Abstract
Spontaneous combustion coal gangue (SCCG) is considered to be an aluminosilicate-based solid waste containing various toxic ions. The alkali-activation method for this material can not only fully use its potential hydration activity but also solidify the hazardous components to some extent. Through introducing additional Pb2+, the solidification behavior of heavy metal Pb2+ for an SCCG-based geopolymer was studied in the present paper. The solidification efficiencies were evaluated by Pb2+ leaching rates under neutral and acidic conditions, while its mechanism was explained by the methods of XRD, TG, FT-IR, SEM, and MIP. The results show that the Pb2+ solidification efficiency increases along with the curing age, and acidic rather than neutral conditions lead to a more intensive solidification capacity. Judging by the permissive maximum value of 5 mg/L, the Pb2+ original concentrations under neutral and acidic circumstances should be lower at 2.0 wt.% and 3.0 wt.%, respectively. The Pb2+ absorption is dominated by the physical process, due to the formation of no new hydration products. However, the Pb2+ addition would interrupt the reconstruction of the Si-Al network structure, slowing the accumulation of N-A-S-H gel and the densifying of the matrix. When the Pb2+ concentration grows, the sizes of hydration productions shrink continuously, more defects appear in the microstructure of the geopolymer, and the pore structure deteriorates rapidly, all of which accelerate the diffusion of toxic ions to the external condition.
1. Introduction
Spontaneous combustion of coal gangue (SCCG) is a natural phenomenon that occurs when coal gangue exposed to the open air and subject to long-term wind and sunshine reaches combustible temperatures [1,2,3]. The combustion of coal gangue would release a considerable amount of toxic gases and fly ash, which contain multiple hazardous elements, including SO2, H2S, Pb, Hg, Cd, and so on. Also, among the fly ash exists combustible hydrocarbon polymers, which, at high temperatures, generate suspended particulates after reactions like oxidative decomposition and dehydrocarbonized polymerization; such particulates can bring about haze [4,5]. However, the elements generated by SCCG in complicated and diverse environments are found to be insidious and uncertain, and when these toxic and radioactive elements diffuse into the atmosphere and soil, problems such as human disease and environmental degradation ensue [6,7,8,9,10]. The safe functioning of society and the sustainable development of the natural environment can be seriously jeopardized.
Traditional treatment methods for SCCG fly ash mainly include high-temperature sintering, high-temperature melting, washing, solidification/stabilization, and low-temperature pyrolysis [11,12,13]. Solidification/stabilization is one of the most commonly used fly ash treatment methods internationally [14]. Through capturing or wrapping the heavy metal elements in the cured fly ash during geopolymerization reactions [15,16,17,18], the pollution caused by SCCG is effectively dealt with, and resources are fully maneuvered and utilized to improve the performance of the gangue geopolymer [19,20,21,22]. Wardhono et al. [23] found that SCCG polymerization occurs under the effect of an alkaline activator, and the polymerization products feature good cementitious properties. Shen et al. [24] investigated the early polymerization process of alkaline-activated gangue geopolymers and revealed that the generated geopolymers feature dense structures and good performance. Liu et al. [25] synthesized geopolymer recycled concrete by taking SCCG, slag, fly ash, and recycled aggregates as raw materials, and revealed through micro-structure analysis that the key factors affecting the mechanical properties of recycled concrete included the internal pore structure, microcracks, and bonding interface performance between the geopolymer and recycled coarse aggregate.
Geopolymers can effectively solidify various heavy metals, like Pb2+, Cu2+, Zn2+, and Cd2+, and the solidification efficiency can achieve 96.86% to 99.86% [26]. Compared with solidified cement, geopolymer solidified bodies possess higher compressive strength, lower toxic leaching concentration of heavy metals, and higher acid corrosion resistance [27,28,29]. Lv et al. [30] proposed a magnesium coal-based backfill material (MCB) with coal gasification coarse slag (CGCS) and coal gangue (CS) as aggregates. Through the in-depth study of As and Pb, it was delineated that MCB can serve as a potential solidification/stabilization agent for the solidification of the heavy metal elements like Cd, As, and Pb contained in materials. While facilitated with good mechanical properties, MCB can effectively realize resource recycling and has huge potential for safe application in backfill. Zhao [31] produced a geopolymer composite with gangue and blast furnace slag as materials for the curing of Pb2+ and Zn2+. It was proved that heavy materials in Pb and Zn tailing could be effectively solidified through physical and chemical methods.
In this study, a solidified body of Pb in SCCG was prepared in conjunction with existing theoretical studies on geopolymer production and geopolymer fly ash/heavy metal. The solidification ability was assessed by analysis of the leaching concentration of Pb2+ in neutral and acidic conditions through heavy metal ion leaching concentration tests. Meanwhile, methods including XR, TG, FT-IR, SEM, and MIP were adopted to further delve into the structural and morphological changes, reaction process, and solidification mechanism of SCCG at the microscopic level. This new approach will help to achieve resource recycling and protect the ecological environment, while at the same time contributing to the development of heavy industry and the preparation green building materials.
2. Experiments
2.1. Materials
The SCCG employed in this study came from Fuxin City, Liaoning Province. The SCCG powder used in this study was obtained after being processed in the ball mill for 8 h, with a moisture content of 0.45%. The main chemical composition of the powder is shown in Table 1. The average particle size was 33.84 um, measured by a laser particle size distribution meter (Bettersize2600, Dandong Baite Instrument Co., Ltd., Dandong, China) (Figure 1). XRD was adopted for the phase test, and the measured mineral composition of SCCG mainly included quartz, illite, anorthite/albite, and kaolinite (Figure 2). The chemical bonding and functional group tests of SCCG powder by Fourier transform infrared spectroscopy (FT-IR) revealed that 3394 cm−1 and 1627 cm−1 corresponded to the H-O-H telescopic vibration peak and bending vibration peak, respectively, 1078 cm−1 corresponded to the Si-O-Si asymmetric vibration peak, 796 cm−1 and 777 cm−1 corresponded to the Si-O-Si symmetric stretching vibration peak, 694 cm−1 corresponded to the Si-O-Si bending vibration peak, and 471 cm−1 corresponded to Si-O bending vibration peak.

