Optimal Quantity Investigation of Metakaolin and Silica Fume in Production of Durable Acid Resistance Alkali Activated Slag Concrete
Abstract
:1. Introduction
2. Materials, Methods, and Experimental Program
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Mechanical Properties and Water Absorption
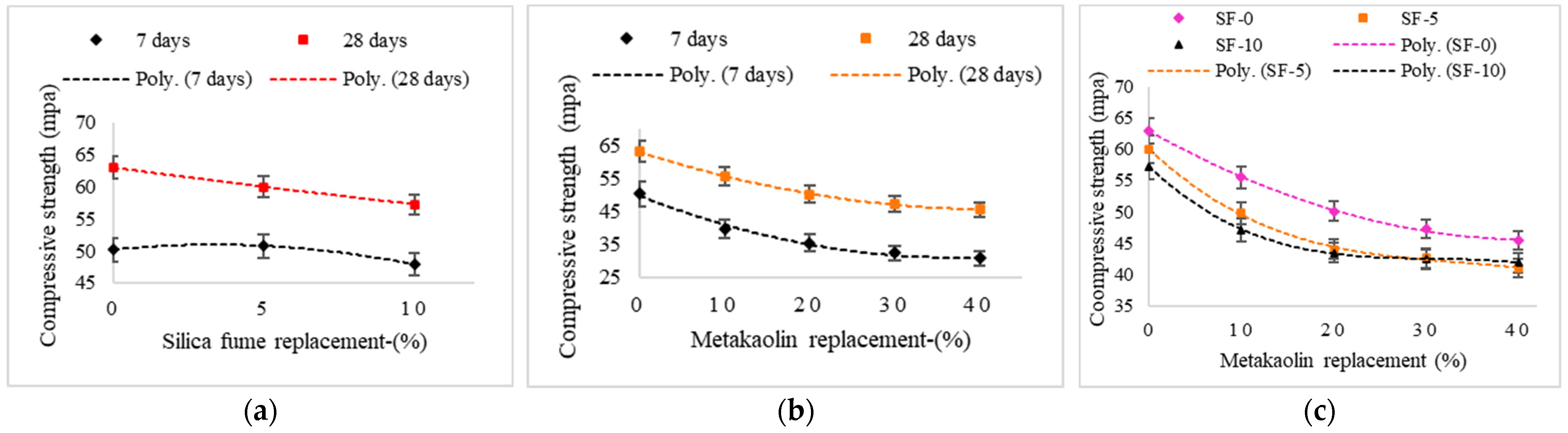
3.1.1. Compressive Strength
