AAR-Reactive Fillers in Concrete: Current Understanding and Knowledge Gaps
Abstract
1. Introduction
2. Evaluation of Systems Containing Reactive AMFs
2.1. Test Methods for Assessing AAR-Induced Expansion
2.1.1. Accelerated Mortar Bar Test (AMBT)
2.1.2. Concrete Prism Test (CPT)
2.1.3. Accelerated Concrete Prism Test (ACPT)
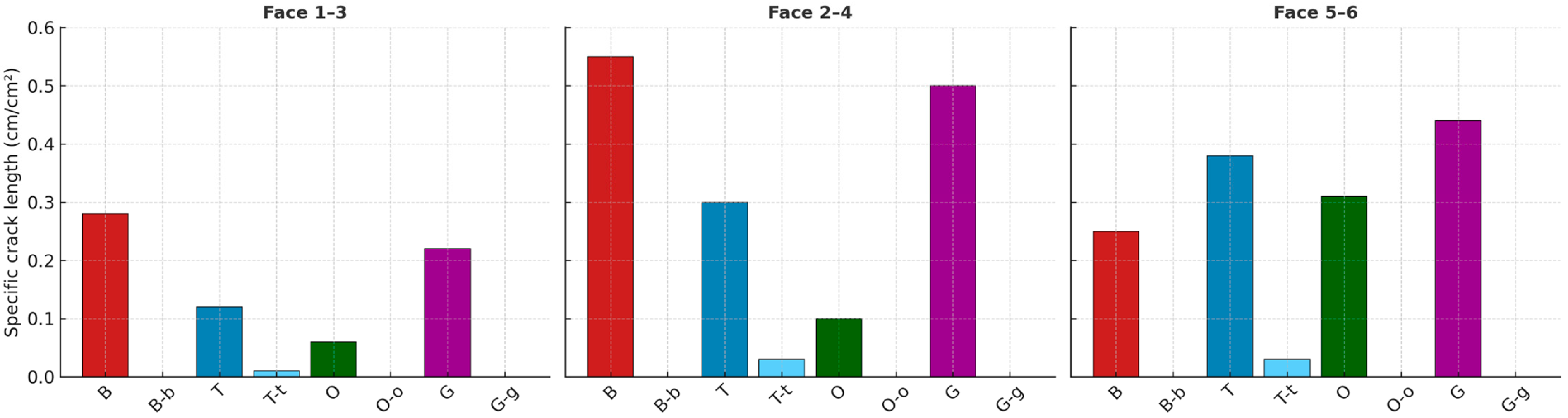
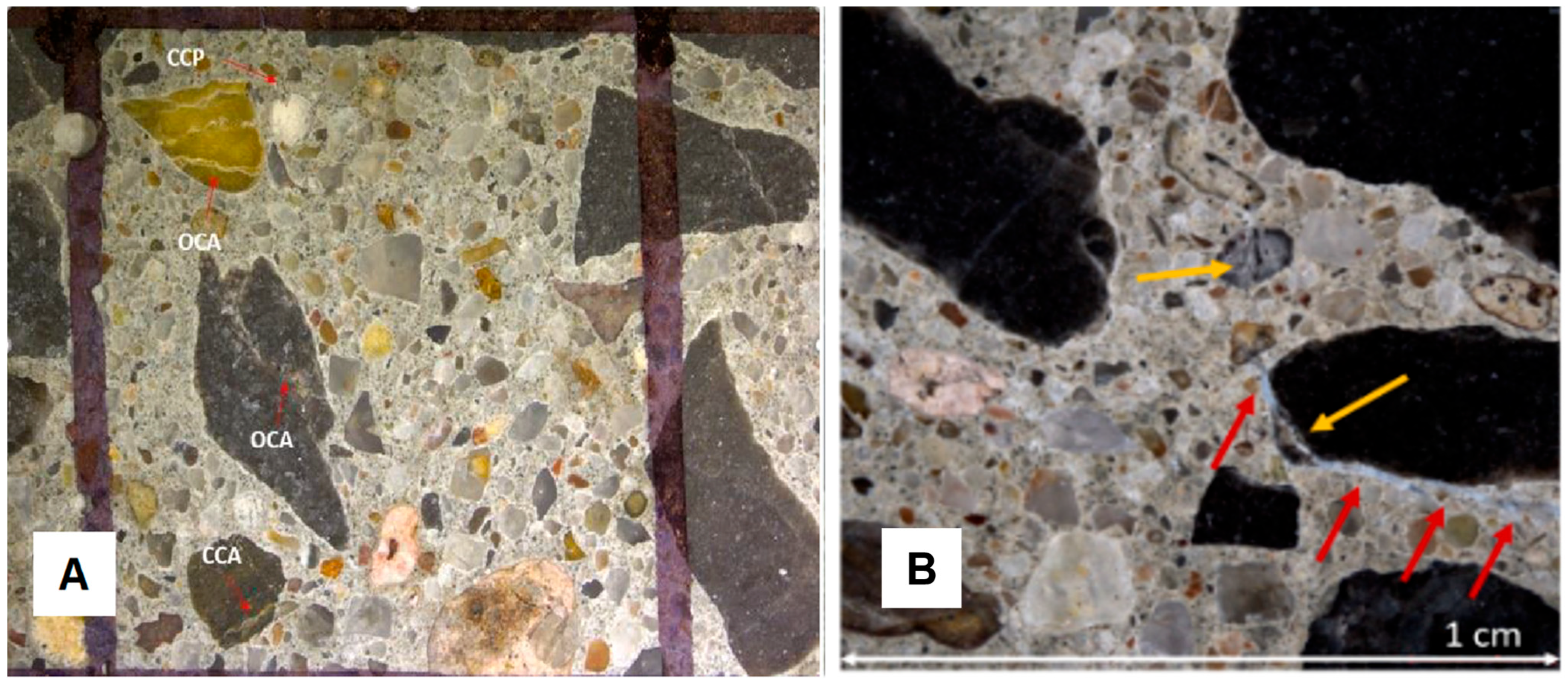
2.2. Test Methods for Assessing AAR-Induced Deterioration
2.3. Summary of Current Knowledge
3. Discussion
3.1. Role of AMF Mineralogy in AAR
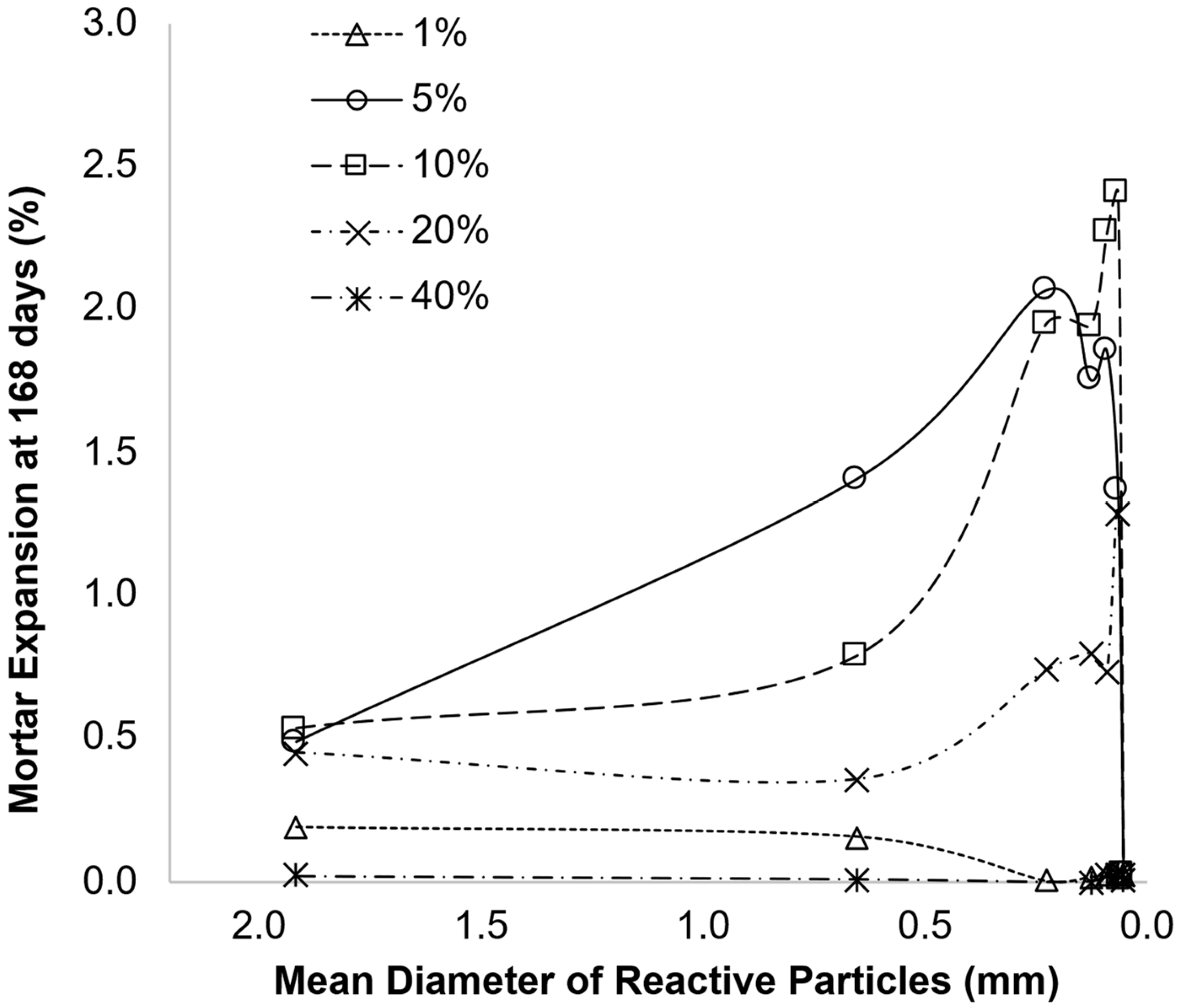
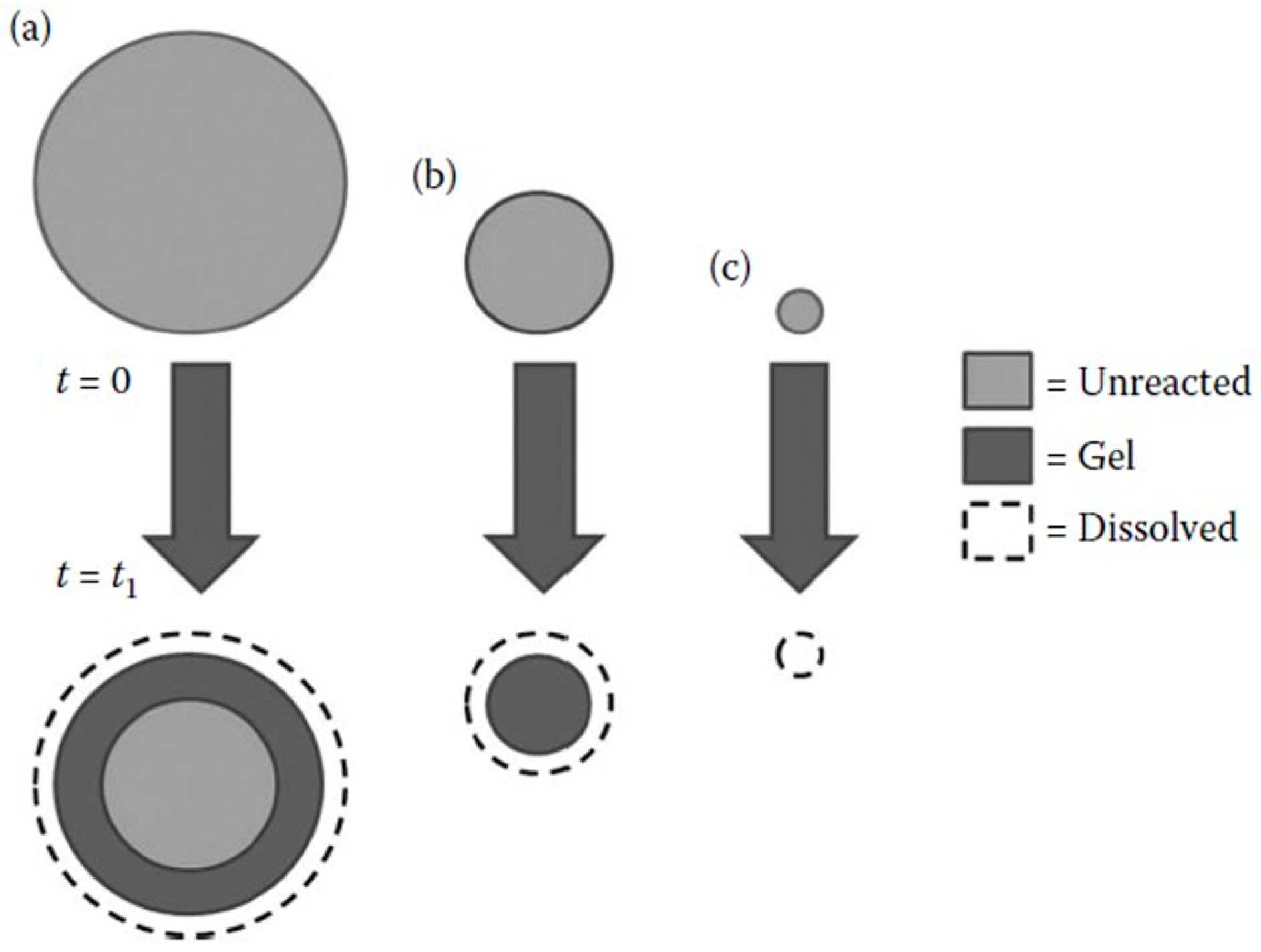
3.2. Role of AMF Particle Size in AAR
3.3. Role of Replacement Content
3.4. Current Gaps and Research Perspectives
4. Conclusions
- The kinetics and ultimate expansion of systems containing AAR-reactive AMFs vary depending on the test used and the mortar/concrete system (e.g., containing reactive coarse aggregates, reactive fine aggregates, or non-reactive aggregates). Therefore, the evaluation of the same AMF in different types of systems and using a long-term test would be beneficial to better understand the influence of AMFs;
- The progress of deterioration has barely been addressed in previous studies, and it has been evaluated only at the ultimate expansion. Therefore, evaluating this at different ages would be beneficial to understand the deterioration progress over time;
