Temperature-Driven Degradation Mechanisms of Steel–Concrete Interfaces in NaCl Solution Environments: Nanoscale Insights from Molecular Dynamics Simulations
Abstract
1. Introduction
2. Simulation Method
2.1. Model Construction
2.2. Force Field and Molecular Dynamics Algorithm
3. Results and Discussions
3.1. Adsorption Properties
3.2. Local Structural Properties
3.2.1. NaCl Solution
3.2.2. Interfacial Solution and the Matrix
3.3. Properties of Surface Calcium Ions
3.3.1. Surface Calcium Ions and the Matrix
3.3.2. Surface Calcium (Inter), Interlayer Calcium (Intra) in C-S-H Gel
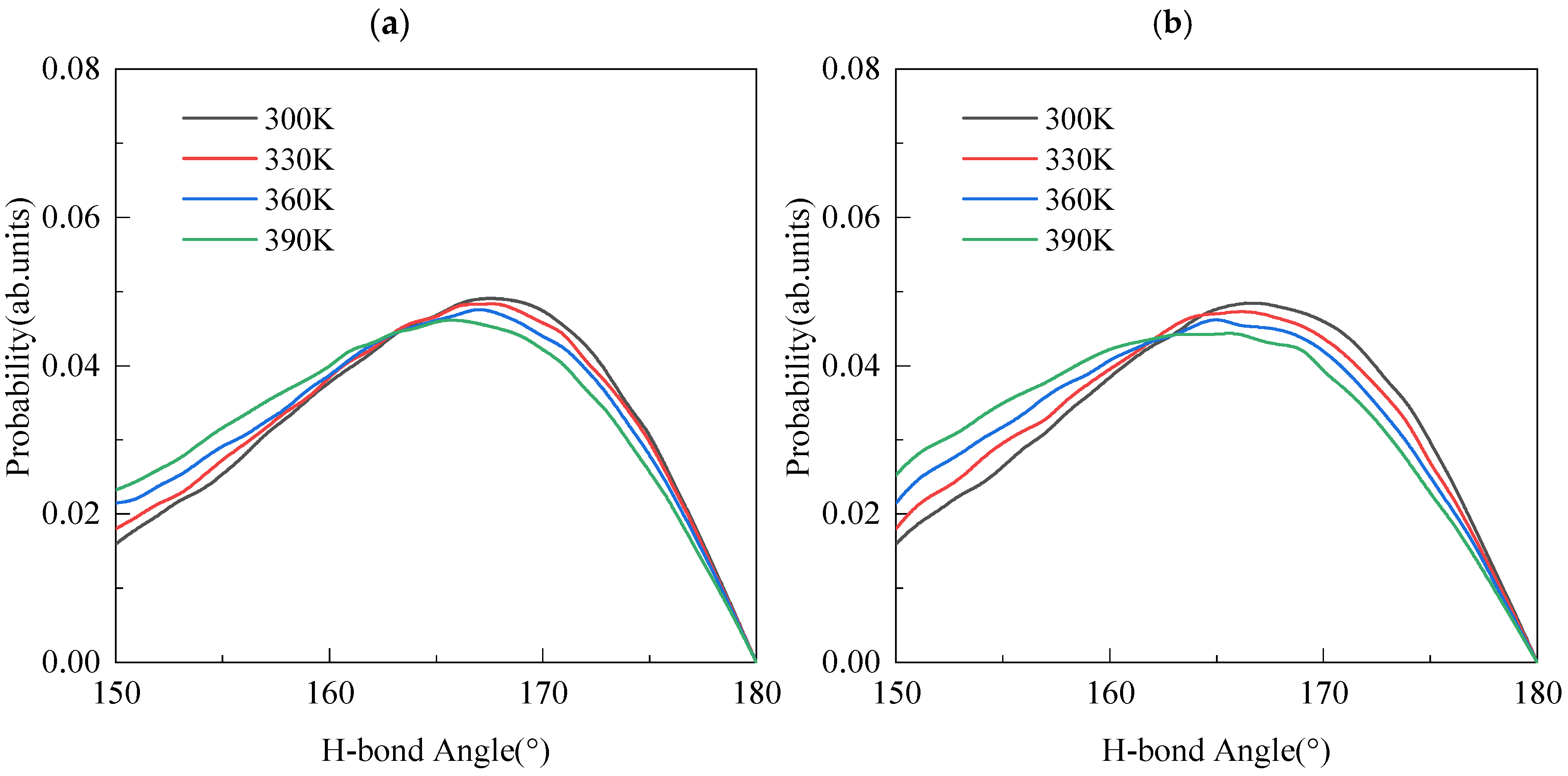
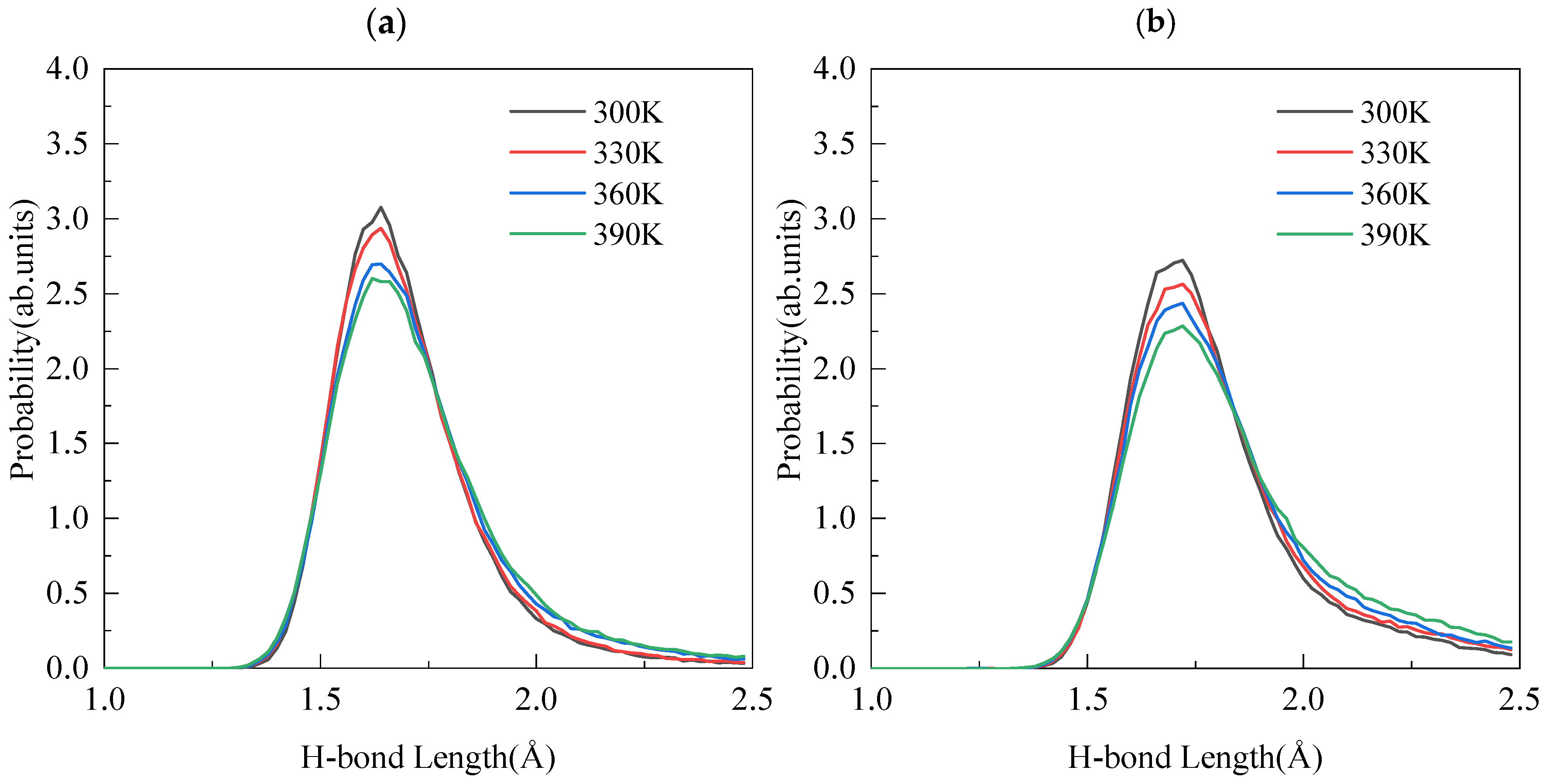
3.4. Hydrogen Bond Properties
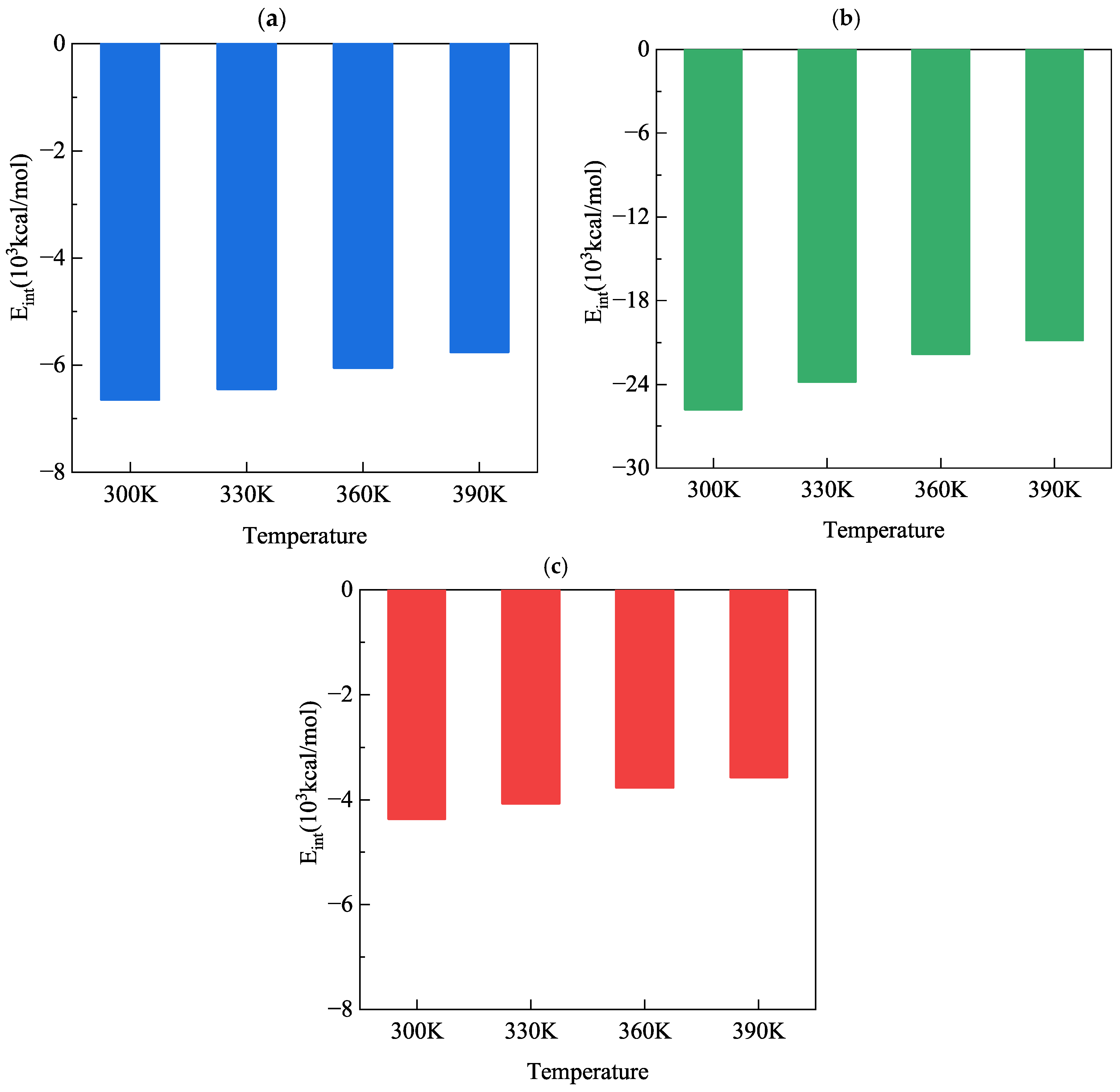
3.5. Interfacial Adsorption Energy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Du, F.Y.; Jin, Z.Q.; She, W.; Xiong, C.S.; Feng, G.Y.; Fan, J.F. Chloride ions migration and induced reinforcement corrosion in concrete with cracks: A comparative study of current acceleration and natural marine exposure. Constr. Build. Mater. 2020, 263, 120099. [Google Scholar] [CrossRef]
- Pour-Ghaz, M.; Isgor, O.B.; Ghods, P. The effect of temperature on the corrosion of steel in concrete. Part 1: Simulated polarization resistance tests and model development. Corros. Sci. 2009, 51, 415–425. [Google Scholar] [CrossRef]
- Nasir, A.; Butt, F.; Ahmad, F. Enhanced mechanical and axial resilience of recycled plastic aggregate concrete reinforced with silica fume and fibers. Innov. Infrastruct. Solut. 2025, 10, 4. [Google Scholar] [CrossRef]