Table 1.
Chemical composition of SCCG (wt.%).

Figure 1.
Particle size distribution of SCCG.

Figure 2.
Mineral composition and molecular structure of SCCG.
NaOH, Ca(OH)2, and Pb(NO3)2 in this study were pure reagents for chemical analysis. Water (H2O) was deionized water produced by the Dalian Bono Chemical Reagent Factory. The water glass solution from Zhejiang Jiashan Yourui Refractories Co., Ltd., Jiaxin, China, was Na2O·nSiO2 comprised of Na2O, SiO2, and H2O, the content of which was 13.75%, 30.00%, and 56.25%, respectively. The water glass modulus was 2.3.
2.2. Experimental Procedures
2.2.1. Mixture Proportions
In this study, Pb2+ was introduced into the SCCG geopolymer in the form of Pb(NO3)2, and the dosage of Pb2+ (in terms of the mass ratio of Pb2+ to the mass of the geopolymer) was designed to be 0.1%, 0.2%, 0.4%, 0.6%, 0.8%, 1.0%, 1.5%, 2.0%, 2.5%, and 3.0%. The Na2SiO3 dosage (alkali equivalent, in terms of Na2O) was 6%, Ca(OH)2 was 10%, and the water–binder ratio was 0.40. The test mix ratio is given in Table 2.

Table 2.
Test mix ratio design and material table.
2.2.2. Experimental Methods
The tests were conducted according to the specifications for SCCG geopolymer solidified body specimens (size 40 mm × 40 mm × 40 mm) given in GB/T17671-2021 [32] Test Method of Cement Mortar Strength (ISO method). The raw materials were weighed according to Table 2 to prepare and age the alkaline excitation solution. Then, SCCG powder, alkali activator solution, deionized water, and Pb (NO3)2 were poured into the cement slurry mixing pot sequentially. The prepared SCCG geopolymer in the form of solidified slurry was poured into the mold, stirred on the vibration table, covered with the protective film, and then placed in the curing box for 24 h before demolding. The preparation process of SCCG is shown in Figure 3. Finally, the slurry specimens were put into the curing box for 3 days, 7 days, and 28 days in preparation for tests.

Figure 3.
Flow chart of geopolymer preparation of SCCG.
Heavy Metal Ion Leaching Solution Test
The heavy metal ion leaching concentration test in this study was conducted with reference to GB5085.3-2007 [33], Identification Standards for Hazardous Wastes—Identification for Extraction Toxicity, and GB14569.1-2011 [34], Performance Requirements for Low and Intermediate Level Radioactive Waste Form—Cemented Waste Form. The required leaching solution preparation method to simulate neutral and acidic circumstances was based on HJ557-2010 [35], Solid Waste-Extraction Procedure for Leaching Toxicity—Horizontal Vibration Method, and HJ/T299-2007 [36], Solid Waste-Extraction Procedure for Leaching Toxicity—Sulphuric Acid & Nitric Acid Method.
Phase Assembly
This study adopted a D8 advance X-ray diffractometer (XRD, Brucker AG, Bremen, Germany) and TGA-DSC I thermalgravimetric analyzer (TGA, Swiss Mettler Toledo Company, Greifensee, Switzerland) for qualitative investigation of the SCCG geopolymer. Meanwhile, the chemical bonding and functional groups of solidified specimens were analyzed using a Nicolet iS10 Fourier transform infrared spectrometer (FT-IR, Thermo Fisher Scientific, Waltham, MA, USA).
Microscopic Test
The micro-morphology and pore structure of the specimens were analyzed using a NovaNano SEM-50 scanning electron microscope (SEM, FEI Corporation, Hillsboro, OR, USA) and AutoPore IV9500 mercury-in-press (MIP, McMurdoch Instruments, Norcross, GA, USA).
3. Results
3.1. Leaching Experiments
3.1.1. Neutral Circumstance
Figure 4 shows the results of the metal leaching concentrations of the geopolymer solidified bodies for different curing periods under different Pb2+ dosages in a neutral condition.

Figure 4.
Metal leaching concentrations of cured polymer at different ages (neutral circumstance).
As can be seen from the Figure, with the growth of the curing period, the Pb2+ leaching concentration of the SCCG geopolymer solidified bodies with different dosages of Pb2+ showed a decreasing trend, and Pb2+ trapping efficiency showed a growing trend. At 1.0 wt.% Pb2+, the leaching concentration for the curing period of 3 d was 2.190 mg/L, and the trapping efficiency was 99.76%. When the curing period increased to 7 d and 28 d, the leaching concentration dropped to 1.6420 mg/L and 1.4276 mg/L, and the trapping efficiency increased to 99.84% and 99.96%, respectively, indicating that the longer the curing period, the better the curing effect of the geopolymer solidified bodies on Pb2+. As Pb2+ dosage increased from 0.10 wt.% to 0.20 wt.%, Pb2+ leaching concentration of geopolymer solidified bodies with a 28 d curing period decreased from 0.1291 mg/L to 0.1056 mg/L, and Pb2+ trapping efficiency increased from 99.87% to 99.95%, which may be due to the fact that Pb2+ dosage within a certain range could optimize the effect of geopolymer trapping on Pb2+ [37]. With further increase in the dosage of Pb2+, the heavy metal leaching concentration of the geopolymer solidified bodies showed a growing trend, and the trapping efficiency of the curing on Pb2+ decreased. After the dosage of Pb2+ exceeded 2.00 wt.%, the metal leaching concentrations of the cured polymer grew faster; when the dosage reached 3.00 wt.%, the heavy metal leaching concentration of the geopolymer curing with a 28 d curing period reached 9.4354 mg/L, which is higher than the leaching concentration limit of 5 mg·L−1 delineated in GB5085.3-2007, Identification Standards for Hazardous Wastes—Identification for Extraction Toxicity. Therefore, it can be seen that the effective curing dosage limit of Pb2+ in SCCG geopolymer solidified bodies under neutral conditions is 2.00 wt.%.
