Microstructural, Mechanical and Fresh-State Performance of BOF Steel Slag in Alkali-Activated Binders: Experimental Characterization and Parametric Mix Design Method
Abstract
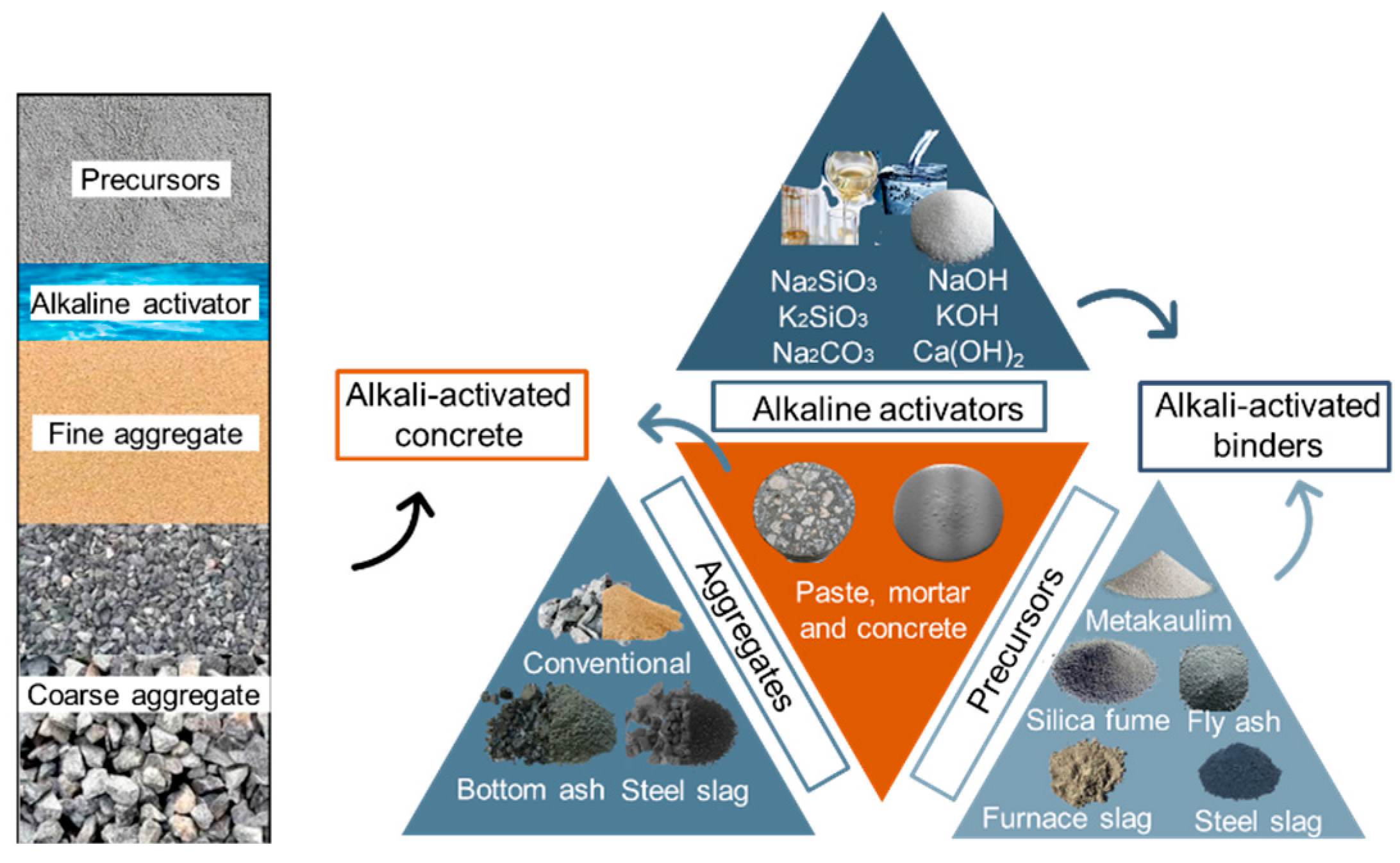
1. Introduction
2. Materials
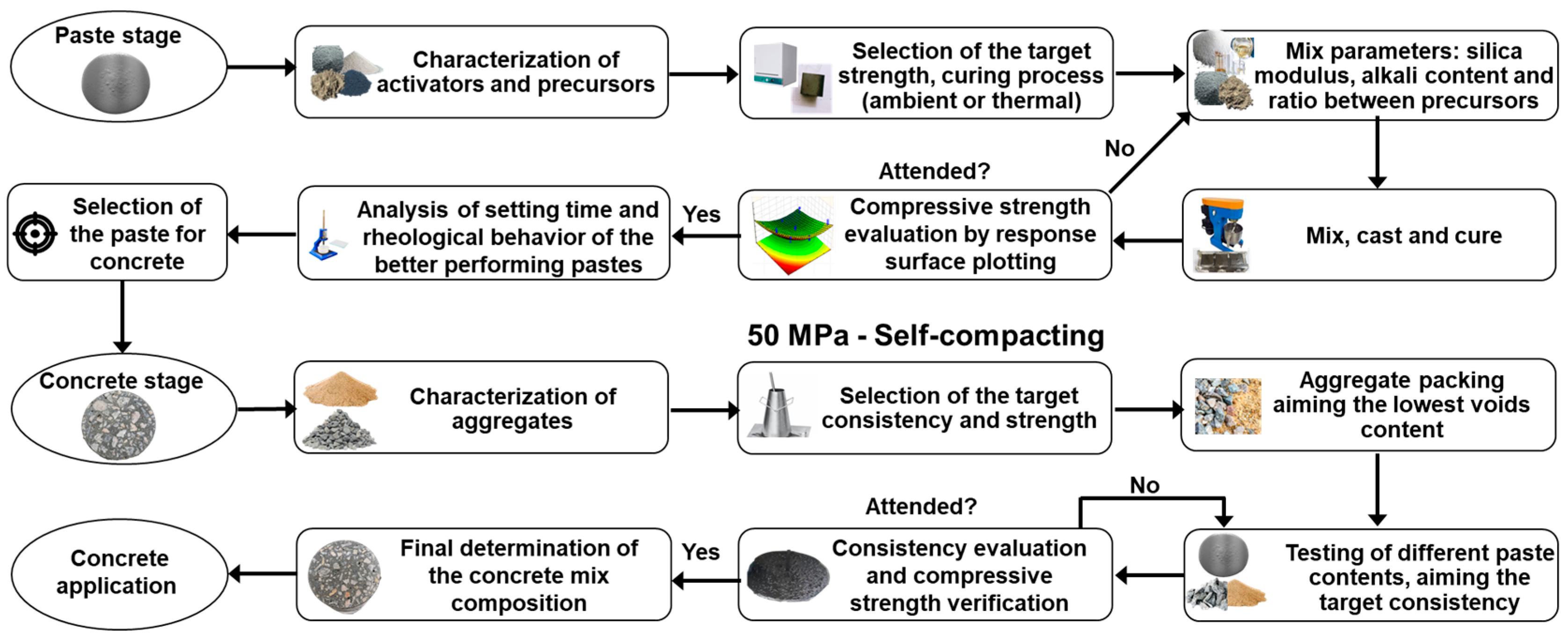
3. Systematic Parametric Validation and Compressive Strength Response Surface Methodology (RSM)
3.1. Methodology Abstract
3.2. Systematic Parametric Validation for AAB Pastes
3.3. Concrete Formulation and Binder Content Optimization
3.4. Specifics on Mixing, Specimen Manufacturing, Curring Conditions and Testing Procedure
3.4.1. AAB Paste
3.4.2. AAB Concretes
4. Results and Discussions
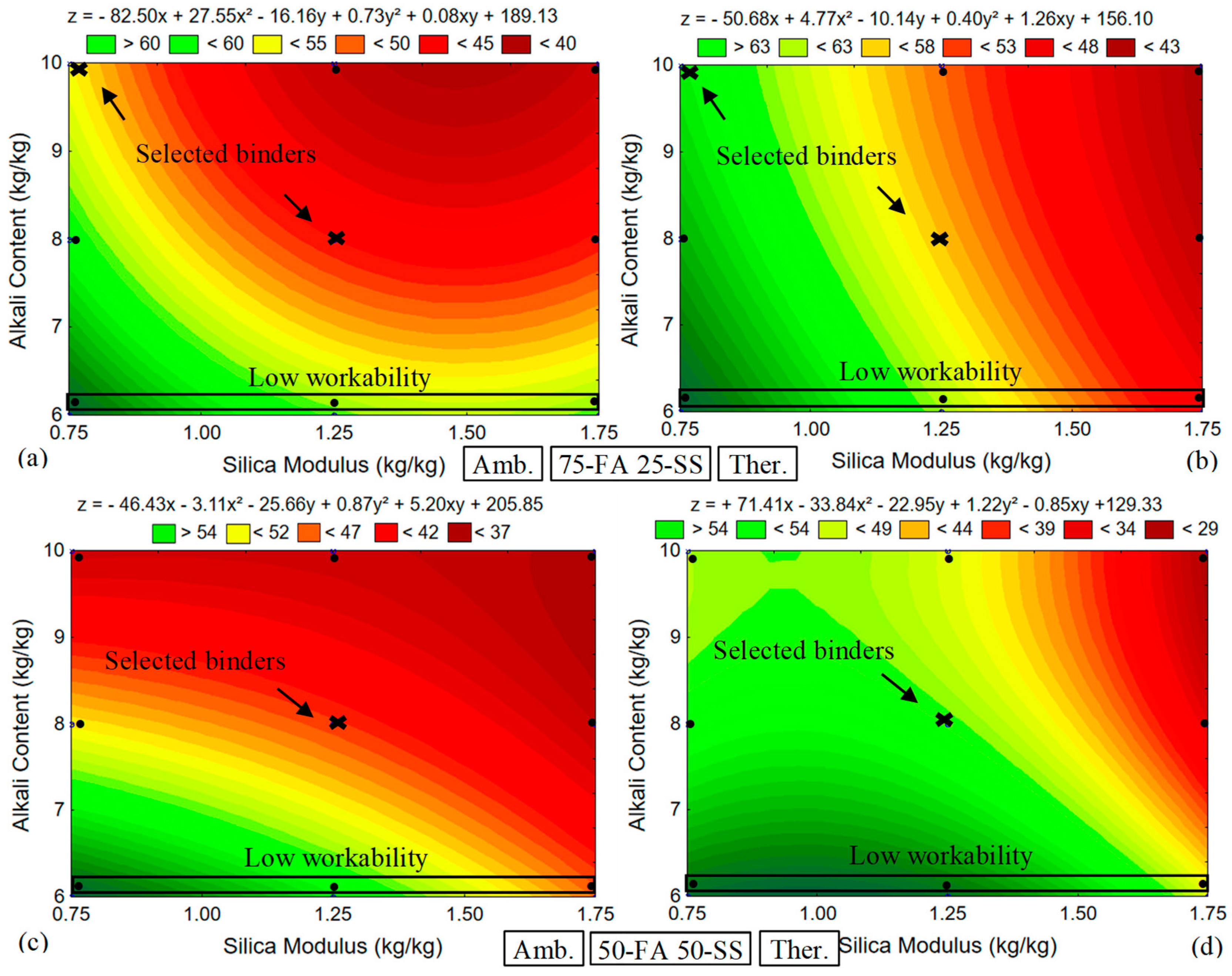
4.1. Systematic Parametric Validation and Paste Compressive Strength SRM
4.1.1. Pastes Compressive Strength Results
4.1.2. Paste Compressive Strength RSM Model
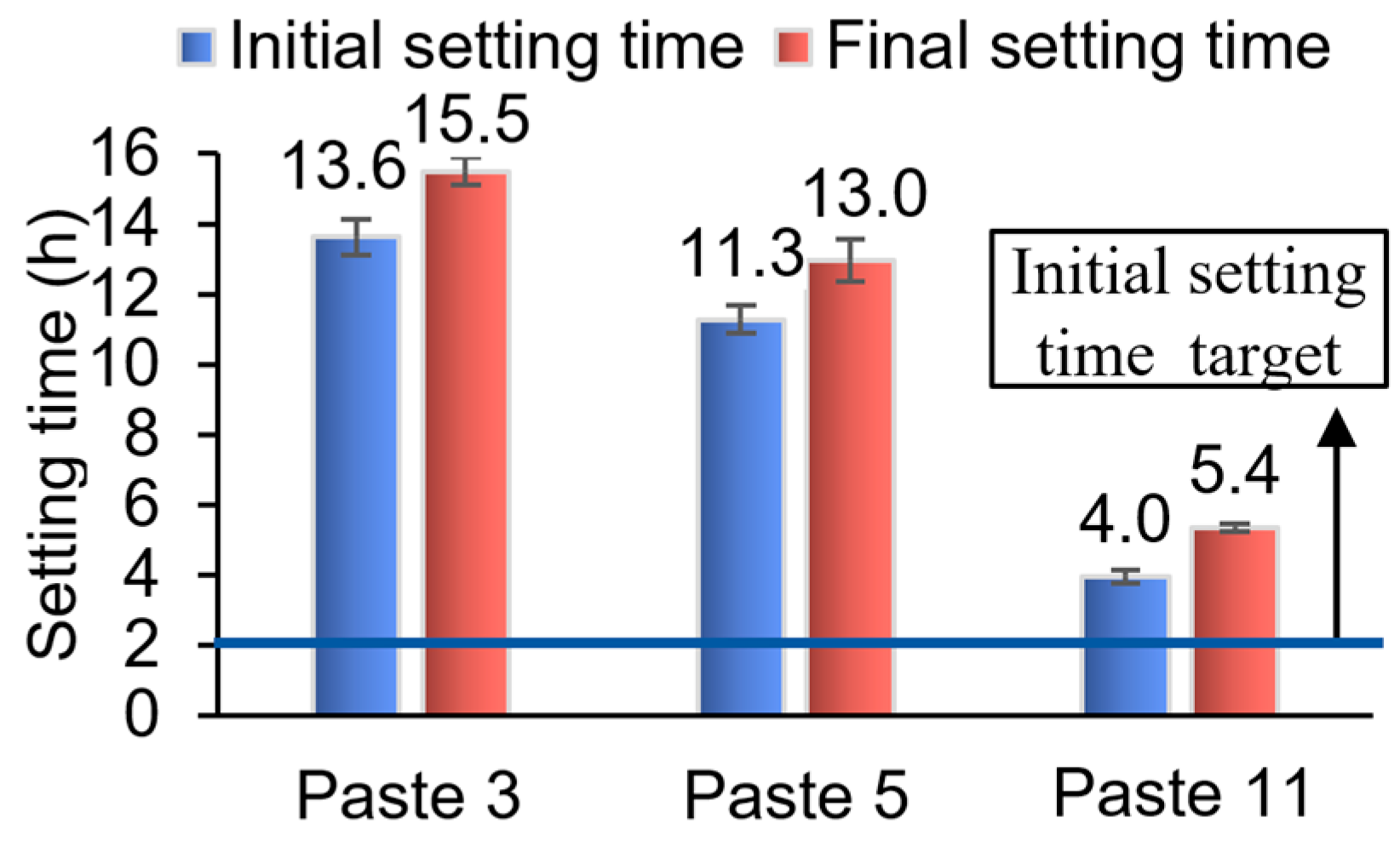
4.1.3. Paste Setting Time, Monotonic Flowability and Early Compressive Strength Results
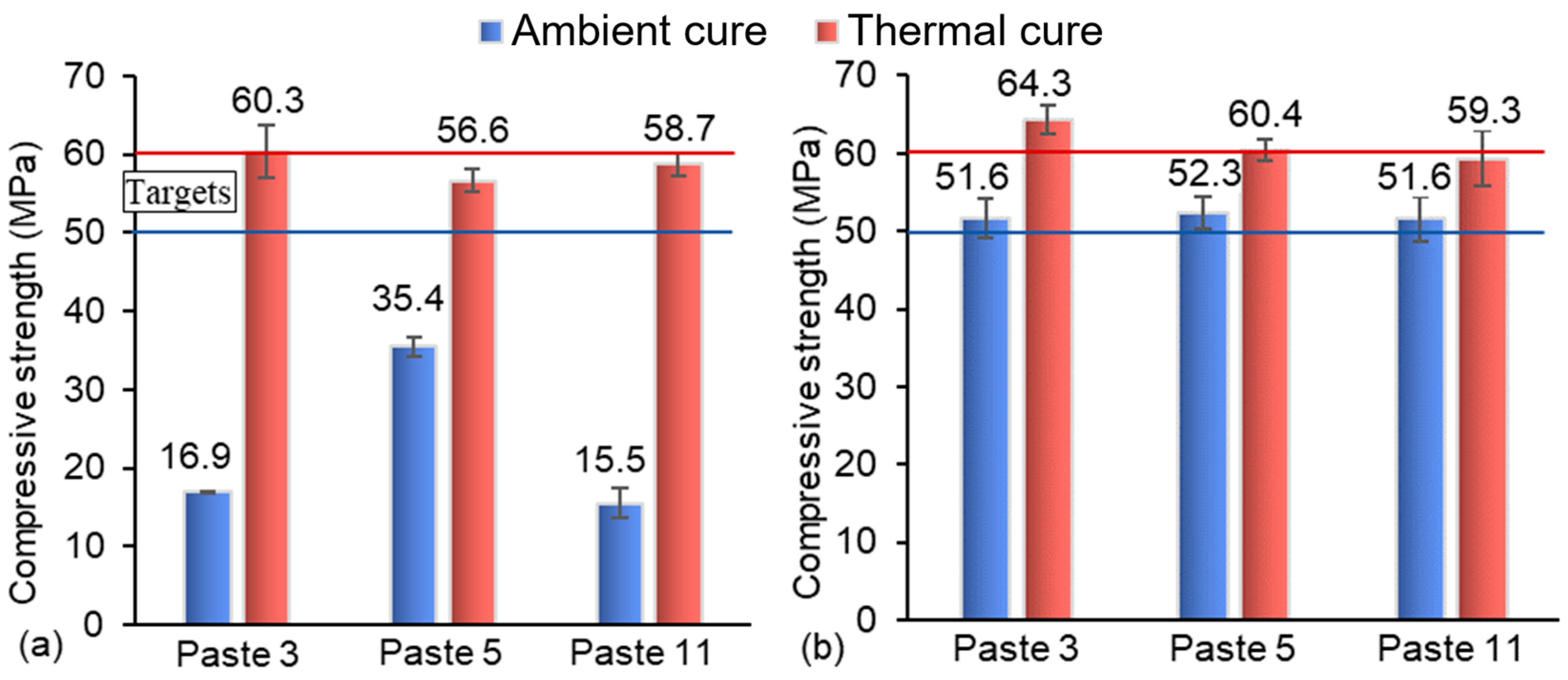
4.1.4. AAB Paste Curing Conditions’ Influence on Compressive Strength Evolution
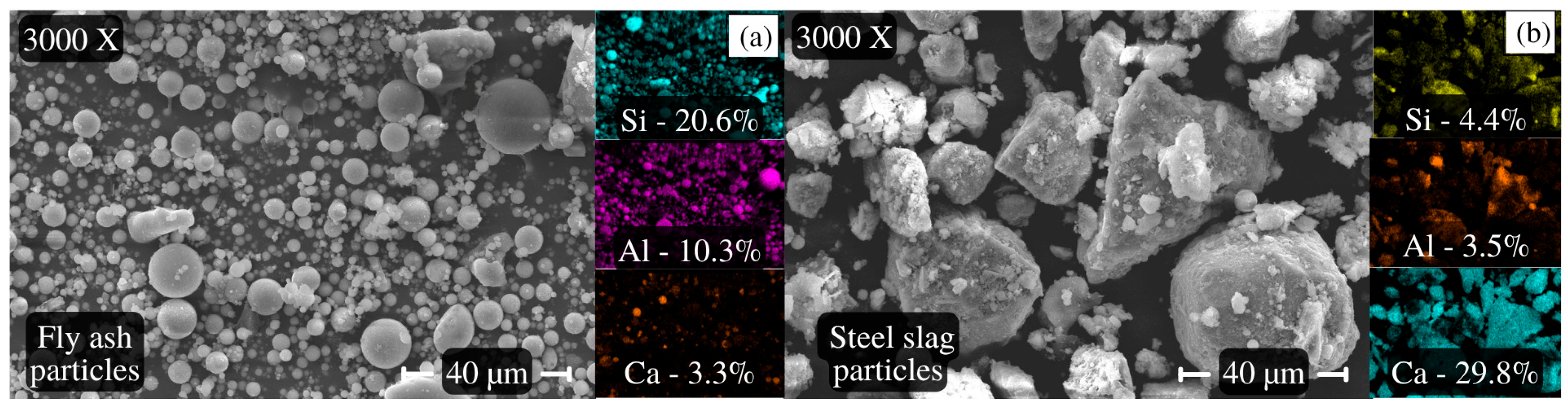
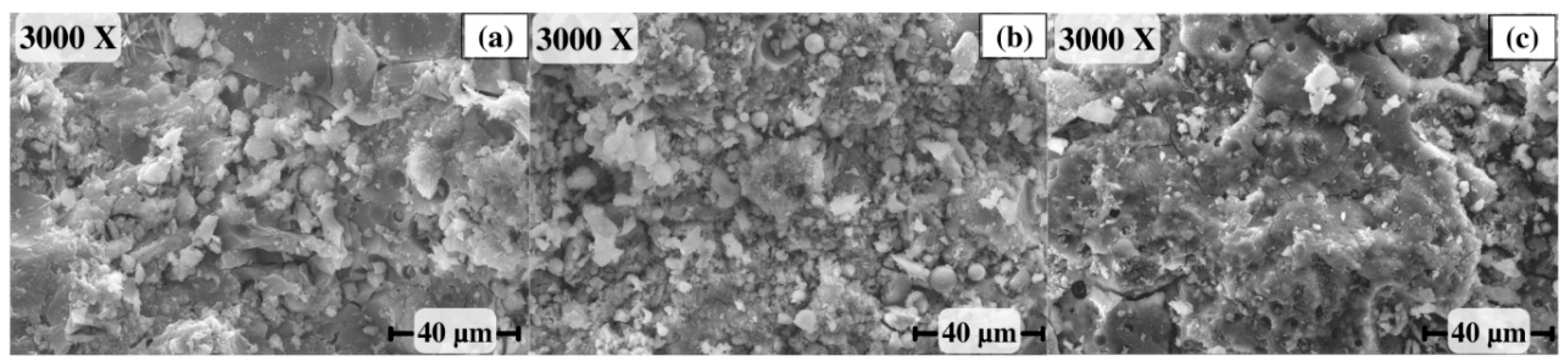
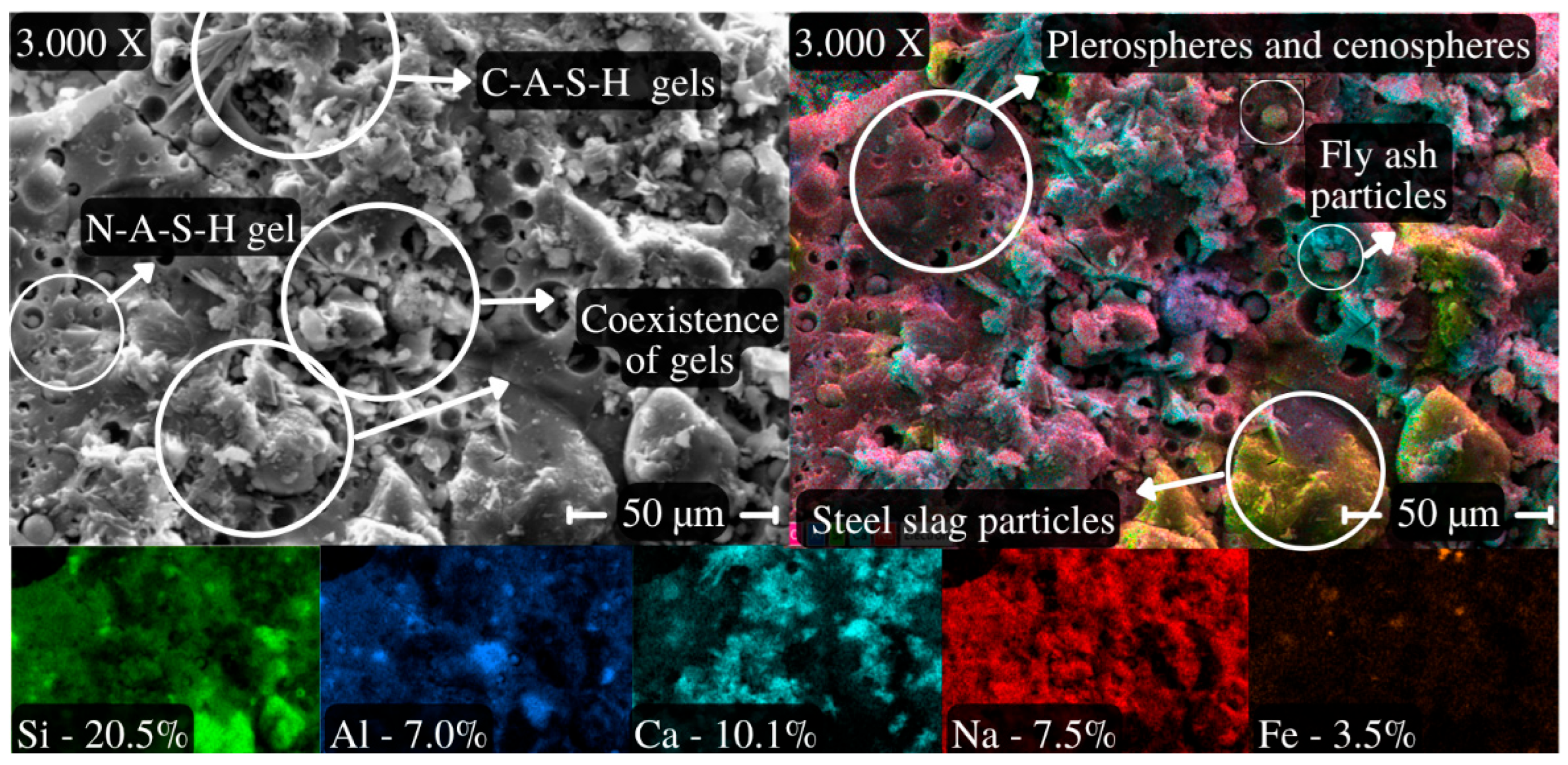
4.1.5. AAB Paste Microstructural Analysis Based on SEM/EDS and XRD
4.2. Alkali-Activated Concrete Results
4.2.1. Fresh-State Self-Compacting AAB Concrete Characterization
4.2.2. AAB Concretes’ Compressive Strength and Young’s Modulus Results
5. Conclusions
- A lower S/N parameter (0.75) demonstrated higher compressive strength results. This result differs from the findings often presented in the literature, where FA and GGBFS-based AAB usually exhibit optimal S/N ratios between 1.25 and 1.75;
- Thermal curing increased compressive strength at early ages, with results exceeding 50 MPa by 3 days and reaching 63.6 MPa at 28 days, but formulations with higher S/N (1.75) showed limited benefits, suggesting the need for parameter-specific adjustments;
