Impact of Electronic Waste Glass on the Properties of Cementitious Materials
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
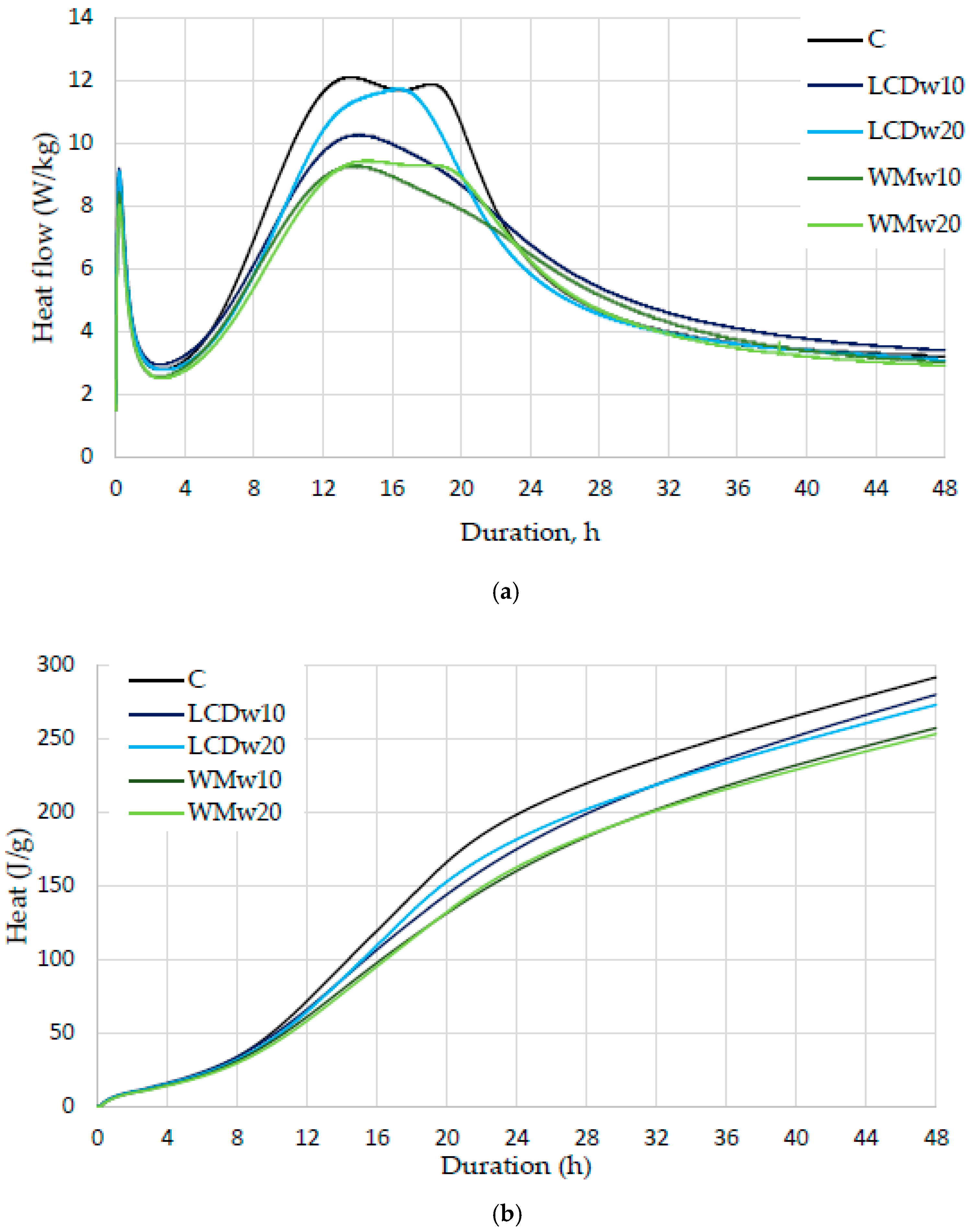
- Both types of waste glass from various displays and washing machines retard cement hydration (from 13.1 h till 15.9 h) and reduce the amount of heat released (from 291.8 J/g to 273.1 J/g with 20% LCDw and 253.3 J/g with 20% WMw). However, different effects were observed when cement was replaced at proportions of 10% and 20%: the degree of hydration decreased by only 4% and 6% in LCDw samples, while in WMw samples it decreased by 12% and 13%, respectively. This indicates that the Na2O present in WMw glass is insufficient to accelerate cement hydration. The time to the maximum heat flow was longer in LCDw samples because more aluminate phases are formed when 20% of LCDw glass is added to the mix, and thus, more heat is released at a later time due to aluminate reactions.