3.1.2. Acid Circumstance
Figure 5 shows the test results of metal leaching concentrations of geopolymer solidified bodies at different curing periods under different Pb2+ dosages in acidic circumstances. It was demonstrated that with the increase of the curing period, the Pb2+ leaching concentration of SCCG geopolymer solidified bodies at various curing periods tended to decrease, and Pb2+ trapping efficiency tended to increase. With the increase in Pb2+ dosage, the metal leaching concentrations of geopolymer solidified bodies showed a growing trend, and Pb2+ trapping efficiency showed a decreasing trend. When the Pb2+ dosage exceeded 2.00 wt.%, the metal leaching concentrations of geopolymer solidified bodies featured a sharper increase; when the Pb2+ dosage exceeded 3.00 wt.%, the metal leaching concentrations of geopolymer solidified bodies at 28 d reached 5.1650 mg/L, a bit higher than the leaching concentration limit of 5 mg·L−1 designated in GB5085.3-2007, Identification Standards for Hazardous Wastes—Identification for Extraction Toxicity. Therefore, it can be seen that the effective curing dosage limit of Pb2+ in SCCG geopolymer solidified bodies under acid conditions is lower than 3.00 wt.%.
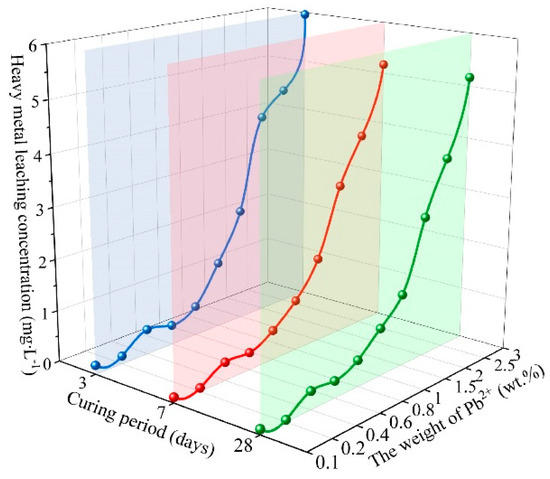
Figure 5.
Metal leaching concentrations of cured polymer at different ages (acid circumstance).
Combining the metal leaching concentrations of SCCG geopolymer solidified bodies in neutral and acid circumstances, it can be concluded that for geopolymers with structural integrity, a moderate Pb2+ dosage has limited influence on the structure of the polymerization products of geopolymer solidified bodies. However, excessive dosage of Pb2+ will destroy the polymerization structure of geopolymers, resulting in an increase in metal leaching concentration of geopolymer solidified bodies and a decrease in Pb2+ trapping efficiency. Compared with neutral environments, the leaching concentration of heavy metals in geopolymer solidified bodies is lower in acidic environments.
3.2. Phase Assembly
3.2.1. XRD
Figure 6a demonstrates the XRD pattern of SCCG geopolymer solidified with a 28 d curing period under different dosages of Pb2+. As can be seen from the Figure, the main crystalline phases of the SCCG geopolymer include quartz, aluminosilicate, zeolite, gypsum, and Ca(OH)2. Under such conditions, geopolymerization products mainly existed in the form of the amorphous gel structure N-A-S-H and C-A-S-H, and the dosage of Pb2+ had little influence on the distribution of the main diffraction peaks of the SCCG geopolymer. In the Figure, geopolymer solidified bodies in the range of 20° to 40° (2θ) appeared as “bulging peaks”, indicating that amorphous gel products had formed inside the geopolymer, and the larger the peak area of the “bulge”, the more geopolymer gel phase products. With the increase in the dosage of Pb2+, the peak area of the “bulging peak” gradually shrunk, and the calcium zeolite crystal peak also gradually weakened, indicating that excessive doping of Pb2+ can destroy the structure of the geopolymer and slow down the process of the geopolymerization reaction. However, Pb elements were not found in the XRD diagrams, indicating Pb was rarely solidified by the geopolymer in the form of chemical bonding, but mainly in the form of physical encapsulation and structural absorption, which aligns with the findings of previous scholars [38].
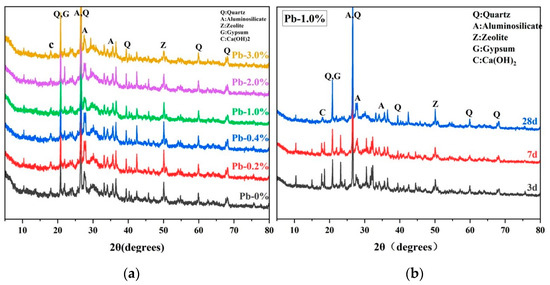
Figure 6.
XRD of SCCG geopolymer solidified bodies: (a) Pb2+ with different dosages at 28 days; (b) 1.0 wt.% Pb2+ content at different ages.
Figure 6b presents the XRD diagram of SCCG geopolymer solidified bodies at 1.0 wt.% Pb2+ with the curing periods of 3 d, 7 d, and 28 d. It can be observed that with the increase of the curing period, the “bulging peak” area of SCCG geopolymer solidified bodies in the range of 20° to 40° (2θ) increased, and the peak value of the crystal phase weakened. This indicates that the structure of SCCG geopolymer solidified bodies is compact, with good curing effects of Pb2+ and low metal leaching concentration. In addition, it was also observed that the crystal peak of the zeolite-like phase increased with the curing period increase, which may contribute to improving the mechanical properties of SCCG geopolymer solidified bodies.