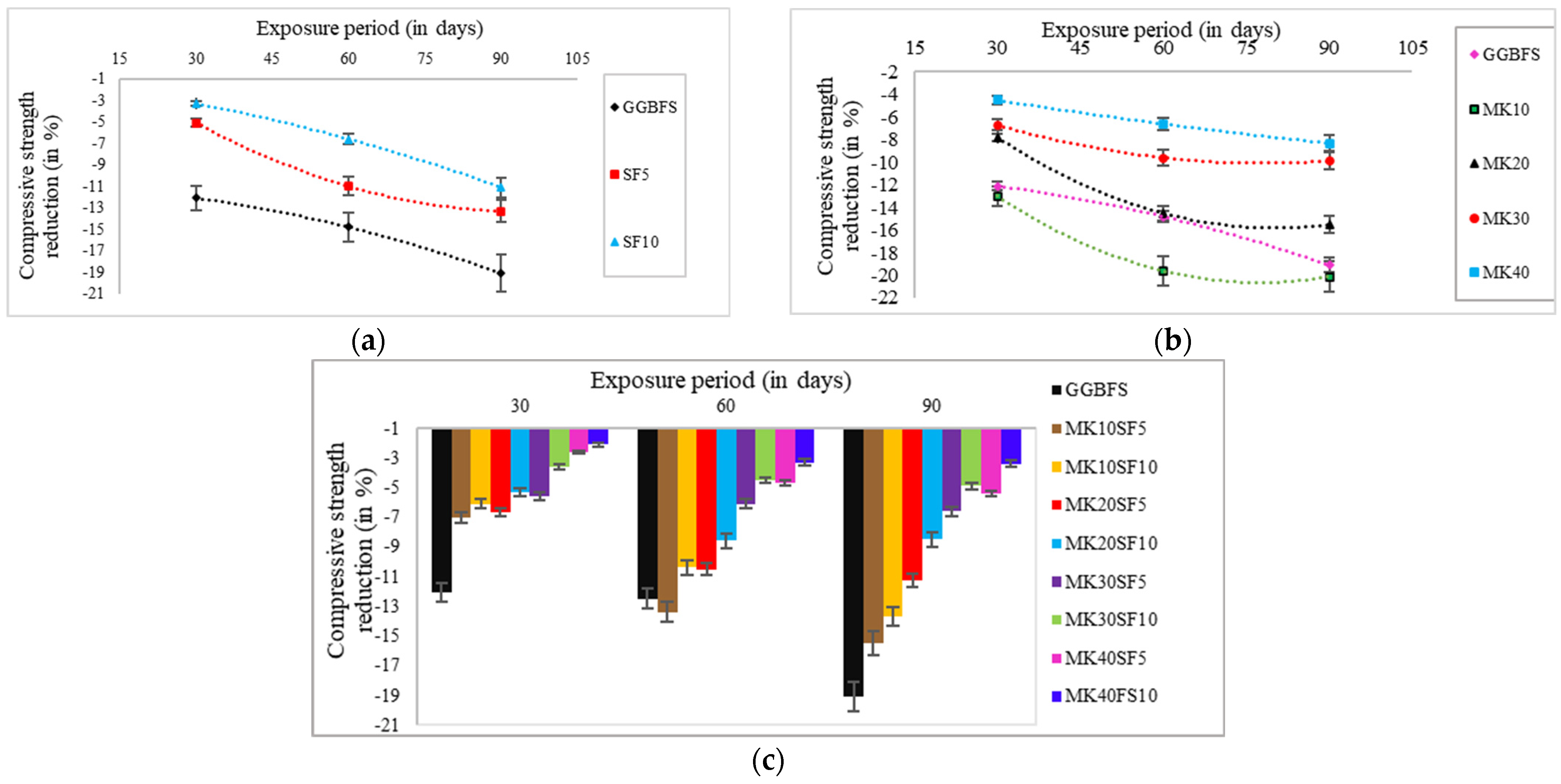
3.1.2. Reduction in Compressive Strength
3.2. Mass Changes
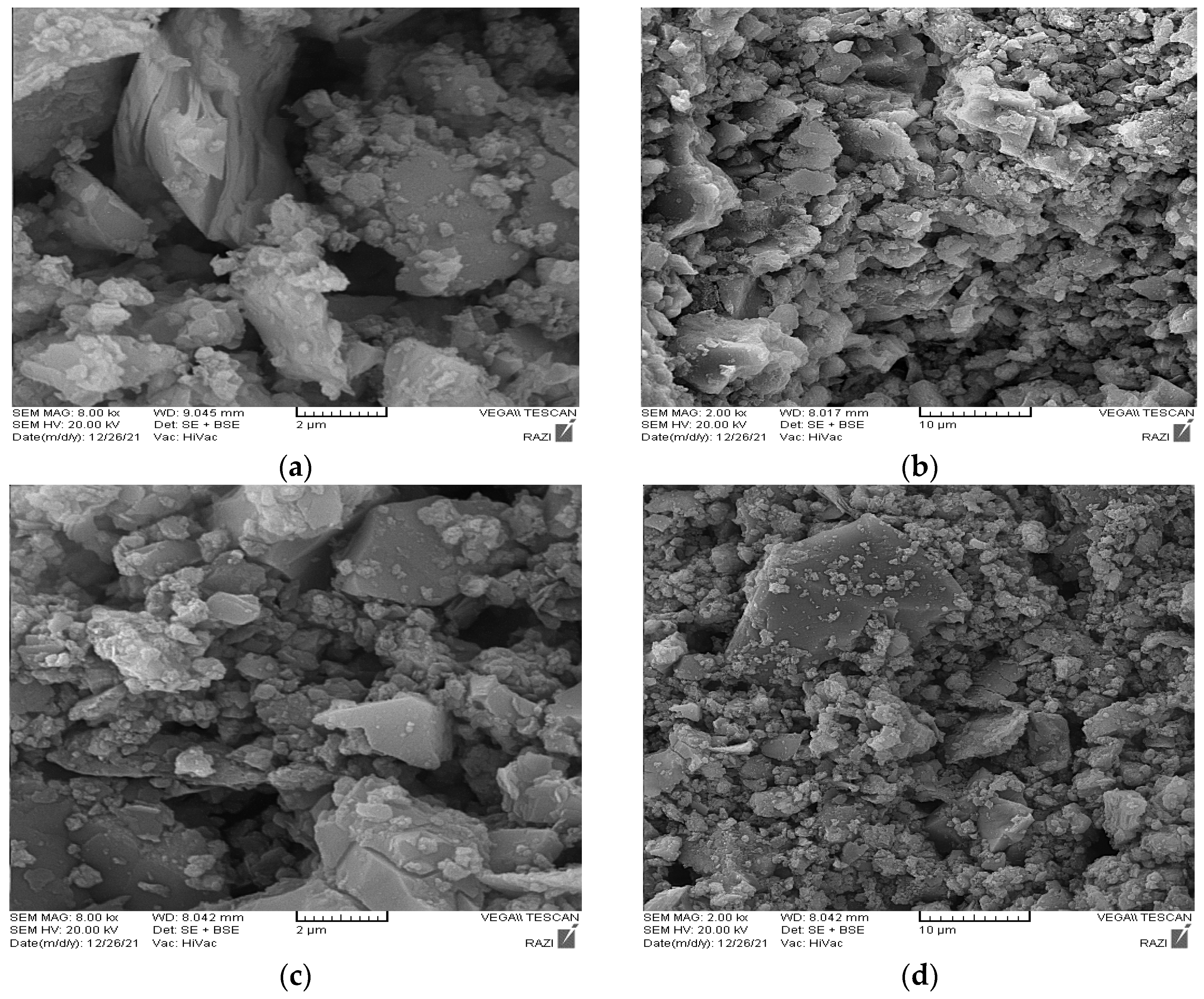
3.3. SEM and EDS Test Results
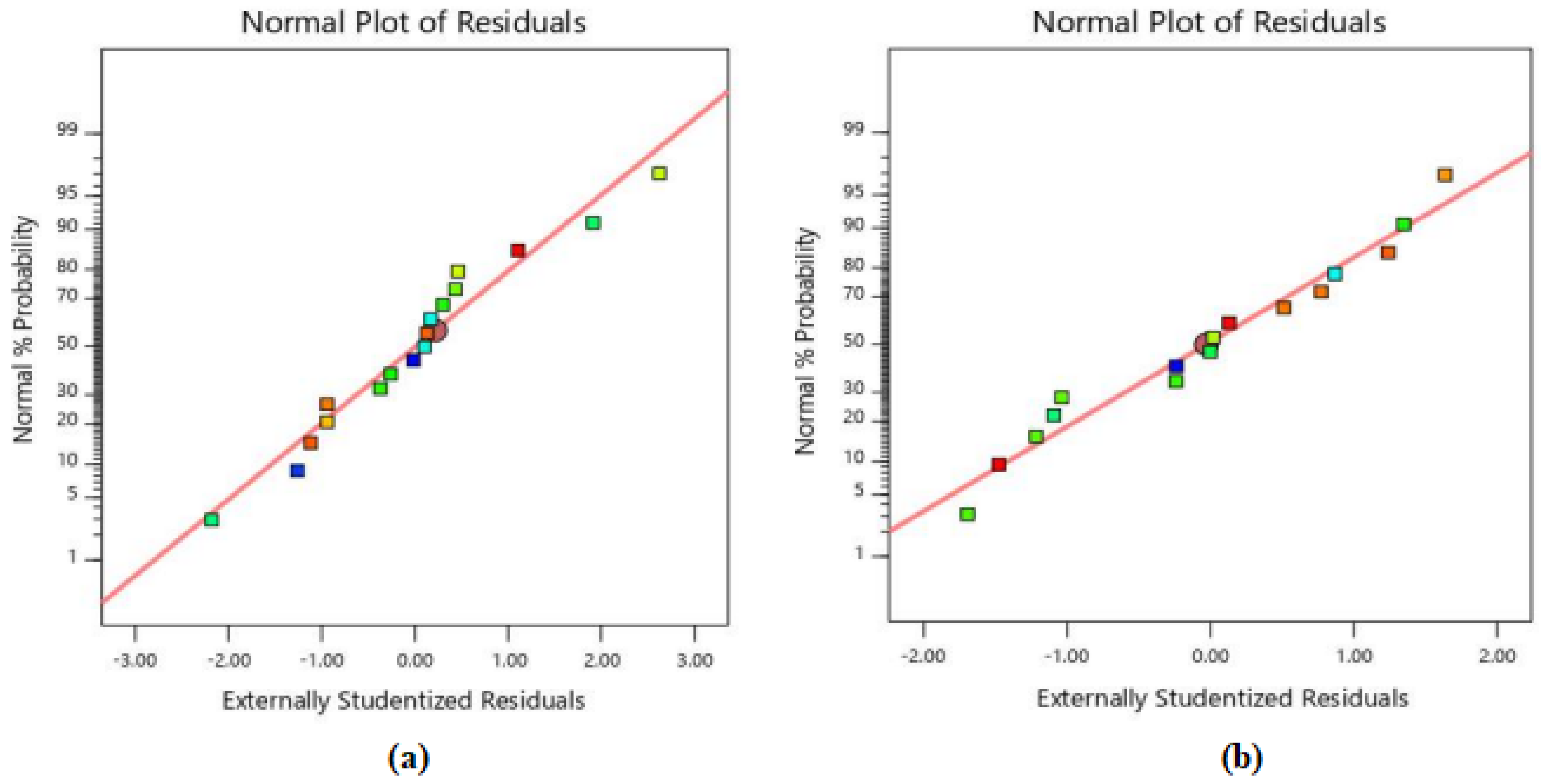
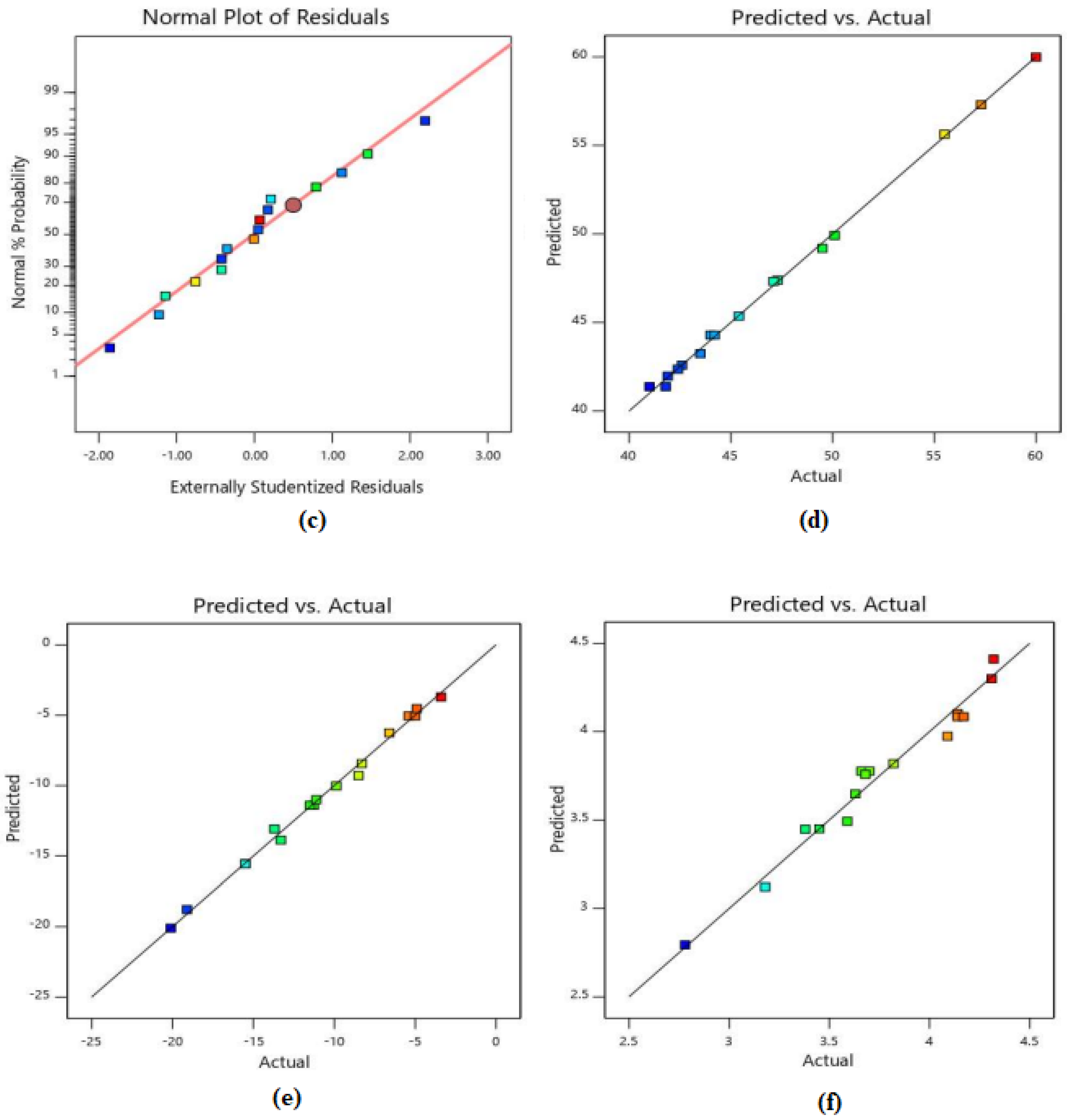
3.4. Statistical Interpretation of Test Results
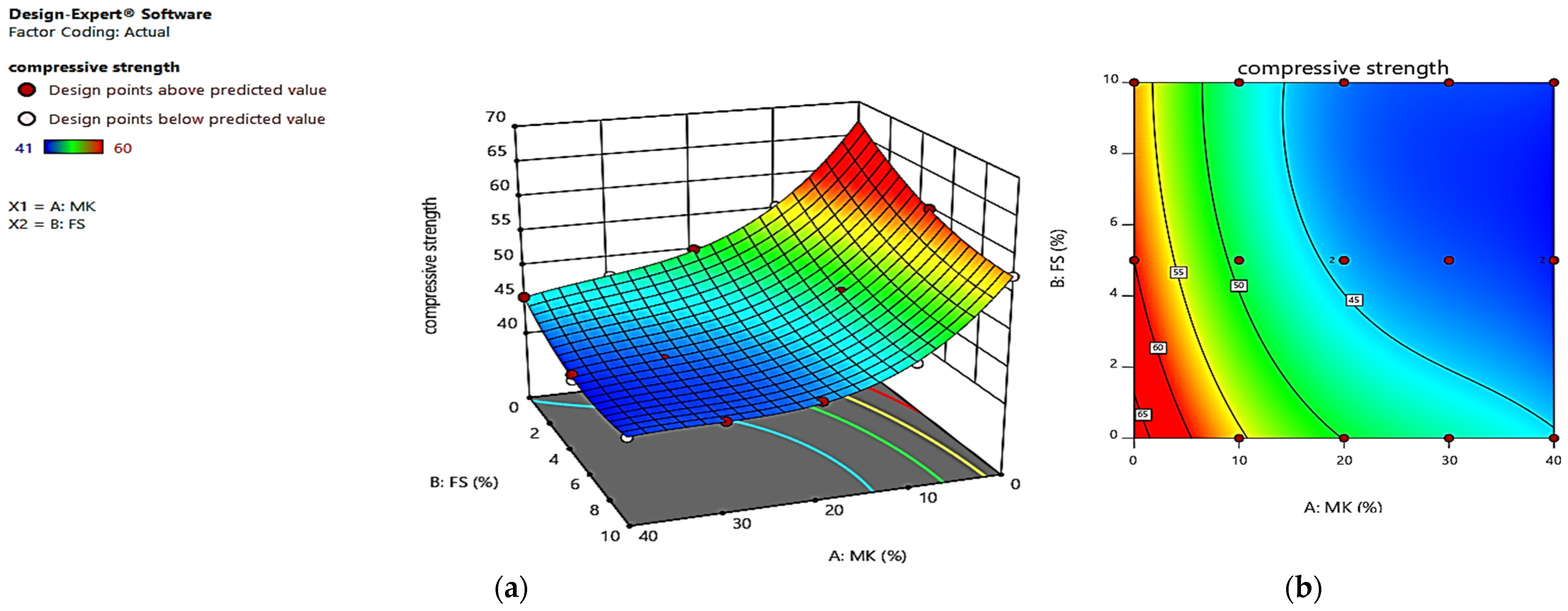
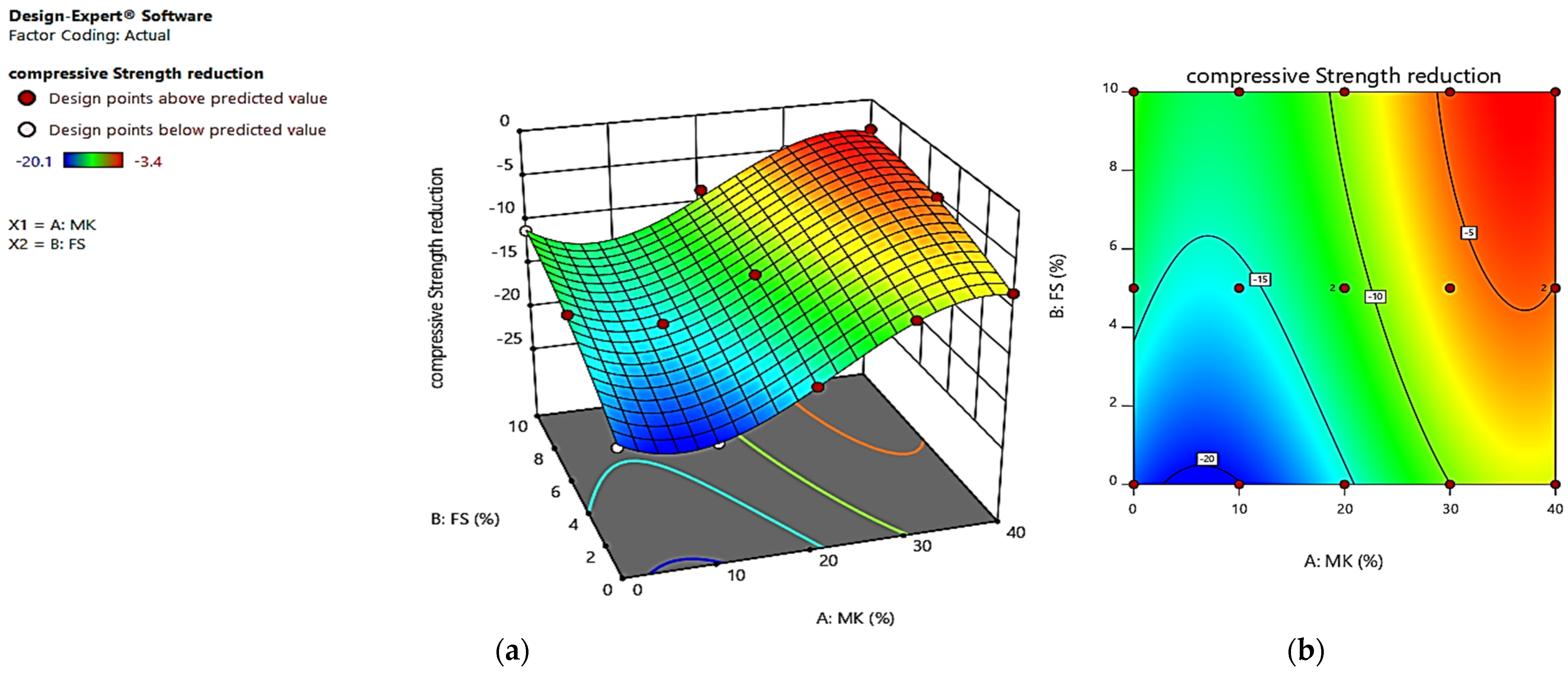