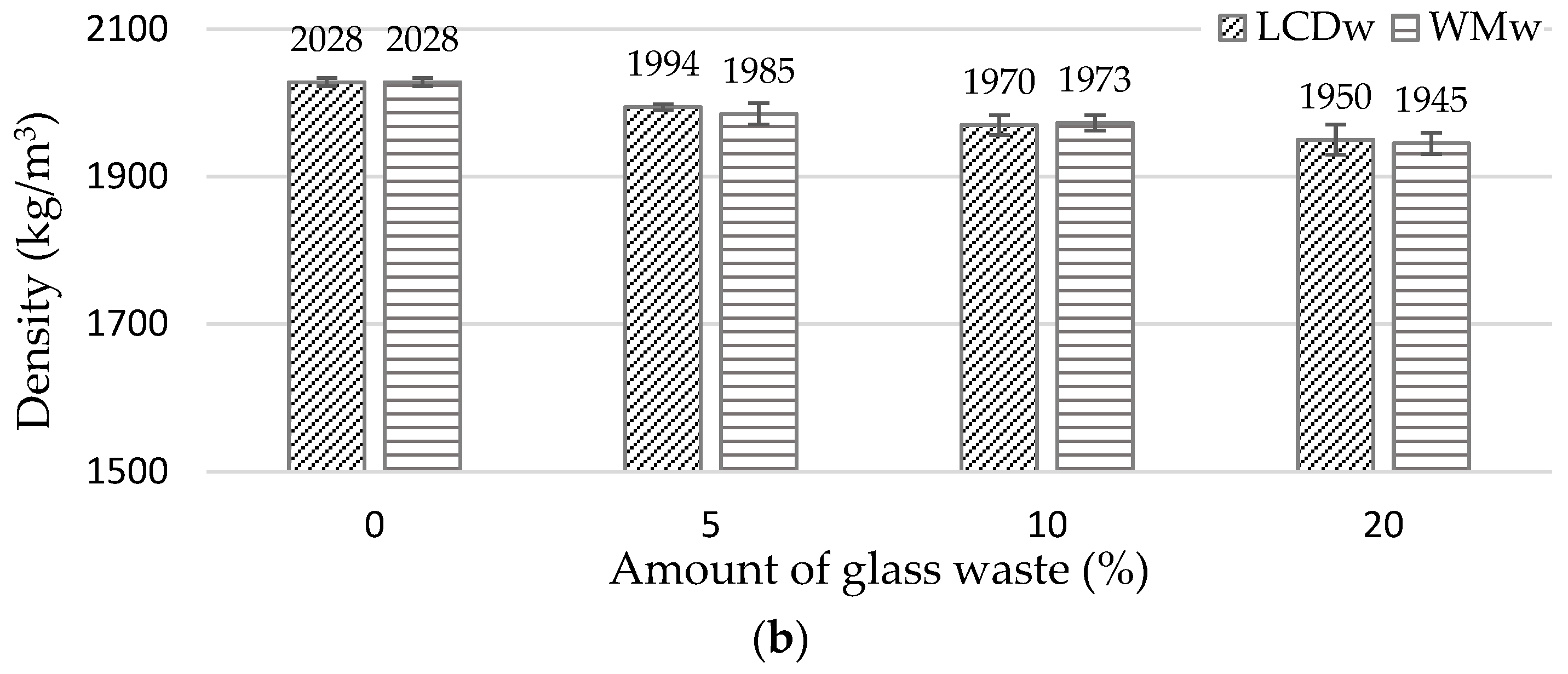
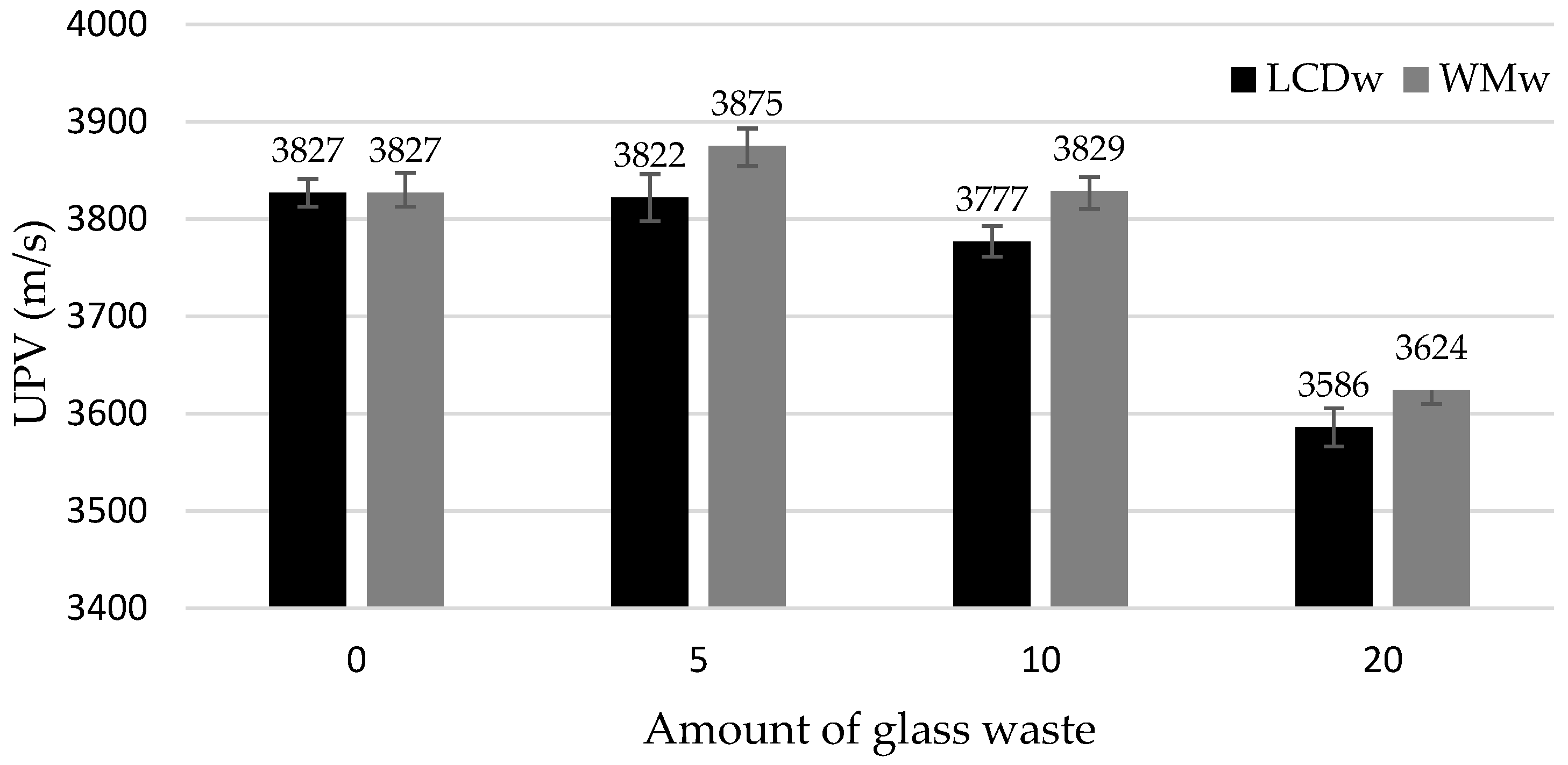
- At 7 and 28 days, the density of the binder was reduced by approx. 2% to 6% in the samples modified with LCDw and WMw. A more uniform structure of the samples modified with up to 10% of waste glass, especially WMw, produced UPV values similar to or slightly higher than the UPV of reference samples (for example, 3827 m/s of a reference sample and 3829 m/s of WMw10). With the increase in glass content of up to 20%, UPV dropped by about 6%.
