Author Contributions
Conceptualization, X.Z. and H.X.; methodology, X.Z. and W.Y.; software, X.Z. and S.M.; validation, J.S. and H.X.; formal analysis, W.Y.; investigation, X.Z.; resources, S.M.; data curation, X.Z. and H.X.; writing—original draft preparation, X.Z. and W.Y.; writing—review and editing, J.S.; visualization, W.L.; supervision, S.M.; project administration, S.M.; funding acquisition, S.M. and W.L. All authors have read and agreed to the published version of the manuscript.
Figure 1.
Particle size distributions of sandstones (a) and other raw materials (b).
Figure 1.
Particle size distributions of sandstones (a) and other raw materials (b).
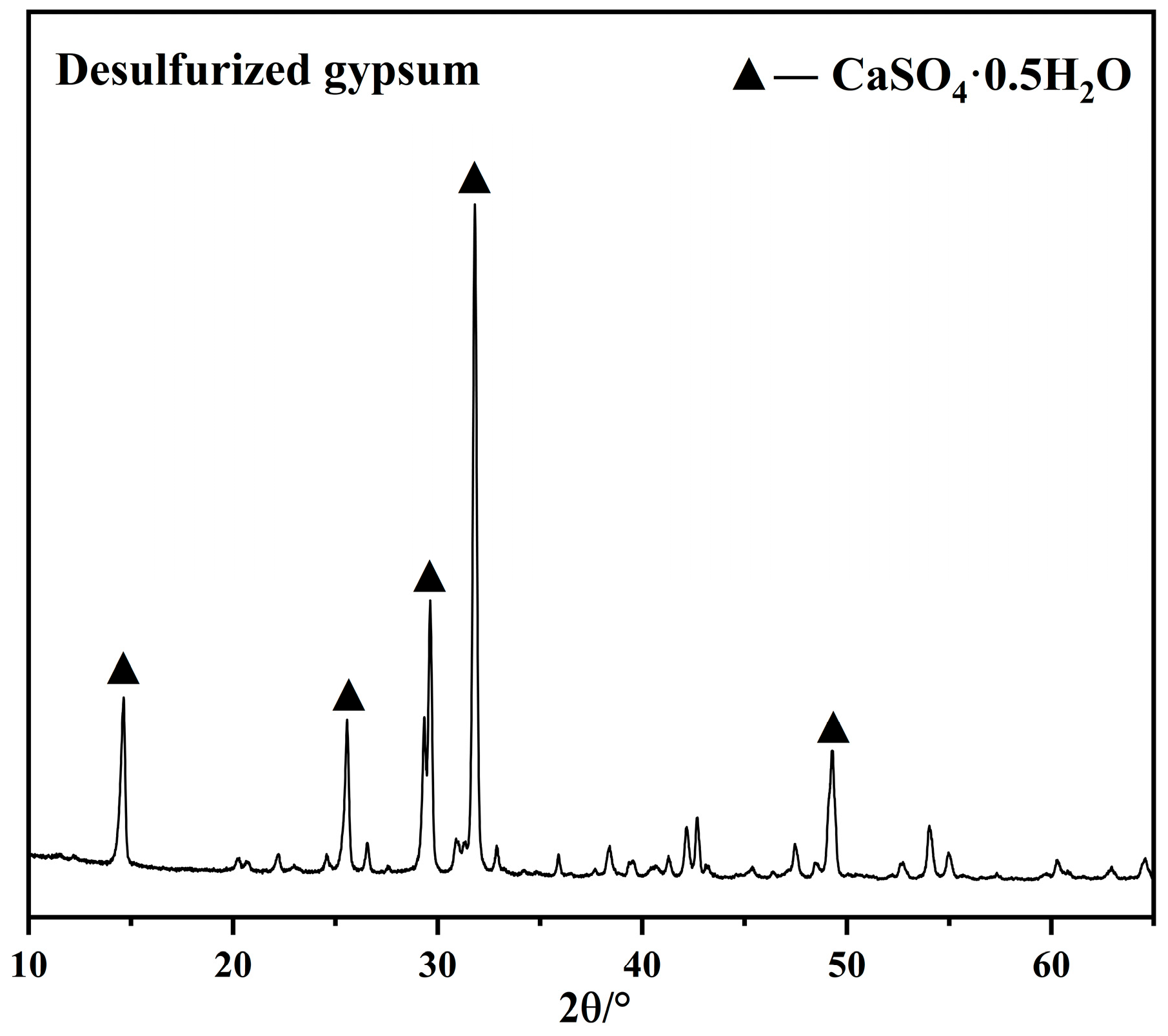
Figure 2.
XRD pattern of desulfurized gypsum.
Figure 2.
XRD pattern of desulfurized gypsum.
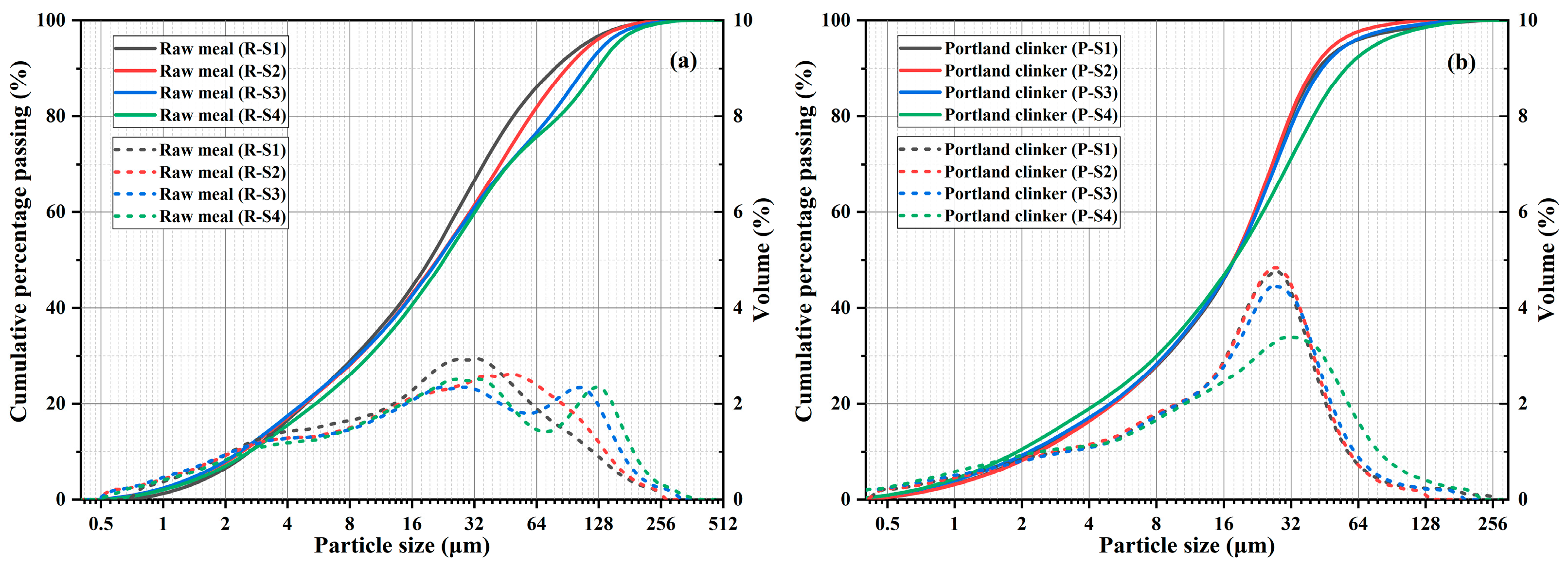
Figure 3.
Particle size distributions of raw meals (a) and Portland clinkers (b).
Figure 3.
Particle size distributions of raw meals (a) and Portland clinkers (b).
