Early-Age Properties and Reaction of Hydrophobic Portland Cement and Alkali-Activated Fly Ash–Slag Pastes with Alkyl Silanes
Abstract
1. Introduction
2. Experimental Program
2.1. Raw Materials
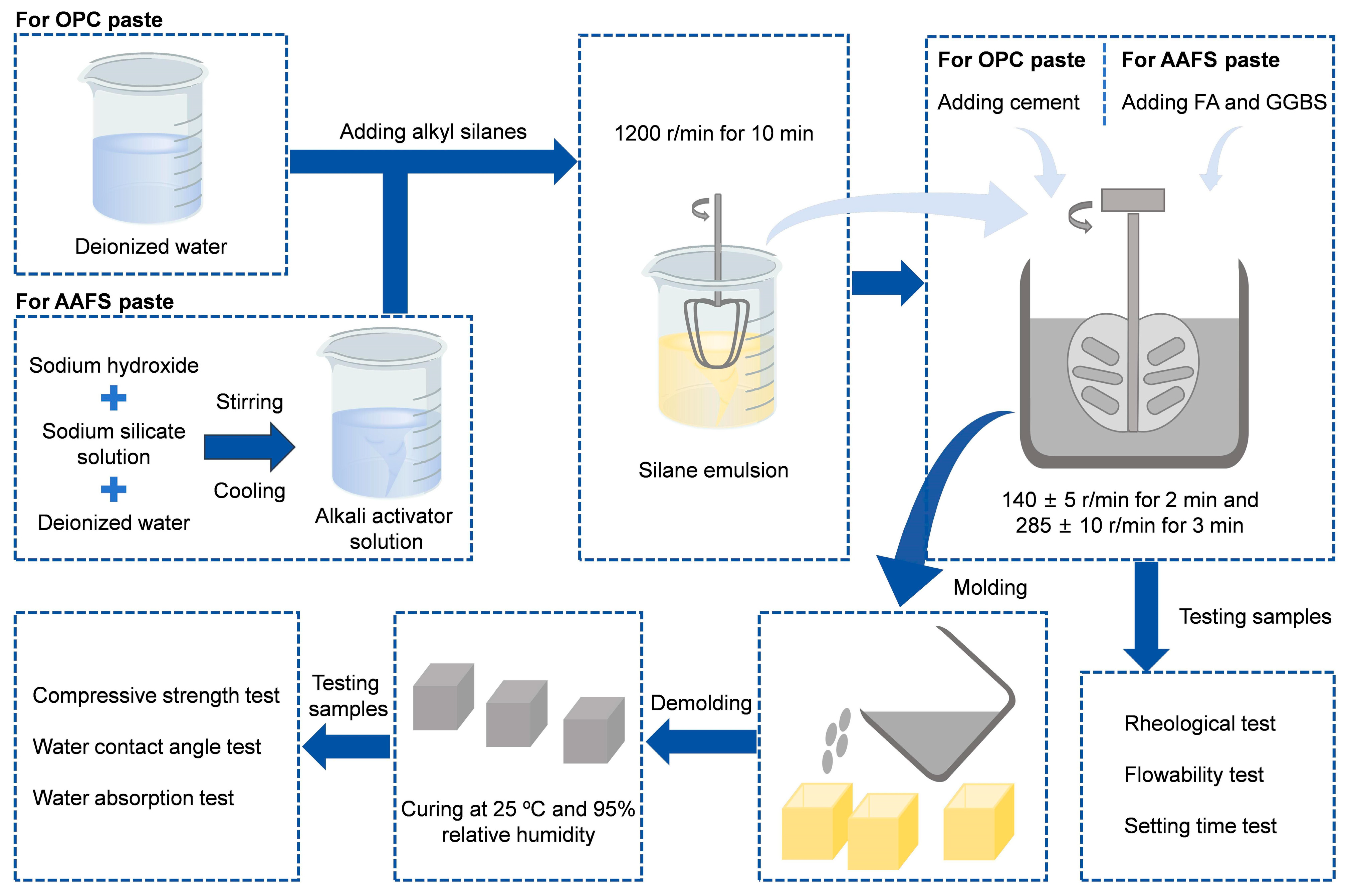
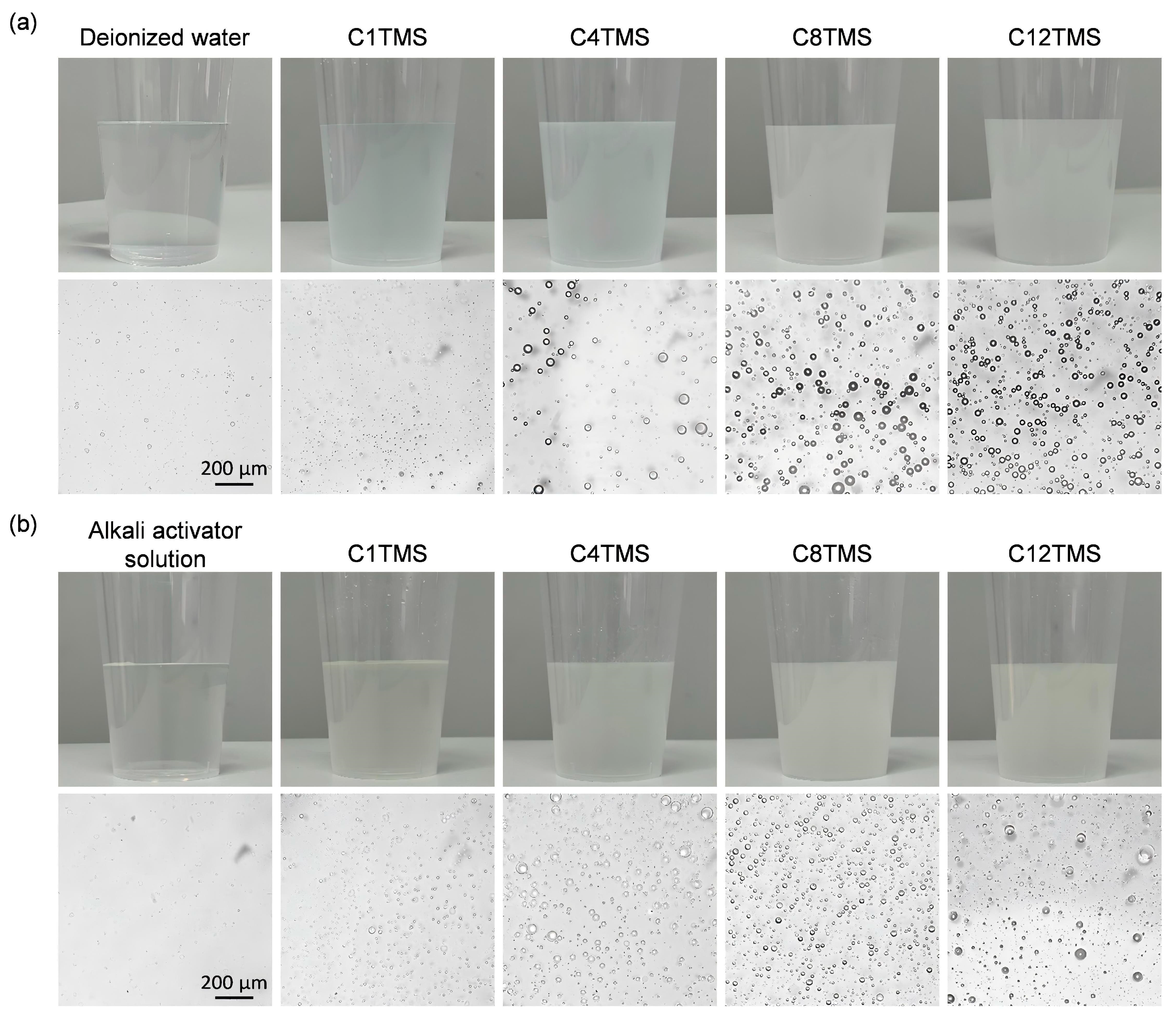
2.2. Sample Preparation
2.3. Testing Methods
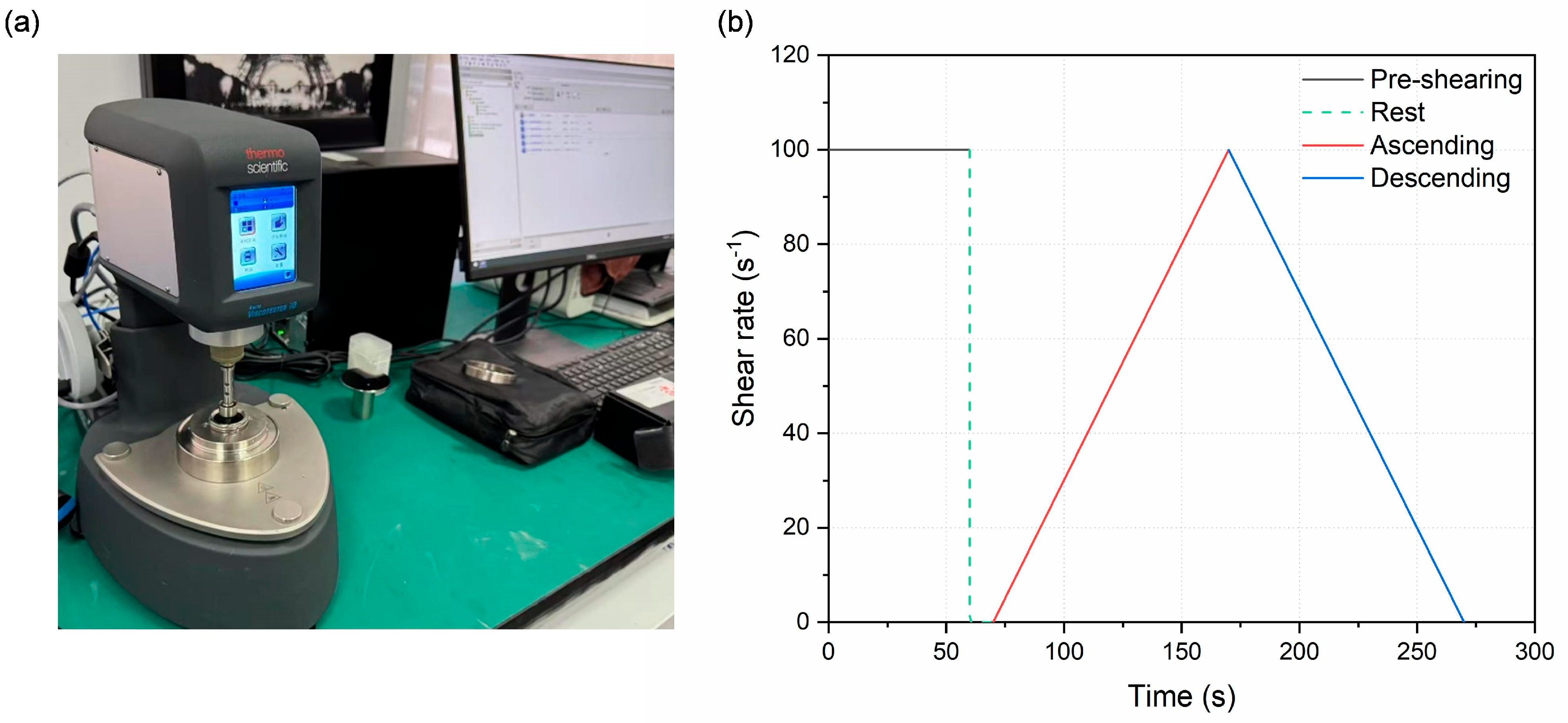
2.3.1. Rheological Test
2.3.2. Flowability Test
2.3.3. Setting Time Test
