A Preliminary Investigation on the Adsorption of Cu2+ by Sawdust/Foamed Geopolymer Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Raw Materials
- The metakaolin (1250 mesh) was purchased from CHENYI Refractory Abrasive Co., Ltd. (Gongyi, China). Its components are shown in Ref. [23], where the mass ratio of SiO2 is 55.06%, and the mass ratio of Al2O3 is 44.12%;
- The sodium hydroxide (NaOH, white granulated) was supplied by ZHIYUAN Chemical Reagent Equipment Co., Ltd. (Tianjin, China);
- The sodium silicate solution (53.07 wt.%, n(SiO2/Na2O): 2.12) was obtained from TIANLIAN Chemical Co., Ltd. (Langfang, China);
- The hydrogen peroxide solution (H2O2, 30 wt.%) was used as a foaming agent and produced by YIHENG Chemical Glass Equipment Co., Ltd. (Changsha, China);
- A soap powder surfactant was used as a stabilizing agent, aiming to reduce the surface tension and increase the stability of the foam to obtain a more homogeneous distribution;
- The distilled water used in the experiment was obtained by NANDAI Trading Co., Ltd. (Wenzhou, China).
2.1.2. Sample Preparation
2.2. Experimental Methods
2.2.1. Adsorption Tests
2.2.2. Characterization Methodologies
3. Results and Discussion
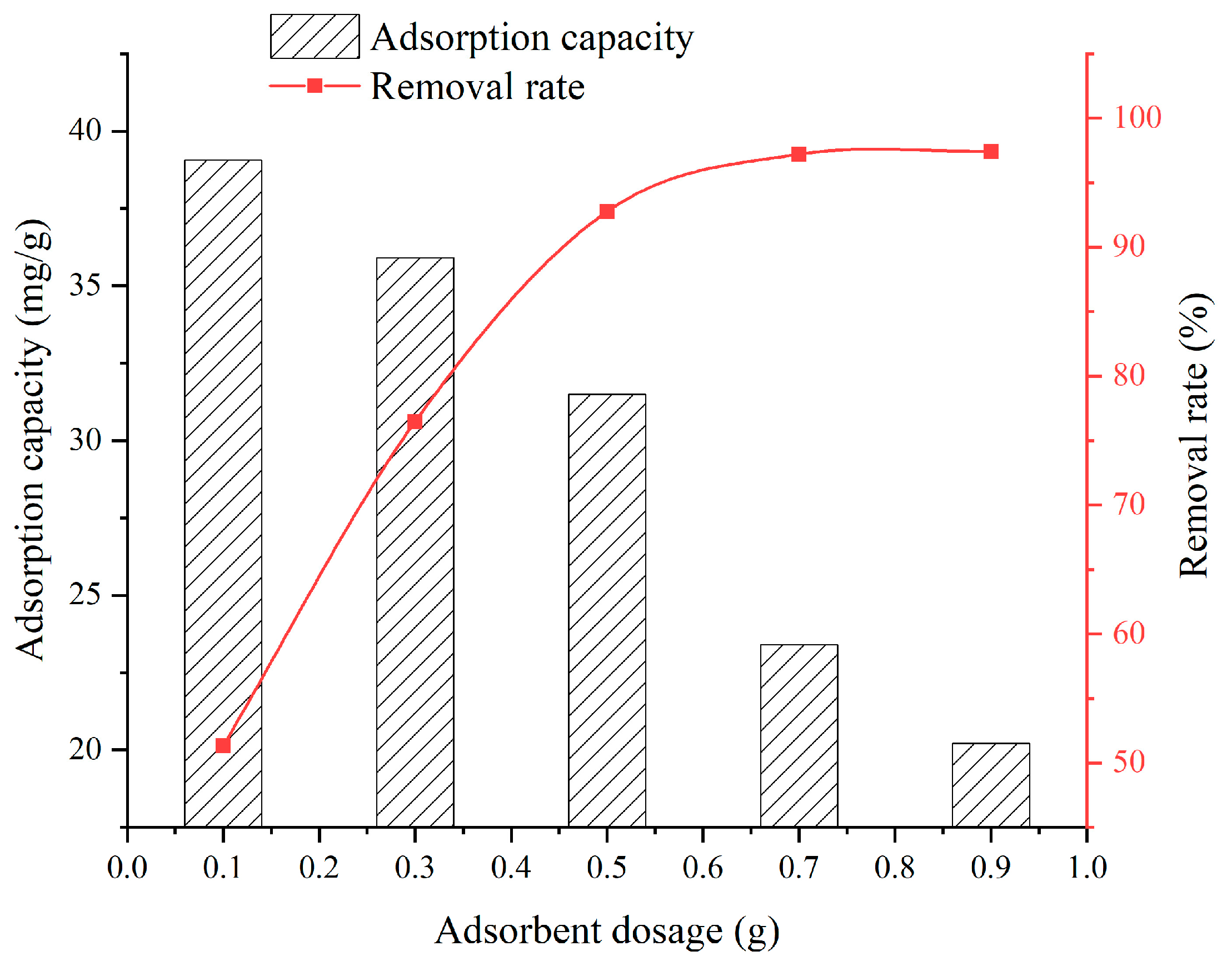
3.1. Effect of SFG Dosage
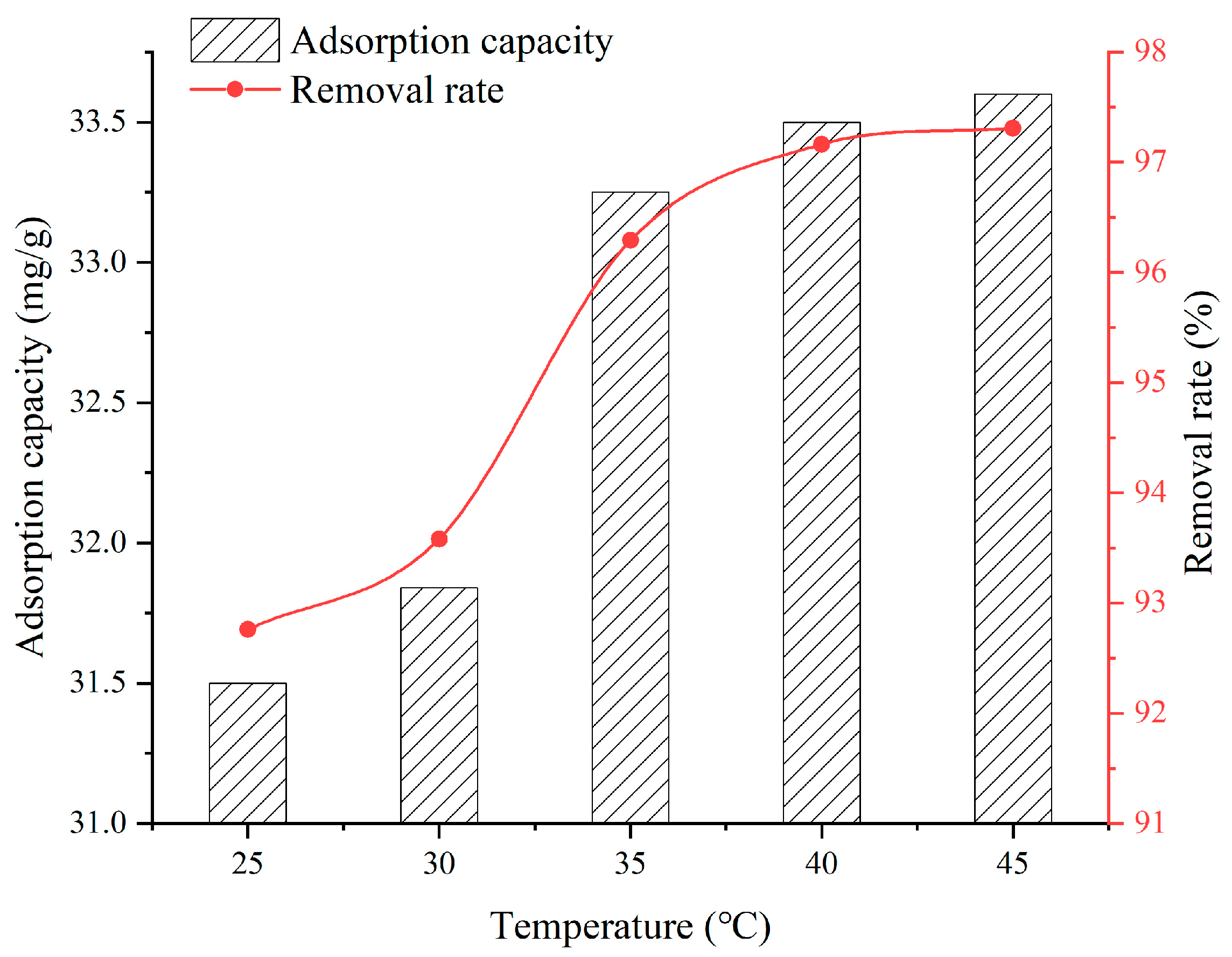