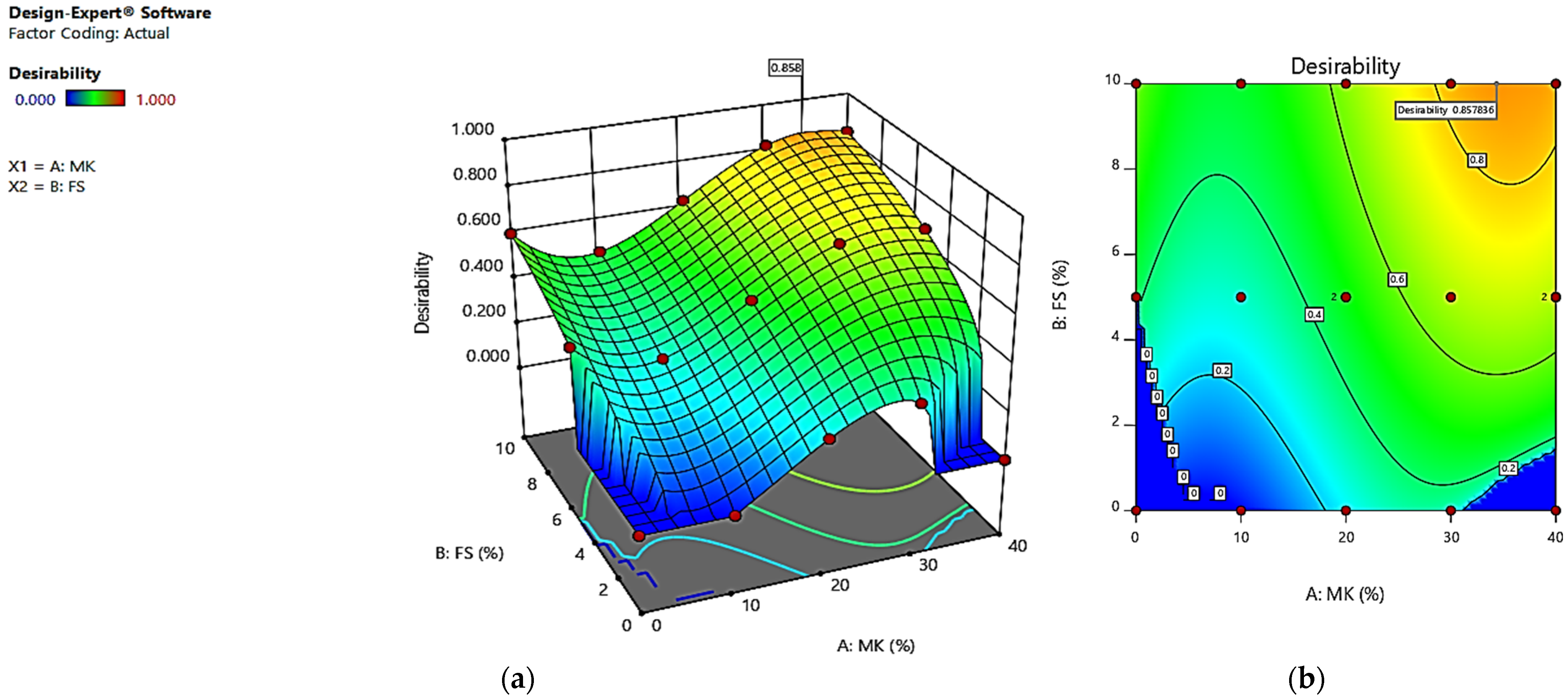
4. Optimization
5. Conclusions
- In the studied specimens, the compressive strength has increased by increasing the slag content in the mix design due to the high amounts of calcium oxide. Also, 34.9 and 9% compressive strength reduction, the maximum values, have been observed in the specimens with 40, and 10% metakaolin and silica fume replacement.