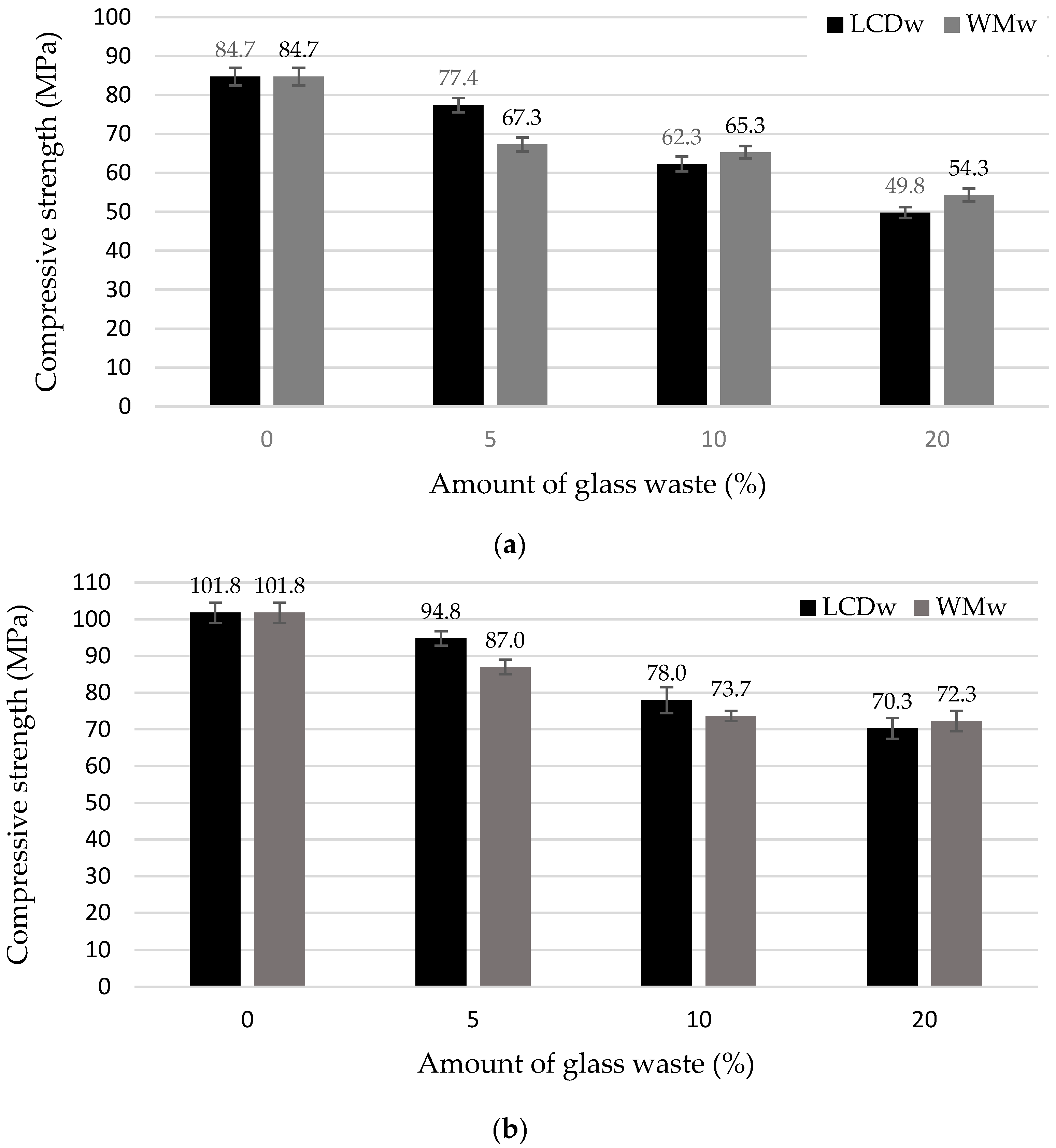
- At 7 days, the compressive strength of all samples modified with waste glass was lower than the strength of the reference samples. The strength in LCDw samples was reduced by 9.4%, 36.0%, and 70.0%, whereas in WMw samples it fell by 25.9%, 29.7%, and 56.0%. At 28 days, the difference in strength in comparison with the reference sample fell, but the decrease in LCDw sample strength of (7.4%, 30.5%, 44.8%) was still lower than the decrease in WMw samples (17.0%, 38.1%, 40.8%). The PAI results show that the recalculated coefficient to the similar amount of cement is from 73.5–98.0%. The strength decrease is caused not only by the reduced cement content but also by retardation of cement hydration, and according to SEM results, it is also due to the agglomeration of ultrafine glass particles, which led to the formation of distinct porous zones throughout the sample, particularly in the samples modified with 20% waste glass.