- Ji, Y.S.; Zhan, G.M.; Tan, Z.C.; Hu, Y.J.; Gao, F.R. Process control of reinforcement corrosion in concrete. Part 1: Effect of corrosion products. Constr. Build. Mater. 2015, 79, 214–222. [Google Scholar] [CrossRef]
- Ji, Y.S.; Wu, M.; Tan, Z.C.; Gao, F.R.; Liu, F. Process control of reinforcement corrosion in concrete. Part 2: Time-dependent dominating factors under different environmental conditions. Constr. Build. Mater. 2014, 73, 214–221. [Google Scholar] [CrossRef]
- Ogunsanya, I.G.; Hansson, C.M. Influence of chloride and sulphate anions on the electronic and electrochemical properties of passive films formed on steel reinforcing bars. Materialia 2019, 8, 100491. [Google Scholar] [CrossRef]
- Wu, J.; Sang, W.; Li, D.; Jin, L. Molecular dynamics simulation of temperature effects on sodium chloride solution adsorption in γ-FeOOH nanopores. Constr. Build. Mater. 2024, 449, 138410. [Google Scholar] [CrossRef]
- Ahmad, F.; Rawat, S.; Yang, R.C.; Zhang, L.H.; Zhang, Y. Fire resistance and thermal performance of hybrid fibre-reinforced magnesium oxychloride cement-based composites. Constr. Build. Mater. 2025, 472, 140867. [Google Scholar] [CrossRef]
- Jin, L.; Yang, J.; Wu, J.; Du, X. Probabilistic prediction model of fatigue life of RC structures considering the meso-scale inhomogeneity of concrete. Mater. Rep. 2024, 38, 23090009. (In Chinese) [Google Scholar]
- Alizade, E.; Jandaghi, A.F.; Zabihi, S. Effect of steel fiber corrosion on mechanical properties of steel fiber reinforced concrete. Asian J. Civ. Eng. 2016, 17, 147–158. [Google Scholar]
- Yang, D.; Ren, X.; Gao, Y.; Fan, T.; Li, M.; Lv, H. Study on the Basic Mechanical Properties of Waste Steel Fiber Reinforced Concrete After High-Temperature Exposure. Buildings 2025, 15, 1025. [Google Scholar] [CrossRef]