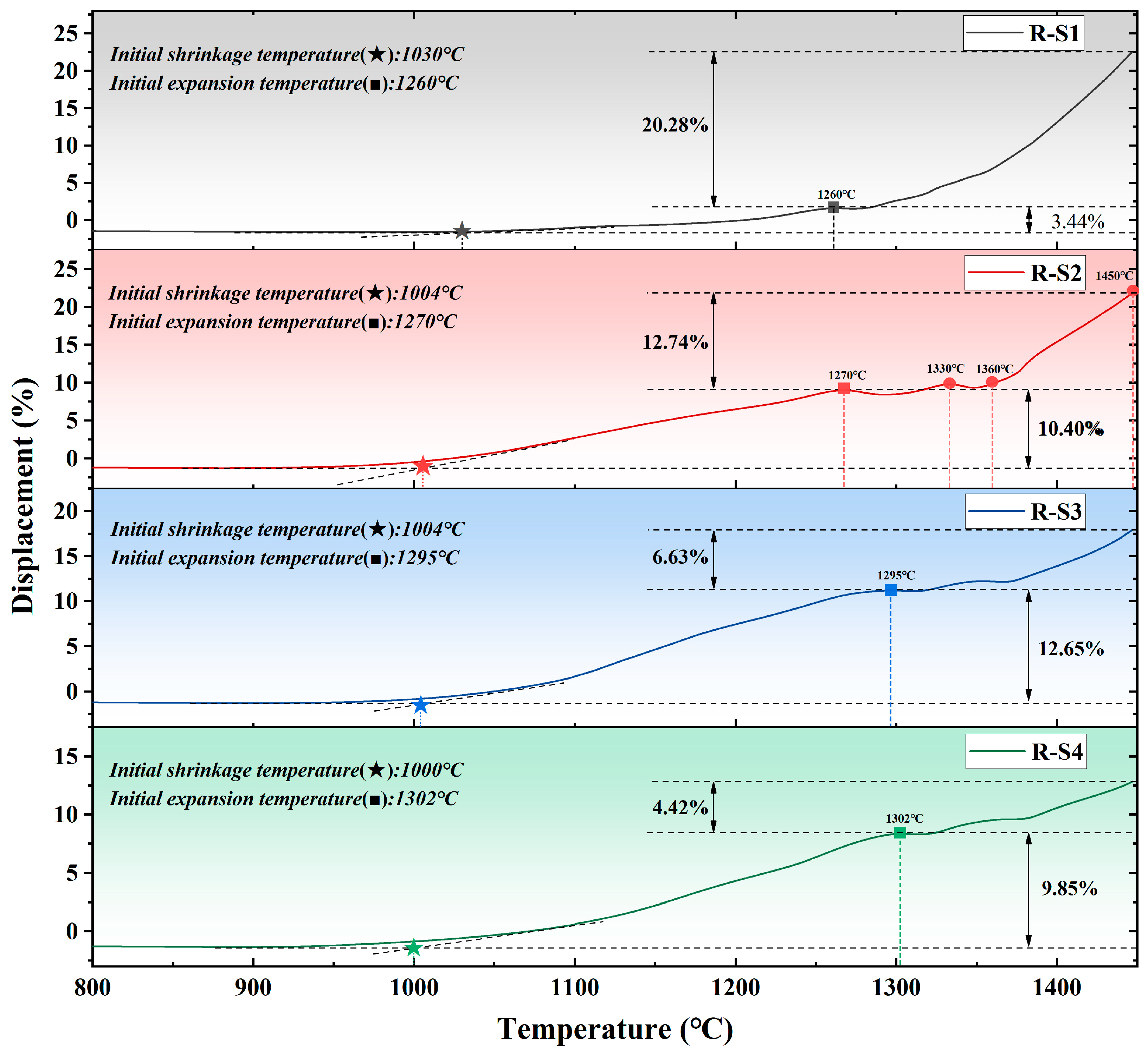
Figure 4.
Thermomechanical curve of raw meals.
Figure 4.
Thermomechanical curve of raw meals.
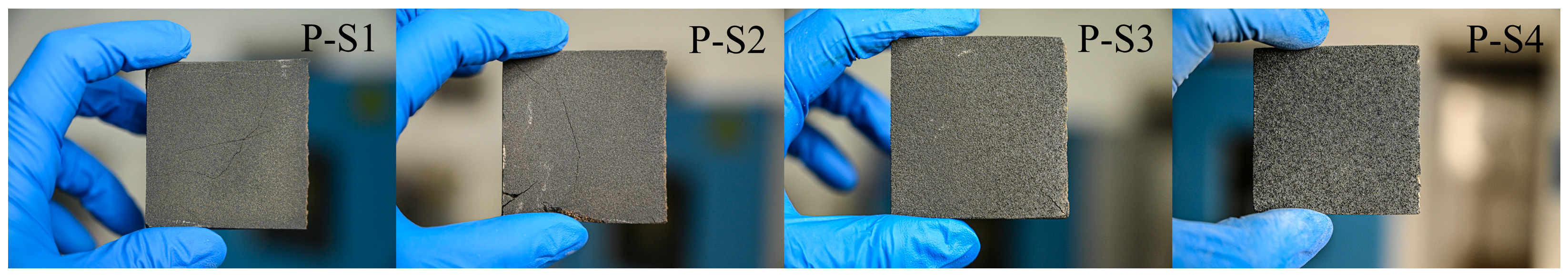
Figure 5.
Diagram of clinker morphology.
Figure 5.
Diagram of clinker morphology.
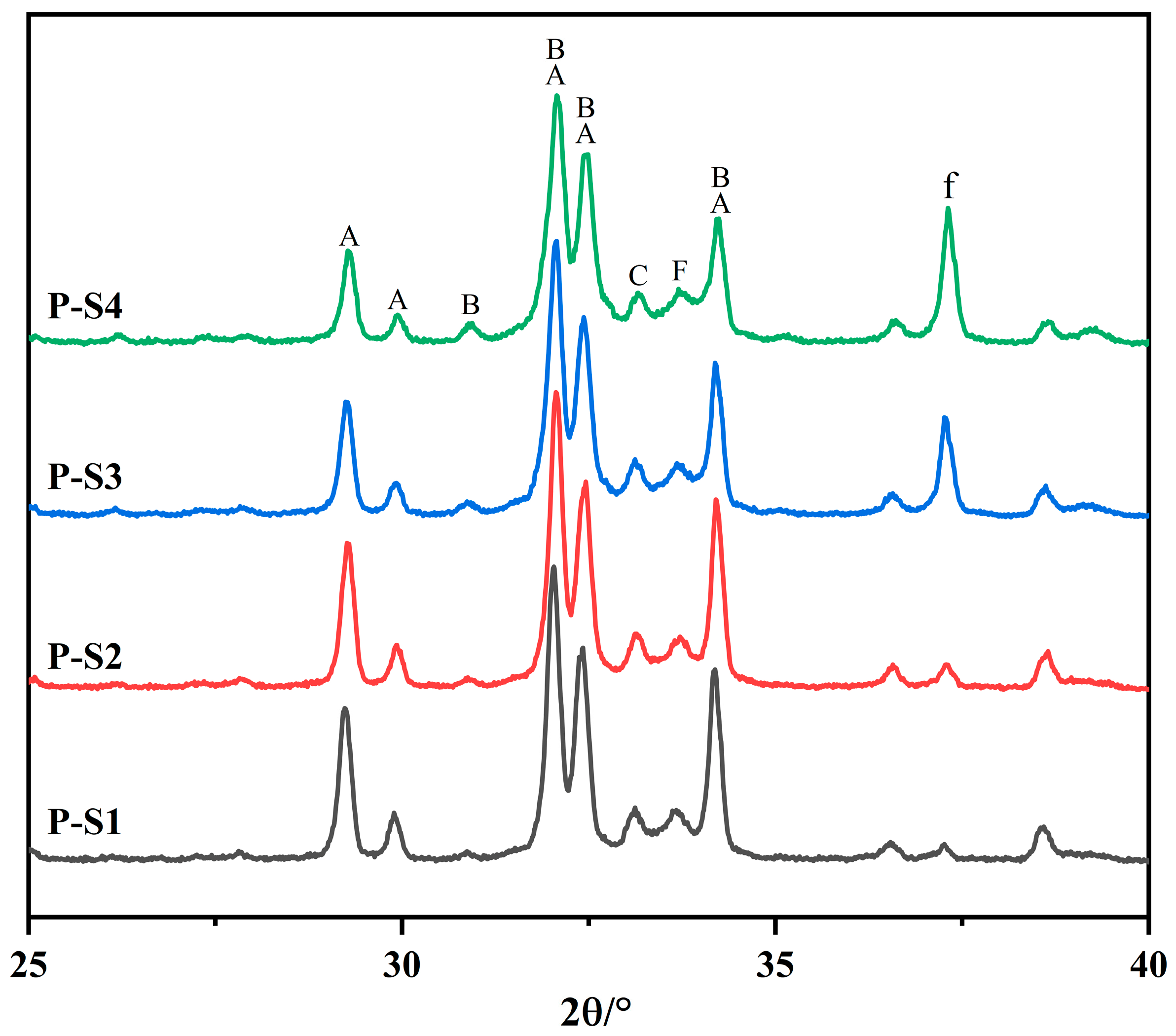
Figure 6.
