The Early Age Hydration Products and Mechanical Properties of Cement Paste with Steel Slag Powder as Additive under Steam Curing Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Test Methods
3. Results
3.1. Water Requirement, Soundness and Setting Time
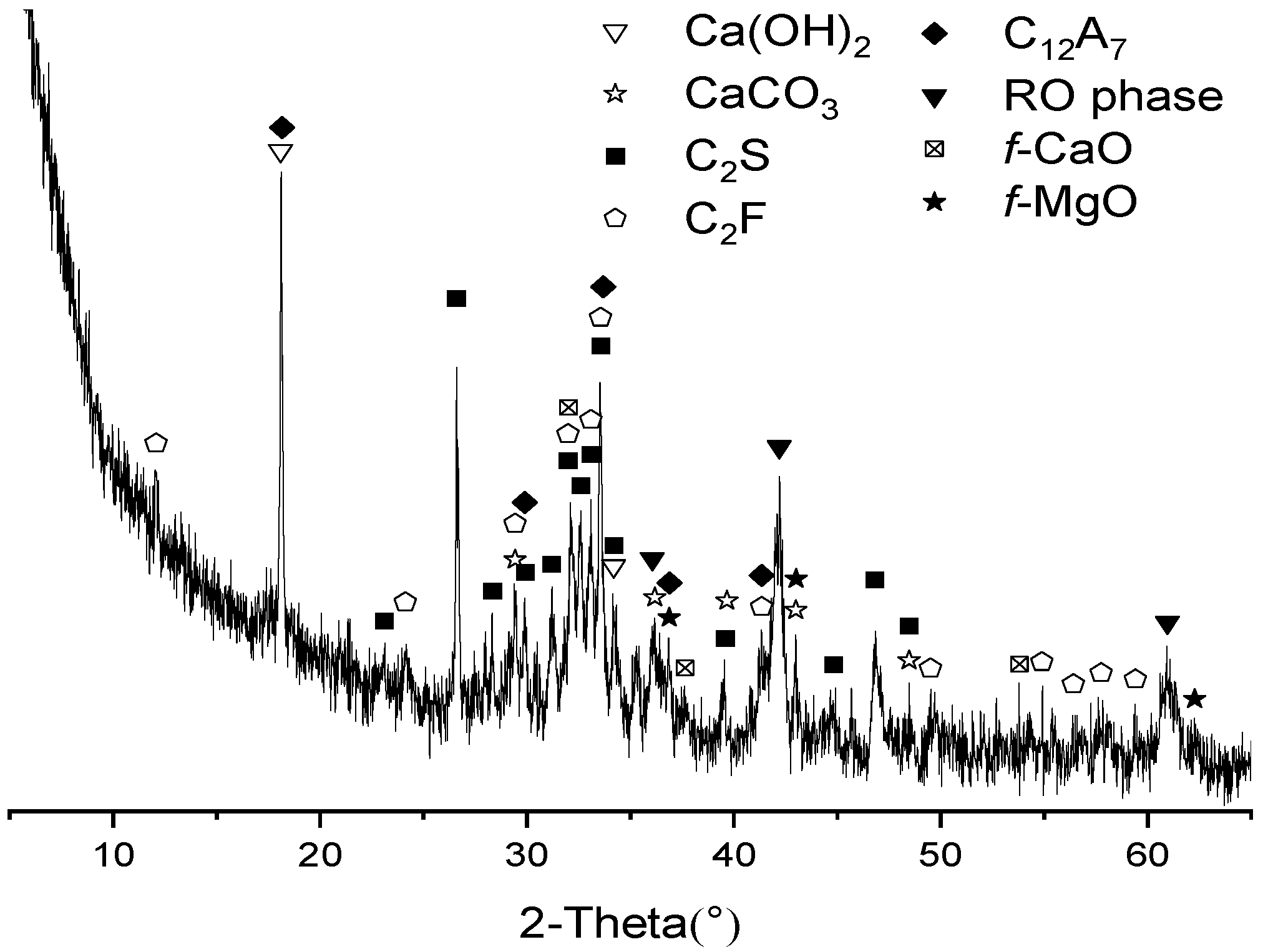
3.2. XRD Analysis of Hydrates
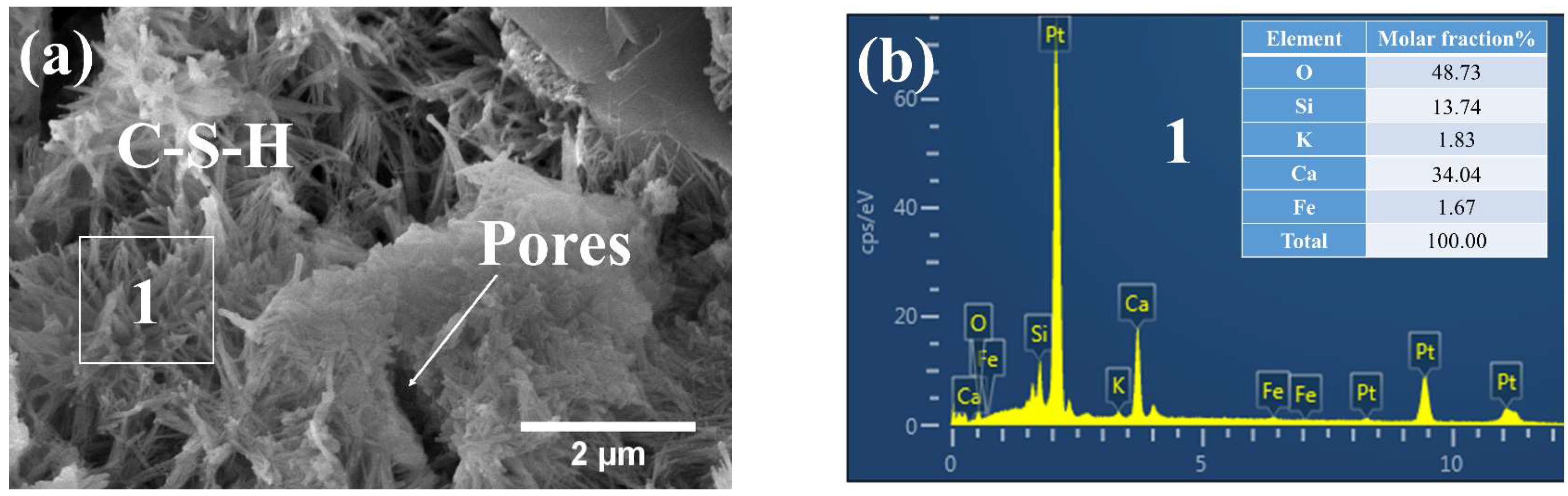
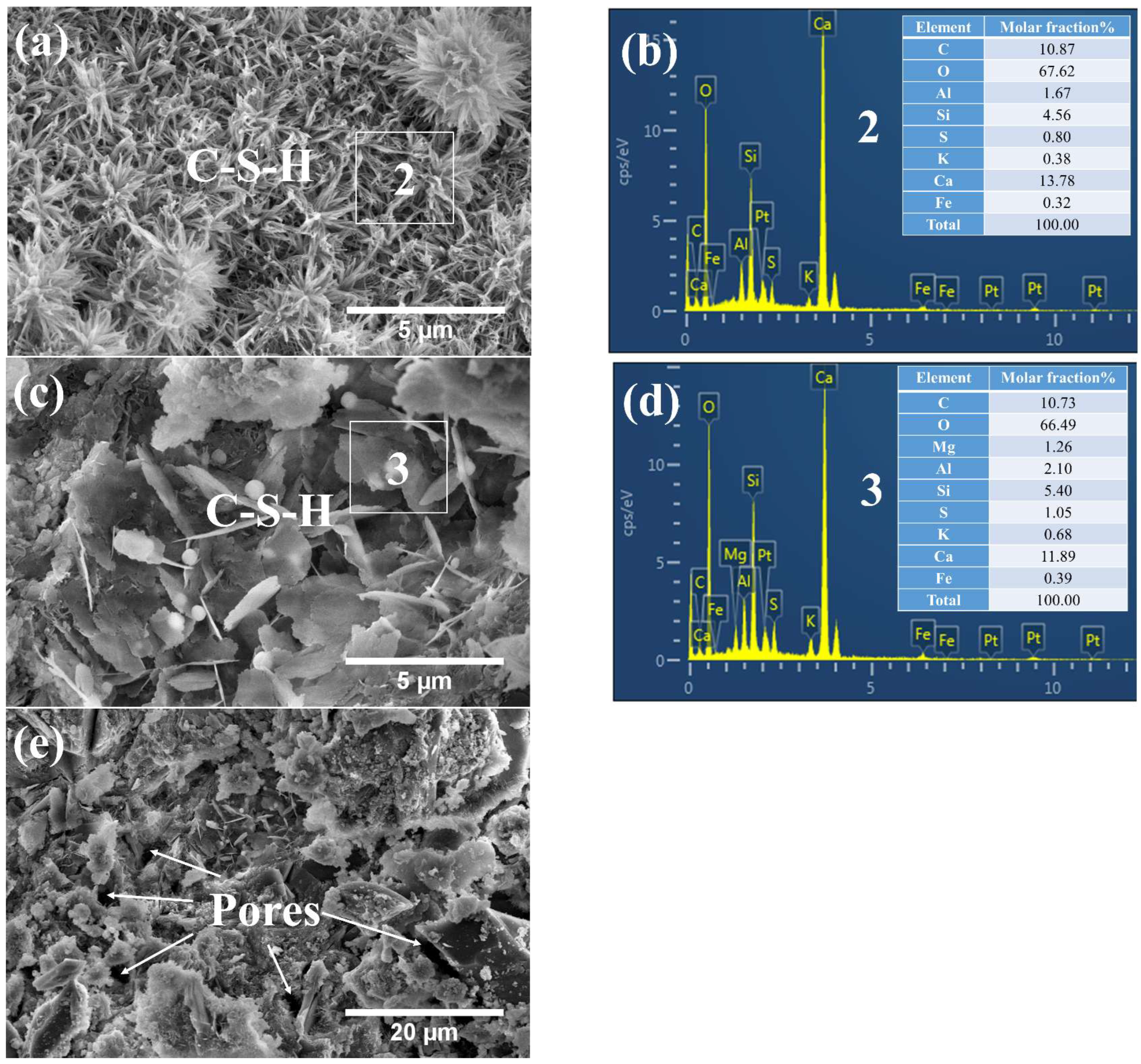
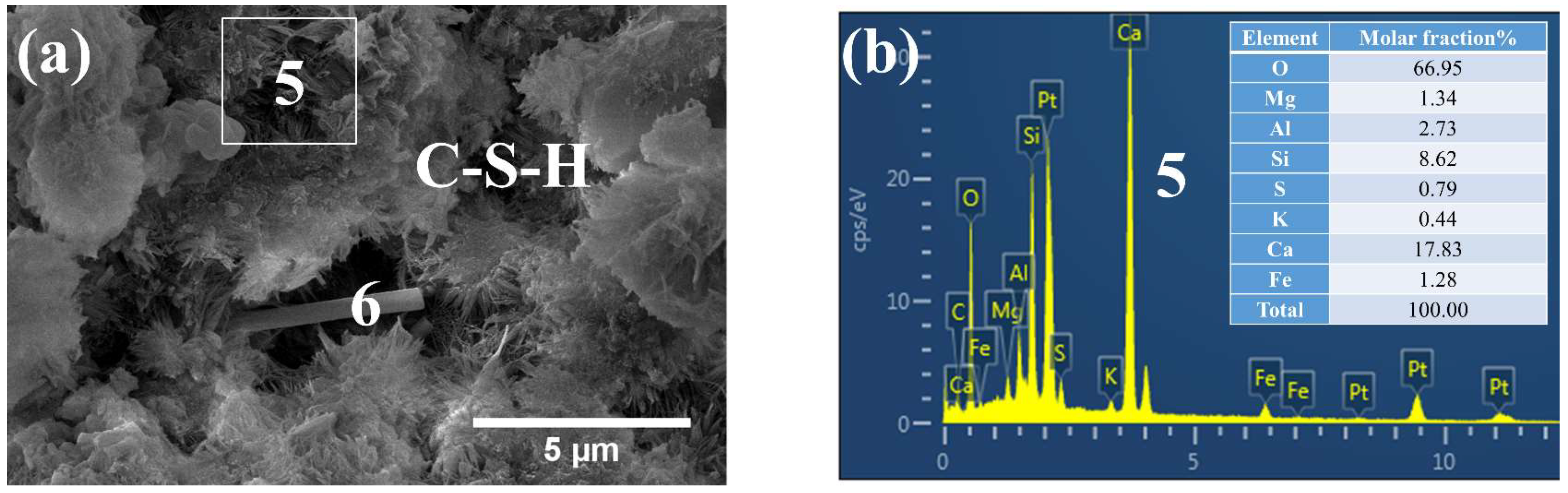
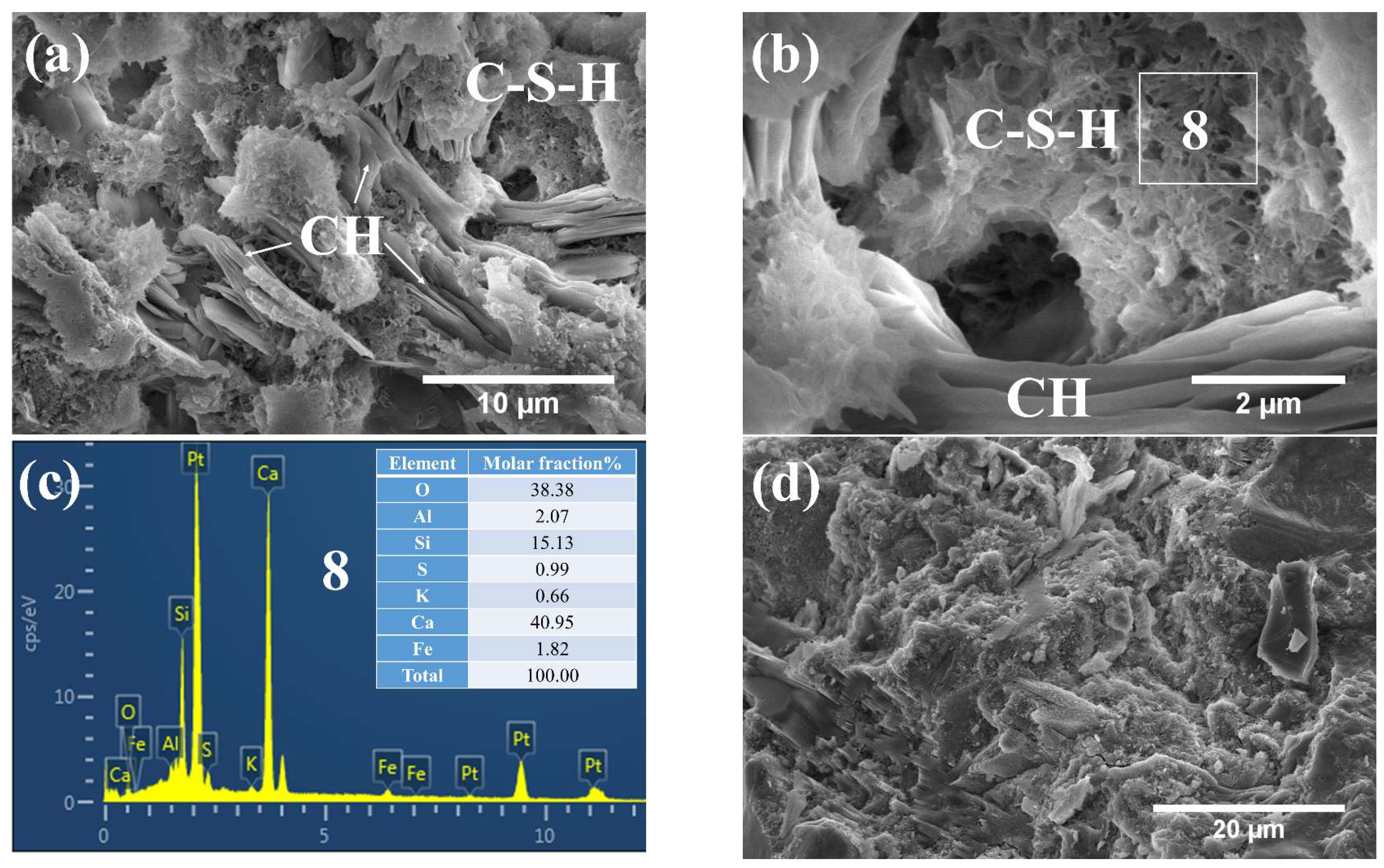
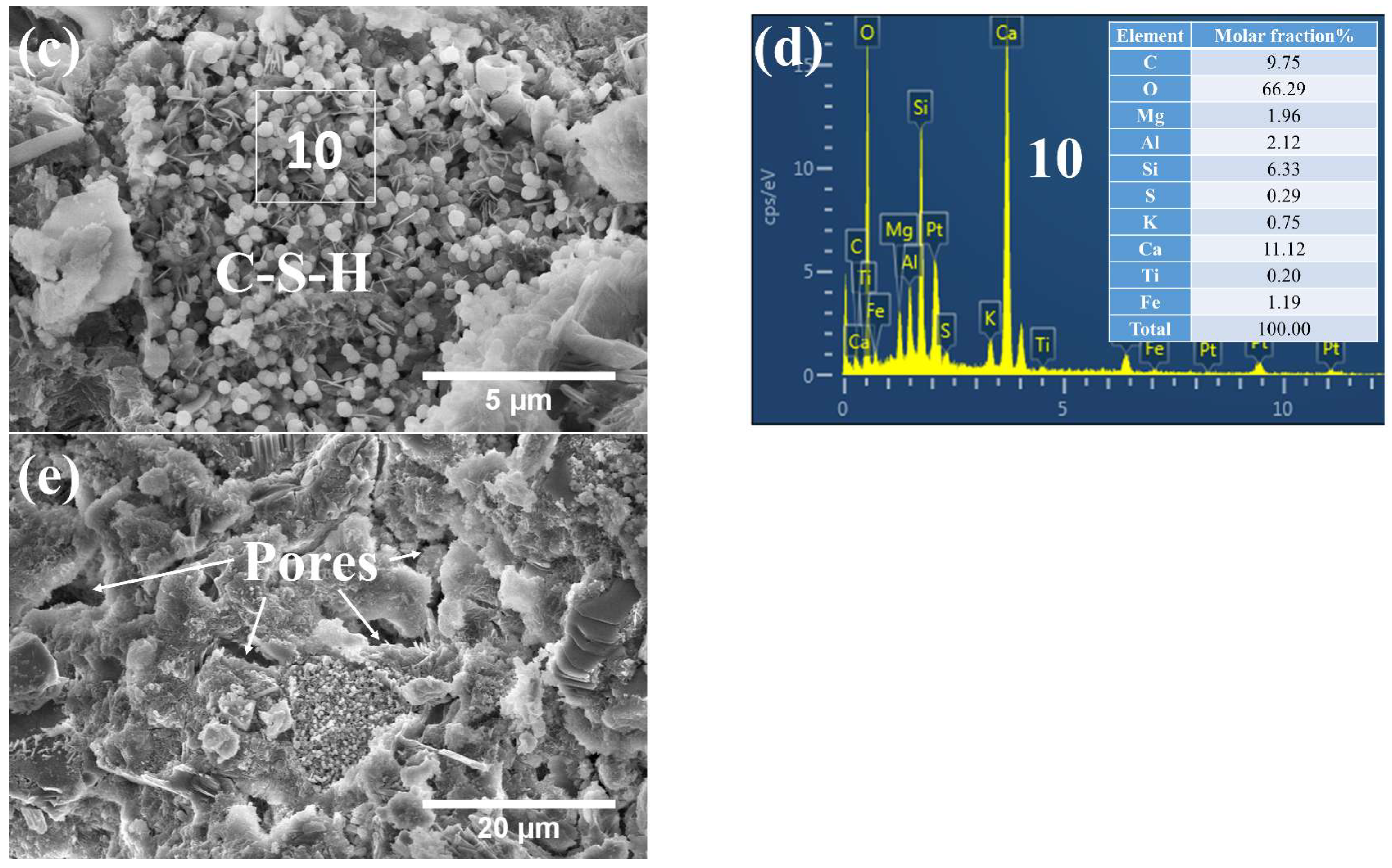
3.3. Phases and Microstructure under SEM-EDS
3.3.1. Phases and Microstructure under S7h
3.3.2. Phases and Microstructure under S7d
3.3.3. Phases and Microstructure under N28d
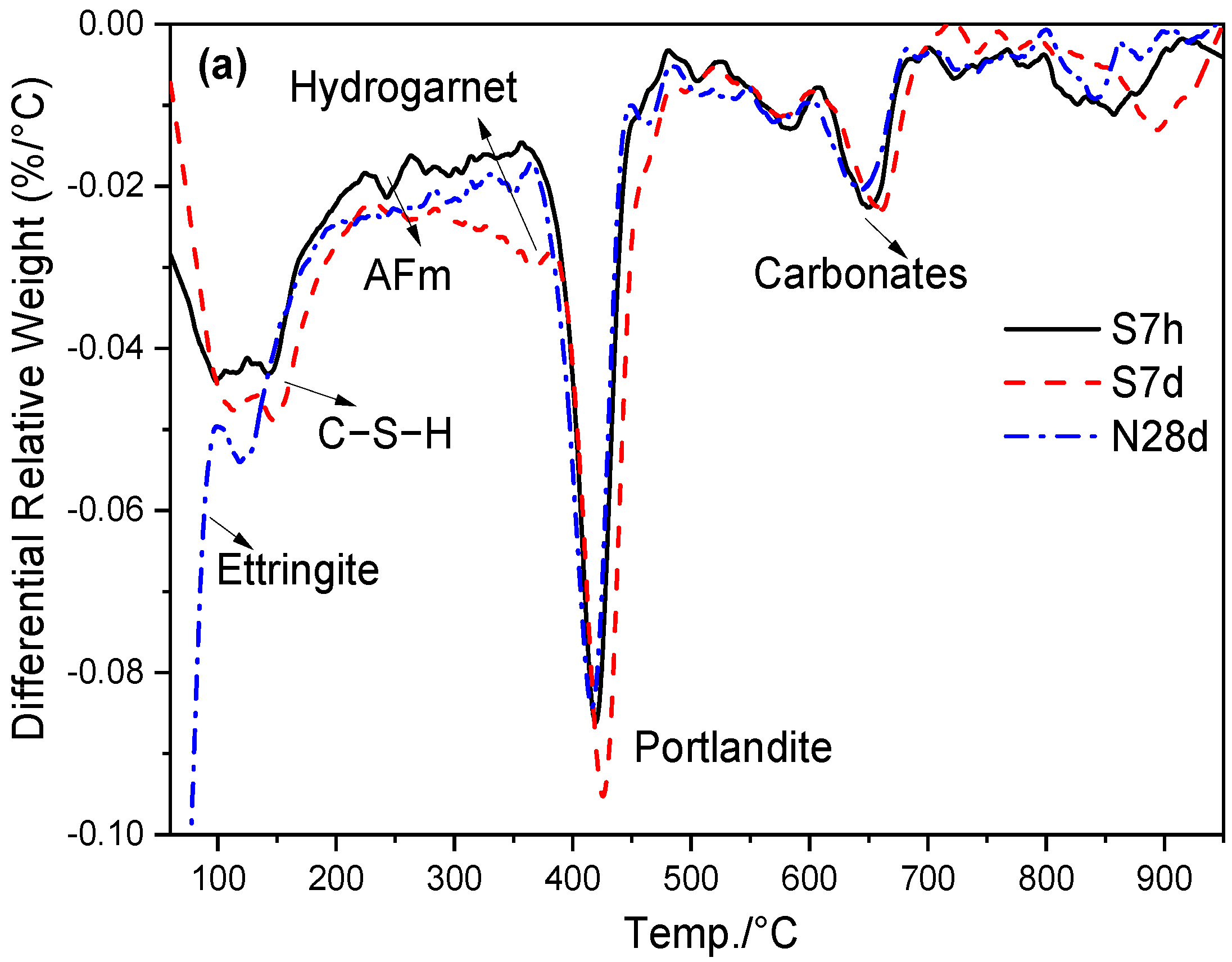
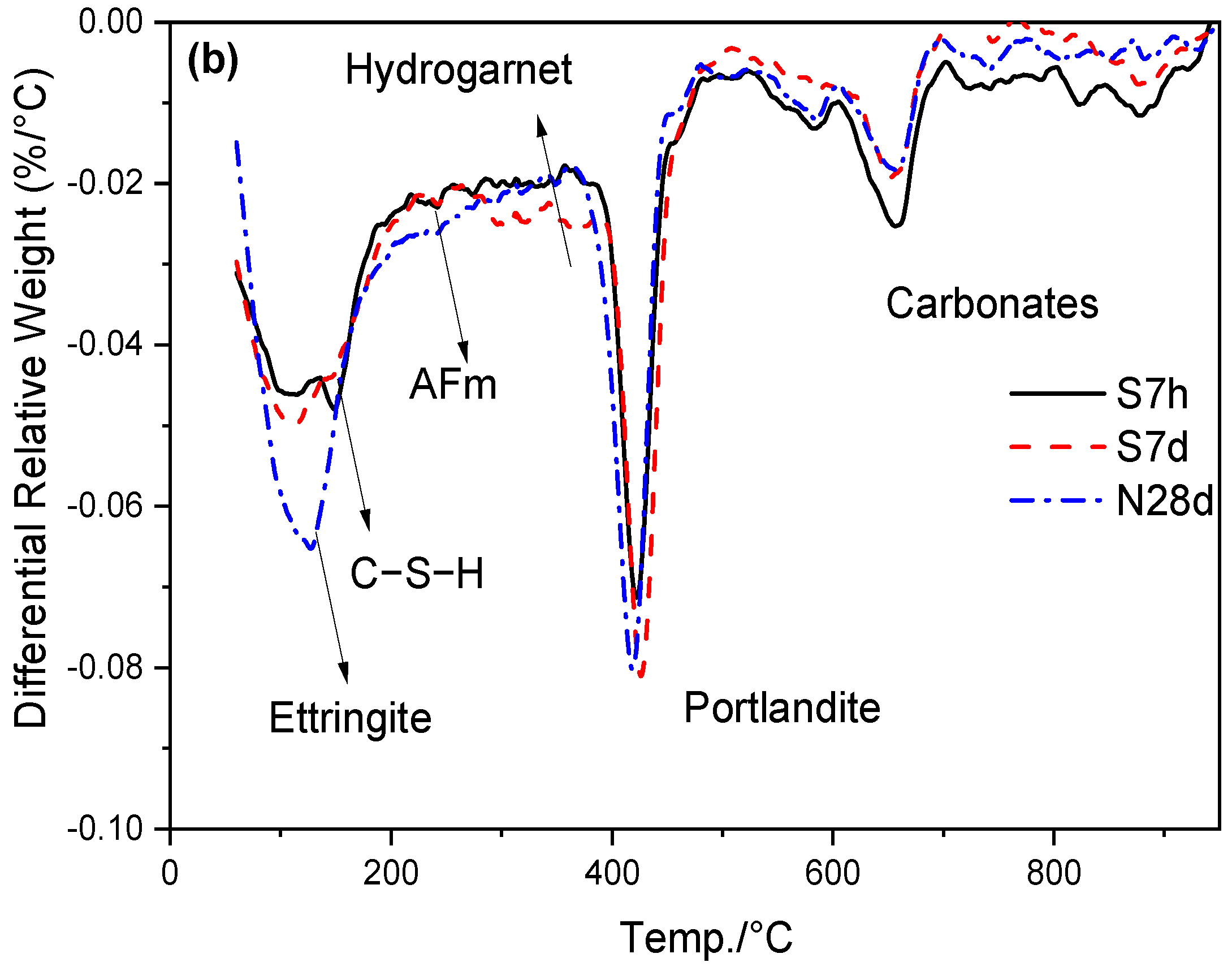
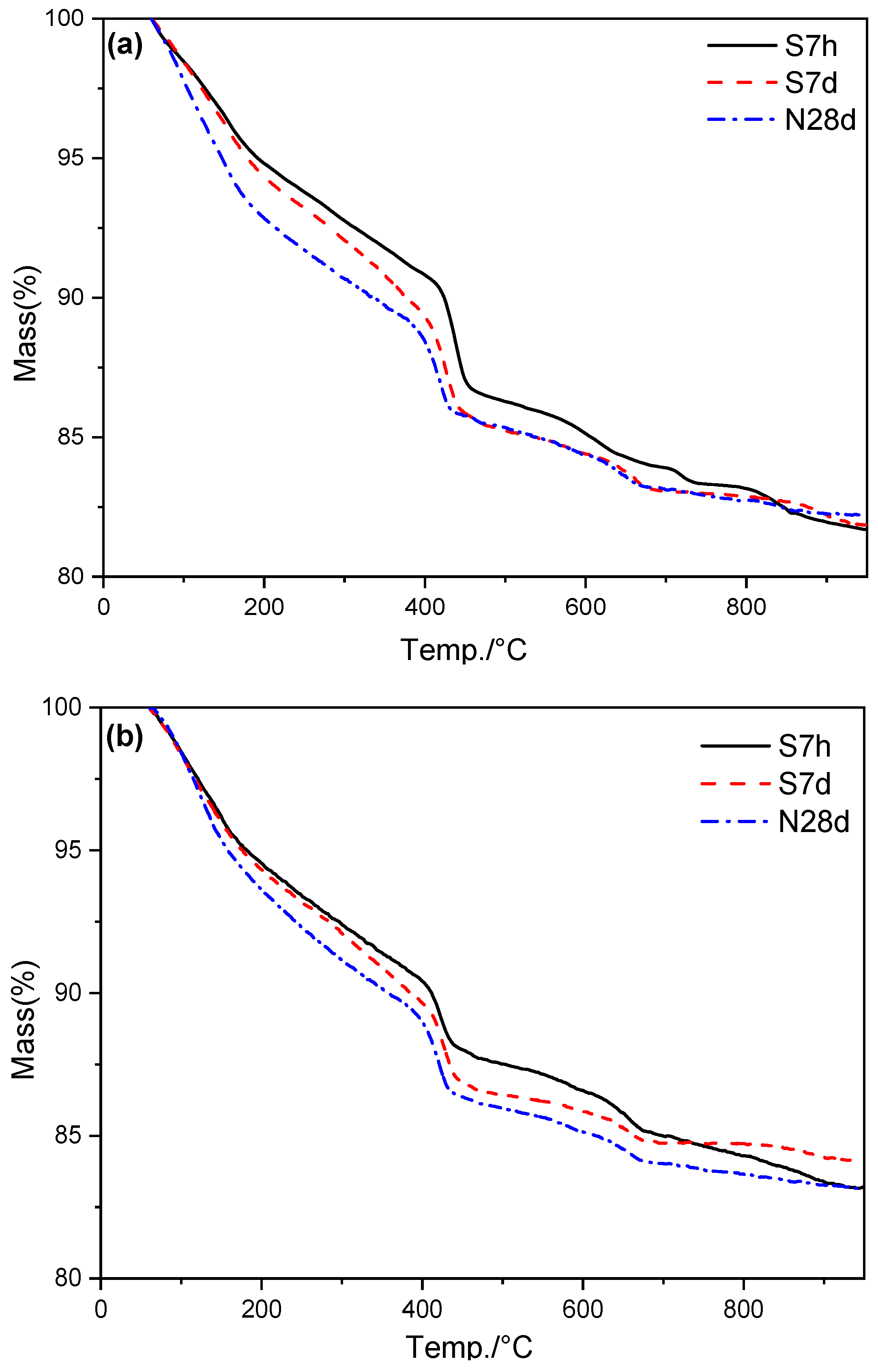
3.4. Hydrates Measured by TG/DTG
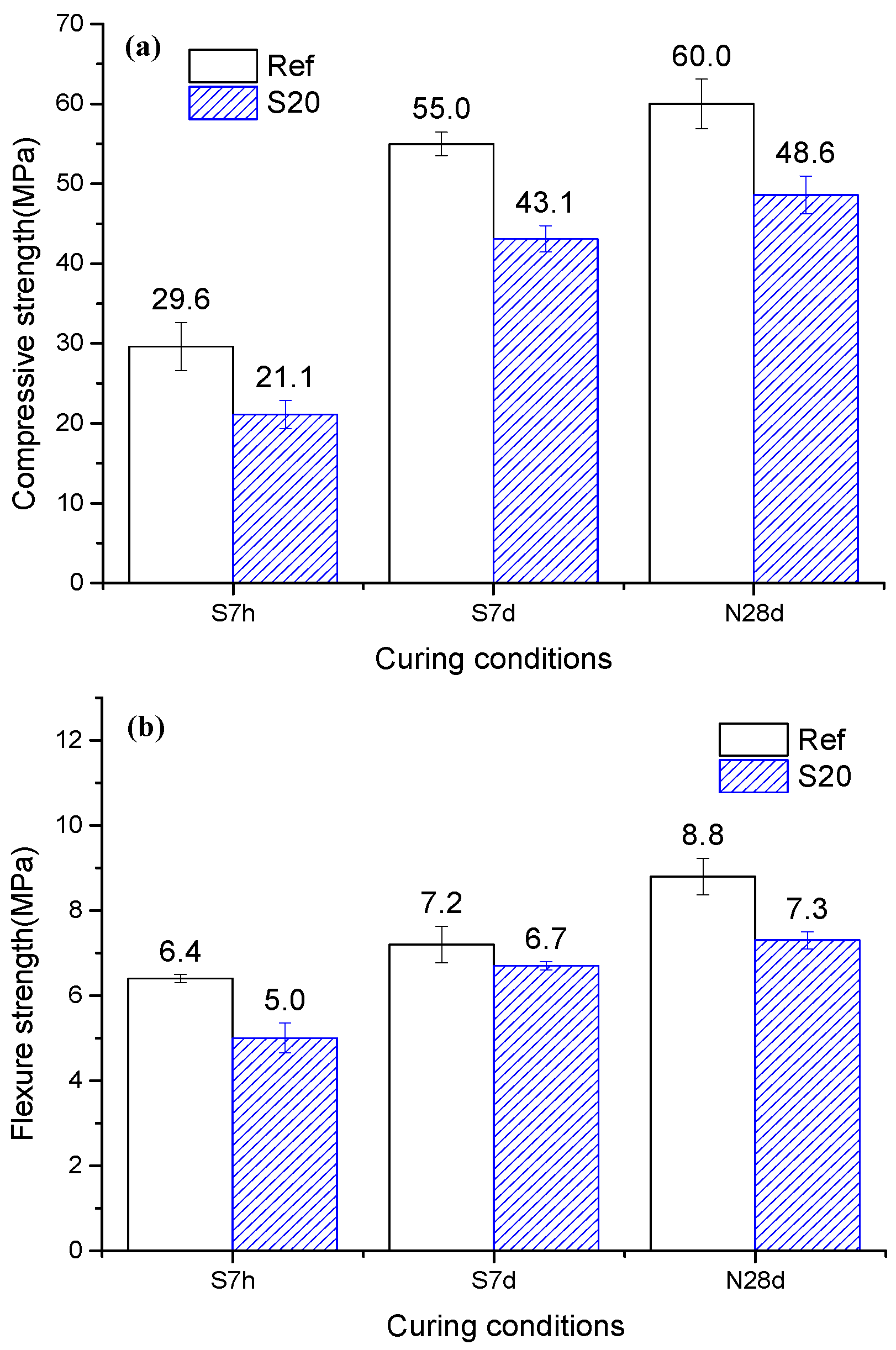
3.5. Compressive Strength and Flexural Strength
4. Discussion
4.1. Phases and Microstructure
4.2. Mechanical Property
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, Y.; Ling, T.; Shi, C.; Pan, S. Characteristics of steel slags and their use in cement and concrete—A review. Resour. Conserv. Recycl. 2018, 136, 187–197. [Google Scholar] [CrossRef]
- Zhang, T.; Yu, Q.; Wei, J.; Li, J.; Zhang, P. Preparation of high performance blended cements and reclamation of iron concentrate from basic oxygen furnace steel slag. Resour. Conserv. Recycl. 2011, 56, 48–55. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, G.; Cheng, H.; Wang, J.; Wan, Y.; Chen, H. An overview of utilization of steel slag. Procedia Environ. Sci. 2012, 16, 791–801. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, S.; Wang, L.; Chen, P.; Shao, D.; Tang, S.; Li, J. High-ferrite Portland cement with slag: Hydration, microstructure, and resistance to sulfate attack at elevated temperature. Cem. Concr. Compos. 2022, 130, 104560. [Google Scholar] [CrossRef]
- Martins, A.; De Carvalho, J.; Costa, L.; Andrade, H.; de Melo, T.; Ribeiro, J.; Pedroti, L.; Peixoto, R. Steel slags in cement-based composites: An ultimate review on characterization, applications and performance. Constr. Build. Mater. 2021, 291, 123265. [Google Scholar] [CrossRef]
- Franco, L.; Mendes, J.; Costa, L.; Pira, R.; Peixoto, R. Design and thermal evaluation of a social housing model conceived with bioclimatic principles and recycled aggregates. Sustain. Cities Soc. 2019, 51, 101725. [Google Scholar] [CrossRef]
- Dong, Q.; Wang, G.; Chen, X.; Tan, J.; Gu, X. Recycling of steel slag aggregate in portland cement concrete: An overview. J. Clean. Prod. 2021, 282, 124447. [Google Scholar] [CrossRef]
- Ozturk, M.; Akgol, O.; Sevim, U.K.; Karaaslan, M.; Demirci, M.; Unal, E. Experimental work on mechanical, electromagnetic and microwave shielding effectiveness properties of mortar containing electric arc furnace slag. Constr. Build. Mater. 2018, 165, 58–63. [Google Scholar] [CrossRef]
- Li, P.; Jiang, J.; Liu, G.; Ren, Z. Physical, mechanical, thermal and sustainable properties of UHPC with converter steel slag aggregates. Case Stud. Constr. Mater. 2022, 17, e01458. [Google Scholar] [CrossRef]