- Ismail, R.; Zakwan, F.A.A.; Petrus, C.; Marzuki, N.A.; Hashim, N.H.; Mustafa, M.F. Compressive Behavior of Steel Fiber Reinforced Concrete After Exposed to High Temperatures; Springer: Singapore, 2014; pp. 731–740. [Google Scholar]
- Yang, J.Y.; Shen, A.Q.; Lyu, Z.H.; Wang, L.S.; Wu, H.; Tian, F. Chloride permeability and deterioration mechanism of pavement concrete under load-temperature coupling. Arab. J. Sci. Eng. 2023, 48, 4227–4243. [Google Scholar] [CrossRef]
- Zhou, Y.; Hou, D.S.; Jiang, J.Y.; Liu, L.; She, W.; Yu, J. Experimental and molecular dynamics studies on the transport and adsorption of chloride ions in the nano-pores of calcium silicate phase: The influence of calcium to silicate ratios. Microporous Mesoporous Mater. 2018, 255, 23–35. [Google Scholar] [CrossRef]
- Kumar Mehta, P.; Monteiro, P.J.M. Concrete: Microstructure, Properties, and Materials; McGraw-Hill Professional: New York, NY, USA, 2006; p. 28. [Google Scholar]
- Vayer, M.; Reynaud, I.; Erre, R. XPS characterisations of passive films formed on martensitic stainless steel: Qualitative and quantitative investigations. J. Mater. Sci. 2000, 35, 2581–2587. [Google Scholar] [CrossRef]
- Deng, H.Y.; He, Z. Interactions of sodium chloride solution and calcium silicate hydrate with different calcium to silicon ratios: A molecular dynamics study. Constr. Build. Mater. 2021, 268, 121067. [Google Scholar] [CrossRef]
- Wang, F.J.; Zhang, Y.; Jiang, J.Y.; Yin, B.; Li, Z.J. Effect of temperature on the capillary transport of sodium sulfate solution in calcium silicate hydrate nanopore: A molecular dynamics study. Constr. Build. Mater. 2020, 231, 117111. [Google Scholar] [CrossRef]
