Non-Ferrous Metal Smelting Slags for Thermal Energy Storage: A Mini Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Copper Slag
2.2. Nickel Slag

| Samples | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | SO3 | Sum | Reference |
|---|---|---|---|---|---|---|---|---|---|
| EFNS-W | 58.10 | 2.29 | 11.10 | 0.29 | 26.50 | 0.06 | 0.04 | 98.38 | [109] |
| EFNS-A | 62.80 | 1.95 | 7.13 | 2.07 | 24.70 | 0.02 | 0.03 | 98.7 | [109] |
| EFNS-W | 46.10 | 4.46 | 12.25 | 6.75 | 27.12 | 0.07 | 0.14 | 96.89 | [110] |
| EFNS-W | 45.23 | 5.91 | 9.74 | 8.93 | 24.17 | - | - | 93.98 | [111] |
| EFNS-W | 32.74 | 8.32 | 43.83 | 3.73 | 2.76 | - | - | 91.38 | [112] |
| EFNS-W | 42.96 | 12.09 | 12.52 | 4.88 | 26.52 | 0.11 | - | 99.08 | [113] |
| BFNS-W | 27.31 | 21.82 | 1.57 | 32.72 | 8.64 | - | - | 92.06 | [114] |
| BFNS-W | 29.95 | 26.31 | 1.55 | 25.19 | 8.93 | 0.4 | 0.9 | 93.23 | [110] |
| BFNS-W | 31.76 | 14.84 | 0.6 | 36.44 | 9.08 | - | 0.04 | 92.76 | [115] |
| BFNS-A | 41.14 | 5.69 | 6.87 | 25.96 | 15.40 | 0.54 | 1.29 | 96.89 | [116] |
2.3. Lead Slag
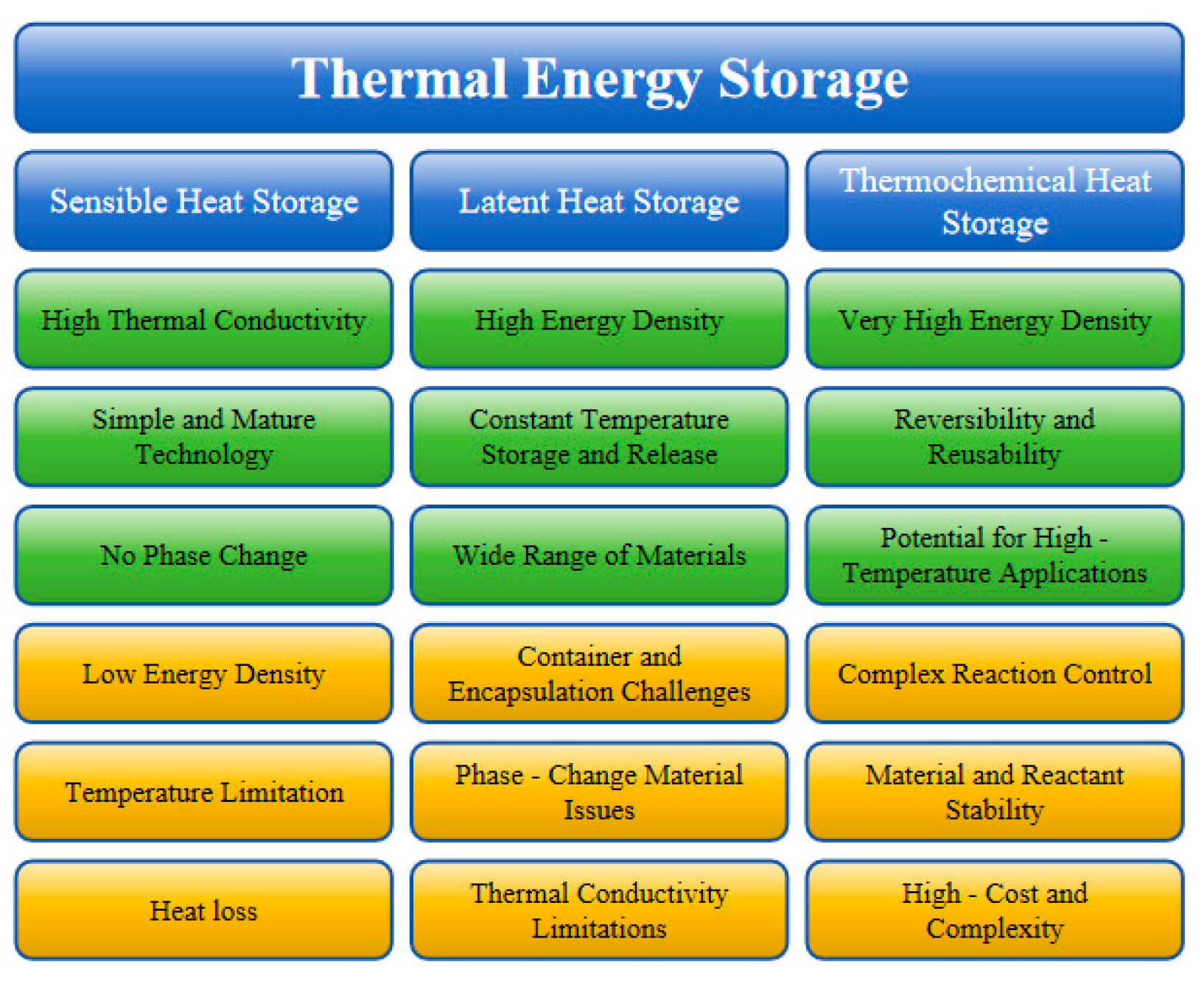
3. Application of Sensible Heat Storage
3.1. The Application of Copper Slag in Sensible Heat Storage
3.2. The Application of Nickel Slag in Sensible Heat Storage
3.3. The Application of Lead Slag in Sensible Heat Storage
3.4. Analysis and Comparisons for Sensible Heat Storage
- (1)
- Copper Slag: It is favored for its low cost and high thermal performance. This makes it a popular choice for developing sensible heat storage materials. Researchers have more experience in this area and it has been widely used in relevant applications.
- (2)
- Nickel Slag: Rich in SiO2, Al2O3, Fe2O3, and MgO, nickel slag has good cementing and durability. It is commonly used in building materials like concrete and road base materials. However, its thermophysical properties for SHS need more research.
- (3)
- Lead Slag: Containing heavy metals like lead and cadmium, lead slag is under-researched in SHS. Its potential applications are hindered. Researchers are particularly interested in its behavior in alkaline conditions. It is found that proper alkaline conditions can significantly reduce heavy metal leaching. This paves the way for its potential uses in other fields.
4. Application of Latent Heat Storage
4.1. The Application of Copper Slag in Latent Heat Storage
4.2. The Application of Nickel Slag in Latent Heat Storage
4.3. The Application of Lead Slag in Latent Heat Storage
4.4. Analysis and Comparisons for Latent Heat Storage
- (1)
- Copper Slag: It has extensive research and practical applications in the realm of sensible heat storage materials. Importantly, its role in latent heat storage applications has also gained significant attention. Research has demonstrated that through various modification techniques, including grinding, sieving, and heat treatment, the sensible heat storage performance of copper slag can be substantially enhanced. Moreover, by integrating copper slag with other high-performance materials, it is possible to develop composite materials that exhibit remarkably improved thermal conductivity and heat storage capacity. These advancements not only broaden the application scope of copper slag in thermal energy storage but also offer innovative solutions for enhancing the efficiency of energy storage systems.
- (2)
- Nickel Slag: As a major solid waste, the excessive accumulation of nickel slag in China poses significant disposal challenges. Yet, its potential social and economic benefits have spurred extensive scholarly exploration. Notably, studies on creating composite phase change thermal storage materials from nickel slag and other solid wastes have grown, achieving promising lab results that have improved nickel slag utilization. These innovations robustly underpin the sustainable development of China’s metallurgical industry and solidify the foundation for future theoretical research.
- (3)
- Lead Slag: Current research on lead slag shows limited direct applications in sensible and LHS. However, the above studies indicate that glass–ceramic materials prepared from lead slag possess both high-temperature resistance and excellent heavy metal leaching resistance, meeting the basic requirements for skeleton materials in high-temperature composite phase change heat storage materials. Specific applications in this field require further exploration.