XRD patterns of Portland clinkers (A: C3S, B: C2S, C: C3A, F: C4AF, and f: f-CaO).
Figure 6.
XRD patterns of Portland clinkers (A: C3S, B: C2S, C: C3A, F: C4AF, and f: f-CaO).
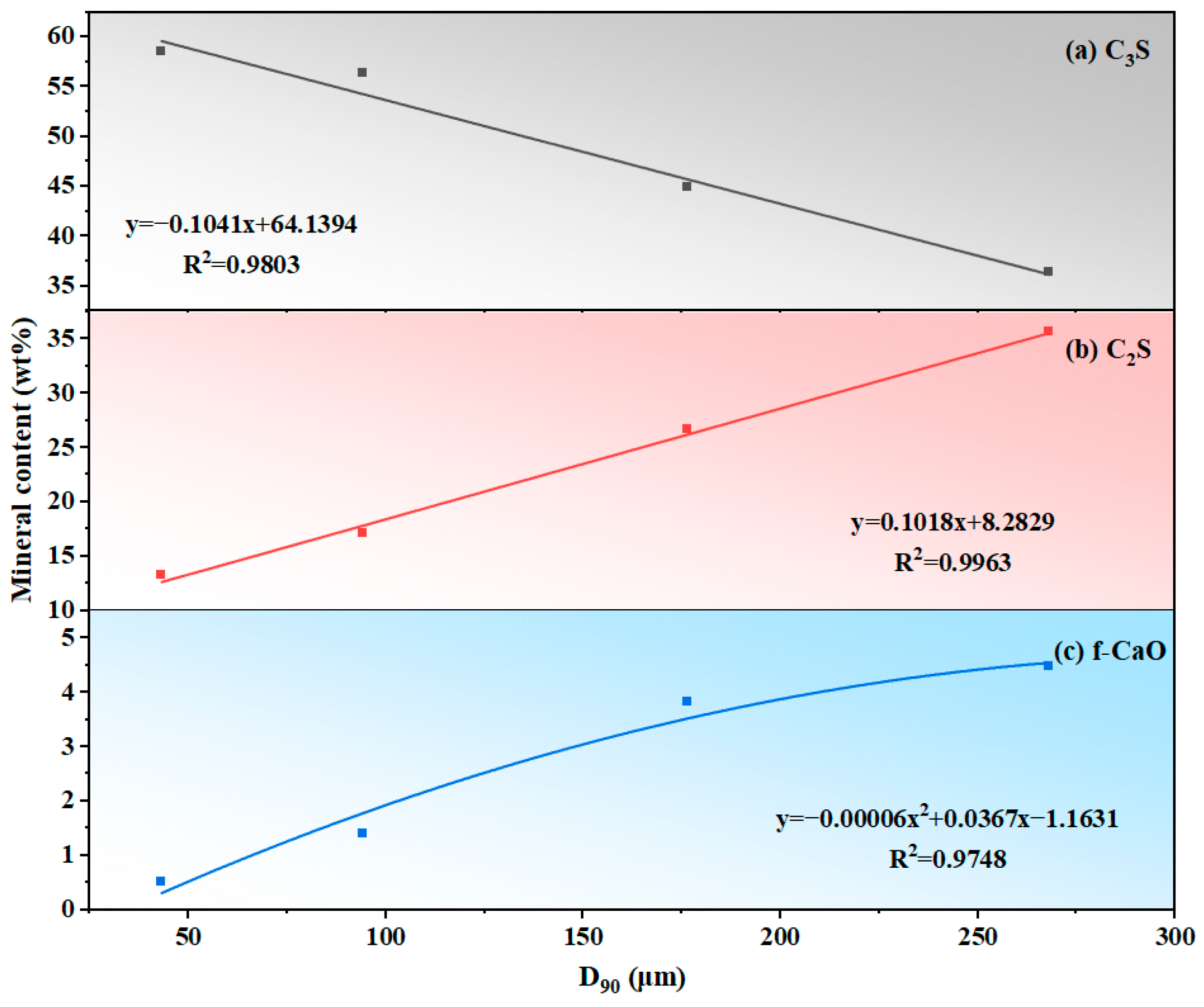
Figure 7.
Quantitative relationship between sandstone fineness (D90) and clinker mineral content: (a) C3S, (b) C2S, and (c) f-CaO.
Figure 7.
Quantitative relationship between sandstone fineness (D90) and clinker mineral content: (a) C3S, (b) C2S, and (c) f-CaO.
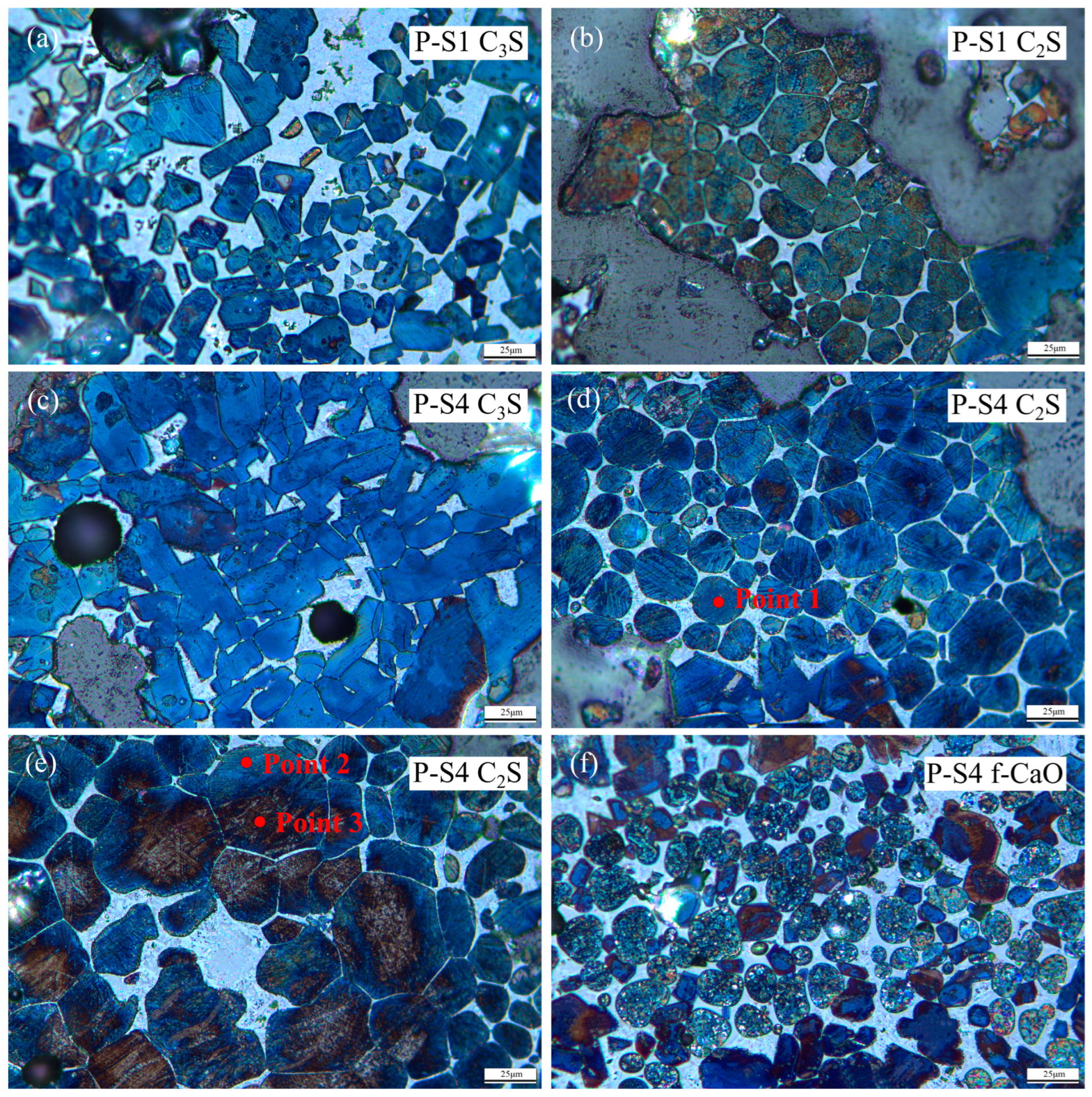
Figure 8.
