Evaluating the Heat of Hydration, Conductivity, and Microstructural Properties of Cement Composites with Recycled Concrete Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. Cement
2.2. Preparation of RCP
2.3. Microcalorimetry Sample Preparation
2.4. Electrical Conductivity Measurements
2.5. TGA and FT-IR Measurements
2.6. SEM Analysis
2.7. Mechanical Properties of RCP-Cement Composites
3. Results and Discussion
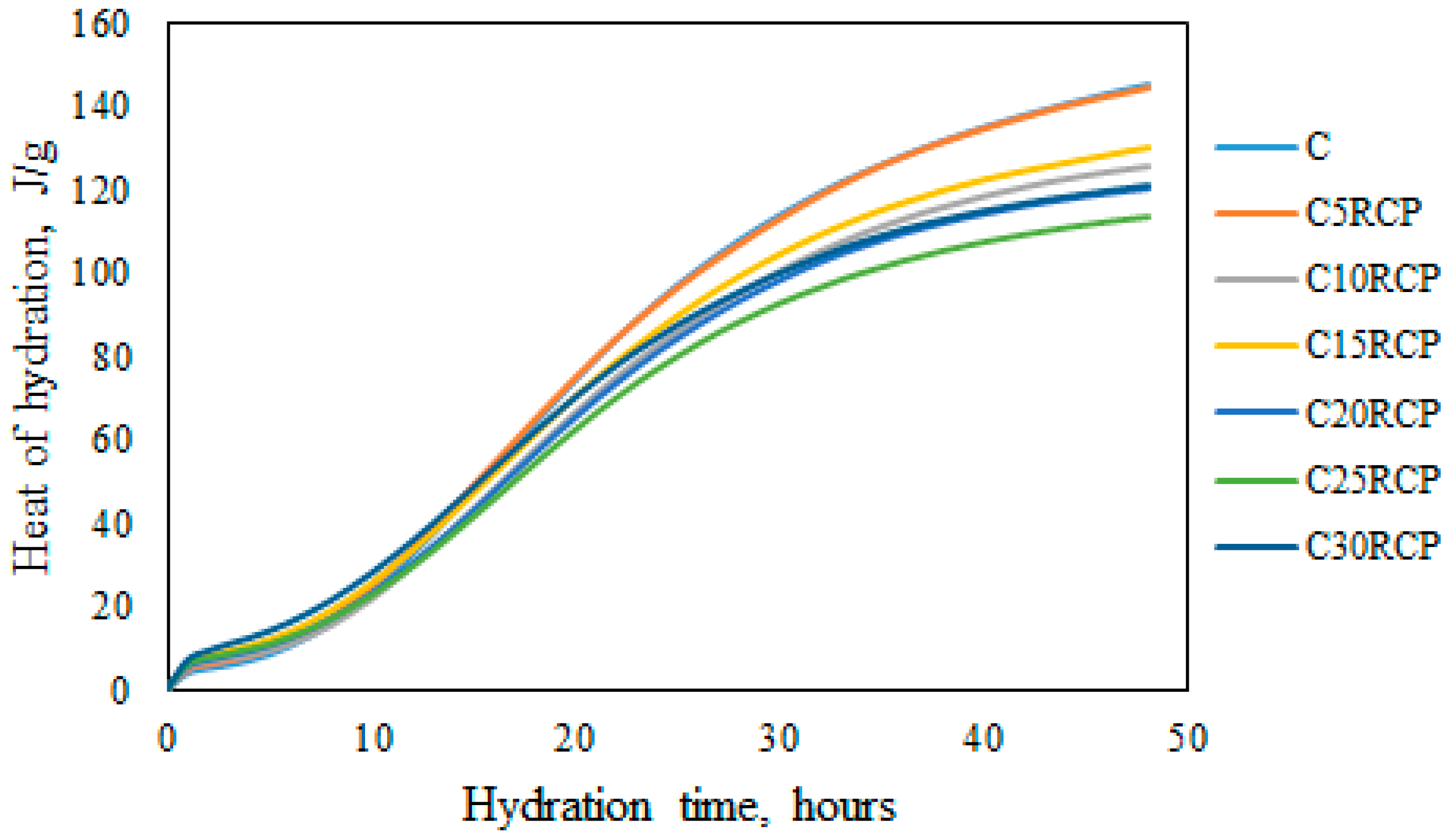
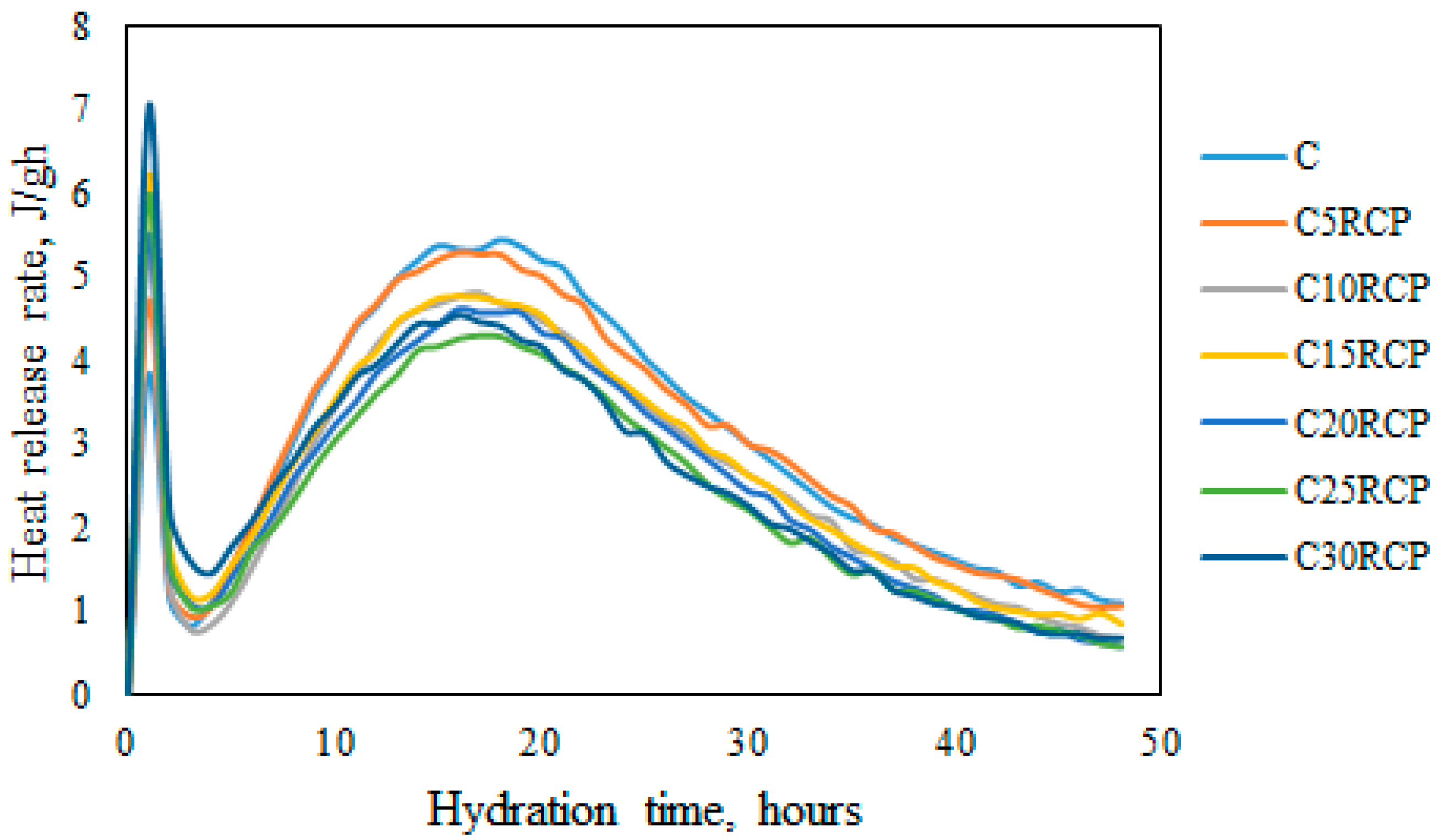
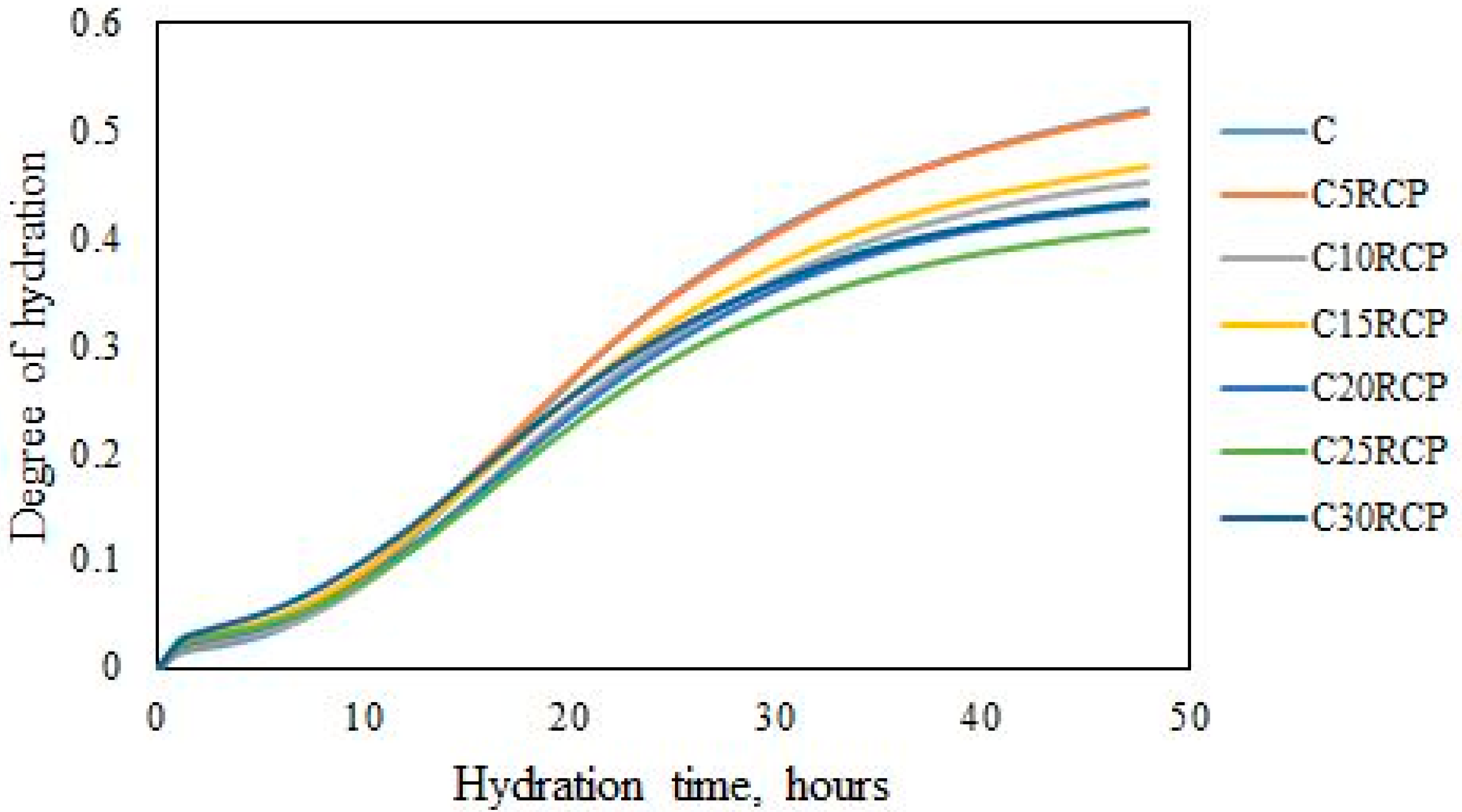
3.1. Microcalorimetrical Analysis
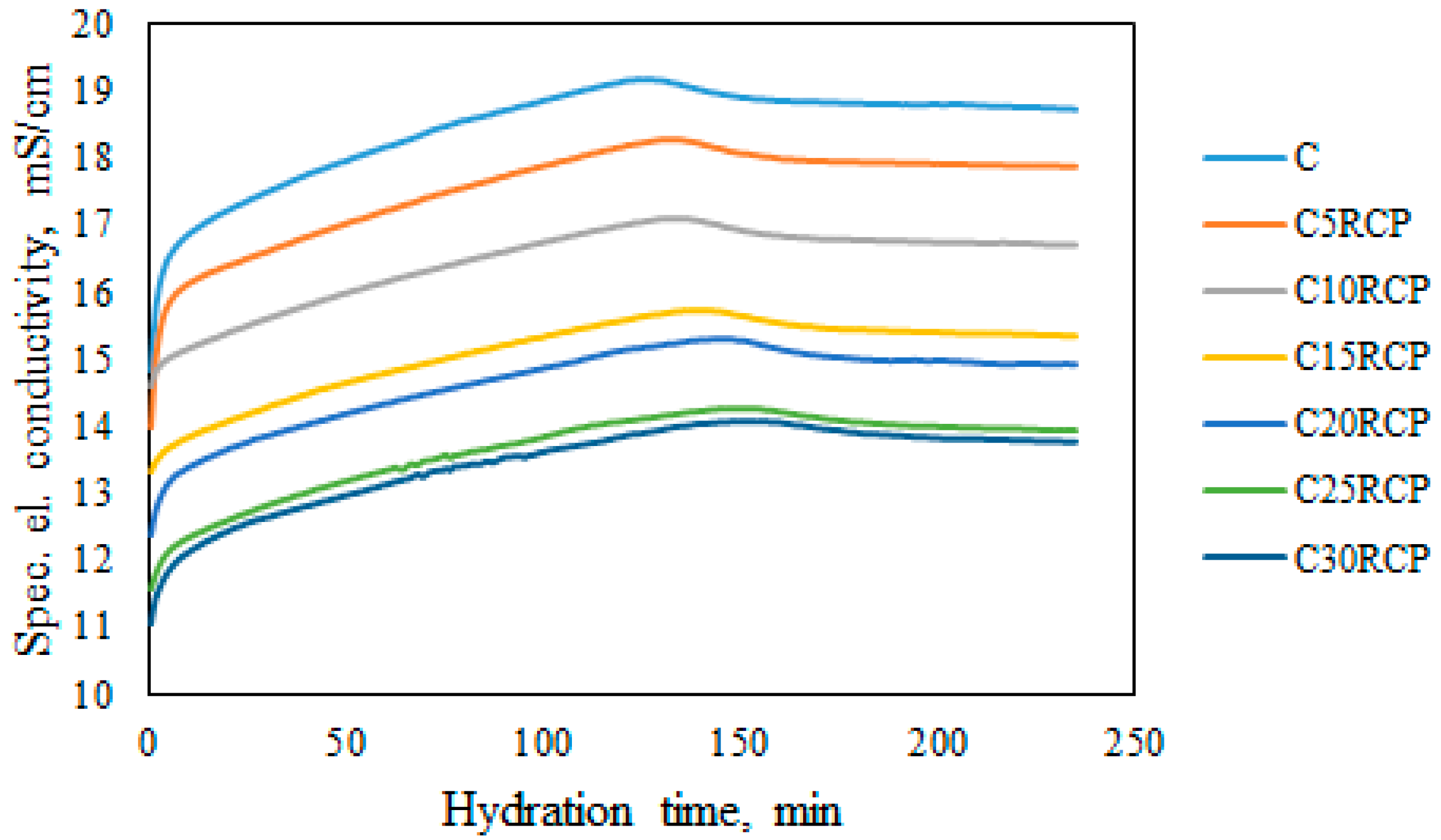
3.2. Electrical Conductivity Results
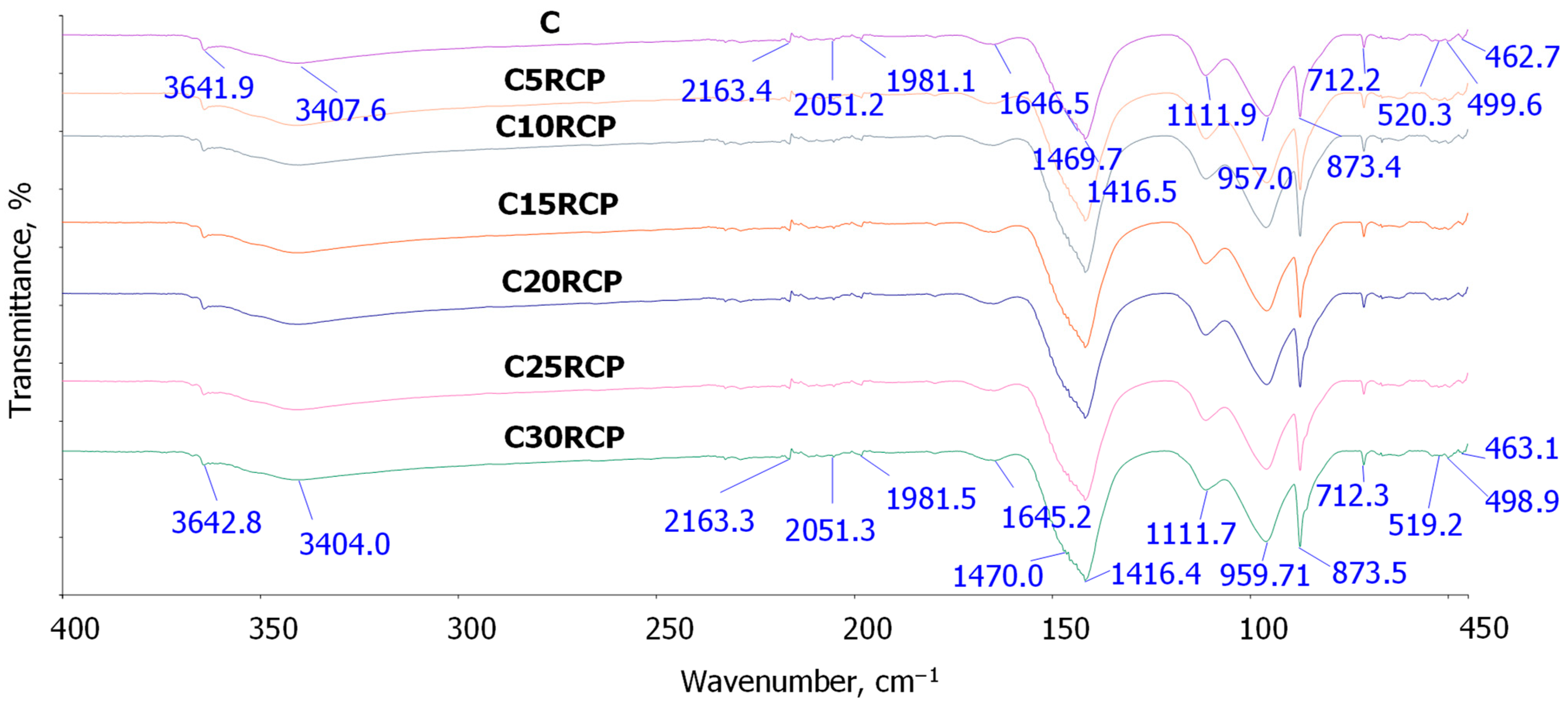
3.3. FT-IR Analysis of RCP in Cement Composites
