Characterization of Steel Industry Byproducts as Precursors in Alkali-Activated Binders
Abstract
1. Introduction
2. Materials and Methods
2.1. Constituent Materials
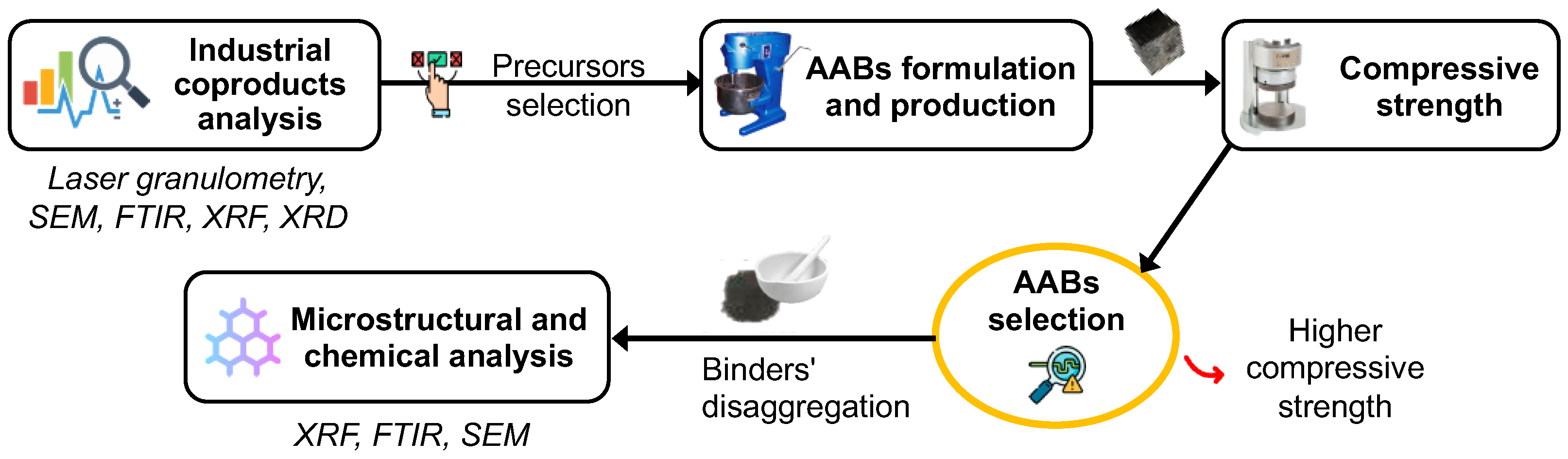
2.2. Methods
2.3. Industrial Byproducts Analysis
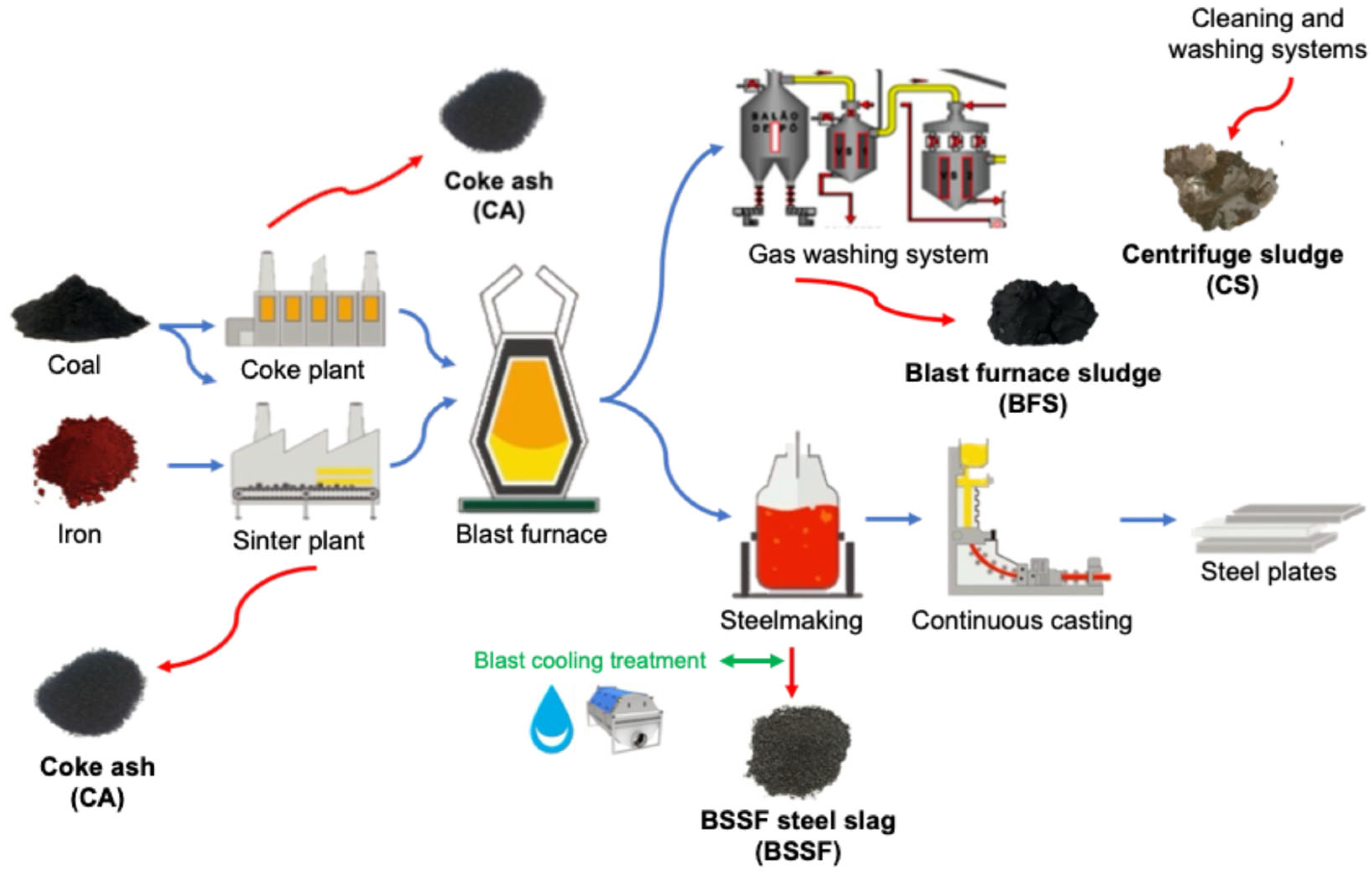
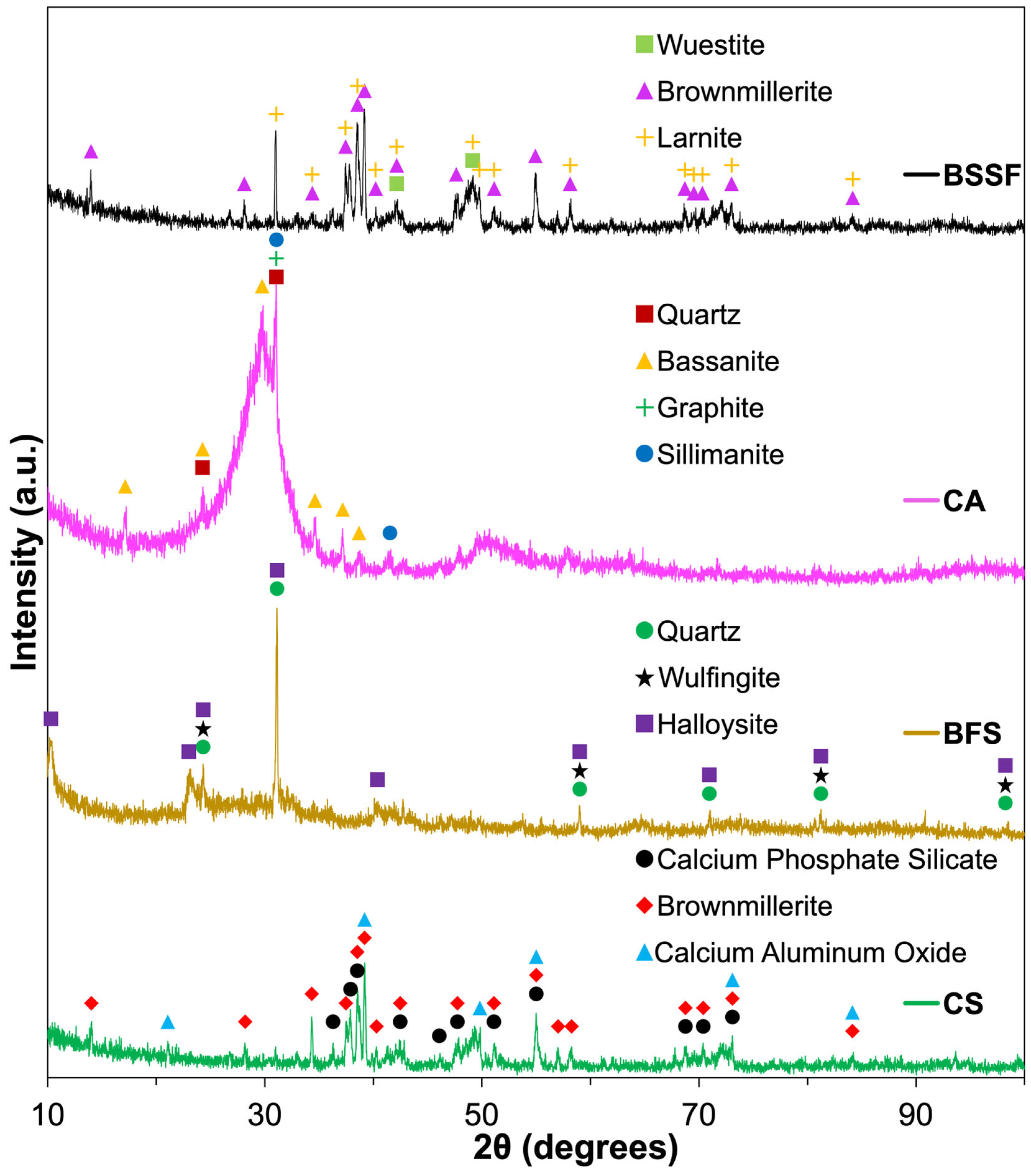
2.3.1. Steel Industry Byproducts
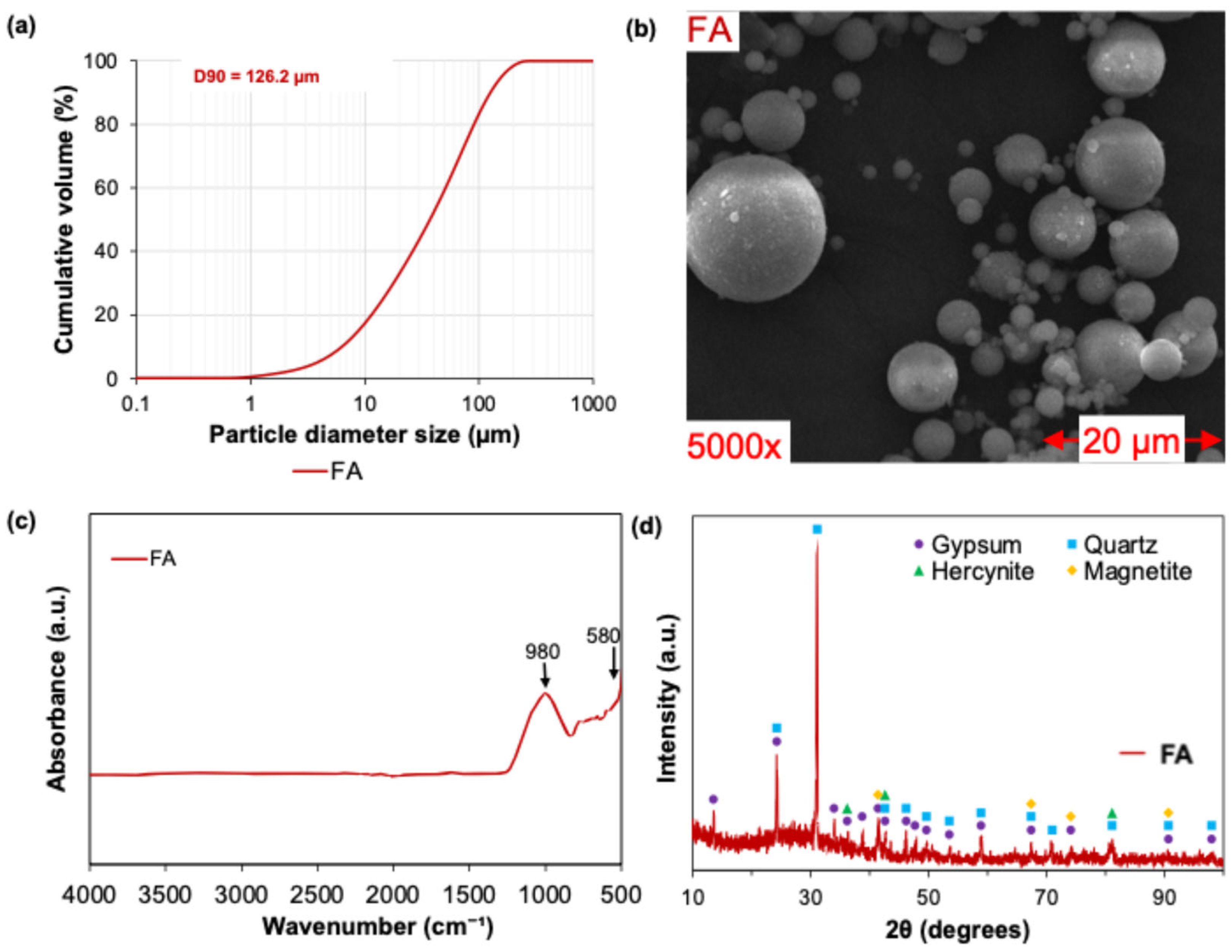
2.3.2. Thermoelectric Byproduct—Fly Ash (FA)
2.4. Formulation of AABs
2.5. Compressive Strength
2.6. Microstructural and Chemical Analysis
2.6.1. XRF
2.6.2. FTIR
2.6.3. SEM
3. Results
3.1. Compressive Strength
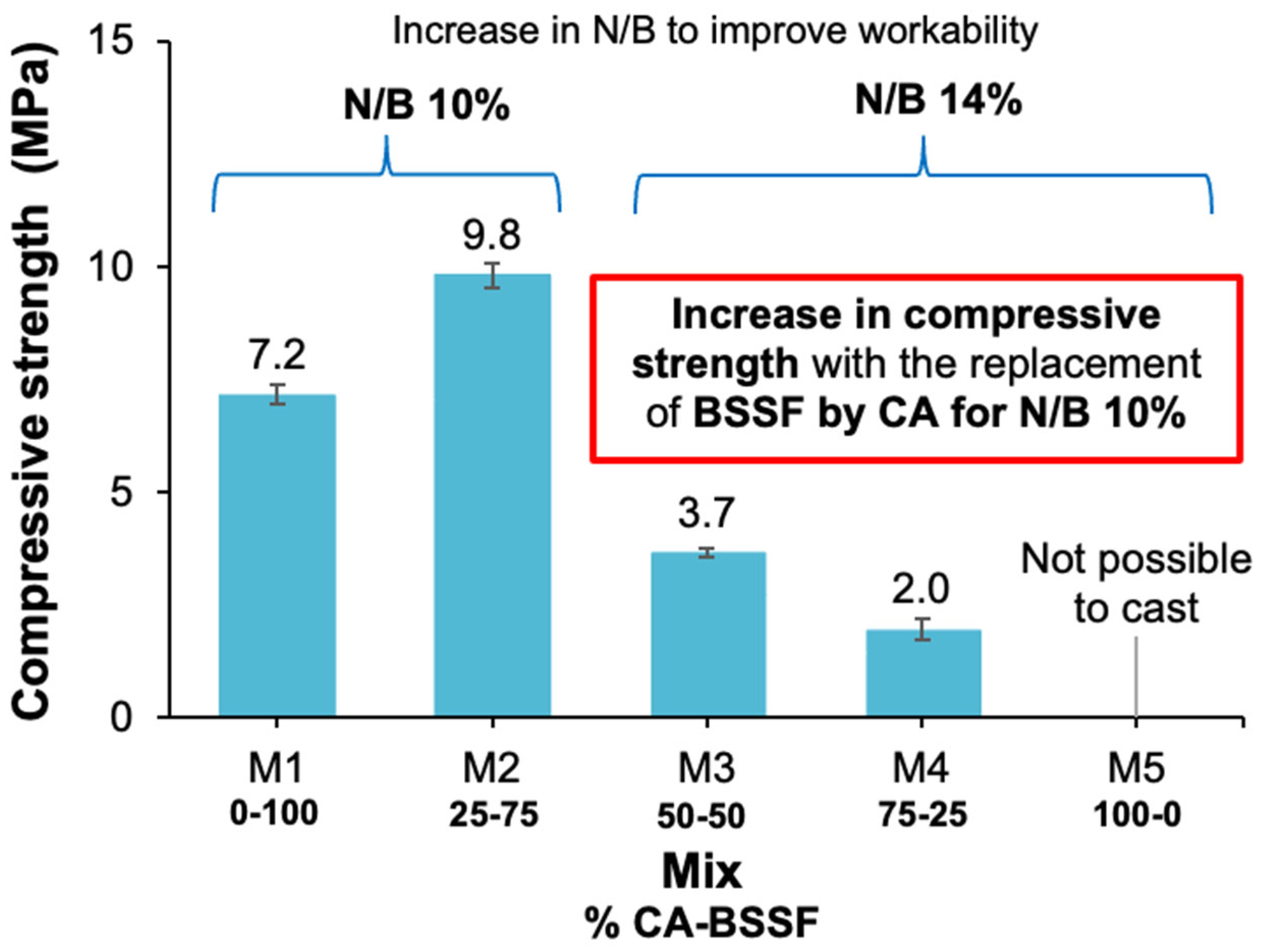
3.1.1. AAB Based on CA and BSSF
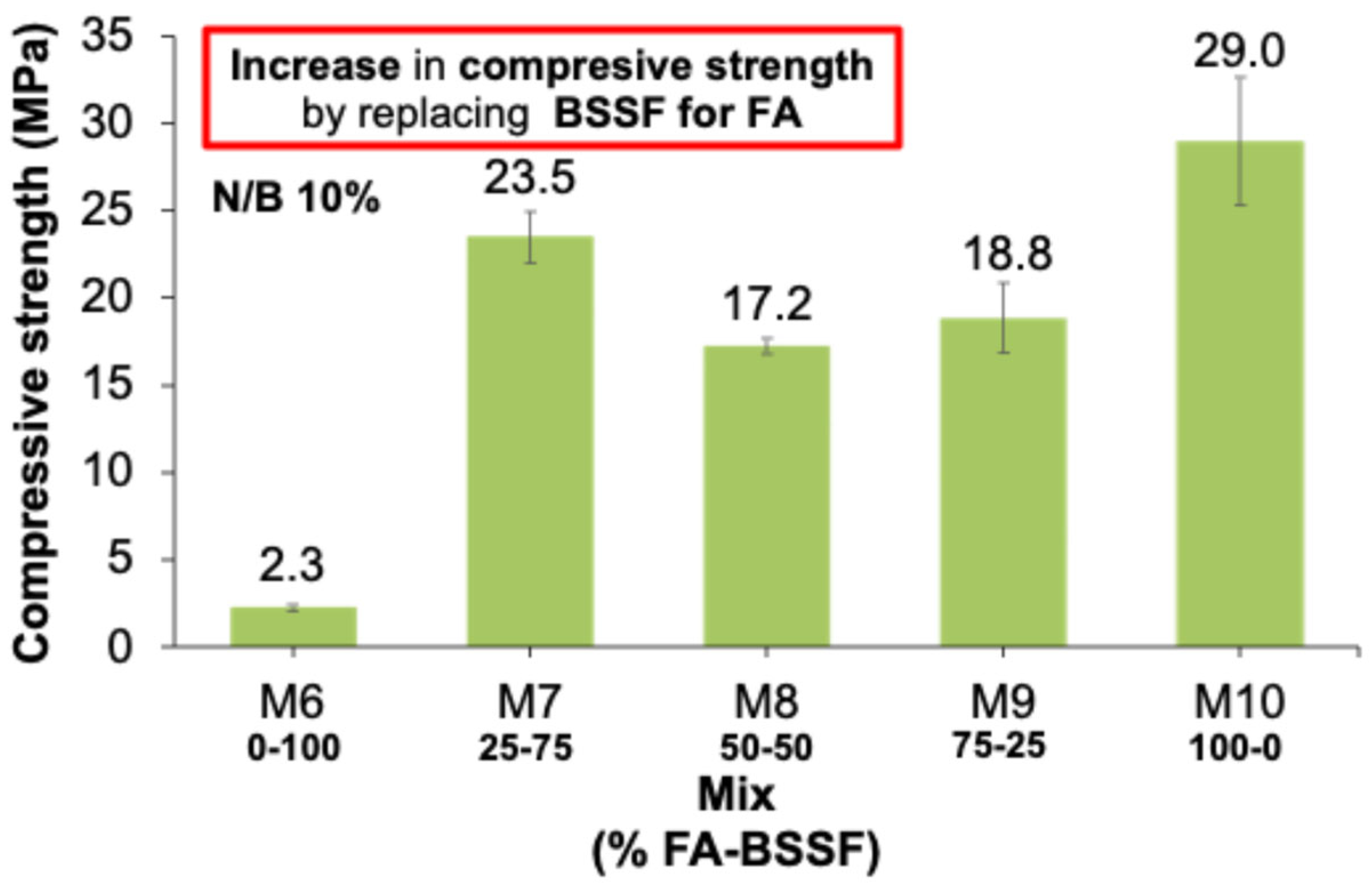
3.1.2. AAB Based on FA and BSSF
3.2. Microstructural and Chemical Analysis
3.2.1. XRF
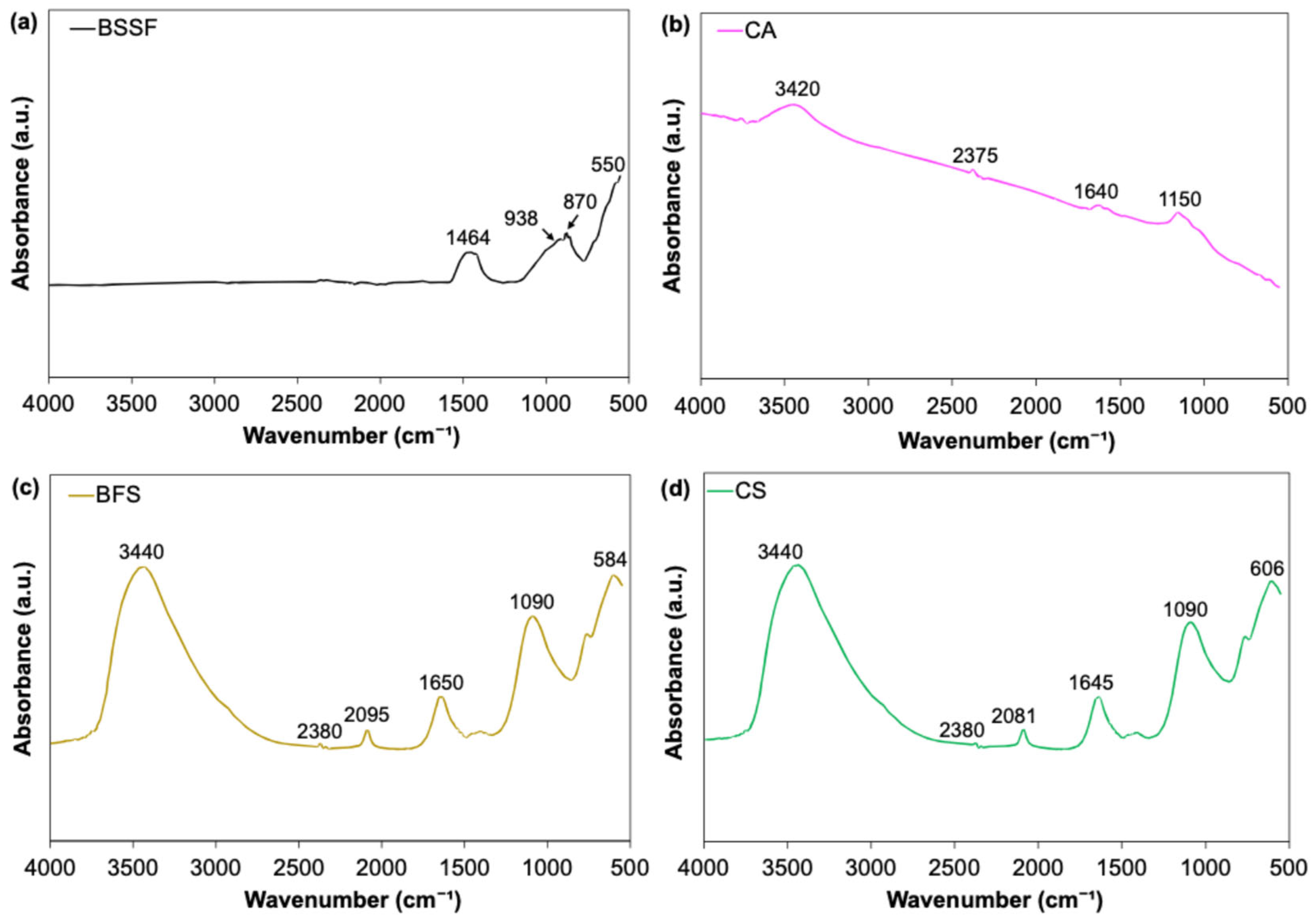
3.2.2. FTIR
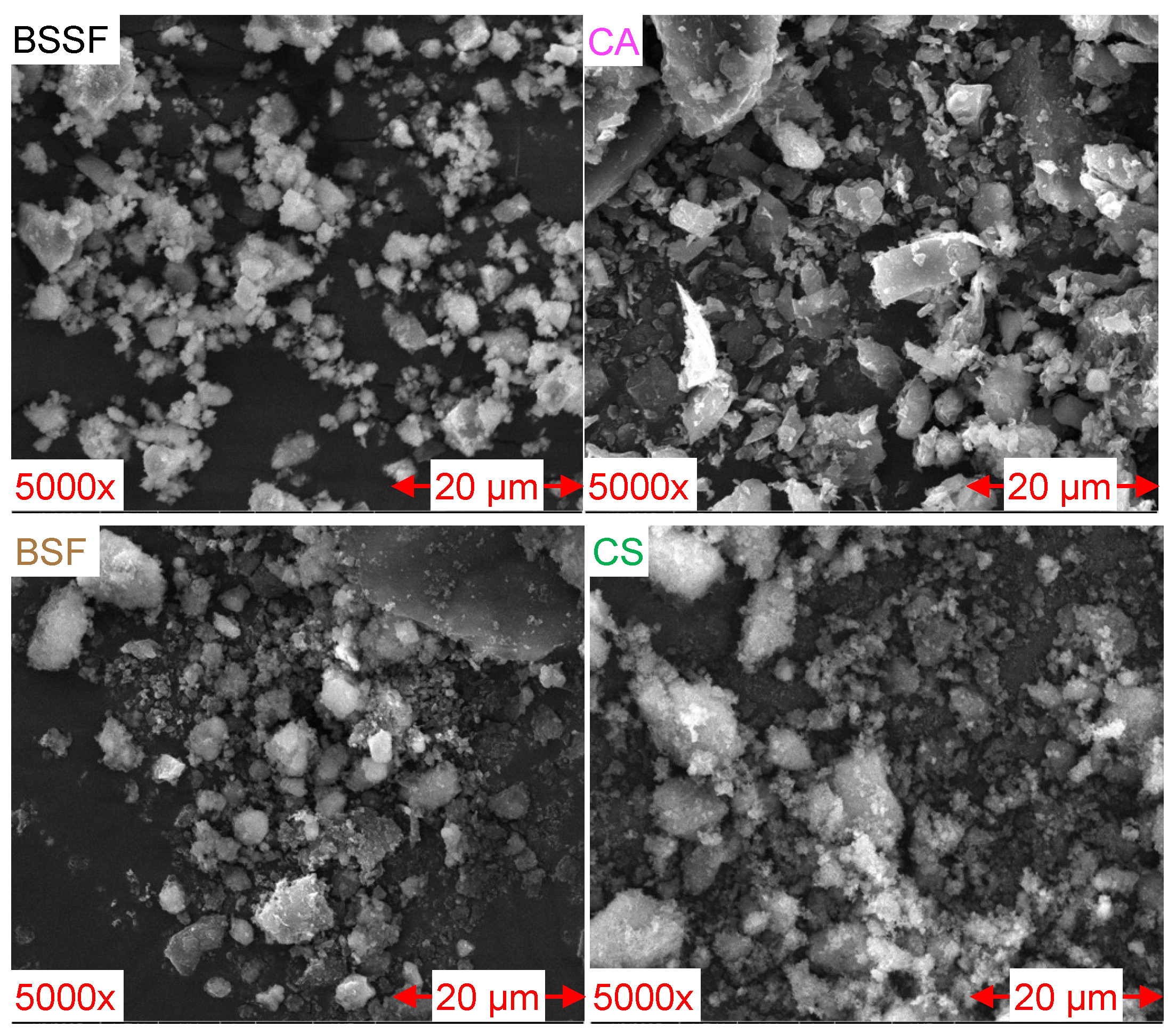
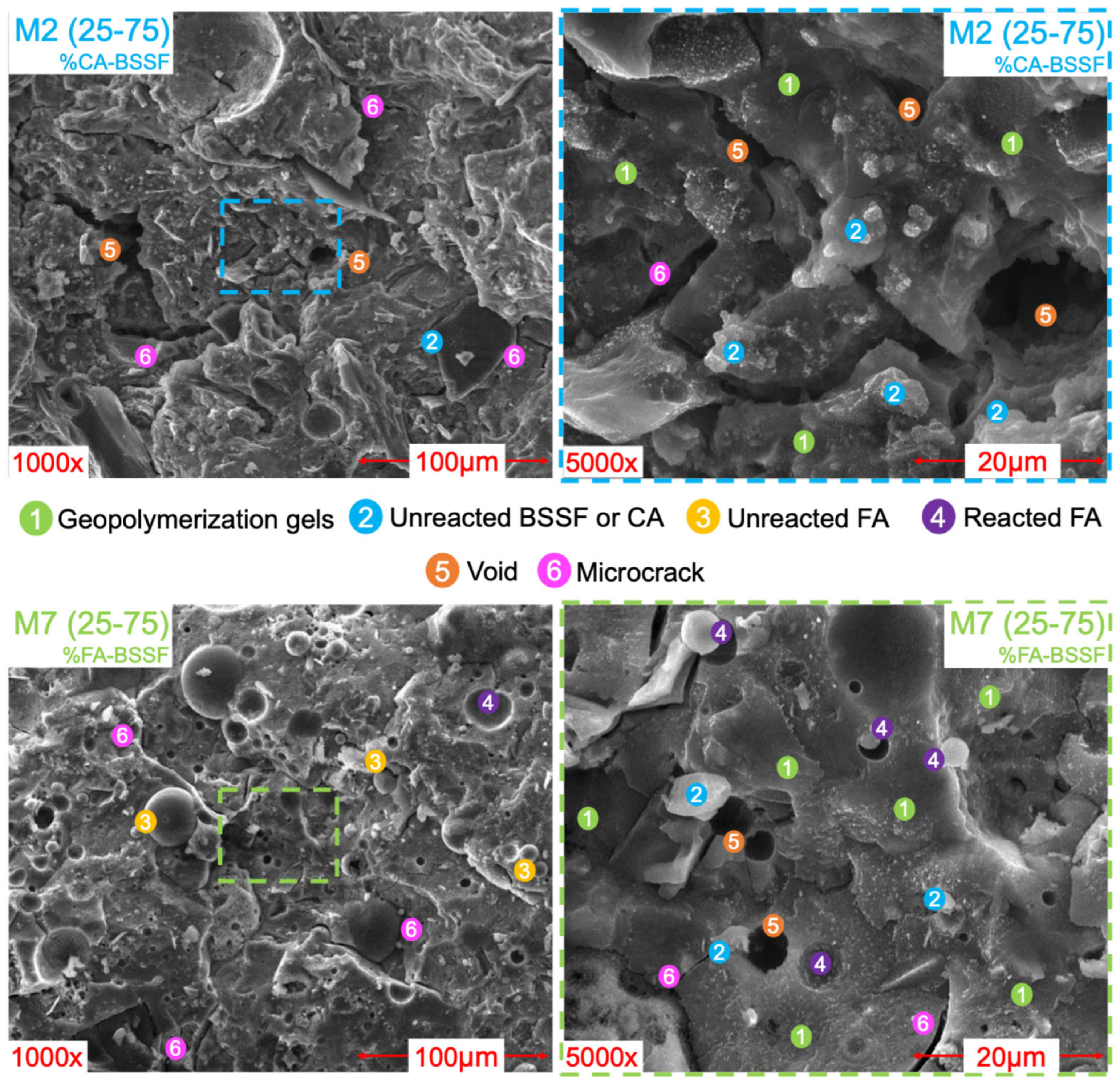
3.2.3. SEM
4. Conclusions
- Among the four steel industry byproducts, CA demonstrated the highest potential for alkali activation due to its finer particle sizes (D90 of 112.4 μm), specific surface area (0.252 m2/g), and favorable aluminosilicate content. BSSF was confirmed as a calcium-rich precursor, exhibiting phases such as brownmillerite and larnite, which support gel formation and accelerate early strength development;
- FA presented a higher content of aluminosilicates and predominantly spherical particles, which enhanced the resulting flowability, packing density, and dissolution kinetics. Its incorporation into BSSF-based AABs contributed to the formation of dense, well-connected gel networks, improving both the mechanical properties and matrix homogeneity of the resulting AABs;
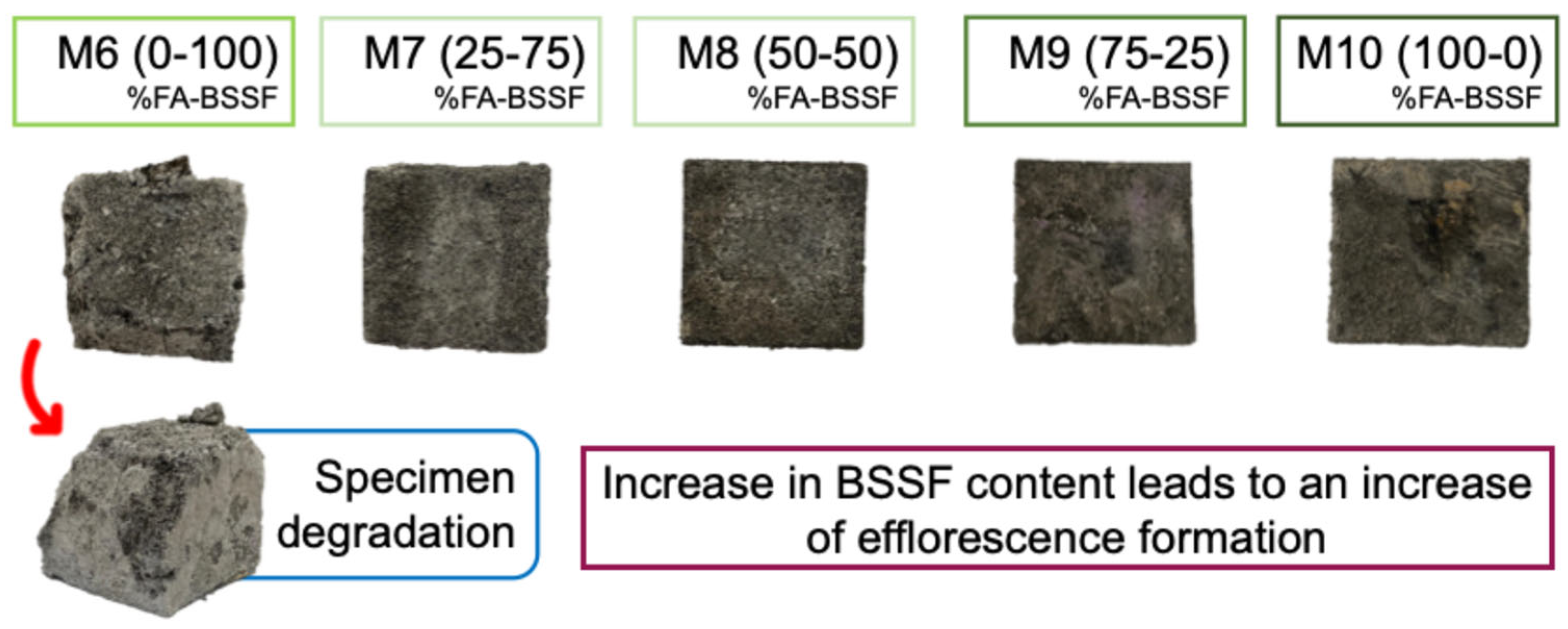
- The increase in BSSF content in the FA-based AABs led to greater efflorescence salt formation, likely due to the excess of CaO;
- Achieving a higher CA content in AABs required higher alkali dosages, which reduced the workability of the AABs and led to a reduction in compressive strength;
- AABs produced solely from steel industry byproducts (BSSF and CA) achieved compressive strengths up to 9.8 MPa. In contrast, the incorporation of FA markedly improved their performance, allowing the compressive strength to reach 23.5 MPa. Both AAB systems exhibited optimal performance at a 75–25 ratio of BSSF to ash (either CA or FA), achieving the best balance between precursor reactivity and mechanical strength;