5. Other Metallurgical Industry Waste
6. Discussion
7. Conclusions and Prospect
- (1)
- Metal slag is rich in a variety of metal elements or metal oxides, showing superior SHS performance, which makes it a research hotspot in the field of heat storage materials.
- (2)
- Different metal slags can be blended with other slags to produce porous ceramic skeleton materials due to their varying oxides and metal elements. For instance, SS, FNS, and FA are utilized to create anorthite cordierite porous ceramics. Mixtures of lead slag, lead–zinc tailings, borax water-quenched slag, and albite form glass ceramics. Nickel slag combined with waste glass powder yields foam ceramics. Secondary aluminum slag and FNS added to FA produce skeleton materials, while copper slag mixed with bauxite results in porous skeleton materials. These materials exhibit excellent thermal stability and mechanical properties, making them ideal for high-temperature thermal storage applications. Additionally, metal slags can be pressed into thermal insulation concrete bricks for building insulation, promoting energy conservation and environmental protection
- (3)
- Although metal slag has broad application prospects in the field of heat storage, previous research shows that the application research of zinc slag, lead slag and other metal slag in the field of heat storage is still relatively scarce. Part of the research on slag mainly focuses on oxidation–reduction extraction and so on, but its potential in heat storage is still insufficient.
- (1)
- The focus will be on the analysis of the components and properties of various metal slags, enhancing their energy storage capacity and thermal stability, and exploring novel processing techniques and additives.
- (2)
- The pursuit of high cost effectiveness and eco-efficiency in slag-based energy storage systems involves applying new slag-based heat storage materials to renewable energy fields. This offers a novel research avenue for developing innovative materials with combined energy storage and other functional properties.
Author Contributions
Funding
Conflicts of Interest
References
- Feng, P.H.; Zhao, B.C.; Wang, R.Z. Thermophysical heat storage for cooling, heating, and power generation: A review. Appl. Therm. Eng. 2020, 166, 114728. [Google Scholar] [CrossRef]
- Gur, I.; Sawyer, K.; Prasher, R. Searching for a better thermal battery. Science 2012, 335, 1454–1455. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.; Gupta, A.K.; Mochida, S. High temperature air combustion (HiTAC): How it all started for applications in industrial furnaces and future prospects. Appl. Energy 2020, 278, 115551. [Google Scholar] [CrossRef]
- Thonon, M.; Fraisse, G.; Zalewski, L.; Pailha, M. Simultaneous charging and discharging processes in latent heat thermal energy storage: A review. Therm. Sci. Eng. Prog. 2024, 47, 102299. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, A.; Zhao, Y.; Xu, Q.; Ding, Y. A Mini Review on Sewage Sludge and Red Mud Recycling for Thermal Energy Storage. Energies 2024, 17, 2079. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Gonzalez-Aguilar, J.; Romero, M.; Coronado, J.M. Solar Energy on Demand: A Review on High Temperature Thermochemical Heat Storage Systems and Materials. Chem. Rev. 2019, 119, 4777–4816. [Google Scholar] [CrossRef]
- Liu, M.; Saman, W.; Bruno, F. Review on storage materials and thermal performance enhancement techniques for high temperature phase change thermal storage systems. Renew. Sustain. Energy Rev. 2012, 16, 2118–2132. [Google Scholar] [CrossRef]
- Asegun Henry, R.P. The Prospect of High Temperature Solid State Energy Conversion to reduce the cost of concentrated solar power. Energy Environ. Sci. 2014, 7, 1819–1828. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, C.Y.; Markides, C.N.; Wang, H.; Li, W. Medium- and high-temperature latent and thermochemical heat storage using metals and metallic compounds as heat storage media: A technical review. Appl. Energy 2020, 280, 115950. [Google Scholar] [CrossRef]
- Kumar, L.; Hasanuzzaman, M.; Rahim, N.A. Global advancement of solar thermal energy technologies for industrial process heat and its future prospects: A review. Energy Convers. Manag. 2019, 195, 885–908. [Google Scholar] [CrossRef]
- Yang, C.Y.; Reijonen, I.; Yu, H.; Dharmarajan, R.; Seshadri, B.; Bolan, N.S. Back to _basic slags as a phosphorous source and liming material. In Soil Amendments For Sustainability: Challenges and Perspectives; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2018. [Google Scholar]
- Seetharaman, S.; Guthrie, R.; McLean, A.; Seetharaman, S.; Sohn, H.Y. Treatise on Process Metallurgy; Elsevier: Amsterdam, The Netherlands, 2013; Volume 3: Industrial Processer. [Google Scholar]
- US Geological Survey. Mineral Commodity Summaries: Iron and Steel Slag. 2025. Available online: https://www.usgs.gov/centers/nmic/iron-and-steel-slag-statistics-and-information?qt-science_support_page_related_con=0#qt-science_support_page_related_con (accessed on 3 July 2025).
- Biava, G.; Depero, L.E.; Bontempi, E. Accelerated Carbonation of Steel Slag and Their Valorisation in Cement Products: A Review. Span. J. Soil Sci. 2024, 14, 12908. [Google Scholar] [CrossRef]
- Gao, W.; Zhou, W.; Lyu, X.; Liu, X.; Su, H.; Li, C.; Wang, H. Comprehensive utilization of steel slag: A review. Powder Technol. 2023, 422, 118449. [Google Scholar] [CrossRef]
- Klaffenbach, E.; Montenegro, V.; Guo, M.; Blanpain, B. Sustainable and Comprehensive Utilization of Copper Slag: A Review and Critical Analysis. J. Sustain. Metall. 2023, 9, 468–496. [Google Scholar] [CrossRef]