2.3.4. Reaction Kinetic Test
2.3.5. Compressive Strength Test
2.3.6. Water Contact Angle Test
2.3.7. Water Absorption Test
3. Results and Discussion
3.1. Rheology
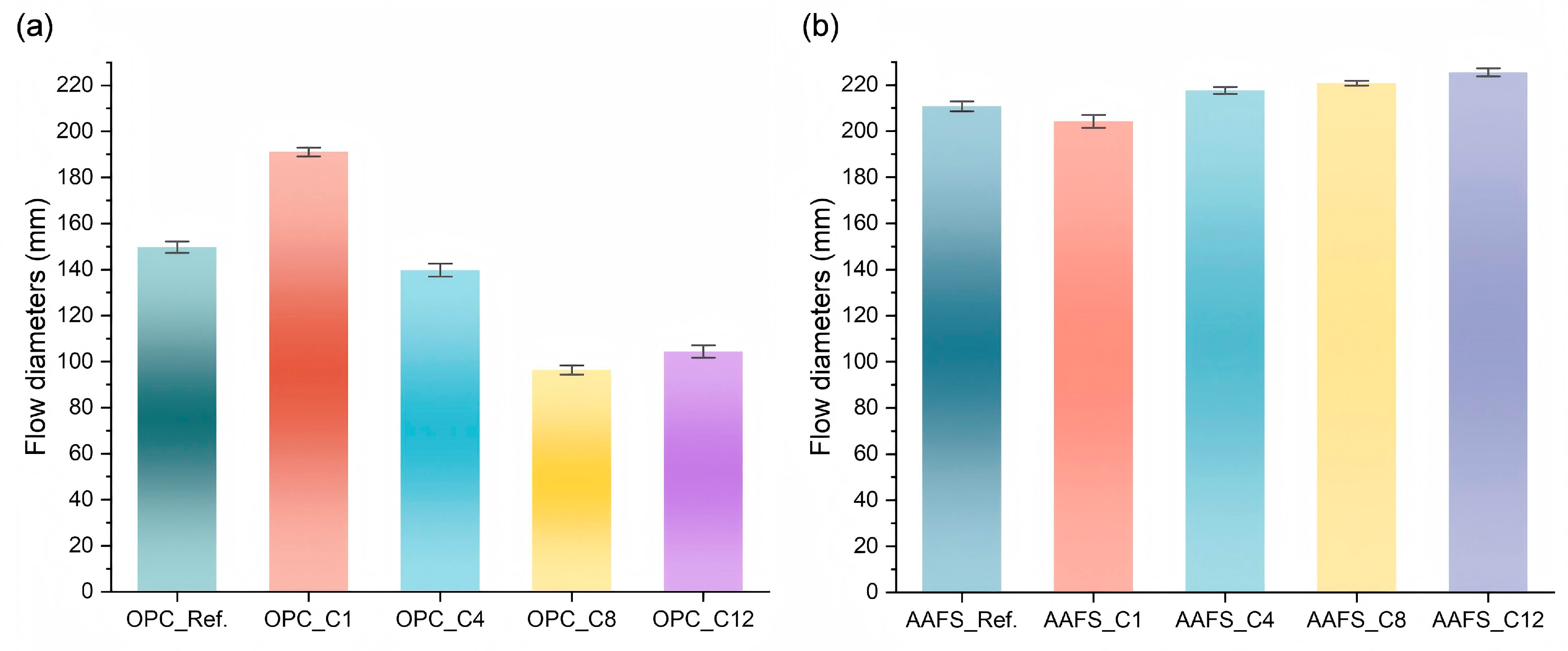
3.2. Flowability
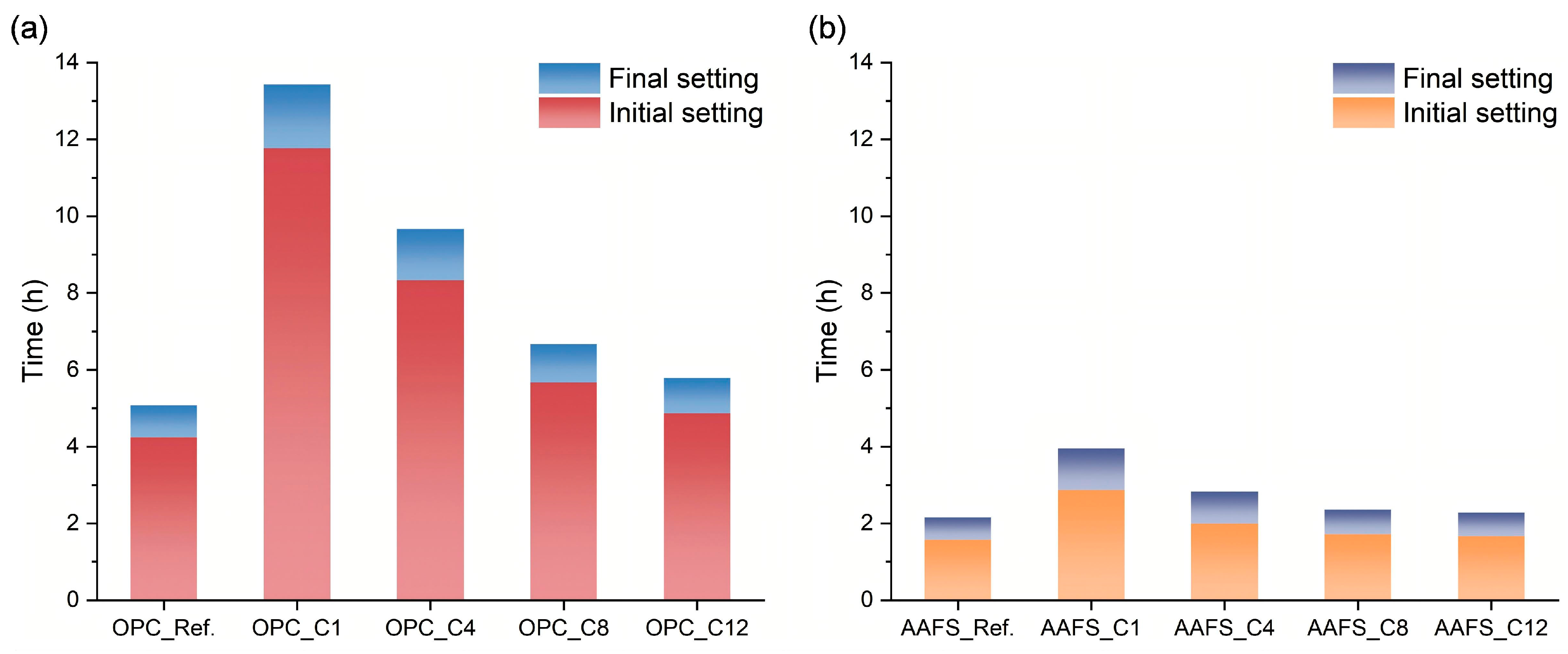
3.3. Setting Time
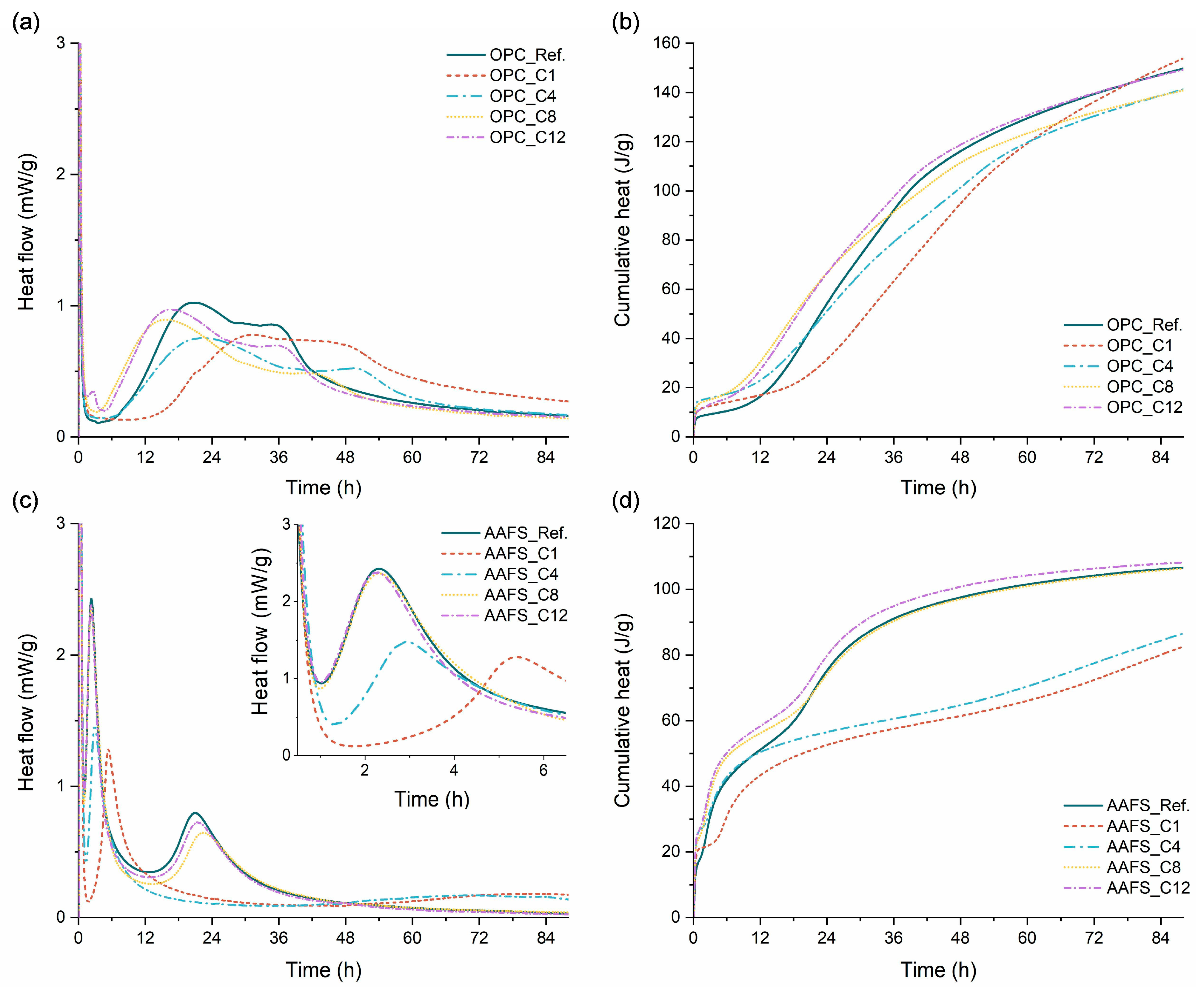
3.4. Reaction Kinetics
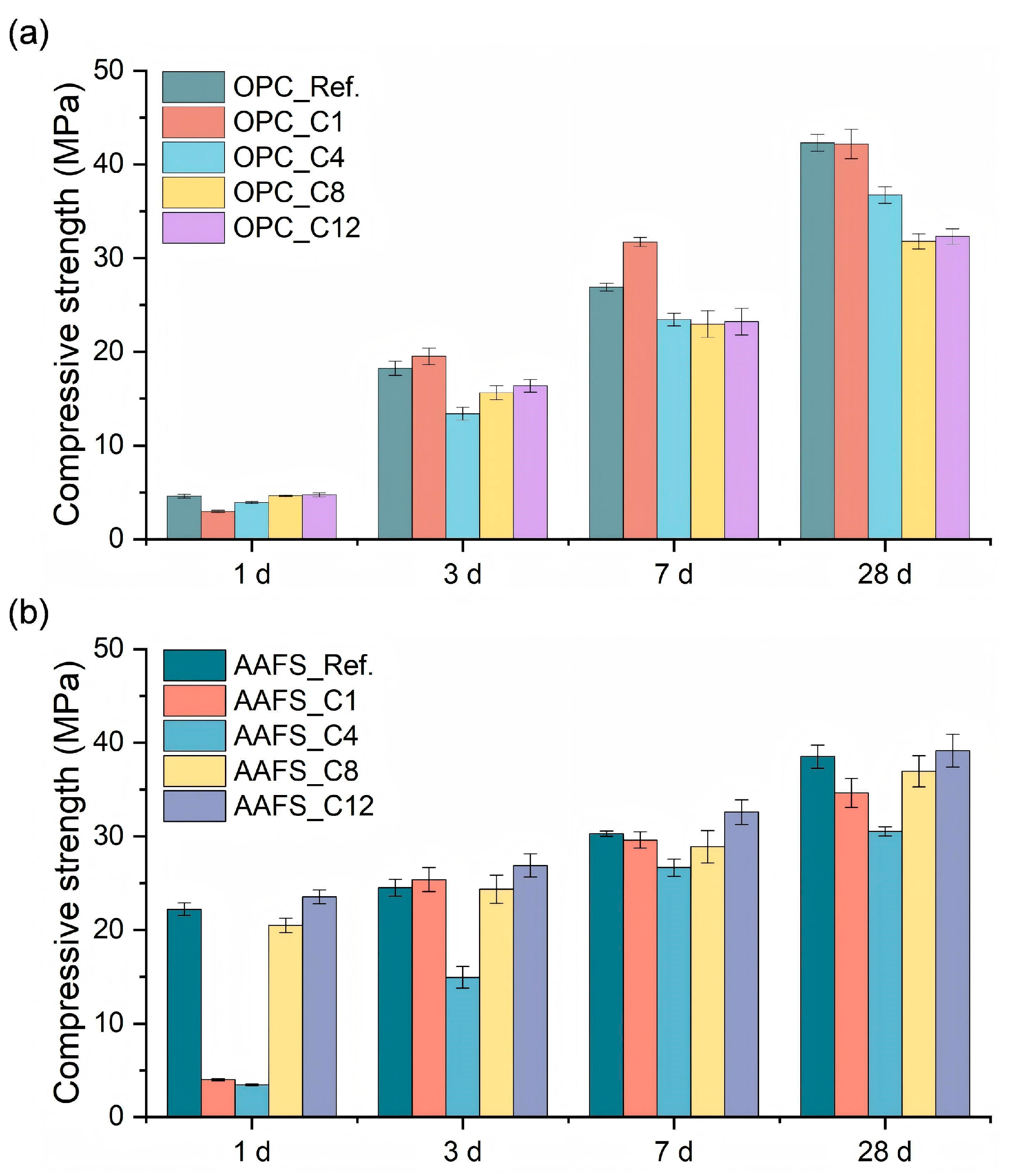
3.5. Compressive Strength
3.6. Matrix Wettability
