A Concise Grid-Based Model Revealing the Temporal Dynamics in Indoor Infection Risk
Abstract
1. Introduction
2. Materials and Methods
2.1. Model Development
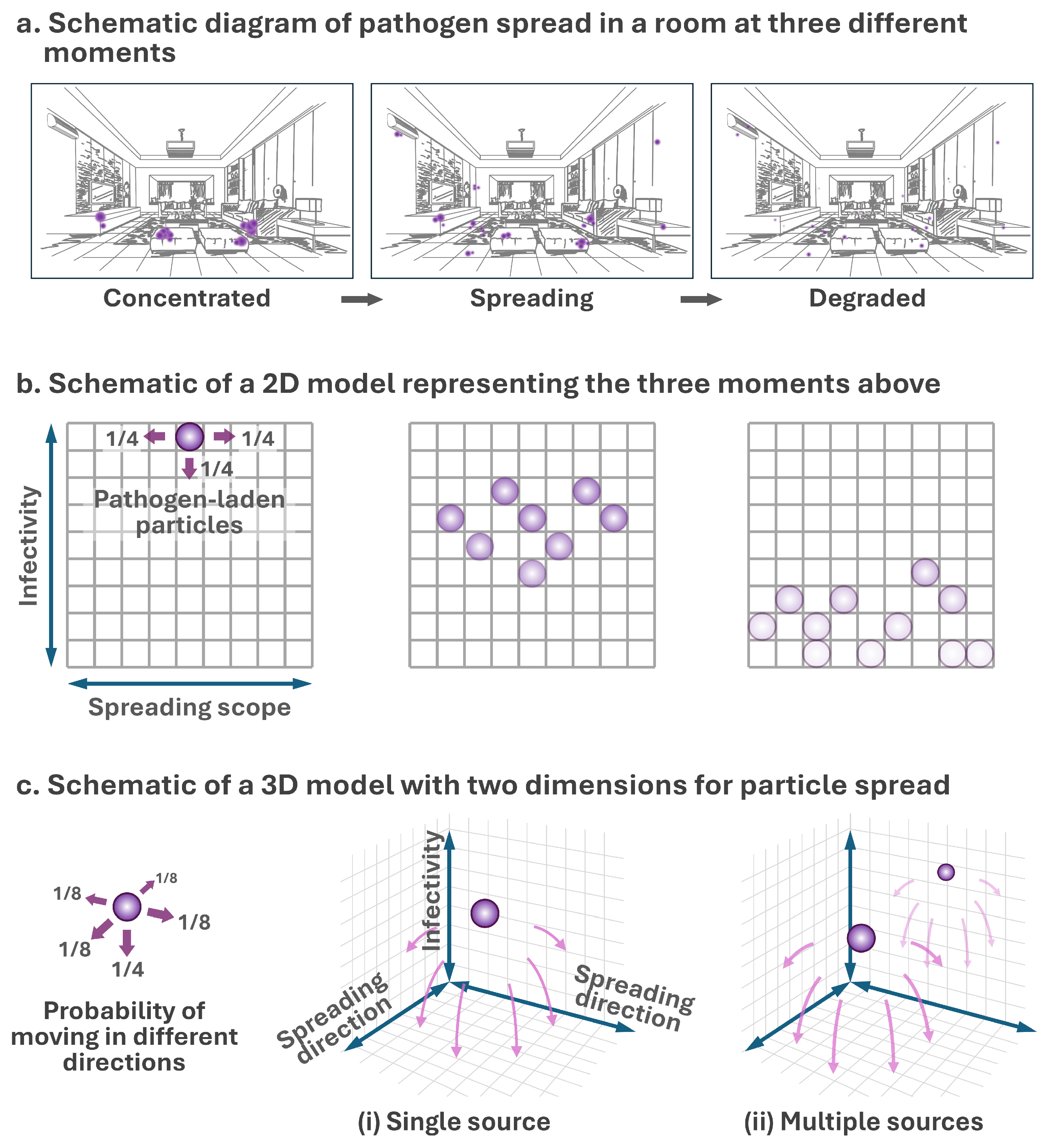
2.1.1. Model Framework
2.1.2. Infection Risk in an Indoor Environment
2.2. Simulation of Pathogen Spread
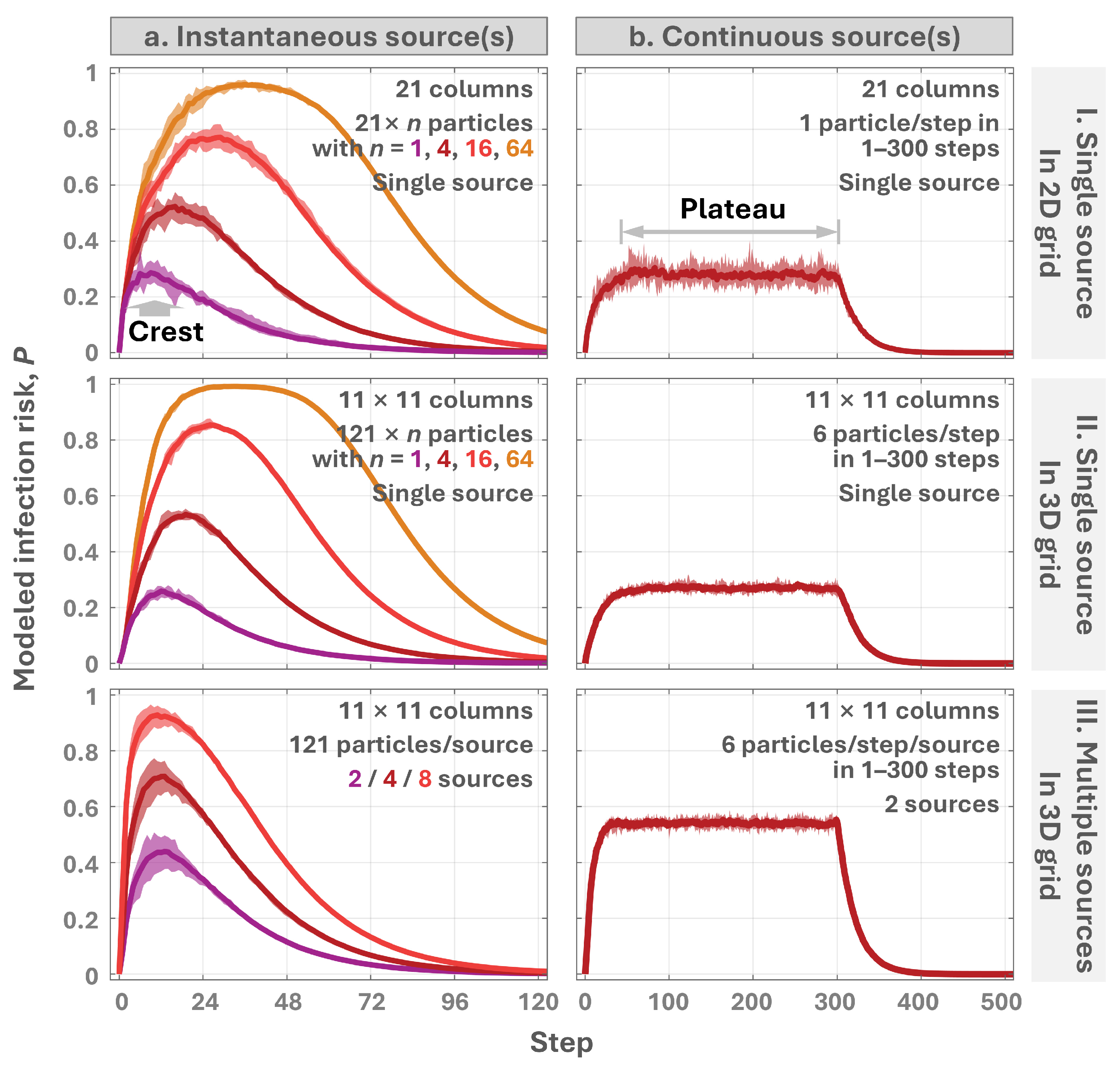
2.2.1. Particle Spread in Idealized Grids
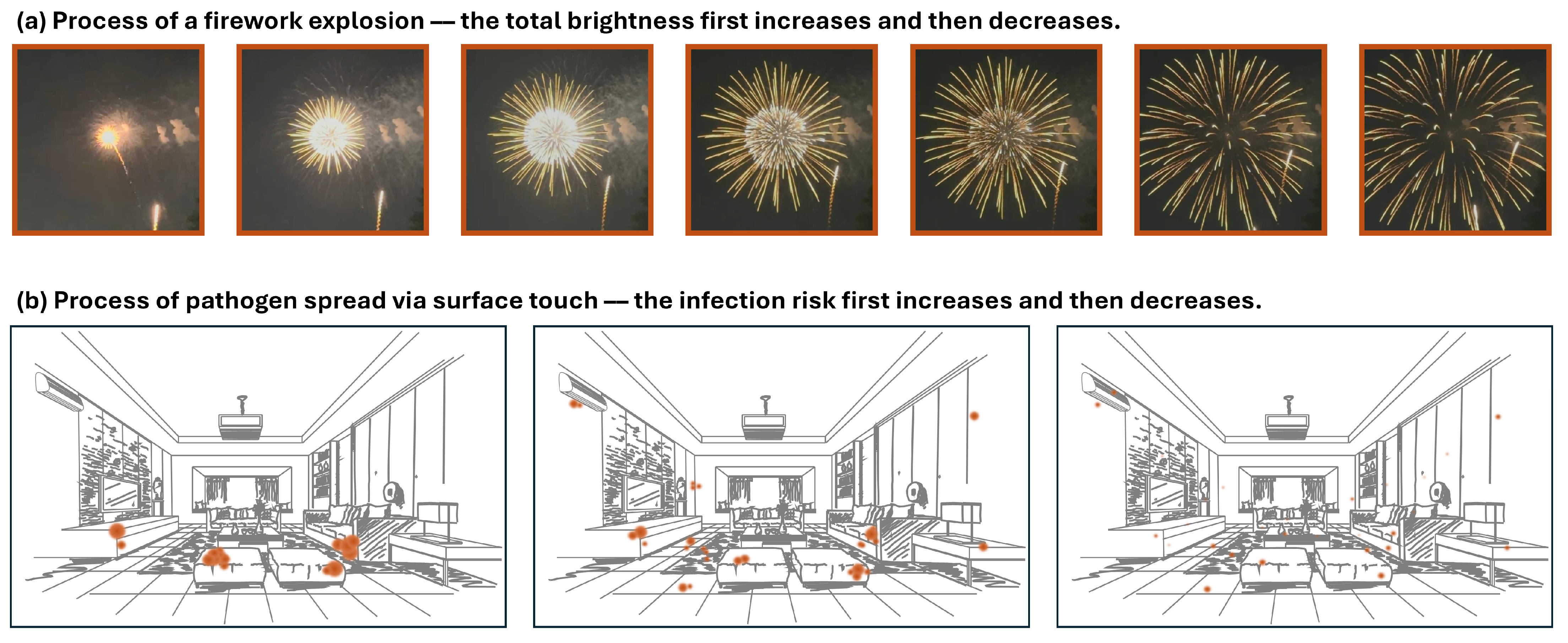
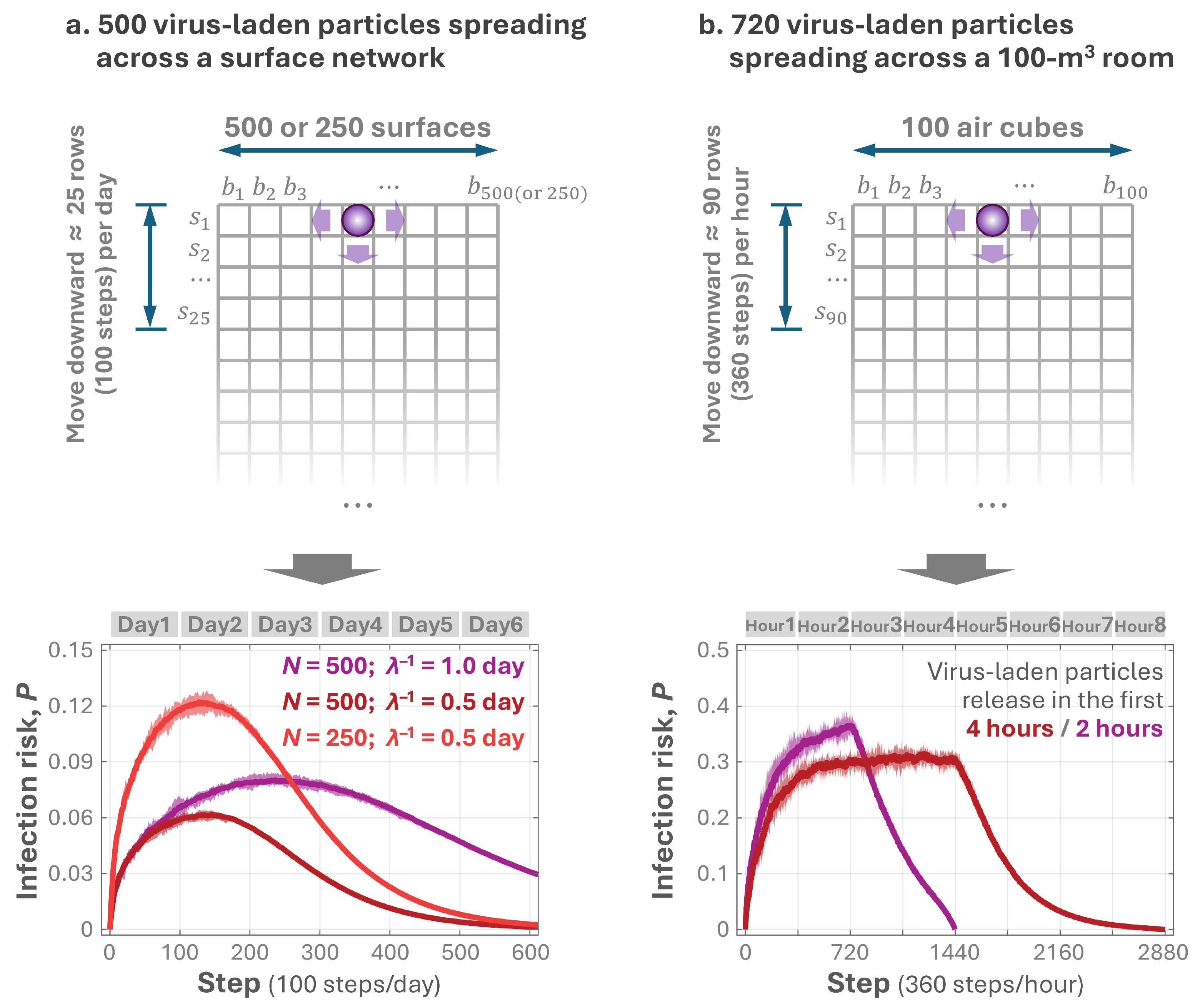
2.2.2. Pathogen Spread via Surface Touch
2.2.3. Pathogen Diffusion in Indoor Air
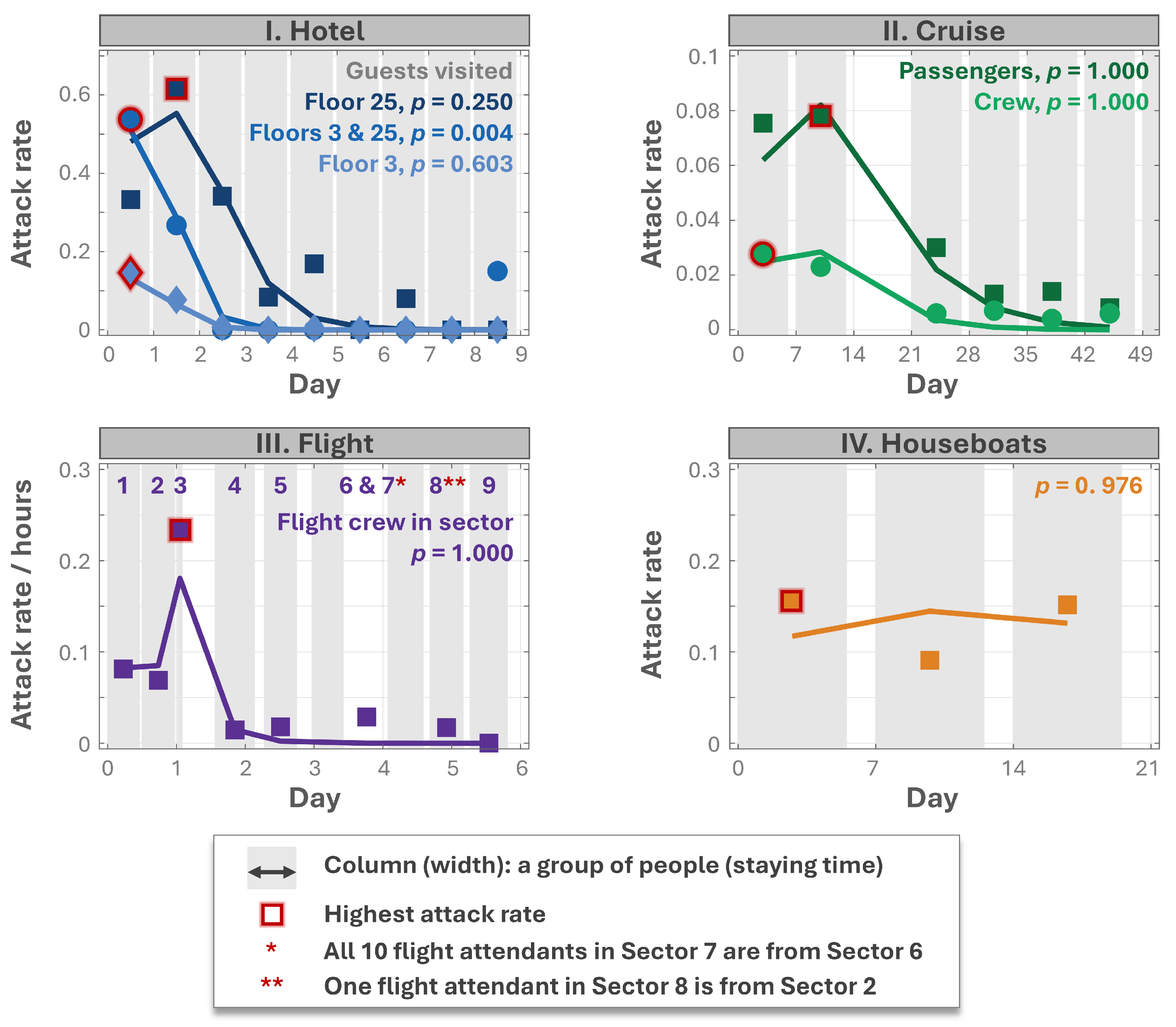
2.3. Model Validation
| No. | Study | Location | Time | Onset/Total Interviewed People |
|---|---|---|---|---|
| I. | Kimura et al., 2011 [26] | A hotel | 2–10 December 2006 | 345/approx. 2700 |
| II. | Isakbaeva et al., 2005 [27] | A cruise ship | 16 November 2002–4 January 2003 | 587/approx. 20,000 |
| III. | Thornley et al., 2011 [28] | A flight cabin | 13–18 October 2009 | 27/63 |
| IV. | Jones et al., 2007 [29] | Four houseboats | 9–29 May 2004 | 20/27 |
2.4. Statistical Analysis
3. Results