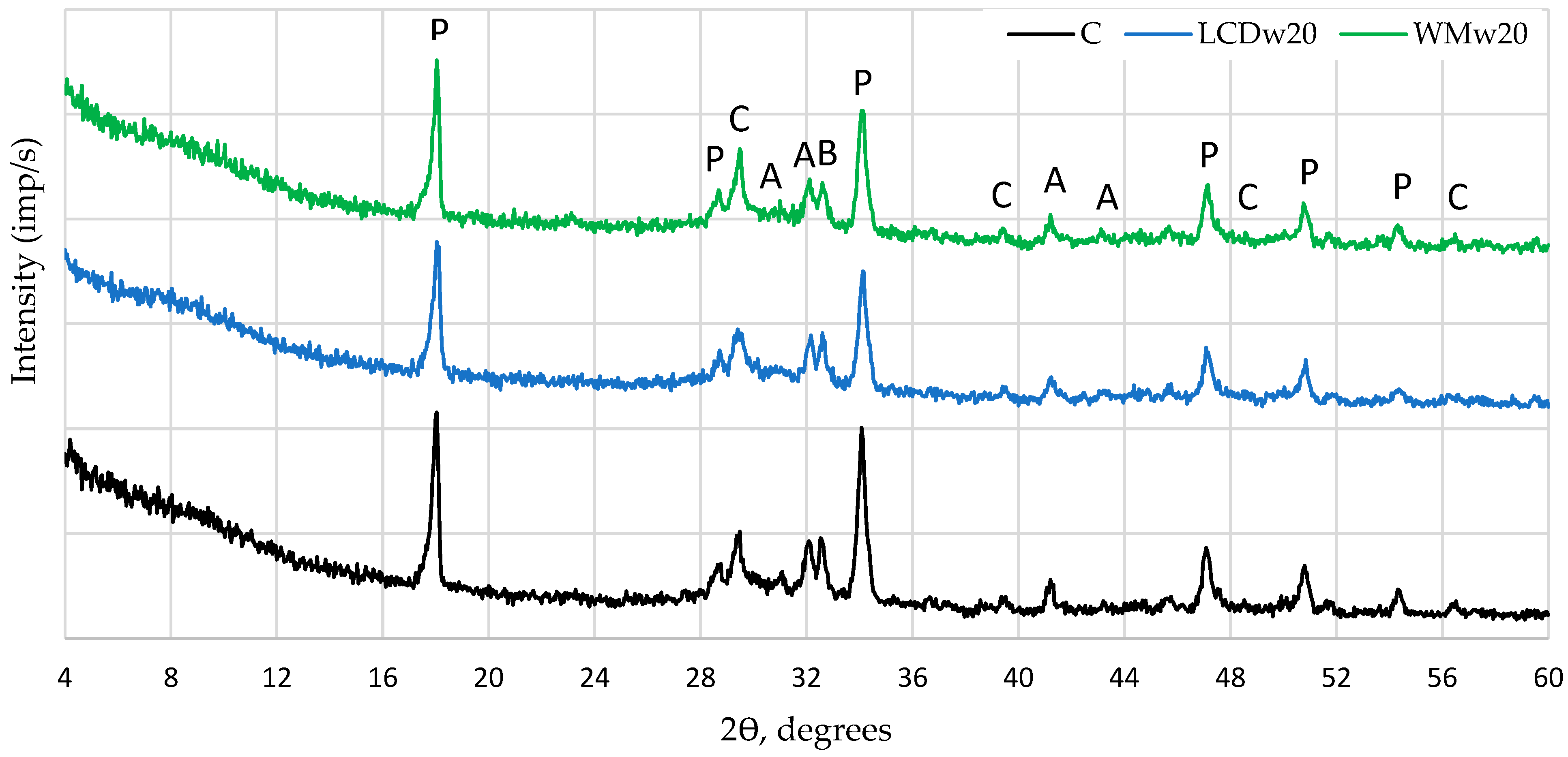
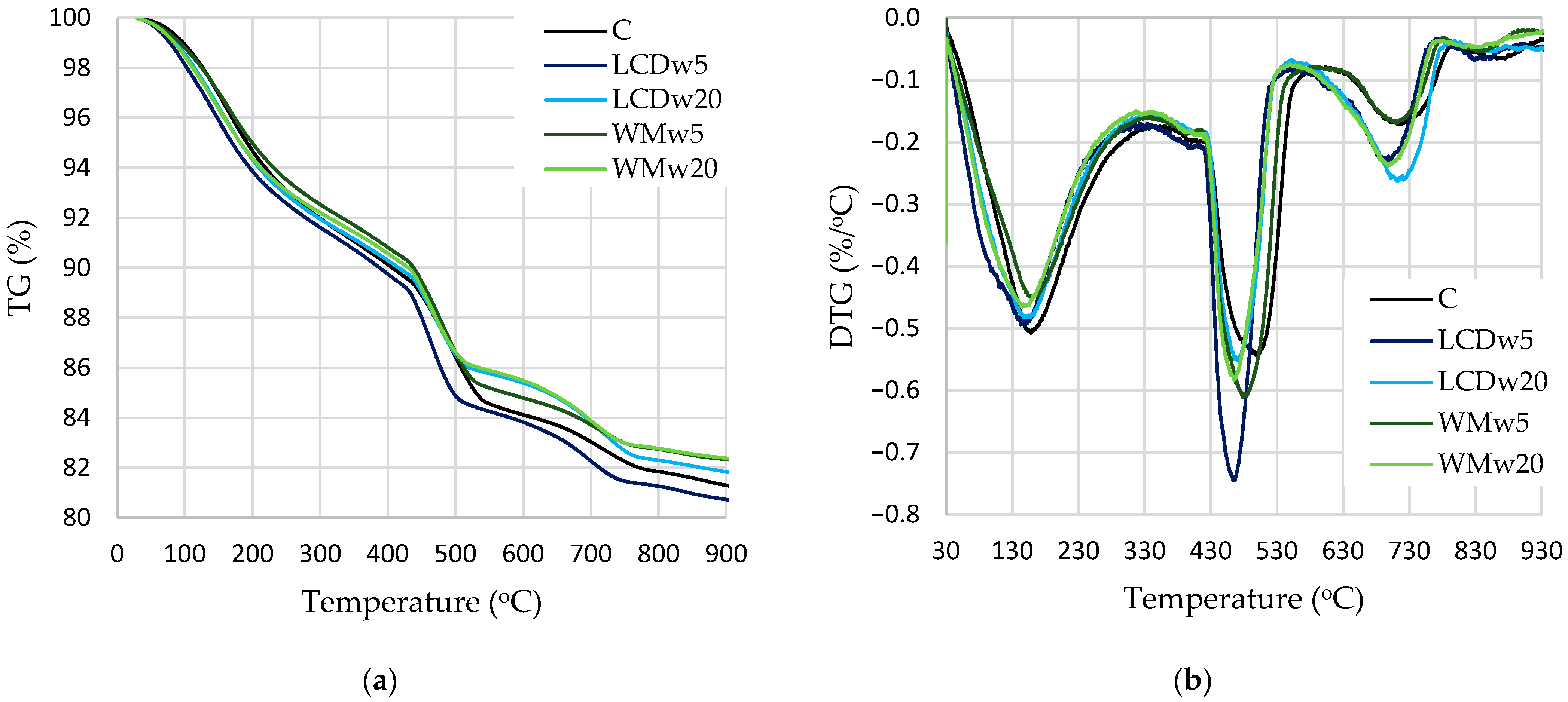
- XRD and TG analyses showed that reference samples contained the highest amounts of portlandite and alite, whereas the modified samples had a higher content of poorly crystallized calcium carbonate. Conversion into equal cement content showed that, at 7 days, control samples had the highest portlandite content (20.6%, and with glass waste, from 18.0–19.9%), but at 28 days, modified samples with waste had the highest amount of portlandite at 20.5–21.6%, compared to the 19.3% of the reference sample. The same was observed with the change in calcium hydrosilicate content according to mass loss in the 110–350 °C temperature range: at 7 days, for the reference sample 6.1%, and for samples with glass waste, 5.6–5.9%; at 28 days, 7.1 and 6.3–8.4, respectively.
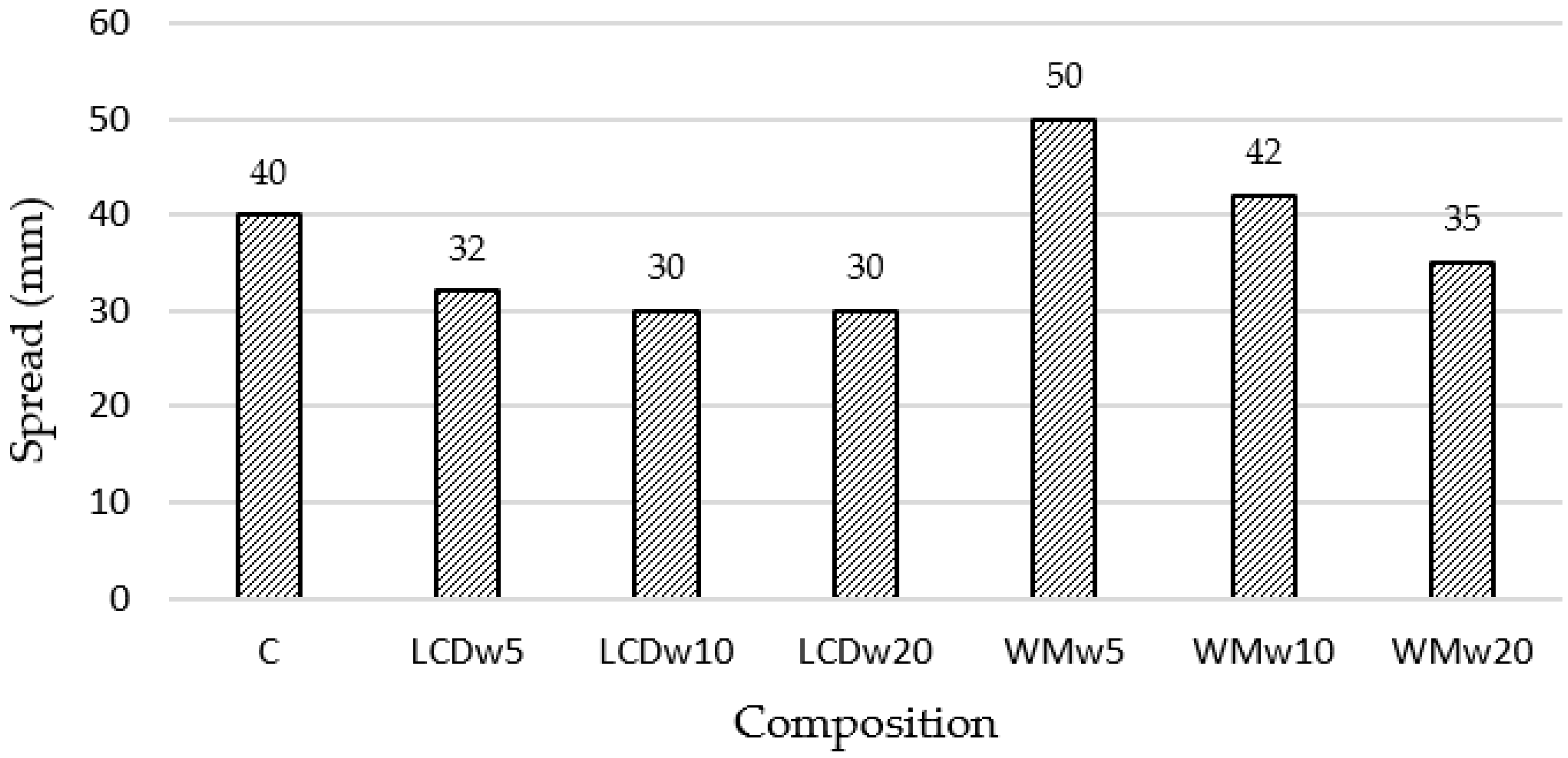
- Waste glass of different chemical compositions had a similar effect on the physical–mechanical properties and mineral compositions of the modified samples. More significant differences were observed in the workability of the modified mixture, where the WMw glass increased the spread by up to 25%, while the LCDw reduced it by approximately 20% compared to the reference sample. Different types of waste glass also behaved differently in terms of early hydration, where a higher degree of hydration (96.0%) was observed in LCDw samples due to higher water absorption.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Badarloo, B.; Lehner, P.; Koubov, L.; Pirizadeh, M. Correlation study of physical and mechanical properties of concretes with crushed LCD glass. J. Clean. Prod. 2023, 385, 135756. [Google Scholar] [CrossRef]
- Amato, A.; Rocchetti, L.; Beolchini, F. Environmental impact assessment of different end-of-life LCD management strategies. Waste Manag. 2017, 59, 432–441. [Google Scholar] [CrossRef]
- Bereketli, I.; Erol Genevois, M.; Esra Albayrak, Y.; Ozyol, M. WEEE treatment strategies’ evaluation using fuzzy LINMAP method. Expert Syst. Appl. 2011, 38, 71–79. [Google Scholar] [CrossRef]
- Tsydenova, O.; Bengtsson, M. Chemical hazards associated with treatment of waste electrical and electronic equipment. Waste Manag. 2011, 31, 45–58. [Google Scholar] [CrossRef]
- Meyer, C. The greening of the concrete industry. Cem. Concr. Compos. 2009, 31, 601–605. [Google Scholar] [CrossRef]
- Hanein, T.; Galvez-Martos, J.L.; Bannerman, M.N. Carbon footprint of calcium sulfoaluminate clinker production. J. Clean. Prod. 2018, 172, 2278–2287. [Google Scholar] [CrossRef]
- Assi, L.; Carter, K.; Deaver, E.; Anay, R.; Ziehl, P. Sustainable concrete: Building a greener future. J. Clean. Prod. 2018, 198, 1641–1651. [Google Scholar] [CrossRef]
- Harrison, E.; Berenjian, A.; Seifan, M. Recycling of waste glass as aggregate in cement-based materials. Environ. Sci. Ecotechnol. 2020, 4, 100064. [Google Scholar] [CrossRef]