- Tian, H.; Guo, Z.; Pan, J.; Zhu, D.; Yang, C.; Xue, Y.; Li, S.; Wang, D. Comprehensive review on metallurgical recycling and cleaning of copper slag. Resour. Conserv. Recycl. 2021, 168, 105366. [Google Scholar] [CrossRef]
- Madheswaran, C.K.; Ambily, P.S.; Dattatreya, J.K.; Rajamane, N.P. Studies on use of Copper Slag as Replacement Material for River Sand in Building Constructions. J. Inst. Eng. (India) Ser. A 2014, 95, 169–177. [Google Scholar] [CrossRef]
- Shen, H.; Forssberg, E. An overview of recovery of metals from slags. Waste Manag. 2003, 23, 933–949. [Google Scholar] [CrossRef]
- Gorai, B.; Jana, R.K.; Premchand. Characteristics and utilisation of copper slag—A review. Resour. Conserv. Recycl. 2003, 39, 299–313. [Google Scholar] [CrossRef]
- González, C.; Parra, R.; Klenovcanova, A.; Imris, I.; Sánchez, M. Reduction of Chilean copper slags: A case of waste management project. Scand. J. Metall. 2005, 34, 143–149. [Google Scholar] [CrossRef]
- Edraki, M.; Baumgartl, T.; Manlapig, E.; Bradshaw, D.; Franks, D.M.; Moran, C.J. Designing mine tailings for better environmental, social and economic outcomes: A review of alternative approaches. J. Clean. Prod. 2014, 84, 411–420. [Google Scholar] [CrossRef]
- Phiri, T.C.; Singh, P.; Nikoloski, A.N. The potential for copper slag waste as a resource for a circular economy: A review—Part II. Miner. Eng. 2021, 172, 107150. [Google Scholar] [CrossRef]
- Busolic, D.; Parada, F.; Parra, R.; Sanchez, M.; Palacios, J.; Hino, M. Recovery of iron from copper flash smelting slags. Miner. Process. Extr. Metall. 2013, 120, 32–36. [Google Scholar] [CrossRef]
- Bulut, G. Recovery of copper and cobalt from ancient slag. Waste Manag. Res. 2006, 24, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Gabasiane, T.S.; Danha, G.; Mamvura, T.A.; Mashifana, T.; Dzinomwa, G. Environmental and Socioeconomic Impact of Copper Slag—A Review. Crystals 2021, 11, 1504. [Google Scholar] [CrossRef]
- Alp, I.; Deveci, H.; Sungun, H. Utilization of flotation wastes of copper slag as raw material in cement production. J. Hazard. Mater. 2008, 159, 390–395. [Google Scholar] [CrossRef]
- Rasoul Abdar Esfahani, S.M.; Zareei, S.A.; Madhkhan, M.; Ameri, F.; Rashidiani, J.; Taheri, R.A. Mechanical and gamma-ray shielding properties and environmental benefits of concrete incorporating GGBFS and copper slag. J. Build. Eng. 2021, 33, 101615. [Google Scholar] [CrossRef]
- Sridharan, M.; Madhavi, T.C. Investigating the influence of copper slag on the mechanical behaviour of concrete. Mater. Today Proc. 2021, 46, 3225–3232. [Google Scholar] [CrossRef]
- Al-Jabri, K.S.; Al-Saidy, A.H.; Taha, R. Effect of copper slag as a fine aggregate on the properties of cement mortars and concrete. Constr. Build. Mater. 2011, 25, 933–938. [Google Scholar] [CrossRef]
- Potysz, A.; van Hullebusch, E.D.; Kierczak, J.; Grybos, M.; Lens, P.N.L.; Guibaud, G. Copper Metallurgical Slags—Current Knowledge and Fate: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2424–2488. [Google Scholar] [CrossRef]
- Kundu, T.; Senapati, S.; Das, S.K.; Angadi, S.I.; Rath, S.S. A comprehensive review on the recovery of copper values from copper slag. Powder Technol. 2023, 426, 118693. [Google Scholar] [CrossRef]
- Coelho, L.M.; Guimarães, A.C.R.; Alves Moreira, C.R.C.L.; dos Santos, G.P.P.; Monteiro, S.N.; da Silveira, P.H.P.M. Feasibility of Using Ferronickel Slag as a Sustainable Alternative Aggregate in Hot Mix Asphalt. Sustainability 2024, 16, 8642. [Google Scholar] [CrossRef]
- Yanning, S.; Qiao, H.; Qiong, F.; Chao, W.; Jianghua, Z. Application of metallurgical ferronickel slag in building materials: A review. J. Build. Eng. 2024, 96, 110632. [Google Scholar] [CrossRef]
- Wang, D.; Ma, B.; Pang, L.; Wang, Q. Alkali-activated blast furnace ferronickel slag for Cr immobilization. Cem. Concr. Compos. 2024, 150, 105560. [Google Scholar] [CrossRef]
- Xi, B.; Li, R.; Zhao, X.; Dang, Q.; Zhang, D.; Tan, W. Constraints and opportunities for the recycling of growing ferronickel slag in China. Resour. Conserv. Recycl. 2018, 139, 15–16. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Castel, A.; Kim, T.; Khan, M.S.H. Performance of fly ash concrete with ferronickel slag fine aggregate against alkali-silica reaction and chloride diffusion. Cem. Concr. Res. 2021, 139, 106265. [Google Scholar] [CrossRef]
- Gu, Y.-c.; Li, J.-L.; Peng, J.-K.; Xing, F.; Long, W.-J.; Khayat, K.H. Immobilization of hazardous ferronickel slag treated using ternary limestone calcined clay cement. Constr. Build. Mater. 2020, 250, 118837. [Google Scholar] [CrossRef]
- Nowinska, K.; Adamczyk, Z. Zinc and Lead Metallurgical Slags as a Potential Source of Metal Recovery: A Review. Materials 2023, 16, 7295. [Google Scholar] [CrossRef]
- Pan, D.a.; Li, L.; Tian, X.; Wu, Y.; Cheng, N.; Yu, H. A review on lead slag generation, characteristics, and utilization. Resour. Conserv. Recycl. 2019, 146, 140–155. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, S.; Zhang, P.; Elmaadawy, K.; Ke, Y.; Li, J.; Li, M.; Hu, J.; Liu, B.; Yang, J.; et al. Microwave enhanced solidification/stabilization of lead slag with fly ash based geopolymer. J. Clean. Prod. 2020, 272, 122957. [Google Scholar] [CrossRef]
- Tukker, A.; Buist, H.; van Oers, L.; van der Voet, E. Risks to health and environment of the use of lead in products in the EU. Resour. Conserv. Recycl. 2006, 49, 89–109. [Google Scholar] [CrossRef]
- Mor, S.; Ravindra, K.; Dahiyaa, R.P.; Chandra, A. Leachate Characterization and assessment of groundwater pollution near municipal solid waste landfill site. Environ. Monit. Assess. 2006, 118, 435–456. [Google Scholar] [CrossRef]
