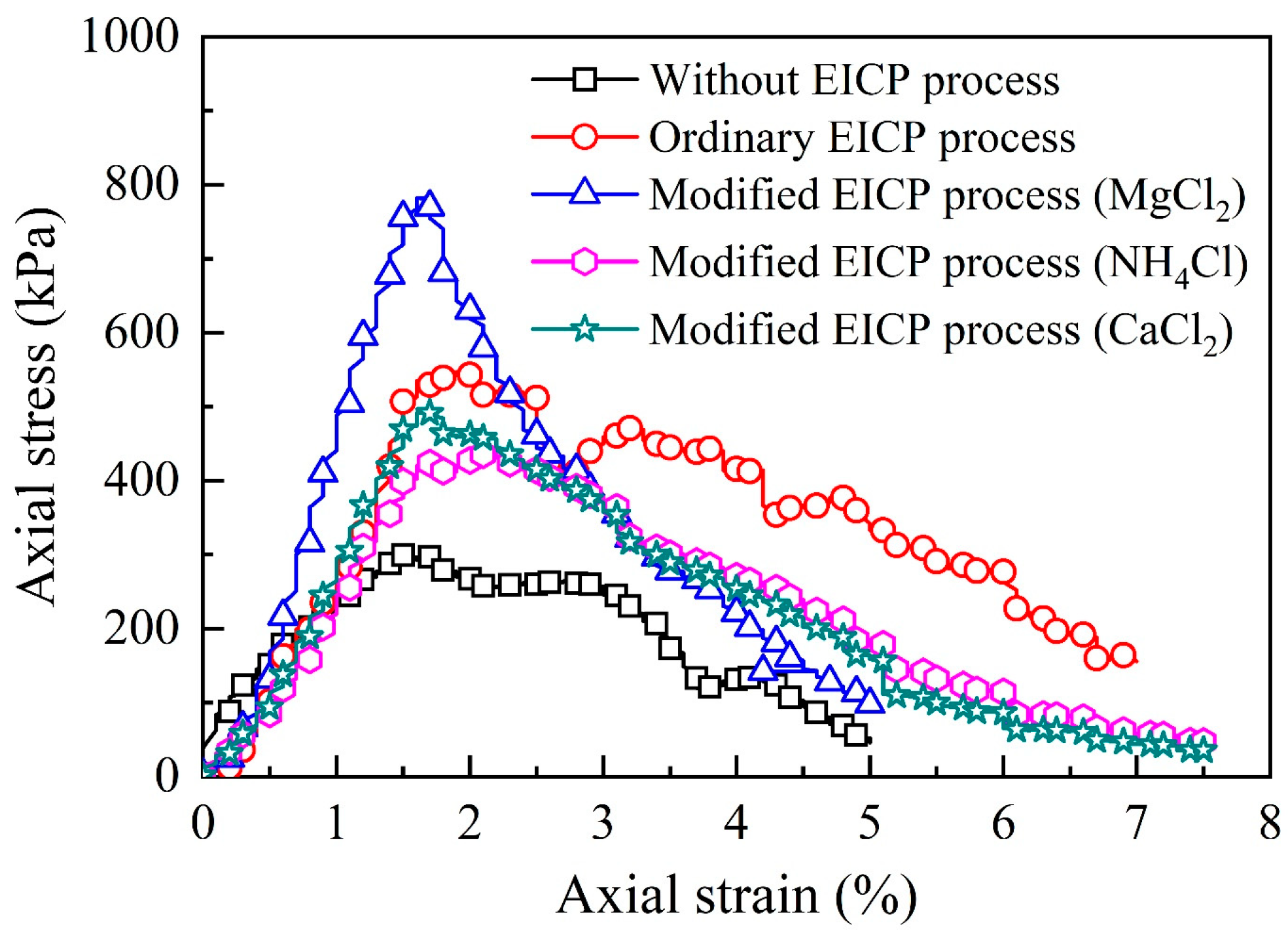
3.3. UCS of the Modified Loess
3.4. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Du, P.F.; Chen, Y.; Zhao, Y.; Qu, L.Q.; Liu, H.Y.; Liu, Z.L.; Xu, J.J.; Tian, X.Y. Formation process and depositional characteristics of mudballs in the Loess Plateau during extreme rainstorms: Dual-threshold constraints on material sources and dynamic conditions. J. Environ. Manag. 2025, 391, 126590. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Jiao, J.Y.; Ma, X.W.; Zhao, W.T.; Ling, Q.; Zhang, X.H.; Han, J.Q.; Du, P.F.; Chen, Y.; Chen, H. Distribution and formation of soil balls under heavy rainstorm conditions in the northern Loess Plateau. J. Hydrol. 2023, 625, 130103. [Google Scholar] [CrossRef]
- Hu, W.L.; Cheng, W.C.; Wen, S.J.; Rahman, M.M. Effects of chemical contamination on microscale structural characteristics of intact loess and resultant macroscale mechanical properties. CATENA 2021, 203, 105361. [Google Scholar] [CrossRef]
- Xu, P.P.; Qian, H.; Li, S.Q.; Li, W.Q.; Chen, J.; Liu, Y.X. Geochemical evidence of fluoride behavior in loess and its influence on seepage characteristics: An experimental study. Sci. Total Environ. 2023, 882, 163564. [Google Scholar] [CrossRef]
- Xu, P.P.; Qian, H.; Chen, J.; Wang, L.B.; Abliz, X.; He, X.Q.; Ma, G.X.; Liu, Y. New insights into microstructure evolution mechanism of compacted loess and its engineering implications. Bull. Eng. Geol. Environ. 2023, 82, 36. [Google Scholar] [CrossRef]
- Hao, Z.T.; Li, X.A.; An, M.X.; Gao, R.R.; Hu, W.; Yao, W.; Zheng, H.; Zhang, Y.T. Characteristics of loess wind sorting and its structural mechanical significance. Eng. Geol. 2023, 326, 107328. [Google Scholar] [CrossRef]
- Ni, W.K.; Nie, Y.P.; Lv, X.F.; Fan, M. Mechanical behavior and microstructure evolution of Malan loess under dynamic compaction. Environ. Earth Sci. 2024, 83, 76. [Google Scholar] [CrossRef]
- Yang, J.D.; Li, X.A.; Li, L.C.; Jing, Z.; Wang, W.P. Formation mechanism of metastable internal support microstructure in Malan Loess and its implications for collapsibility. Eng. Geol. 2025, 346, 107892. [Google Scholar] [CrossRef]
- Li, H.R.; Yang, M.; Guo, X.H. Study of the disintegration of loess modified with fly ash and Roadyes. Sci. Rep. 2023, 13, 7253. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Xu, J.; Wang, Z.F.; Wang, B. Experimental study on microstructure and hydraulic performance of bentonite modified loess. KSCE J. Civ. Eng. 2023, 27, 2778–2791. [Google Scholar] [CrossRef]