- Jani, Y.; Hogland, W. Waste glass in the production of cement and concrete—A review. J. Environ. Chem. Eng. 2014, 2, 1767–1775. [Google Scholar] [CrossRef]
- Kaya, M. Current WEEE recycling solutions. In Waste Electrical and Electronic Equipment Recycling; Vegliò, F., Birloaga, I., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 33–93. [Google Scholar] [CrossRef]
- Siddique, R. Waste Glass. In Waste Materials and By-Products in Concrete; Springer: Berlin/Heidelberg, Germany, 2008; pp. 147–175. [Google Scholar] [CrossRef]
- Islam, G.M.S.; Rahman, M.H.; Kazi, N. Waste glass powder as partial replacement of cement for sustainable concrete practice. Int. J. Sustain. Built Environ. 2017, 6, 37–44. [Google Scholar] [CrossRef]
- Góra, J.; Franus, M.; Barnat-Hunek, D.; Franus, W. Utilization of Recycled Liquid Crystal Display (LCD) Panel Waste in Concrete. Materials 2019, 12, 2941. [Google Scholar] [CrossRef]
- Kim, S.K.; Yang, H.J. Utilization of liquid crystal display (LCD) waste glass powder as cementitious binder in mortar for enhancing neutron shielding performance. Constr. Build. Mater. 2021, 270, 121859. [Google Scholar] [CrossRef]
- ASTM C618-22; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM International: West Conshohocken, PA, USA, 2022.
- Jiang, X.; Xiao, R.; Bai, Y.; Huang, B.; Ma, Y. Influence of waste glass powder as a supplementary cementitious material (SCM) on physical and mechanical properties of cement paste under high temperatures. J. Clean. Prod. 2022, 340, 130778. [Google Scholar] [CrossRef]
- Nassar, R.U.D.; Soroushian, P. Strength and durability of recycled aggregate concrete containing milled glass as partial replacement for cement. Constr. Build. Mater. 2012, 29, 368–377. [Google Scholar] [CrossRef]
- Matos, A.M.; Sousa-Coutinho, J. Durability of mortar using waste glass powder as cement replacement. Constr. Build. Mater. 2012, 36, 205–215. [Google Scholar] [CrossRef]
- Yang, H.J.; Usman, M.; Hanif, A. Suitability of liquid crystal display (LCD) glass waste as supplementary cementing material (SCM): Assessment based on strength, porosity, and durability. J. Build. Eng. 2021, 42, 102793. [Google Scholar] [CrossRef]
- Wang, W.J.; Wang, C.D.; Lee, T.C.; Chang, C.C. Characterization of a mortar made with cement and sludge from the light-emitting diode manufacturing process. Constr. Build. Mater. 2014, 56, 106–112. [Google Scholar] [CrossRef]
- Kim, S.K.; Kang, S.T.; Kim, J.K.; Jang, I.Y. Effects of Particle Size and Cement Replacement of LCD Glass Powder in Concrete. Adv. Mater. Sci. Eng. 2017, 2017, 3928047. [Google Scholar] [CrossRef]
- Wang, H.Y. The effect of the proportion of thin film transistor–liquid crystal display (TFT–LCD) optical waste glass as a partial substitute for cement in cement mortar. Constr. Build. Mater. 2011, 25, 791–797. [Google Scholar] [CrossRef]
- Raju, A.S.; Anand, K.B.; Rakesh, P. Partial replacement of Ordinary Portland cement by LCD glass powder in concrete. Mater. Today Proc. 2020, 46, 5131–5137. [Google Scholar] [CrossRef]