- (1)
- Hydroxyl and water region (>1600 cm−1)
- (2)
- Carbonate region (1400–1470 cm−1)
- (3)
- Sulfate region (1100–1150 cm−1)
- (4)
- Silicate region (<1000 cm−1)
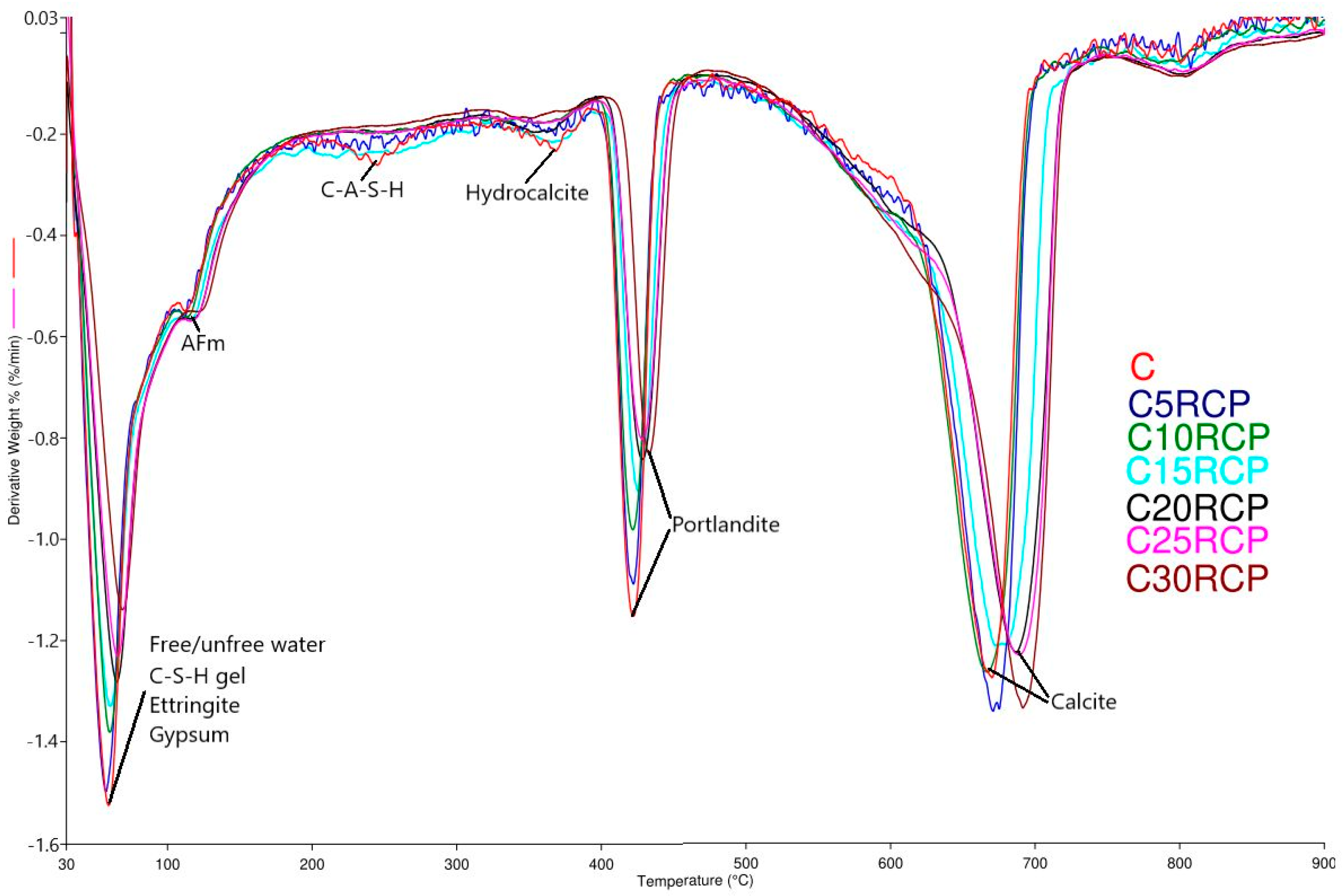
3.4. Thermogravimetric Analysis (TGA)
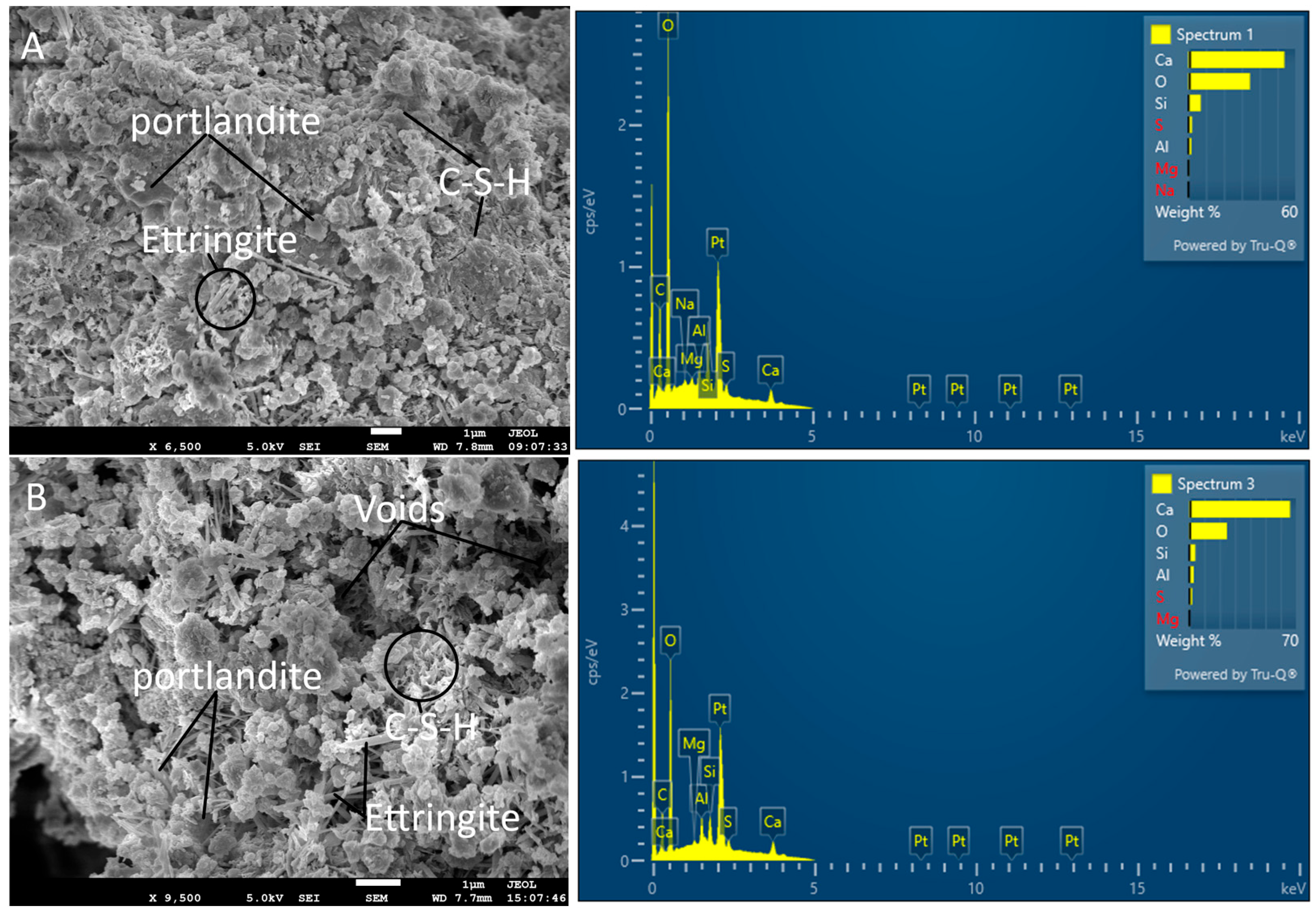
3.5. Scanning Electron Microscopy
- (1)
- At the microscale (<1 μm), RCP particles densified the matrix by filling capillary pores between cement grains and providing nucleation sites for C–S–H growth (as evidenced by FT-IR peak shifts).
- (2)
- At the mesoscale (>1 μm), higher RCP loading (20–30%) reduced the cement content available for hydration products and created weak interfacial zones around RCP agglomerates (Figure 9B). This aligns with findings by Xiao et al. [13], who reported a critical threshold at 25% RCP, beyond which net porosity increased. Additionally, the interstitial porosity (i.e., air void content) increased significantly with RCP additions. This observation is critical, as higher porosity may negatively influence the mechanical properties and durability of recycled cement-based composites [43].