- Hilts, S.R. Effect of smelter emission reductions on children’s blood lead levels. Sci. Total Environ. 2003, 303, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ogundiran, M.B.; Ogundele, D.T.; Afolayan, P.G.; Osibanjo, O. Heavy Metals Levels in Forage Grasses, Leachate and Lactating CowsReared around Lead Slag Dumpsites in Nigeria. Int. J. Environ. Res. 2012, 63, 695–702. [Google Scholar]
- Saca, N.; Radu, L.; Fugaru, V.; Gheorghe, M.; Petre, I. Composite materials with primary lead slag content: Application in gamma radiation shielding and waste encapsulation fields. J. Clean. Prod. 2018, 179, 255–265. [Google Scholar] [CrossRef]
- Yan, P.; Chen, B.; Aminul Haque, M.; Liu, T. Influence of red mud on the engineering and microstructural properties of sustainable ultra-high performance concrete. Constr. Build. Mater. 2023, 396, 132404. [Google Scholar] [CrossRef]
- Liu, X.; Han, Y.; He, F.; Gao, P.; Yuan, S. Characteristic, hazard and iron recovery technology of red mud—A critical review. J. Hazard. Mater. 2021, 420, 126542. [Google Scholar] [CrossRef]
- Wu, P.; Liu, X.; Zhang, Z.; Wei, C. Properties of red mud-filled and modified resin composites. Constr. Build. Mater. 2023, 409, 133984. [Google Scholar] [CrossRef]
- Xue, S.; Li, M.; Jiang, J.; Millar, G.J.; Li, C.; Kong, X. Phosphogypsum stabilization of bauxite residue: Conversion of its alkaline characteristics. J. Environ. Sci. 2019, 77, 1–10. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Z.; Zhu, H.; Wang, Z.; Guo, J.; Zhang, X.; Yu, H.; Yue, X.; Ning, P.; Li, B. The roles of red mud as desulfurization and denitrification in flue gas: A review. J. Environ. Chem. Eng. 2023, 11, 109770. [Google Scholar] [CrossRef]
- Nganda, A.; Srivastava, P.; Lamba, B.Y.; Pandey, A.; Kumar, M. Advances in the fabrication, modification, and performance of biochar, red mud, calcium oxide, and bentonite catalysts in waste-to-fuel conversion. Environ. Res. 2023, 232, 116284. [Google Scholar] [CrossRef]
- Occhicone, A.; Graziuso, S.G.; De Gregorio, E.; Montagnaro, F.; Ricciotti, L.; Tarallo, O.; Roviello, G.; Ferone, C. Synthesis and characterization of new acid-activated red mud-metakaolin geopolymers and comparison with their alkaline counterparts. J. Clean. Prod. 2024, 435, 140492. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Liu, Z. Red mud-based catalysts for the catalytic removal of typical air pollutants: A review. J. Environ. Sci. 2023, 127, 628–640. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Cordeiro, E.; Scaratti, G.; de Souza, D.C.S.; Nickel, C.D.M.; José, H.J.; de Fátima Peralta Muniz Moreira, R.; De Noni, A. Red mud as catalyst for the treatment of pharmaceuticals compounds by advanced oxidation processes—A review. Environ. Nanotechnol. Monit. Manag. 2024, 21, 100938. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, Z.; Wang, K.; Wang, F.; Jiang, H.; Liang, M.; Wei, J.; Airey, G. Sustainable utilization of bauxite residue (Red Mud) as a road material in pavements: A critical review. Constr. Build. Mater. 2021, 270, 121419. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, J.; Li, H.; Zhao, P.; Chen, Q. Recycling red mud from the production of aluminium as a red cement-based mortar. Waste Manag. Res. 2017, 35, 500–507. [Google Scholar] [CrossRef]
- Ke, W.; Li, C.; Zhu, F.; Luo, X.; Feng, J.; Li, X.; Jiang, Y.; Wu, C.; Hartley, W.; Xue, S. Effect of potentially toxic elements on soil multifunctionality at a lead smelting site. J. Hazard. Mater. 2023, 454, 131525. [Google Scholar] [CrossRef]
- Yang, S.; Yao, X.; Li, J.; Wang, X.; Zhang, C.; Wu, S.; Wang, K.; Wang, W. Preparation and properties of ready-to-use low-density foamed concrete derived from industrial solid wastes. Constr. Build. Mater. 2021, 287, 122946. [Google Scholar] [CrossRef]
- Ren, C.; Wang, W.; Li, G. Preparation of high-performance cementitious materials from industrial solid waste. Constr. Build. Mater. 2017, 152, 39–47. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, D.; Dong, L.; Xu, Y.; Liu, J. Pollution Status and Environmental Sound Management (ESM) Trends on Typical General Industrial Solid Waste. Procedia Environ. Sci. 2016, 31, 615–620. [Google Scholar] [CrossRef]
- Ananth, S.; Selvakumar, P. Experimental investigation of sand-based sensible heat energy storage system. Appl. Therm. Eng. 2025, 264, 125390. [Google Scholar] [CrossRef]
- Abubakar, S.; Umaru, S.; Kaisan, M.U.; Umar, U.A.; Ashok, B.; Nanthagopal, K. Development and performance comparison of mixed-mode solar crop dryers with and without thermal storage. Renew. Energy 2018, 128, 285–298. [Google Scholar] [CrossRef]
- Kedida, D.K.; Amibe, D.A.; Birhane, Y.T. Performance of a Pebble Bed Thermal Storage Integrated with Concentrating Parabolic Solar Collector for Cooking. J. Renew. Energy 2019, 2019, 4238549. [Google Scholar] [CrossRef]
- Wang, S.; Abdulridha, A.; Naito, C.; Quiel, S.; Suleiman, M.; Romero, C.; Neti, S. Enhancement of conventional concrete mix designs for sensible thermal energy storage applications. J. Energy Storage 2023, 61, 106735. [Google Scholar] [CrossRef]
- Lakshmi, D.V.N.; Layek, A.; Kumar, P.M. Performance Analysis of Trapezoidal Corrugated Solar Air Heater with Sensible Heat Storage Material. Energy Procedia 2017, 109, 463–470. [Google Scholar] [CrossRef]
- Hu, B.; Wang, H.; Yang, J.; Li, J.; Li, C.; Ren, X. Experimental and numerical investigation on heating performance comparison of air conditioning heating systems based on water-phase change material heat storage system and water heat storage system. Energy 2025, 331, 137005. [Google Scholar] [CrossRef]