- Ambient-cured AAB pastes demonstrated progressive strength development, surpassing thermally cured specimens at later ages (e.g., 68 MPa at 112 days), highlighting the viability of this lower-energy and more sustainable curing strategy;
- AAB concretes exhibited lower Young’s modulus values compared to what is expected for Portland cement concretes with similar strength levels and aggregates, consistent with the geopolymer literature. The modulus ranged from 20.2 GPa (ambient curing) to 26.2 GPa (thermal curing), influenced by the coexistence of N-A-S-H and C-A-S-H gels and the precursor’s calcium content;
- The microstructures of the AABs were dense and homogeneous as compared to conventional concretes, and were characterized by low porosity (9.4%) and optimal gel proportions.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delgado, J.M.D.; Oyedele, L.; Ajayi, A.; Akanbi, L.; Akinade, O.; Bilal, M.; Owolabi, H. Robotics and automated systems in construction: Understanding industry-specific challenges for adoption. J. Build. Eng. 2019, 26, 100868. [Google Scholar] [CrossRef]
- Baic, I.R.; Kozioł, W.; Miros, A. Dependence of extraction and production of construction aggregates on selected indicators of economic development in Poland. Arch. Civ. Eng. 2021, 67, 437–453. [Google Scholar] [CrossRef]
- Singh, N.B.; Middendorf, B. Geopolymers as an alternative to Portland cement: An overview. Constr. Build. Mater. 2019, 237, 117455. [Google Scholar] [CrossRef]
- Li, N.; Shi, C.; Zhang, Z.; Zhu, D.; Hwang, H.J.; Zhu, Y.; Sun, T. A mixture proportioning method for the development of performance-based alkali-activated slag-based concrete. Cem. Concr. Comp. 2018, 93, 163–174. [Google Scholar] [CrossRef]