- Fei, X.P.; Guo, L.P.; Wu, J.D.; Lyu, B.C.; Chu, Y.J.; Shen, X.Y. Impact of temperature, pH value and multiple ions on the physisorption of chloride ion on C-S-H gel surface. Constr. Build. Mater. 2023, 392, 131967. [Google Scholar]
- Tong, T.T.; Li, Z.L.; Shang, H.B. Penetration characteristics of water in calcium silicate hydrate nanoslit and temperature effect. Mol. Simul. 2023, 49, 1502–1511. [Google Scholar] [CrossRef]
- Im, S.; Jee, H.; Suh, H.; Kanematsu, M.; Morooka, S.; Taku, K.; Yuhei, N.; Machida, A.; Kim, J.; Bae, S. Temperature effects on local structure, phase transformation, and mechanical properties of calcium silicate hydrates. J. Am. Ceram. Soc. 2021, 104, 4803–4818. [Google Scholar] [CrossRef]
- Wang, X.F.; Li, T.R.; Wei, P.; Li, D.W.; Han, N.X.; Xing, F.; Gan, Y.; Chen, Z. Computational study of the nanoscale mechanical properties of C-S-H composites under different temperatures. Comput. Mater. Sci. 2018, 146, 42–53. [Google Scholar] [CrossRef]
- Tu, Y.M.; Yuan, L.; Liu, D.Y.; Cao, J.; Ding, Y.H.; Das, O.; Försth, M.; Sas, G.; Elfgren, L. Molecular dynamics simulations of chloride and sulfate ion transport in C-S-H gel and γ-FeOOH nanopores. J. Adv. Concr. Technol. 2022, 20, 720–731. [Google Scholar] [CrossRef]
- Tian, Z.S.; Kang, X.J.; Ji, H.D.; Ye, H.L. Quantitative relationship between microstructure of steel-concrete interface and chloride-induced corrosion rate of steel in unsaturated cementitious materials. Cem. Concr. Res. 2025, 188, 107736. [Google Scholar] [CrossRef]