- Several aspects related to the mineralogy of the source rock need to be considered when evaluating the use of AAR-reactive AMFs, such as the crushing process, which influences the dispersion of mineral grains and morphology of particles, as well as alkali release;
- The effects of the size of particles have not been completely understood, as the results are conflicting. One hypothesis to explain such behaviour is the pessimum effect, which has also been studied, with some models proposed to explain it. When considering the studies in which AAR-reactive AMFs were used, different parameters were adopted as a measure of particle size, which hinders comparison;
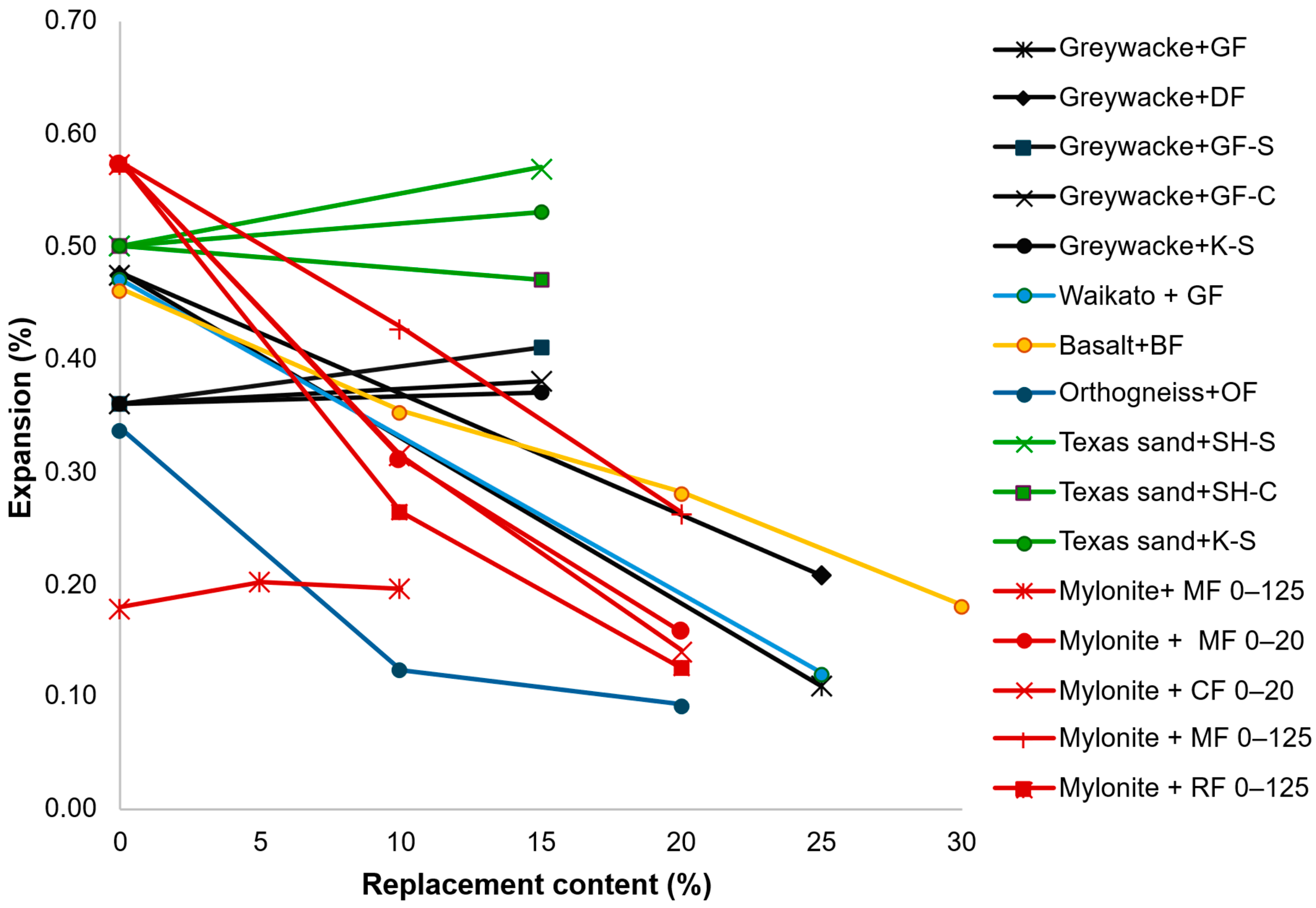
- Considering the percentage of cement replaced, in general, the expansions are reduced when the percentage increases, whereas the opposite occurs when sand is replaced;
- Several tests were used to assess the effects of AAR-reactive AMFs in mortar and concrete. In general, accelerated results indicated a reduction in expansion with the use of AMFs, whereas longer tests indicated the same or slightly increased expansions. Moreover, the test methods and parameters tended to vary owing to the different standards applied. Therefore, even when using the same test, the results are not comparable, as the standards and the specifications are different. Thus, a comprehensive evaluation of several aspects previously analyzed while maintaining the same tests and parameters would be essential to better understand the effects of AAR-reactive AMFs.