- Provis, J.L. Geopolymers and other alkali activated materials: Why, how, and what? Mater. Struct. 2014, 47, 11–25. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and related alkali-activated materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Farooq, F.; Jin, X.; Javed, M.F.; Akbar, A.; Shah, M.I.; Aslam, F.; Alyousef, R. Geopolymer concrete as sustainable material: A state of the art review. Constr. Build. Mater. 2021, 306, 124762. [Google Scholar] [CrossRef]
- Xie, T.; Visintin, P.; Zhao, X.; Gravina, R. Mix design and mechanical properties of geopolymer and alkali activated concrete: Review of the state-of-the-art and the development of a new unified approach. Constr. Build. Mater. 2020, 256, 119380. [Google Scholar] [CrossRef]
- Provis, J.L.; Van Deventer, J.S.J. (Eds.) Alkali Activated Materials: State-of-the-Art Report, RILEM TC 224-AAM; RILEM State-of-the-Art Reports; Springer: Dordrecht, The Netherlands, 2014; Volume 13, ISBN 978-94-007-7671-5. [Google Scholar]
- Li, N.; Shi, C.; Zhang, Z.; Wang, H.; Liu, Y. A review on mixture design methods for geopolymer concrete. Comp. Part B Eng. 2019, 178, 107490. [Google Scholar] [CrossRef]
- Soutsos, M.; Boyle, A.P.; Vinai, R.; Hadjierakleous, A.; Barnett, S.J. Factors influencing the compressive strength of fly ash based geopolymers. Constr. Build. Mater. 2016, 110, 355–368. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.; Zhang, Z.; Ou, Z. Durability of alkali-activated materials in aggressive environments: A review on recent studies. Constr. Build. Mater. 2017, 152, 598–613. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L. Durability of alkali-activated materials: Progress and perspectives. J. Am. Ceram. Soc. 2014, 97, 997–1008. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, K.; Li, Q.; Wang, J.; Ling, Y. Fabrication and engineering properties of concretes based on geopolymers/alkali-activated binders-A review. J. Clean. Prod. 2020, 258, 120896. [Google Scholar] [CrossRef]