3.2. Effect of Solution Temperature
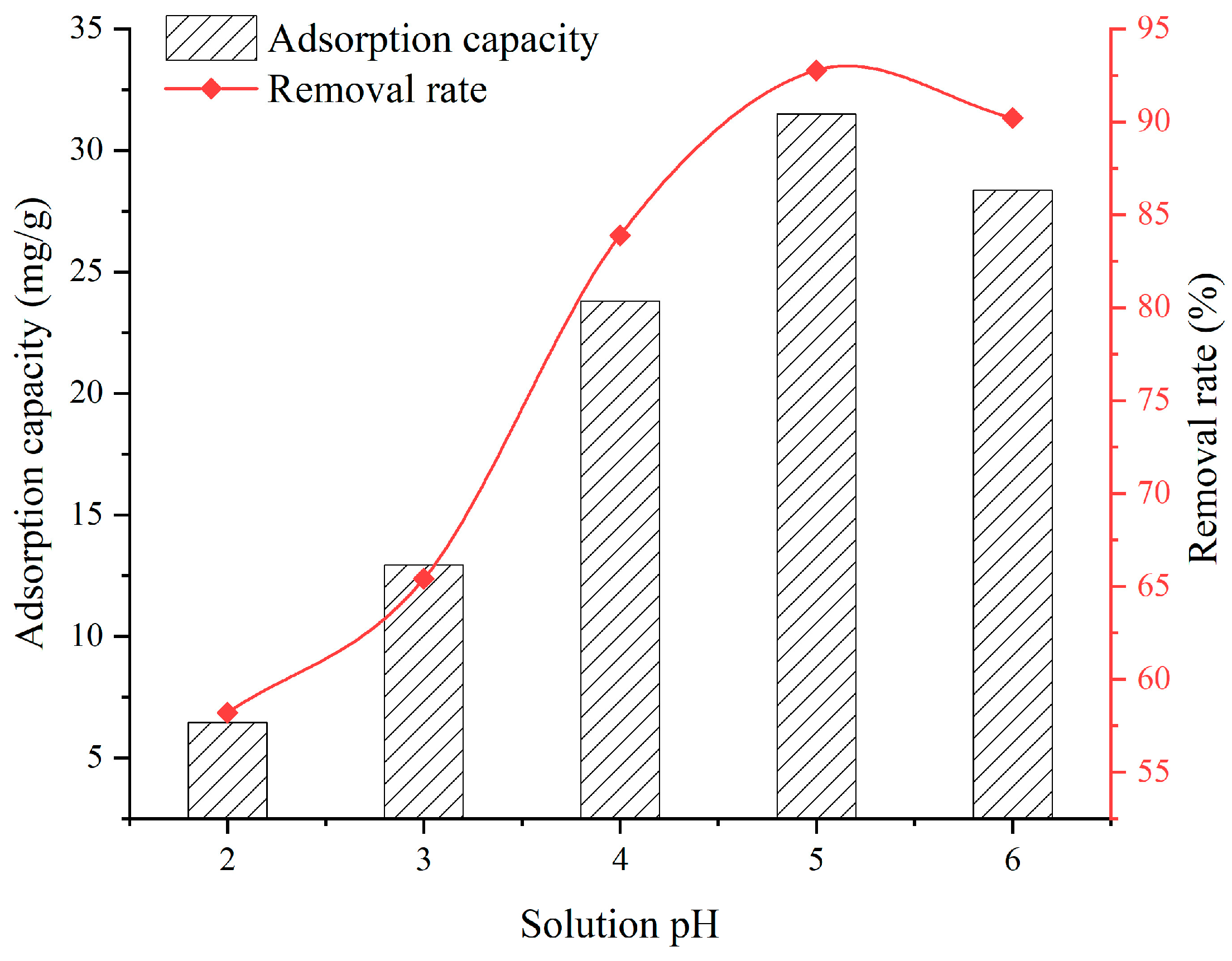
3.3. Effect of Solution pH
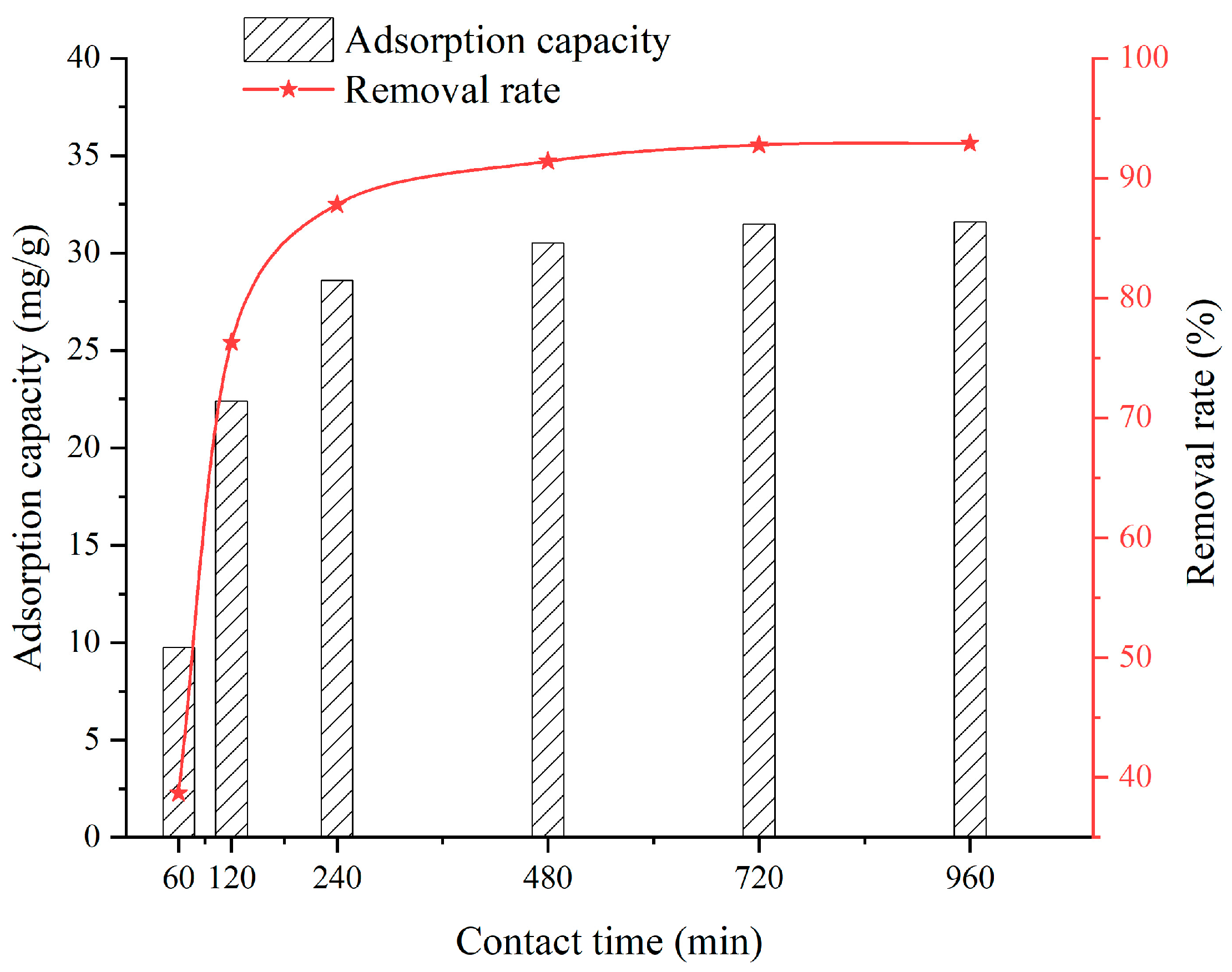
3.4. Effect of Contact Time
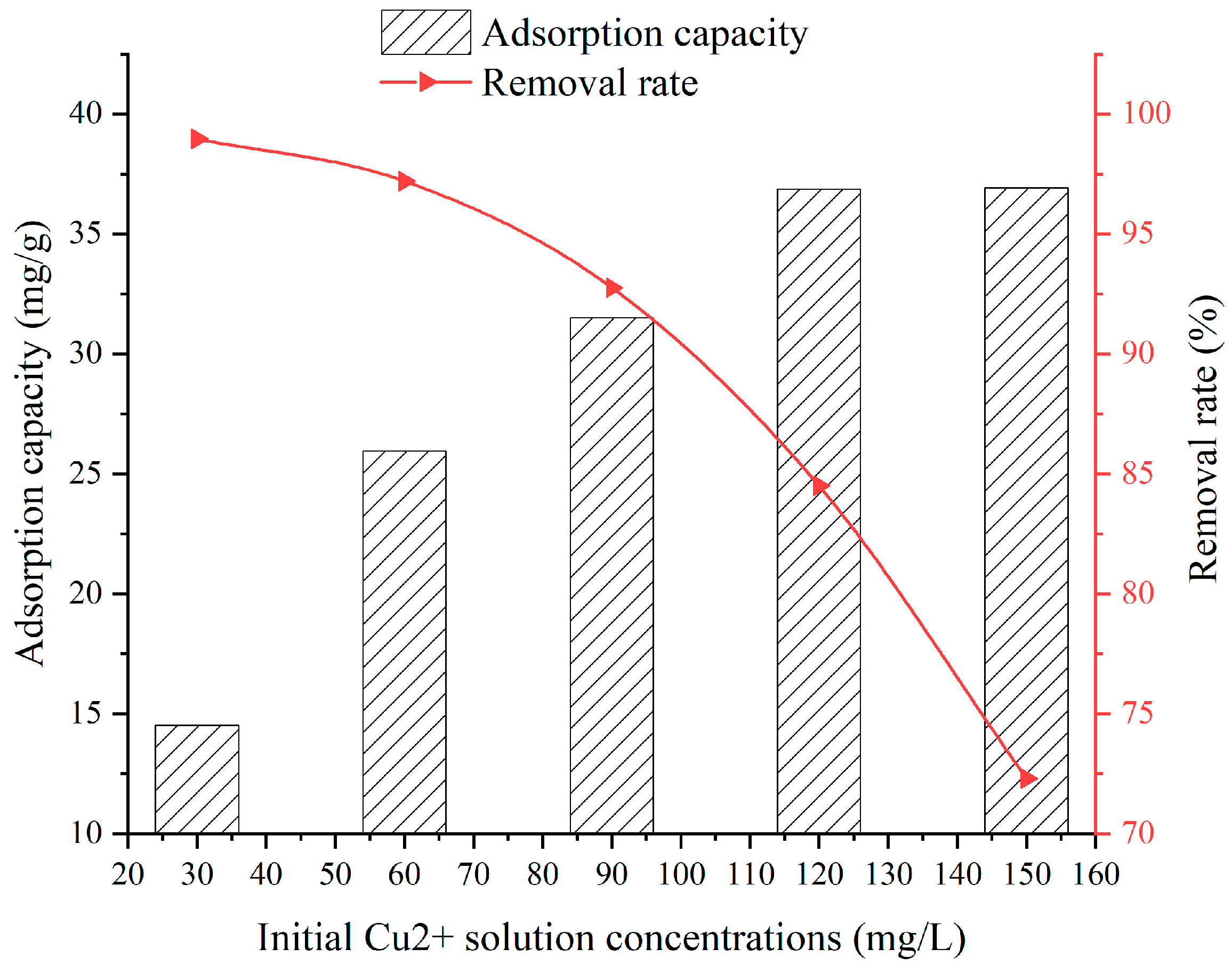
3.5. Effect of Initial Cu2+ Solution Concentrations
3.6. EDS and XRD Analysis
4. Comparison of Adsorption Properties with Sawdust and Foamed Geopolymer
| Category | Adsorbent | Type | Compressive Strength (MPa) | Metal Ions | Adsorption Capacity (mg/g) | Removal Rate (%) | Test Conditions | Ref. |
|---|---|---|---|---|---|---|---|---|
| Adsorbents from sawdust and foamed geopolymer | Sawdust/foamed geopolymer (SFG) | Block | 1.25 | Cu2+ | 31.5 | 92.76 | C0: 90 mg/L; pH: 5; Te: 25 °C; tc: 720 min; mb: 0.5 g | This work |
| Adsorbents from sawdust | Modified sawdust by KOH | Powder | / | Cu2+ | 7.64 | 85 | C0: 30 mg/L; pH: 4. | [32] |
| Natural sawdust | Powder | / | Cu2+ | 3.88 | 74 | |||
| Sawdust by calcination | Powder | / | Cu2+ | 15 | / | C0: 50 mg/L; pH: 5; Te: 25 °C; tc: 4320 min. | [35] | |
| Sawdust–chitosan nanocomposite beads | Powder | / | Cu2+ | 1.8 | 90.32 | C0: 50 mg/L; pH: 5; Te: 30 °C; tc: 70 min. | [36] | |
| Sawdust-based biochar | Powder | / | Cu2+ | / | 90.8 | C0: 50 mg/L; tc: 720 min. | [37] | |