- An important aspect that may have hindered the development of knowledge on this topic is the nomenclature, as the term used to refer to reactive AMFs varies across studies. Therefore, this study proposes the term AAR-reactive AMFs (which could be ASR- or ACR-reactive AMFs) for clarity.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAR | Alkali–aggregate reaction |
| BF | Basalt filler |
| ACPT | Accelerated concrete prism test |
| ACR | Alkali–carbonate reaction |
| AMBT | Accelerated mortar bar test |
| AMF | Aggregate mineral filler |
| ASR | Alkali–silica reaction |
| BET | Brunauer–Emmett–Teller |
| CCA | Closed crack in the aggregate |
| CCP | Crack in cement paste |
| CF | Cataclasite filler |
| CPT | Concrete prism test |
| DF | Dacite filler |
| DRI | Damage rating index |
| GF | Greywacke filler |
| GrF | Granite filler |
| K | Kingston |
| MF | Mylonite filler |
| OCA | Open crack in aggregate |
| OF | Orthogneiss filler |
| PSD | Particle size distribution |
| RF | Rhyolite filler |
| RH | Relative humidity |
| SCM | Supplementary cementitious material |
| SDI | Stiffness damage index |
| SDT | Stiffness damage test |
| SF | Sandstone filler |
| SH | Springhill |
| SSA | Specific surface area |
| TX | Texas sand |
Appendix A
| Ultimate Expansion | Reduction | Test | Condition | |
|---|---|---|---|---|
| Greywacke [62] | 0.11 (coarse) | 77% | AMBT | 25% replacing cement Systems with coarse and fine reactive aggregates |
| 0.12 (fine) | 74% | |||
| Dacite [62] | 0.21 | 56% | AMBT | 25% replacing cement |
| Sandstone Capanda [63] | 0.17 (20%) | 7% | NBRI method modified | Replacement of cement |
| 0.11 (40%) | 39% | |||
| 0.05 (60%) | 72% | |||
| Sandstone Formosa [63] | 0.40 (20%) | 19% | NBRI method modified | Replacement of cement |
| 0.28 (40%) | 43% | |||
| 0.11 (60%) | 77% | |||
| 0.04 (80%) | 91% | |||
| Orthogneiss [64] | 0.12 (10%) | 65% | AMBT | Replacement of cement |
| 0.09 (20%) | 74% | |||
| Basalt [65] | 0.35 (10%) | 24% | NBRI method | Replacement of cement |
| 0.28 (20%) | 39% | |||
| 0.18 (30%) | 61% | |||
| Greywacke [67] | 0.41 (15% sand—coarse) | −14% (increment) | Accelerated CPT | Replacement of sand and cement. Systems with coarse and fine reactive aggregates |
| 0.38 (15% cement—coarse) | −6% (increment) | |||
| 0.57 (15% sand—fine) | −14% (increment) | |||
| 0.47 (15% sand—fine) | 6% | |||
| Dolomitic argillaceous limestone [67] | 0.37 (coarse) | −3% (increment) | Accelerated CPT | 15% replacing sand. Systems with coarse and fine reactive aggregates |
| 0.53 (fine) | −6% (increment) | |||
| Siliceous limestone [69] | 0.03 (15%) | 49% | CPT (French standard) | 50% replacing cement and 50% replacing sand |
| 0.03 (30% | 55% | |||
| Metaquartzite [70] | 0.03 | 89% | CPT (French standard) | 20% replacing sand Blaine fineness: 400 m2/kg |
| Siliceous limestone [70] | 0.128 | 36% | CPT (French standard) | 20% replacing sand Blaine fineness: 600 m2/kg |
| Opaline aggregate [70] | 0.01 | 96% | CPT (French standard) | 20% replacing sand Blaine fineness: 650 m2/kg Only 36% of the aggregate used was opal, the remainder was non-reactive aggregate |
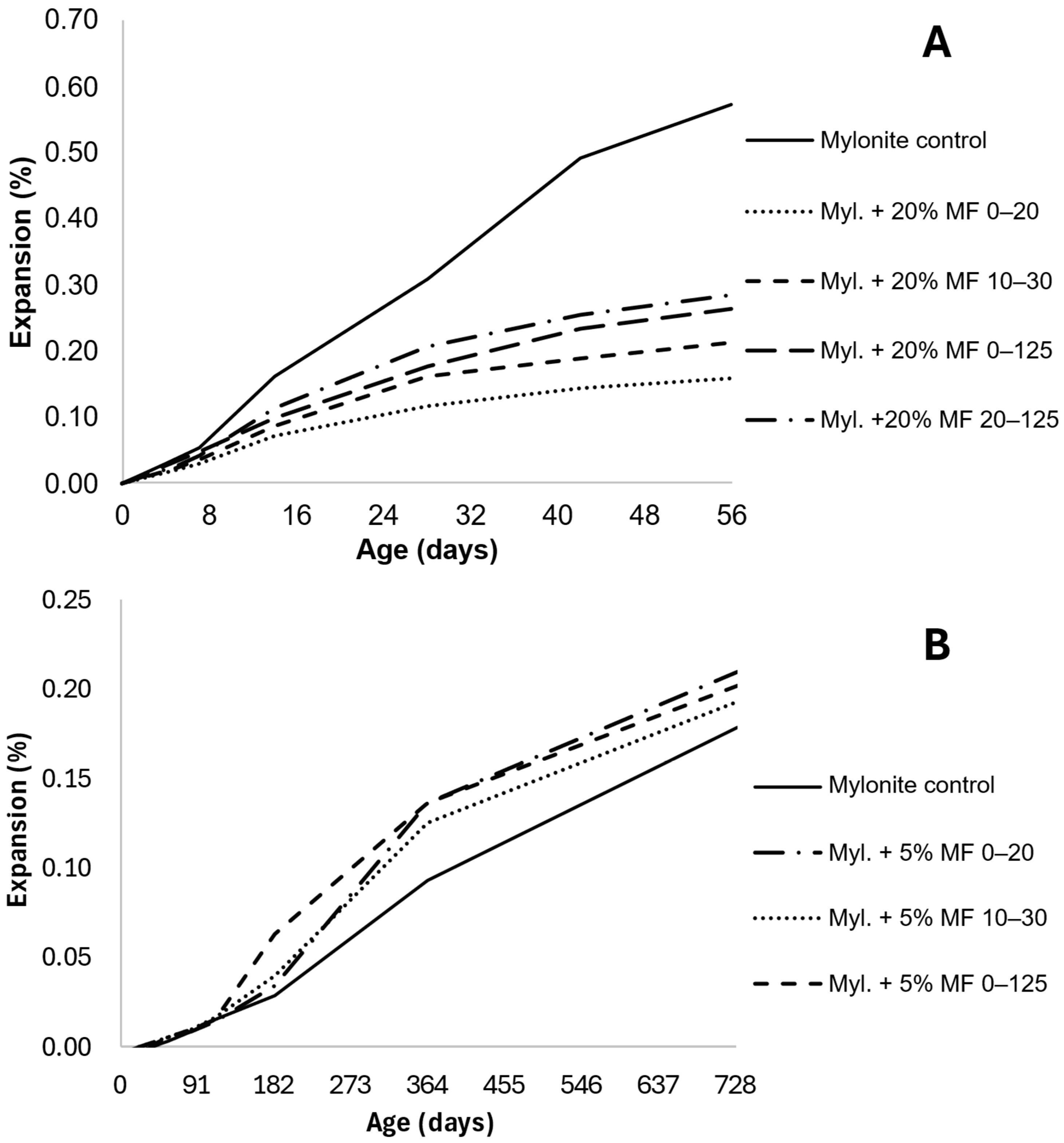
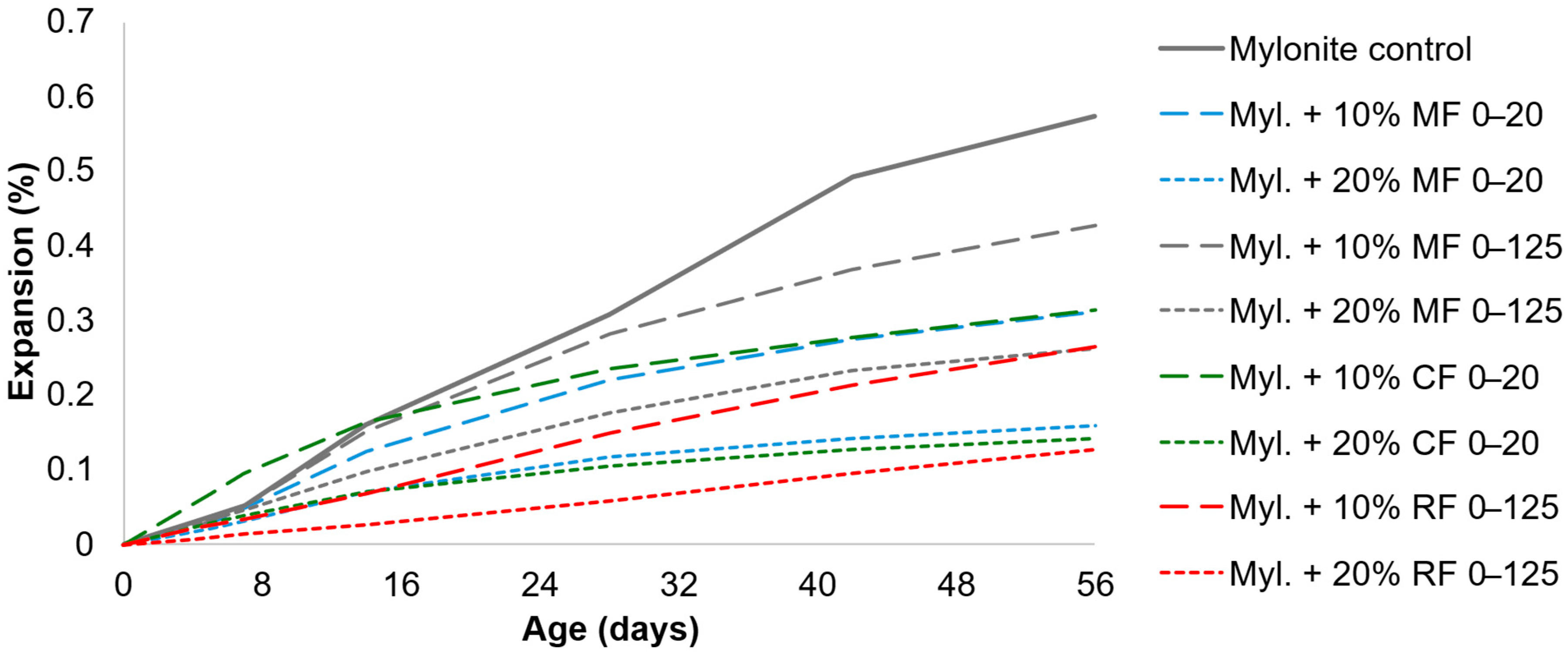
| Mylonite [66] | 0.311 (10% 0–20) | 46% | AMBT (Norwegian standard) | When not mentioned, the coarse aggregate is mylonite |
| 0.158 (20% 0–20) | 72% | |||
| 0.212 (20% 10–30) | 63% | |||
| 0.109 (Gran. Agg. 20% 0–125) | 51% | |||
| 0.286 (20% 20–125) | 50% | |||
| 0.166 (Cat. Agg. 20% 0–20) | 68% | |||
| 0.427 (10% 0–125) | 26% | |||
| 0.263 (20% 0–125) | 54% | |||
| Cataclasite [66] | 0.314 (10% 0–20) | 45% | AMBT (Norwegian standard) | Replacement of sand The coarse aggregate is mylonite |
| 0.141 (20% 0–20) | 75% | |||
| 0.265 (20% 10–40) | 54% | |||
| Icelandic Rhyolite [66] | 0.264 (10% 0–125) | 54% | AMBT (Norwegian standard) | Replacement of sand The coarse aggregate is mylonite |
| 0.041 (20% 0–20) | 93% | |||
| 0.088 (20% 10–40) | 85% | |||
| 0.126 (20% 0–125) | 78% | |||
| Mylonite [66] | 0.210 (5% 0–20) | −17% (increment) | CPT (Norwegian standard) | Replacement of sand The coarse aggregate is mylonite |
| 0.193 (5% 10–30) | −8% (increment) | |||
| 0.202 (5% 0–125) | −13% (increment) | |||
| 0.200 (10% 0–125) | −12% (increment) | |||
| Cataclasite [66] | 0.191 (5% 0–125) | −7% (increment) | CPT (Norwegian standard) | Replacement of sand The coarse aggregate is mylonite |
| Icelandic Rhyolite [66] | 0.041 (5% 0–125) | 77% | CPT (Norwegian standard) | Replacement of sand The coarse aggregate is mylonite |
| Maximum Dimension/ Range | D10 (µm) | D50 (µm) | D90 (µm) | Blaine Fineness (m2/kg) | BET (m2/kg) | |
|---|---|---|---|---|---|---|
| Greywacke filler [62] | - | 2.44 | 30.50 | 99.21 | - | - |
| Dacite filler [62] | - | 2.14 | 41.19 | 96.22 | - | - |
| Orthogneiss [31,64] | <150 µm | 41.84 | 105.36 | 200.01 | 173.79 | 1892.4 |
| Greywacke [67] | - | - | 30.00 | - | - | - |
| Dolomitic argillaceous limestone [67] | - | - | 19.00 | - | - | - |
| Siliceous limestone [69] | <100 µm | - | ~16 µm | - | 450 | - |
| Metaquartzite [70] | 80 µm | - | - | - | 100, 200, and 400 | - |
| Siliceous limestone [70] | 80 µm | - | - | - | 200, 400, and 600 | - |
| Opaline aggregate [70] | 80 µm | - | - | - | 200, 400, and 650 | - |
| Sandstone [75] | - | - | - | - | 210, 400, 610, and 860 | - |
| Andesite [83] | - | - | - | - | 780 m2/kg | - |
| Basalt [74] | <75 µm | - | - | - | 170–200 | - |
| Mylonite [66] | 0–20, 10–30, 20–125, 0–125 µm | - | - | - | - | - |
| Cataclasite [66] | 0–20, 10–40, 0–125 µm | - | - | - | - | - |
| Icelandic Rhyolite [66] | 0–20, 10–40, 0–125 µm | - | - | - | - | - |
| Oxide | Dolomitic Argillaceous Limestone [67] | Siliceous Limestone [69] | Siliceous Limestone [70] | Andesite [83] | Greywacke [62] | Greywacke [67] | Dacite [62] | Orthogneiss [64] | Sandstone [75] | Metaquartzite [70] | Opaline Aggregate [70] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 9.47 | 16.15 | 15.7 | 66.2 | 66.85 | 60.45 | 68.4 | 58.15 | 63.04 | 87.7 | 92.7 |
| Al2O3 | 2.66 | 1.71 | 1.7 | 16.1 | 14.24 | 12.17 | 13.3 | 15.89 | 10.65 | 4.0 | 0.0 |
| Fe2O3 | 0.90 | 0.76 | 1.1 | 3.4 | 3.8 | 5.21 | 3.3 | 7.44 | 3.23 | 1.0 | 0.3 |
| CaO | 41.51 | 43.12 | 43.6 | 3.3 | 1.94 | 5.21 | 2.4 | 5.19 | 8.78 | 0.4 | 0.2 |
| K2O | 0.82 | 0.58 | 0.5 | 2.6 | 3.11 | 2.67 | 3.8 | 4.26 | 1.97 | 0.9 | 0.1 |
| Na2O | 0.17 | 0.05 | 0.5 | 3.5 | 4.25 | 1.41 | 2.4 | 3.16 | 1.54 | 0.1 | 0.2 |
| MgO | 5.48 | 1.29 | 1.5 | 2.0 | 1.58 | 3.50 | 1.3 | 2.47 | 2.56 | 0.2 | 0.1 |
| Traces | 0.41 | 1.18 | 0.2 | 1.00 | 1.94 | 1.13 | 1.00 | 2.72 | 7.33 | 0.1 | 1.1 |
| Na2Oeq | 0.71 | 0.43 | 0.83 | 5.21 | 6.30 | 3.17 | 4.9 | 5.96 | 2.84 | 0.69 | 0.23 |
| L.O.I. | 38.58 | 35.16 | 34.9 | 1.9 | 2.29 | 8.25 | 4.1 | 0.70 | - | 1.1 | 6.0 |
References
- IEA. Net Zero by 2050—A Roadmap for the Global Energy Sector; IEA: Paris, France, 2021. [Google Scholar]
- IEA. Energy Technology Perspectives 2020; IEA: Paris, France, 2020. [Google Scholar]