3.1. Model Framework and Simulation Insights
3.2. Temporal Variation in the Infection Risk for Surface and Air Pathogens
3.3. Validation of the New Model
4. Discussion
4.1. Mechanism of the Rise-and-Fall Pattern
4.2. Conciseness and Flexibility of the New Model
4.3. Occurrence of the Crest as an Indicator of Fomite Transmission
4.4. Limitation and Potential Applications
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Assumptions in Modeling Indoor Pathogen Spread
Appendix A.2. Firework Explosion
References
- Wang, C.C.; Prather, K.A.; Sznitman, J.; Jimenez, J.L.; Lakdawala, S.S.; Tufekci, Z.; Marr, L.C. Airborne transmission of respiratory viruses. Science 2021, 373, eabd9149. [Google Scholar] [CrossRef] [PubMed]
- Lopman, B.; Gastanaduy, P.; Park, G.W.; Hall, A.J.; Parashar, U.D.; Vinje, J. Environmental transmission of norovirus gastroenteritis. Curr. Opin. Virol. 2012, 2, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Muder, R.R.; Victor, L.Y.; Woo, A.H. Mode of transmission of Legionella pneumophila: A critical review. Arch. Intern. Med. 1986, 146, 1607–1612. [Google Scholar] [CrossRef]
- Leung, N.H.L. Transmissibility and transmission of respiratory viruses. Nat. Rev. Microbiol. 2021, 19, 528–545. [Google Scholar] [CrossRef]
- Otter, J.A.; Yezli, S.; Salkeld, J.A.; French, G.L. Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. Am. J. Infect. Control 2013, 41, S6–S11. [Google Scholar] [CrossRef]
- Wang, Q.; Lin, Z.; Niu, J.; Choi, G.K.-Y.; Fung, J.C.; Lau, A.K.; Louie, P.; Leung, K.K.; Huang, J.; Cheng, P. Spread of SARS-CoV-2 aerosols via two connected drainage stacks in a high-rise housing outbreak of COVID-19. J. Hazard. Mater. 2022, 430, 128475. [Google Scholar] [CrossRef]
- Asadi, S.; Gaaloul Ben Hnia, N.; Barre, R.S.; Wexler, A.S.; Ristenpart, W.D.; Bouvier, N.M. Influenza A virus is transmissible via aerosolized fomites. Nat. Commun. 2020, 11, 4062. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, N.; Miao, T.; Chan, J.P.T.; Huang, H.; Lee, P.K.H.; Li, Y. Surface touch network structure determines bacterial contamination spread on surfaces and occupant exposure. J. Hazard. Mater. 2021, 416, 126137. [Google Scholar] [CrossRef]