- Wang, H.Y.; Huang, W.L. Durability of self-consolidating concrete using waste LCD glass. Constr. Build. Mater. 2010, 24, 1008–1013. [Google Scholar] [CrossRef]
- Wang, H.Y.; Zeng, H.; Wu, J.Y. A study on the macro and micro properties of concrete with LCD glass. Constr. Build. Mater. 2014, 50, 664–670. [Google Scholar] [CrossRef]
- Terro, M.J. Properties of concrete made with recycled crushed glass at elevated temperatures. Build. Environ. 2006, 41, 633–639. [Google Scholar] [CrossRef]
- Wang, H.Y. A study of the effects of LCD glass sand on the properties of concrete. Waste Manag. 2009, 29, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Her-Yung, W. A study of the engineering properties of waste LCD glass applied to controlled low strength materials concrete. Constr. Build. Mater. 2009, 23, 2127–2131. [Google Scholar] [CrossRef]
- Wang, C.C.; Wang, H.Y. Assessment of the compressive strength of recycled waste LCD glass concrete using the ultrasonic pulse velocity. Constr. Build. Mater. 2017, 137, 345–353. [Google Scholar] [CrossRef]
- Ling, T.C.; Poon, C.S. A comparative study on the feasible use of recycled beverage and CRT funnel glass as fine aggregate in cement mortar. J. Clean. Prod. 2012, 29–30, 46–52. [Google Scholar] [CrossRef]
- Ling, T.-C.; Poon, C.S.; Kou, S.C. Feasibility of using recycled glass in architectural cement mortars. Cem. Concr. Compos. 2011, 33, 848–854. [Google Scholar] [CrossRef]
- Zele, S.; Joshi, A.; Gogate, N.; Marathe, D.; Shitole, A. Experimental investigation on utilization of crushed solar panel waste as sand replacement in concrete. Solar Energy 2024, 269, 112338. [Google Scholar] [CrossRef]
- Al-Awabdeh, F.W.; Al-Kheetan, M.J.; Jweihan, Y.S.; Al-Hamaiedeh, H.; Ghaffar, S.H. Comprehensive investigation of recycled waste glass in concrete using silane treatment for performance improvement. Results Eng. 2022, 16, 100790. [Google Scholar] [CrossRef]
- LST EN 12706; Adhesives-Test Methods for Hydraulic Setting Floor Smoothing and/or Levelling Compounds—Determination of Flow Characteristics. Lietuvos Standartizacijos Departamentas: Vilnius, Lithuania, 2004.
- Malaiskiene, J.; Costa, C.; Baneviciene, V.; Antonovic, V.; Vaiciene, M. The effect of nano SiO2 and spent fluid catalytic cracking catalyst on cement hydration and physical mechanical properties. Constr. Build. Mater. 2021, 299, 124281. [Google Scholar] [CrossRef]
- LST EN 196-1; Methods of Testing Cement—Part 1: Determination of Strength. Lietuvos Standartizacijos Departamentas: Vilnius, Lithuania, 2016.
- Spychał, E.; Stepien, P. Effect of Cellulose Ether and Starch Ether on Hydration of Cement Processes and Fresh-State Properties of Cement Mortars. Materials 2022, 15, 8764. [Google Scholar] [CrossRef]
- Lothenbach, B.; Durdzinski, P.; Weerdt, K. Thermogravimetric analysis. In A Practical Guide to Microstructural Analysis of Cementitious Materials, 1st ed.; Scrivener, K., Snellings, R., Lothenbach, B., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 177–212. [Google Scholar]