3.2.2. TG-DTG
Thermogravimetric-differential thermal analysis (TG-DTG) allows a better investigation into the variation of physical properties (weight, temperature, enthalpy, etc.) of SCCG geopolymer solidified bodies with outside temperature. Figure 7 shows the TG-DTG test results of SCCG geopolymer solidified bodies with different Pb2+ dosages for a 28 d curing period and 1.0 wt.% Pb2+ dosage at different curing periods.
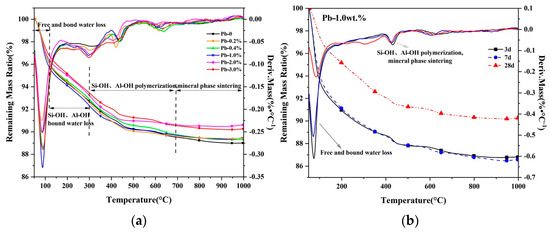
Figure 7.
TG-DTG of SCCG geopolymer solidified bodies: (a) Pb2+ with different dosages at 28 days; (b) 1.0 wt.% Pb2+ content at different ages.
It can be seen from Figure 7a that the doping of Pb2+ made no difference in the high-temperature weight loss course of the SCCG geopolymer. The thermal shrinkage of SCCG geopolymer solidified bodies covered four stages [39]: ➀ below 100 °C, the loss of free water came first; ➁ from 100 °C to 300 °C, there was a loss of binding water produced from the polymerization of Si-OH and Al-OH; ➂ 300 °C to 700 °C; ➃ 700 °C to 950 °C. The shrinkage in stages ➂ and ➃ may result from the shrinkage of capillaries due to the polymerization of Si-OH and Al-OH and the sintering of clay minerals. TG curves in the figure show that the doping of Pb2+ led to a decrease in the mass loss of the geopolymer solidified bodies. It is speculated that the entry of Pb2+ into the geopolymerization reaction system disrupted the geopolymer structure, resulting in a decrease in the amount of geopolymerized gel. Therefore, the mass loss of the geopolymer solidified bodies was less than that of the pure geopolymer during the continuous temperature increase. Meanwhile, with the increase in temperature, the thermogravimetric curve of the geopolymer solidified bodies gradually decreased with the continuous loss of structural water as the temperature increased. From the DTG curves, it can be observed that when the temperature reached 100 °C, there was an obvious heat absorption peak of geopolymer solidified bodies for each dosage of Pb2+; when the temperature reached 300 °C, the area of heat absorption peak increased with the increase of Pb2+ dosage; as the temperature was near 640 °C, there was a weak peak of heat absorption, and the area of the peak of the smaller dosage of Pb2+ turned out to be slightly larger than that of larger dosage of Pb2+; as the temperature continued to go up to 900 °C, no obvious heat absorption peak was observed, indicating that the geopolymer solidified bodies were not crystallized to generate leucogranite, and the SCCG geopolymer solidified bodies had good performance in high-temperature resistance.
As can be seen from Figure 7b, with the growth of the curing period, the weight loss of the geopolymer solidified bodies was reduced after a continuous temperature increase. This showed that with the increase of the curing period, the geopolymer solidified bodies featured excellent high-temperature resistance; also, the free water and bound water inside the geopolymer solidified bodies were reduced, and the geopolymerization reaction was better, which led to a better encapsulation effect on Pb2+.
3.2.3. FT-IR
Figure 8a shows the FT-IR results of SCCG solidified bodies with different dosages of Pb2+ for the curing period of 28 d. As can be seen from the Figure, the broad peak at 3440 cm−1 was the O-H hydroxyl stretching vibration peak of H2O, the weaker absorption peak at 1654 cm−1 was the hydroxyl (OH) bending vibration peak combined with H, and that at 1450 cm−1 was the O-H bending vibration peak, all of which indicated that the geopolymerization produces chemically bonded H2O in the course of the reaction. The absorption peaks at 400 cm−1 to 500 cm−1 were attributed to the Si-O bonding in the silica-oxygen tetrahedra and the Al-O bonding in the aluminum-oxygen tetrahedra. The absorption peaks at 600 cm−1 to 800 cm−1 were symmetric telescopic vibration peaks of Si-O-Si and Al-O-Si.
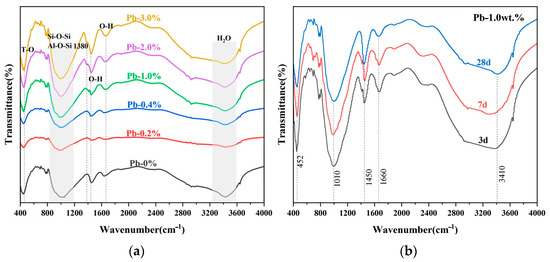
Figure 8.
FT-IR analysis of SCCG geopolymer solidified bodies: (a) Pb2+ with different dosages at 28 days; (b) 1.0 wt.% Pb2+ content at different ages.
When the dosage of Pb2+ was small, the telescopic vibration peak patterns of Si-O-Si and Al-O-Si in the range of 800 cm−1 to 1200 cm−1 gradually weakened and featured lower frequencies with the increase in Pb2+. As the dosage of Pb2+ continued to increase, the telescopic vibration peaks were strengthened instead and moved to still lower frequencies. This illustrates that Pb2+ participated in the polymerization process of the geopolymer, bringing about the reorganization of the Al-Si structure.