- XRF analysis confirmed that the FA-based AAB (M7) contained higher amounts of SiO2 (23.9% vs. 9.3% for M2) and Al2O3 (3.9% vs. 1.0% for M2), achieving more effective gel formation and, consequently, improved mechanical strength. In contrast, the aCA-based AAB (M2) presented higher CaO (32.9% vs. 28.0% for M7) and Fe2O3 (49.1% vs. 35.2% for M7) contents. SEM and FTIR analyses revealed that M7 developed a denser, more homogeneous matrix with fewer unreacted particles, whereas M2 showed incomplete precursor dissolution, a discontinuous matrix, and higher porosity, which is consistent with its lower compressive strength;
- The combination of BSSF with FA offers a pathway to valorize industrial byproducts while producing eco-efficient binders with mechanical properties that are suitable for structural and paving applications. CA–BSSF systems, although less reactive, may still be applicable for non-structural uses, such as low-strength construction elements (e.g., drywall panels), soil stabilization, and asphalt binder modification.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trindade, E.; Lima, L.; Alencar, L.; Alencar, M. Identification of Obstacles to Implementing Sustainability in the Civil Construction Industry Using Bow-Tie Tool. Buildings 2020, 10, 165. [Google Scholar] [CrossRef]
- Gartner, E. Industrially Interesting Approaches to “Low-CO2” Cements. Cem. Concr. Res. 2004, 34, 1489–1498. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. Geopolymers and Other Alkali-Activated Materials. In Lea’s Chemistry of Cement and Concrete; Elsevier: Amsterdam, The Netherlands, 2019; pp. 779–805. [Google Scholar]
- Zerzouri, M.; Bouchenafa, O.; Hamzaoui, R.; Ziyani, L.; Alehyen, S. Physico-Chemical and Mechanical Properties of Fly Ash Based-Geopolymer Pastes Produced from Pre-Geopolymer Powders Obtained by Mechanosynthesis. Constr. Build. Mater. 2021, 288, 123135. [Google Scholar] [CrossRef]
- Xie, T.; Visintin, P.; Zhao, X.; Gravina, R. Mix Design and Mechanical Properties of Geopolymer and Alkali Activated Concrete: Review of the State-of-the-Art and the Development of a New Unified Approach. Constr. Build. Mater. 2020, 256, 119380. [Google Scholar] [CrossRef]
- Nunes, V.A.; Suraneni, P.; Bezerra, A.C.S.; Thomas, C.; Borges, P.H.R. Influence of Activation Parameters on the Mechanical and Microstructure Properties of an Alkali-Activated BOF Steel Slag. Appl. Sci. 2022, 12, 12437. [Google Scholar] [CrossRef]