| Adsorbents from foamed geopolymer | Geopolymer foams | Block | / | Cu2+ | 5.53~5.93 | 94.9 | C0: 50 mg/L; pH: 5; tc: 1440 min. | [33] |
| Geopolymer foams | Block | 0.86 | Cu2+ | 64.9 | 58 | C0: 200 mg/L; pH: 5; Te: 25 °C; tc: 1440 min. | [30] | |
| Foamed geopolymer | Sphere | / | Cu2+ | 24.69 | 92.8 | C0: 200 mg/L; pH: 5; tc: 2880 min. | [21] | |
| Porous geopolymer | Block | 0.35 | Cu2+ | 19.59 | 97.96 | C0: 100 mg/L; pH: 5; Te: 20 °C; tc: 1440 min. | [34] |
5. Conclusions
- (1)
- The novel SFG porous material exhibits excellent adsorption performance in removing heavy metal Cu2+. Meanwhile, it has the advantages of high mechanical strength, high recyclability, and high moldability, which allows it to be made into different block or spherical shapes according to user needs;
- (2)
- When the SFG dosage varies from 0.1 g to 0.9 g, the adsorption capacity and removal rate are 20.22~39.06 mg/g and 51.32~98.09%, respectively. When the solution temperature varies from 25 °C to 45 °C, the adsorption capacity and removal rate are 31.5~33.6 mg/g and 92.76~97.31%, respectively. When the solution pH varies from 2 to 6, the adsorption capacity and removal rate are 6.45~28.36 mg/g and 58.2~90.2%, respectively. When the contact time varies from 60 min to 960 min, the adsorption capacity and removal rate are 9.75~31.6 mg/g and 38.7~92.9%, respectively. When the initial Cu2+ solution concentration varies from 30 mg/L to 150 mg/L, the adsorption capacity and removal rate are 14.52~36.92 mg/g and 72.3~98.96%, respectively;
- (3)
- Considering the economy, environmental friendliness, and comprehensive performance, a desirable SFG adsorbent with an SFG dosage of 0.5 g, temperature of 25 °C, pH of 5, contact time of 720 min, and initial Cu2+ solution concentrations of 90 mg/L, is recommended, of which the adsorption capacity is 31.5 mg/g with the removal rate being 92.76%;
- (4)
- It is feasible to replace a portion of metakaolin with natural sawdust to prepare SFG adsorbent. Although the adsorption performance of the SFG adsorbent prepared is similar to that of foamed geopolymer, this method fully utilizes waste sawdust and has the advantages of low carbon, energy saving, and environmental protection.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- China Statistical Yearbook, Compiled by National Bureau of Statistics of China; China Statistics Press: Beijing, China, 2022. Available online: https://www.stats.gov.cn/sj/ndsj/2022/indexch.htm (accessed on 16 October 2022).
- China Forestry and Grassland Statistical Yearbook 2022; China Forestry Publishing House: Beijing, China, 2022; pp. 16–17.