- Harvey, A.P.; Fuhrmeister, E.R.; Cantrell, M.E.; Pitol, A.K.; Swarthout, J.M.; Powers, J.E.; Nadimpalli, M.L.; Julian, T.R.; Pickering, A.J. Longitudinal monitoring of SARS-CoV-2 RNA on high-touch surfaces in a community setting. Environ. Sci. Technol. Lett. 2021, 8, 168–175. [Google Scholar] [CrossRef]
- Hu, X.; Ni, W.; Wang, Z.; Ma, G.; Pan, B.; Dong, L.; Gao, R.; Jiang, F. The distribution of SARS-CoV-2 contamination on the environmental surfaces during incubation period of COVID-19 patients. Ecotoxicol. Environ. Saf. 2021, 208, 111438. [Google Scholar] [CrossRef] [PubMed]
- Chia, P.Y.; Coleman, K.K.; Tan, Y.K.; Ong, S.W.X.; Gum, M.; Lau, S.K.; Lim, X.F.; Lim, A.S.; Sutjipto, S.; Lee, P.H.; et al. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat. Commun. 2020, 11, 2800. [Google Scholar] [CrossRef]
- Meyerowitz, E.A.; Richterman, A.; Gandhi, R.T.; Sax, P.E. Transmission of SARS-CoV-2: A review of viral, host, and environmental factors. Ann. Intern. Med. 2021, 174, 69–79. [Google Scholar] [CrossRef]