Microstructure of clinker minerals ((a): C3S of P-S1, (b): C2S of P-S1, (c): C3S of P-S4, (d,e): C2S of P-S4, and (f): f-CaO of P-S4).
Figure 8.
Microstructure of clinker minerals ((a): C3S of P-S1, (b): C2S of P-S1, (c): C3S of P-S4, (d,e): C2S of P-S4, and (f): f-CaO of P-S4).
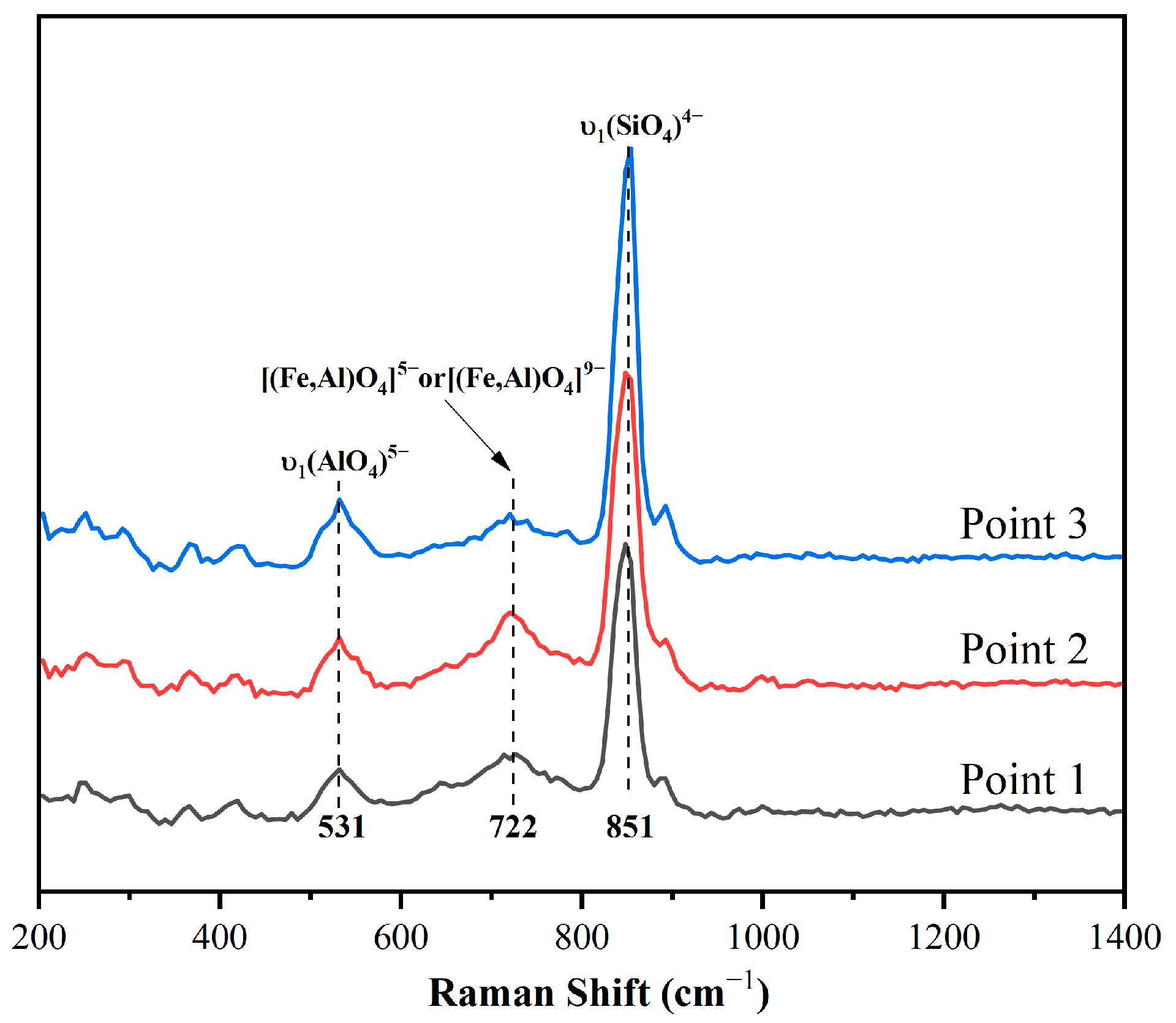
Figure 9.
Raman spectra of clinker minerals.
Figure 9.
Raman spectra of clinker minerals.
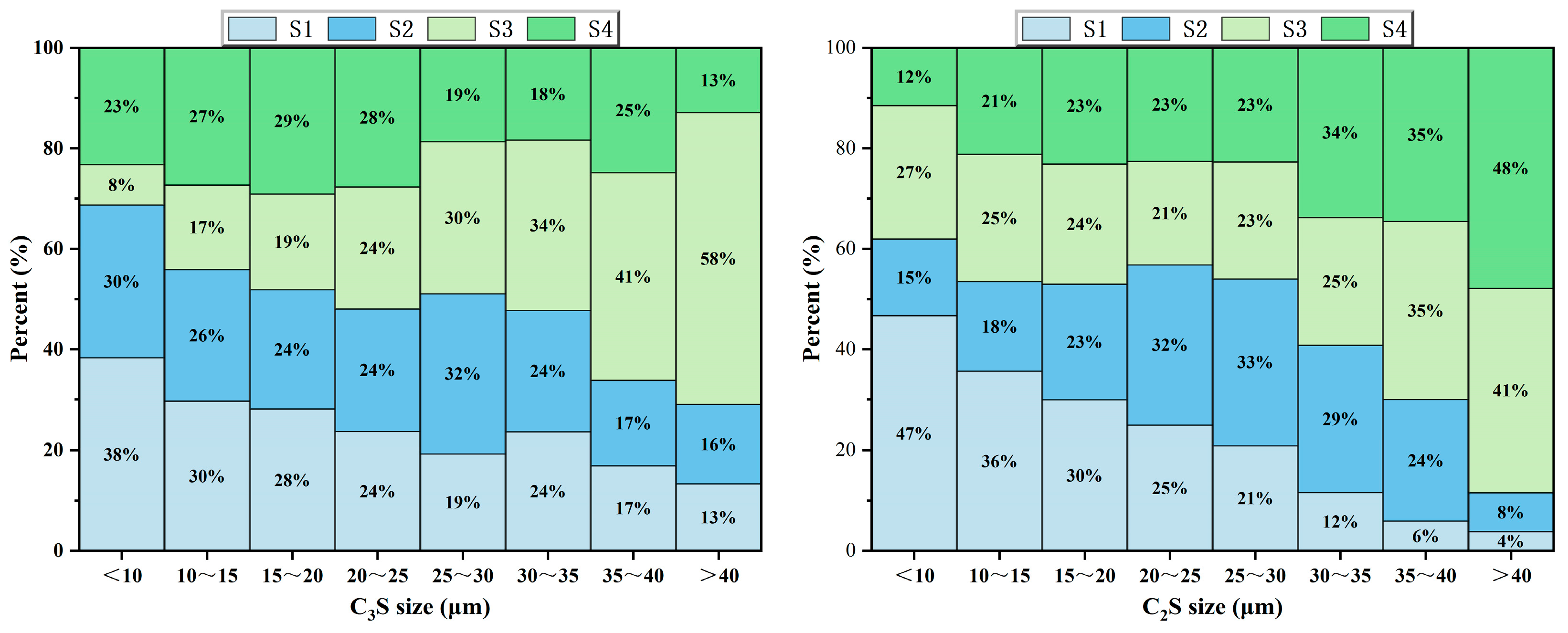
Figure 10.
Proportion of crystal content in the same particle size range of C3S and C2S in all clinkers.
Figure 10.