- Gao, L.N.; Zhang, Y.B.; Shi, H.; Duan, Q.; Xu, S.Y.; Qin, W.J.; Duan, Z.X. Effect of modified materials on the hydraulic conductivity of loess soil. Sci. Rep. 2025, 15, 12340. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Lin, Q.W.; Fang, L.Y. Study on the mechanical properties of loess improved by lignosulfonate and its mechanism analysis and prospects. Appl. Sci. 2022, 12, 9843. [Google Scholar] [CrossRef]
- Chen, J.L.; Wang, Y.J.; Shao, L.M.; Lv, F.; Zhang, H.; He, P.J. In-situ removal of odorous NH3 and H2S by loess modified with biologically stabilized leachate. J. Environ. Manag. 2022, 323, 116248. [Google Scholar] [CrossRef]
- Cheng, Z.; Cheng, X.R.; Xie, Y.C.; Ma, Z.; Liu, Y.H. Strength tests and numerical simulations of loess modified by desulfurization ash and fly ash. Materials 2022, 15, 512. [Google Scholar] [CrossRef]
- Avramenko, M.; Nakashima, K.; Kawasaki, S. State-of-the-Art Review on Engineering Uses of Calcium Phosphate Compounds: An Eco-Friendly Approach for Soil Improvement. Materials 2022, 15, 6878. [Google Scholar] [CrossRef]
- Maston, O.; Ouahbi, T.; Taibi, S.; Hajjar, A.E.; Sapin, L.; Filet, A.E.; Duchemin, B.; Fleureau, J.M. Effect of MICP treatment on the mechanical properties of clay soils. Transp. Geotech. 2025, 50, 101483. [Google Scholar] [CrossRef]
- Hao, L.; Lin, P.; Garg, A. Unsaturated soil properties of MICP treated granitic residual soil of Shantou region of China. Acta Geophys. 2023, 71, 1885–1894. [Google Scholar] [CrossRef]
- Yuan, X.Q.; Zhu, T.K.; Wang, Q.; Chen, H.E.; Lin, S.; Wang, X.; Xu, X. An experimental investigation of dispersive soils treated by microbially induced calcium carbonate precipitation (MICP). Constr. Build. Mater. 2024, 446, 137941. [Google Scholar] [CrossRef]
- Gao, Y.Q.; He, J.; Gao, Y.F.; Wang, L.Y.; Ren, J. Denitrification-based MICP for cementation of soil: Treatment process and mechanical performance. Acta Geotech. 2022, 17, 3799–3815. [Google Scholar] [CrossRef]
- Tang, C.S.; Yin, L.Y.; Jiang, N.J.; Zhu, C.; Zeng, H.; Li, H.; Shi, B. Factors affecting the performance of microbial-induced carbonate precipitation (MICP) treated soil: A review. Environ. Earth Sci. 2020, 79, 94. [Google Scholar] [CrossRef]
- Su, F.; Yang, Y.Y.; Qi, Y.; Zhang, H.N. Combining microbially induced calcite precipitation (MICP) with zeolite: A new technique to reduce ammonia emission and enhance soil treatment ability of MICP technology. J. Environ. Chem. Eng. 2022, 10, 107770. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, Y.; Jin, Y.H.; Xiong, Y.; Chen, G.N.; Ruan, F.Y. Behaviour and mechanism of cadmium immobilization in contaminated soil by calcium carbide residue-enhanced MICP. Sci. Rep. 2024, 14, 25409. [Google Scholar] [CrossRef] [PubMed]