- Al-Kheetan, M.J.; Byzyka, J.; Ghaffar, S.H. Sustainable Valorisation of Silane-Treated Waste Glass Powder in Concrete Pavement. Sustainability 2021, 13, 4949. [Google Scholar] [CrossRef]
- Mejdi, M.; Wilson, W.; Saillio, M.; Chaussadent, T.; Divet, L.; Tagnit-Hamou, A. Investigating the pozzolanic reaction of post-consumption glass powder and the role of portlandite in the formation of sodium-rich C-S-H. Cem. Concr. Res. 2019, 123, 105790. [Google Scholar] [CrossRef]
- Lia, T.; Tier, L. Microscopic Mechanism Analysis of Glass Powder with Different Replacement Rate on Concrete Performance. Glass Phys. Chem. 2023, 49, 245–255. [Google Scholar] [CrossRef]
- Bignozzi, M.C.; Saccani, A.; Barbieri, L.; Lancellotti, I. Glass waste as supplementary cementing materials: The effects of glass chemical composition. Cem. Concr. Compos. 2015, 55, 45–52. [Google Scholar] [CrossRef]
- Mostafa, N.Y.; Brown, P.W. Heat of hydration of high reactive pozzolans in blended cements: Isothermal conduction calorimetry. Thermochim. Acta 2005, 435, 162–167. [Google Scholar] [CrossRef]
- Mikhailenko, P.; Cassagnabere, F.; Emam, A.; Lachemi, M. Influence of physico- chemical characteristics on the carbonation of cement paste at high reploacement rates of metakaolin. Constr. Build. Mater. 2018, 158, 164–172. [Google Scholar] [CrossRef]
- Snoeck, D.; Velasco, L.F.; Mignon, A.; Van Vlierberghe, S.; Dubruel, P.; Lodewyckx, P.; De Belie, N. The influence of different drying techniques on the water sorption properties of cement-based materials. Cem. Concr. Res. 2014, 64, 54–62. [Google Scholar] [CrossRef]
- Tahwia, A.M.; Essam, A.; Tayeh, B.A.; Elrahman, M.A. Enhancing sustainability of ultra-high performance concrete utilizing high-volume waste glass powder. Case Stud. Constr. Mater. 2022, 17, e01648. [Google Scholar] [CrossRef]
- Serelis, E.; Vaitkevicius, V. Effect of waste glass powder and liquid glass on the Physico-Chemistry of Aluminum-Based Ultra-Lightweight concrete. Constr. Build. Mater. 2023, 390, 131615. [Google Scholar] [CrossRef]
- Abellan-Garcia, J.; Khan, M.I.; Abbas, Y.M.; Martínez-Lirón, V.; Carvajal-Muñoz, J.S. The drying shrinkage response of recycled-waste-glass-powder-and calcium-carbonate-based ultrahigh-performance concrete. Constr. Build. Mater. 2023, 379, 131163. [Google Scholar] [CrossRef]
- Redondo-Mosquera, J.D.; Sánchez-Angarita, D.; Redondo-Pérez, M.; Gómez-Espitia, J.C.; Abellán-García, J. Development of high-volume recycled glass ultra-high-performance concrete with high C3A cement. Case Stud. Constr. Mater. 2023, 18, e01906. [Google Scholar] [CrossRef]
- Liu, G.; Tang, Y.; Wang, J. Recycling and valorization of hydrated cement blends in mortars viasemi-dry carbonation—The role of waste glass, granulated blast furnace slag and fly ash. Constr. Build. Mater. 2023, 401, 132987. [Google Scholar] [CrossRef]