- European Cement Research Academy. CSI/ECRA—Technology Papers 2017 Development of State of the Art Techniques in Cement Manufacturing: Trying to Look Ahead; European Cement Research Academy: Geneva, Switzerland, 2017. [Google Scholar]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary Cementitious Materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Scrivener, K.; John, V.; Gartner, E. Eco-Efficient Cements: Potential Economically Viable Solutions for a Low-CO2 Cement-Based Materials Industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Gartner, E.; Hirao, H. A Review of Alternative Approaches to the Reduction of CO2 Emissions Associated with the Manufacture of the Binder Phase in Concrete. Cem. Concr. Res. 2015, 78, 126–142. [Google Scholar] [CrossRef]
- Ludwig, H.M.; Zhang, W. Research Review of Cement Clinker Chemistry. Cem. Concr. Res. 2015, 78, 24–37. [Google Scholar] [CrossRef]
- Damtoft, J.S.; Lukasik, J.; Herfort, D.; Sorrentino, D.; Gartner, E.M. Sustainable Development and Climate Change Initiatives. Cem. Concr. Res. 2008, 38, 115–127. [Google Scholar] [CrossRef]
- Ballan, J.; Paone, P. Supplementary Cementitious Materials: Concepts for the Treatment of Raw Materials. IEEE Ind. Appl. Mag. 2014, 20, 61–65. [Google Scholar] [CrossRef]
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined Clay Limestone Cements (LC3). Cem. Concr. Res. 2018, 114, 49–56. [Google Scholar] [CrossRef]
- Miller, S.A.; Habert, G.; Myers, R.J.; Harvey, J.T. Achieving Net Zero Greenhouse Gas Emissions in the Cement Industry via Value Chain Mitigation Strategies. One Earth 2021, 4, 1398–1411. [Google Scholar] [CrossRef]
- Amran, M.; Makul, N.; Fediuk, R.; Lee, Y.H.; Vatin, N.I.; Lee, Y.Y.; Mohammed, K. Global Carbon Recoverability Experiences from the Cement Industry. Case Stud. Constr. Mater. 2022, 17, e01439. [Google Scholar] [CrossRef]
- von Greve-Dierfeld, S.; Lothenbach, B.; Vollpracht, A.; Wu, B.; Huet, B.; Andrade, C.; Medina, C.; Thiel, C.; Gruyaert, E.; Vanoutrive, H.; et al. Understanding the Carbonation of Concrete with Supplementary Cementitious Materials: A Critical Review by RILEM TC 281-CCC. Mater. Struct./Mater. Constr. 2020, 53, 136. [Google Scholar] [CrossRef]
- ACI. Guide for Proportioning Concrete Mixtures with Ground Calcium Carbonate and Other Mineral Fillers; ACI: Farmington Hills, MI, USA, 2020; ISBN 9781641951227. [Google Scholar]
- John, V.M.; Damineli, B.L.; Quattrone, M.; Pileggi, R.G. Fillers in Cementitious Materials—Experience, Recent Advances and Future Potential. Cem. Concr. Res. 2018, 114, 65–78. [Google Scholar] [CrossRef]
- Korpa, A.; Kowald, T.; Trettin, R. Hydration Behaviour, Structure and Morphology of Hydration Phases in Advanced Cement-Based Systems Containing Micro and Nanoscale Pozzolanic Additives. Cem. Concr. Res. 2008, 38, 955–962. [Google Scholar] [CrossRef]
- Moosberg-Bustnes, H.; Lagerblad, B.; Forssberg, E. The Function of Fillers in Concrete. Mater. Struct. 2004, 37, 74–81. [Google Scholar] [CrossRef]
- Aqel, M.; Panesar, D.K. Hydration Kinetics and Compressive Strength of Steam-Cured Cement Pastes and Mortars Containing Limestone Filler. Constr. Build. Mater. 2016, 113, 359–368. [Google Scholar] [CrossRef]
- Li, C.; Jiang, L.; Xu, N.; Jiang, S. Pore Structure and Permeability of Concrete with High Volume of Limestone Powder Addition. Powder Technol. 2018, 338, 416–424. [Google Scholar] [CrossRef]
- Craeye, B.; De Schutter, G.; Desmet, B.; Vantomme, J.; Heirman, G.; Vandewalle, L.; Cizer, Ö.; Aggoun, S.; Kadri, E.H. Effect of Mineral Filler Type on Autogenous Shrinkage of Self-Compacting Concrete. Cem. Concr. Res. 2010, 40, 908–913. [Google Scholar] [CrossRef]
- Poppe, A.M.; De Schutter, G. Cement Hydration in the Presence of High Filler Contents. Cem. Concr. Res. 2005, 35, 2290–2299. [Google Scholar] [CrossRef]
- Kadri, E.H.; Aggoun, S.; De Schutter, G.; Ezziane, K. Combined Effect of Chemical Nature and Fineness of Mineral Powders on Portland Cement Hydration. Mater. Struct./Mater. Constr. 2010, 43, 665–673. [Google Scholar] [CrossRef]
- Rahhal, V.; Talero, R. Early Hydration of Portland Cement with Crystalline Mineral Additions. Cem. Concr. Res. 2005, 35, 1285–1291. [Google Scholar] [CrossRef]
- Xie, D.; Liu, Q.; Zhou, Z.; Gao, J.; Liu, C. Rheology and Hardened Properties of Eco-Friendly Ultra-High Performance Concrete Paste: Role of Waste Stone Powder Fillers. Constr. Build. Mater. 2024, 447, 138163. [Google Scholar] [CrossRef]
- Soroka, I.; Setter, N. The Effect of Fillers on Strength of Cement Mortars. Cem. Concr. Res. 1977, 7, 449–456. [Google Scholar] [CrossRef]
- Dobiszewska, M.; Schindler, A.K.; Pichór, W. Mechanical Properties and Interfacial Transition Zone Microstructure of Concrete with Waste Basalt Powder Addition. Constr. Build. Mater. 2018, 177, 222–229. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, X.; Zhou, J.; Shi, Y.; Umar, H.A.; Long, G.; Xie, Y. Development of an Eco-Friendly Ultra-High Performance Concrete Based on Waste Basalt Powder for Sichuan-Tibet Railway. J. Clean. Prod. 2021, 312, 127775. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, P.; Guo, H.; Lou, R.; Ye, W.; Liu, Y.; Liu, K. Effect of Dry Process Manufactured Sands Dust on the Mechanical Property and Durability of Recycled Concrete. J. Build. Eng. 2024, 87, 108942. [Google Scholar] [CrossRef]
- Multon, S.; Cyr, M.; Sellier, A.; Diederich, P.; Petit, L. Effects of Aggregate Size and Alkali Content on ASR Expansion. Cem. Concr. Res. 2010, 40, 508–516. [Google Scholar] [CrossRef]
- Vardhan, K.; Goyal, S.; Siddique, R.; Singh, M. Mechanical Properties and Microstructural Analysis of Cement Mortar Incorporating Marble Powder as Partial Replacement of Cement. Constr. Build. Mater. 2015, 96, 615–621. [Google Scholar] [CrossRef]
- Coutinho, Y. Influence of the Grinding Process of Aggregates on AAR Expansions. Master’s Thesis, Universidade Federal de Pernambuco, Recife, Brazil, 2019. (In Portuguese). [Google Scholar]
- Ramos, T.; Matos, A.M.; Schmidt, B.; Rio, J.; Sousa-Coutinho, J. Granitic Quarry Sludge Waste in Mortar: Effect on Strength and Durability. Constr. Build. Mater. 2013, 47, 1001–1009. [Google Scholar] [CrossRef]
- Mármol, I.; Ballester, P.; Cerro, S.; Monrós, G.; Morales, J.; Sánchez, L. Use of Granite Sludge Wastes for the Production of Coloured Cement-Based Mortars. Cem. Concr. Compos. 2010, 32, 617–622. [Google Scholar] [CrossRef]
- Nixon, P.J.; Sims, I. RILEM Recommendations for the Prevention of Damage by Alkali-Aggregate Reactions in New Concrete Structures; State-of-the-Art-Report of the RILEM Technical Committee 219 ACS; Springer: Dordrecht, The Netherlands, 2016. [Google Scholar]
- Leemann, A.; Góra, M.; Lothenbach, B.; Heuberger, M. Alkali Silica Reaction in Concrete—Revealing the Expansion Mechanism by Surface Force Measurements. Cem. Concr. Res. 2024, 176, 107392. [Google Scholar] [CrossRef]
- Fournier, B.; Berubé, M.A. Alkali-Aggregate Reaction in Concrete: A Review of Basic Concepts and Engineering Implications. Can. J. Civil. Eng. 2000, 27, 167–191. [Google Scholar] [CrossRef]
- Sims, I.; Poole, A. Alkali-Aggregate Reaction in Concrete—A World Review; CRC Press: London, UK, 2017; ISBN 9781138027565. [Google Scholar]
- Thomas, M.D.A.; Fournier, B.; Folliard, K.J. Alkali-Aggregate Reactivity (AAR) Facts Book; U.S. Department of Transportation, Federal Highway Administration: Washington, DC, USA, 2013; ISBN FHWA-HIF-13-019.