Figure 8b shows the FT-IR spectra of 1.0 wt.% Pb2+ SCCG geopolymer solidified bodies for different curing periods. It can be seen that the vibration peaks were the bending vibration peaks of T-O bonds in the silica-oxygen tetrahedra or aluminum-oxygen tetrahedra, which were greatly affected by heavy metals; the Si-O-Si and Al-O-Si telescopic vibration peaks at 1010 cm−1 were the internal structures of Si and Al tetrahedra, which were moderately affected by heavy metals. With the increase of the curing period, the areas of these two peaks shrunk slightly, and the vibration peaks moved in the direction of lower frequencies. It is postulated that with the passage of curing time, the geopolymerization reaction became more pronounced, and the solidification effect on Pb2+ was greater.
3.3. Microstructure
3.3.1. SEM
Figure 9 showcases the micro-morphology of SCCG geopolymer solidified bodies at 28 d under different Pb2+ dosages (0, 0.20%, 0.40%, 1.00%, 2.00%, 3.00%). It can be seen that the doping of Pb2+ affected the microscopic morphology of the geopolymerization products. With the increase in Pb2+ doping, the microstructure of SCCG geopolymer solidified bodies gradually grew loose and porous, and the size of the geopolymer gel products gradually decreased, but no new crystalline phase appeared. Tian [40] pointed out that the curing mechanism of geopolymers on heavy metal ions mainly includes chemical bonding and physical encapsulation. Fan [41] indicated that geopolymers can effectively immobilize heavy metals through structural adsorption, i.e., adsorption of heavy metals by zeolite-like phases generated through geopolymerization reactions.
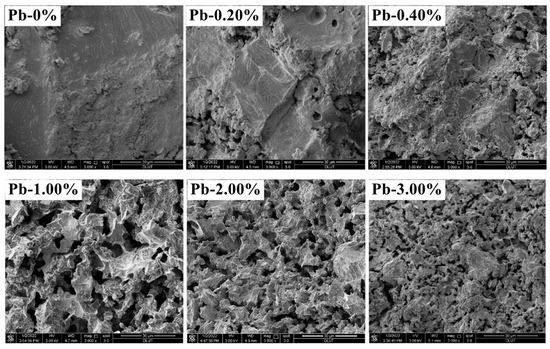
Figure 9.
SEM images of solidified aggregates of SCCG with different Pb2+ for a curing period of 28 days.
Figure 10 shows the distribution of Pb elements in geopolymer solidified bodies at different Pb2+ doping levels. It can be observed that the content of Pb was low at 0.00 wt.% Pb2+ doping level. With the increase in Pb2+ doping, the distribution density of Pb in the geopolymer increased, and when the doping of Pb2+ reached 3.00 wt.%, the distribution of Pb in the geopolymer became dense. When the dosage of Pb2+ was small, the curing form of Pb2+ in the SCCG geopolymer was mainly chemical bonding and physical encapsulation; however, with the increase in the dosage of Pb2+, less Pb2+ became involved in the chemical bonding, and the rest of the Pb2+ was physically encapsulated in the three-dimensional reticulated gel structure of the geopolymer or solidified by structural adsorption in the geopolymerized structure. Such a circumstance may result in the destruction of the geopolymer structure, a decrease in geopolymer solidification strength, and an increase in the leaching concentration of heavy metals.
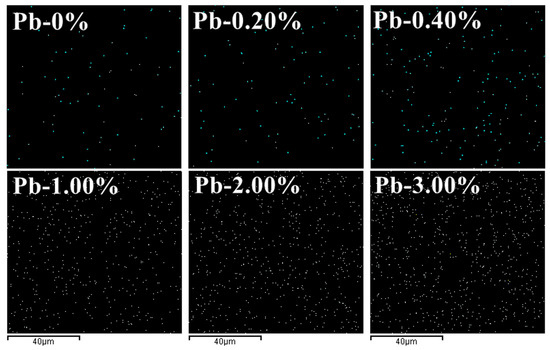
Figure 10.
Distribution of Pb elements in geopolymer solidified bodies with different Pb2+.
3.3.2. MIP
The introduction of heavy metal ions changes the pore characteristics of aggregate structures of geopolymers. The pore size of cementitious materials can be divided into four categories [42]: ➀ gel pores, with a diameter less than 10 nm; ➁ transition pores, with a diameter in the range of 10 to 100 nm; ➂ capillary pores, with a diameter in the range of 100 to 1000 nm; ➃ macropores, with a diameter more than 1000 nm. Figure 11 offers a demonstration of diameter distribution and cumulative pore areas of SCCG geopolymer solidified bodies for a 28 d curing period with different Pb2+ dosages. Table 3 presents the results of the pore structure analysis of geopolymer solidified bodies with different Pb2+ doping.

Figure 11.
MIP of geopolymer solidified form of lead at different dosages for 28 d curing period: (a) pore size distribution; (b) accumulated pore area.

Table 3.
Pore structure analysis of polymeric solidified bodies of spontaneous combustion coal gangue.
As can be seen from Figure 11 and Table 3, most of the pores inside SCCG geopolymer solidified bodies were gel pores and transition pores. With the increase in Pb2+, the total porosity of polymeric solidified bodies showed an increasing trend. The porosity of geopolymer solidified bodies with 0.00 wt.% Pb2+ was 26.92%, that with 0.20 wt.% Pb2+ was 16.60%, and that with 3.0 wt.% Pb2+ was 38.79%; meanwhile, the cumulative pore area of the geopolymer solidified bodies increased with Pb2+ doping increase.
As the dosage of Pb2+ was small, the porosity of geopolymer solidified bodies was less than that at 0.00 wt.% Pb2+, which may be because small dosages of Pb2+ participate in the geopolymerization reaction process by chemical bonding, and Pb(NO3)2 was soluble in alkali solution, rendering Pb2+ amorphous in the network structure of the geopolymer. At the same time, the geopolymerization product had a zeolite-like phase structure, and the cyclic molecules in the polymerization products were intertwined and polymerized to form a cage-like cavity [43], which encapsulated Pb2+ without destroying the overall structure of the geopolymer. As a result, the structure of the geopolymer was denser, and the porosity was lower with the increase in Pb2+ within a small dosage.