| Materials | CaO | SiO2 | SO3 | Al2O3 | Fe2O3 | MgO | K2O | Na2O | P2O5 | SrO | TiO2 | BaO | CO2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cement | 70.4 | 22.8 | 2.67 | 2.18 | 0.29 | 0.65 | 0.07 | 0.20 | 0.28 | 0.17 | 0.06 | – | – |
| LCDw | 7.59 | 57.3 | 0.07 | 14.0 | 0.79 | 1.36 | 0.68 | 2.14 | 0.02 | 7.53 | – | 1.27 | 6.10 |
| WMw | 10.1 | 68.2 | 0.05 | 2.20 | 0.12 | 1.32 | 1.12 | 12.2 | 0.01 | 0.01 | – | 0.04 | 4.53 |
| Paste Designation | Time of the Second Maximum (h) | Heat after Hours of Hydration (J/g) | |||
|---|---|---|---|---|---|
| 12 | 24 | 36 | 48 | ||
| C | 13.11 | 71.8 | 198.4 | 251.8 | 291.8 |
| LCDw10 | 13.75 | 66.1 | 175.0 | 236.4 | 280.0 |
| LCDw20 | 15.93 | 64.6 | 181.7 | 233.8 | 273.1 |
| WMw10 | 13.42 | 61.0 | 160.2 | 218.1 | 257.3 |
| WMw20 | 14.25 | 58.3 | 162.6 | 215.8 | 253.3 |
| Paste Designation | Degree of Hydration | |||
|---|---|---|---|---|
| 12 | 24 | 36 | 48 | |
| C | 24.6 | 68.0 | 86.3 | 100 |
| LCDw10 | 22.7 | 60.0 | 81.0 | 96.0 |
| LCDw20 | 22.1 | 62.3 | 80.1 | 93.6 |
| WMw10 | 20.9 | 54.9 | 74.7 | 88.2 |
| WMw20 | 20.0 | 55.7 | 74.0 | 86.8 |
| Amount of Replaced Cement, % | After 7 Days | After 28 Days | ||
|---|---|---|---|---|
| LCDw | WMw | LCDw | WMw | |
| 5 | 91.4 (96.2) | 79.5 (83.7) | 93.1 (98.0) | 85.5 (90.0) |
| 10 | 73.6 (81.8) | 77.1 (85.7) | 76.6 (85.1) | 72.4 (80.4) |
| 20 | 58.8 (73.5) | 64.1 (80.1) | 69.1 (86.4) | 71.0 (88.8) |
| Mark | 110–170 °C, % | 180–350 °C, % | 110–350 °C, % at Equal Cement Content | 420–530 °C, % | CH Content in Sample, % | CH Content at Equal Cement Content, % | At 610–770 °C, % |
|---|---|---|---|---|---|---|---|
| After 7 days | |||||||
| C | 2.2 | 3.9 | 6.1 | 5.0 | 20.6 | 20.6 | 1.8 |
| LCDw5 | 2.1 | 3.4 | 5.8 | 4.4 | 18.1 | 19.1 | 2.9 |
| LCDw20 | 1.8 | 2.9 | 5.9 | 3.6 | 14.8 | 18.5 | 2.4 |
| WMw5 | 2.0 | 3.3 | 5.6 | 4.6 | 18.9 | 19.9 | 1.9 |
| WMw20 | 1.6 | 3.1 | 5.9 | 3.5 | 14.4 | 18.0 | 3.9 |
| After 28 days | |||||||
| C | 2.6 | 4.5 | 7.1 | 4.7 | 19.3 | 19.3 | 2.0 |
| LCDw5 | 2.7 | 3.9 | 6.9 | 4.9 | 20.1 | 21.2 | 2.4 |
| LCDw20 | 2.7 | 4.0 | 8.4 | 4.0 | 16.4 | 20.5 | 2.9 |
| WMw5 | 2.3 | 4.0 | 6.3 | 5.0 | 20.6 | 21.6 | 1.9 |
| WMw20 | 2.6 | 3.7 | 7.6 | 4.0 | 16.4 | 20.5 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malaiškienė, J.; Bekerė, K. Impact of Electronic Waste Glass on the Properties of Cementitious Materials. Buildings 2024, 14, 1218. https://doi.org/10.3390/buildings14051218
Malaiškienė J, Bekerė K. Impact of Electronic Waste Glass on the Properties of Cementitious Materials. Buildings. 2024; 14(5):1218. https://doi.org/10.3390/buildings14051218
Chicago/Turabian StyleMalaiškienė, Jurgita, and Karolina Bekerė. 2024. "Impact of Electronic Waste Glass on the Properties of Cementitious Materials" Buildings 14, no. 5: 1218. https://doi.org/10.3390/buildings14051218
APA StyleMalaiškienė, J., & Bekerė, K. (2024). Impact of Electronic Waste Glass on the Properties of Cementitious Materials. Buildings, 14(5), 1218. https://doi.org/10.3390/buildings14051218