- Zou, S.; Li, H.; Wang, S.; Jiang, R.; Zou, J.; Zhang, X.; Liu, L.; Zhang, G. Experimental research on an innovative sawdust biomass-based insulation material for buildings. J. Clean. Prod. 2020, 260, 121029. [Google Scholar] [CrossRef]
- Rani, C.N. Feasibility study on the utilization of low-cost sawdust for adsorption of caffeine: Equilibrium, optimization, and response surface modeling. Desalination Water Treat. 2022, 276, 271–281. [Google Scholar] [CrossRef]
- Adegoke, K.A.; Adesina, O.O.; Okon-Akan, O.A.; Adegoke, O.R.; Olabintan, A.B.; Ajala, O.A.; Olagoke, H.; Maxakato, N.W.; Bello, O.S. Sawdust-biomass based materials for sequestration of organic and inorganic pollutants and potential for engineering applications. Curr. Res. Green Sustain. Chem. 2022, 5, 100274. [Google Scholar] [CrossRef]
- Orozco, C.I.; Freire, M.S.; Gómez-Díaz, D.; González-Álvarez, J. Removal of copper from aqueous solutions by biosorption onto pine sawdust. Sustain. Chem. Pharm. 2023, 32, 101016. [Google Scholar] [CrossRef]
- Faisal, M.L.; Sachit, D.E.; Faisal, F. Cadmium removal efficiency from synthetic wastewater using sawdust as a sustainable adsorbent. Desalination Water Treat. 2024, 318, 100321. [Google Scholar] [CrossRef]
- Samal, P.P.; Qaiyum, A.; Ghosh, A.; Kumari, R.; Mohanta, J.; Das, S.; Swain, J.; Dey, B.; Dey, S. Acacia auriculiformis leaf extract mediated green synthesis of goethite and boehmite embedded activated sawdust for Cr (VI) adsorption. J. Hazard. Mater. Adv. 2024, 13, 100405. [Google Scholar] [CrossRef]
- Mishra, D.; Bansal, P.; Singh, S.K.; Adhikari, A.; Chatterjee, S. Undoped polyaniline-modified sawdust as an adsorbent for lead removal. Mater. Today Proc. 2024, 111, 137–146. [Google Scholar] [CrossRef]
- El Hajam, M.; Kandri, N.I.; Plavan, G.-I.; Harrath, A.H.; Mansour, L.; Boufahja, F.; Zerouale, A. Pb2+ ions adsorption onto raw and chemically activated Dibetou sawdust: Application of experimental designs. J. King Saud Univ.-Sci. 2020, 32, 2176–2189. [Google Scholar] [CrossRef]
- Ji, Z.; Zhang, G.; Liu, R.; Qu, J.; Liu, H. Potential applications of solid waste-based geopolymer materials: In wastewater treatment and greenhouse gas emission reduction. J. Clean. Prod. 2024, 443, 141144. [Google Scholar] [CrossRef]
- Nasreddine, H.; Salem, T.; Djerbi, A.; Dujardin, N.; Gautron, L. Enhanced thermal insulation behavior of metakaolin-based geopolymer reinforced by miscanthus fibers. Appl. Clay Sci. 2024, 258, 107496. [Google Scholar] [CrossRef]
- Tatlisu, G.C.; Aciksari, C.; Celebi, S.; Turan, S. Developing a hollow glass microsphere/geopolymer thermal insulation composite for hot metal surface coating. Ceram. Int. 2022, 48, 11924–11939. [Google Scholar] [CrossRef]