- Mehla, N.; Kumar, A. Experimental evaluation of used engine oil based thermal energy storage coupled with novel evacuated tube solar air collector (NETAC). J. Energy Storage 2021, 39, 102656. [Google Scholar] [CrossRef]
- Ndukwu, M.C.; Onyenwigwe, D.; Abam, F.I.; Eke, A.B.; Dirioha, C. Development of a low-cost wind-powered active solar dryer integrated with glycerol as thermal storage. Renew. Energy 2020, 154, 553–568. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Ganapathiraman, S.; Manickam, P. Experimental studies on evacuated tube collector with in-built energy storage—Waste car engine oil as sensible heat storage medium. J. Energy Storage 2024, 84, 110929. [Google Scholar] [CrossRef]
- Abddaim, E.; Sakami, S.; El Hassnaoui, A.; Boukhattem, L. Impact of temperature on chemical, thermo-physical, and mechanical properties of four rock materials for sensible thermal energy storage. J. Energy Storage 2024, 89, 111602. [Google Scholar] [CrossRef]
- Grirate, H.; Agalit, H.; Zari, N.; Elmchaouri, A.; Molina, S.; Couturier, R. Experimental and numerical investigation of potential filler materials for thermal oil thermocline storage. Sol. Energy 2016, 131, 260–274. [Google Scholar] [CrossRef]
- Liu, J.; Chang, Z.; Wang, L.; Xu, J.; Kuang, R.; Wu, Z. Exploration of Basalt Glasses as High-Temperature Sensible Heat Storage Materials. ACS Omega 2020, 5, 19236–19246. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Almeida, F.; Neto, F. Development of rock sensible heat storage system: Modeling of a hematite reservoir. J. Energy Storage 2021, 40, 102818. [Google Scholar] [CrossRef]
- Khare, S.; Dell’Amico, M.; Knight, C.; McGarry, S. Selection of materials for high temperature sensible energy storage. Sol. Energy Mater. Sol. Cells 2013, 115, 114–122. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Li, H.; Zhou, J.; Cen, K. Thermal properties and friction behaviors of slag as energy storage material in concentrate solar power plants. Sol. Energy Mater. Sol. Cells 2018, 182, 21–29. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Z.; Zhu, Y.; Zhang, H.; Yan, M.; Liu, D.; Hao, J. Preparation and characterization of novel low-cost sensible heat storage materials with steel slag. J. Energy Storage 2024, 76, 109643. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, K.; Ding, Y.; Liang, X.; Wang, S.; Gao, X.; Zhang, Z. Fabrication and thermal properties of CaCl2·6H2O–CO(NH2)2/SiO2 as room-temperature shape-stable composite PCM for building thermal insulation. Sol. Energy Mater. Sol. Cells 2021, 232, 111355. [Google Scholar] [CrossRef]
- Harikrishnan, S.; Devaraju, A.; Sivasamy, P.; Kalaiselvam, S. Experimental Investigation of Improved Thermal Characteristics of SiO2/myristic acid Nanofluid as Phase Change Material (PCM). Mater. Today Proc. 2019, 9, 397–409. [Google Scholar] [CrossRef]
- Yoo, D.H.; Jeon, I.K.; Kim, H.G.; Lee, J.S.; Ryou, J.-S. Experimental evaluation of fire resistance performance of cement mortar with PCM/Mg(OH)2-based composite fine aggregate. Constr. Build. Mater. 2021, 287, 123018. [Google Scholar] [CrossRef]
- Yu, Q.; Jiang, Z.; Cong, L.; Lu, T.; Suleiman, B.; Leng, G.; Wu, Z.; Ding, Y.; Li, Y. A novel low-temperature fabrication approach of composite phase change materials for high temperature thermal energy storage. Appl. Energy 2019, 237, 367–377. [Google Scholar] [CrossRef]
- Chai, Z.; Chen, X.; Fang, M.; Min, X.; Huang, Z.; Wang, Q.; Liu, C.; Liu, Y.; Huang, F. Fabrication and properties of high-thermal-storage RTO ceramics using bauxite tailings and red mud. Ceram. Int. 2023, 49, 31342–31350. [Google Scholar] [CrossRef]
- Jeong, S.-G.; Jeon, J.; Lee, J.-H.; Kim, S. Optimal preparation of PCM/diatomite composites for enhancing thermal properties. Int. J. Heat Mass Transf. 2013, 62, 711–717. [Google Scholar] [CrossRef]
- Xiao, J.; Huang, J.; Zhu, P.; Wang, C.; Li, X. Preparation, characterization and thermal properties of binary nitrate salts/expanded graphite as composite phase change material. Thermochim. Acta 2014, 587, 52–58. [Google Scholar] [CrossRef]
- Dora, S.; Mini, K.M. Performance assessment of capric acid-ethyl alcohol/expanded vermiculite phase change material incorporated cement mortar for thermal insulation in buildings. J. Energy Storage 2023, 72, 108550. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, Z.; Zhang, T.; Zhang, D. Preparation and thermophysical properties of three-dimensional attapulgite based composite phase change materials. J. Energy Storage 2020, 32, 101847. [Google Scholar] [CrossRef]
- Buonomo, B.; Rita Golia, M.; Manca, O.; Nardini, S. A review on thermal energy storage with phase change materials enhanced by metal foams. Therm. Sci. Eng. Prog. 2024, 53, 102732. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, J.; Zhang, W.; Xu, J.; Wang, B.; Wang, X.; Zhou, J.; Guo, H.; Liu, Y. Composites with a Novel Core–shell Structural Expanded Perlite/Polyethylene glycol Composite PCM as Novel Green Energy Storage Composites for Building Energy Conservation. Appl. Energy 2023, 330, 120363. [Google Scholar] [CrossRef]
- Ren, Y.; Xu, C.; Yuan, M.; Ye, F.; Ju, X.; Du, X. Ca(NO3)2-NaNO3/expanded graphite composite as a novel shape-stable phase change material for mid- to high-temperature thermal energy storage. Energy Convers. Manag. 2018, 163, 50–58. [Google Scholar] [CrossRef]
- Wen, R.; Xu, Y.; Xu, Y.; Sun, Z.; Zhang, L.; Qiao, J.; Wu, X.; Min, X.; Huang, Z. Attapulgite: A promising natural mineral as carrier material for fatty acids phase change material. J Therm Anal Calorim 2022, 147, 7203–7212. [Google Scholar] [CrossRef]