- Xiao, S.; Jones, R.M.; Zhao, P.; Li, Y. The dynamic fomite transmission of Methicillin-resistant Staphylococcus aureus in hospitals and the possible improved intervention methods. Build. Environ. 2019, 161, 106246. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, X.; Jia, W.; Jin, T.; Xiao, S.; Chen, W.; Hang, J.; Ou, C.; Lei, H.; Qian, H.; et al. Evidence for lack of transmission by close contact and surface touch in a restaurant outbreak of COVID-19. J. Infect. 2021, 83, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Sober, E. The principle of parsimony. Br. J. Philos. Sci. 1981, 32, 145–156. [Google Scholar] [CrossRef]
- Zhao, P.; Li, Y. Modeling and experimental validation of microbial transfer via surface touch. Environ. Sci. Technol. 2021, 55, 4148–4161. [Google Scholar] [CrossRef]
- Sze To, G.N.; Chao, C.Y.H. Review and comparison between the Wells-Riley and dose-response approaches to risk assessment of infectious respiratory diseases. Indoor Air 2010, 20, 2–16. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, P.; Miao, T.; Chan, P.-T.; Jia, W.; Zhao, P.; Su, B.; Chen, X.; Li, Y. Real human surface touch behavior based quantitative analysis on infection spread via fomite route in an office. Build. Environ. 2021, 191, 107578. [Google Scholar] [CrossRef]
- Li, Y.; Qian, H.; Hang, J.; Chen, X.; Cheng, P.; Ling, H.; Wang, S.; Liang, P.; Li, J.; Xiao, S.; et al. Probable airborne transmission of SARS-CoV-2 in a poorly ventilated restaurant. Build. Environ. 2021, 196, 107788. [Google Scholar] [CrossRef]
- Zhang, N.; Li, Y.; Huang, H. Surface touch and its network growth in a graduate student office. Indoor Air 2018, 28, 963–972. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, P.; Lam, T.; Zhang, N.; Wang, H.; Zhang, H.; Liu, J.; Li, Y. High-touch surfaces with moderate contamination levels as key nodes in microbial dissemination. J. Hazard. Mater. 2025, 495, 138834. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.; Knight, A.; Richards, G.P. Persistence and elimination of human Norovirus in food and on food contact surfaces: A critical review. J. Food Prot. 2016, 79, 1273–1294. [Google Scholar] [CrossRef]
- ANSI/ASHRAE Standard 55; Thermal Environmental Conditions for Human Occupancy. American Society of Heating, Refrigerating and Air-Conditioning Engineers: Atlanta, GA, USA, 1992.