- Hori, K.; Tsutsumi, N.; Kato, T.; Kitano, Y.; Sugahara, K. Overview of Iron/Steel Slag Application and Development of New Utilization Technologies. Nippon. Steel Sumitomo Met. Tech. Rep. 2015, 109, 5–11. [Google Scholar]
- ArcelorMittal Pecém (AMP). Co-Produtos. Available online: https://brasil.arcelormittal.com/a-arcelormittal/quem-somos/arcelormittal-pecem (accessed on 4 December 2024).
- Instituto Aço Brasil. Anuário Estatístico 2023; Instituto Aço Brasil: Brasília, Brazil, 2023; Available online: https://acobrasil.org.br/site/wp-content/uploads/2023/07/AcoBrasil_Anuario_2023.pdf (accessed on 20 January 2024).
- Maciel, F.W.F.; Sousa, M.; Cabral, A.E.B. Avaliação do Uso de Escória de Aciaria em Blocos Intertravados de Concreto para Pavimentação. 2020. Available online: https://repositorio.ufc.br/handle/riufc/57587 (accessed on 27 August 2025).
- Dias, A.R.O.; Amancio, F.A.; Sousa, I.L.X.; Lucas, S.O.; Lima, D.A.; Cabral, A.E.B. Efeitos da Substituição do Cimento Portland por Escória de Aciaria BSSF nas Propriedades Físicas e Mecânicas do Concreto. Matéria 2020, 25, e-1190. [Google Scholar] [CrossRef]
- Benittez, L.H.; Marques Neto, J.D.C.; Ferreira, F.G.S.; Chotoli, F.F.; Santos, R.F.C.; Guilge, M.S. Bloco de Concreto com Incorporação de Escória de Aciaria BSSF: Um Estudo para Substituição de Agregados Naturais. Rev. Principia Divulgação Científica Tecnológica IFPB 2022, 59, 785. [Google Scholar] [CrossRef]
- Pereira, A.P.S.; Ramos, F.J.H.T.V.; Silva, M.H.P. Caracterização Estrutural de Geopolímeros Sustentáveis de Escória de Aciaria LD e Escória de Aciaria LF com KOH. Matéria 2020, 25, e-12827. [Google Scholar] [CrossRef]
- Caetano, P.; Batista, T.; Nogueira, R.; Cabral, A.; Costa, H. Tiempo de Configuración y Propiedades Mecánicas de Cementos de Ceniza/Escoria Activados con Álcali Curados a Temperatura Ambiente. Rev. Ing. Constr. 2023, 38, 104–113. [Google Scholar] [CrossRef]
- Araújo, L.B.R. Rheological, Mechanical and Durability Evaluation of Alkali-Activated Pastes and Concretes Designed Based on Fly Ash and Steel Slag. Master’s Thesis, Universidade Federal do Ceará, Fortaleza, Brazil, 2023. [Google Scholar]
- Milad, A.; Ali, A.S.B.; Babalghaith, A.M.; Memon, Z.A.; Mashaan, N.S.; Arafa, S.; Md. Yusoff, N.I. Utilisation of Waste-Based Geopolymer in Asphalt Pavement Modification and Construction—A Review. Sustainability 2021, 13, 3330. [Google Scholar] [CrossRef]
- Hamid, A.; Baaj, H.; El-Hakim, M. Temperature and Aging Effects on the Rheological Properties and Performance of Geopolymer-Modified Asphalt Binder and Mixtures. Materials 2023, 16, 1012. [Google Scholar] [CrossRef]