- Lee, N.K.; Lee, H.K. Setting and mechanical properties of alkali-activated fly ash/slag concrete manufactured at room temperature. Constr. Build. Mater. 2013, 47, 1201–1209. [Google Scholar] [CrossRef]
- Rafeet, A.; Vinai, R.; Soutsos, M.; Sha, W. Guidelines for mix proportioning of fly ash/GGBS based alkali activated concretes. Constr. Build. Mater. 2017, 147, 130–142. [Google Scholar] [CrossRef]
- Thomas, R.J.; Peethamparan, S. Stepwise regression modeling for compressive strength of alkali-activated concrete. Constr. Build. Mater. 2017, 141, 315–324. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, C.-J.; Li, N. Fracture properties of slag/fly ash-based geopolymer concrete cured in ambient temperature. Constr. Build. Mater. 2018, 190, 787–795. [Google Scholar] [CrossRef]
- Bondar, D.; Nanukuttan, S.; Provis, J.L.; Soutsos, M. Efficient mix design of alkali-activated slag concretes based on packing fraction of ingredients and paste thickness. J. Clean. Prod. 2019, 218, 438–449. [Google Scholar] [CrossRef]
- Zhong, Q.; Su, M.; Tian, X.; Peng, H. Workability and mechanical properties for GGBFS–MK geopolymer synthesis: Influencing factor analysis and a mix design method. Mater. Struct. 2023, 56, 144. [Google Scholar] [CrossRef]
- Naidu, T.S.; Sheridan, C.M.; Van Dyk, L.D. Basic oxygen furnace slag: Review of current and potential uses. Miner. Eng. 2020, 149, 106234. [Google Scholar] [CrossRef]
- Nunes, V.A.; Borges, P.H.R. Recent advances in the reuse of steel slags and future perspectives as binder and aggregate for alkali-activated materials. Constr. Build. Mater. 2021, 281, 122605. [Google Scholar] [CrossRef]
- Crude Steel Production and 2021 Global Crude Steel Production Totals. December 2021. Available online: https://worldsteel.org/ (accessed on 9 June 2025).