Proportion of crystal content in the same particle size range of C3S and C2S in all clinkers.
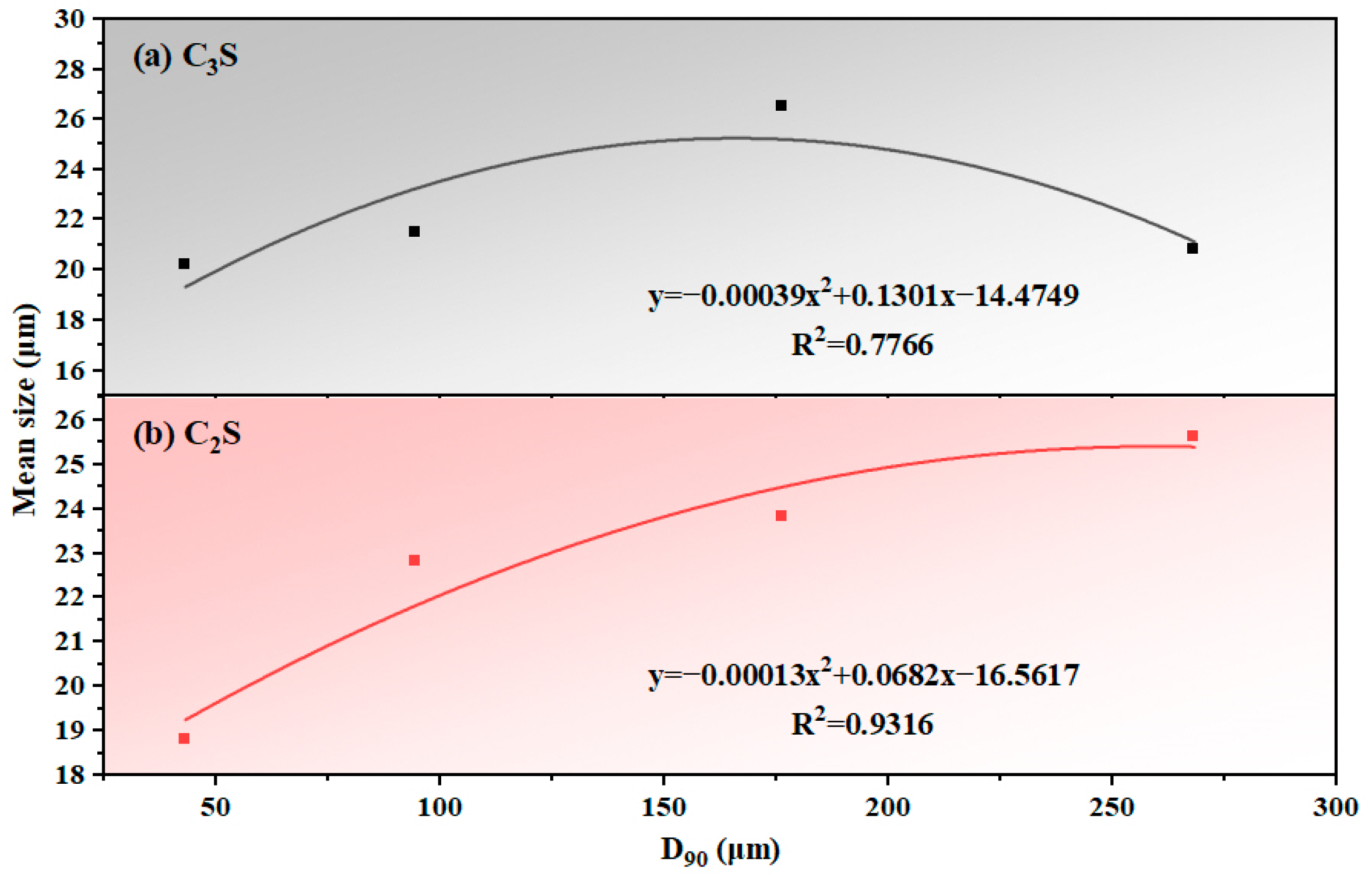
Figure 11.
Quantitative relationship between sandstone fineness (D90) and mean grain size: (a) C3S and (b) C2S.
Figure 11.
Quantitative relationship between sandstone fineness (D90) and mean grain size: (a) C3S and (b) C2S.
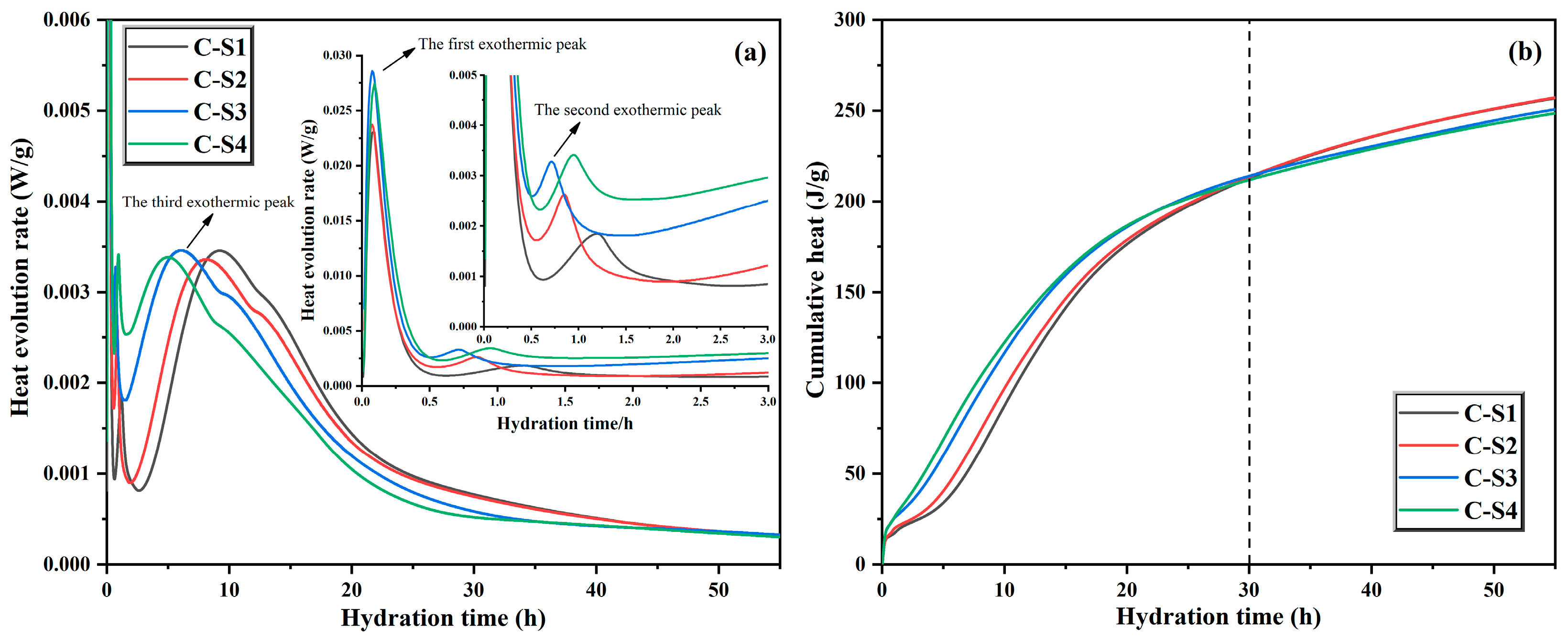
Figure 12.
Hydration heat curve (a) and cumulative heat curve (b) of cement.
Figure 12.
Hydration heat curve (a) and cumulative heat curve (b) of cement.