- Hossiney, N.; Sepuri, H.K.; Mohan, M.K.; Chandra, S.K.; Kumar, S.L.; HK, T. Geopolymer Concrete Paving Blocks Made with Recycled Asphalt Pavement (RAP) Aggregates towards Sustainable Urban Mobility Development. Cogent Eng. 2020, 7, 1824572. [Google Scholar] [CrossRef]
- Tahir, M.F.M.; Abdullah, M.M.A.B.; Rahim, S.Z.A.; Hasan, M.R.M.; Sandu, A.V.; Vizureanu, P.; Ghazali, C.M.R.; Kadir, A.A. Mechanical and Durability Analysis of Fly Ash Based Geopolymer with Various Compositions for Rigid Pavement Applications. Materials 2022, 15, 3458. [Google Scholar] [CrossRef]
- Walkley, B.; San Nicolas, R.; Sani, M.-A.; Rees, G.J.; Hanna, J.V.; van Deventer, J.S.J.; Provis, J.L. Phase Evolution of C-(N)-A-S-H/N-A-S-H Gel Blends Investigated via Alkali-Activation of Synthetic Calcium Aluminosilicate Precursors. Cem. Concr. Res. 2016, 89, 120–135. [Google Scholar] [CrossRef]
- Soutsos, M.; Boyle, A.P.; Vinai, R.; Hadjierakleous, A.; Barnett, S.J. Factors Influencing the Compressive Strength of Fly Ash Based Geopolymers. Constr. Build. Mater. 2016, 110, 355–368. [Google Scholar] [CrossRef]
- Zhang, P.; Zheng, Y.; Wang, K.; Zhang, J. A Review on Properties of Fresh and Hardened Geopolymer Mortar. Compos. B Eng. 2018, 152, 79–95. [Google Scholar] [CrossRef]
- Nazari, A.; Bagheri, A.; Riahi, S. Properties of Geopolymer with Seeded Fly Ash and Rice Husk Bark Ash. Mater. Sci. Eng. A 2011, 528, 7395–7401. [Google Scholar] [CrossRef]
- Li, Z.; Xu, G.; Shi, X. Reactivity of Coal Fly Ash Used in Cementitious Binder Systems: A State-of-the-Art Overview. Fuel 2021, 301, 121031. [Google Scholar] [CrossRef]
- Matsunaga, T.; Kim, J.K.; Hardcastle, S.; Rohatgi, P.K. Crystallinity and Selected Properties of Fly Ash Particles. Mater. Sci. Eng. A 2002, 325, 333–343. [Google Scholar] [CrossRef]
- Doucet, F.J. Effective CO2-Specific Sequestration Capacity of Steel Slags and Variability in Their Leaching Behaviour in View of Industrial Mineral Carbonation. Miner. Eng. 2010, 23, 262–269. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J.M. Concrete: Microstructure, Properties, and Materials, 4th ed.; McGraw-Hill Education: New York, NY, USA, 2014. [Google Scholar]
- Provis, J.L.; van Deventer, J.S.J. Alkali Activated Materials: State-of-the-Art Report, RILEM TC 224-AAM; Springer: Berlin/Heidelberg, Germany, 2014; p. 13. [Google Scholar]
- Fernández-Jiménez, A.; Palomo, A. Characterisation of Fly Ashes. Potential Reactivity as Alkaline Cements. Fuel 2003, 82, 2259–2265. [Google Scholar] [CrossRef]