With the increase in Pb2+ doping, Pb2+ and OH− reacted to generate hydroxyl complexes, which decreased the alkali concentration of the geopolymerization reaction system and increased the structural viscosity, hindering the dissolution of Si and Al. Meanwhile, hydroxyl complex ions of heavy metal may affect the condensation reaction of [SiO4] and [AlO4] tetrahedra, resulting in slower growth of the Si and Al network skeleton and reducing the gel structure of the system. This finally led to an increase in porosity, a decrease in compressive strength, and an increase in the concentration of heavy metal leaching of geopolymerized solidified bodies.
4. Discussion
The solidification of Pb2+ by SCCG geopolymer solidified bodies mainly includes chemical bonding, physical encapsulation, and adsorption of the structure. After combustion, the internal carbon content of coal gangue decreases, while the content of SiO2 and Al2O3 significantly increases. Similar to materials such as fly ash and granulated blast furnace slag, it has high activity and has a certain effect on adsorbing Pb2+. The reaction course of how SCCG geopolymer cured Pb2+ is shown in Figure 12a, and the reaction equation is given in Figure 12b.
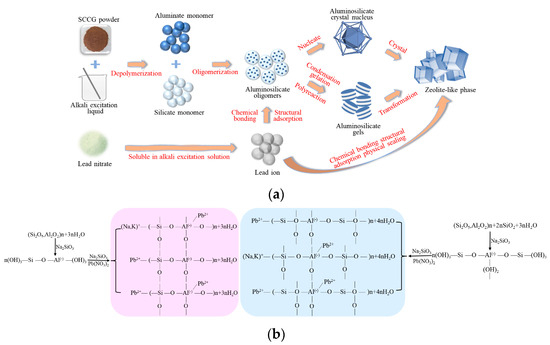
Figure 12.
Reaction process and equation of curing Pb2+ with aggregates from SCCG: (a) reaction process of curing Pb2+ with aggregates from SCCG; (b) reaction equation of polymers solidified by SCCG.
In the geopolymerization reaction system, aluminate monomers could react with silicate monomers or silicate oligomers to form silica-aluminate oligomers through oligomerization reaction in alkaline environments. Pb2+ dissolved in alkaline solution through Pb(NO3)2, and part of it could replace the structural sites of Na+, Ca2+, and K+ in the silica-aluminate oligomer, participating in the geopolymerization reaction in the form of chemical bonding. Also, due to the negative charge of the aluminum-oxygen tetrahedron, a small amount of Pb2+ would bind with the anionic group, and the geopolymerization structure would be incorporated into the structure of the silica-aluminate oligomer through neutral chemical bonding.
As the reaction continued, the silicoaluminate oligomer continued to polymerize to produce an amorphous silicoaluminate polymer, and the polymer condensed to form amorphous silicoaluminate gel. At this time, part of Pb2+ in the geopolymerized structure combined with the amorphous structure to form the gel phase, while part of the silicialuminate oligomer formed silicoaluminate nuclei in the reaction. As the geopolymerization reaction continued, the amorphous gel phase and the silicoaluminate nuclei further crystallized into the zeolite phase of the SCCG polymer. In this stage, part of the free Pb2+ was wrapped in the geopolymer cage structure, and a small proportion of Pb2+ was absorbed by the aluminum ion in the geopolymerization products’ zeolite phase and zeolite-like phase. The adsorption of Pb2+ by the SCCG geopolymer is beneficial for the removal of heavy metal toxic ions. The preparation of geopolymer cementitious materials using SCCG as raw material is not only beneficial for reducing the emission of toxic ions during the preparation process but also for addressing the problem of coal gangue accumulation pollution and lowering costs, having significance for environmental energy protection and producing economic benefits.
5. Conclusions
In this study, the leaching rate of Pb2+ under neutral and acidic conditions was analyzed to evaluate the curing efficiency. The mechanism was explained by XRD, TG, FT-IR, SEM, and MIP. The results indicated the following:
- (1)
- The solidification efficiency of Pb2+ increases with the curing period increase, and the efficiency in the acidic condition is more prominent than that in the neutral condition. Under the specification requirement to achieve a leaching concentration of 5 mg/L−1, the Pb2+ doping for SCCG geopolymerization is 2.0 wt% and 3.0 wt%. Efficiency in the acidic condition is more prominent than that in the neutral condition.
- (2)
- The doping of Pb2+ would interrupt the reconstruction of the Si-Al network structure, slow the accumulation of N-A-S-H gel, and densify the structure of solidified bodies. Since there appeared no new crystalline phases containing Pb elements in the XRD diagrams, it is concluded that a large amount of Pb is solidified in the form of physical encapsulation and structural adsorption. However, the doping of Pb2+ does not change the high-temperature weight loss course of the SCCG geopolymer, and the incorporation of Pb2+ reduces the mass loss of geopolymer solidified bodies.
- (3)
- With the increase in Pb2+ dosage, the density of Pb gradually increases, and the size of the hydration product decreases. Also, more defects appear in the microstructure of the SCCG geopolymer, and the pore structure deteriorates rapidly, resulting in faster diffusion of toxic ions to the outside.
- (4)
- A moderate amount of Pb2+ can optimize the geopolymer solidification effect and render the structure more stable. In contrast, excessive incorporation of Pb2+ can lead to imbalance and damage to the internal structure of the geopolymer.