- Horii, K.; Kato, T.; Sugahara, K.; Tsutsumi, N.; Kitano, Y. Overview of iron/steel slag application and development of new utilization technologies. Nippon. Steel Sumitomo Tech. Rep. 2015, 109, 5–11. [Google Scholar]
- Elahi, M.M.A.; Hossain, M.M.; Karim, M.R.; Zain, M.F.M.; Shearer, C. A review on alkali-activated binders: Materials composition and fresh properties of concrete. Constr. Build. Mater. 2020, 260, 119788. [Google Scholar] [CrossRef]
- Jin, S.; Zhao, Z.; Jiang, S.; Sun, J.; Pan, H.; Jiang, L. Comparison and summary of relevant standards for comprehensive utilization of fly ash at home and abroad. IOP Conf. Ser. Earth Environ. Sci. 2021, 621, 012006. [Google Scholar] [CrossRef]
- Song, W.; Zhu, Z.; Peng, Y.; Wan, Y.; Xu, X.; Pu, S.; Song, S.; Wei, Y. Effect of steel slag on fresh, hardened and microstructural properties of high-calcium fly ash based geopolymers at standard curing condition. Constr. Build. Mater. 2019, 229, 116933. [Google Scholar] [CrossRef]
- Cristelo, N.; Coelho, J.; Miranda, T.; Palomo, Á.; Fernández-Jiménez, A. Alkali activated composites–An innovative concept using iron and steel slag as both precursor and aggregate. Cem. Concr. Comp. 2019, 103, 11–21. [Google Scholar] [CrossRef]
- Adesanya, E.; Ohenoja, K.; Kinnunen, P.; Illikainen, M. Properties and durability of alkali-activated ladle slag. Mater. Struct. 2017, 50, 255. [Google Scholar] [CrossRef]
- Nassar, A.K.; Kathirvel, P. Effective utilization of agricultural waste in synthesizing activator for sustainable geopolymer technology. Constr. Build. Mater. 2022, 362, 129681. [Google Scholar] [CrossRef]
- Nassar, A.K.; Kathirvel, P.; Murali, G.; Alqemlas, T.; Azab, M. Innovative one-part Alkali activated binder from activator derived from agricultural waste: Synthesis and application for sustainable construction. Results Eng. 2024, 22, 101975. [Google Scholar] [CrossRef]
- ASTM C618; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use as a Mineral Admixture in Concrete. American Society For Testing And Materials: West Conshohocken, PA, USA, 2019.
- Yusuf, M.O.; Johari, M.A.M.; Ahmad, Z.A.; Maslehuddin, M. Evolution of alkaline activated ground blast furnace slag–ultrafine palm oil fuel ash based concrete. Mater. Des. 2014, 55, 387–393. [Google Scholar] [CrossRef]
- Gomes, P.C.C.; Barros, A.R. Métodos de Dosagem de Concreto Autoadensável; PINI: São Paulo, Brazil, 2009. [Google Scholar]
- Nath, P.; Sarker, P.K. Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef]
- Costa, H.N. Cimentos Álcali-Ativados à Base de Cinzas do Carvão Mineral e de Escórias Siderúrgicas. Ph.D. Thesis, Engineering and Materials Science Program, Federal University of Ceará, Fortaleza, Brazil, 2022. [Google Scholar]
- ABNT NBR 7.212; Ready-Mixed Concrete—Preparation, Supply and Control. Associação Brasileira De Normas Técnicas: Rio de Janeiro, Brazil, 2021.
- ASTM C191; Standard Test Methods for Time of Setting of Hydraulic Cement by Vicat Needle. American Society for Testing and Materials: West Conshohocken, PA, USA, 2021.
- Luo, Z.; Li, W.; Wang, K.; Castel, A.; Shah, S.P. Comparison on the properties of ITZs in fly ash-based geopolymer and Portland cement concretes with equivalent flowability. Cem. Concr. Res. 2021, 143, 106392. [Google Scholar] [CrossRef]
- Tavares, M.L.; Targino, D.L.L.; Cabral, A.E.B.; Nogueira, H. Evaluation of the effect of curing conditions on compressive strength and microstructure of alkali-activated binders. Labor Eng. 2024, 18, e024006. [Google Scholar] [CrossRef]
- Roussel, N.; Coussot, P. “Fifty-cent rheometer” for yield stress measurements: From slump to spreading flow. J. Rheol. 2005, 49, 705–718. [Google Scholar] [CrossRef]
- Tan, Z.; Bernal, S.A.; Provis, J.L. Reproducible mini-slump test procedure for measuring the yield stress of cementitious pastes. Materials and Struct. 2017, 50, 235. [Google Scholar] [CrossRef]
- European Federation of National Associations Representing for Concrete (EFNARC). European Guidelines for Self-Compacting Concrete: Specification, Production and Use, EFNARC—Publication Index|NBS; EFNARC (European Federation of National Associations Representing for Concrete): Flums, Switzerland, 2005; p. 68. [Google Scholar]
- Bernal, S.A.; De Gutiérrez, R.M.; Pedraza, A.L.; Provis, J.L.; Rodriguez, E.D.; Delvasto, S. Effect of binder content on the performance of alkali-activated slag concretes. Cem. Concr. Res. 2011, 41, 1–8. [Google Scholar] [CrossRef]
- ASTM C143; Standard Test Method for Slump of Hydraulic-Cement Concrete. American Society For Testing And Materials: West Conshohocken, PA, USA, 2015.
- ASTM C1611; Standard Test Method for Slump Flow of Self-Consolidating Concrete. American Society For Testing And Materials: West Conshohocken, PA, USA, 2010.
- ASTM C349; Standard Test Method for Compressive Strength of Hydraulic-Cement Mortars (Using Portions of Prisms Broken in Flexure). American Society For Testing And Materials: West Conshohocken, PA, USA, 2018.
- ASTM C39; Standard test method for compressive strength of cylindrical concrete specimens. American Society For Testing And Materials: West Conshohocken, PA, USA, 2020.
- ASTM C469; Standard Test Method for Static Modulus of Elasticity and Poisson’s Ratio of Concrete in Compression. American Society for Testing and Materials: West Conshohocken, PA, USA, 2022.