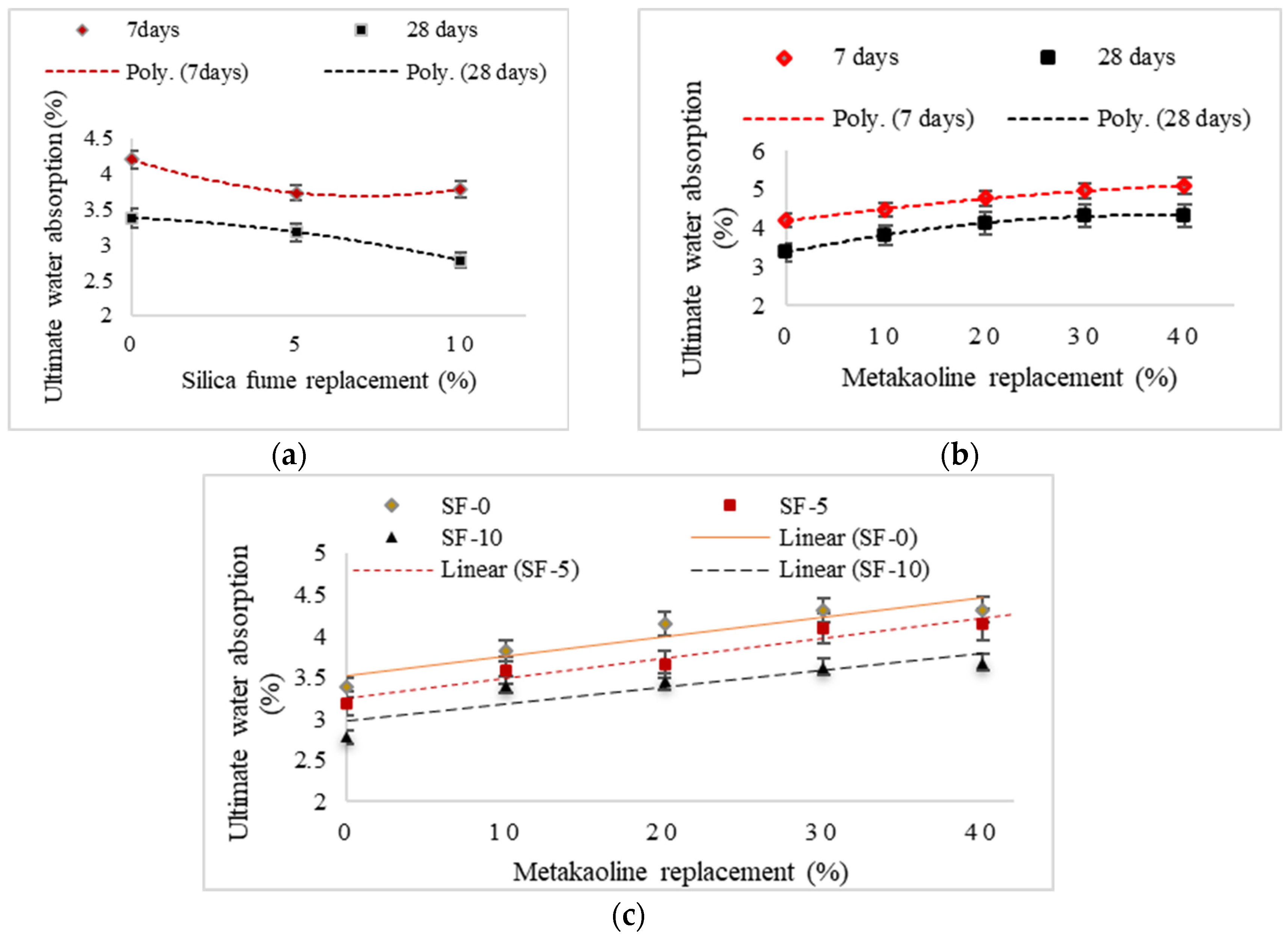
- Replacing slag with metakaolin (up to 40%) increases the specimens’ short-term, final, and capillary water absorption to 28.1%, 27.8%, and 10%, respectively. This is due to plate-shaped metakaolin particles with high specific surfaces which create cavities that increase water absorption.