- Ho, J.; Liang, Y.; Wang, Y.; Lai, M.; Huang, Z.; Yang, D.; Zhang, Q. Residual properties of steel slag coarse aggregate concrete after exposure to elevated temperatures. Constr. Build. Mater. 2022, 316, 125751. [Google Scholar] [CrossRef]
- Pomaro, B.; Gramegna, F.; Cherubini, R.; De Nadal, V.; Salomoni, V.; Faleschini, F. Gamma-ray shielding properties of heavyweight concrete with Electric Arc Furnace slag as aggregate: An experimental and numerical study. Construct. Build. Mater. 2019, 200, 188–197. [Google Scholar] [CrossRef]
- Costa, L.; Nogueira, M.; Andrade, H.; de Carvalho, J.; da Fonseca Eloi, F.; Brigolini, G.; Peixoto, R. Mechanical and durability performance of concretes produced with steel slag aggregate and mineral admixtures. Construct. Build. Mater. 2022, 318, 126152. [Google Scholar] [CrossRef]
- Pang, B.; Zhou, Z.; Cheng, X.; Du, P.; Xu, H. ITZ properties of concrete with carbonated steel slag aggregate in salty freeze-thaw environment. Construct. Build. Mater. 2016, 114, 162–171. [Google Scholar] [CrossRef]
- Cheng, X.; Tian, W.; Gao, J.; Gao, Y. Performance evaluation and lifetime prediction of steel slag coarse aggregate concrete under sulfate attack. Construct. Build. Mater. 2022, 344, 128203. [Google Scholar] [CrossRef]
- Özkök, E.; Davis, A.P.; Aydilek, A.H. Treatment Methods for Mitigation of High Alkalinity in Leachates of Aged Steel Slag. J. Environ. Eng. 2016, 142, 04015063. [Google Scholar] [CrossRef]