- Boquet, E.; Boronat, A.; Ramos-Cormenzana, A. Production of calcite (calcium carbonate) crystals by soil bacteria is a general phenomenon. Nature 1973, 246, 527–529. [Google Scholar] [CrossRef]
- Whiffin, V.S.; Van-Paassen, L.A.; Harkes, M.P. Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Van Paassen, L.A. Biogrout Ground Improvement by Microbial Induced Carbonate Precipitation. Ph.D. Thesis, Murdoch University, Perth, Australia, 2009. [Google Scholar]
- Zhang, Y.; Guo, H.X.; Cheng, X.H. Role of calcium sources in the strength and microstructure of microbial mortar. Constr. Build. Mater. 2015, 77, 160–167. [Google Scholar] [CrossRef]
- Li, J.; Zhu, F.Q.; Wu, F.S.; Chen, Y.X.; Richards, J.; Li, T.X.; Li, P.; Shang, D.J.; Yu, J.; Viles, H.; et al. Impact of soil density on biomineralization using EICP and MICP techniques for earthen sites consolidation. J. Environ. Manag. 2024, 363, 121410. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.H.; Miao, L.C.; Wang, H.X.; Wu, L.Y.; Shi, W.B.; Kawasaki, S. State-of-the-art review of soil erosion control by MICP and EICP techniques: Problems, applications, and prospects. Sci. Total Environ. 2024, 912, 169016. [Google Scholar] [CrossRef]
- Lai, H.J.; Cui, M.J.; Chu, J. Effect of pH on soil improvement using one-phase-low-pH MICP or EICP biocementation method. Acta Geotech. 2023, 18, 3259–3272. [Google Scholar] [CrossRef]
- Almajed, A.; Lateef, M.A.; Moghal, A.A.B.; Lemboye, K. State-of-the-Art Review of the Applicability and Challenges of Microbial-Induced Calcite Precipitation (MICP) and Enzyme-Induced Calcite Precipitation (EICP) Techniques for Geotechnical and Geoenvironmental Applications. Crystals 2021, 11, 370. [Google Scholar] [CrossRef]
- Ahenkorah, I.; Rahman, M.M.; Karim, M.R.; Beecham, S. Enzyme induced calcium carbonate precipitation and its engineering application: A systematic review and meta-analysis. Constr. Build. Mater. 2021, 308, 125000. [Google Scholar] [CrossRef]
- Ahenkorah, I.; Rahman, M.M.; Karim, M.R.; Beecham, S. Unconfined compressive strength of MICP and EICP treated sands subjected to cycles of wetting-drying, freezing-thawing and elevated temperature: Experimental and EPR modelling. J. Rock Mech. Geotech. Eng. 2023, 15, 1226–1247. [Google Scholar] [CrossRef]