- Soleimanpour, S.; Sadrameli, S.M.; Mousavi, S.A.H.S.; Jafaripour, M. Preparation and characterization of high temperature shape stable NaNO3/diatomite phase change materials with nanoparticles for solar energy storage applications. J. Energy Storage 2022, 45, 103735. [Google Scholar] [CrossRef]
- Rathore, P.K.S.; Shukla, S.k. Improvement in thermal properties of PCM/Expanded vermiculite/expanded graphite shape stabilized composite PCM for building energy applications. Renew. Energy 2021, 176, 295–304. [Google Scholar] [CrossRef]
- Xiong, Y.; Fan, Y.; Wu, Y.; Ren, J.; Li, X.; Yao, C.; Zhao, Y.; Xu, Q.; Ding, Y. Low-carbon shape-stable phase change composite utilizing semi-coke ash for building thermal energy storage. Sol. Energy Mater. Sol. Cells 2024, 270, 112823. [Google Scholar] [CrossRef]
- Xiong, Y.; Yang, Y.; Zhang, A.; Ren, J.; Xu, Q.; Wu, Y.; Zhao, Y.; Ding, Y. Investigation on low-carbon shape-stable phase change composite by steel slag and carbide slag for solar thermal energy storage. J. Energy Storage 2024, 76, 109736. [Google Scholar] [CrossRef]
- Zheng, W.; He, D.; Wang, Y.; Chen, J.; Xue, M.; Li, H. Preparation of cement-based color facing mortar by copper pyrometallurgical slag modification: Efficient utilization of high-iron-content slag. J. Environ. Chem. Eng. 2021, 9, 105888. [Google Scholar] [CrossRef]
- Li, Z.; Ma, G.; Zheng, D.; Zhang, X. Effect of Fe2O3 on the crystallization behavior, microstructure and properties of glass-ceramics prepared from the modified tailings after reduction of copper slag. Mater. Today Commun. 2022, 31, 103516. [Google Scholar] [CrossRef]
- Heo, J.H.; Kim, B.-S.; Park, J.H. Effect of CaO Addition on Iron Recovery from Copper Smelting Slags by Solid Carbon. Metall. Mater. Trans. B 2013, 44, 1352–1363. [Google Scholar] [CrossRef]
- Calderón-Vásquez, I.; Segovia, V.; Cardemil, J.M.; Barraza, R. Assessing the use of copper slags as thermal energy storage material for packed-bed systems. Energy 2021, 227, 120370. [Google Scholar] [CrossRef]
- Navarro, M.E.; Martínez, M.; Gil, A.; Fernández, A.I.; Cabeza, L.F.; Olives, R.; Py, X. Selection and characterization of recycled materials for sensible thermal energy storage. Sol. Energy Mater. Sol. Cells 2012, 107, 131–135. [Google Scholar] [CrossRef]
- Ortega, I.; Faik, A.; Gil, A.; Rodríguez-Aseguinolaza, J.; D’Aguanno, B. Thermo-physical Properties of a Steel-making by-product to be used as Thermal Energy Storage Material in a Packed-bed System. Energy Procedia 2015, 69, 968–977. [Google Scholar] [CrossRef]
- Ortega-Fernández, I.; Calvet, N.; Gil, A.; Rodríguez-Aseguinolaza, J.; Faik, A.; D’Aguanno, B. Thermophysical characterization of a by-product from the steel industry to be used as a sustainable and low-cost thermal energy storage material. Energy 2015, 89, 601–609. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Zang, X.; Xing, X. Structure and Phase Changes of Nickel Slag in Oxidation Treatment. Minerals 2020, 10, 313. [Google Scholar] [CrossRef]
- Hussain, A.; Abidi, I.H.; Tso, C.Y.; Chan, K.C.; Luo, Z.; Chao, C.Y.H. Thermal management of lithium ion batteries using graphene coated nickel foam saturated with phase change materials. Int. J. Therm. Sci. 2018, 124, 23–35. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Zhu, H.; Wu, Q.; Hu, Z.; Feng, Z.; Jia, Z. Preparation and properties of foam ceramic from nickel slag and waste glass powder. Ceram. Int. 2020, 46, 23623–23628. [Google Scholar] [CrossRef]
- Liu, J.; Xu, J.; Zhang, Y.; Su, Z.; Xu, C.; Jiang, T. Co-utilization of secondary aluminum dross and ferronickel slag for preparation of cordierite–mullite insulating ceramic. J. Am. Ceram. Soc. 2022, 106, 2049–2060. [Google Scholar] [CrossRef]
- Chen, S.; Chen, F.; Zheng, C.; Wu, Z. Dynamic mechanical properties of ferronickel slag powder-modified soil-cement using split-hopkinson pressure bar testing. Mater. Res. Express 2023, 10, 115503. [Google Scholar] [CrossRef]
- Liu, P.; Li, B.; Cheung, S.C.P.; Wu, W. Material and energy flows in rotary kiln-electric furnace smelting of ferronickel alloy with energy saving. Appl. Therm. Eng. 2016, 109, 542–559. [Google Scholar] [CrossRef]
- Choi, Y.C.; Choi, S. Alkali–silica reactivity of cementitious materials using ferro-nickel slag fine aggregates produced in different cooling conditions. Constr. Build. Mater. 2015, 99, 279–287. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Q.; Shi, M. Characteristics and reactivity of ferronickel slag powder. Constr. Build. Mater. 2017, 156, 773–789. [Google Scholar] [CrossRef]
- Cao, R.; Jia, Z.; Zhang, Z.; Zhang, Y.; Banthia, N. Leaching kinetics and reactivity evaluation of ferronickel slag in alkaline conditions. Cem. Concr. Res. 2020, 137, 106202. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Bartzas, G. Effect of sulphate and nitrate anions on heavy metal immobilisation in ferronickel slag geopolymers. Appl. Clay Sci. 2013, 73, 103–109. [Google Scholar] [CrossRef]
- Wang, M.; Feng, C.; Liu, Y.; Zhang, D.; Xiong, D.; Zhang, X. Enhancement of pozzolanic activity of high-magnesium nickel slag via phase separation by adding CaO. Mater. Today Commun. 2023, 34, 105427. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Q.; Lu, J. The effects of cations and concentration on reaction mechanism of alkali-activated blast furnace ferronickel slag. Compos. Part B Eng. 2022, 236, 109825. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Q.; Zhuang, S.; Yang, J. Evaluation of alkali-activated blast furnace ferronickel slag as a cementitious material: Reaction mechanism, engineering properties and leaching behaviors. Constr. Build. Mater. 2018, 188, 860–873. [Google Scholar] [CrossRef]