- Maximum damage was observed in the GGBFS specimens having the most mass increase, equal to 2.31%, owing to expanding crystals forming, such as calcium sulfate (gypsum) as a reaction product of calcium oxide and sulfuric acid.
- Although increasing the slag in alkali-activated slag concrete has increased the compressive strength and decreased the permeability, the compressive strength of specimens exposed to sulfuric acid solution has reduced by 19.1% due to the solubility of Ca(OH)2 in the acid solution and migration of OH- ions. Therefore, with increasing the slag, the damage increases owing to the reaction of Ca(OH)2 from calcium oxide in the slag with the sulfuric acid solution.
- The interfacing of alkali-activated concrete (AAC) with sulfuric acid (H2SO4) instigates a cascade of chemical reactions that can substantially deteriorate the structural integrity of the concrete. These reactions commence with neutralizing alkali activators (NaOH or KOH), dissolution of the C-S-H gel, and precipitation of gypsum (CaSO4·2H2O) at the initial interfacing stage. As the degradation process progresses, further dissolution of the C-S-H gel, formation of ettringite (CaAl2(SO4)3·3H2O), and sulfate attack (CaSO4·2H2O → Ca(OH)2 + 2H2SO4) ensue, leading to a marked reduction in compressive strength, a surge in porosity and permeability, and the onset of cracking and spalling. These detrimental effects ultimately curtail the AAC specimens’ properties, which were exposed to acidic environments.
- Based on the RSM optimization results of the studied specimens, a mix design consisting of 34.4% MK and 10% SF appears to be the most effective in achieving minimum mass change, optimal permeability, and compressive strength of alkali-activated slag-based concrete when exposed to sulfuric acid.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Farhan, N.A.; Sheikh, M.N.; Hadi, M.N. Investigation of engineering properties of normal and high strength fly ash based geopolymer and alkali-activated slag concrete compared to ordinary Portland cement concrete. Constr. Build. Mater. 2019, 196, 26–42. [Google Scholar] [CrossRef]
- Aliabdo, A.A.; Abd Elmoaty, M.; Emam, M.A. Factors affecting the mechanical properties of alkali activated ground granulated blast furnace slag concrete. Constr. Build. Mater. 2019, 197, 339–355. [Google Scholar] [CrossRef]
- Mehta, A.; Siddique, R. Sulfuric acid resistance of fly ash based geopolymer concrete. Constr. Build. Mater. 2017, 146, 136–143. [Google Scholar] [CrossRef]
- Ariffin, M.A.M.; Bhutta, M.A.R.; Hussin, M.W.; Tahir, M.M.; Aziah, N. Sulfuric acid resistance of blended ash geopolymer concrete. Constr. Build. Mater. 2013, 43, 80–86. [Google Scholar] [CrossRef]
- Manjunath, R.; Narasimhan, M.C. Alkali-activated concrete systems: A state of art. In New Materials in Civil Engineering; Butterworth-Heinemann: Oxford, UK, 2020; pp. 459–491. [Google Scholar] [CrossRef]
- Geraldo, R.H.; Gonçalves, J.P.; Camarini, G. Mechanical properties of an eco-friendly one-part alkali-activated binder: Influence of metakaolin and water content. Ceram. Int. 2023, 49, 11854–11864. [Google Scholar] [CrossRef]
- Jindal, B.B.; Alomayri, T.; Hasan, A.; Kaze, C.R. Geopolymer concrete with metakaolin for sustainability: A comprehensive review on raw material’s properties, synthesis, performance, and potential application. Environ. Sci. Pollut. Res. 2022, 30, 25299–25324. [Google Scholar] [CrossRef] [PubMed]
- Davidovits, J. Geopolymers: Inorganic polymeric new materials. J. Therm. Anal. Calorim. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Shilar, F.A.; Ganachari, S.V.; Patil, V.B.; Reddy, I.N.; Shim, J. Preparation and validation of sustainable metakaolin based geopolymer concrete for structural application. Constr. Build. Mater. 2023, 371, 130688. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Resistance of alkali-activated slag concrete to acid attack. Cem. Concr. Res. 2003, 33, 1607–1611. [Google Scholar] [CrossRef]
- Alzeebaree, R.; Çevik, A.; Nematollahi, B.; Sanjayan, J.; Mohammedameen, A.; Gülşan, M.E. Mechanical properties and durability of unconfined and confined geopolymer concrete with fiber reinforced polymers exposed to sulfuric acid. Constr. Build. Mater. 2019, 215, 1015–1032. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Labrincha, J.; Leonelli, C.; Palomo, A.; Chindaprasit, P. (Eds.) Handbook of Alkali-Activated Cements, Mortars and Concretes; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Davidovits, J. Geopolymer Cement. A Review; Technical Papers; Geopolymer Institute: Saint-Quentin, France, 2013; Volume 21, pp. 1–11. [Google Scholar]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Taylor, H.F. Cement Chemistry, 2nd ed.; Thomas Telford: London, UK, 1997. [Google Scholar]
- Neville, A.M. Properties of Concrete; Longman: London, UK, 1995. [Google Scholar]
- Ibrahim, M.; Johari, M.A.M.; Rahman, M.K.; Maslehuddin, M. Effect of alkaline activators and binder content on the properties of natural pozzolan-based alkali activated concrete. Constr. Build. Mater. 2017, 147, 648–660. [Google Scholar] [CrossRef]
- Chen, W.; Brouwers, H.J.H. The hydration of slag, part 1: Reaction models for alkali-activated slag. J. Mater. Sci. 2007, 42, 428–443. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.C.; Van Deventer, J.S. The coexistence of geopolymeric gel and calcium silicate hydrate at the early stage of alkaline activation. Cem. Concr. Res. 2005, 35, 1688–1697. [Google Scholar] [CrossRef]
- Bernal, S.A.; Rodríguez, E.D.; de Gutiérrez, R.M.; Gordillo, M.; Provis, J.L. Mechanical and thermal characterisation of geopolymers based on silicate-activated metakaolin/slag blends. J. Mater. Sci. 2011, 46, 5477–5486. [Google Scholar] [CrossRef]