- Murthy, N.S.; Minor, H. General Procedure for Evaluating Amorphous Scattering and Crystallinity from X-Ray Diffraction Scans of Semicrystalline Polymers. Polymer 1990, 31, 996–1002. [Google Scholar] [CrossRef]
- Puertas, F.; Martínez-Ramírez, S.; Alonso, S.; Vázquez, T. Alkali-Activated Fly Ash/Slag Cements: Strength Behaviour and Hydration Products. Cem. Concr. Res. 2000, 30, 1625–1632. [Google Scholar] [CrossRef]
- He, M.; Li, B.; Zhou, W.; Chen, H.; Liu, M.; Zou, L. Preparation and Characteristics of Steel Slag Ceramics from Converter Slag. In Proceedings of the Characterization of Minerals, Metals, and Materials 2018 (TMS 2018), Phoenix, AZ, USA, 11–15 March 2018; pp. 13–20. [Google Scholar]
- Klug, J.L.; Medeiros, S.L.S.; Caldas, H.; Bentes, M.; Becker, H. Separation of Iron and Calcium from a BSSF Steelmaking Slag Through Acid Leaching. Mater. Res. 2022, 25, e20210571. [Google Scholar] [CrossRef]
- Tirado González, J.G.; Reyes Segura, B.T.; Esguerra-Arce, J.; Bermúdez Castañeda, A.; Aguilar, Y.; Esguerra-Arce, A. An Innovative Magnetic Oxide Dispersion-Strengthened Iron Compound Obtained from an Industrial Byproduct, with a View to Circular Economy. J. Clean. Prod. 2020, 268, 122362. [Google Scholar] [CrossRef]
- Zambrano, O.A.; Coronado, J.J.; Rodríguez, S.A. Mechanical Properties and Phases Determination of Low Carbon Steel Oxide Scales Formed at 1200 °C in Air. Surf. Coat. Technol. 2015, 282, 155–162. [Google Scholar] [CrossRef]
- Tseng, Y.-H.; Kuo, Y.-H.; Tsai, M.-H. Industrial-Scale Brownmillerite Formation in Oxygen-Blown Basic Oxygen Furnace Slag: A Novel Stabilization Approach for Sustainable Utilization. Materials 2025, 18, 2182. [Google Scholar] [CrossRef]
- Kaja, A.M.; Melzer, S.; Brouwers, H.J.H. On the Optimization of BOF Slag Hydration Kinetics. Cem. Concr. Compos. 2021, 124, 104262. [Google Scholar] [CrossRef]
- Van Setten, B.A.A.L.; Makkee, M.; Moulijn, J.A. Science and Technology of Catalytic Diesel Particulate Filters. Catal. Rev. 2001, 43, 489–564. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman Microspectroscopy of Soot and Related Carbonaceous Materials: Spectral Analysis and Structural Information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Cantarino, M.V.; de Carvalho Filho, C.; Mansur, M.B. Selective Removal of Zinc from Basic Oxygen Furnace Sludges. Hydrometallurgy 2012, 111, 124–128. [Google Scholar] [CrossRef]