- Yanning, S.; Qiong, F.; Hongxia, Q.; Chao, W. Study on mechanically activated ferrous extraction tailing of nickel slag as cementitious materials: Physical and chemical properties, mechanical properties and reaction mechanism. Constr. Build. Mater. 2024, 422, 135748. [Google Scholar] [CrossRef]
- Sobanska, S.; Deneele, D.; Barbillat, J.; Ledésert, B. Natural weathering of slags from primary Pb–Zn smelting as evidenced by Raman microspectroscopy. Appl. Geochem. 2016, 64, 107–117. [Google Scholar] [CrossRef]
- Scheinert, M.; Kupsch, H.; Bletz, B. Geochemical investigations of slags from the historical smelting in Freiberg, Erzgebirge (Germany). Geochemistry 2009, 69, 81–90. [Google Scholar] [CrossRef]
- de Andrade Lima, L.R.P.; Bernardez, L.A. Characterization of the lead smelter slag in Santo Amaro, Bahia, Brazil. J. Hazard. Mater. 2011, 189, 692–699. [Google Scholar] [CrossRef]
- Ettler, V.; Komarkova, M.; Jehlicka, J.; Coufal, P.; Hradil, D.; Machovic, V.; Delorme, F. Leaching of lead metallurgical slag in citric solutions--implications for disposal and weathering in soil environments. Chemosphere 2004, 57, 567–577. [Google Scholar] [CrossRef]
- Seignez, N.; Gauthier, A.; Bulteel, D.; Buatier, M.; Recourt, P.; Damidot, D.; Potdevin, J.L. Effect of Pb-rich and Fe-rich entities during alteration of a partially vitrified metallurgical waste. J. Hazard. Mater. 2007, 149, 418–431. [Google Scholar] [CrossRef]
- Seignez, N.; Gauthier, A.; Bulteel, D.; Damidot, D.; Potdevin, J.-L. Leaching of lead metallurgical slags and pollutant mobility far from equilibrium conditions. Appl. Geochem. 2008, 23, 3699–3711. [Google Scholar] [CrossRef]
- Ettler, V.; Johan, Z.; Kříbek, B.; Šebek, O.; Mihaljevič, M. Mineralogy and environmental stability of slags from the Tsumeb smelter, Namibia. Appl. Geochem. 2009, 24, 1–15. [Google Scholar] [CrossRef]
- de Andrade Lima, L.R.P.; Bernardez, L.A. Evaluation of the chemical stability of a landfilled primary lead smelting slag. Environ. Earth Sci. 2012, 68, 1033–1040. [Google Scholar] [CrossRef]
- Ettler, V.; Johan, Z. 12 years of leaching of contaminants from Pb smelter slags: Geochemical/mineralogical controls and slag recycling potential. Appl. Geochem. 2014, 40, 97–103. [Google Scholar] [CrossRef]
- Yin, N.H.; Sivry, Y.; Guyot, F.; Lens, P.N.; van Hullebusch, E.D. Evaluation on chemical stability of lead blast furnace (LBF) and imperial smelting furnace (ISF) slags. J. Environ. Manag. 2016, 180, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Onisei, S.; Pontikes, Y.; Van Gerven, T.; Angelopoulos, G.N.; Velea, T.; Predica, V.; Moldovan, P. Synthesis of inorganic polymers using fly ash and primary lead slag. J. Hazard. Mater. 2012, 205–206, 101–110. [Google Scholar] [CrossRef]
- Segovia, A.V.; Cardemil, J.M.; Sancy, M.; Escobar, R. Analysis of copper slag from a Chilean foundry for application as filler material in thermal energy storage systems. J. Energy Storage 2025, 111, 115281. [Google Scholar] [CrossRef]
- Sibarani, D.; Hamuyuni, J.; Luomala, M.; Lindgren, M.; Jokilaakso, A. Thermal Conductivity of Solidified Industrial Copper Matte and Fayalite Slag. JOM 2020, 72, 1927–1934. [Google Scholar] [CrossRef]
- Khare, S.; Dell’Amico, M.; Knight, C.; McGarry, S. Selection of materials for high temperature latent heat energy storage. Sol. Energy Mater. Sol. Cells 2012, 107, 20–27. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, B.; Wu, Q.; Li, Y.; Feng, Z.; Wang, Y.; Hu, Z. Physical properties and microstructure of nickel slag/metakaolin-based geopolymer with different contents of nickel slag. Adv. Cem. Res. 2020, 32, 244–250. [Google Scholar] [CrossRef]
- Buzatu, T.; Talpoş, E.; Petrescu, M.I.; Ghica, V.G.; Iacob, G.; Buzatu, M. Utilization of granulated lead slag as a structural material in roads constructions. J. Mater. Cycles Waste Manag. 2014, 17, 707–717. [Google Scholar] [CrossRef]
- Barna, R.; Moszkowicz, P.; Gervais, C. Leaching assessment of road materials containing primary lead and zinc slags. Waste Manag. 2004, 24, 945–955. [Google Scholar] [CrossRef]
- Carvalho, S.Z.; Vernilli, F.; Almeida, B.; Demarco, M.; Silva, S.N. The recycling effect of BOF slag in the portland cement properties. Resour. Conserv. Recycl. 2017, 127, 216–220. [Google Scholar] [CrossRef]
- Alwaeli, M. Application of granulated lead–zinc slag in concrete as an opportunity to save natural resources. Radiat. Phys. Chem. 2013, 83, 54–60. [Google Scholar] [CrossRef]
- Atzeni, C.; Massidda, L.; Sanna, U. Use of granulated slag from lead and zinc processing in concrete technology. Cem. Concr. Res. 1996, 26, 1381–1388. [Google Scholar] [CrossRef]
- Pelino, M. Recycling of zinc-hydrometallurgy wastes in glass and glass ceramic materials. Waste Manag. 2000, 20, 561–568. [Google Scholar] [CrossRef]
- Kritikaki, A.; Zaharaki, D.; Komnitsas, K. Valorization of Industrial Wastes for the Production of Glass–Ceramics. Waste Biomass Valorization 2016, 7, 885–898. [Google Scholar] [CrossRef]
- Hao, J.; Dou, Z.-h.; Zhang, T.-a.; Wan, X.-y.; Wang, K. Energy conservation and emission reduction through utilization of latent heat of copper slag for iron and copper recovery. J. Clean. Prod. 2024, 436, 140602. [Google Scholar] [CrossRef]
- Ye, C.L.; Zhang, M.J.; Yang, S.; Mweemba, S.; Huang, A.; Liu, X.; Zhang, X.L. Application of copper slags in encapsulating high-temperature phase change thermal storage particles. Sol. Energy Mater. Sol. Cells 2023, 254, 112257. [Google Scholar] [CrossRef]
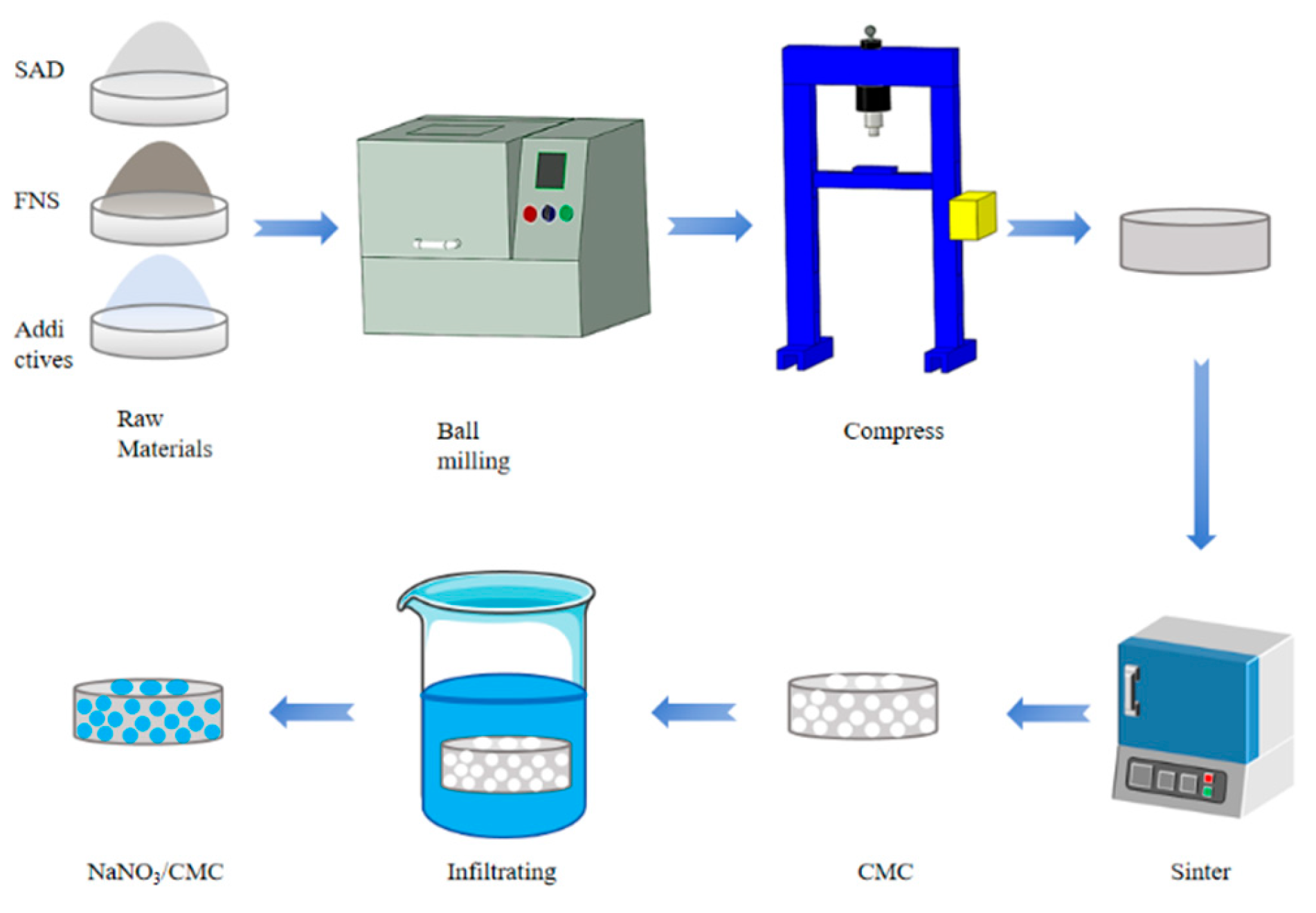