- Nazaroff, W.W. Residential air-change rates: A critical review. Indoor Air 2021, 31, 282–313. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Morawska, L.; Gilbert, D. Particle deposition rates in residential houses. Atmos. Environ. 2005, 39, 3891–3899. [Google Scholar] [CrossRef]
- Kimura, H.; Nagano, K.; Kimura, N.; Shimizu, M.; Ueno, Y.; Morikane, K.; Okabe, N. A norovirus outbreak associated with environmental contamination at a hotel. Epidemiol. Infect. 2011, 139, 317–325. [Google Scholar] [CrossRef]
- Isakbaeva, E.T.; Widdowson, M.A.; Beard, R.S.; Bulens, S.N.; Mullins, J.; Monroe, S.S.; Bresee, J.; Sassano, P.; Cramer, E.H.; Glass, R.I. Norovirus transmission on cruise ship. Emerg. Infect. Dis. 2005, 11, 154–157. [Google Scholar] [CrossRef]
- Thornley, C.N.; Emslie, N.A.; Sprott, T.W.; Greening, G.E.; Rapana, J.P. Recurring norovirus transmission on an airplane. Clin. Infect. Dis. 2011, 53, 515–520. [Google Scholar] [CrossRef]
- Jones, E.L.; Kramer, A.; Gaither, M.; Gerba, C.P. Role of fomite contamination during an outbreak of norovirus on houseboats. Int. J. Environ. Health Res. 2007, 17, 123–131. [Google Scholar] [CrossRef]
- Cheesbrough, J.S.; Green, J.; Gallimore, C.I.; Wright, P.A.; Brown, D.W. Widespread environmental contamination with Norwalk-like viruses (NLV) detected in a prolonged hotel outbreak of gastroenteritis. Epidemiol. Infect. 2000, 125, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.E.; Heisey-Grove, D.; Beach, M.; Dicker, R.C.; Matyas, B.T. Prolonged outbreak of giardiasis with two modes of transmission. Epidemiol. Infect. 2006, 134, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Hu, T.; Zhang, J.; Mai, W.; Jian, M.; Li, J.; Chen, F.; Zhu, M.; Zhuang, L.; Jin, T.; et al. Probable close contact transmission in a restaurant in China. J. Infect. 2022, 85, 573–607. [Google Scholar] [CrossRef]
- Xiao, S.; Hao, Z.; Zhao, F.; Zhao, P.; Zhang, N.; Li, Y. Residence time of particles in indoor surface networks. J. Hazard. Mater. 2025, 488, 137267. [Google Scholar] [CrossRef]
- Xiao, S.; Li, Y.; Sung, M.; Wei, J.; Yang, Z. A study of the probable transmission routes of MERS-CoV during the first hospital outbreak in the Republic of Korea. Indoor Air 2018, 28, 51–63. [Google Scholar] [CrossRef]
- Bulfone, T.C.; Malekinejad, M.; Rutherford, G.W.; Razani, N. Outdoor Transmission of SARS-CoV-2 and Other Respiratory Viruses: A Systematic Review. J. Infect. Dis. 2021, 223, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Luo, K.; Xiao, S.; Yang, H.; Hang, J.; Ou, C.; Cowling, B.J.; Yen, H.L.; Hui, D.S.; Hu, S.; et al. Predominant airborne transmission and insignificant fomite transmission of SARS-CoV-2 in a two-bus COVID-19 outbreak originating from the same pre-symptomatic index case. J. Hazard. Mater. 2022, 425, 128051. [Google Scholar] [CrossRef] [PubMed]
- Khanh, N.C.; Thai, P.Q.; Quach, H.L.; Thi, N.H.; Dinh, P.C.; Duong, T.N.; Mai, L.T.Q.; Nghia, N.D.; Tu, T.A.; Quang, N.; et al. Transmission of SARS-CoV 2 during long-haul flight. Emerg. Infect. Dis. 2020, 26, 2617–2624. [Google Scholar] [CrossRef]
- Groves, L.M. Community transmission of SARS-CoV-2 at three fitness facilities—Hawaii, June–July 2020. Morb. Mortal. Wkly. Rep. 2021, 70, 316–320. [Google Scholar] [CrossRef]