- Rodriguez Rodriguez, N.; Gijsemans, L.; Bussé, J.; Roosen, J.; Önal, M.A.R.; Masaguer Torres, V.; Binnemans, K. Selective Removal of Zinc from BOF Sludge by Leaching with Mixtures of Ammonia and Ammonium Carbonate. J. Sustain. Metall. 2020, 6, 680–690. [Google Scholar] [CrossRef]
- Elgersma, F.; Kamst, G.F.; Witkamp, G.J.; Van Rosmalen, G.M. Acidic Dissolution of Zinc Ferrite. Hydrometallurgy 1992, 29, 173–189. [Google Scholar] [CrossRef]
- Zafar, I.; Rashid, K.; Hameed, R.; Aslam, K. Correlating Reactivity of Fly Ash with Mechanical Strength of the Resultant Geopolymer. Arab. J. Sci. Eng. 2022, 47, 12469–12478. [Google Scholar] [CrossRef]
- Lu, C.; Wang, Q.; Liu, Y.; Xue, T.; Yu, Q.; Chen, S. Influence of New Organic Alkali Activators on Microstructure and Strength of Fly Ash Geopolymer. Ceram. Int. 2022, 48, 12442–12449. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, B.; Chen, J.; Li, Z.; Liang, X.; De Lima, L.M.; Liu, C.; Yin, S.; Yu, Q.; Lothenbach, B.; et al. Thermodynamic Modeling of Alkali-Activated Fly Ash Paste. Cem. Concr. Res. 2024, 186, 107699. [Google Scholar] [CrossRef]
- Si, R.; Zhan, Y.; Zang, Y.; Sun, Y.; Huang, Y. Effect of Basalt Fiber on Fracture Properties and Drying Shrinkage of Alkali-Activated Slag with Different Silicate Modulus. J. Mater. Res. Technol. 2023, 25, 552–569. [Google Scholar] [CrossRef]
- Balaguera, C.A.C.; Botero, M.A.G. Characterization of Steel Slag for the Production of Chemically Bonded Phosphate Ceramics (CBPC). Constr. Build. Mater. 2020, 241, 118138. [Google Scholar] [CrossRef]
- Ghorbani, S.; Stefanini, L.; Sun, Y.; Walkley, B.; Provis, J.L.; De Schutter, G.; Matthys, S. Characterisation of Alkali-Activated Stainless Steel Slag and Blast-Furnace Slag Cements. Cem. Concr. Compos. 2023, 143, 105230. [Google Scholar] [CrossRef]
- Wang, C.; Shaw, L.L. On Synthesis of Fe2SiO4/SiO2 and Fe2O3/SiO2 Composites through Sol–Gel and Solid-State Reactions. J. Sol-Gel Sci. Technol. 2014, 72, 602–614. [Google Scholar] [CrossRef]
- Bohra, V.K.J.; Nerella, R.; Madduru, S.R.C.; Rohith, P. Microstructural Characterization of Fly Ash Based Geopolymer. Mater. Today Proc. 2020, 27, 1625–1629. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, K.; Li, Q.; Wang, J.; Ling, Y. Fabrication and Engineering Properties of Concretes Based on Geopolymers/Alkali-Activated Binders: A Review. J. Clean Prod. 2020, 258, 120896. [Google Scholar] [CrossRef]
- ASTM C618-22; Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM International: West Conshohocken, PA, USA, 2022.