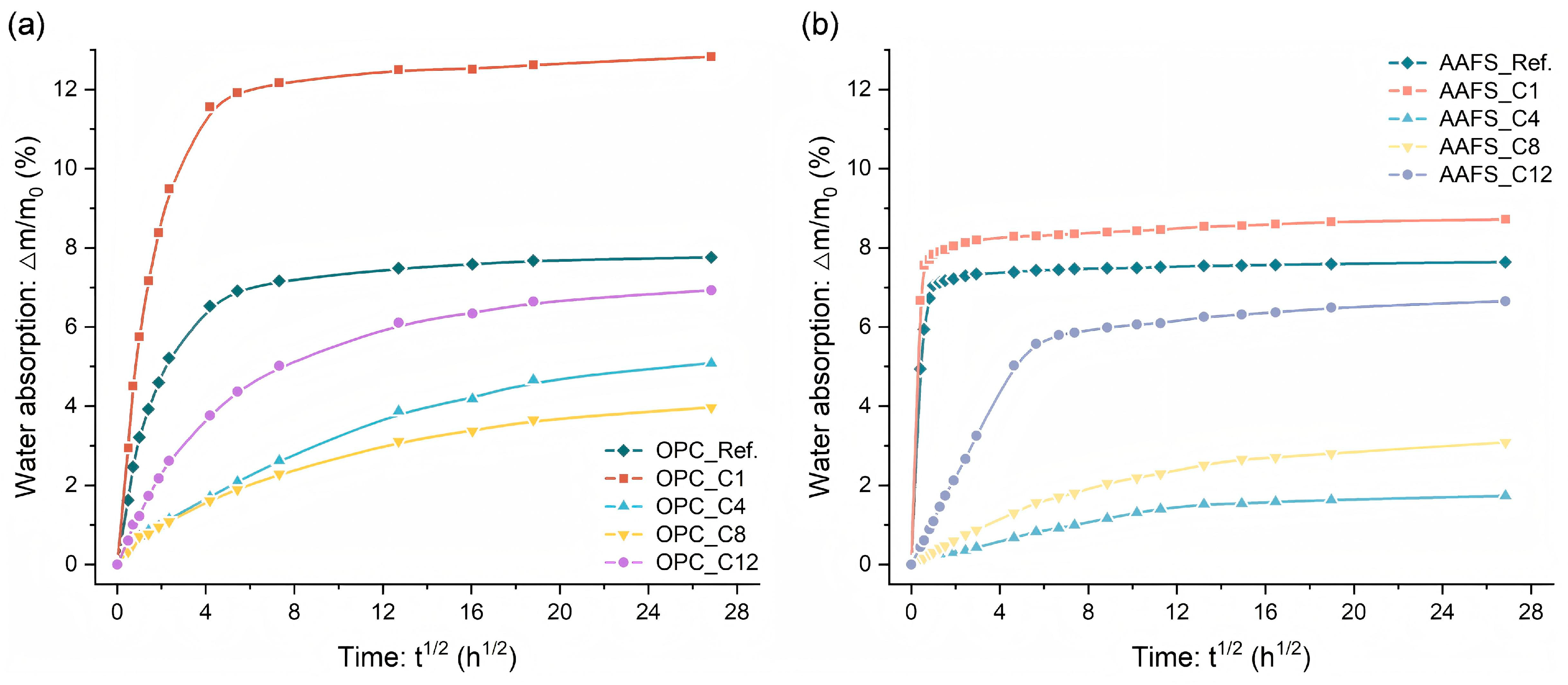
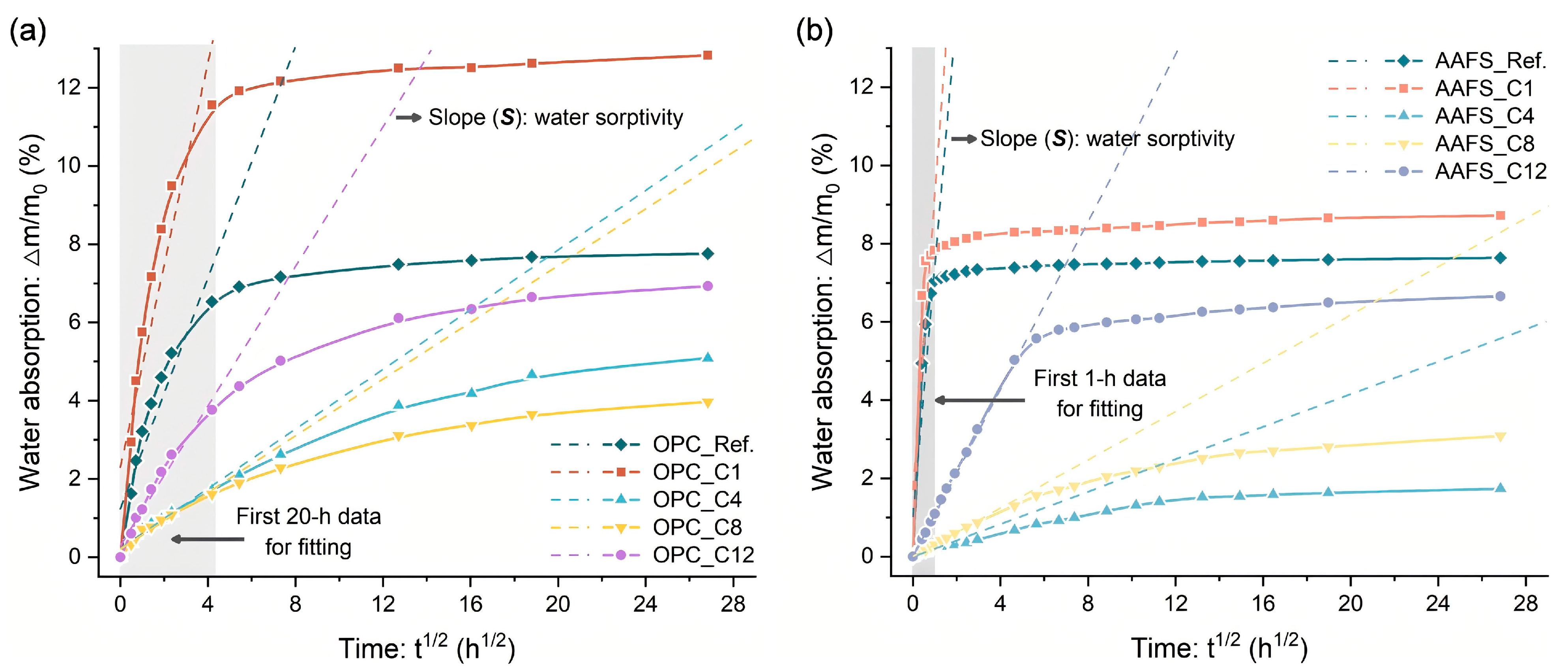
3.7. Water Absorption Behavior
4. Conclusions
- The effects of alkyl silanes on the paste rheology and flowability were affected by both alkyl chain length of silanes and binder systems. In OPC, C1TMS reduced the yield stress and plastic viscosity by 33.6% and 21.0%, respectively, and enhanced flowability by 27.6%. However, C4TMS, C8TMS, and C12TMS showed the opposite effect compared to C1TMS due to improved hydrophobicity, with 54.2–314.6% increase in yield stress and 11.0–70.0% in plastic viscosity. In contrast, the plastic viscosity of AAFS just changed within 5.2–13.1% due to alkyl silane incorporation. Additionally, although AAFS pastes showed higher plastic viscosity than OPC, its lower yield stress and the “ball-bearing” effect of FA particles contributed to superior flowability.