- Grattan-Bellew, P.E.; Mitchell, L.D.; Margeson, J.; Min, D. Is Alkali-Carbonate Reaction Just a Variant of Alkali–Silica Reaction ACR = ASR? Cem. Concr. Res. 2010, 40, 556–562. [Google Scholar] [CrossRef]
- Katayama, T. The So-Called Alkali-Carbonate Reaction (ACR)—Its Mineralogical and Geochemical Details, with Special Reference to ASR. Cem. Concr. Res. 2010, 40, 643–675. [Google Scholar] [CrossRef]
- Katayama, T. A Critical Review of Carbonate Rock Reactions—Is Their Reactivity Useful or Harmful? In Proceedings of the 9th International Conference on Alkali-Aggregate Reaction, London, UK, 27–31 July 1992; pp. 508–517. [Google Scholar]
- Medeiros, R.; Sanchez, L.; dos Santos, A.C. Assessing Alkali-Carbonate Reaction-Induced Damage in Critical Concrete Infrastructure: The First ACR-Affected Field Structure Reported in Brazil. In Proceedings of the 17th International Conference on Alkali-Aggregate Reaction in Concrete (ICAAR 2024), Ottawa, ON, Canada, 18–24 May 2024; RILEM Bookseries. Sanchez, L.F., Trottier, C., Eds.; Springer: Cham, Switzerland, 2024; Volume 50, pp. 445–452. [Google Scholar] [CrossRef]
- Leemann, A.; Münch, B.; Trottier, C.; Sanchez, L. Microstructural Consequences of Alkali-Carbonate Reaction. In Proceedings of the 17th International Conference on Alkali-Aggregate Reaction in Concrete (ICAAR 2024), Ottawa, ON, Canada, 18–24 May 2024; RILEM Bookseries. Sanchez, L.F., Trottier, C., Eds.; Springer: Cham, Switzerland, 2024; Volume 49, pp. 87–94. [Google Scholar] [CrossRef]
- Katayama, T.; Grattan-Bellew, P.E. Petrography of the Kingston Experimental Sidewalk at Age 22 Years—ASR as the Cause of Deleteriously Expansive, So-Called Alkali-Carbonate Reaction. In Proceedings of the 14th International Conference on Alkali-Aggregate Reaction in Concrete, Austin, TX, USA, 20–25 May 2012. [Google Scholar]
- ASTM C1260; Test Method for Potential Alkali Reactivity of Aggregates (Mortar-Bar Method). ASTM International: West Conshohocken, PA, USA, 2023.
- ABNT NBR 15577-4; Aggregates–Alkali-Aggregate Reactivity. Part 4: Determination of Expansion on Mortar Bars by Accelerated Mortar Bar Method. ABNT: Rio de Janeiro, Brazil, 2018.
- ASTM C1293; Test Method for Determination of Length Change of Concrete Due to Alkali-Silica Reaction. ASTM International: West Conshohocken, PA, USA, 2023.
- CSA A23.1:24/CSA A23.2:24; Concrete Materials and Methods of Concrete Construction/Test Methods and Standard Practices for Concrete. CSA Group: Toronto, ON, Canada, 2024.
- ABNT NBR 15577-6; Aggregates–Alkali-Aggregate Reactivity. Part 6: Determination of Mitigation of Expansion on Concrete Prisms. ABNT: Rio de Janeiro, Brazil, 2018.
- Sanchez, L.F.M.; Drimalas, T.; Fournier, B.; Mitchell, D.; Bastien, J. Comprehensive Damage Assessment in Concrete Affected by Different Internal Swelling Reaction (ISR) Mechanisms. Cem. Concr. Res. 2018, 107, 284–303. [Google Scholar] [CrossRef]
- Bérubé, M.; Founier, B. Canadian Experience with Testing for Alkali-Aggregate Reactivity in Concrete. Cem. Concr. Compos. 2003, 15, 27–47. [Google Scholar] [CrossRef]
- Demerchant, D.P.; Fournier, B.; Strang, F. Alkali-Aggregate Research in New Brunswick. Can. J. Civ. Eng. 2000, 27, 212–225. [Google Scholar] [CrossRef]
- Golmakani, F.; Hooton, R.D. Comparison of Laboratory Performance Tests Used to Assess Alkali-Silica Reactivity. In Proceedings of the Annual Conference—Canadian Society for Civil Engineering, London, ON, Canada, 1–4 June 2016; Volume 2, pp. 1–7. [Google Scholar]
- Grattan-Bellew, P.E. A Critical Review of Ultra-Accelerated Alkali-Silica Reactivity. Cem. Concr. Compos. 1997, 19, 403–414. [Google Scholar] [CrossRef]
- Ideker, J.H.; Bentivegna, A.F.; Folliard, K.J.; Juenger, M.C.G. Do Current Laboratory Test Methods Accurately Predict Alkali-Silica Reactivity? ACI Mater. J. 2012, 109, 395–402. [Google Scholar] [CrossRef]
- Thomas, M.; Fournier, B.; Folliard, K.; Ideker, J.; Shehata, M. Test Methods for Evaluating Preventive Measures for Controlling Expansion Due to Alkali-Silica Reaction in Concrete. Cem. Concr. Res. 2006, 36, 1842–1856. [Google Scholar] [CrossRef]
- Lindgård, J.; Andiç-Çakır, Ö.; Fernandes, I.; Rønning, T.F.; Thomas, M.D.A. Alkali–Silica Reactions (ASR): Literature Review on Parameters Influencing Laboratory Performance Testing. Cem. Concr. Res. 2012, 42, 223–243. [Google Scholar] [CrossRef]
- Ideker, J.H.; East, B.L.; Folliard, K.J.; Thomas, M.D.A.; Fournier, B. The Current State of the Accelerated Concrete Prism Test. Cem. Concr. Res. 2010, 40, 550–555. [Google Scholar] [CrossRef]
- ABNT NBR 15577-7; Aggregates–Alkali-Aggregate Reactivity. Part 7: Determination of Concrete Prism by Accelerated Method. ABNT: Rio de Janeiro, Brazil, 2018.
- AASHTO T 380-22; Standard Method of Test for Potential Alkali Reactivity of Aggregates and Effectiveness of ASR Mitigation Measures (Miniature Concrete Prism Test, MCPT). AASHTO: Washington, DC, USA, 2022.