- Rattanasak, U.; Chindaprasirt, P. Influence of NaOH Solution on the Synthesis of Fly Ash Geopolymer. Miner. Eng. 2009, 22, 1073–1078. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Q.; Wang, B.; Wang, P.; Zou, J. Morphology and Composition of Microspheres in Fly Ash from the Luohuang Power Plant, Chongqing, Southwestern China. Minerals 2016, 6, 30. [Google Scholar] [CrossRef]
- Risdanareni, P.; Puspitasari, P.; Januarti Jaya, E. Chemical and Physical Characterization of Fly Ash as Geopolymer Material. MATEC Web Conf. 2017, 97, 01031. [Google Scholar] [CrossRef]
- Sun, Q.; Zhao, S.; Zhao, X.; Song, Y.; Ban, X.; Zhang, N. Influence of Different Grinding Degrees of Fly Ash on Properties and Reaction Degrees of Geopolymers. PLoS ONE 2023, 18, e0282927. [Google Scholar] [CrossRef] [PubMed]
- Rafeet, A.; Vinai, R.; Soutsos, M.; Sha, W. Guidelines for Mix Proportioning of Fly Ash/GGBS Based Alkali Activated Concretes. Constr. Build. Mater. 2017, 147, 130–142. [Google Scholar] [CrossRef]
- Hadi, M.N.S.; Zhang, H.; Parkinson, S. Optimum Mix Design of Geopolymer Pastes and Concretes Cured in Ambient Condition Based on Compressive Strength, Setting Time and Workability. J. Build. Eng. 2019, 23, 301–313. [Google Scholar] [CrossRef]
- Li, N.; Shi, C.; Zhang, Z.; Zhu, D.; Hwang, H.-J.; Zhu, Y.; Sun, T. A Mixture Proportioning Method for the Development of Performance-Based Alkali-Activated Slag-Based Concrete. Cem. Concr. Compos. 2018, 93, 163–174. [Google Scholar] [CrossRef]
- Thomas, R.J.; Peethamparan, S. Stepwise Regression Modeling for Compressive Strength of Alkali-Activated Concrete. Constr. Build. Mater. 2017, 141, 315–324. [Google Scholar] [CrossRef]
- Song, W.; Zhu, Z.; Peng, Y.; Wan, Y.; Xu, X.; Pu, S.; Song, S.; Wei, Y. Effect of Steel Slag on Fresh, Hardened and Microstructural Properties of High-Calcium Fly Ash Based Geopolymers at Standard Curing Condition. Constr. Build. Mater. 2019, 229, 116933. [Google Scholar] [CrossRef]
- Cristelo, N.; Coelho, J.; Miranda, T.; Palomo, Á.; Fernández-Jiménez, A. Alkali Activated Composites—An Innovative Concept Using Iron and Steel Slag as Both Precursor and Aggregate. Cem. Concr. Compos. 2019, 103, 11–21. [Google Scholar] [CrossRef]
- ASTM C349-18; Test Method for Compressive Strength of Hydraulic-Cement Mortars (Using Portions of Prisms Broken in Flexure). ASTM: West Conshohocken, PA, USA, 2018.
- NBR 13279; Argamassa Para Assentamento e Revestimento de Paredes e Tetos—Determinação Da Resistência à Tração Na Flexão e à Compressão. Associação Brasileira de Normas Técnicas (ABNT): Rio de Janeiro, Brazil, 2005.
- Ismail, I.; Bernal, S.A.; Provis, J.L.; Nicolas, R.S.; Hamdan, S.; Van Deventer, J.S. Modification of Phase Evolution in Alkali-Activated Blast Furnace Slag by the Incorporation of Fly Ash. Cem. Concr. Compos. 2013, 45, 125–135. [Google Scholar] [CrossRef]
- Alonso, M.M.; Gismera, S.; Blanco, M.T.; Lanzón, M.; Puertas, F. Alkali-Activated Mortars: Workability and Rheological Behaviour. Constr. Build. Mater. 2017, 145, 576–587. [Google Scholar] [CrossRef]
- Vance, K.; Dakhane, A.; Sant, G.; Neithalath, N. Observations on the Rheological Response of Alkali Activated Fly Ash Suspensions: The Role of Activator Type and Concentration. Rheol. Acta 2014, 53, 843–855. [Google Scholar] [CrossRef]
- Popovicheva, O.; Persiantseva, N.M.; Shonija, N.K.; DeMott, P.; Koehler, K.; Petters, M.; Suzanne, J. Water Interaction with Hydrophobic and Hydrophilic Soot Particles. Phys. Chem. Chem. Phys. 2008, 10, 2332–2344. [Google Scholar] [CrossRef]
- Ueda, S.; Mori, T.; Iwamoto, Y.; Ushikubo, Y.; Miura, K. Wetting Properties of Fresh Urban Soot Particles: Evaluation Based on Critical Supersaturation and Observation of Surface Trace Materials. Sci. Total Environ. 2022, 811, 152274. [Google Scholar] [CrossRef]
- Mahfoud, E.; Maherzi, W.; Benzerzour, M.; Ndiaye, K.; Aggoun, S. Effet Du Sédiment de Dragage et Du Rapport SiO2/Al2O3 Sur La Résistance et La Porosité Des Mortiers «One Part Geopolymer». In Proceedings of the 40èmes Rencontres Universitaires de Génie Civil—RUGC2022, Villeneuve d’Ascq, France, 23–25 May 2022. [Google Scholar]
- Chen, X.; Huang, W.; Zhou, J. Effect of Moisture Content on Compressive and Split Tensile Strength of Concrete. Indian J. Eng. Mater. Sci. 2012, 19, 427–435. [Google Scholar]
- Allahverdi, A.; Kani, E.N.; Hossain, K.M.A.; Lachemi, M. Methods to Control Efflorescence in Alkali-Activated Cement-Based Materials. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Woodhead Publishing: Cambridge, UK, 2015; pp. 463–483. [Google Scholar]
- Marvila, M.T.; de Azevedo, A.R.G.; Vieira, C.M.F. Reaction Mechanisms of Alkali-Activated Materials. Rev. IBRACON Estrut. Mater. 2021, 14, 3. [Google Scholar] [CrossRef]
- Gonzalez, P.L.L.; Novais, R.M.; Labrincha, J.A.; Blanpain, B.; Pontikes, Y. Modifications of Basic-Oxygen-Furnace Slag Microstructure and Their Effect on the Rheology and the Strength of Alkali-Activated Binders. Cem. Concr. Compos. 2018, 97, 143–153. [Google Scholar] [CrossRef]
- Kaya, M.; Koksal, F.; Gencel, O.; Munir, M.J.; Kazmi, S.M.S. Influence of Micro Fe2O3 and MgO on the Physical and Mechanical Properties of the Zeolite and Kaolin Based Geopolymer Mortar. J. Build. Eng. 2022, 52, 104443. [Google Scholar] [CrossRef]
- Rafeet, A.; Vinai, R.; Soutsos, M.; Sha, W. Effects of Slag Substitution on Physical and Mechanical Properties of Fly Ash-Based Alkali Activated Binders (AABs). Cem. Concr. Res. 2019, 122, 118–135. [Google Scholar] [CrossRef]