- Siyal, A.A.; Shamsuddin, M.R.; Khan, M.I.; Rabat, N.E.; Zulfiqar, M.; Man, Z.; Siame, J.; Azizli, K.A. A review on geopolymers as emerging materials for the adsorption of heavy metals and dyes. J. Environ. Manag. 2018, 224, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, B.; Zhang, Q.; Wen, Q.; Lu, X.; Xiao, K.; Ekberg, C.; Zhang, S. Application of geopolymers for treatment of industrial solid waste containing heavy metals: State-of-the-art review. J. Clean. Prod. 2023, 390, 136053. [Google Scholar] [CrossRef]
- Tan, T.H.; Mo, K.H.; Ling, T.-C.; Lai, S.H. Current development of geopolymer as alternative adsorbent for heavy metal removal. Environ. Technol. Innov. 2020, 18, 100684. [Google Scholar] [CrossRef]
- Novais, R.M.; Pullar, R.C.; Labrincha, J.A. Geopolymer foams: An overview of recent advancements. Prog. Mater. Sci. 2020, 109, 100621. [Google Scholar] [CrossRef]
- Ettahiri, Y.; Bouargane, B.; Fritah, K.; Akhsassi, B.; Pérez-Villarejo, L.; Aziz, A.; Bouna, L.; Benlhachemi, A.; Novais, R.M. A state-of-the-art review of recent advances in porous geopolymer: Applications in adsorption of inorganic and organic contaminants in water. Constr. Build. Mater. 2023, 395, 132269. [Google Scholar] [CrossRef]
- Liang, K.; Yang, G.; Wang, X.Q.; Chow, C.L.; Lau, D. Development of effective porous geopolymer adsorbent with high strength for copper (II) ion removal. J. Clean. Prod. 2024, 449, 141752. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, Y.; Qiu, X.; Zhou, F.; Wang, H.; Zhou, S.; Yan, C. Novel porous phosphoric acid-based geopolymer foams for adsorption of Pb (II), Cd (II) and Ni (II) mixtures: Behavior and mechanism. Ceram. Int. 2023, 49, 7030–7039. [Google Scholar] [CrossRef]
- Tan, T.H.; Mo, K.H.; Lai, S.H.; Ling, T.-C. Investigation on the copper ion removal potential of a facile-fabricated foamed geopolymer sphere for wastewater remediation. Clean. Mater. 2022, 4, 100088. [Google Scholar] [CrossRef]
- Ji, Z.; Su, L.; Pei, Y. Characterization and adsorption performance of waste-based porous open-cell geopolymer with one-pot preparation. Ceram. Int. 2021, 47, 12153–12162. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Liu, L. Development of a multifunctional bio-based insulation material with corncob and silica aerogel. Energy Build. 2024, 323, 114817. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Zou, S.; Liu, L.; Bai, C.; Zhang, G.; Fang, L. Experimental study on durability and acoustic absorption performance of biomass geopolymer-based insulation materials. Constr. Build. Mater. 2022, 361, 129575. [Google Scholar] [CrossRef]