- Lindgård, J.; Andiç-Çakır, Ö.; Borchers, I.; Broekmans, M.; Brouard, E.; Fernandes, I.; Giebson, C.; Pedersen, B.; Pierre, C.; Rønning, T.F.; et al. RILEM TC 219-ACS-P: Literature Survey on Performance Testing; Lindgård, J., Ed.; SINTEF Building and Infrastructure: Trondheim, Norway, 2011. [Google Scholar]
- Tapas, M.J.; Thomas, P.; Vessalas, K.; Nsiah-Baafi, E.; Martin, L.; Sirivivatnanon, V. Comparative Study of the Efficacy of Fly Ash and Reactive Aggregate Powders in Mitigating Alkali-Silica Reaction. J. Build. Eng. 2023, 63, 105571. [Google Scholar] [CrossRef]
- Castro, C.H.; Santos, M.C.; Traboulsi, M.A.; Bittencourt, R.M. Influence of Pulverized Aggregate on Alkali-Aggregate Reaction. In Proceedings of the Simpósio Sobre Reatividade Álcali-Agregado em estruturas de Concreto, Goiânia, Brazil, 1997. (In Portuguese). [Google Scholar]
- Coutinho, Y.; Montefalco, L.; Carneiro, A. Evaluation of ASR-Reactive Aggregate Powder on ASR Expansions of Mortars and Concretes Using AMBT and MCPT. In Proceedings of the 17th International Conference on Alkali-Aggregate Reaction in Concrete (ICAAR 2024), Ottawa, ON, Canada, 18–24 May 2024; RILEM Bookseries. Sanchez, L.F., Trottier, C., Eds.; Springer: Cham, Switzerland, 2024; Volume 50, pp. 604–611. [Google Scholar] [CrossRef]
- Oliveira, P.J.; Salles, F.M.; Andriolo, F.R. Crushed Powder Filler—The Use on RCC and the Reduction of Expansion Due to the Alkali-Aggregate Reaction. In Proceedings of the International Symposium of Roller Compacted Concrete Dams, Santander, Spain, 2–4 October 1995. [Google Scholar]
- Pedersen, B. Alkali-Reactive and Inert Fillers in Concrete. Rheology of Fresh Mixtures and Expansive Reactions. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2004. [Google Scholar]
- Antunes, L.R. The Influence of Alternative Materials on Alkali Aggregate Reaction (AAR) Induced Development in Concrete. Master’s Thesis, University of Ottawa, Ottawa, ON, USA, 2021. [Google Scholar]
- Sanchez, L.F.M.; Fournier, B.; Jolin, M.; Bastien, J.; Mitchell, D. Tools for assessing damage in concrete affected by AAR coming from fine and coarse aggregates. Rev. IBRACON Estrut. Mater. 2017, 10. [Google Scholar] [CrossRef]
- Guédon-Dubied, J.-S.; Cadoret, G.; Durieux, V.; Martineau, F.; Fasseu, P.; Van Overbeke, V. Etude Du Calcaire Tournaisien de La Carrière Cimescaut À (Belgique) Analyse Pétrographique et Chimique et réactivité Aux Alcalins. Bull. Lab. Ponts Chaussées 2000, 226, 57–66. [Google Scholar]
- Carles-Gibergues, A.; Cyr, M.; Moisson, M.; Ringot, E. A Simple Way to Mitigate Alkali-Silica Reaction. Mater. Struct./Mater. Constr. 2008, 41, 73–83. [Google Scholar] [CrossRef]
- Sanchez, L.; Fournier, B.; Jolin, M.; Bedoya, M.A.B.; Bastien, J.; Duchesne, J. Use of Damage Rating Index to Quantify Alkali-Silica Reaction Damage in Concrete: Fine versus Coarse Aggregate. ACI Mater. J. 2016, 113, 395. [Google Scholar] [CrossRef]
- Sanchez, L.F.M. Contribution to the Assessment of Damage in Aging Concrete Infrastructures Affected by Alkali-Aggregate Reaction. Ph.D. Thesis, Universite Laval, Québec, QC, Canada, 2014. [Google Scholar]
- De Souza, D.J.; Antunes, L.R.; Bezerra, A.C.; Sanchez, L.F.M. Influence of Mineral Fillers (MF) on ASR-Induced Expansion and Deterioration. In RILEM Bookseries; Springer: Cham, Switzerland; Volume 50, pp. 612–619. [CrossRef]
- Salles, F.M.; Oliveira, P.J.R.; Andriolo, F.R. Use of Crushing Fines to Reduce AAR-Expansions. In Proceedings of the Simpósio Sobre Reatividade Álcali-Agregado em Estruturas de Concreto, Goiânia, Brazil, 1997. (In Portuguese). [Google Scholar]
- Li, Y.; He, Z.; Hu, S. Mechanism of Suppressing ASR Using Ground Reactive Sandstone Powders Instead of Cement. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2015, 30, 344–351. [Google Scholar] [CrossRef]
- Räisänen, M.; Mertamo, M. An Evaluation of the Procedure and Results of Laboratory Crushing in Quality Assessment of Rock Aggregate Raw Materials. Bull. Eng. Geol. Environ. 2004, 63, 33–39. [Google Scholar] [CrossRef]
- Diógenes, L.; Maia, R.; Bessa, I.; Castelo Branco, V.; Nogueira Neto, J.; Silva, F. The Influence of Crushing Processes and Mineralogy of Aggregates on Their Shape Properties and Susceptibility to Degradation. Constr. Build. Mater. 2021, 284, 122745. [Google Scholar] [CrossRef]
- Bouquety, M.N.; Descantes, Y. Experimental Study of Crushed Aggregate Shape. Constr. Build. Mater. 2007, 21, 865–872. [Google Scholar] [CrossRef]
- Åkesson, U.; Stigh, J.; Lindqvist, J.E.; Göransson, M. The Influence of Foliation on the Fragility of Granitic Rocks, Image Analysis and Quantitative Microscopy. Eng. Geol. 2003, 68, 275–288. [Google Scholar] [CrossRef]
- Pang, L.; Wu, S.; Zhu, J.; Wan, L. Relationship between Petrographical and Physical Properties of Aggregates. J. Wuhan Univ. Technol.-Mater. Sci. 2010, 25, 678–681. [Google Scholar] [CrossRef]
- Coutinho, Y.; Montefalco, L.; Carneiro, A. Influence of Aggregate Crushing on the Results of Accelerated Alkali-Silica Reactivity Tests. Constr. Build. Mater. 2022, 325, 126737. [Google Scholar] [CrossRef]
- Valduga, L. Influence of Procedure Conditions of ASTM C 1260 in the Verification of Alkali-Aggregate Reation. Ph.D. Thesis, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, 2007. (In Portuguese). [Google Scholar]
- Qinghan, B.; Xuequan, W.; Mingshu, T.; Nishibayashi, S.; Kuroda, T.; Tiecheng, W. Effect of Reactive Aggregate Powder on Suppressing Expansion Due to Alkali-Silica Reaction. In Proceedings of the 10th International Conference on AAR in Concrete, Melbourne, Australia, 18–23 August 1996; pp. 546–553. [Google Scholar]