- Bellum, R.R.; Reddy, K.H.K.; Reddy, G.C.; Reddy, M.R.K.; Gamini, S. Influence of steel slag on strength and microstructural characteristics of fly ash-based geopolymer concrete. Multiscale and Multidisciplinary Modeling, Exp. Des. 2024, 7, 5499–5514. [Google Scholar] [CrossRef]
- Guo, X.; Pan, X. Mechanical properties and mechanisms of fiber reinforced fly ash–steel slag based geopolymer mortar. Constr. Build. Mater. 2018, 179, 633–641. [Google Scholar] [CrossRef]
- Lee, W.H.; Cheng, T.W.; Lin, K.Y.; Lin, K.L.; Wu, C.C.; Tsai, C.T. Geopolymer technologies for stabilization of basic oxygen furnace slags and sustainable application as construction materials. Sustainability 2020, 12, 5002. [Google Scholar] [CrossRef]
- Mcalexander, M.; Bharadwaj, K.; Weiss, J.W.; Isgor, O.B. Reactivity of waterglass in cementitious systems. Cement 2023, 12, 100067. [Google Scholar] [CrossRef]
- Choi, S.C.; Lee, W.K. Effect of Fe2O3 on the physical property of geopolymer paste. In: Advanced materials research. Adv. Mater. Res. 2012, 586, 126–129. [Google Scholar] [CrossRef]
- Hadi, M.N.S.; Zhang, H.; Parkinson, S. Optimum mix design of geopolymer pastes and concretes cured in ambient condition based on compressive strength, setting time and workability. J. Build. Eng. 2019, 23, 301–313. [Google Scholar] [CrossRef]
- Geddes, D.A.; Walkley, B.; Matsuda, T.; Provis, J.P. Multi-year cementitious hydrate product formation in non-Portland high performance concretes. Cement 2024, 18, 100111. [Google Scholar] [CrossRef]
- Dai, X.; Aydin, S.; Yardimci, M.Y.; Sun, Y.; Schutter, G. Effect of temperature on the fresh and hardened state properties of alkali-activated slag/fly ash mixtures. Mater. Struct. 2023, 56, 107. [Google Scholar] [CrossRef]
- Ma, G.; Yan, Y.; Zhang, M.; Sanjayan, J. Effect of Steel Slag on 3d Concrete Printing of Geopolymer with Quaternary Binders. Ceram. Int. 2016, 48, 26233–26247. [Google Scholar] [CrossRef]
- Lee, W.H.; Wang, J.H.; Ding, Y.C.; Cheng, T.W. A study on the characteristics and microstructures of GGBS/FA based geopolymer paste and concrete. Constr. Build. Mater. 2019, 211, 807–813. [Google Scholar] [CrossRef]
- Huang, G.; Huang, Y.; Zheng, X.; Zhang, F.; Xu, J.; Qi, J.; Chen, Z. Study on the Characteristics of Crystal Formation and Transformation of Alkali-Activated Slag Minerals Induced by Weak Alkali. Crystals 2024, 14, 1231. [Google Scholar] [CrossRef]
- Zhu, C.; Wan, Y.; Wang, L.; Ye, Y.; Yu, H.; Yang, J. Strength Characteristics and Microstructure Analysis of Alkali-Activated Slag–Fly Ash Cementitious Material. Materials 2022, 15, 5998. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, H.; Mostofinejad, D. High-performance concrete based on alkaline earth metal ions-activated slag at ambient temperature: Mechanical and microstructure properties. J. Mater. Res. Technol. 2023, 24, 4192–4207. [Google Scholar] [CrossRef]
- Li, N.; Shi, C.; Wang, Q.; Zhang, Z. Composition design and performance of alkali-activated cements. Mater. Struct. 2017, 50, 178. [Google Scholar] [CrossRef]
- Turkoglu, M.; Bayraktar, O.Y.; Benli, A.; Kaplan, G. Effect of cement clinker type, curing regime and activator dosage on the performance of one-part alkali-activated hybrid slag/clinker composites. J. Build. Eng. 2023, 68, 106164. [Google Scholar] [CrossRef]
- Nunes, V.A.; Suraneni, P.; Bezerra, A.C.; Thomas, C.; Borges, P.H. Influence of activation parameters on the mechanical and microstructure properties of an alkali-activated BOF steel slag. Appl. Sci. 2022, 12, 12437. [Google Scholar] [CrossRef]
- Fang, Y.; Kayali, O. The fate of water in fly ash-based geopolymers. Constr. Build. Mater. 2013, 39, 89–94. [Google Scholar] [CrossRef]
- Puligilla, S.; Mondal, P. Role of slag in microstructural development and hardening of fly ash-slag geopolymer. Cem. Concr. Res. 2013, 43, 70–80. [Google Scholar] [CrossRef]
- Lloyd, R.R.; Provis, J.L.; Smeaton, K.J.; Van Deventer, J.S. Spatial distribution of pores in fly ash-based inorganic polymer gels visualised by Wood’s metal intrusion. Microporous Mesoporous Mater. 2009, 126, 32–39. [Google Scholar] [CrossRef]