- Smith, A.J.; McCarthy, N.; Saldana, L.; Ihekweazu, C.; McPhedran, K.; Adak, G.K.; Iturriza-Gomara, M.; Bickler, G.; O’Moore, E. A large foodborne outbreak of norovirus in diners at a restaurant in England between January and February 2009. Epidemiol. Infect. 2012, 140, 1695–1701. [Google Scholar] [CrossRef][Green Version]
- Thornley, C.N.; Hewitt, J.; Perumal, L.; Van Gessel, S.M.; Wong, J.; David, S.A.; Rapana, J.P.; Li, S.; Marshall, J.C.; Greening, G.E. Multiple outbreaks of a novel norovirus GII.4 linked to an infected post-symptomatic food handler. Epidemiol. Infect. 2013, 141, 1585–1597. [Google Scholar] [CrossRef]
- Hirakata, Y.; Arisawa, K.; Nishio, O.; Nakagomi, O. Multiprefectural spread of gastroenteritis outbreaks attributable to a single genogroup II norovirus strain from a tourist restaurant in Nagasaki, Japan. J. Clin. Microbiol. 2005, 43, 1093–1098. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marks, P.J.; Vipond, I.B.; Carlisle, D.; Deakin, D.; Fey, R.E.; Caul, E.O. Evidence for airborne transmission of Norwalk-like virus (NLV) in a hotel restaurant. Epidemiol. Infect. 2000, 124, 481–487. [Google Scholar] [CrossRef]
- Sabria, M.; Alvarez, J.; Dominguez, A.; Pedrol, A.; Sauca, G.; Salleras, L.; Lopez, A.; Garcia-Nunez, M.A.; Parron, I.; Barrufet, M.P. A community outbreak of Legionnaires’ disease: Evidence of a cooling tower as the source. Clin. Microbiol. Infect. 2006, 12, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Greig, J.E.; Carnie, J.A.; Tallis, G.F.; Ryan, N.J.; Tan, A.G.; Gordon, I.R.; Zwolak, B.; Leydon, J.A.; Guest, C.S.; Hart, W.G. An outbreak of Legionnaires’ disease at the Melbourne Aquarium, April 2000: Investigation and case-control studies. Med. J. Aust. 2004, 180, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.W.; Sheppard, A.E.; Madder, H.; Moir, I.; Moroney, R.; Quan, T.P.; Griffiths, D.; George, S.; Butcher, L.; Morgan, M.; et al. A Candida auris outbreak and its control in an intensive care setting. N. Engl. J. Med. 2018, 379, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases. Science Brief: SARS-CoV-2 and Surface (Fomite) Transmission for Indoor Community Environments; CDC COVID-19 Science Briefs; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2021.
- Li, Y.; Wei, Z. Dynamics and optimal control of a stochastic coronavirus (COVID-19) epidemic model with diffusion. Nonlinear Dyn. 2022, 109, 91–120. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, P.; Zheng, X. A Concise Grid-Based Model Revealing the Temporal Dynamics in Indoor Infection Risk. Buildings 2025, 15, 2786. https://doi.org/10.3390/buildings15152786
Zhao P, Zheng X. A Concise Grid-Based Model Revealing the Temporal Dynamics in Indoor Infection Risk. Buildings. 2025; 15(15):2786. https://doi.org/10.3390/buildings15152786
Chicago/Turabian StyleZhao, Pengcheng, and Xiaohong Zheng. 2025. "A Concise Grid-Based Model Revealing the Temporal Dynamics in Indoor Infection Risk" Buildings 15, no. 15: 2786. https://doi.org/10.3390/buildings15152786
APA StyleZhao, P., & Zheng, X. (2025). A Concise Grid-Based Model Revealing the Temporal Dynamics in Indoor Infection Risk. Buildings, 15(15), 2786. https://doi.org/10.3390/buildings15152786