- Lizarazo-Marriaga, J.; Claisse, P.; Ganjian, E. Effect of steel slag and Portland cement in the rate of hydration and strength of blast furnace slag pastes. J. Mater. Civil Eng. 2011, 23, 153–160. [Google Scholar] [CrossRef]
- Tsakiridis, P.E.; Papadimitriou, G.D.; Tsivilis, S.; Koroneos, C. Utilization of steel slag for Portland cement clinker production. J. Hazard. Mater. 2008, 152, 805–811. [Google Scholar] [CrossRef]
- Pan, Z.; Zhou, J.; Jiang, X.; Xu, Y.; Jin, R.; Ma, J.; Zhuang, Y.; Diao, Z.; Zhang, S.; Si, Q.; et al. Investigating the effects of steel slag powder on the properties of self-compacting concrete with recycled aggregates. Constr. Build. Mater. 2019, 200, 570–577. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H.; Wu, K. Effects of steel slag powder on workability and durability of concrete. J. Wuhan Univ. Technol. 2014, 29, 733–739. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Q.; Zheng, Y.; Cang, Z.; Gisele, K.; Yu, C.; Cang, D. Synergistic effect and mechanism of waste glass on the mechanical properties and autoclave stability of cementitious materials containing steel slag. Constr. Build. Mater. 2021, 311, 125295. [Google Scholar] [CrossRef]
- Sun, X.; Liu, J.; Zhao, Y.; Zhao, J.; Li, Z.; Sun, Y.; Qiu, J.; Zheng, P. Mechanical activation of steel slag to prepare supplementary cementitious materials: A comparative research based on the particle size distribution, hydration, toxicity assessment and carbon dioxide emission. J. Build. Eng. 2022, 60, 105200. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, J.; Qi, L. Effects of temperature and carbonation curing on the mechanical properties of steel slag-cement binding materials. Constr. Build. Mater. 2016, 124, 999–1006. [Google Scholar] [CrossRef]