- Hou, D.S.; Xu, X.Q.; Wang, M.H.; Chen, Z.; Zhang, J.R.; Dong, B.Q.; Miao, J.; Liu, C. Nanoscale insights on the interface between passive film of steel and cement hydrate: Diffusion, kinetics and mechanics. Appl. Surf. Sci. 2020, 514, 145898. [Google Scholar] [CrossRef]
- Zhang, L.Z.; Wang, R.A.; Luan, Y.L.; Wang, H.; Tong, H. Molecular dynamics study on bonding behaviors of γ-FeOOH/CSH interface under aggressive conditions. Appl. Surf. Sci. 2025, 680, 161342. [Google Scholar] [CrossRef]
- Pellenq, R.J.M.; Kushima, A.; Shahsavari, R.; Van Vliet, K.J.; Buehler, M.J.; Yip, S.; Ulm, F.J. A realistic molecular model of cement hydrates. Proc. Natl. Acad. Sci. USA 2009, 106, 16102–16107. [Google Scholar] [CrossRef]
- Hou, D.S.; Zhang, J.R.; Li, Z.J.; Zhu, Y. Uniaxial tension study of calcium silicate hydrate (C-S-H): Structure, dynamics and mechanical properties. Mater. Struct. 2015, 48, 3811–3824. [Google Scholar] [CrossRef]
- Pan, T.Y.; Lu, Y. Quantum-chemistry based studying of rebar passivation in alkaline concrete environment. Int. J. Electrochem. Sci. 2011, 6, 4967–4983. [Google Scholar] [CrossRef]
- Pan, T.Y.; van Duin, A.C.T. Passivation of steel surface: An atomistic modeling approach aided with X-ray analyses. Mater. Lett. 2011, 65, 3223–3226. [Google Scholar] [CrossRef]
- Christensen, H.; Christensen, A.N. Hydrogen-Bonds of Gamma-Feooh. Acta Chem. Scand. 1978, 32, 87–88. [Google Scholar] [CrossRef]
- Hou, D.; Zhang, K.; Hong, F.; Wu, S.R.; Wang, Z.; Li, M.; Wang, M. The corrosion deterioration of reinforced passivation Film: The impact of defects. Appl. Surf. Sci. 2022, 582, 152408. [Google Scholar] [CrossRef]