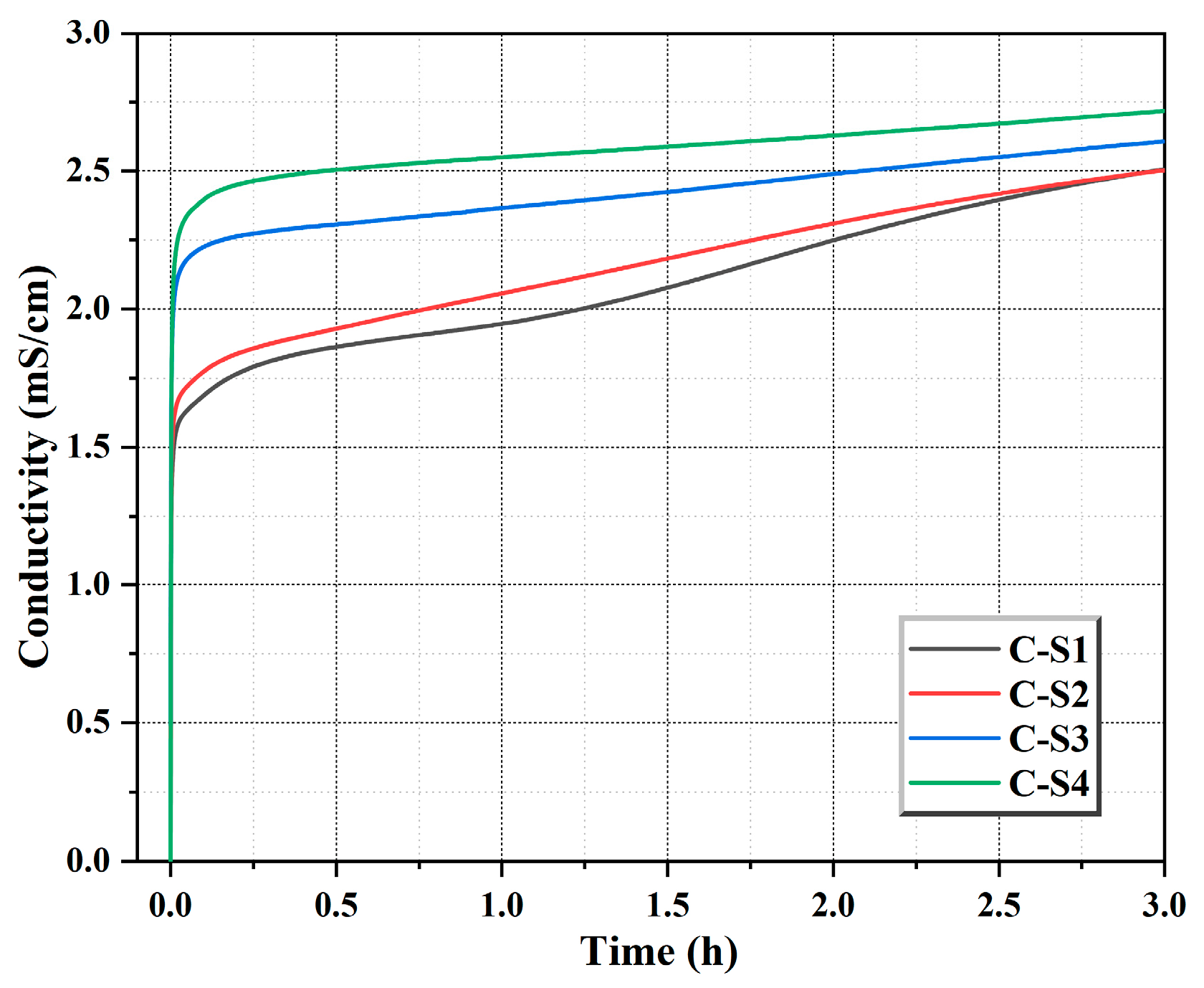
Figure 13.
Conductivity measurements of cement during the induction period.
Figure 13.
Conductivity measurements of cement during the induction period.
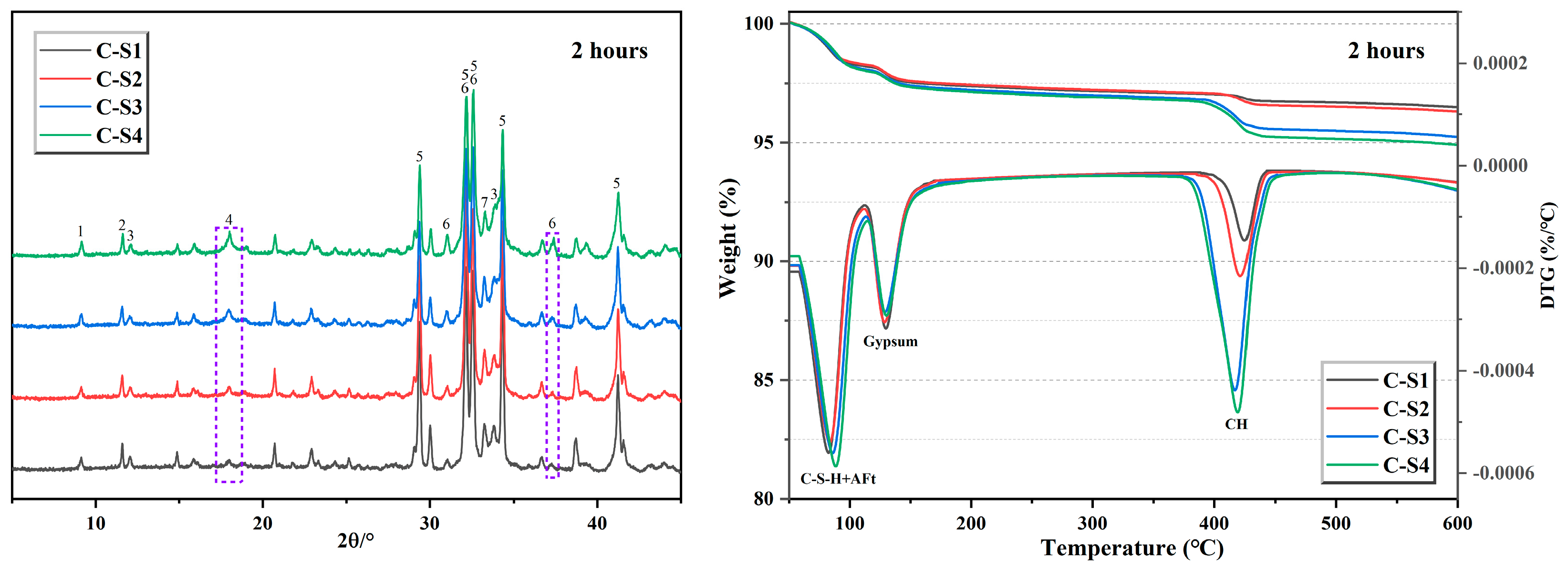
Figure 14.
XRD patterns and TG-DTG curves of cement hydration for 2 h (1-AFt, 2-CaSO4·2H2O, 3-C4AF, 4-Ca(OH)2, 5-C3S, 6-C2S, and 7-C3A).
Figure 14.
XRD patterns and TG-DTG curves of cement hydration for 2 h (1-AFt, 2-CaSO4·2H2O, 3-C4AF, 4-Ca(OH)2, 5-C3S, 6-C2S, and 7-C3A).
Figure 15.
XRD patterns and TG-DTG curves of cement hydration for 8 h (1-AFt, 2-CaSO4·2H2O, 3-C4AF, 4-Ca(OH)2, 5-C3S, 6-C2S, and 7-C3A).
Figure 15.
XRD patterns and TG-DTG curves of cement hydration for 8 h (1-AFt, 2-CaSO4·2H2O, 3-C4AF, 4-Ca(OH)2, 5-C3S, 6-C2S, and 7-C3A).
Figure 16.
XRD patterns and TG-DTG curves of cement hydration for 12 h (1-AFt, 2-C4AF, 3-Ca(OH)2, 4-C3S, 5-C2S, and 6-C3A).
Figure 16.
XRD patterns and TG-DTG curves of cement hydration for 12 h (1-AFt, 2-C4AF, 3-Ca(OH)2, 4-C3S, 5-C2S, and 6-C3A).