- Bernal, S.A.; Rodríguez, E.D.; de Gutiérrez, R.M.; Provis, J.L.; Delvasto, S. Activation of metakaolin/slag blends using alkaline solutions based on chemically modified silica fume and rice husk ash. Waste Biomass Valorization 2012, 3, 99–108. [Google Scholar] [CrossRef]
- Albidah, A.; Alghannam, M.; Alghamdi, H.; Abbas, H.; Almusallam, T.; Al-Salloum, Y. Influence of GGBFS and silica fume on characteristics of alkali-activated Metakaolin-based concrete. Eur. J. Environ. Civ. Eng. 2023, 27, 3260–3283. [Google Scholar] [CrossRef]
- Shi, C.; Shi, Z.; Hu, X.; Zhao, R.; Chong, L. A review on alkali-aggregate reactions in alkali-activated mortars/concretes made with alkali-reactive aggregates. Mater. Struct. 2015, 48, 621–628. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, K.; Li, Q.; Wang, J.; Ling, Y. Fabrication and Engineering Properties of Concretes Based on Geopolymers/Alkali-activated Binders—A Review. J. Clean. Prod. 2020, 258, 120896. [Google Scholar] [CrossRef]
- Mobili, A.; Telesca, A.; Marroccoli, M.; Tittarelli, F. Calcium sulfoaluminate and alkali-activated fly ash cements as alternative to Portland cement: Study on chemical, physical-mechanical, and durability properties of mortars with the same strength class. Constr. Build. Mater. 2020, 246, 118436. [Google Scholar] [CrossRef]
- Shahani, A.; Ahmadi, J.; Saeedi Razavi, B. The Effect of Using Alkaline Adhesives on the Durability of Lightweight Structural Concrete against Harmful Environments’. Sci. Q. J. Iran. Assoc. Eng. Geol. 2022, 15, 49–64. [Google Scholar]
- Xu, H.; Van Deventer, J.S.J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef]
- Lee, N.K.; Lee, H.K. Setting and mechanical properties of alkali-activated fly ash/slag concrete manufactured at room temperature. Constr. Build. Mater. 2013, 47, 1201–1209. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Rose, V.; De Gutierrez, R.M. Evolution of binder structure in sodium silicate-activated slag-metakaolin blends. Cem. Concr. Compos. 2011, 33, 46–54. [Google Scholar] [CrossRef]
- Wang, J.; Wu, X.L.; Wang, J.X.; Liu, C.Z.; Lai, Y.M.; Hong, Z.K.; Zheng, J.P. Hydrothermal synthesis and characterization of alkali-activated slag–fly ash–metakaolin cementitious materials. Microporous Mesoporous Mater. 2012, 155, 186–191. [Google Scholar] [CrossRef]
- Ahmed, H.U.; Mohammed, A.S.; Faraj, R.H.; Abdalla, A.A.; Qaidi, S.M.; Sor, N.H.; Mohammed, A.A. Innovative modeling techniques including MEP, ANN and FQ to forecast the compressive strength of geopolymer concrete modified with nanoparticles. Neural Comput. Appl. 2023, 35, 12453–12479. [Google Scholar] [CrossRef]
- Ahmed, H.U.; Mohammed, A.S.; Qaidi, S.M.; Faraj, R.H.; Hamah Sor, N.; Mohammed, A.A. Compressive strength of geopolymer concrete composites: A systematic comprehensive review, analysis and modeling. Eur. J. Environ. Civ. Eng. 2023, 27, 1383–1428. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Li, L. A review: The comparison between alkali-activated slag (Si+Ca) and metakaolin (Si+Al) cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Shi, C.; Stegemann, J.A. Acid corrosion resistance of different cementing materials. Cem. Concr. Res. 2000, 30, 803–808. [Google Scholar] [CrossRef]
- Aiken, T.A.; Kwasny, J.; Sha, W.; Soutsos, M.N. Effect of slag content and activator dosage on the resistance of fly ash geopolymer binders to sulfuric acid attack. Cem. Concr. Res. 2018, 111, 23–40. [Google Scholar] [CrossRef]
- Li, Z.; Lu, D.; Gao, X. Multi-objective optimization of gap-graded cement paste blended with supplementary cementitious materials using response surface methodology. Constr. Build. Mater. 2020, 248, 118552. [Google Scholar] [CrossRef]
- Verran, G.O.; Mendes, R.P.K.; Dalla Valentina, L.V.O. DOE applied to optimization of aluminum alloy die castings. J. Mater. Process. Technol. 2008, 200, 120–125. [Google Scholar] [CrossRef]
- Mohammed, B.S.; Haruna, S.; Liew, M.S. Optimization and characterization of cast in-situ alkali-activated pastes by response surface methodology. Constr. Build. Mater. 2019, 225, 776–787. [Google Scholar] [CrossRef]
- Zhang, L.; Yue, Y. Influence of waste glass powder usage on the properties of alkali-activated slag mortars based on response surface methodology. Constr. Build. Mater. 2018, 181, 527–534. [Google Scholar] [CrossRef]
- C 127; Standard Test Method for Density, Relative Density and Absorption of Coarse Aggregate. American Society for Testing and Materials: West Conshohocken, PA, USA, 2003.
- ASTM C33-03; Standard Specification for Concrete Aggregate. Annual Book of ASTM Standards. American Society for Testing and Materials: West Conshohocken, PA, USA, 2003.
- Rilem, T.C. 116-PCD. Permeability of concrete as a criterion of its durability. Mater. Struct. 1999, 2, 174–179. [Google Scholar]
- BS 1881:Part 116:1983; Method for Determination of Compressive Strength of Concrete Cubes. British Standards Institution: London, UK, 1983.
- BS 1881:Part 122:1983; Method of Determine of Water Absorption. British Standards Institution: London, UK, 1983.
- ASTM C 642-90; Standard Test Method for Specific Gravity, Absorption, and Voids in Hardened Concrete. Annual Book of ASTM Standard. American Society for Testing and Materials: West Conshohocken, PA, USA, 1990.
- Xiang, J.; He, Y.; Liu, L.; Zheng, H.; Cui, X. Exothermic behavior and drying shrinkage of alkali-activated slag concrete by low temperature-preparation method. Constr. Build. Mater. 2020, 262, 120056. [Google Scholar] [CrossRef]
- Zamanabadi, S.N.; Zareei, S.A.; Shoaei, P.; Ameri, F. Ambient-cured alkali-activated slag paste incorporating micro-silica as repair material: Effects of alkali activator solution on physical and mechanical properties. Constr. Build. Mater. 2019, 229, 116911. [Google Scholar] [CrossRef]