- Valentim, B.; Flores, D.; Guedes, A.; Shreya, N.; Paul, P.; Colin, R. Ward Notes on the occurrence of char plerospheres in fly ashes derived from Bokaro and Jharia coals (Jharkhand, India) and the influence of the combustion conditions on their genesis. Int. J. Coal Geol. 2016, 158, 29–43. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; Macphee, D.E. Compatibility studies between NASH and CASH gels. Study in the ternary diagram Na2O–CaO–Al2O3–SiO2–H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Zhu, X.; Li, W.; Du, Z.; Zhou, S.; Zhang, Y.; Li, F. Recycling and utilization assessment of steel slag in metakaolin based geopolymer from steel slag by-product to green geopolymer. Constr. Build. Mater. 2021, 305, 124654. [Google Scholar] [CrossRef]
- Kamath, M.; Prashanth, S.; Kumar, M. Micro-characterisation of alkali activated paste with fly ash-GGBS-metakaolin binder system with ambient setting characteristics. Constr. Build. Mater. 2021, 277, 122323. [Google Scholar] [CrossRef]
- Araújo, L.B.R.; Targino, D.L.; Babadopulos, L.F.A.L.; Fabbri, A.; Cabral, A.E.B.; Chehade, R.; Costa, H.N. Impact of Curing Temperature and Steel Slag Aggregates on High-Strength Self-Compacting Alkali-Activated Concrete. Buildings 2025, 15, 457. [Google Scholar] [CrossRef]
- Lin, C.; Liu, Z.; Gao, Y.; Li, Z.; Zhang, J.; Niu, H. Study on the effect and mechanism of cement-based material retarder on red mud-based hybrid alkali activated cement. J. Build. Eng. 2023, 70, 106353. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.; Ding, Z.; Shan, R.; Zhao, M. Construction of a Molecular Dynamics Model of NASH Geopolymer Based on XRD Analysis. Materials 2024, 17, 6103. [Google Scholar] [CrossRef]
- Izadifar, M.; Valencia, N.C.; Xiao, P.; Ukrainczyk, N.; Koenders, E. 3D off-lattice coarse-grained Monte Carlo simulations for nucleation of alkaline aluminosilicate gels. Materials 2023, 16, 1863. [Google Scholar] [CrossRef] [PubMed]
- Saini, G.; Vattipalli, U. Assessing properties of alkali activated GGBS based self-compacting geopolymer concrete using nano-silica. Case Stud. Constr. Mater. 2020, 12, e00352. [Google Scholar] [CrossRef]
- Huseien, G.F.; Sam, A.R.M.; Alyousef, R. Texture, morphology and strength performance of self-compacting alkali-activated concrete: Role of fly ash as GBFS replacement. Constr. Build. Mater. 2021, 270, 121368. [Google Scholar] [CrossRef]
- Zheng, Z.; Deng, P. Mechanical and fracture properties of slag/steel slag-based geopolymer fully recycled aggregate concrete. Constr. Build. Mater. 2024, 413, 134533. [Google Scholar] [CrossRef]
- Katman, H.Y.B.; Khai, W.J.; Bheel, N.; Kırgız, M.S.; Kumar, A.; Khatib, J.; Benjeddou, O. Workability, Strength, Modulus of Elasticity, and Permeability Feature of Wheat Straw Ash-Incorporated Hydraulic Cement Concrete. Buildings 2022, 12, 1363. [Google Scholar] [CrossRef]
- Bezerra, A.K.; Melo, A.R.; Freitas, I.L.; Babadopulos, L.F.; Carret, J.C.; Soares, J.B. Determination of modulus of elasticity and Poisson’s ratio of cementitious materials using S-wave measurements to get consistent results between static, ultrasonic and resonant testing. Constr. Build. Mater. 2023, 398, 132456. [Google Scholar] [CrossRef]
- Bahmani, H.; Mostofinejad, D. Microstructure of Ultra-High-Performance Concrete (UHPC)—A Review Study. J. Build. Eng. 2022, 50, 104118. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Flexural strength and elastic modulus of ambient-cured blended low-calcium fly ash geopolymer concrete. Constr. Build. Mater. 2017, 130, 22–31. [Google Scholar] [CrossRef]
- Němeček, J.; Šmilauer, V.; Kopecký, L. Nanoindentation characteristics of alkali-activated aluminosilicate materials. Cem. Concr. Comp. 2011, 33, 163–170. [Google Scholar] [CrossRef]
- Waqas, R.M.; Butt, F.; Zhu, X.; Jiang, T.; Tufail, R.F.A. Comprehensive Study on the Factors Affecting the Workability and Mechanical Properties of Ambient Cured Fly Ash and Slag Based Geopolymer Concrete. Appl. Sci. 2021, 11, 8722. [Google Scholar] [CrossRef]
- Palankar, N.; Ravi, S.A.U.; Mithun, B.M. Investigations on Alkali-Activated Slag/Fly Ash Concrete with steel slag coarse aggregate for pavement structures. Int. J. Pavement Eng. 2017, 18, 500–512. [Google Scholar] [CrossRef]
- Yang, K.H.; Song, J.K.; Song, K.I. Assessment of CO2 reduction of alkali-activated concrete. J. Clean. Prod. 2012, 39, 265–272. [Google Scholar] [CrossRef]




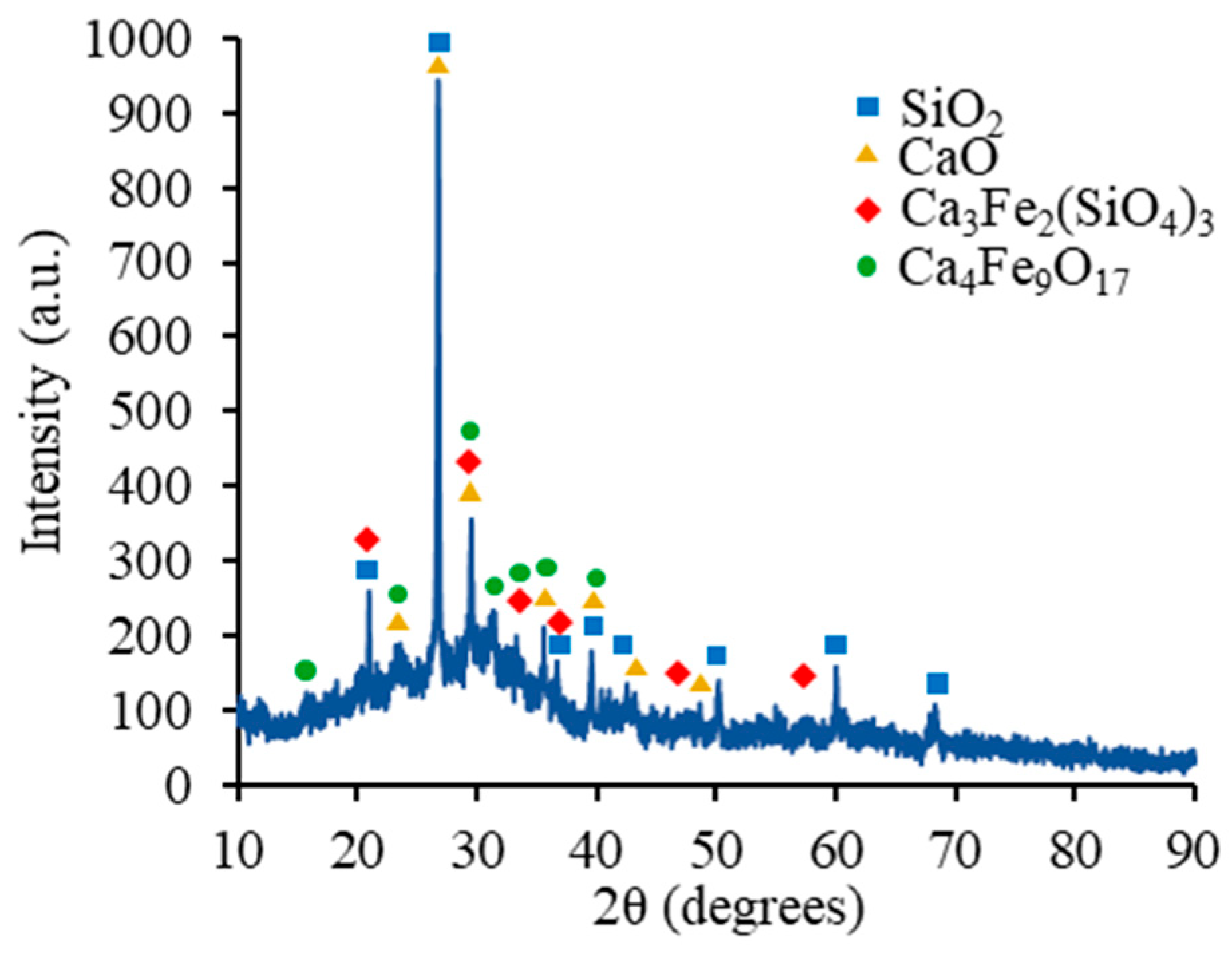
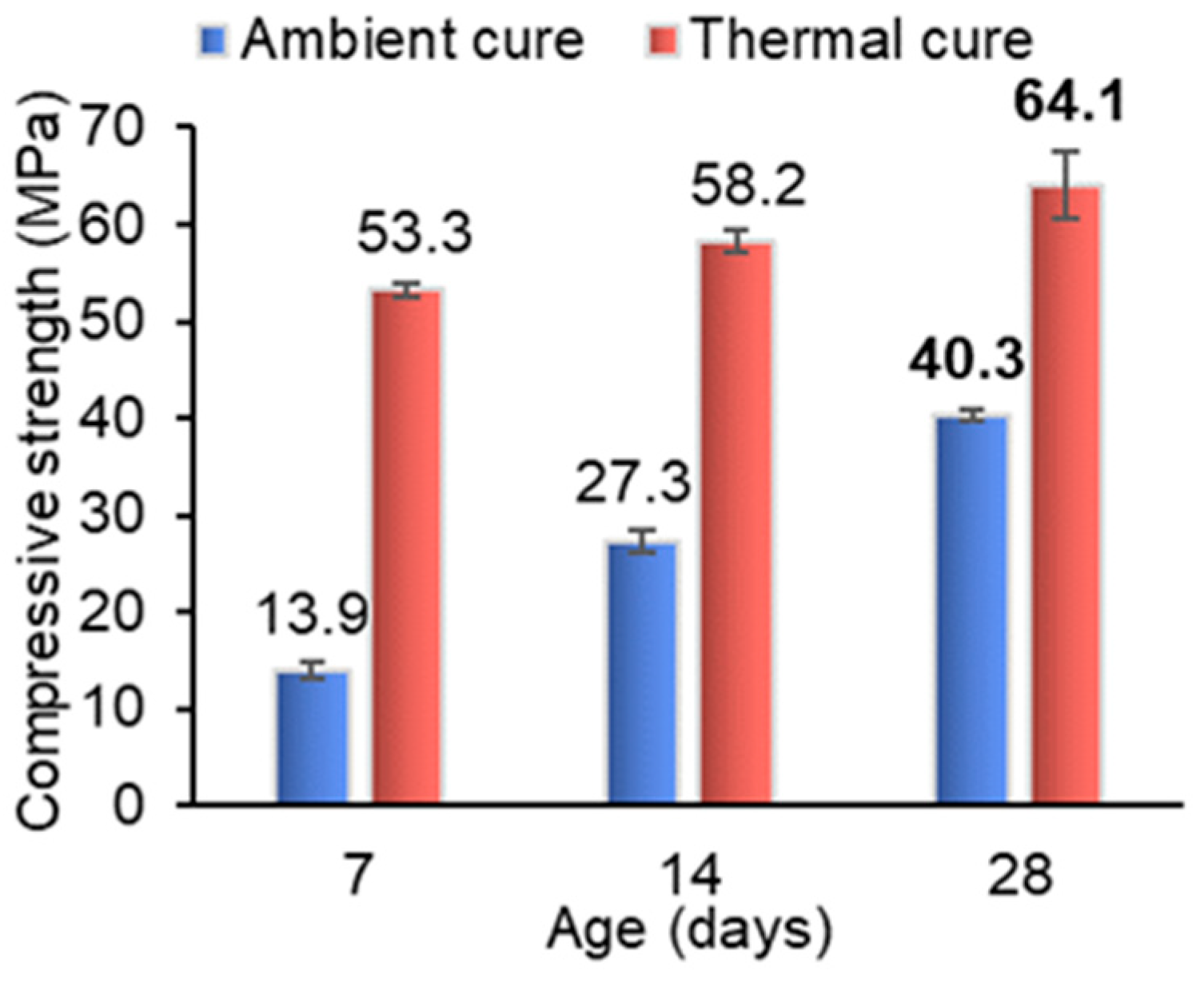
| Material | Al2O3 | SiO2 | P2O5 | SO3 | Cl | K2O | CaO | TiO2 | MnO | Fe2O3 |