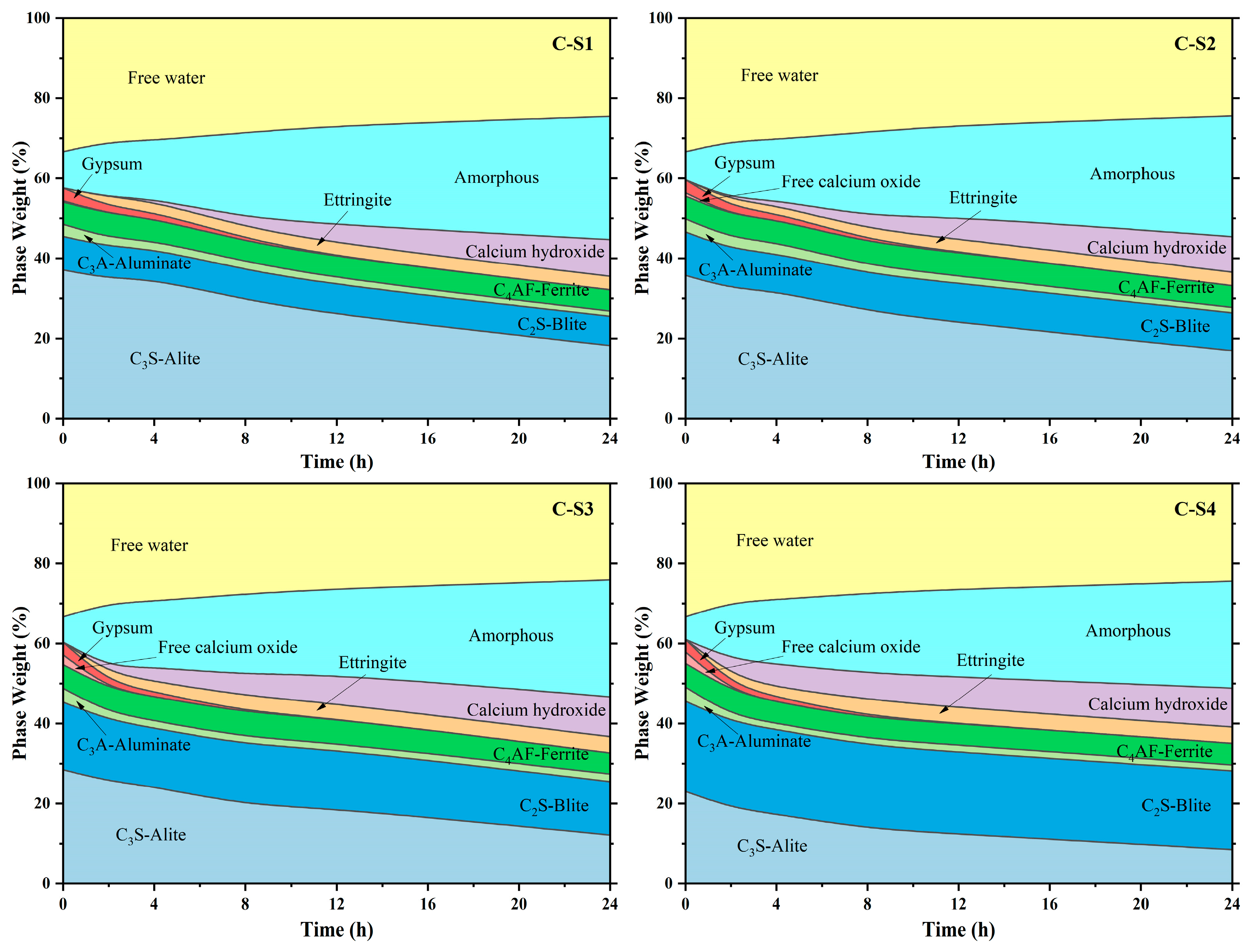
Figure 17.
Quantitative analysis of minerals of cement pastes at different ages.
Figure 17.
Quantitative analysis of minerals of cement pastes at different ages.
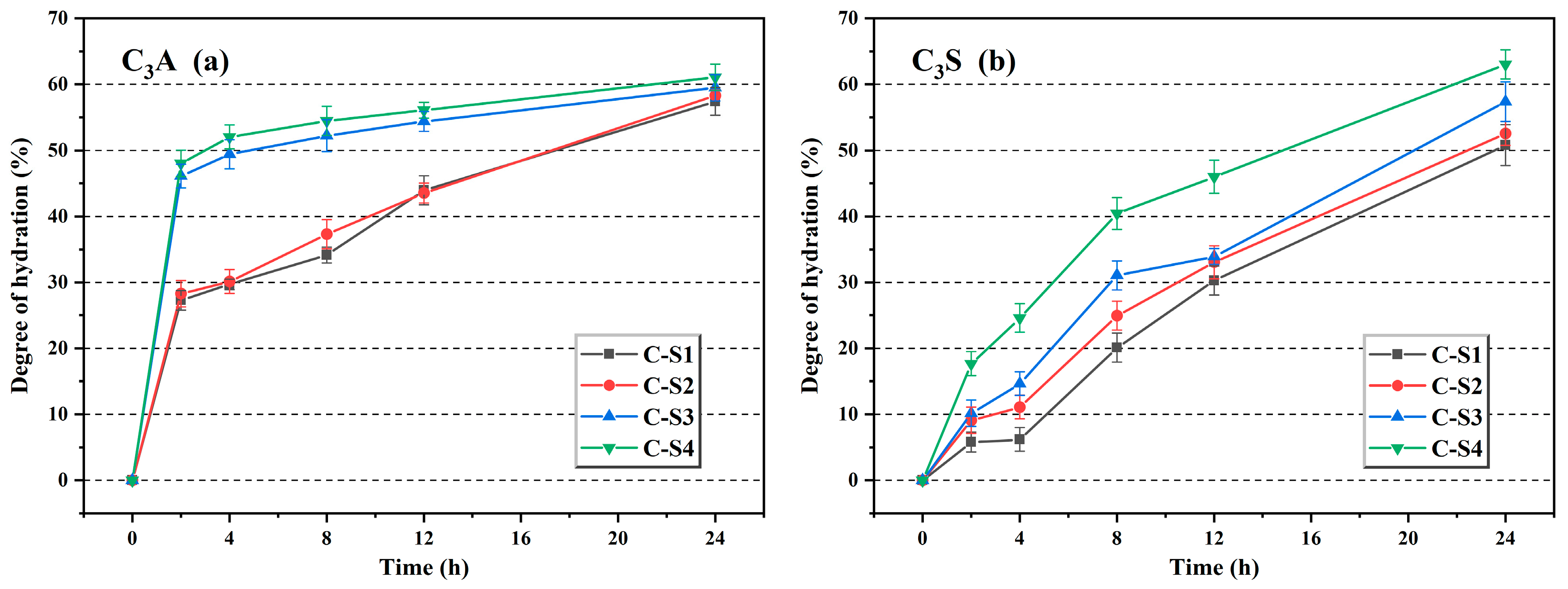
Figure 18.
The hydration degree of C3A (a) and C3S (b).
Figure 18.
The hydration degree of C3A (a) and C3S (b).
Figure 19.
Compressive strength of Portland cement at different ages.
Figure 19.
Compressive strength of Portland cement at different ages.