- All alkyl silanes delayed setting of OPC and AAFS pastes, with shorter chains causing stronger retardation. C1TMS and C4TMS postponed the main exothermic peaks of both binder systems and suppressed early gel formation but promoted reaction recovery at later stages. C8TMS and C12TMS had relatively minor effects on the setting time and the early heat release.
- Compressive strength development correlated well with early-age reaction kinetics. AAFS pastes showed higher 1-day strength than OPC. C1TMS and C4TMS suppressed early strength development, reducing the 1-day strength of OPC by 14.8–35.7% and AAFS by 82.0–84.5%. However, C1TMS-modified OPC and AAFS pastes showed accelerated strength development after 3 d.
- Hydrophobic performance was mainly governed by alkyl chain length and dispersion uniformity of silanes. In OPC, C8TMS showed the best hydrophobic modification effect, yielding a WCA of 145° and reducing water sorptivity by 75.7%. C4TMS and C12TMS also improved hydrophobicity. C1TMS, with limited hydrophobic methyl groups and abundant hydrophilic Si–OH, increased water sorptivity and decreased WCA. Similar trends were observed in AAFS pastes, with C4TMS showing the best hydrophobicity, followed by C8TMS and C12TMS.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Monteiro, P.J.; Miller, S.A.; Horvath, A. Towards Sustainable Concrete. Nat. Mater. 2017, 16, 698–699. [Google Scholar] [CrossRef]
- Habert, G.; Miller, S.A.; John, V.M.; Provis, J.L.; Favier, A.; Horvath, A.; Scrivener, K.L. Environmental Impacts and Decarbonization Strategies in the Cement and Concrete Industries. Nat. Rev. Earth Environ. 2020, 1, 559–573. [Google Scholar] [CrossRef]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in Understanding Alkali-Activated Materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Shi, C.; Qu, B.; Provis, J.L. Recent Progress in Low-Carbon Binders. Cem. Concr. Res. 2019, 122, 227–250. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-Activated Materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, A.; Bao, X.; Ni, T.; Ling, J. A Review on Geopolymer in Potential Coating Application: Materials, Preparation and Basic Properties. J. Build. Eng. 2020, 32, 101734. [Google Scholar] [CrossRef]
- Amran, Y.M.; Alyousef, R.; Alabduljabbar, H.; El-Zeadani, M. Clean Production and Properties of Geopolymer Concrete; A Review. J. Clean. Prod. 2020, 251, 119679. [Google Scholar] [CrossRef]
- Zhu, H.; Ren, J. Effects of Nano-SiO2 and Mixing Water on Early-Strength Mechanisms of High-Fluidity Slag and Desulfurization Gypsum Composite Alkali Activated Materials. Constr. Build. Mater. 2025, 492, 142959. [Google Scholar] [CrossRef]
- Luo, Y.; Li, S.; Klima, K.; Brouwers, H.; Yu, Q. Degradation Mechanism of Hybrid Fly Ash/Slag Based Geopolymers Exposed to Elevated Temperatures. Cem. Concr. Res. 2022, 151, 106649. [Google Scholar] [CrossRef]
- Hojati, M.; Radlińska, A. Shrinkage and Strength Development of Alkali-Activated Fly Ash-Slag Binary Cements. Constr. Build. Mater. 2017, 150, 808–816. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, K.; Li, Q.; Wang, J.; Ling, Y. Fabrication and Engineering Properties of Concretes Based on Geopolymers/Alkali-Activated Binders-A Review. J. Clean. Prod. 2020, 258, 120896. [Google Scholar] [CrossRef]
- Shen, C.; Zhu, Y.; Shi, W.; He, K.; Xiao, X.; Xu, X.; Shi, J.; Xu, G. Mechanically Stable Superhydrophobic Surface on Cement-Based Materials. Chem. Phys. 2020, 538, 110912. [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, H.; Wu, Q.; Yang, T.; Yin, Z.; Tian, L. Investigation of the Hydrophobicity and Microstructure of Fly Ash-Slag Geopolymer Modified by Polydimethylsiloxane. Constr. Build. Mater. 2023, 369, 130540. [Google Scholar] [CrossRef]
- Jiang, C.; Jiang, L.; Li, S.; Tang, X.; Zhang, L. Impact of Cation Type and Fly Ash on Deterioration Process of High Belite Cement Pastes Exposed to Sulfate Attack. Constr. Build. Mater. 2021, 286, 122961. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, G.; Qi, Y.; Zeng, Q. In-Situ Assessment of the Water-Penetration Resistance of Polymer Modified Cement Mortars by μ-XCT, SEM and EDS. Cem. Concr. Compos. 2020, 114, 103821. [Google Scholar] [CrossRef]
- Wen, J.; Wan, Y.; Xu, C.; Yang, Y. A Review of New Methods for Measuring Saturation of Concrete and Its Impact on Concrete Properties. J. Build. Eng. 2024, 96, 110664. [Google Scholar] [CrossRef]
- Yi, Y.; Zhu, D.; Guo, S.; Zhang, Z.; Shi, C. A Review on the Deterioration and Approaches to Enhance the Durability of Concrete in the Marine Environment. Cem. Concr. Compos. 2020, 113, 103695. [Google Scholar] [CrossRef]
- Li, Q.; Yang, K.; Yang, C. An Alternative Admixture to Reduce Sorptivity of Alkali-Activated Slag Cement by Optimising Pore Structure and Introducing Hydrophobic Film. Cem. Concr. Compos. 2019, 95, 183–192. [Google Scholar] [CrossRef]
- Liang, G.; Liu, T.; Li, H.; Wu, K. Shrinkage Mitigation, Strength Enhancement and Microstructure Improvement of Alkali-Activated Slag/Fly Ash Binders by Ultrafine Waste Concrete Powder. Compos. Part B Eng. 2022, 231, 109570. [Google Scholar] [CrossRef]
- Li, H.; Guo, X. Fabricating Hydrophobic Silica Fume to Improve Mechanical Strength and Anti-Corrosion of Integral Hydrophobic Cement Mortar and Its Carbon Emission Assessment. J. Clean. Prod. 2024, 439, 140857. [Google Scholar] [CrossRef]
- Sidhu, J.; Kumar, P. Development of Hydrophobicity in Geopolymer Composites-Progress and Perspectives. Constr. Build. Mater. 2023, 396, 132344. [Google Scholar] [CrossRef]
- Wu, B.; Qiu, J. Enhancing the Hydrophobic PP Fiber/Cement Matrix Interface by Coating Nano-AlOOH to the Fiber Surface in a Facile Method. Cem. Concr. Compos. 2022, 125, 104297. [Google Scholar] [CrossRef]
- Manabe, K.; Saikawa, M.; Sato, I.; Loo, C.S.; Takashima, K.; Norikane, Y. Biomimetic 3D-Printed Armored Structures for Durable Superhydrophobic Surfaces: Integrating Macroprotection and Nanofunctionality. ACS Appl. Polym. Mater. 2024, 6, 13701–13709. [Google Scholar] [CrossRef]
- Ruan, S.; Chen, S.; Liu, Y.; Yan, D.; Sun, Z. Investigation on the Effect of Fiber Wettability on Water Absorption Kinetics of Geopolymer Composites. Ceram. Int. 2022, 48, 36678–36689. [Google Scholar] [CrossRef]
- Sánchez, R.Z.; Gonzalez-Coneo, J.; Luna, M.; Diaz, A.; Mosquera, M.J. Studying the Bulk Hydrophobization of Cement Mortars by the Combination of Alkylalkoxysilane Admixture and Fluoropolymer-Functionalized Aggregate. J. Build. Eng. 2023, 65, 105771. [Google Scholar] [CrossRef]
- Wang, F.; Lei, S.; Ou, J.; Li, W. Effect of PDMS on the Waterproofing Performance and Corrosion Resistance of Cement Mortar. Appl. Surf. Sci. 2020, 507, 145016. [Google Scholar] [CrossRef]
- Muzenski, S.; Flores-Vivian, I.; Sobolev, K. Hydrophobic Engineered Cementitious Composites for Highway Applications. Cem. Concr. Compos. 2015, 57, 68–74. [Google Scholar] [CrossRef]