Author Contributions
F.L.: Writing—review and editing, methodology; R.T.: writing—original draft, software; B.W.: conceptualization, revision; J.Y.: data curation. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the Joint Funds of the National Natural Science Foundation of China (U20A20324), National Natural Science Foundation of China (51902270, 52272016); Scientific Research Program Funded by the Education Department of Shaanxi Provincial Government (Program No. 23JS059); the Special Fund for the Launch of Scientific Research in Xijing University (XJ21T01); the Youth Innovation Team of Shaanxi Universities.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, F.; Tang, R.; Wang, B.; Yuan, X. Experimental Study on Solidification of Pb2+ in Fly Ash-Based Geopolymers. Sustainability 2021, 13, 12621. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, X.; Hu, S.; Shao, H.; Liao, Q.; Fan, Y. Experimental study of the effects of stacking modes on the spontaneous combustion of coal gangue. Process Saf. Environ. Prot. 2019, 123, 39–47. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Shi, X.; Liu, S.; Shu, P.; Xia, S. Investigation of thermal behavior and hazards quantification in spontaneous combustion fires of coal and coal gangue. Sci. Total Environ. 2022, 843, 157072. [Google Scholar] [CrossRef]
- Fan, C.; Wu, Z.; Wang, B.; Zheng, W. Solidification of municipal solid waste incineration fly ash with alkali-activated technology. J. Environ. Manag. 2023, 348, 119404. [Google Scholar] [CrossRef]
- Shao, X.; Qin, B.; Shi, Q.; Yang, Y.; Ma, Z.; Xu, Y.; Hao, M.; Jiang, Z.; Jiang, W. Study on the sequestration capacity of fly ash on CO2 and employing the product to prevent spontaneous combustion of coal. Fuel 2023, 334, 126378. [Google Scholar] [CrossRef]
- Guo, W.; Chen, B.; Li, G.; Liu, M.; Liu, X.; Chen, Q.; Zhang, X.; Li, S.; Chen, S.; Feng, W.; et al. Ambient PM2.5 and Related Health Impacts of Spontaneous Combustion of Coal and Coal Gangue. Environ. Sci. Technol. 2021, 55, 5763–5771. [Google Scholar] [CrossRef]
- Li, J.; Wang, J. Comprehensive utilization and environmental risks of coal gangue: A review. J. Clean. Prod. 2019, 239, 117946. [Google Scholar] [CrossRef]
- Liu, Y.; Qi, X.; Luo, D.; Zhang, Y.; Qin, J. Detection of Spontaneous Combustion Areas of Coal Gangue Dumps and Comprehensive Governance Technologies: A Case Study. ACS Omega 2023, 8, 47690–47700. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, C.; Chen, C. Characteristics of polycyclic aromatic hydrocarbon release during spontaneous combustion of coal and gangue in the same coal seam. J. Loss Prev. Process Ind. 2018, 55, 392–399. [Google Scholar] [CrossRef]
- Wang, S.; Luo, K.; Wang, X.; Sun, Y. Estimate of sulfur, arsenic, mercury, fluorine emissions due to spontaneous combustion of coal gangue: An important part of Chinese emission inventories. Environ. Pollut. 2016, 209, 107–113. [Google Scholar] [CrossRef]
- Kong, B.; Li, Z.; Yang, Y.; Liu, Z.; Yan, D. A review on the mechanism, risk evaluation, and prevention of coal spontaneous combustion in China. Environ. Sci. Pollut. Res. 2017, 24, 23453–23470. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Xie, G.; Li, Z.; Fan, X.; Zhang, W.; Xing, F.; Tang, L.; Ren, J. Resource utilization of municipal solid waste incineration fly ash-Cement and alkali-activated cementitious materials: A review. Sci. Total Environ. 2022, 852, 158254. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhao, Q.; Qiu, W.; Xue, Y.; Li, N. Microstructure and Strength of Alkali-Activated Bricks Containing Municipal Solid Waste Incineration (MSWI) Fly Ash Developed as Construction Materials. Sustainability 2019, 11, 1283. [Google Scholar] [CrossRef]
- Dermatas, D.; Meng, X. Utilization of fly ash for stabilization/solidification of heavy metal contaminated soils. Eng. Geol. 2003, 70, 377–394. [Google Scholar] [CrossRef]
- Fan, C.; Wang, B.; Ai, H.; Qi, Y.; Liu, Z. A comparative study on solidification/stabilization characteristics of coal fly ash-based geopolymer and Portland cement on heavy metals in MSWI fly ash. J. Clean. Prod. 2021, 319, 128790. [Google Scholar] [CrossRef]
- Luna Galiano, Y.; Fernández Pereira, C.; Vale, J. Stabilization/solidification of a municipal solid waste incineration residue using fly ash-based geopolymers. J. Hazard. Mater. 2011, 185, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Ryu, G.S.; Lee, Y.B.; Koh, K.T.; Chung, Y.S. The mechanical properties of fly ash-based geopolymer concrete with alkaline activators. Constr. Build. Mater. 2013, 47, 409–418. [Google Scholar] [CrossRef]
- Zhang, P.; Gao, Z.; Wang, J.; Guo, J.; Hu, S.; Ling, Y. Properties of fresh and hardened fly ash/slag based geopolymer concrete: A review. J. Clean. Prod. 2020, 270, 122389. [Google Scholar] [CrossRef]
- Han, X.; Zhang, P.; Zheng, Y.; Wang, J. Utilization of municipal solid waste incineration fly ash with coal fly ash/metakaolin for geopolymer composites preparation. Constr. Build. Mater. 2023, 403, 133060. [Google Scholar] [CrossRef]
- Li, Q.; Sun, Z.; Tao, D.; Xu, Y.; Li, P.; Cui, H.; Zhai, J. Immobilization of simulated radionuclide 133Cs+ by fly ash-based geopolymer. J. Hazard. Mater. 2013, 262, 325–331. [Google Scholar] [CrossRef]