- Kriskova, L.; Pontikes, Y.; Zhang, F.; Cizer, Ö.; Jones, P.T.; Van Balen, K.; Blanpain, B. Influence of mechanical and chemical activation on the hydraulic properties of gamma dicalcium silicate. Cem. Concr. Res. 2014, 55, 59–68. [Google Scholar] [CrossRef]
- Nunes, V.; Borges, P. Recent advances in the reuse of steel slags and future perspectives as binder and aggregate for alkali-activated materials. Constr. Build. Mater. 2021, 281, 122605. [Google Scholar] [CrossRef]
- Huo, B.; Li, B.; Huang, S.; Chen, C.; Zhang, Y.; Banthia, N. Hydration and soundness properties of phosphoric acid modified steel slag powder. Constr. Build. Mater. 2020, 254, 119319. [Google Scholar] [CrossRef]
- Huo, B.; Li, B.; Chen, C.; Zhang, Y. Surface etching and early age hydration mechanisms of steel slag powder with formic acid. Constr. Build. Mater. 2021, 280, 122500. [Google Scholar] [CrossRef]
- Li, B.; Tang, Z.; Huo, B.; Liu, Z.; Cheng, Y.; Ding, B.; Zhang, P. The early age hydration products and mechanical properties of cement paste containing GBFS under steam curing condition. Buildings 2022, 12, 1746. [Google Scholar] [CrossRef]
- Tomek, R. Advantages of precast concrete in highway infrastructure construction. Procedia Eng. 2017, 196, 176–180. [Google Scholar] [CrossRef]
- Zeyad, A.; Tayeh, B.; Adesina, A.; de Azevedo, A.; Amin, M.; Hadzima-Nyarko, M.; Agwa, I. Review on effect of steam curing on behavior of concrete. Cleaner Mater. 2022, 3, 100042. [Google Scholar] [CrossRef]