- Ahmad, F.; Rawat, S.; Yang, R.; Zhang, L.; Fanna, D.J.; Soe, K.; Zhang, Y.X. Effect of metakaolin and ground granulated blast furnace slag on the performance of hybrid fibre-reinforced magnesium oxychloride cement-based composites. Int. J. Civ. Eng. 2025, 23, 853–868. [Google Scholar] [CrossRef]
- Fedotova, M.V. Effect of temperature and pressure on structural self-organization of aqueous sodium chloride solutions. J. Mol. Liq. 2010, 153, 9–14. [Google Scholar] [CrossRef]
- Dügenci, O.; Haktanir, T.; Altun, F. Experimental research for the effect of high temperature on the mechanical properties of steel fiber-reinforced concrete. Constr. Build. Mater. 2015, 75, 82–88. [Google Scholar] [CrossRef]
- Çelik, Z.; Urtekin, Y. Effects of high temperature and water re-curing on the flexural behavior and mechanical properties of steel-basalt hybrid fiber-reinforced concrete. Appl. Sci. 2025, 15, 1587. [Google Scholar] [CrossRef]
- Kim, J.; Lee, G.P.; Moon, D.Y. Evaluation of mechanical properties of steel-fibre-reinforced concrete exposed to high temperatures by double-punch test. Constr. Build. Mater. 2015, 79, 182–191. [Google Scholar] [CrossRef]
- Xiao, J.Z.; Xie, Q.H.; Xie, W.G. Study on high-performance concrete at high temperatures in China (2004–2016)—An updated overview. Fire Saf. J. 2018, 95, 11–24. [Google Scholar] [CrossRef]
- Ahmad, F.; Qureshi, M.I.; Rawat, S.; Alkharisi, M.K.; Alturki, M. E-waste in concrete construction: Recycling, applications, and impact on mechanical, durability, and thermal properties—A review. Innov. Infrastruct. Solut. 2025, 10, 246. [Google Scholar] [CrossRef]