- Wen, J.; Wan, Y.; Yang, Y. Sustainable Synthesis of Hydrophobic Waste Glass Powder for Enhanced Water and Frost Resistance in Mortar. Constr. Build. Mater. 2025, 487, 142097. [Google Scholar] [CrossRef]
- Yao, H.; Xie, Z.; Huang, C.; Yuan, Q.; Yu, Z. Recent Progress of Hydrophobic Cement-Based Materials: Preparation, Characterization and Properties. Constr. Build. Mater. 2021, 299, 124255. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, Y.; Shen, Z.; Chen, H. Fabrication of Self-Healing Superhydrophobic Surfaces from Water-Soluble Polymer Suspensions Free of Inorganic Particles through Polymer Thermal Reconstruction. Coatings 2018, 8, 144. [Google Scholar] [CrossRef]
- Gao, R.; Mao, J.; Ruan, S.; Qiu, Y.; Ye, S.; Chen, S.; Liu, Y.; Yan, D. A Comparative Study of Metakaolin-Based Geopolymers with Immobilised Organic Liquids: Vegetable Oil, Mineral Oil, and Organosilicon Liquids. J. Build. Eng. 2025, 108, 112860. [Google Scholar] [CrossRef]
- Feng, B.; Liu, J.; Chen, Y.; Tan, X.; Zhang, M.; Sun, Z. Properties and Microstructure of Self-Waterproof Metakaolin Geopolymer with Silane Coupling Agents. Constr. Build. Mater. 2022, 342, 128045. [Google Scholar] [CrossRef]
- John, S.K.; Nadir, Y.; Cascardi, A.; Arif, M.M.; Girija, K. Effect of Addition of Nanoclay and SBR Latex on Fly Ash-Slag Geopolymer Mortar. J. Build. Eng. 2023, 66, 105875. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, M.; Feng, Y. A Review on Nanomaterials and Polymers Modified Cementitious Materials with High Performances. J. Build. Eng. 2025, 104, 112331. [Google Scholar] [CrossRef]
- Di Mundo, R.; Labianca, C.; Carbone, G.; Notarnicola, M. Recent Advances in Hydrophobic and Icephobic Surface Treatments of Concrete. Coatings 2020, 10, 449. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, X.; Chen, S.; Lin, H.; Li, Z.; Lin, X. Hydrophobic or Superhydrophobic Modification of Cement-Based Materials: A Systematic Review. Compos. Part B Eng. 2022, 243, 110104. [Google Scholar] [CrossRef]
- Sandrolini, F.; Franzoni, E.; Pigino, B. Ethyl Silicate for Surface Treatment of Concrete–Part I: Pozzolanic Effect of Ethyl Silicate. Cem. Concr. Compos. 2012, 34, 306–312. [Google Scholar] [CrossRef]
- Ruan, S.; Gao, R.; Tu, W.; Li, G.; Lu, J.-X.; Yan, D.; Poon, C.S. Hydration Products and Hybridisation Mechanisms of Hydrophobic Cement Pastes with Alkyl-Organosilanes. Cem. Concr. Compos. 2025, 163, 106208. [Google Scholar] [CrossRef]
- Pape, P.G. Silane Coupling Agents: Enhancements of Physical Properties of Plastics. Eng. Plast. 1996, 9, 109–115. [Google Scholar]
- Otte, M.; Schweinitz, A.; Bonus, M.; Enke, U.; Schumann, C.; Gohlke, H.; Benndorf, K. Hydrophobic Alkyl Chains Substituted to the 8-Position of Cyclic Nucleotides Enhance Activation of CNG and HCN Channels by an Intricate Enthalpy-Entropy Compensation. Sci. Rep. 2018, 8, 14960. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, S.; Yu, J.; Kong, X. Water Absorption Behavior of Hydrophobized Concrete Using Silane Emulsion as Admixture. Cem. Concr. Res. 2022, 154, 106738. [Google Scholar] [CrossRef]
- She, W.; Zheng, Z.; Zhang, Q.; Zuo, W.; Yang, J.; Zhang, Y.; Zheng, L.; Hong, J.; Miao, C. Predesigning Matrix-Directed Super-Hydrophobization and Hierarchical Strengthening of Cement Foam. Cem. Concr. Res. 2020, 131, 106029. [Google Scholar] [CrossRef]
- Wang, D.; Wu, X.; Yuan, L.; Wu, D.; Zhao, Q.; Pan, H.; Qi, W. Oil Absorption and Plant Symbiosis Capacity of Hydrophobic Modified Concrete: Preparation and Performance Analysis. Constr. Build. Mater. 2024, 413, 134897. [Google Scholar] [CrossRef]
- Li, F.; Liu, L.; Liu, K.; Zheng, A.; Liu, J. Investigation on Waterproof Mechanism and Micro-Structure of Cement Mortar Incorporated with Silicane. Constr. Build. Mater. 2020, 239, 117865. [Google Scholar] [CrossRef]
- She, Y.; Chen, Y.; Li, L.; Xue, L.; Yu, Q. Understanding the Generation and Evolution of Hydrophobicity of Silane Modified Fly Ash/Slag Based Geopolymers. Cem. Concr. Compos. 2023, 142, 105206. [Google Scholar] [CrossRef]
- Yang, J.; Zuo, W.; She, W. Towards a Further Understanding of Cement Hydration at the Early-Age Stage in the Presence of Hydrophobic Silane IBTEO. Cem. Concr. Compos. 2024, 153, 105712. [Google Scholar] [CrossRef]
- Koohestani, B. Effect of Saline Admixtures on Mechanical and Microstructural Properties of Cementitious Matrices Containing Tailings. Constr. Build. Mater. 2017, 156, 1019–1027. [Google Scholar] [CrossRef]
- Chen, B.; Shao, H.; Li, B.; Li, Z. Influence of Silane on Hydration Characteristics and Mechanical Properties of Cement Paste. Cem. Concr. Compos. 2020, 113, 103743. [Google Scholar] [CrossRef]
- Xie, M.; Zhong, Y.; Li, Z.; Lei, F.; Jiang, Z. Study on Alkylsilane-Incorporated Cement Composites: Hydration Mechanism and Mechanical Properties Effects. Cem. Concr. Compos. 2021, 122, 104161. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.; Rhee, K. Hydrophilicity and Hydrophobicity Consideration of Organic Surfactant Compounds: Effect of Alkyl Chain Length on Corrosion Protection. Adv. Colloid Interface Sci. 2022, 306, 102723. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, Y.; Shu, X.; Dong, L.; Qiao, M.; Ran, Q. Micelle Conformation of Sodium Alkyl Sulfate Surfactants with Different Hydrophobic Chain Length: A Molecular Dynamics Study. Comput. Mater. Sci. 2023, 229, 112452. [Google Scholar] [CrossRef]
- Ruan, S.; Gao, R.; Tu, W.; Yan, D.; Zhang, M. Alkali-Activated Materials with Organics: A Critical Review. Compos. Part B Eng. 2024, 284, 111712. [Google Scholar] [CrossRef]
- Dong, D.; Huang, Y.; Pei, Y.; Zhang, X.; Cui, N.; Zhao, P.; Hou, P.; Lu, L. Effect of Spherical Silica Fume and Fly Ash on the Rheological Property, Fluidity, Setting Time, Compressive Strength, Water Resistance and Drying Shrinkage of Magnesium Ammonium Phosphate Cement. J. Build. Eng. 2023, 63, 105484. [Google Scholar] [CrossRef]
- Dai, X.; Aydin, S.; Yardimci, M.Y.; Qiang, R.; Lesage, K.; De Schutter, G. Rheology, Early-Age Hydration and Microstructure of Alkali-Activated GGBFS-Fly Ash-Limestone Mixtures. Cem. Concr. Compos. 2021, 124, 104244. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, C.; Hu, X.; Shi, C. Effect of Silica Fume on Rheology of Slag-Fly Ash-Silica Fume-Based Geopolymer Pastes with Different Activators. Cem. Concr. Res. 2023, 174, 107336. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, S.; Rahul, A.; Tao, Y.; Van Bockstaele, F.; Dewettinck, K.; Ye, G.; De Schutter, G. Rheology of Alkali-Activated Slag Pastes: New Insight from Microstructural Investigations by Cryo-SEM. Cem. Concr. Res. 2022, 157, 106806. [Google Scholar] [CrossRef]
- GB/T 8077 2023; Methods for Testing Uniformity of Concrete Admixtures. Standardization Administration of the People’s Republic of China: Beijing, China, 2023.