- Davidovits, J. Why Alkali-Activated Materials (AAM) Are Not Geopolymers; Publication Technical Paper; Geopolymer Institute Library: Saint-Quentin, France, 2018; Volume 25. [Google Scholar]
- Liu, J.; Doh, J.H.; Ong, D.E.; Liu, Z.; Hadi, M.N. Methods to evaluate and quantify the geopolymerization reactivity of waste-derived aluminosilicate precursor in alkali-activated material: A state-of-the-art review. Constr. Build. Mater. 2023, 362, 129784. [Google Scholar] [CrossRef]
- Marvila, M.T.; Azevedo, A.R.; Vieira, C.M. Reaction mechanisms of alkali-activated materials. Rev. IBRACON Estrut. Mater. 2021, 14, e14309. [Google Scholar] [CrossRef]
- Hojati, M.; Radlińska, A. Shrinkage and strength development of alkali-activated fly ash-slag binary cements. Constr. Build. Mater. 2017, 150, 808–816. [Google Scholar] [CrossRef]
- Lee, N.K.; Jang, J.G.; Lee, H.K. Shrinkage characteristics of alkali-activated fly ash/slag paste and mortar at early ages. Cem. Concr. Compos. 2014, 53, 239–248. [Google Scholar] [CrossRef]
- Najimi, M.; Ghafoori, N. Engineering properties of natural pozzolan/slag based alkali-activated concrete. Constr. Build. Mater. 2019, 208, 46–62. [Google Scholar] [CrossRef]
- Wang, A.; Zheng, Y.; Zhang, Z.; Liu, K.; Li, Y.; Shi, L.; Sun, D. The durability of alkali-activated materials in comparison with ordinary Portland cements and concretes: A review. Engineering 2020, 6, 695–706. [Google Scholar] [CrossRef]
- Mohammed, B.S.; Achara, B.E.; Nuruddin, M.F.; Yaw, M.; Zulkefli, M.Z. Properties of nano-silica-modified self-compacting engineered cementitious composites. J. Clean. Prod. 2017, 162, 1225–1238. [Google Scholar] [CrossRef]
- Aydın, S. A ternary optimization of mineral additives of alkali activated cement mortars. Constr. Build. Mater. 2013, 43, 131–138. [Google Scholar] [CrossRef]







| Material | SiO2 | Al2O3 | CaO | Fe2O3 | MgO | SO3 | MnO | Etc. |
|---|---|---|---|---|---|---|---|---|
| GGBFS | 35.43 | 9.17 | 38.23 | 0.81 | 8.67 | 2.5 | 1.34 | 3.85 |
| Metakaolin | 48.00 | 41.00 | 3.10 | 1.30 | 1.80 | 0.20 | - | 4.6 |
| Silica fume | 96.4 | 1.32 | 0.49 | 0.87 | 0.97 | 0.10 | - | - |
| Mixture | Slag | Metakaolin | Silica Fume |
|---|---|---|---|
| GGBFS | 408 | - | - |
| SF5 | 387.6 | - | 20.4 |
| SF10 | 367.2 | - | 40.8 |
| MK10 | 367.2 | 40.8 | - |
| MK10SF5 | 346.8 | 40.8 | 20.4 |
| MK10SF10 | 326.4 | 40.8 | 40.8 |
| MK20 | 326.4 | 81.6 | - |
| MK20SF5 | 306 | 81.6 | 20.4 |
| MK20SF10 | 285.6 | 81.6 | 40.8 |
| MK30 | 285.6 | 122.4 | - |
| MK30SF5 | 265.2 | 122.4 | 20.4 |
| MK30SF10 | 244.8 | 122.4 | 40.8 |
| MK40 | 244.8 | 163.2 | - |
| MK40SF5 | 214 | 163.2 | 20.4 |
| MK40SF10 | 204 | 163.2 | 40.8 |
| Mix | Compressive Strength (MPa) | Water Absorption (%) | ||||
|---|---|---|---|---|---|---|
| Short Time | Ultimate | |||||
| 7 Days | 28 Days | 7 Days | 28 Days | 7 Days | 28 Days | |
| GGBFS | 50.2 | 63 | 3.77 | 3.09 | 4.2 | 3.38 |
| SF5 | 50.8 | 60 | 3.4 | 2.89 | 3.73 | 3.18 |
| SF10 | 47.9 | 57.3 | 3.43 | 2.41 | 3.78 | 2.78 |
| MK10 | 39.7 | 55.5 | 3.99 | 3.43 | 4.47 | 3.82 |
| MK10SF5 | 37.4 | 49.8 | 4.01 | 3.37 | 4.41 | 3.59 |
| MK10SF10 | 34.4 | 47.1 | 3.68 | 3.11 | 4.01 | 3.41 |
| MK20 | 35.4 | 50.1 | 4.39 | 3.73 | 4.78 | 4.14 |
| MK20SF5 | 31.8 | 44 | 3.96 | 3.26 | 4.36 | 3.66 |
| MK20SF10 | 31.2 | 43.5 | 3.78 | 3.11 | 4.18 | 3.45 |
| MK30 | 32.3 | 47.3 | 4.54 | 3.89 | 4.96 | 4.31 |
| MK30SF5 | 29.8 | 42.6 | 4.31 | 3.71 | 4.79 | 4.09 |
| MK30SF10 | 29.8 | 42.4 | 4.05 | 3.41 | 4.42 | 3.63 |
| MK40 | 30.7 | 45.4 | 4.66 | 3.96 | 5.1 | 4.32 |
| MK40SF5 | 28.9 | 41 | 4.41 | 3.81 | 4.98 | 4.14 |
| MK40SF10 | 30 | 41.9 | 3.95 | 3.39 | 4.38 | 3.68 |
| Mix Design | 7 Days (×10−2) | i (×10−3) | S | 28 Days (×10−2) | i (×10−3) | S | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gr | Gr/cm2 | Gr/cm2 | Gr | Gr/cm2 | Gr/cm2 | |||||||
| 3 h | 6 h | 24 h | 72 h | /min | 3 h | 6 h | 24 h | 72 h | /min | |||
| GGBFS | 10,150 | 11,150 | 11,750 | 11,325 | 5.63 | 1.13 | 4575 | 6800 | 8050 | 8775 | 2.54 | 0.87 |
| SF5 | 10,000 | 10,200 | 10,425 | 10,650 | 5.55 | 1.06 | 4500 | 6000 | 6250 | 6475 | 2.50 | 0.64 |
| SF10 | 9600 | 9800 | 10,000 | 10,150 | 5.33 | 1.01 | 4800 | 6700 | 7050 | 7175 | 2.66 | 0.71 |
| MK10 | 9400 | 10,550 | 11,450 | 11,525 | 5.22 | 1.15 | 4900 | 6950 | 8750 | 9250 | 2.72 | 0.92 |
| MK10SF5 | 10,900 | 11,750 | 11,850 | 11,900 | 6.05 | 1.19 | 6225 | 8500 | 8650 | 8800 | 3.45 | 0.88 |
| MK10SF10 | 11,000 | 11,300 | 11,400 | 11,400 | 6.11 | 1.14 | 6025 | 8850 | 9300 | 9450 | 3.34 | 0.94 |
| MK20 | 10,850 | 11,275 | 11,400 | 11,500 | 6.02 | 1.15 | 6050 | 9150 | 9500 | 9675 | 3.36 | 0.96 |
| MK20SF5 | 10,550 | 11,300 | 11,600 | 11,700 | 5.86 | 1.17 | 6450 | 8800 | 9050 | 9200 | 3.58 | 0.92 |
| MK20SF10 | 11,150 | 11,300 | 11,400 | 11,450 | 6.19 | 1.14 | 5700 | 7900 | 8250 | 8500 | 3.16 | 0.85 |
| MK30 | 11,600 | 11,750 | 11,950 | 12,000 | 6.44 | 1.20 | 6275 | 6375 | 9700 | 9850 | 3.48 | 0.98 |
| MK30SF5 | 10,800 | 11,100 | 11,300 | 11,450 | 6.00 | 1.14 | 5775 | 8550 | 8775 | 9000 | 3.20 | 0.90 |
| MK30SF10 | 10,875 | 11,300 | 11,450 | 11,650 | 6.04 | 1.16 | 6150 | 9150 | 9275 | 9375 | 3.41 | 0.937 |
| MK40 | 10,950 | 11,375 | 11,775 | 11,950 | 6.08 | 1.19 | 6250 | 9150 | 9375 | 9650 | 3.47 | 0.96 |
| MK40SF5 | 10,675 | 11,100 | 11,350 | 11,500 | 5.93 | 1.15 | 5775 | 8675 | 8800 | 9300 | 3.20 | 0.93 |
| MK40SF10 | 10,750 | 11,150 | 11,400 | 11,600 | 5.97 | 1.16 | 5900 | 8850 | 8950 | 9175 | 3.27 | 0.91 |
| Oxygen (O) | Sodium (Na) | Aluminum (Al) | Silicon (Si) | Calcium (Ca) | Magnesium (Mg) | Sulfur (S) | Potassium (K) | Titanium (Ti) | Iron (Fe) | |
|---|---|---|---|---|---|---|---|---|---|---|