- Rawat, S.; Saliba, P.; Estephan, P.C.; Ahmad, F.; Zhang, Y. Mechanical performance of hybrid fibre reinforced magnesium oxychloride cement-based composites at ambient and elevated temperature. Buildings 2024, 14, 270. [Google Scholar] [CrossRef]
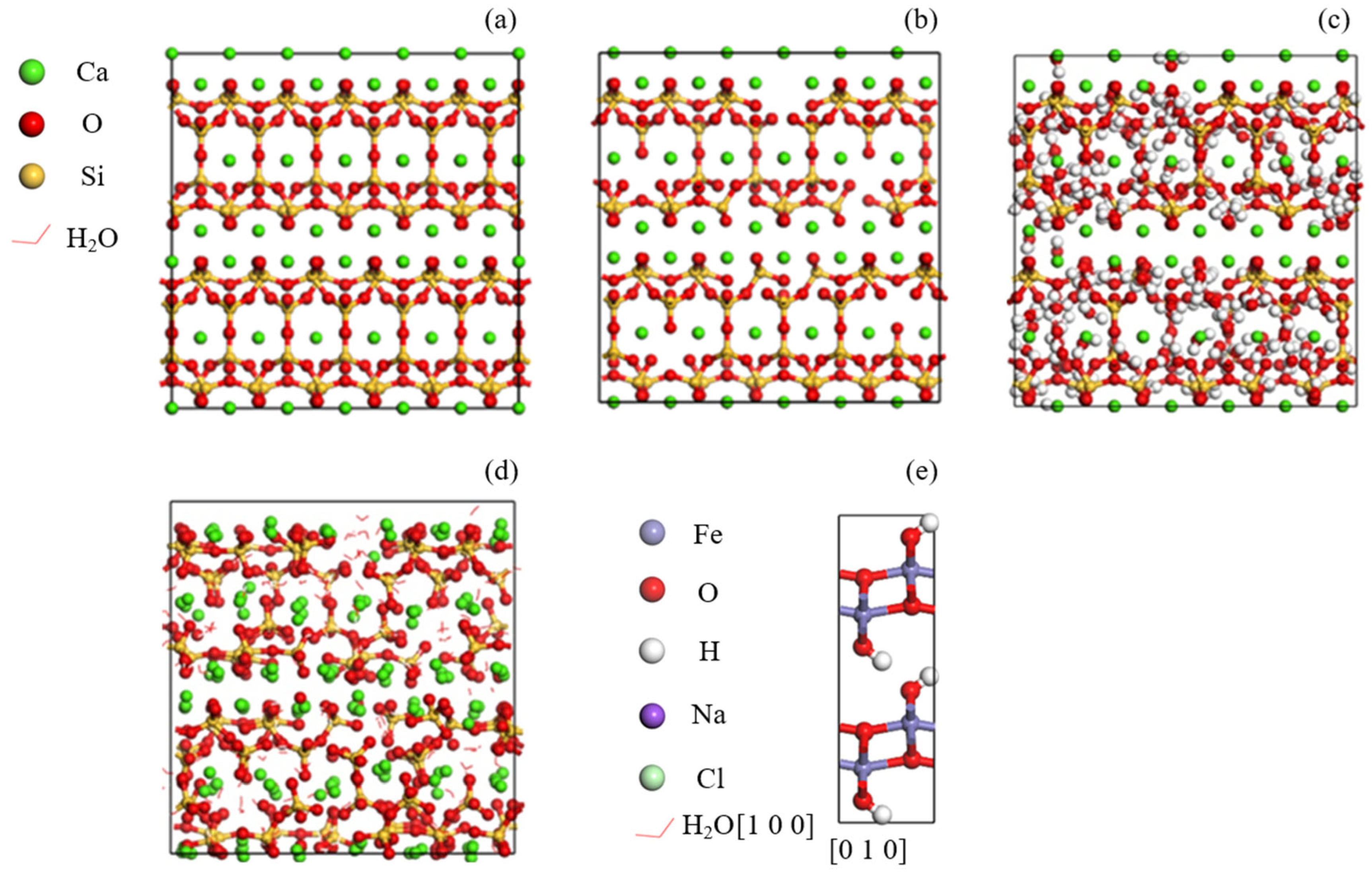

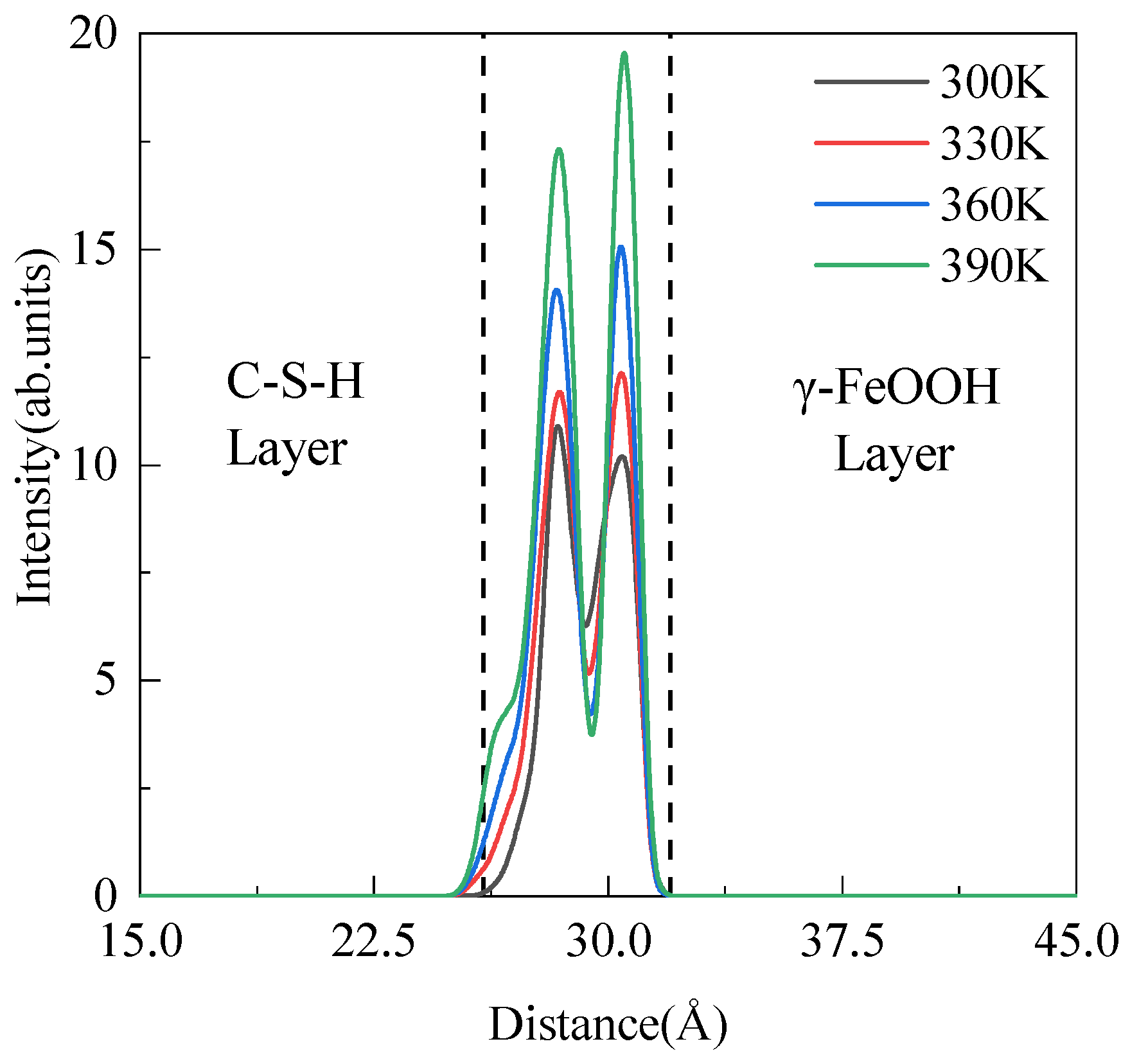

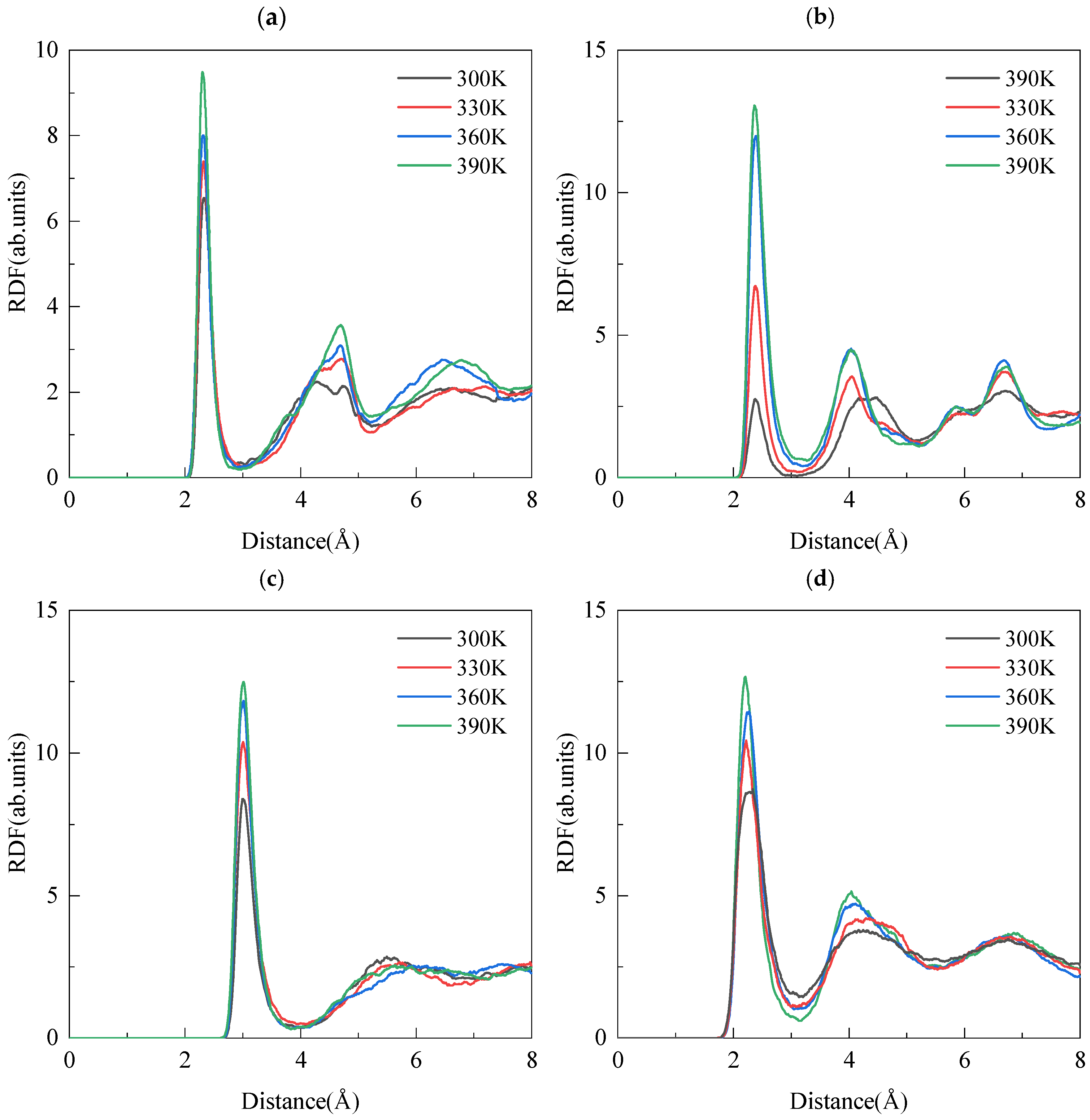

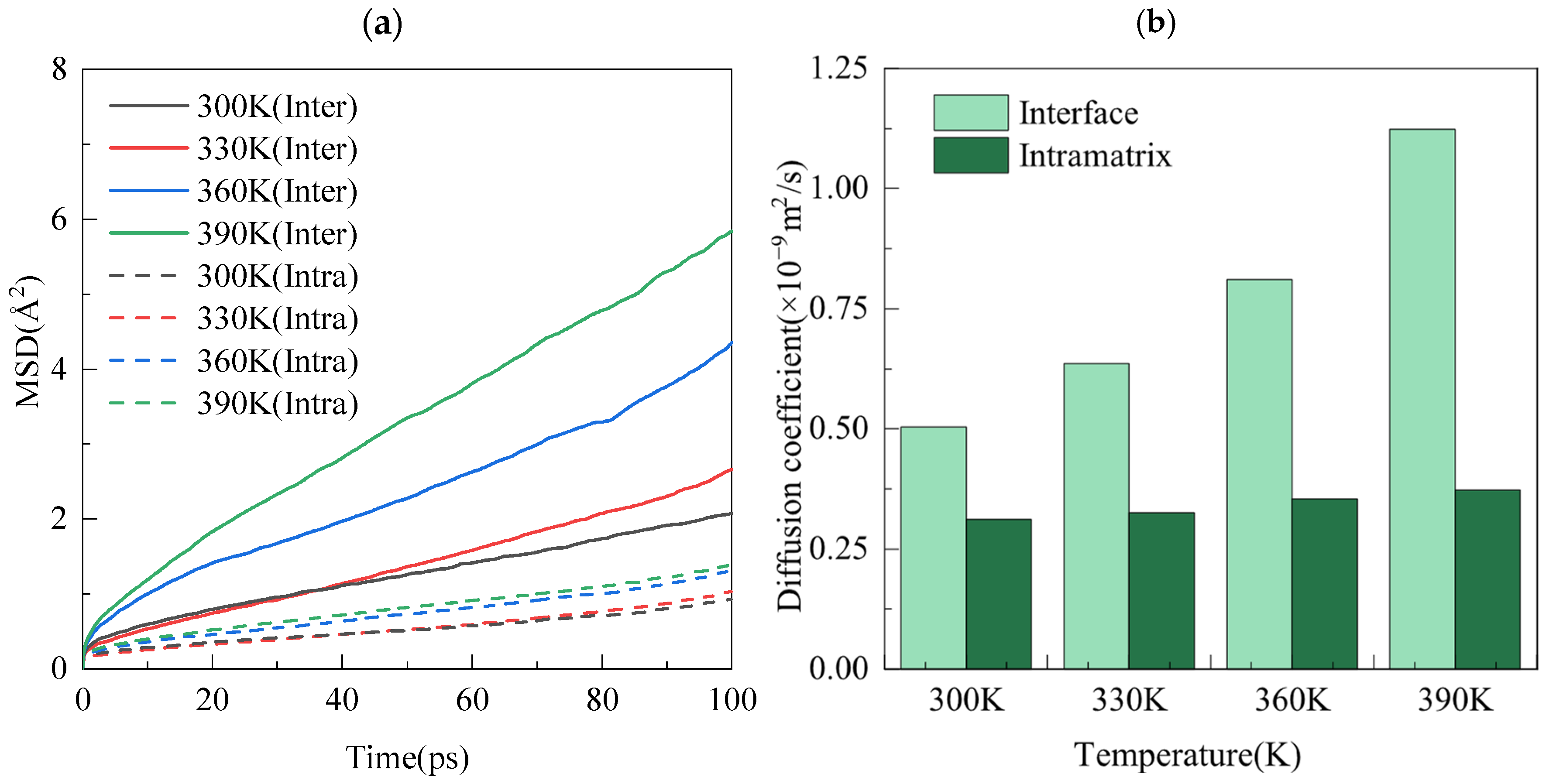
| Species | Symbol | Charge (e) | D0 (kcal/mol) | R0 (Å) |
|---|---|---|---|---|
| Water hydrogen | Hw | 0.4100 | ||
| Hydroxyl hydrogen | Ho | 0.4250 | ||
| Water oxygen | Ow | −0.8200 | 0.1554 | 3.5532 |
| Hydroxyl oxygen | Oh | −0.9500 | 0.1554 | 3.5532 |
| Octahedral iron | Fe | 1.5750 | 9.0298 × 10−6 | 5.5070 |
| Aqueous sodium ion | Na | 1.0000 | 0.1000 | 2.6378 |
| Aqueous chloride ion | Cl | −1.0000 | 0.1001 | 4.9388 |
| Bridging oxygen | Ob | −1.0500 | 0.1554 | 3.5532 |
| Setrahedral silicon | St | 2.1000 | 1.8405 × 10−6 | 3.7064 |
| Hydroxide calcium | Cah | 1.0500 | 5.0298 × 10−6 | 6.2428 |
| Aqueous calcium ion | Ca | 2.0000 | 0.1000 | 3.2237 |
| Temperature | Diffusion Coefficients (10−9 m2/s) | |
|---|---|---|
| Interface | Intramatrix | |
| 300 K | 0.5002 | 0.3159 |
| 330 K | 0.6364 | 0.3288 |
| 360 K | 0.8126 | 0.3559 |
| 390 K | 1.1234 | 0.3736 |
| Temperature | C-S-H/Solution | γ-FeOOH/Solution | ||||
|---|---|---|---|---|---|---|
| Angle(°) | Length(Å) | Number | Angle(°) | Length(Å) | Number | |
| 300 K | 165.04 | 1.699 | 199 | 164.98 | 1.786 | 233 |
| 330 K | 164.84 | 1.702 | 182 | 164.45 | 1.799 | 221 |
| 360 K | 164.52 | 1.721 | 173 | 163.98 | 1.809 | 199 |
| 390 K | 164.09 | 1.727 | 170 | 163.53 | 1.828 | 179 |