- Su, Y.; Luo, B.; Luo, Z.; Xu, F.; Huang, H.; Long, Z.; Shen, C. Mechanical characteristics and solidification mechanism of slag/fly ash-based geopolymer and cement solidified organic clay: A comparative study. J. Build. Eng. 2023, 71, 106459. [Google Scholar] [CrossRef]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.H.; Tong, D.S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly ash-based geopolymer: Clean production, properties and applications. J. Clean. Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- Wardhono, A.; Gunasekara, C.; Law, D.W.; Setunge, S. Comparison of long term performance between alkali activated slag and fly ash geopolymer concretes. Constr. Build. Mater. 2017, 143, 272–279. [Google Scholar] [CrossRef]
- Shen, J.; Li, Y.; Lin, H.; Lv, J.; Feng, S.; Ci, J. Early properties and chemical structure analysis of alkali-activated brick geopolymer with varied alkali dosage. J. Build. Eng. 2022, 60, 105186. [Google Scholar] [CrossRef]
- Liu, C.; Deng, X.; Liu, J.; Hui, D. Mechanical properties and microstructures of hypergolic and calcined coal gangue based geopolymer recycled concrete. Constr. Build. Mater. 2019, 221, 691–708. [Google Scholar] [CrossRef]
- Muhammad, F.; Huang, X.; Li, S.; Xia, M.; Zhang, M.; Liu, Q.; Shehzad Hassan, M.A.; Jiao, B.; Yu, L.; Li, D. Strength evaluation by using polycarboxylate superplasticizer and solidification efficiency of Cr6+, Pb2+ and Cd2+ in composite based geopolymer. J. Clean. Prod. 2018, 188, 807–815. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, F.; Zhou, F.; Lu, M.; Hou, H.; Li, J.; Liu, D.; Wang, T. Early solidification/stabilization mechanism of heavy metals (Pb, Cr and Zn) in Shell coal gasification fly ash based geopolymer. Sci. Total Environ. 2022, 802, 149905. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H. Self-Solidification/Stabilization of Heavy Metal Wastes of Class C Fly Ash–Based Geopolymers. J. Mater. Civ. Eng. 2013, 25, 491–496. [Google Scholar] [CrossRef]
- Cui, C.-Y.; Meng, K.; Xu, C.-S. Vertical vibration of a floating pile considering the incomplete bonding effect of the pile-soil interface. Comput. Geotech. 2022, 150, 104–894. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, L.; Yang, P.; Xie, G.; Zhang, C.; Deng, S. Study on leaching and curing mechanism of heavy metals in magnesium coal based backfill materials. Process Saf. Environ. Prot. 2023, 177, 1393–1402. [Google Scholar] [CrossRef]
- Zhao, S.; Xia, M.; Yu, L.; Huang, X.; Jiao, B.; Li, D. Optimization for the preparation of composite geopolymer using response surface methodology and its application in lead-zinc tailings solidification. Constr. Build. Mater. 2021, 266, 120969. [Google Scholar] [CrossRef]
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). China National Standards: Shenzhen, China, 2021. (In Chinese)
- GB5085.3-2007; Identification Standards for Hazardous Wastes-Identification for Extraction Toxicity. China National Standards: Shenzhen, China, 2007. (In Chinese)
- GB14569.1-2011; Performance Requirements for Low and Intermediate Level Radioactive Waste Form-Cemented Waste Form. China National Standards: Shenzhen, China, 2021. (In Chinese)
- HJ557-2010; Solid Waste-Extraction Procedure for Leaching Toxicity- Horizontal Vibration Method. China National Standards: Shenzhen, China, 2010. (In Chinese)
- HJ/T299-2007; Solid Waste-Extraction Procedure for Leaching Toxicity-Sulphuric Acid & Nitric Acid Method. China National Standards: Shenzhen, China, 2007. (In Chinese)
- Wang, H.; Zhu, Z.; Pu, S.; Song, W. Solidification/Stabilization of Pb2+ and Cd2+ Contaminated Soil Using Fly Ash and GGBS Based Geopolymer. Arab. J. Sci. Eng. 2021, 47, 4385–4400. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Dai, J.-G.; Wang, L.; Tsang, D.C.W.; Poon, C.S. Influence of lead on stabilization/solidification by ordinary Portland cement and magnesium phosphate cement. Chemosphere 2018, 190, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Wang, Y.; Yang, F.; Rossi, M. A Review on the Application of Circulating Fluidized Bed Fly Ash in Building Materials. Adv. Mater. Sci. Eng. 2022, 2022, 7099430. [Google Scholar] [CrossRef]
- Tian, Q.; Bai, Y.; Pan, Y.; Chen, C.; Yao, S.; Sasaki, K.; Zhang, H. Application of Geopolymer in Stabilization/Solidification of Hazardous Pollutants: A Review. Molecules 2022, 27, 4570. [Google Scholar] [CrossRef]
- Fan, J.; Yan, J.; Zhou, M.; Xu, Y.; Lu, Y.; Duan, P.; Zhu, Y.; Zhang, Z.; Li, W.; Wang, A.; et al. Heavy metals immobilization of ternary geopolymer based on nickel slag, lithium slag and metakaolin. J. Hazard. Mater. 2023, 453, 131380. [Google Scholar] [CrossRef]
- Yang, M.; Paudel, S.R.; Asa, E. Comparison of pore structure in alkali activated fly ash geopolymer and ordinary concrete due to alkali-silica reaction using micro-computed tomography. Constr. Build. Mater. 2020, 236, 117524. [Google Scholar] [CrossRef]
- Khalid, H.R.; Lee, N.K.; Choudhry, I.; Wang, Z.; Lee, H.K. Evolution of zeolite crystals in geopolymer-supported zeolites: Effects of composition of starting materials. Mater. Lett. 2019, 239, 33–36. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).