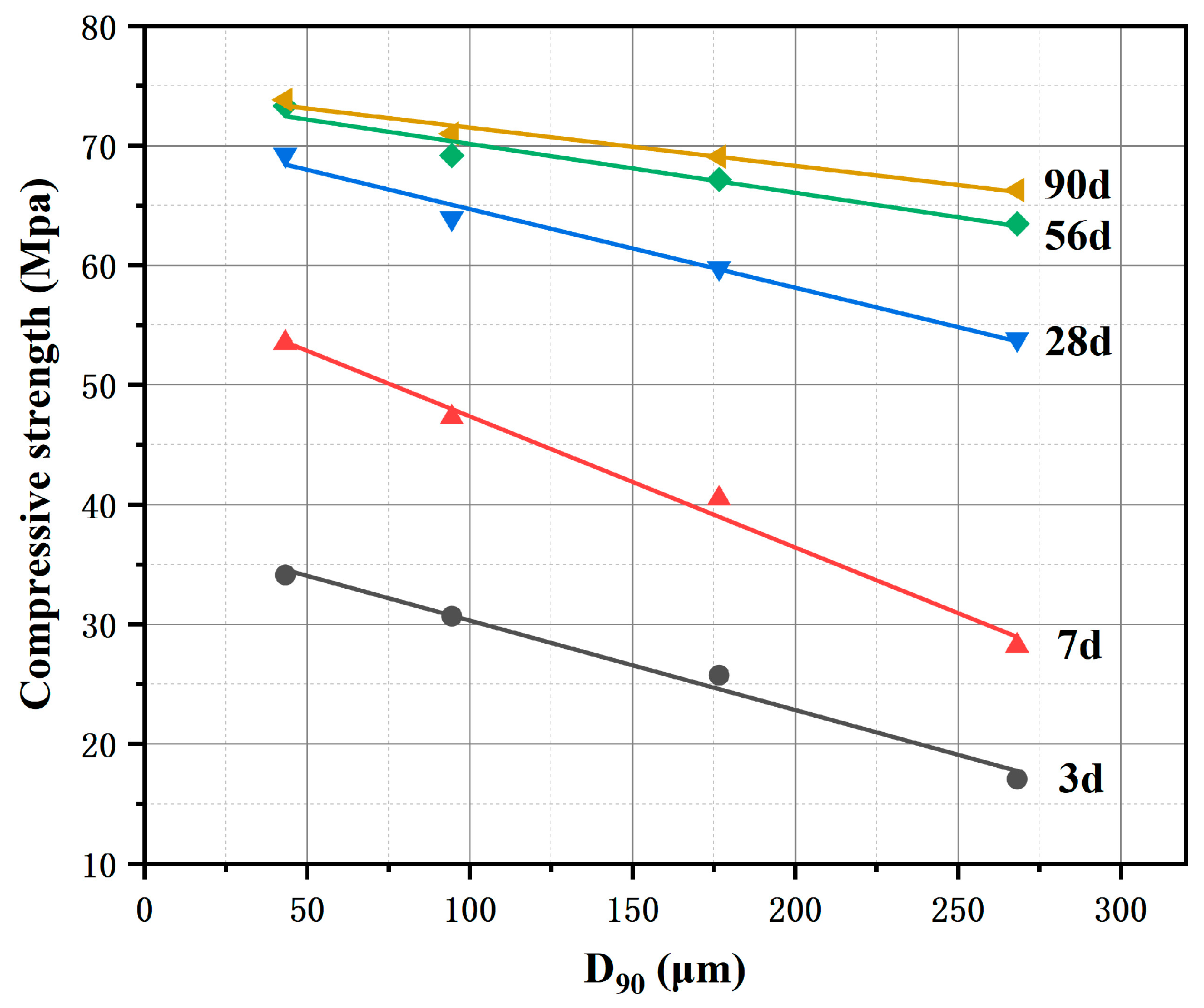
Figure 20.
Quantitative relationship between sandstone fineness (D90) and cement compressive strength.
Figure 20.
Quantitative relationship between sandstone fineness (D90) and cement compressive strength.
Table 1.
The chemical compositions of raw materials and Portland clinker (wt.%).
Table 1.
The chemical compositions of raw materials and Portland clinker (wt.%).
| | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | SO3 | LOI 1 |
|---|
| Low-grade limestone | 7.63 | 1.87 | 0.98 | 49.02 | 1.22 | 0.26 | 0.03 | 0.22 | 38.90 |
| Sandstone | 62.35 | 6.56 | 2.04 | 14.23 | 0.47 | 1.72 | 0.13 | - | 11.24 |
| Bauxite | 31.45 | 56.57 | 5.52 | 1.37 | 0.20 | 0.40 | 0.06 | - | 1.31 |
| Iron slag | 28.81 | 4.19 | 59.1 | 2.27 | 1.20 | 1.15 | 0.43 | 0.51 | 2.40 |
| Desulfurized gypsum | 2.05 | 0.70 | 0.27 | 36.21 | 0.41 | 0.09 | 0.10 | 44.99 | 13.87 |
| Portland clinker (P-S1) | 21.71 | 5.27 | 3.42 | 66.37 | 1.35 | 0.14 | 0.19 | 0.41 | 0.28 |
Table 2.
Particle size distributions of sandstones with varying fineness (μm).
Table 2.
Particle size distributions of sandstones with varying fineness (μm).
| | D10 1 | D20 | D30 | D40 | D50 | D60 | D70 | D80 | D90 |
|---|
| S1 | 1.7 | 3.3 | 5.8 | 9.5 | 14.8 | 20.6 | 26.2 | 32.9 | 43.2 |
| S2 | 10.4 | 30. | 41.2 | 48.4 | 54.7 | 61.2 | 68.6 | 78.3 | 94.4 |
| S3 | 34.4 | 83.2 | 99.7 | 111.3 | 121.7 | 131.9 | 143.0 | 156. | 176.6 |
| S4 | 41.7 | 136.0 | 157.9 | 174.0 | 188.4 | 202. | 218.9 | 238.3 | 268.2 |
Table 3.
The proportion of raw meals (wt%).
Table 3.
The proportion of raw meals (wt%).
| Raw Material | Low-Grade Limestone | Sandstone | Bauxite | Iron Slag |
|---|
| R-S1 to R-S4 | 86.1 | 10.1 | 2.0 | 1.8 |
Table 4.
The calculated parameters of the clinker’s specific surface area.
Table 4.
The calculated parameters of the clinker’s specific surface area.
| | Standard Sample 1 | P-S1 | P-S2 | P-S3 | P-S4 |
|---|
| Density (g/cm3) | 3.03 | 3.20 | 3.20 | 3.20 | 3.20 |
| Permeation time (s) | 76.8 | 80.0 | 81.7 | 82.3 | 79.0 |
| Specific surface area (m2/kg) | 358.2 | 346.2 | 349.8 | 351.1 | 343.9 |
Table 5.
Crystallographic reference identifiers (ICSD) for Rietveld refinement components.
Table 5.
Crystallographic reference identifiers (ICSD) for Rietveld refinement components.
| Phase | ICSD No. | Ref. | Phase | ICSD No. | Ref. |
|---|
| C3S-M3 | 94742 | [15] | CaO | 28905 | [16] |
| C2S-β | 81096 | [17] | AFt | 155395 | [18] |
| C4AF | 9197 | [19] | CaSO4·2H2O | 27876 | [20] |
| C3A | 1841 | [21] | Ca(OH)2 | 15471 | [22] |
Table 6.
The volume change of reactions during clinker calcination (cm3).
Table 6.
The volume change of reactions during clinker calcination (cm3).
| Number | Reaction Formula | Volume | Volume Change |
|---|
| Before | After |
|---|
| 1 | | 60.70 | 52.12 | −8.58 |
| 2 | | 75.65 | 64.14 | −11.51 |
| 3 | | 122.84 | 128.91 | 6.07 |
| 4 | | 68.84 | 71.92 | 3.08 |
Table 7.
Quantitative results of clinkers by external standard method.
Table 7.
Quantitative results of clinkers by external standard method.
| | C3S | C2S | C3A | C4AF | CaO | ACn 1 |
|---|
| P-S1 | 58.45 | 13.19 | 4.77 | 8.72 | 0.50 | 14.38 |
| P-S2 | 56.33 | 17.04 | 5.20 | 8.87 | 1.40 | 11.16 |
| P-S3 | 44.84 | 26.62 | 5.41 | 9.39 | 3.81 | 9.92 |
| P-S4 | 36.29 | 35.56 | 5.48 | 9.34 | 4.47 | 8.87 |
Table 8.
C3S and C2S size statistics.
Table 8.
C3S and C2S size statistics.
| | C3S Size (μm) | | | C2S Size (μm) | | |
|---|
| | S1 | S2 | S3 | S4 | S1 | S2 | S3 | S4 |
|---|
| N total | 365 | 432 | 326 | 339 | 355 | 348 | 400 | 395 |
| Minimum | 4.1 | 6.3 | 7.0 | 7.5 | 7.0 | 6.8 | 7.4 | 8.1 |
| Media | 18.3 | 20.1 | 23.6 | 19.0 | 18.0 | 22.4 | 21.5 | 22.7 |
| Maximum | 52.2 | 63.3 | 89.3 | 61.1 | 41.9 | 45.6 | 62.3 | 79.0 |
| Mean | 20.2 | 21.5 | 26.5 | 20.8 | 18.8 | 22.8 | 23.8 | 25.6 |
Table 9.
Regression equations and coefficients of determination between sandstone fineness (D90) and cement strength at different curing ages.
Table 9.
Regression equations and coefficients of determination between sandstone fineness (D90) and cement strength at different curing ages.
| Ages | Regression Equation | Coefficient of Determination (R2) |
|---|
| 3 days | | 0.9878 |
| 7 days | | 0.9904 |
| 28 days | | 0.9842 |
| 56 days | | 0.9564 |
| 90 days | | 0.9751 |