- Rees, C.A.; Provis, J.L.; Lukey, G.C.; Van Deventer, J.S.J. In Situ ATR-FTIR Study of the Early Stages of Fly Ash Geopolymer Gel Formation. Langmuir 2007, 23, 9076–9082. [Google Scholar] [CrossRef] [PubMed]
- Puligilla, S.; Mondal, P. Co-Existence of Aluminosilicate and Calcium Silicate Gel Characterized through Selective Dissolution and FTIR Spectral Subtraction. Cem. Concr. Res. 2015, 70, 39–49. [Google Scholar] [CrossRef]
- Yunsheng, Z.; Wei, S.; Qianli, C.; Lin, C. Synthesis and Heavy Metal Immobilization Behaviors of Slag Based Geopolymer. J. Hazard. Mater. 2006, 143, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Robayo, R.A.; Mulford, A.; Munera, J.; de Gutiérrez, R.M. Alternative Cements Based on Alkali-Activated Red Clay Brick Waste. Constr. Build. Mater. 2016, 128, 163–169. [Google Scholar] [CrossRef]
- Yang, T.; Zhu, H.; Zhang, Z.; Gao, X.; Zhang, C.; Wu, Q. Effect of Fly Ash Microsphere on the Rheology and Micro-Structure of Alkali-Activated Fly Ash/Slag Pastes. Cem. Concr. Res. 2018, 109, 198–207. [Google Scholar] [CrossRef]
- Puligilla, S.; Mondal, P. Role of Slag in Microstructural Development and Hardening of Fly Ash-Slag Geopolymer. Cem. Concr. Res. 2013, 43, 70–80. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, J.; Liao, Y. Influence of Ground Granulated Blast Furnace Slag on the Early Hydration and Microstructure of Alkali-Activated Converter Steel Slag Binder. J. Therm. Anal. Calorim. 2022, 147, 243–252. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Z.; Zhuang, S.; He, W. Hydration Properties and Microstructure Characteristics of Alkali–Activated Steel Slag. Constr. Build. Mater. 2020, 241, 118141. [Google Scholar] [CrossRef]
- Ahmad, M.R.; Qian, L.P.; Fang, Y.; Wang, A.; Dai, J.G. A Multiscale Study on Gel Composition of Hybrid Alkali-Activated Materials Partially Utilizing Air Pollution Control Residue as an Activator. Cem. Concr. Compos. 2023, 136, 104856. [Google Scholar] [CrossRef]
- Ahmad, M.R.; Khan, M.; Wang, A.; Zhang, Z.; Dai, J.G. Alkali-Activated Materials Partially Activated Using Flue Gas Residues: An Insight into Reaction Products. Constr. Build. Mater. 2023, 371, 130760. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Criado, M. Microstructure Development of Alkali-Activated Fly Ash Cement: A Descriptive Model. Cem. Concr. Res. 2005, 35, 1204–1209. [Google Scholar] [CrossRef]
- Provis, J.L.; Myers, R.J.; White, C.E.; Rose, V.; Van Deventer, J.S.J. X-Ray Microtomography Shows Pore Structure and Tortuosity in Alkali-Activated Binders. Cem. Concr. Res. 2012, 42, 855–864. [Google Scholar] [CrossRef]
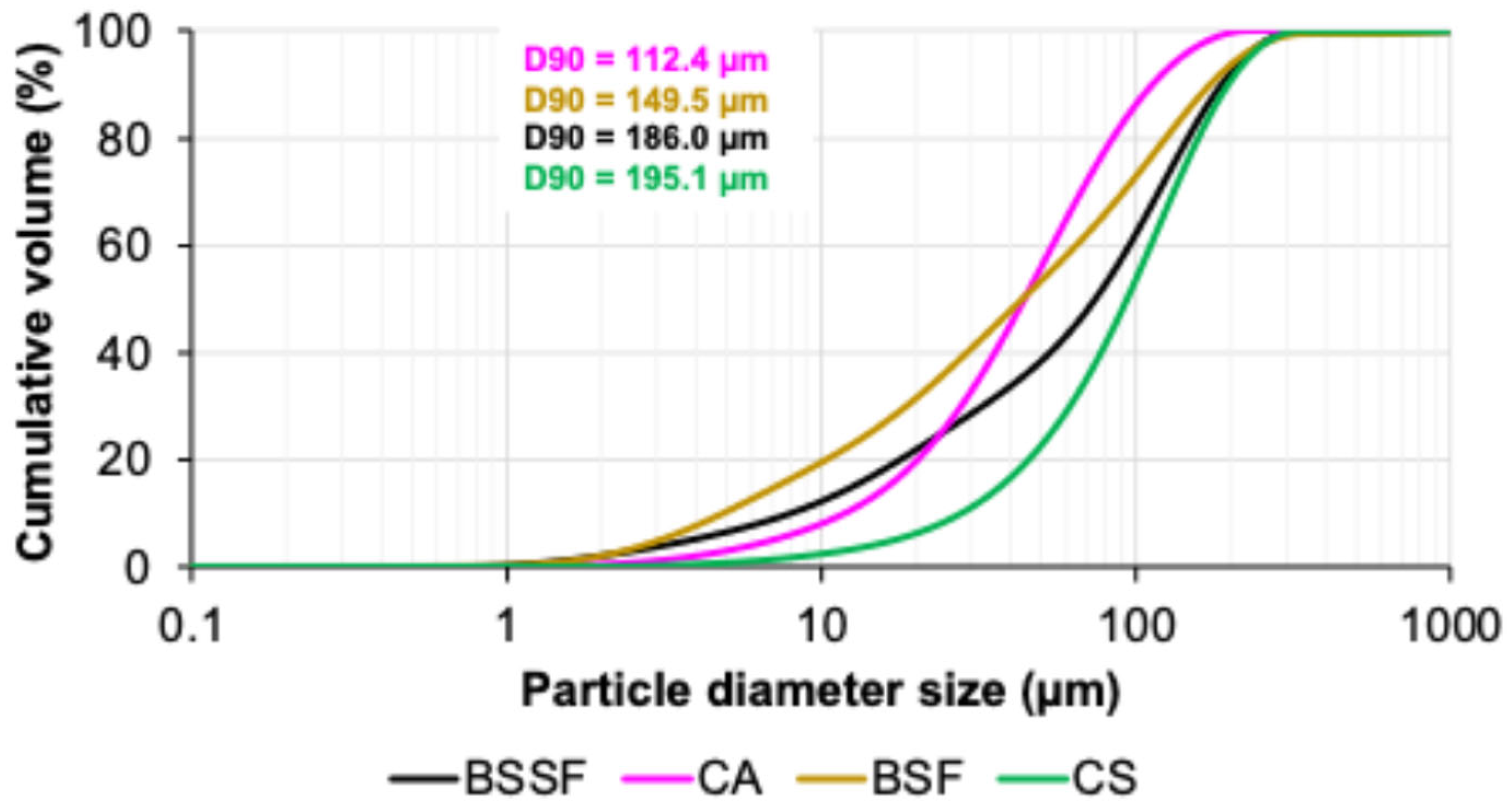

| Material | Al2O3 | SiO2 | P2O5 | SO3 | Cl | K2O | CaO | TiO2 | ZnO | MnO | Fe2O3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BSSF | 1.3 | 6.9 | 2.2 | - | - | - | 45.1 | 0.4 | - | 3.2 | 39.9 |
| CA | 6.0 | 15.4 | - | 24.8 | 4.5 | 2.9 | 20.4 | 2.6 | - | - | 22.0 |
| BFS | 3.1 | 6.3 | 0.5 | 12.2 | 0.7 | - | 3.7 | - | 35.4 | 0.2 | 34.5 |
| CS | 37.3 | 2.5 | 11.7 | 11.1 | 1.8 | 0.7 | 7.8 | - | - | 0.4 | 20.7 |
| Material | Al2O3 | SiO2 | P2O5 | SO3 | Cl | K2O | CaO | TiO2 | MnO | Fe2O3 |
|---|---|---|---|---|---|---|---|---|---|---|
| FA | 15.6 | 46.8 | 0.4 | 1.9 | 0.0 | 3.7 | 8.1 | 1.8 | 0.1 | 21.0 |
| ID | CA–BSSF (%) | FA–BSSF (%) | NaOH Solution | Na2SiO3 Solution | CA | FA | BSSF |
|---|---|---|---|---|---|---|---|
| M1 | 0-100 | - | 396.4 | 350.1 | 0.0 | - | 1485.7 |
| M2 | 25-75 | - | 392.6 | 346.8 | 367.9 | - | 1103.7 |
| M3 | 50-50 | - | 482.2 | 378.2 | 573.2 | - | 573.2 |
| M4 | 75-25 | - | 404.3 | 357.1 | 811.8 | - | 270.6 |
| M5 | 100-0 | 382.9 | 338.2 | 1023.3 | - | 0.0 | |
| M6 | - | 0-100 | 441.5 | 366.0 | - | 0.0 | 1610.4 |
| M7 | - | 25-75 | 410.8 | 340.6 | - | 374.7 | 1123.9 |
| M8 | - | 50-50 | 384.2 | 318.5 | - | 700.7 | 700.7 |
| M9 | - | 75-25 | 360.8 | 299.1 | - | 986.9 | 329.0 |
| M10 | - | 100-0 | 340.0 | 281.9 | - | 1240.3 | - |
| Material | Al2O3 | SiO2 | P2O5 | SO3 | K2O | CaO | TiO2 | MnO | Fe2O3 | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| M2 (25–75) %CA–BSSF | 1.0 | 9.3 | 2.3 | 1.2 | 0.2 | 32.9 | 0.8 | 2.8 | 49.1 | 0.4 |
| M7 (25–75) %FA–BSSF | 3.9 | 23.9 | 3.2 | 0.8 | 1.6 | 28.0 | 1.5 | 1.8 | 35.2 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, M.L.d.; Melo, A.R.S.; Prévitali, L.; Babadopulos, L.F.d.A.L.; Bastos, J.B.d.S.; Bessa, I.S. Characterization of Steel Industry Byproducts as Precursors in Alkali-Activated Binders. Buildings 2025, 15, 3119. https://doi.org/10.3390/buildings15173119
Souza MLd, Melo ARS, Prévitali L, Babadopulos LFdAL, Bastos JBdS, Bessa IS. Characterization of Steel Industry Byproducts as Precursors in Alkali-Activated Binders. Buildings. 2025; 15(17):3119. https://doi.org/10.3390/buildings15173119
Chicago/Turabian StyleSouza, Madson Lucas de, Abcael Ronald Santos Melo, Laura Prévitali, Lucas Feitosa de Albuquerque Lima Babadopulos, Juceline Batista dos Santos Bastos, and Iuri Sidney Bessa. 2025. "Characterization of Steel Industry Byproducts as Precursors in Alkali-Activated Binders" Buildings 15, no. 17: 3119. https://doi.org/10.3390/buildings15173119
APA StyleSouza, M. L. d., Melo, A. R. S., Prévitali, L., Babadopulos, L. F. d. A. L., Bastos, J. B. d. S., & Bessa, I. S. (2025). Characterization of Steel Industry Byproducts as Precursors in Alkali-Activated Binders. Buildings, 15(17), 3119. https://doi.org/10.3390/buildings15173119