- Liu, J.; Su, Z.; Xu, J.; Zhang, Y. Thermal storage performance of NaNO3/cordierite-mullite ceramic (CMC) composites deriving from metallurgical slag. Ceram. Int. 2023, 49, 38094–38102. [Google Scholar] [CrossRef]
- Leng, G.; Qiao, G.; Jiang, Z.; Xu, G.; Qin, Y.; Chang, C.; Ding, Y. Micro encapsulated & form-stable phase change materials for high temperature thermal energy storage. Appl. Energy 2018, 217, 212–220. [Google Scholar] [CrossRef]
- Liu, J.; Su, Z.; Xu, J.; Zhang, Y. Novel value-add recycling route of industry solid waste for acting as supporting skeleton of NaNO3 phase change materials (PCMs). J. Environ. Chem. Eng. 2024, 12, 112332. [Google Scholar] [CrossRef]
- Francis, A.A. Conversion of blast furnace slag into new glass-ceramic material. J. Eur. Ceram. Soc. 2004, 24, 2819–2824. [Google Scholar] [CrossRef]
- Pan, D.a.; Zhang, S.; Bao, H.; Guo, B.; Liu, B. A Method for Preparing Hedenbergite Glass Ceramics by Lead Slag. Patent CN104773958A, 24 November 2017. [Google Scholar]
- Chinnam, R.K.; Francis, A.A.; Will, J.; Bernardo, E.; Boccaccini, A.R. Review. Functional glasses and glass-ceramics derived from iron rich waste and combination of industrial residues. J. Non-Cryst. Solids 2013, 365, 63–74. [Google Scholar] [CrossRef]
- Karamanov, A.; Taglieri, G.; Pelino, M. Sintering in nitrogen atmosphere of iron-rich glass-ceramics. J. Am. Ceram. Soc. 2004, 87, 1354–1357. [Google Scholar] [CrossRef]
- Carlini, R.; Alfieri, I.; Zanicchi, G.; Soggia, F.; Gombia, E.; Lorenzi, A. Synthesis and Characterization of Iron-Rich Glass Ceramic Materials: A Model for Steel Industry Waste Reuse. J. Mater. Sci. Technol. 2016, 32, 1105–1110. [Google Scholar] [CrossRef]
- Naimi, K.M.A.; Delclos, T.; Calvet, N. Industrial Waste Produced in the UAE, Valuable High-temperature Materials for Thermal Energy Storage Applications. Energy Procedia 2015, 75, 2087–2092. [Google Scholar] [CrossRef]
- Koçak, B.; Fernández, A.I.; Paksoy, H. Characterization of demolition waste powder to be processed as sensible thermal energy storage material. Sol. Energy Mater. Sol. Cells 2021, 230, 111283. [Google Scholar] [CrossRef]
- Agalit, H.; Zari, N.; Maaroufi, M. Suitability of industrial wastes for application as high temperature thermal energy storage (TES) materials in solar tower power plants—A comprehensive review. Sol. Energy 2020, 208, 1151–1165. [Google Scholar] [CrossRef]
- Py, X.; Calvet, N.; Olives, R.; Meffre, A.; Echegut, P.; Bessada, C.; Veron, E.; Ory, S. Recycled Material for Sensible Heat Based Thermal Energy Storage to be Used in Concentrated Solar Thermal Power Plants. J. Sol. Energy Eng. 2011, 133, 031008. [Google Scholar] [CrossRef]


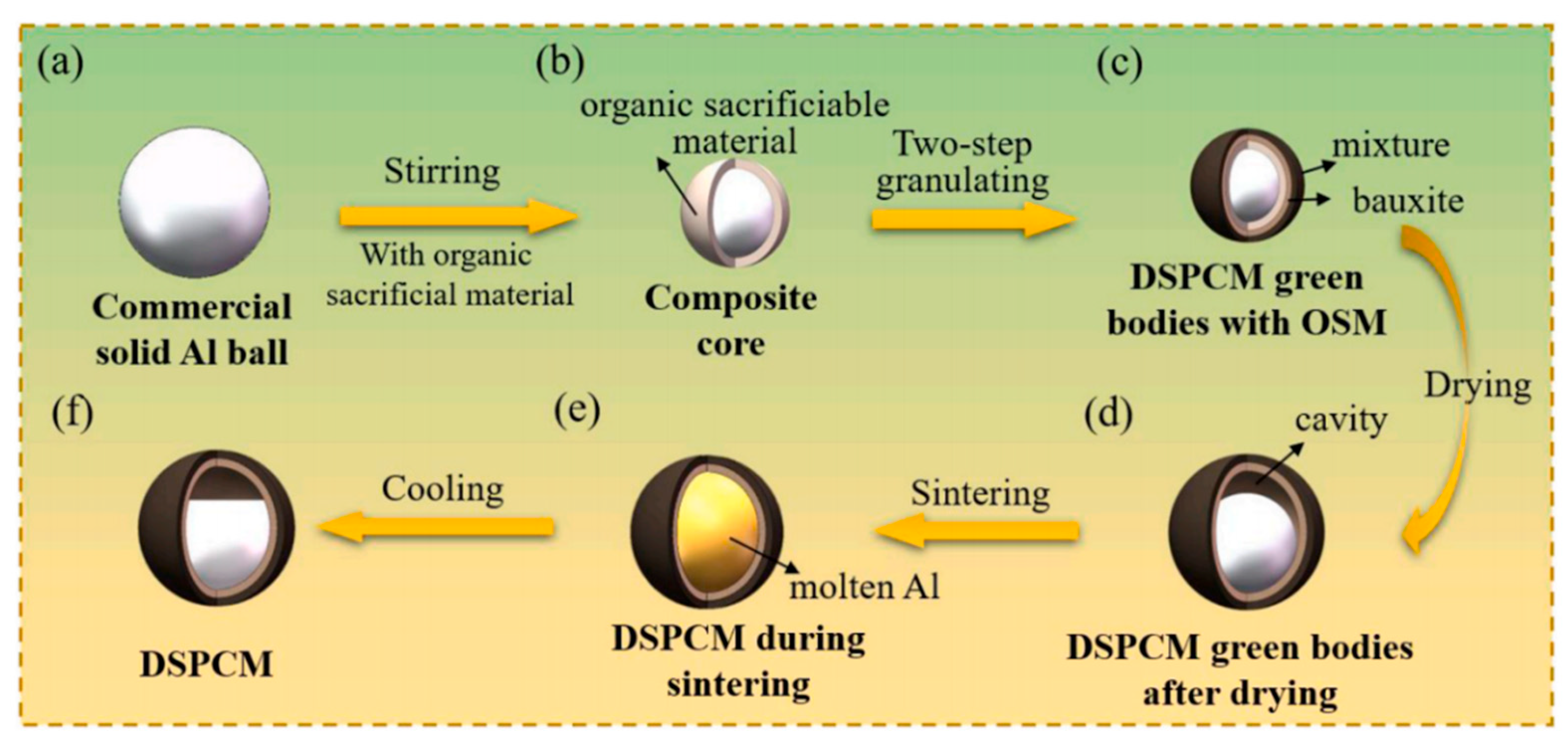

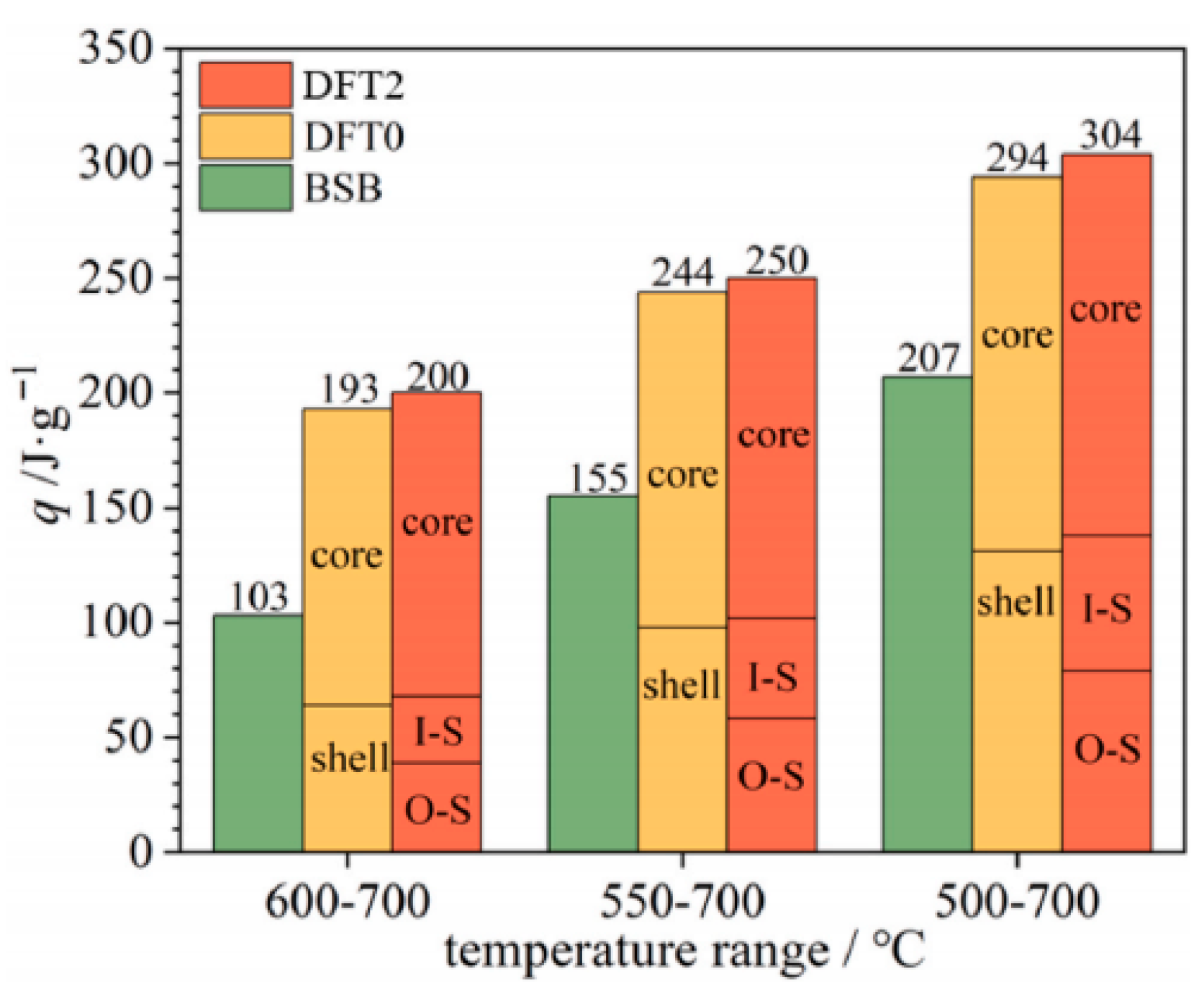




| Slag Type | Sample ID | Density ρ [kg/m3] | Thermal Conductivity K [W/(m·k)] | Specific Heat Capacity Cp [J/(g·k)] | Thermal Stability [℃] | Reference |
|---|---|---|---|---|---|---|
| Copper slag | ES-N a,b | 3500 | 1.595 | 0.7–1.1 | up to800 | [99] |
| Copper slag | ES-A a | 3700 | 2.173 | 1.4–1.5 | up to 800 | [99] |
| Copper slag | Curto C. Slag | 4350 | - | 0.670–1.004 | up to 1200 | - |
| Copper slag | Slag P a | 3600 | 0.8 | 0.571–1.180 | up to 800 | [100] |
| Copper slag | Slag B | 3700 | 1.1 | 0.650–0.990 | up to 800 | [100] |
| Steel slag | EAF 1 a | 3430 | 1.47 | 0.865 | up to 1000 | [101] |
| Steel slag | EAF 2 | 4110 | 1.51 | 0.837 | up to 1000 | [101] |
| Steel slag | Slag 1 | 3430 | 1.65–1.23 | 0.710–0.950 | up to 1100 | [102] |
| Steel slag | Slag 2 | 3770 | 1.50–1.73 | 0.690–0.890 | up to 1100 | [102] |
| Steel slag | S slag | 3600 | 1.695–1.74 | 0.713–0.858 | up to 1000 | [77] |
| Steel slag | C slag | 3700 | 1.84–1.75 | 0.717–0.975 | up to 1000 | [77] |
| Fe2O3 | FeO | CaO | SiO2 | Al2O3 | MgO | ZnO | PbO | CuO | S | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 2.07 | 22.91 | 20.5 | 34.92 | 2.07 | 2.36 | 3.63 | 1.12 | 0.10 | 1.11 | [120] |
| 10.38 | 23.32 | 22.10 | 24.33 | 2.46 | 2.71 | 11.11 | 3.63 | - | 0.39 | [121] |
| - | 14.99 | 23.05 | 43.09 | 6.22 | 1.58 | 4.01 | - | - | - | [122] |
| 32.47 | 9.49 | 4.5 | 14.68 | 4.7 | 1.43 | 2.82 | 10.34 | 2.75 | 6.51 | [123] |
| 28.81 | 17.56 | 11.53 | 35.5 | 3.85 | 4.65 | 6.02 | 4.03 | 0.79 | 0.24 | [123] |
| 7.63 | 20.47 | 23.11 | 21.39 | 3.56 | 5.44 | 9.47 | 4.06 | - | 0.37 | [119] |
| 28.10 | - | 23.11 | 21.39 | 3.56 | 5.44 | 9.47 | 4.06 | - | 0.37 | [124] |
| 3.36 | 28.90 | 18.34 | 31.34 | 4.26 | 1.72 | 8.20 | 2.69 | - | 1.19 | [125] |
| 8.11 | 23.27 | 22.14 | 24.88 | 2.46 | 2.71 | 10.77 | 3.74 | - | - | [126] |
| 31.57 | - | 3.05 | 21.56 | 1.73 | 0.15 | 6.18 | 12.28 | 1.64 | 8.01 | [127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, M.; Xiong, Y.; Zhang, A.; Li, X.; Wu, Y.; Zhang, C.; Zhao, Y.; Ding, Y. Non-Ferrous Metal Smelting Slags for Thermal Energy Storage: A Mini Review. Buildings 2025, 15, 2376. https://doi.org/10.3390/buildings15132376
Yin M, Xiong Y, Zhang A, Li X, Wu Y, Zhang C, Zhao Y, Ding Y. Non-Ferrous Metal Smelting Slags for Thermal Energy Storage: A Mini Review. Buildings. 2025; 15(13):2376. https://doi.org/10.3390/buildings15132376
Chicago/Turabian StyleYin, Meichao, Yaxuan Xiong, Aitonglu Zhang, Xiang Li, Yuting Wu, Cancan Zhang, Yanqi Zhao, and Yulong Ding. 2025. "Non-Ferrous Metal Smelting Slags for Thermal Energy Storage: A Mini Review" Buildings 15, no. 13: 2376. https://doi.org/10.3390/buildings15132376
APA StyleYin, M., Xiong, Y., Zhang, A., Li, X., Wu, Y., Zhang, C., Zhao, Y., & Ding, Y. (2025). Non-Ferrous Metal Smelting Slags for Thermal Energy Storage: A Mini Review. Buildings, 15(13), 2376. https://doi.org/10.3390/buildings15132376