- Cui, M.J.; Lai, H.J.; Wu, S.F.; Chu, J. Comparison of soil improvement methods using crude soybean enzyme, bacterial enzyme or bacteria-induced carbonate precipitation. Géotechnique 2022, 74, 18–26. [Google Scholar] [CrossRef]
- Hu, W.L.; Cheng, W.C.; Wen, S.J.; Yuan, K. Revealing the Enhancement and Degradation Mechanisms Affecting the Performance of Carbonate Precipitation in EICP Process. Front. Bioeng. Biotechnol. 2021, 9, 750258. [Google Scholar] [CrossRef]
- Neupane, D.; Yasuhara, H.; Kinoshita, N.; Unno, T. Applicability of Enzymatic Calcium Carbonate Precipitation as a Soil-Strengthening Technique. J. Geotech. Geoenviron. Eng. 2013, 139, 2201–2211. [Google Scholar] [CrossRef]
- Almajed, A.; Tirkolaei, H.K.; Eward, K.J. Baseline Investigation on Enzyme-Induced Calcium Carbonate Precipitation. J. Geotech. Geoenviron. Eng. 2018, 144, 04018081. [Google Scholar] [CrossRef]
- Carmona, J.P.S.F.; Oliveira, P.J.V.; Lemos, L.J.L. Biostabilization of a Sandy Soil Using Enzymatic Calcium Carbonate Precipitation. Procedia Eng. 2016, 143, 1301–1308. [Google Scholar] [CrossRef]
- Zhang, Q.; Ye, W.M.; Liu, Z.R.; Wang, Q.; Chen, Y.G. Influence of injection methods on calcareous sand cementation by EICP technique. Constr. Build. Mater. 2023, 363, 129724. [Google Scholar] [CrossRef]
- Yuan, L.; Li, G.; Liu, J.; Wang, P.; Liu, C.; Zhang, J. Study on Mechanical Properties of Sandy Soil Solidified by Enzyme-Induced Calcium Carbonate Precipitation (EICP). Buildings 2024, 14, 1977. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, S.X. Feasibility study of applying enzyme-induced carbonate precipitation (EICP) without calcium source for remediation of lead-contaminated loess. Buildings 2024, 14, 1810. [Google Scholar] [CrossRef]
- Shen, D.; Liu, Z.; Song, Z.; Wu, C. Reinforcement mechanism and erosion resistance of loess slope using enzyme induced calcite precipitation technique. Sustainability 2023, 15, 1044. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, J. Laboratory study on the use of urease-induced calcium carbonate precipitation for stabilization of coal fly ash. Minerals 2023, 13, 185. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, Y.; Chen, J. Comparison of solidification characteristics between polymer-cured and bio-cured fly ash in the laboratory. Polymers 2023, 15, 1107. [Google Scholar] [CrossRef] [PubMed]
- Hammes, F.; Boon, N.; Villiers, J.D. Strain-specific ureolytic microbial calcium carbonate precipitation. Appl. Environ. Microbiol. 2003, 29, 4901–4909. [Google Scholar] [CrossRef]