- Ramezanianpour, A.; Esmaeili, K.; Ghahari, S. Influence of initial steam curing and different types of mineral additives on mechanical and durability properties of self-compacting concrete. Constr. Build. Mater. 2014, 73, 187–194. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, W.; Peng, Y.; Tang, S.; Wang, L.; Shi, Y.; Li, Y.; Wang, Y.; Geng, Z.; Wu, K. Hydration and Fractal Analysis on Low-Heat Portland Cement Pastes Using Thermodynamics-Based Methods. Fractal Fract. 2023, 7, 606. [Google Scholar] [CrossRef]
- Nandhini, K.; Karthikeyan, J. The early-age prediction of concrete strength using maturity models: A review. J. Build. Pathol. Rehabil. 2021, 6, 7. [Google Scholar] [CrossRef]
- GB/T 17671-1999; Method of Testing Cements–Determination of Strength. The National Standards of the People’s Republic of China: Beijing, China, 1999. (In Chinese)
- GB/T 1346-2011; Test Methods for Water Requirement of Normal Consistency, Setting Time and Soundness of the Portland Cement. The National Standards of the People’s Republic of China: Beijing, China, 2011. (In Chinese)
- GB/T 750-1992; Autoclave Method for Soundness of Portland Cement. The National Standards of the People’s Republic of China: Beijing, China, 1992. (In Chinese)
- GB/T 2419-2005; Test Method for Fluidity of Cement Mortar. The National Standards of the People’s Republic of China: Beijing, China, 2005. (In Chinese)