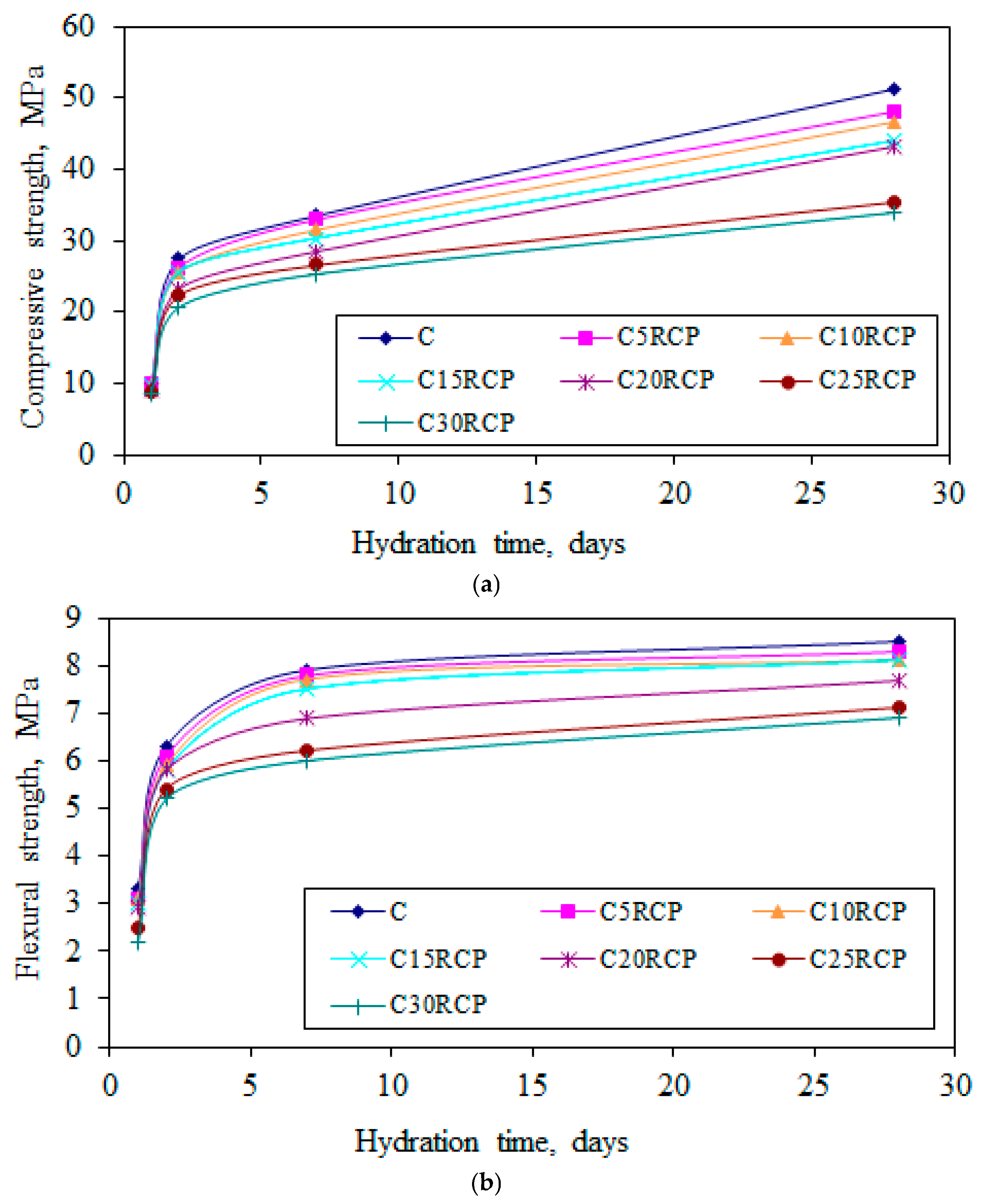
3.6. Compressive and Flexural Strength
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| RCP | Recycled Concrete Powder |
| TGA | Thermogravimetric Analysis |
| CDW | Construction and Demolition Waste |
| RCA | Recycled Concrete Aggregate |
| ITZ | Interfacial Transition Zone |
| SCM | Supplementary Cementitious Material |
| FT-IR | Fourier-Transform Infrared Spectroscopy |
| SEM | Scanning Electron Microscope |
| DTG | Derivative Thermogravimetry |
References
- Al-Kheetan, M.J.; Rahman, M.M.; Balakrishna, M.N.; Chamberlain, D.A. Performance enhancement of self-compacting concrete in saline environment by hydrophobic surface protection. Can. J. Civ. Eng. 2019, 46, 677–686. [Google Scholar] [CrossRef]
- Chen, C.-F.; Wu, C.-T.; Lin, J.-Y. Challenges and Opportunities of Aging Houses and Construction and Demolition Waste in Taiwan. Buildings 2025, 15, 595. [Google Scholar] [CrossRef]
- Gherman, I.-E.; Lakatos, E.-S.; Clinci, S.D.; Lungu, F.; Constandoiu, V.V.; Cioca, L.I.; Rada, E.C. Circularity Outlines in the Construction and Demolition Waste Management: A Literature Review. Recycling 2023, 8, 69. [Google Scholar] [CrossRef]
- Likes, L.; Markandeya, A.; Haider, M.M.; Bollinger, D.; McCloy, J.S.; Nassiri, S. Recycled concrete and brick powders as supplements to Portland cement for more sustainable concrete. J. Clean. Prod. 2022, 364, 132651. [Google Scholar] [CrossRef]
- Rocha, J.H.A.; Filho, R.D.T. The utilization of recycled concrete powder as supplementary cementitious material in cement-based materials: A systematic literature review. J. Build. Eng. 2023, 76, 107319. [Google Scholar] [CrossRef]
- Li, S.; Gao, J.; Li, Q.; Zhao, X. Investigation of using recycled powder from the preparation of recycled aggregate as a supplementary cementitious material. Constr. Build. Mater. 2021, 267, 120976. [Google Scholar] [CrossRef]
- Rocha, J.H.A.; Filho, R.D.T. Physical-mechanical assessment to mortars including recycled concrete powder and metakaolin. Case Stud. Constr. Mater. 2024, 21, e03996. [Google Scholar] [CrossRef]
- Rocha, J.H.A.; Chileno, N.G.C.; Filho, R.D.T. Enhancing mortar performance: A comparative study on particle size of recycled concrete powder and metakaolin in binary and ternary blends. J. Build. Eng. 2024, 98, 111280. [Google Scholar] [CrossRef]
- Lin, Y.; He, T.; Da, Y.; Yang, R.; Zheng, D. Effects of recycled micro-powders mixing methods on the properties of recycled concrete. J. Build. Eng. 2023, 80, 107994. [Google Scholar] [CrossRef]
- Wang, R.; Yu, N.; Li, Y. Methods for improving the microstructure of recycled concrete aggregate: A review. Constr. Build. Mater. 2020, 242, 118164. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J.; Hu, X.; Ran, B.; Wu, T.; Zhou, X.; Xiong, Y. Physical performance, durability, and carbon emissions of recycled cement concrete and fully recycled concrete. Constr. Build. Mater. 2024, 447, 138128. [Google Scholar] [CrossRef]
- Guo, H.; Shi, C.; Guan, X.; Zhu, J.; Ding, Y.; Ling, T.-C.; Zhang, H.; Wang, Y. Durability of recycled aggregate concrete—A review. Cem. Concr. Compos. 2018, 89, 251–259. [Google Scholar] [CrossRef]
- Li, J.; Zhan, B.; Gao, P.; Hu, L.; Qiao, M.; Sha, H.; Yu, Q. Effects of recycled concrete powders on the rheology, setting and early age strength of cement paste. Constr. Build. Mater. 2023, 401, 132899. [Google Scholar] [CrossRef]
- Xiao, J.; Ma, Z.; Sui, T.; Akbarnezhad, A.; Duan, Z. Mechanical properties of concrete mixed with recycled powder produced from construction and demolition waste. J. Clean. Prod. 2018, 188, 720–731. [Google Scholar] [CrossRef]
- Liu, X.; Liu, L.; Lyu, K.; Li, T.; Zhao, P.; Liu, R.; Zuo, J.; Fu, F.; Shah, S.P. Enhanced early hydration and mechanical properties of cement-based materials with recycled concrete powder modified by nano-silica. J. Build. Eng. 2022, 50, 104175. [Google Scholar] [CrossRef]