- Al Ghafri, E.; Al Tamimi, N.; El-Hassan, H.; Maraqa, M.A.; Hamouda, M. Synthesis and multi-objective optimization of fly ash-slag geopolymer sorbents for heavy metal removal using a hybrid Taguchi-TOPSIS approach. Environ. Technol. Innov. 2024, 35, 103721. [Google Scholar] [CrossRef]
- Yu, Y. New Adsorbing Materials of Water Phase; Science Press: Beijing, China, 2016; pp. 121–122. (In Chinese) [Google Scholar]
- Duan, P.; Yan, C.; Zhou, W.; Ren, D. Development of fly ash and iron ore tailing based porous geopolymer for removal of Cu(II) from wastewater. Ceram. Int. 2016, 42, 13507–13518. [Google Scholar] [CrossRef]
- Goyal, M.; Rattan, V.K.; Aggarwal, D.; Bansal, R.C. Removal of copper from aqueous solutions by adsorption on activated carbons. Colloids Surf. A Physicochem. Eng. Asp. 2001, 190, 229–238. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.S.; Al Zboon, K.; Al-Makhadmeh, L.; Hararah, M.; Mahasneh, M. Fly ash based geopolymer for heavy metal removal: A case study on copper removal. J. Environ. Chem. Eng. 2015, 3, 1669–1677. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Ma, M.; Sun, Y.; Ma, C. Rapid performance optimization strategy of MK-FA-GBFS based geopolymer foam heavy-metal adsorbent. Constr. Build. Mater. 2023, 394, 132161. [Google Scholar] [CrossRef]
- Bai, C.; Zheng, K.; Sun, F.; Wang, X.; Zhang, L.; Zheng, T.; Colombo, P.; Wang, B. A review on metakaolin-based porous geopolymers. Appl. Clay Sci. 2024, 258, 107490. [Google Scholar] [CrossRef]
- Kovacova, Z.; Demcak, S.; Balintova, M.; Pla, C.; Zinicovscaia, I. Influence of wooden sawdust treatments on Cu(II) and Zn(II) removal from water. Materials 2020, 13, 3575. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, S.; Qiu, X.; Meng, Y.; Wang, H.; Zhou, S.; Qiao, Q.; Yan, C. Clinoptilolite based zeolite-geopolymer hybrid foams: Potential application as low-cost sorbents for heavy metals. J. Environ. Manag. 2023, 330, 117167. [Google Scholar] [CrossRef]
- Mingming, S.; Hengze, Z.; Ye, L.; Lingqi, Z. The adsorption properties of steel slag-based porous geopolymer for Cu2+ removal. Miner. Eng. 2023, 201, 108225. [Google Scholar] [CrossRef]
- Forgionny, A.; Acelas, N.Y.; Ocampo-Pérez, R.; Padilla-Ortega, E.; Pérez, S.; Flórez, E. Mechanism adsorption analysis during the removal of Cd2+ and Cu2+ onto cedar sawdust via experiment coupled with theoretical calculation: Mono-and multicomponent systems. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100715. [Google Scholar] [CrossRef]
- Kayalvizhi, K.; Alhaji, N.; Saravanakkumar, D.; Mohamed, S.B.; Kaviyarasu, K.; Ayeshamariam, A.; Al-Mohaimeed, A.M.; AbdelGawwad, M.R.; Elshikh, M.S. Adsorption of copper and nickel by using sawdust chitosan nanocomposite beads—A kinetic and thermodynamic study. Environ. Res. 2022, 203, 111814. [Google Scholar] [CrossRef]