- Altun, I.; Yılmaz, İ. Study on Steel Furnace Slags with High MgO as Additive in Portland Cement. Cem. Concr. Res. 2002, 32, 1247–1249. [Google Scholar] [CrossRef]
- Hu, S.; He, Y.; Lu, L.; Ding, Q. Effect of fine steel slag powder on the early hydration process of Portland cement. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 2006, 21, 147–149. [Google Scholar] [CrossRef]
- Péra, J.; Ambroise, J.; Chabannet, M. Properties of Blast-furnace Slags Containing High Amounts of Manganese. Cem. Concr. Res. 1999, 29, 171–177. [Google Scholar] [CrossRef]
- Zhuang, S.; Wang, Q.; Luo, T. Effect of C12A7 in steel slag on the early-age hydration of cement. Cem. Concr. Res. 2022, 162, 107010. [Google Scholar] [CrossRef]
- Erdem, T.; Turanli, L.; Erdogan, T. Setting time: An important criterion to determine the length of the delay period before steam curing of concrete. Cem. Concr. Res. 2003, 33, 741–745. [Google Scholar] [CrossRef]
- Quillin, K.; Osborne, G.; Majumdar, A.; Singh, B. Effects of w/c ratio and curing conditions on strength development in BRECEM concretes. Cem. Concr. Res. 2001, 31, 627–632. [Google Scholar] [CrossRef]
- Jappy, T.; Glasser, F. Synthesis and stability of silica-substituted hydrogarnet Ca3Al2Si3-xO12-4x(OH)4x. Adv. Cem. Res. 1991, 4, 1–8. [Google Scholar] [CrossRef]
- Taylor, H.F. Cement Chemistry, 2nd ed.; Thomas Telford: London, UK, 1997; pp. 170–171. [Google Scholar]
- Sha, W. Differential scanning calorimetry study of the hydration products in portland cement pastes with metakaolin replacement. Adv. Build. Technol. 2002, 1, 881–888. [Google Scholar] [CrossRef]