|---|---|---|---|---|---|---|---|---|---|---|
| BOF Steel slag precursor (%m.) | 1.94 | 5.64 | 0.84 | 0.83 | 0.04 | 0.14 | 53.14 | - | 2.97 | 34.40 |
| BSSF Steel slag aggregate (%m.) | 0.70 | 4.78 | 0.96 | - | 0.01 | 0.05 | 40.46 | 0.42 | 4.13 | 50.14 |
| Fly ash (%m.) | 11.14 | 42.17 | 0.53 | 1.08 | 0.06 | 3.97 | 10.25 | 2.74 | 0.27 | 26.98 |
| Parameters | Min. | Max. | Var. | Total |
|---|---|---|---|---|
| S/N (ad) | 0.75 | 1.75 | 0.5 | 3 |
| N/B (%) | 6.0 | 10.0 | 2.0 | 3 |
| Precursors (FA-SS) | 75-25 | 50-50 | - | 2 |
| Curing Method | Ambient (25 °C) | Thermal (65 °C) | - | 2 |
| Paste ID | FA-SS | S/N 1 | N/B 2 | Sodium Hydroxide | Sodium Silicate | Fly Ash | Steel Slag | A/B 3 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| kg/m3 | % vol. | kg/m3 | % vol. | kg/m3 | % vol. | kg/m3 | % vol. | % mass | ||||
| 1 | 75-25 | 0.75 | 6.0 | 261.1 | 20.0 | 230.6 | 14.6 | 1223.3 | 52.3 | 407.8 | 13.0 | 30.1 |
| 2 | 75-25 | 0.75 | 8.0 | 312.0 | 23.9 | 275.6 | 17.4 | 1096.5 | 46.8 | 365.5 | 11.7 | 40.2 |
| 3 | 75-25 | 0.75 | 10.0 | 353.4 | 27.1 | 312.1 | 19.7 | 993.5 | 42.4 | 331.2 | 10.6 | 50.2 |
| 4 | 75-25 | 1.25 | 6.0 | 162.1 | 12.4 | 374.9 | 23.7 | 1193.3 | 51.0 | 397.8 | 12.7 | 33.7 |
| 5 | 75-25 | 1.25 | 8.0 | 192.8 | 14.8 | 445.9 | 28.2 | 1064.5 | 45.5 | 354.8 | 11.3 | 45.0 |
| 6 | 75-25 | 1.25 | 10.0 | 217.5 | 16.7 | 503.1 | 31.8 | 960.8 | 41.0 | 320.3 | 10.2 | 56.2 |
| 7 | 75-25 | 1.75 | 6.0 | 67.8 | 5.2 | 512.3 | 32.4 | 1164.7 | 49.8 | 388.2 | 12.4 | 37.4 |
| 8 | 75-25 | 1.75 | 8.0 | 80.2 | 6.1 | 606.6 | 38.3 | 1034.3 | 44.2 | 344.8 | 11.0 | 49.8 |
| 9 | 75-25 | 1.75 | 10.0 | 90.2 | 6.9 | 681.9 | 43.1 | 930.2 | 39.7 | 310.1 | 9.9 | 62.3 |
| 10 | 50-50 | 0.75 | 6.0 | 273.1 | 20.9 | 241.2 | 15.2 | 852.9 | 36.4 | 852.9 | 27.3 | 30.1 |
| 11 | 50-50 | 0.75 | 8.0 | 324.8 | 24.9 | 286.9 | 18.1 | 760.9 | 32.5 | 760.9 | 24.3 | 40.2 |
| 12 | 50-50 | 0.75 | 10.0 | 366.5 | 28.1 | 323.7 | 20.4 | 686.8 | 29.3 | 686.8 | 22.0 | 50.2 |
| 13 | 50-50 | 1.25 | 6.0 | 169.3 | 13.0 | 391.6 | 24.7 | 831.1 | 35.5 | 831.1 | 26.6 | 33.7 |
| 14 | 50-50 | 1.25 | 8.0 | 200.4 | 15.4 | 463.6 | 29.3 | 737.8 | 31.5 | 737.8 | 23.6 | 45.0 |
| 15 | 50-50 | 1.25 | 10.0 | 225.2 | 17.3 | 521.0 | 32.9 | 663.4 | 28.3 | 663.4 | 21.2 | 56.2 |
| 16 | 50-50 | 1.75 | 6.0 | 70.7 | 5.4 | 534.6 | 33.8 | 810.3 | 34.6 | 810.3 | 25.9 | 37.4 |
| 17 | 50-50 | 1.75 | 8.0 | 83.3 | 6.4 | 629.9 | 39.8 | 716.1 | 30.6 | 716.1 | 22.9 | 49.8 |
| 18 | 50-50 | 1.75 | 10.0 | 93.3 | 7.2 | 705.4 | 44.6 | 641.5 | 27.4 | 641.5 | 20.5 | 62.3 |
| Materials | (kg/m3) | % vol. |
|---|---|---|
| Fly Ash | 382.1 | 16.3 |
| Steel Slag prec. | 127.4 | 4.1 |
| NaOH (solution)—10 mol/L | 135.9 | 10.4 |
| Na2SiO3 (solution) | 120.1 | 7.6 |
| Fine aggr. | 561.6 | 21.8 |
| Coarse aggr. 4.75–12.5 mm | 417.2 | 16.0 |
| Coarse aggr. 9.5–25 mm | 625.8 | 23.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, L.B.R.; Targino, D.L.L.; Babadopulos, L.F.A.L.; Costa, H.N.; Cabral, A.E.B.; Bastos, J.B.S. Microstructural, Mechanical and Fresh-State Performance of BOF Steel Slag in Alkali-Activated Binders: Experimental Characterization and Parametric Mix Design Method. Buildings 2025, 15, 2056. https://doi.org/10.3390/buildings15122056
Araújo LBR, Targino DLL, Babadopulos LFAL, Costa HN, Cabral AEB, Bastos JBS. Microstructural, Mechanical and Fresh-State Performance of BOF Steel Slag in Alkali-Activated Binders: Experimental Characterization and Parametric Mix Design Method. Buildings. 2025; 15(12):2056. https://doi.org/10.3390/buildings15122056
Chicago/Turabian StyleAraújo, Lucas B. R., Daniel L. L. Targino, Lucas F. A. L. Babadopulos, Heloina N. Costa, Antonio E. B. Cabral, and Juceline B. S. Bastos. 2025. "Microstructural, Mechanical and Fresh-State Performance of BOF Steel Slag in Alkali-Activated Binders: Experimental Characterization and Parametric Mix Design Method" Buildings 15, no. 12: 2056. https://doi.org/10.3390/buildings15122056
APA StyleAraújo, L. B. R., Targino, D. L. L., Babadopulos, L. F. A. L., Costa, H. N., Cabral, A. E. B., & Bastos, J. B. S. (2025). Microstructural, Mechanical and Fresh-State Performance of BOF Steel Slag in Alkali-Activated Binders: Experimental Characterization and Parametric Mix Design Method. Buildings, 15(12), 2056. https://doi.org/10.3390/buildings15122056