- Leemann, A.; Holzer, L. Alkali-Aggregate Reaction-Identifying Reactive Silicates in Complex Aggregates by ESEM Observation of Dissolution Features. Cem. Concr. Compos. 2005, 27, 796–801. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, G.; Deng, M.; Tang, M.; Lu, D. The Use of Thermodynamic Analysis in Assessing Alkali Contribution by Alkaline Minerals in Concrete. Cem. Concr. Compos. 2008, 30, 353–359. [Google Scholar] [CrossRef]
- Soares, D.; Silva, A.S.; Mirão, J.; Fernandes, I.; Menéndez, E. Study on the Factors Affecting Alkalis Release from Aggregates into ASR. In Proceedings of the 15th Conference on Alkali-Aggregate Reaction in Concrete, São Paulo, Brazil, 3–7 July 2016; Hasparyk, N.P., Bernardes, H.M., Eds.; São Paulo, Brazil, 2016. [Google Scholar]
- Ferraz, A.R.; Fernandes, I.; Soares, D.; Silva, A.S.; Quinta-Ferreira, M. Assessment of the Alteration of Granitic Rocks and Its Influence on Alkalis Release. IOP Conf. Ser. Earth Environ. Sci. 2017, 95, 022001. [Google Scholar] [CrossRef]
- Berubé, M.A.; Duchesne, J.; Dorion, J.F.; Rivest, M. Laboratory Assessment of Alkali Contribution by Aggregates to Concrete and Application to Concrete Structures Affected by Alkali—Silica Reactivity. Cem. Concr. Res. 2002, 32, 1215–1227. [Google Scholar] [CrossRef]
- Hou, X.; Struble, L.J.; Kirkpatrick, R.J. Formation of ASR Gel and the Roles of C-S-H and Portlandite. Cem. Concr. Res. 2004, 34, 1683–1696. [Google Scholar] [CrossRef]
- Thomas, M. The Effect of Supplementary Cementing Materials on Alkali-Silica Reaction: A Review. Cem. Concr. Res. 2011, 41, 1224–1231. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry, 2nd ed; Thomas Telford: London, UK, 1997; ISBN 0727725920. [Google Scholar]
- Dyer, T. Concrete Durability; 1°; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9780203862117. [Google Scholar]
- Pedersen, B.M.; Wigum, B.J.; Lindgård, J. Influence of Aggregate Particle Size on The Alkali-Silica Reaction-A Literature Review. In Proceedings of the 15th International Conference on Alkali-Aggregate Reaction in Concrete, Sao Paulo, Brazil, 3–7 July 2016; Bernardes, H.M., Hasparyk, N., Eds.; São Paulo, Brazil, 2016. [Google Scholar]
- Boddy, A.M.; Hooton, R.D.; Thomas, M.D.A. The Effect of the Silica Content of Silica Fume on Its Ability to Control Alkali–Silica Reaction. Cem. Concr. Res. 2003, 33, 1263–1268. [Google Scholar] [CrossRef]
- Gudmundsson, G.; Olafsson, H. Alkali-Silica Reactions and Silica Fume: 20 Years of Experience in Iceland. Cem. Concr. Res. 1999, 29, 1289–1297. [Google Scholar] [CrossRef]
- Marusin, S.L.; Shotwell, L.B. Alkali-Silica Reaction in Concrete Caused by Densified Silica Fume Lumps: A Case Study. Cem. Concr. Aggreg. 2000, 20, 90–94. [Google Scholar] [CrossRef]
- Diamond, S. Alkali Silica Reactions-Some Paradoxes. Cem. Concr. Compos. 1997, 19, 391–401. [Google Scholar] [CrossRef]
- Stanton, T. Expansion of Concrete through Reaction between Cement and Aggregate. Proc. Am. Soc. Civil. Eng. 1940, 66, 1781–1811. [Google Scholar] [CrossRef]
- Vivian, H.E. Studies in Cement-Aggregate Reaction. XIX: The Effect on Mortar Expansion of the Particle Size of the Reactive Component in the Aggregate. Aust. J. Appl. Sci. 1951, 2, 488–494. [Google Scholar]
- Diamond, S.; Thaulow, N. A Study of Expansion Due to Alkali—Silica Reaction as Conditioned by the Grain Size of the Reactive Aggregate. Cem. Concr. Res. 1974, 4, 591–607. [Google Scholar] [CrossRef]
- Hobbs, D.W.; Gutteridge, W.A. Particle Size of Aggregate and Its Influence upon the Expansion Caused by the Alkali-Silica Reaction. Mag. Concr. Res. 1979, 31, 235–242. [Google Scholar] [CrossRef]
- Ramyar, K.; Topal, A.; Andiç, Ö. Effects of Aggregate Size and Angularity on Alkali–Silica Reaction. Cem. Concr. Res. 2005, 35, 2165–2169. [Google Scholar] [CrossRef]
- Qiu, X.; Chen, J.; Ye, G.; De Schutter, G. Insights in the Chemical Fundamentals of ASR and the Role of Calcium in the Early Stage Based on a 3D Reactive Transport Model. Cem. Concr. Res. 2022, 157, 106778. [Google Scholar] [CrossRef]
- Kuo, W.T.; Shu, C.Y. Effect of Particle Size and Curing Temperature on Expansion Reaction in Electric Arc Furnace Oxidizing Slag Aggregate Concrete. Constr. Build. Mater. 2015, 94, 488–493. [Google Scholar] [CrossRef]
- Sekrane, N.Z.; Asroun, A. Modelling the Effects of Aggregate Size on Alkali Aggregate Reaction Expansion. Technol. Appl. Sci. Res. 2014, 4, 656–661. [Google Scholar] [CrossRef]
- Zhang, X.; Gravest, G.W. The Alkali-Silica Reaction in OPe/Silica Glass Mortar with Particular Reference to Pessimum Effects. Adv. Cem. Res. 1990, 3, 9–13. [Google Scholar] [CrossRef]
- Suwito, A.; Jin, W.; Xi, Y.; Meyer, C. A Mathematical Model for the Pessimum Size Effect of ASR in Concrete. Concr. Sci. Eng. 2002, 4, 23–34. [Google Scholar]
- Goltermann, P. Mechanical Predictions of Concrete Deterioration-Part 2: Classification of Crack Patterns. ACI Mater. J. 1995, 92, 58–62. [Google Scholar]
- Bažant, Z.P.; Zi, G.; Meyer, C. Fracture Mechanics of ASR in Concretes with Waste Glass Particles of Different Sizes. J. Eng. Mech. 2000, 126, 226–232. [Google Scholar] [CrossRef]
- Bektas, F.; Turanli, L.; Topal, T.; Goncuoglu, M.C. Alkali Reactivity of Mortars Containing Chert and Incorporating Moderate-Calcium Fly Ash. Cem. Concr. Res. 2004, 34, 2209–2214. [Google Scholar] [CrossRef]