- Xue, C.; Tian, G.; Zhang, Y.; Wang, Z.; Qiao, H.; Su, L. Study on the effect of particle size on the basic characteristics of RCP and hydration kinetics of slurry. Constr. Build. Mater. 2025, 470, 140540. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Bai, H.; Ma, L. Utilization of Recycled Concrete Powder in Cement Composite: Strength, Microstructure and Hydration Characteristics. J. Renew. Mater. 2021, 9, 2189–2208. [Google Scholar] [CrossRef]
- Rahimi, E.; Kawasaki, Y.; Yui, A.; Yamachi, Y. Fully Recycled Syntheses Using Recycled Concrete Powder, Oyster Shell and Wood Powder: Effect of Combined Ground Treatment on Mechanical Strength and FTIR, XRD, and SEM Characterization. Open J. Compos. Mater. 2025, 15, 44–57. [Google Scholar] [CrossRef]
- Barbir, D.; Dabić, P.; Krolo, P. Hydration Study of Ordinary Portland Cement in the Presence of Lead(II) Oxide. Chem. Biochem. Eng. Q. 2013, 27, 95–99. [Google Scholar]
- Juenger, M.C.G.; Snellings, R.; Bernal, S.A. Supplementary cementitious materials: New sources, characterization, and performance insights. Cem. Concr. Res. 2019, 122, 257–273. [Google Scholar] [CrossRef]
- De Weerdt, K.; Ben Haha, M.; Le Saout, G.; Kjellsen, K.O.; Justnes, H.; Lothenbach, B. Hydration mechanisms of ternary Portland cements containing limestone powder and fly ash. Cem. Concr. Res. 2011, 41, 279–291. [Google Scholar] [CrossRef]
- Snellings, R. Assessing, Understanding and Unlocking Supplementary Cementitious Materials. RILEM Tech. Lett. 2016, 1, 50–55. [Google Scholar] [CrossRef]
- Oey, T.; Kumar, A.; Bullard, J.W.; Neithalath, N.; Sant, G. The Filler Effect: The Influence of Filler Content and Surface Area on Cementitious Reaction Rates. J. Am. Ceram. Soc. 2013, 96, 1978–1990. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Ma, Y.; Gong, J.; Ouyang, X.; Li, Z.; Wang, H.; Fu, J. Roles of recycled concrete powder on the properties of alkali-activated slag: Reaction products and microstructure development. Compos. Part B Eng. 2025, 301, 112493. [Google Scholar] [CrossRef]
- Kurad, R.; Silvestre, J.D.; de Brito, J.; Ahmed, H. Effect of incorporation of high volume of recycled concrete aggregates and fly ash on the strength and global warming potential of concrete. J. Clean. Prod. 2017, 166, 485–502. [Google Scholar] [CrossRef]
- Rajabipour, F.; Weiss, J. Electrical conductivity of drying cement paste. Mater. Struct. 2007, 40, 1143–1160. [Google Scholar] [CrossRef]
- Snellings, R.; Mertens, G.; Elsen, J. Supplementary Cementitious Materials. Rev. Mineral. Geochem. 2012, 74, 211–278. [Google Scholar] [CrossRef]
- Fabiani, C.; Pisello, A.L.; D’Alessandro, A.; Ubertini, F.; Cabeza, L.F.; Cotana, F. Effect of PCM on the Hydration Process of Cement-Based Mixtures: A Novel Thermo-Mechanical Investigation. Materials 2018, 11, 871. [Google Scholar] [CrossRef]
- Ylmen, R.; Jaglid, U.; Steenari, B.-M.; Panas, I. Early hydration and setting of Portland cement monitored by IR, SEM and Vicat techniques. Cem. Concr. Res. 2009, 39, 433–439. [Google Scholar] [CrossRef]
- Mendoza, R.C.; Grande, J.O.; Acda, M.N. Effect of keratin fibers on setting and hydration characteristics of portland cement. J. Nat. Fibers 2021, 18, 1801–1808. [Google Scholar] [CrossRef]
- Wang, D.; Gong, Q.; Yuan, Q.; Luo, S. Review of the Properties of fiber-reinforced polymer-reinforced seawater-sea sand concrete. J. Mater. Civ. Eng. 2021, 33, 04021285. [Google Scholar] [CrossRef]
- Kuzielová, E.; Slaný, M.; Žemlička, M.; Másilko, J.; Palou, M.T. Phase Composition of Silica Fume-Portland Cement Systems Formed under Hydrothermal Curing Evaluated by FTIR, XRD, and TGA. Materials 2021, 14, 2786. [Google Scholar] [CrossRef] [PubMed]
- Manan, A.; Zhang, P.; Alattyih, W.; Alanazi, H.; Elagan, S.K.; Ahmad, J. Mechanical and microstructural characterization of sustainable concrete containing recycled concrete and waste rubber tire fiber. Mater. Res. Express 2024, 11, 085701. [Google Scholar] [CrossRef]
- Huang, J.; Chen, Y.; Yu, Q. Amorphous calcium carbonate formation from carbonated recycled cement powder: A novel carbonation-activated cementitious material. Compos Part B Eng. 2025, 297, 112336. [Google Scholar] [CrossRef]