- Abo-El-Enein, S.A.; Ali, A.H.; Talkhan, F.N. Utilization of microbial induced calcite precipitation for sand consolidation and mortar crack remediation. HBRC J. 2012, 8, 185–192. [Google Scholar] [CrossRef]
- Putra, H.; Yasuhara, H.; Kinoshita, N.; Neupane, D.; Lu, C.W. Optimization of enzyme-mediated calcite precipitation as a soil-improvement technique: The effect of aragonite and gypsum on the mechanical properties of treated sand. Crystals 2017, 7, 59. [Google Scholar] [CrossRef]
- Putra, H.; Yasuhara, H.; Kinoshita, N.; Neupane, D.; Lu, C.W. Effects of magnesium as substitute material in enzyme-mediated calcite precipitation for soil-improvement technique. Front. Bioeng. Biotechnol. 2016, 4, 37. [Google Scholar] [CrossRef]
- Yuan, H.; Ren, G.Z.; Liu, K.; Zheng, W.; Zhao, Z.L. Experimental study of EICP combined with organic materials for silt improvement in the Yellow River flood area. Appl. Sci. 2020, 10, 7678. [Google Scholar] [CrossRef]
- Putra, H.; Yasuhara, H.; Erizal; Sutoyo; Fauzan, M. Review of Enzyme-Induced Calcite Precipitation as a Ground-Improvement Technique. Infrastructures 2020, 5, 66. [Google Scholar] [CrossRef]
- GB/T 50123-2019; Standard for Geotechnical Test Methods. China Planning Press: Beijing, China, 2019.
- ASTM D2487-17; Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System). ASTM International: West Conshohocken, PA, USA, 2017.
- International Centre for Diffraction Data (ICDD). PDF-4+ 2023 (Powder Diffraction File Database); Release 2023; ICDD: Philadelphia, PA, USA, 2023. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Hu, W.; Chen, K.; Wang, W. Effects of Ion-Regulated Mechanisms on Calcite Precipitation in the Enzyme-Induced Carbonate Precipitation Treatment of Loess. Buildings 2025, 15, 3222. https://doi.org/10.3390/buildings15173222
Wang X, Hu W, Chen K, Wang W. Effects of Ion-Regulated Mechanisms on Calcite Precipitation in the Enzyme-Induced Carbonate Precipitation Treatment of Loess. Buildings. 2025; 15(17):3222. https://doi.org/10.3390/buildings15173222
Chicago/Turabian StyleWang, Xinwen, Wenle Hu, Ke Chen, and Weijing Wang. 2025. "Effects of Ion-Regulated Mechanisms on Calcite Precipitation in the Enzyme-Induced Carbonate Precipitation Treatment of Loess" Buildings 15, no. 17: 3222. https://doi.org/10.3390/buildings15173222
APA StyleWang, X., Hu, W., Chen, K., & Wang, W. (2025). Effects of Ion-Regulated Mechanisms on Calcite Precipitation in the Enzyme-Induced Carbonate Precipitation Treatment of Loess. Buildings, 15(17), 3222. https://doi.org/10.3390/buildings15173222