- Duan, K.; Wang, J.; Liu, Z.; Li, X.; Zhang, J.; Wang, X.; Wang, D. Flowability and In-Situ Phase Evolution of Na2CO3-Carbide Slag-Activated Blast Furnace Slag and Fly Ash. Constr. Build. Mater. 2025, 466, 140341. [Google Scholar] [CrossRef]
- GB/T 1346 2024; Test Methods for Water Requirement of Normal Consistency, Setting Time and Soundness of the Port-land Cement. Standardization Administration of the People’s Republic of China: Beijing, China, 2024.
- GB/T 17671-2021; Method of Testing Cements—Determination of Strength (ISO Method). Standardization Administration of the People’s Republic of China: Beijing, China, 2021.
- Hisakado, T. Effect of Surface Roughness on Contact between Solid Surfaces. Wear 1974, 28, 217–234. [Google Scholar] [CrossRef]
- ASTM C642-13; Standard Test Method for Density, Absorption, and Voids in Hardened Concrete. ASTM International: West Conshohocken, PA, USA, 2013.
- Hall, C. Capillary Imbibition in Cement-Based Materials with Time-Dependent Permeability. Cem. Concr. Res. 2019, 124, 105835. [Google Scholar] [CrossRef]
- Yang, T.; Zhu, H.; Zhang, Z.; Gao, X.; Zhang, C.; Wu, Q. Effect of Fly Ash Microsphere on the Rheology and Microstructure of Alkali-Activated Fly Ash/Slag Pastes. Cem. Concr. Res. 2018, 109, 198–207. [Google Scholar] [CrossRef]
- Alnahhal, M.F.; Kim, T.; Hajimohammadi, A. Distinctive Rheological and Temporal Viscoelastic Behaviour of Alkali-Activated Fly Ash/Slag Pastes: A Comparative Study with Cement Paste. Cem. Concr. Res. 2021, 144, 106441. [Google Scholar] [CrossRef]
- Li, L.; Wei, Y.; Li, Z.; Farooqi, M.U. Rheological and Viscoelastic Characterizations of Fly Ash/Slag/Silica Fume-Based Geopolymer. J. Clean. Prod. 2022, 354, 131629. [Google Scholar] [CrossRef]
- Roussel, N.; Lemaître, A.; Flatt, R.J.; Coussot, P. Steady State Flow of Cement Suspensions: A Micromechanical State of the Art. Cem. Concr. Res. 2010, 40, 77–84. [Google Scholar] [CrossRef]
- Favier, A.; Hot, J.; Habert, G.; de Lacaillerie, J.; Roussel, N. Rheology of Geopolymer: Comparative Study Between Portland Cement and Metakaolin Based Geopolymer. In 1st RILEM International Conference on Rheology and Processing of Construction Materials; RILEM Publications Sarl: Paris, France, 2013; pp. 49–56. [Google Scholar]
- Chen, B.; Wang, M.; Manzano, H.; Zhao, Y.; Li, Y. Molecular Elucidation of Cement Hydration Inhibition by Silane Coupling Agents. Nat. Commun. 2025, 16, 1597. [Google Scholar] [CrossRef]
- Deb, P.S.; Nath, P.; Sarker, P.K. The Effects of Ground Granulated Blast-Furnace Slag Blending with Fly Ash and Activator Content on the Workability and Strength Properties of Geopolymer Concrete Cured at Ambient Temperature. Mater. Des. 2014, 62, 32–39. [Google Scholar] [CrossRef]
- Pan, Z.; Tan, M.; Zheng, G.; Wei, L.; Tao, Z.; Hao, Y. Effect of Silica Fume Type on Rheology and Compressive Strength of Geopolymer Mortar. Constr. Build. Mater. 2024, 430, 136488. [Google Scholar] [CrossRef]
- Lu, Z.; Kong, X.; Jansen, D.; Zhang, C.; Wang, J.; Pang, X.; Yin, J. Towards a Further Understanding of Cement Hydration in the Presence of Triethanolamine. Cem. Concr. Res. 2020, 132, 106041. [Google Scholar] [CrossRef]
- Peng, L.; Jiang, Y.; Ban, J.; Shen, Y.; Ma, Z.; Zhao, Y.; Shen, P.; Poon, C.-S. Mechanism Underlying Early Hydration Kinetics of Carbonated Recycled Concrete Fines-Ordinary Portland Cement (CRCF-OPC) Paste. Cem. Concr. Compos. 2023, 144, 105275. [Google Scholar] [CrossRef]
- Saeed, S.; Al Soubaihi, R.M.; White, L.S.; Bertino, M.F.; Saoud, K.M. Rapid Fabrication of Cross-Linked Silica Aerogel by Laser Induced Gelation. Microporous Mesoporous Mater. 2016, 221, 245–252. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.; Lazaro, A.; Brouwers, H. Investigation on a Green Olivine Nano-Silica Source Based Activator in Alkali Activated Slag-Fly Ash Blends: Reaction Kinetics, Gel Structure and Carbon Footprint. Cem. Concr. Res. 2017, 100, 129–139. [Google Scholar] [CrossRef]
- Brough, A.; Atkinson, A. Sodium Silicate-Based, Alkali-Activated Slag Mortars: Part I. Strength, Hydration and Microstructure. Cem. Concr. Res. 2002, 32, 865–879. [Google Scholar] [CrossRef]