- Wang, C.; Chazallon, C.; Braymand, S.; Hornych, P. Thermogravimetric analysis (TGA) for characterization of self-cementation of recycled concrete aggregates in pavement. Thermochim. Acta 2024, 733, 179680. [Google Scholar] [CrossRef]
- Alarcon-Ruiz, L.; Platret, G.; Massieu, E.; Ehrlacher, A. The use of thermal analysis in assessing the effect of temperature on a cement paste. Cem. Concr. Res. 2005, 35, 609–613. [Google Scholar] [CrossRef]
- Collier, N.C. Transition and decopomposition temperatures of cement phases—A collection of thermal analysis data. Ceram. Silik. 2016, 60, 338–343. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials, 1st ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2016. [Google Scholar]
- Zhang, D.; Zhang, S.; Huang, B.; Yang, Q.; Li, J. Comparison of mechanical, chemical, and thermal activation methods on the utilisation of recycled concrete powder from construction and demolition waste. J. Build. Eng. 2022, 61, 105295. [Google Scholar] [CrossRef]
- Sičáková, A.; Kim, J.; Bálintová, M.; Eštoková, A.; Junáková, N.; Orolin, P.; Ubysz, A. Contribution to the Prediction of the Recycling Potential of Recycled Concrete as a Cement Admixture Based on the Compressive Strength of the Parent Concrete. Int. J. Concr. Struct. Mater. 2024, 18, 80. [Google Scholar] [CrossRef]
- de la Villa Mencía, R.V.; Frías, M.; Ramírez, S.M.; Carrasco, L.F.; Gimenez, R.G. Concrete/glass construction and demolition waste (CDW) synergies in ternary eco-cement-paste mineralogy. Materials 2022, 15, 4661. [Google Scholar] [CrossRef]
- Trigo, A.P.M.; Liborio, J.B.L. Doping technique in the interfacial transition zone between paste and lateritic aggregate for the production of structural concretes. Mater. Res. 2013, 17, 16–22. [Google Scholar] [CrossRef]
- Wu, H.; Yang, D.; Ma, Z. Micro-structure, mechanical and transport properties of cementitious materials with high-volume waste concrete powder and thermal modification. Constr. Build. Mater. 2021, 313, 125477. [Google Scholar] [CrossRef]


| Component | Content (wt.%) | Property | Value |
|---|---|---|---|
| SiO2 | 22.85 | Blaine fineness (cm2/g) | 3300 |
| Al2O3 | 4.81 | Standard consistency (wt.%) | 30 |
| Fe2O3 | 2.79 | Initial setting time (min) | 85 |
| CaO | 65.23 | Final setting time (min) | 150 |
| MgO | 1.61 | Compressive strength (MPa) | |
| SO3 | 3.00 | - 3 days | 33.50 |
| K2O | 1.89 | - 28 days | 50.70 |
| Sample | TG/DTG Data (°C) | ΔW * (%) | ||||
|---|---|---|---|---|---|---|
| Phase 1 | Phase 2 | Phase 3 | T *onset | T *max | ||
| C | 30–411.4 | 411.4–620.9 | 620.9–900 | 411.4 | 422.2 | 74.3 |
| C5RCP | 30–410.9 | 410.9–624.6 | 624.6–900 | 410.9 | 421.7 | 74.3 |
| C10RCP | 30–411.8 | 411.8–638.9 | 638.9–900 | 411.8 | 421.7 | 74.7 |
| C15RCP | 30–416.1 | 416.1–647.4 | 647.4–900 | 416.1 | 426.3 | 72.8 |
| C20RCP | 30–416.9 | 416.9–658.1 | 658.1–900 | 416.9 | 428.3 | 73.4 |
| C25RCP | 30–416.3 | 416.3–660.2 | 660.2–900 | 416.3 | 428.4 | 73.4 |
| C30RCP | 30–422.5 | 422.5–669.2 | 669.2–900 | 422.5 | 433 | 73.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbir, D.; Dabić, P.; Jakić, M.; Weber, I. Evaluating the Heat of Hydration, Conductivity, and Microstructural Properties of Cement Composites with Recycled Concrete Powder. Buildings 2025, 15, 2613. https://doi.org/10.3390/buildings15152613
Barbir D, Dabić P, Jakić M, Weber I. Evaluating the Heat of Hydration, Conductivity, and Microstructural Properties of Cement Composites with Recycled Concrete Powder. Buildings. 2025; 15(15):2613. https://doi.org/10.3390/buildings15152613
Chicago/Turabian StyleBarbir, Damir, Pero Dabić, Miće Jakić, and Ivana Weber. 2025. "Evaluating the Heat of Hydration, Conductivity, and Microstructural Properties of Cement Composites with Recycled Concrete Powder" Buildings 15, no. 15: 2613. https://doi.org/10.3390/buildings15152613
APA StyleBarbir, D., Dabić, P., Jakić, M., & Weber, I. (2025). Evaluating the Heat of Hydration, Conductivity, and Microstructural Properties of Cement Composites with Recycled Concrete Powder. Buildings, 15(15), 2613. https://doi.org/10.3390/buildings15152613