| Temperature | Interfacial Adsorption Energy (103 kcal/mol) | |||||
|---|---|---|---|---|---|---|
| C-S-H/γ-FeOOH | C-S-H/Solution | γ-FeOOH/Solution | ||||
| 100 ps | 300 ps | 100 ps | 300 ps | 100 ps | 300 ps | |
| 300 K | −6.6585 | −7.4944 | −25.9392 | −26.2784 | −4.4239 | −4.7568 |
| 330 K | −6.4644 | −7.2763 | −23.9271 | −24.2928 | −4.1177 | −4.4419 |
| 360 K | −6.0620 | −6.8891 | −21.8759 | −22.2775 | −3.8220 | −4.1543 |
| 390 K | −5.7760 | −6.6704 | −20.9912 | −21.2616 | −3.6102 | −3.9537 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Mo, J.; Sang, W.; Wu, J. Temperature-Driven Degradation Mechanisms of Steel–Concrete Interfaces in NaCl Solution Environments: Nanoscale Insights from Molecular Dynamics Simulations. Buildings 2025, 15, 2894. https://doi.org/10.3390/buildings15162894
Xu J, Mo J, Sang W, Wu J. Temperature-Driven Degradation Mechanisms of Steel–Concrete Interfaces in NaCl Solution Environments: Nanoscale Insights from Molecular Dynamics Simulations. Buildings. 2025; 15(16):2894. https://doi.org/10.3390/buildings15162894
Chicago/Turabian StyleXu, Jianchao, Jiayi Mo, Wenlong Sang, and Jieqiong Wu. 2025. "Temperature-Driven Degradation Mechanisms of Steel–Concrete Interfaces in NaCl Solution Environments: Nanoscale Insights from Molecular Dynamics Simulations" Buildings 15, no. 16: 2894. https://doi.org/10.3390/buildings15162894
APA StyleXu, J., Mo, J., Sang, W., & Wu, J. (2025). Temperature-Driven Degradation Mechanisms of Steel–Concrete Interfaces in NaCl Solution Environments: Nanoscale Insights from Molecular Dynamics Simulations. Buildings, 15(16), 2894. https://doi.org/10.3390/buildings15162894