- Zuo, Y.; Nedeljković, M.; Ye, G. Coupled Thermodynamic Modelling and Experimental Study of Sodium Hydroxide Activated Slag. Constr. Build. Mater. 2018, 188, 262–279. [Google Scholar] [CrossRef]
- Barberena-Fernández, A.; Carmona-Quiroga, P.; Blanco-Varela, M.T. Interaction of TEOS with Cementitious Materials: Chemical and Physical Effects. Cem. Concr. Compos. 2015, 55, 145–152. [Google Scholar] [CrossRef]
- Li, F.; Chen, Q.; Lu, Y.; Zou, Y.; Li, S. Dry Shrinkage and Micro-Structure of Alkali-Activated Fly Ash/Slag Pastes Incorporated with Silane Coupling Agent Modified MWCNTs. Cem. Concr. Res. 2024, 175, 107382. [Google Scholar] [CrossRef]
- Kubissa, W.; Jaskulski, R. Measuring and Time Variability of the Sorptivity of Concrete. Procedia Eng. 2013, 57, 634–641. [Google Scholar] [CrossRef]
- Ruan, S.; Yan, D.; Chen, S.; Jiang, F.; Shi, W. Process and Mechanisms of Multi-Stage Water Sorptivity in Hydrophobic Geopolymers Incorporating Polydimethylsiloxane. Cem. Concr. Compos. 2022, 128, 104460. [Google Scholar] [CrossRef]
- Qu, Z.; Yu, Q.; Ji, Y.; Gauvin, F.; Voets, I.K. Mitigating Shrinkage of Alkali Activated Slag with Biofilm. Cem. Concr. Res. 2020, 138, 106234. [Google Scholar] [CrossRef]
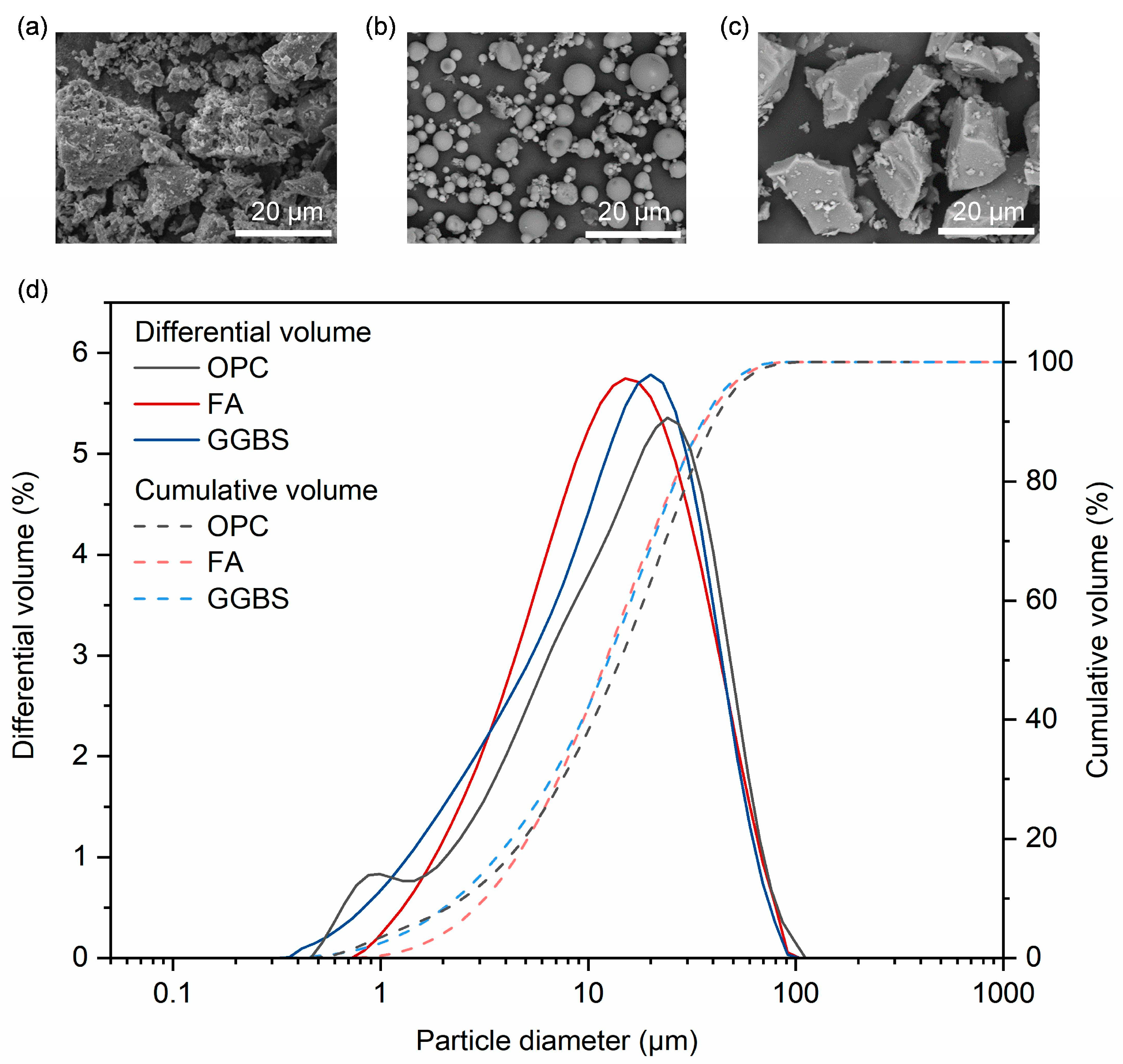


| Oxide | SiO2 | Al2O3 | CaO | Fe2O3 | TiO2 | Na2O | K2O | MgO | P2O5 | SO3 | MnO |
|---|---|---|---|---|---|---|---|---|---|---|---|
| OPC | 22.27 | 6.34 | 56.08 | 4.51 | 0.59 | 0.57 | 1.10 | 3.91 | 0.18 | 4.11 | 0.22 |
| FA | 51.21 | 28.28 | 4.23 | 5.38 | 1.07 | 0.53 | 1.28 | 0.82 | 0.19 | 0.47 | 0.05 |
| GGBS | 28.12 | 14.28 | 44.8 | 0.35 | 0.74 | 0.29 | 0.29 | 7.58 | 0.02 | 1.65 | 0.14 |
| Alkyl Silane | Abbreviation | Molecular Formula | Molecular Structure |
|---|---|---|---|
| Methyl-trimethoxysilane | C1TMS | C4H12O3Si | 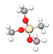 |
| Butyl-trimethoxysilane | C4TMS | C7H18O3Si | 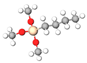 |
| Octyl-trimethoxysilane | C8TMS | C11H26O3Si | 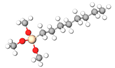 |
| Dodecyl-trimethoxysilane | C12TMS | C15H34O3Si |  |
| Specimen Designation | OPC | FA | GGBS | Water | SS Solution | SH | Alkyl Silanes |
|---|---|---|---|---|---|---|---|
| OPC_Ref. | 100 | - | - | 45 | - | - | 0 |
| OPC_Cn | 100 | - | - | 45 | - | - | 1.45 |
| AAFS_Ref. | - | 50 | 50 | 21.3 | 36.5 | 2.6 | 0 |
| AAFS_Cn | - | 50 | 50 | 21.3 | 36.5 | 2.6 | 1.60 |
| Mixture | Yield Stress | Plastic Viscosity | Mixture | Yield Stress | Plastic Viscosity |
|---|---|---|---|---|---|
| τ0 (Pa) | μp (Pa·s) | τ0 (Pa) | μp (Pa·s) | ||
| OPC_Ref. | 11.60 | 1.00 | AAFS_Ref. | 6.25 | 2.91 |
| OPC_C1 | 7.70 | 0.79 | AAFS_C1 | 6.68 | 3.09 |
| OPC_C4 | 17.89 | 1.11 | AAFS_C4 | 5.61 | 2.76 |
| OPC_C8 | 48.09 | 1.70 | AAFS_C8 | 6.08 | 2.62 |
| OPC_C12 | 37.93 | 1.41 | AAFS_C12 | 5.74 | 2.53 |
| Mixture | Water Sorptivity | Mixture | Water Sorptivity |
|---|---|---|---|
| OPC_Ref. | 1.48 | AAFS_Ref. | 6.99 |
| OPC_C1 | 2.63 | AAFS_C1 | 7.59 |
| OPC_C4 | 0.38 | AAFS_C4 | 0.21 |
| OPC_C8 | 0.36 | AAFS_C8 | 0.30 |
| OPC_C12 | 0.90 | AAFS_C12 | 1.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, R.; Mao, J.; Ruan, S.; Tu, W.; Wang, Y.; Yan, D. Early-Age Properties and Reaction of Hydrophobic Portland Cement and Alkali-Activated Fly Ash–Slag Pastes with Alkyl Silanes. Buildings 2025, 15, 2966. https://doi.org/10.3390/buildings15162966
Gao R, Mao J, Ruan S, Tu W, Wang Y, Yan D. Early-Age Properties and Reaction of Hydrophobic Portland Cement and Alkali-Activated Fly Ash–Slag Pastes with Alkyl Silanes. Buildings. 2025; 15(16):2966. https://doi.org/10.3390/buildings15162966
Chicago/Turabian StyleGao, Rongfeng, Jiaxi Mao, Shengqian Ruan, Wenlin Tu, Yansong Wang, and Dongming Yan. 2025. "Early-Age Properties and Reaction of Hydrophobic Portland Cement and Alkali-Activated Fly Ash–Slag Pastes with Alkyl Silanes" Buildings 15, no. 16: 2966. https://doi.org/10.3390/buildings15162966
APA StyleGao, R., Mao, J., Ruan, S., Tu, W., Wang, Y., & Yan, D. (2025). Early-Age Properties and Reaction of Hydrophobic Portland Cement and Alkali-Activated Fly Ash–Slag Pastes with Alkyl Silanes. Buildings, 15(16), 2966. https://doi.org/10.3390/buildings15162966