| % | ||||||||||
| Phase A | 53.08 | 0.91 | 10.75 | 25.83 | 0.63 | 0.61 | - | 5.09 | - | 3.11 |
| Phase B | 56.40 | 2.79 | 3.66 | 16.25 | 14.8 | 1.4 | 2.91 | 0.5 | 0.87 | 0.39 |
| RUN | Factor 1 | Factor 2 | Response 1 | Response 2 | Response 3 | |||
|---|---|---|---|---|---|---|---|---|
| A:MK | B:SF | Compressive Strength | Compressive Strength Reduction | Water Absorption (Ultimate) | ||||
| % | % | (MPa) | (%) | (%) | ||||
| Actual Value | Predicted Value | Actual Value | Predicted Value | Actual Value | Predicted Value | |||
| 1 | 40 | 0 | 45.4 | 45.36 | −8.3 | −8.44 | 4.32 | 4.41 |
| 2 | 0 | 5 | 60 | 59.99 | −13.3 | −13.87 | 3.18 | 3.12 |
| 3 | 10 | 5 | 49.5 | 49.19 | −15.5 | −15.56 | 3.59 | 3.49 |
| 4 | 10 | 10 | 47.1 | 47.32 | −13.7 | −13.08 | 3.41 | 3.16 |
| 5 | 20 | 5 | 44.2 | 44.29 | −11.5 | −11.39 | 3.7 | 3.78 |
| 6 | 20 | 10 | 43.5 | 43.24 | −8.5 | −9.29 | 3.45 | 3.45 |
| 7 | 10 | 0 | 55.5 | 55.63 | −20.1 | −20.09 | 3.82 | 3.82 |
| 8 | 0 | 10 | 57.3 | 57.3 | −11.1 | −11.00 | 2.78 | 2.79 |
| 9 | 40 | 5 | 41 | 41.38 | −5 | −5.05 | 4.17 | 4.08 |
| 10 | 30 | 10 | 42.4 | 42.36 | −4.9 | −4.53 | 3.63 | 3.65 |
| 11 | 30 | 0 | 47.3 | 47.39 | −9.87 | −10.02 | 4.31 | 4.30 |
| 12 | 40 | 10 | 41.9 | 41.97 | −3.4 | −3.71 | 3.68 | 3.76 |
| 13 | 20 | 0 | 50.1 | 49.91 | −15.5 | −15.54 | 4.14 | 4.10 |
| 14 | 40 | 5 | 41.8 | 41.38 | −5.4 | −5.05 | 4.14 | 4.08 |
| 15 | 20 | 5 | 44 | 44.29 | −11.27 | −11.39 | 3.66 | 3.78 |
| 16 | 30 | 5 | 42.6 | 42.59 | −6.6 | −6.25 | 4.09 | 3.97 |
| 17 | 0 | 0 | 63 | 64.48 | −19.1 | −18.78 | 3.38 | 3.45 |
| Responses | Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|---|
| Compressive strength (MPa) | Model | 523.68 | 8 | 65.46 | 672.94 | <0.0001 | significant |
| A-Mt | 19.55 | 1 | 19.65 | 200.97 | <0.0001 | ||
| B-Sf | 42.62 | 1 | 42.62 | 438.15 | <0.0001 | ||
| AB | 5.58 | 1 | 5.58 | 57.32 | 0.0001 | ||
| A2 | 77.10 | 1 | 77.10 | 792.59 | <0.0001 | ||
| B2 | 16.81 | 1 | 16.81 | 172.82 | <0.0001 | ||
| A2B | 0.0046 | 1 | 0.0046 | 0.0475 | 0.8337 | ||
| AB2 | 0.0033 | 1 | 0.0033 | 0.0340 | 0.8590 | ||
| A3 | 7.10 | 1 | 7.10 | 72.97 | <0.0001 | ||
| B3 | 0.0000 | 0 | |||||
| Residual | 0.6809 | 7 | 0.0973 | ||||
| Lack of Fit | 0.3409 | 5 | 0.0682 | 0.4011 | 0.8226 | not significant | |
| Pure Error | 0.3400 | 2 | 0.1700 | ||||
| Cor Total | 524.36 | 15 | |||||
| Compressive strength reduction (%) | Model | 396.01 | 8 | 49.50 | 282.24 | <0.0001 | significant |
| A-Mt | 77.31 | 1 | 77.31 | 440.81 | <0.0001 | ||
| B-Sf | 39.48 | 1 | 39.48 | 225.07 | <0.0001 | ||
| AB | 2.91 | 1 | 2.91 | 16.60 | 0.0036 | ||
| A2 | 12.02 | 1 | 12.02 | 68.55 | <0.0001 | ||
| B2 | 4.57 | 1 | 4.57 | 26.04 | 0.0009 | ||
| A2B | 0.0066 | 1 | 0.0066 | 0.0377 | 0.8510 | ||
| AB2 | 0.5826 | 1 | 0.5826 | 3.32 | 0.1058 | ||
| A3 | 28.91 | 1 | 28.91 | 164.81 | <0.0001 | ||
| B3 | 0.0000 | 0 | |||||
| Residual | 1.40 | 8 | 0.1754 | ||||
| Lack of Fit | 1.30 | 6 | 0.2161 | 4.06 | 0.2108 | not significant | |
| Pure Error | 0.1065 | 2 | 0.0532 | ||||
| Cor Total | 397.42 | 16 | |||||
| Water absorption ultimate (%) | Model | 2.72 | 5 | 0.5447 | 84.58 | <0.0001 | significant |
| A-Mt | 1.83 | 1 | 1.83 | 284.64 | <0.0001 | ||
| B-Sf | 0.9453 | 1 | 0.9453 | 146.80 | <0.0001 | ||
| AB | 0.0003 | 1 | 0.0003 | 0.0517 | 0.8247 | ||
| A2 | 0.0890 | 1 | 0.0890 | 13.81 | 0.0040 | ||
| B2 | 0.0122 | 1 | 0.0122 | 1.90 | 0.1981 | ||
| Residual | 0.0644 | 10 | 0.0064 | ||||
| Lack of Fit | 0.0631 | 8 | 0.0079 | 12.63 | 0.0754 | not significant | |
| Pure Error | 0.0013 | 2 | 0.0006 | ||||
| Cor Total | 2.79 | 15 | |||||
| Model | 2.72 | 5 | 0.5447 | 84.58 | <0.0001 | significant | |
| A-Mt | 1.83 | 1 | 1.83 | 284.64 | <0.0001 | ||
| B-Sf | 0.9453 | 1 | 0.9453 | 146.80 | <0.0001 | ||
| AB | 0.0003 | 1 | 0.0003 | 0.0517 | 0.8247 |
| Response | Compressive Strength (MPa) | Compressive Strength Reduction (%) | Water Absorption Ultimate (%) |
|---|---|---|---|
| Standard deviation | 0.2777 | 0.4464 | 0.0802 |
| Mean | 47.10 | −10.77 | 3.75 |
| C.V % | 0.5897 | 4.15 | 2.14 |
| R2 | 0.9987 | 0.9950 | 0.9723 |
| Adjusted R2 | 0.9978 | 0.9920 | 0.9654 |
| Predicted R2 | 0.9968 | 0.9844 | 0.9532 |
| Adequate precision | 101.3066 | 57.2138 | 40.2918 |
| Factors and Responses | Goals | Lower Limit | Upper Limit | Importance |
|---|---|---|---|---|
| MK | In range | 0 | 40 | 3 |
| SF | In range | 0 | 10 | 3 |
| Compressive strength (MPa) | In range | 41 | 60 | 3 |
| Strength reduction (%) | Maximize | −20.1 | −3.4 | 5 |
| Water absorption ultimate (%) | Minimize | 2.78 | 4.32 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azmakan, A.; Ahmadi, J.; Shahani, A.; Badarloo, B.; Garbowski, T. Optimal Quantity Investigation of Metakaolin and Silica Fume in Production of Durable Acid Resistance Alkali Activated Slag Concrete. Buildings 2024, 14, 21. https://doi.org/10.3390/buildings14010021
Azmakan A, Ahmadi J, Shahani A, Badarloo B, Garbowski T. Optimal Quantity Investigation of Metakaolin and Silica Fume in Production of Durable Acid Resistance Alkali Activated Slag Concrete. Buildings. 2024; 14(1):21. https://doi.org/10.3390/buildings14010021
Chicago/Turabian StyleAzmakan, Abolfazl, Jamal Ahmadi, Arash Shahani, Baitollah Badarloo, and Tomasz Garbowski. 2024. "Optimal Quantity Investigation of Metakaolin and Silica Fume in Production of Durable Acid Resistance Alkali Activated Slag Concrete" Buildings 14, no. 1: 21. https://doi.org/10.3390/buildings14010021
APA StyleAzmakan, A., Ahmadi, J., Shahani, A., Badarloo, B., & Garbowski, T. (2024). Optimal Quantity Investigation of Metakaolin and Silica Fume in Production of Durable Acid Resistance Alkali Activated Slag Concrete. Buildings, 14(1), 21. https://doi.org/10.3390/buildings14010021