- Leemann, A.; Lothenbach, B. The Influence of Potassium–Sodium Ratio in Cement on Concrete Expansion Due to Alkali-Aggregate Reaction. Cem. Concr. Res. 2008, 38, 1162–1168. [Google Scholar] [CrossRef]
- Komba, J.; Mgangira, M.B.; Mohale, L. Investigation of the Effects of the Type of Crusher on Coarse Aggregate Shape Properties Using the Three-Dimensional Laser Scanning Technique. In Proceedings of the Geo-China: New Frontiers in Civil Infrastructure, Shandong, China, 25–27 July 2016; pp. 125–132. [Google Scholar]
- Braga, J.A.; Zanella, M.R.; Zaleski, J.M.; Andriolo, F.R. Use of Rolled Concrete-Capanda Project-Angola-Special Tests. In Proceedings of the XIX Seminário Nacional de Grandes Barragens, Aracaju, Brazil, 1991; pp. 353–385. [Google Scholar]
- Alves, E.F.R.; Carmo, J.B.M.; Santos, M.C.; Traboulsi, M.A. Comparative Study on the Expansion of Molded Concrete and Mortar. In Proceedings of the Simpósio Sobre Reatividade Álcali-Agregado em Estruturas de Concreto, Goiânia, Brazil, 1997. [Google Scholar]
- Mohammadi, A.; Ghiasvand, E.; Nili, M. Relation between Mechanical Properties of Concrete and Alkali-Silica Reaction (ASR); A Review. Constr. Build. Mater. 2020, 258, 119567. [Google Scholar] [CrossRef]
- Shao, Y.; Lefort, T.; Moras, S.; Rodriguez, D. Studies on Concrete Containing Ground Waste Glass. Cem. Concr. Res. 2000, 30, 91–100. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, A.; Tang, M.; Wu, B.; Zhang, N. Influence of Aggregate Size and Aggregate Size Grading on ASR Expansion. Cem. Concr. Res. 1999, 29, 1393–1396. [Google Scholar] [CrossRef]
- Shafaatian, S.M.H.; Akhavan, A.; Maraghechi, H.; Rajabipour, F. How Does Fly Ash Mitigate Alkali-Silica Reaction (ASR) in Accelerated Mortar Bar Test (ASTM C1567)? Cem. Concr. Compos. 2013, 37, 143–153. [Google Scholar] [CrossRef]
- Shayan, A. The “Pessimum” Effect in an Accelerated Mortar Bar Test Using 1M NaOH Solution at 80 °C. Cem. Concr. Compos. 1992, 14, 249–255. [Google Scholar] [CrossRef]
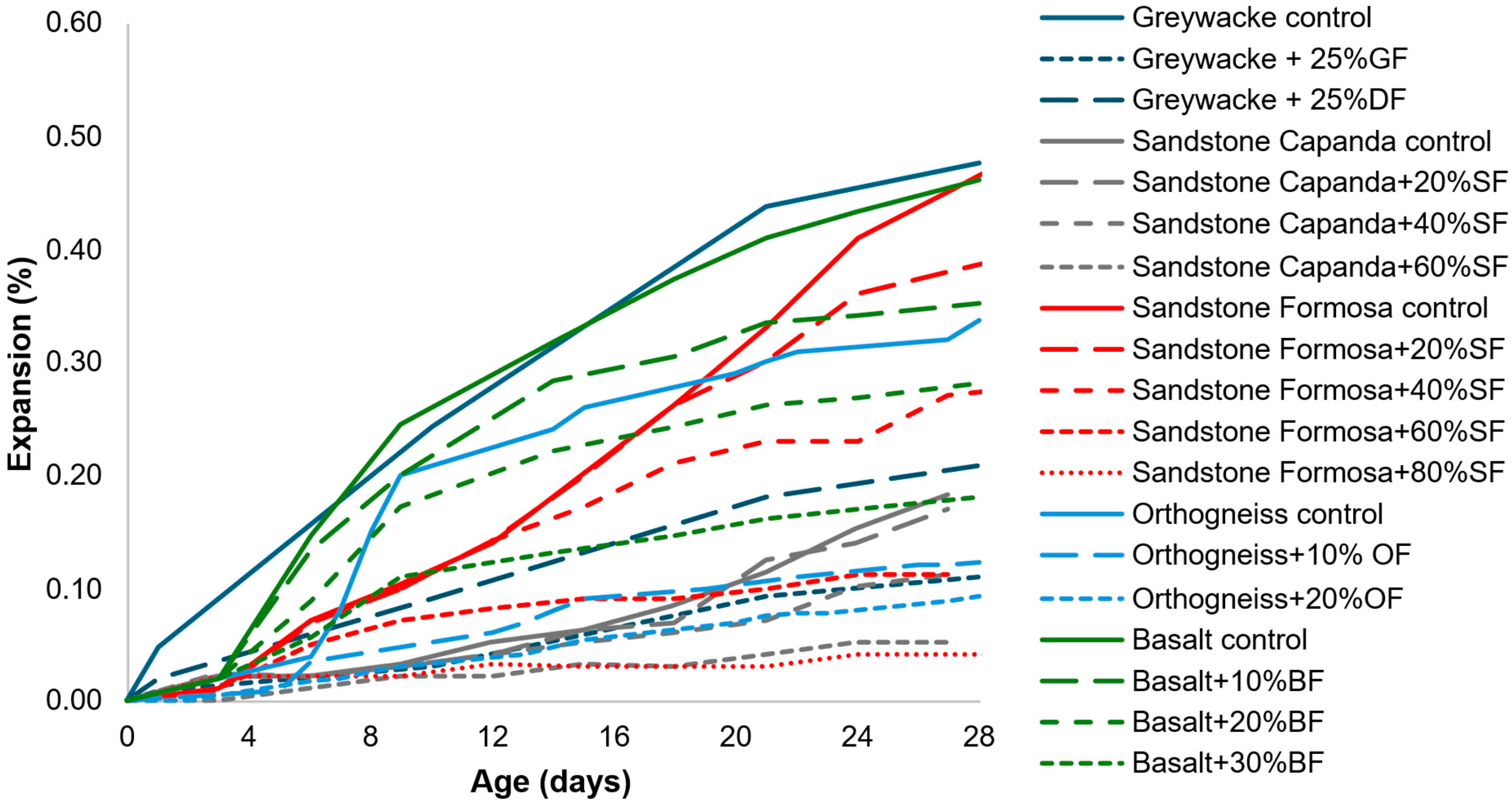

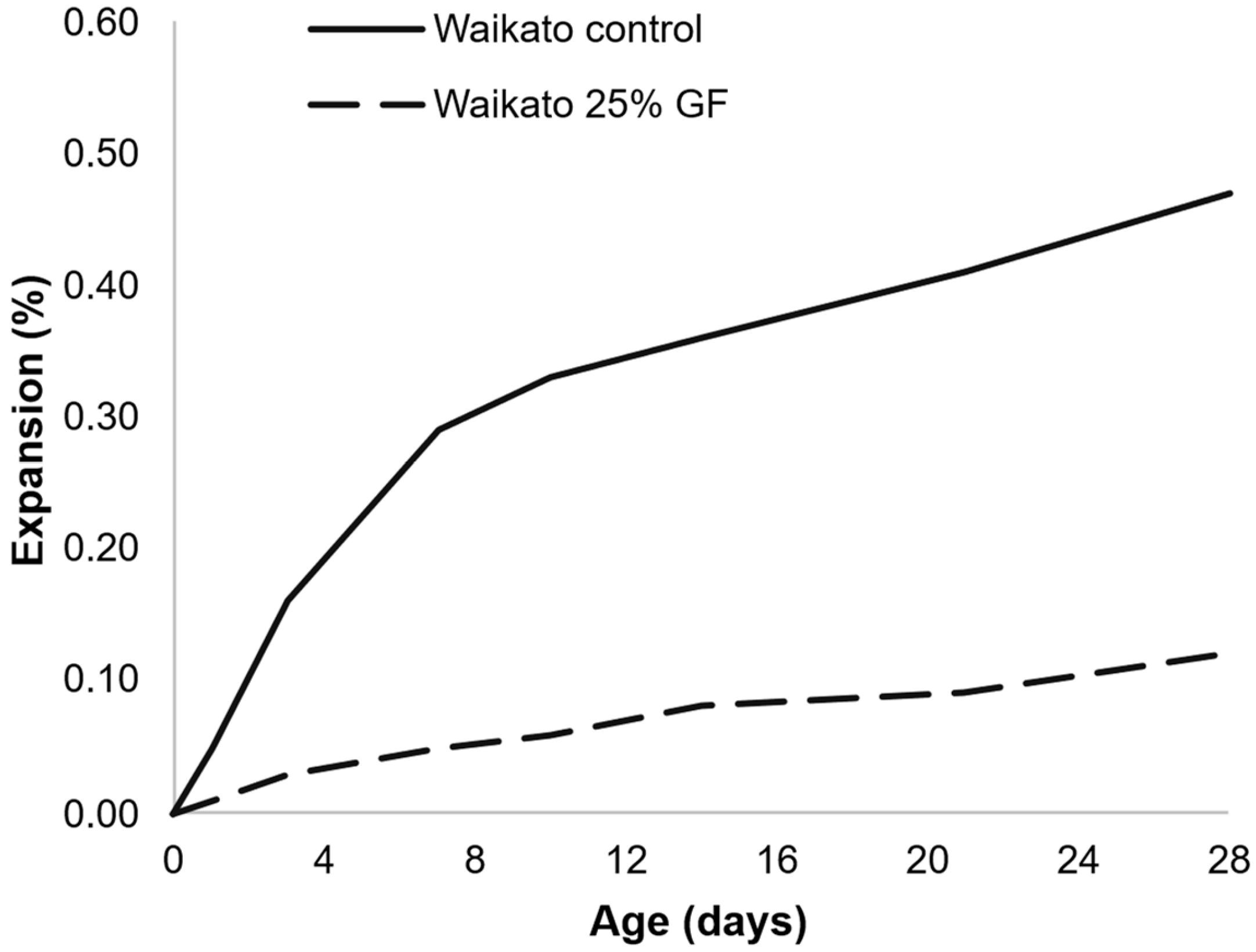
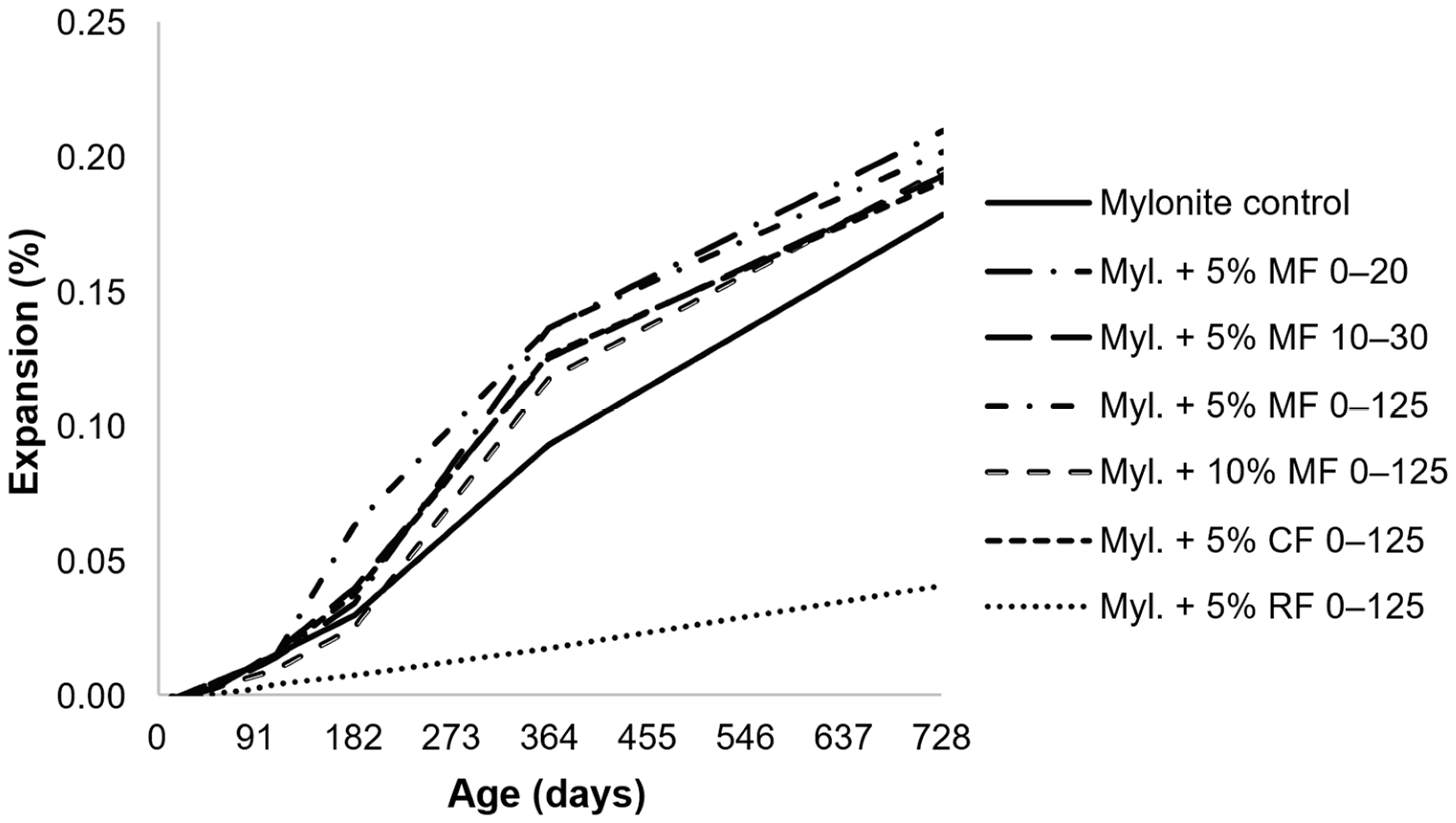
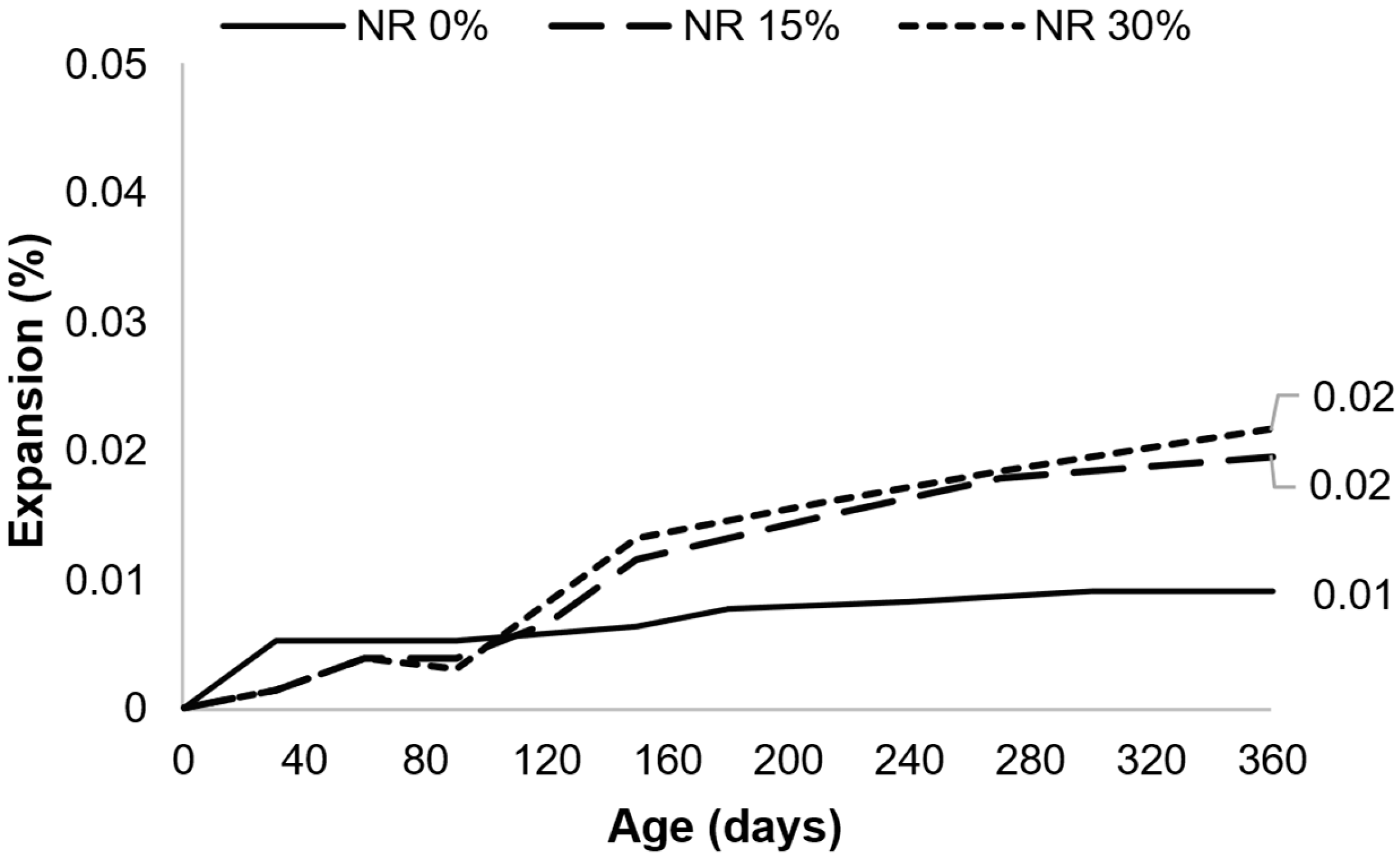

| Year | Filler | Dimension | Crushing Process | % | Tests | Nomenclature | Ref. | Observation |
|---|---|---|---|---|---|---|---|---|
| 1996 | Andesite | Blaine fineness: 780 m2/kg | - | 30, 40, 50, 60, and 70% replacing cement | Autoclave (mortar) | Ground reactive aggregate powder | [83] | Reduction in expansions |
| 1997 | Basalt | <75 µm | - | 10, 20, 30% replacing cement | NBRI method | Aggregate powder | [74] | Reduction in expansions and pozzolanic activity |
| 1997 | Sandstone (Capanda) | <75 µm | - | 20, 40, 60% replacing cement | NBRI method | Powdered aggregate | [63] | Reduction in expansions and pozzolanic activity |
| Sandstone (Formoso) | 20, 40, 60, 80% replacing cement | |||||||
| 2000 | Limestone | <100 µm (D50: ~16 µm) Blaine fineness: 450 m2/kg | - | 15% and 30%, but half replacing cement and half replacing sand | CPT(French standard) | Filler 742 | [69] | Reduction in expansions |
| 2004 | Mylonite | 0–20, 10–30, 20–125, 0–125 µm | - | 2, 5, and 10% replacing sand in volume | AMBT, CPT (Norwegian standards) | Alkali-reactive fillers | [66] | Effect of temperature and amorphous silica content on the pozzolanic reactivity of ASR-reactive fillers |
| Cataclasite | 0–20, 10–40, 0–125 µm | |||||||
| Icelandic Rhyolite | 0–20, 10–40, 0–125 µm | |||||||
| 2008 | Metaquartzite, siliceous limestone, opaline aggregate, crushed waste glass | 80 µm Blaine fineness: 100–650 m2/kg | - | 10% and 20% replacing sand | Autoclave (mortar) Test in concrete (different parameters) | Reactive aggregate powder | [70] | Reduction in expansions |
| 2015 | Sandstone | Blaine fineness: 210–860 m2/kg | - | 10, 20, 30, and 40% replacing cement | AMBT | Reactive powder | [75] | Reduction in expansions |
| 2021 | Greywacke | D50: 30 µm | Crushing and sieving to obtain particles <150 µm | 15% replacing cement and sand | Accelerated CPT | Filler | [67] | Reduction or similar expansion when replacing cement; higher or similar expansions when replacing sand |
| Dolomitic argillaceous limestone | D50: 19 µm | |||||||
| 2023 | Greywacke | D50: 30.50 µm | Ring mill for 5 min | 25% replacing cement | AMBT | Reactive aggregate powder | [62] | Reduction in expansions and pozzolanic activity. No contribution to compressive strength development |
| Dacite | D50: 41.49 µm | |||||||
| 2024 | Orthogneiss | <150 µm D50: 105.36 µm | Crushing and sieving to obtain particles < 150 µm | 10 and 20% replacing cement and sand | AMBT, MCPT | Reactive aggregate powder | [64] | Reduction in expansions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coutinho, Y.; Medeiros, R.; Sanchez, L.; Carneiro, A. AAR-Reactive Fillers in Concrete: Current Understanding and Knowledge Gaps. Buildings 2025, 15, 3025. https://doi.org/10.3390/buildings15173025
Coutinho Y, Medeiros R, Sanchez L, Carneiro A. AAR-Reactive Fillers in Concrete: Current Understanding and Knowledge Gaps. Buildings. 2025; 15(17):3025. https://doi.org/10.3390/buildings15173025
Chicago/Turabian StyleCoutinho, Yane, Rennan Medeiros, Leandro Sanchez, and Arnaldo Carneiro. 2025. "AAR-Reactive Fillers in Concrete: Current Understanding and Knowledge Gaps" Buildings 15, no. 17: 3025. https://doi.org/10.3390/buildings15173025
APA StyleCoutinho, Y., Medeiros, R., Sanchez, L., & Carneiro, A. (2025). AAR-Reactive Fillers in Concrete: Current Understanding and Knowledge Gaps. Buildings, 15(17), 3025. https://doi.org/10.3390/buildings15173025