- Manyuchi, M.; Sukdeo, N.; Stinner, W.; Mutusva, T. Influence of sawdust based biochar on gold tailings wastewater heavy metal contaminants removal. South Afr. J. Chem. Eng. 2021, 37, 81–91. [Google Scholar] [CrossRef]
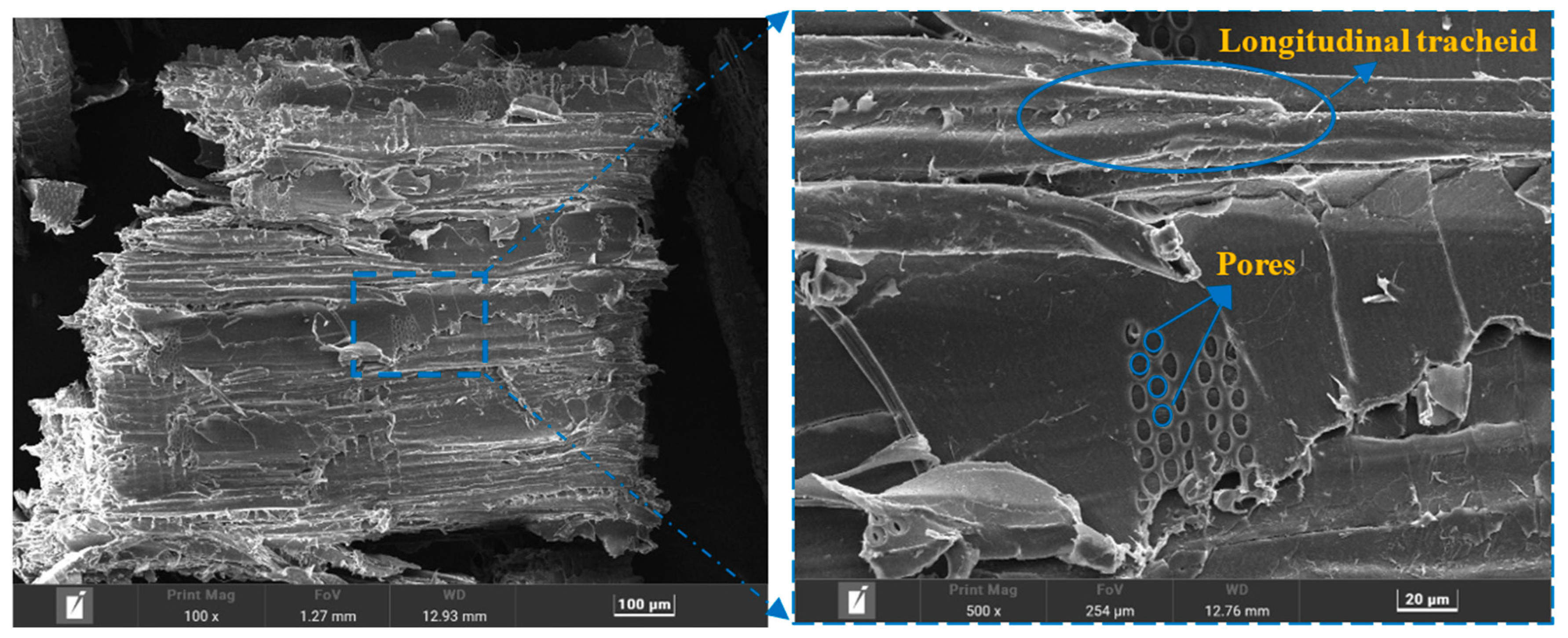

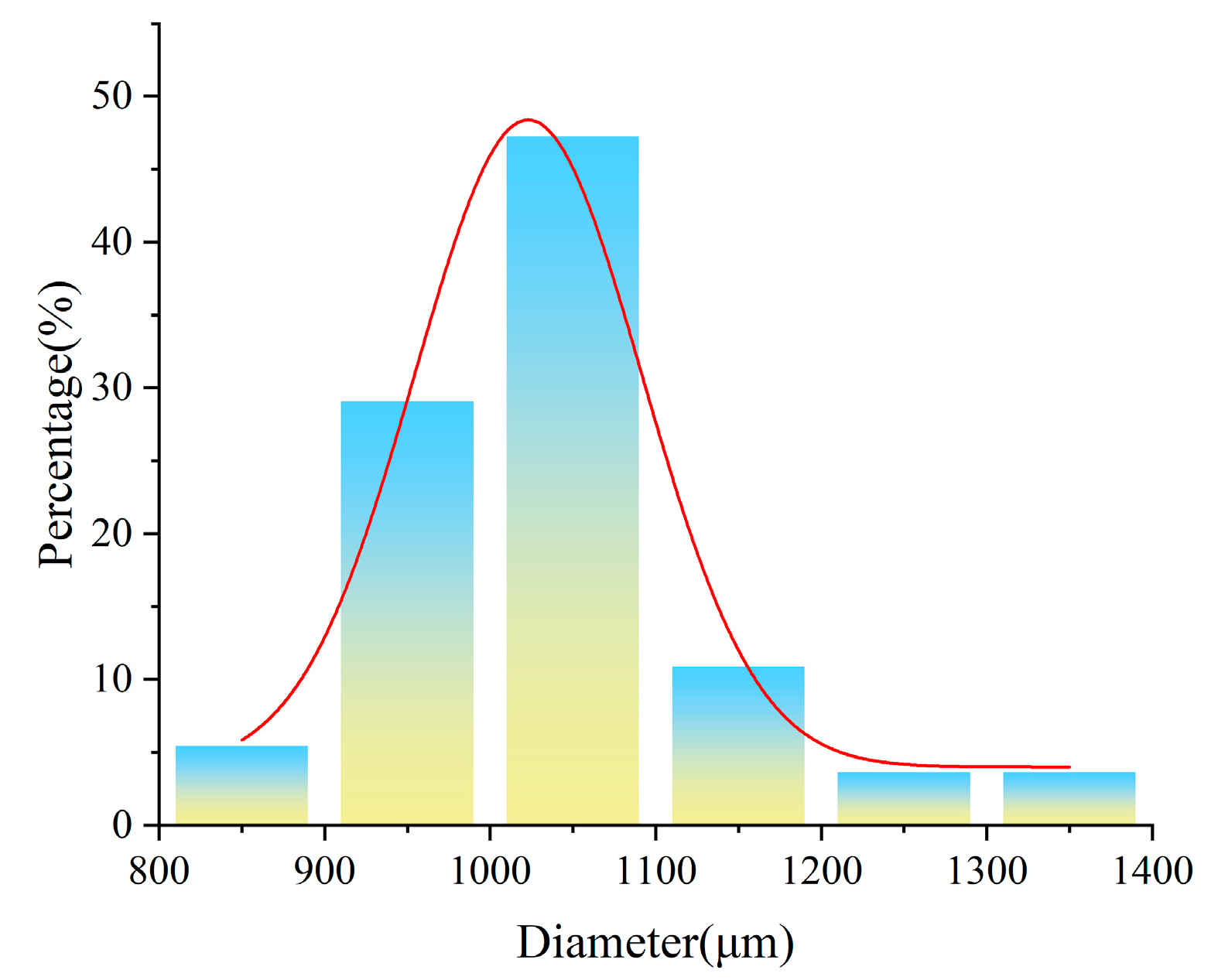



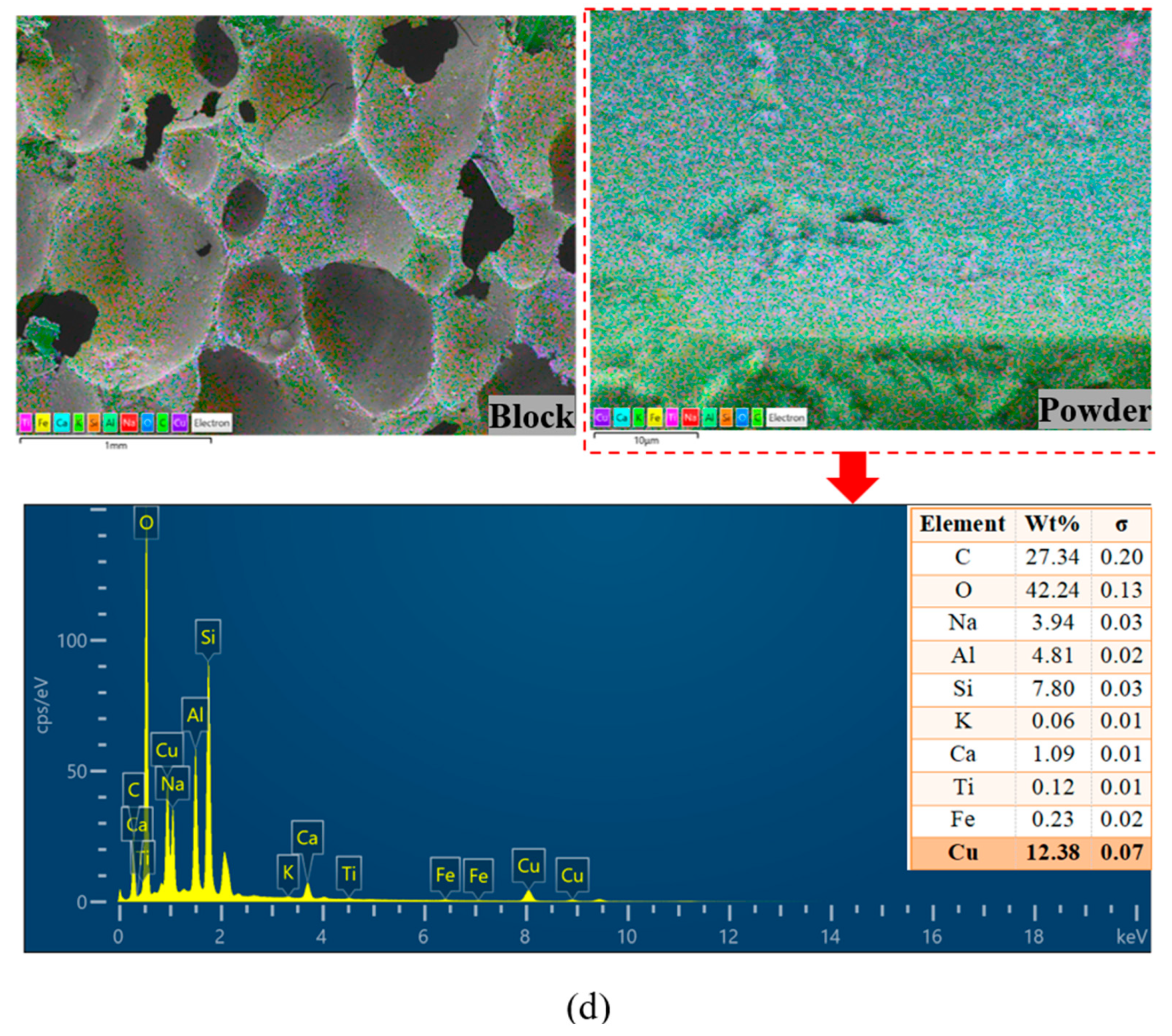
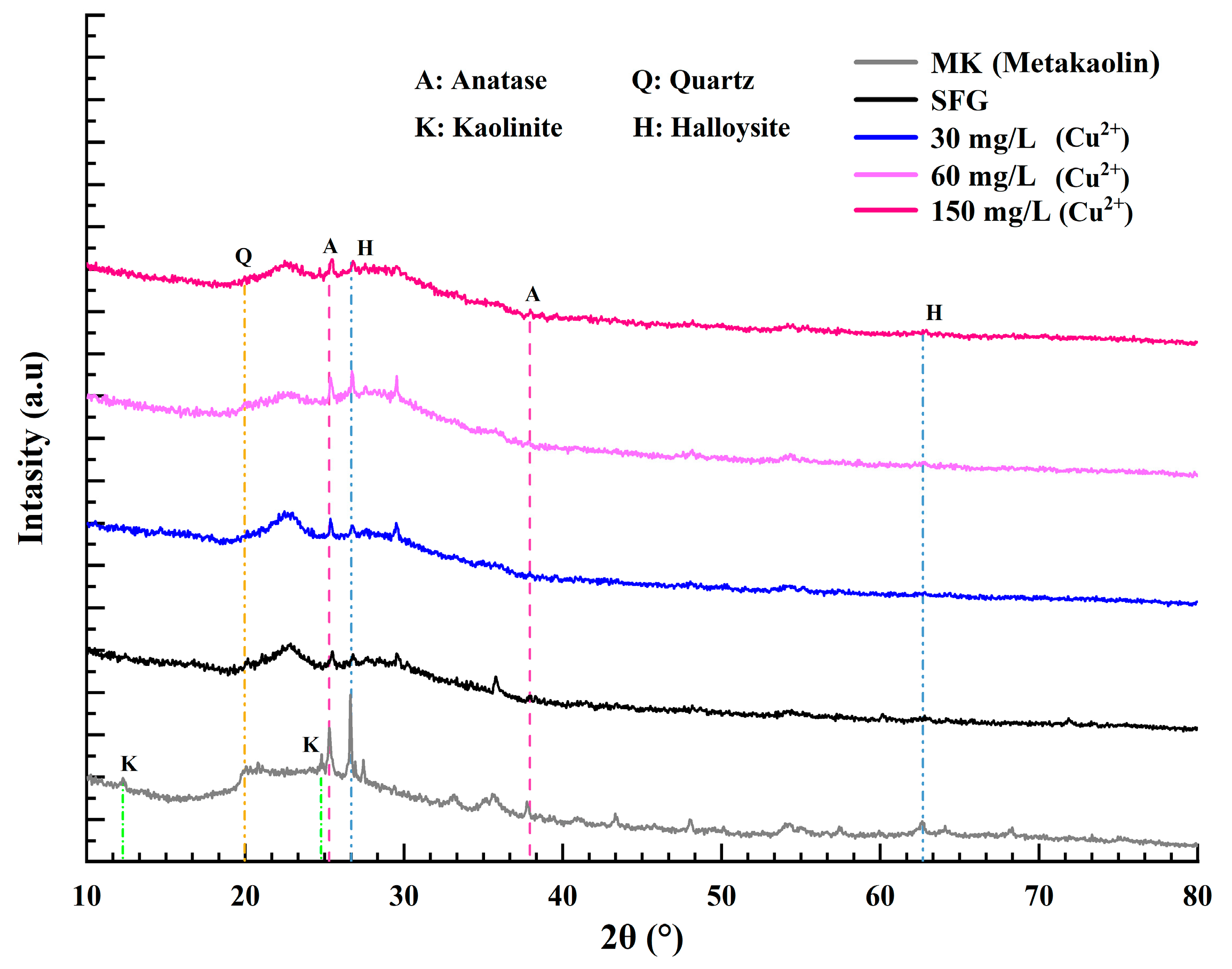
| Sample | Composition (g) | |||||||
|---|---|---|---|---|---|---|---|---|
| Metakaolin | Sawdust | Sodium Silicate Solution | NaOH | Mixing Water | Prewetting Water | H2O2 | Surfactant | |
| SFG adsorbent | 180 | 38.7 | 164.7 | 22.6 | 58.2 | 38.7 | 13.5 | 4.1 |
| Sample Number | Temperature (°C) | pH | Initial Concentration (mg/L) | Contact Time (min) | SFG Dosage (g) |
|---|---|---|---|---|---|
| D1 | 25 | 5 | 90 | 720 | 0.1 |
| D2 | 25 | 5 | 90 | 720 | 0.3 |
| D3 | 25 | 5 | 90 | 720 | 0.5 |
| D4 | 25 | 5 | 90 | 720 | 0.7 |
| D5 | 25 | 5 | 90 | 720 | 0.9 |
| T1 | 25 | 5 | 90 | 720 | 0.5 |
| T2 | 30 | 5 | 90 | 720 | 0.5 |
| T3 | 35 | 5 | 90 | 720 | 0.5 |
| T4 | 40 | 5 | 90 | 720 | 0.5 |
| T5 | 45 | 5 | 90 | 720 | 0.5 |
| P1 | 25 | 2 | 90 | 720 | 0.5 |
| P2 | 25 | 3 | 90 | 720 | 0.5 |
| P3 | 25 | 4 | 90 | 720 | 0.5 |
| P4 | 25 | 5 | 90 | 720 | 0.5 |
| P5 | 25 | 6 | 90 | 720 | 0.5 |
| W1 | 25 | 5 | 90 | 60 | 0.5 |
| W2 | 25 | 5 | 90 | 120 | 0.5 |
| W3 | 25 | 5 | 90 | 240 | 0.5 |
| W4 | 25 | 5 | 90 | 480 | 0.5 |
| W5 | 25 | 5 | 90 | 720 | 0.5 |
| W6 | 25 | 5 | 90 | 960 | 0.5 |
| C1 | 25 | 5 | 30 | 720 | 0.5 |
| C2 | 25 | 5 | 60 | 720 | 0.5 |
| C3 | 25 | 5 | 90 | 720 | 0.5 |
| C4 | 25 | 5 | 120 | 720 | 0.5 |
| C5 | 25 | 5 | 150 | 720 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Li, H.; Zhang, X. A Preliminary Investigation on the Adsorption of Cu2+ by Sawdust/Foamed Geopolymer Composites. Buildings 2025, 15, 2251. https://doi.org/10.3390/buildings15132251
Wang S, Li H, Zhang X. A Preliminary Investigation on the Adsorption of Cu2+ by Sawdust/Foamed Geopolymer Composites. Buildings. 2025; 15(13):2251. https://doi.org/10.3390/buildings15132251
Chicago/Turabian StyleWang, Shuang, Hongqiang Li, and Xiaofeng Zhang. 2025. "A Preliminary Investigation on the Adsorption of Cu2+ by Sawdust/Foamed Geopolymer Composites" Buildings 15, no. 13: 2251. https://doi.org/10.3390/buildings15132251
APA StyleWang, S., Li, H., & Zhang, X. (2025). A Preliminary Investigation on the Adsorption of Cu2+ by Sawdust/Foamed Geopolymer Composites. Buildings, 15(13), 2251. https://doi.org/10.3390/buildings15132251