- Hou, J.; Chen, Z.; Liu, J. Hydration Activity and Expansibility Model for the RO Phase in Steel Slag. Metall. Mater. Trans. B 2020, 51, 1697–1704. [Google Scholar] [CrossRef]
- Yan, J.; Wu, S.; Yang, C.; Zhao, Z.; Xie, J. Influencing mechanisms of RO phase on the cementitious properties of steel slag powder. Constr. Build. Mater. 2022, 350, 128926. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Z.; Zhuang, S.; He, W. Hydration properties and microstructure characteristics of alkali–activated steel slag. Constr. Build. Mater. 2020, 241, 118141. [Google Scholar] [CrossRef]
- Song, Q.; Su, J.; Nie, J.; Li, H.; Hu, Y.; Chen, Y.; Li, R.; Deng, Y. The occurrence of MgO and its influence on properties of clinker and cement: A review. Constr. Build. Mater. 2021, 293, 123494. [Google Scholar] [CrossRef]
- Kasselouris, V.; Ftikos, C.; Parissakis, G. On the hydration of MgO in cement pastes hydrated up to eight years. Cem. Concr. Res. 1985, 15, 758–764. [Google Scholar] [CrossRef]
- Peng, Y.; Tang, S.; Huang, J.; Tang, C.; Wang, L.; Liu, Y. Fractal analysis on pore structure and modeling of hydration of magnesium phosphate cement paste. Fractal Fract. 2022, 6, 337. [Google Scholar] [CrossRef]
- Rahman, M.; Sarker, P.; Shaikh, F.; Saha, A. Soundness and compressive strength of Portland cement blended with ground granulated ferronickel slag. Constr. Build. Mater. 2017, 140, 194–202. [Google Scholar] [CrossRef]
- Mo, L.; Deng, M.; Tang, M.; Al-Tabbaa, A. MgO expansive cement and concrete in China: Past, present and future. Cem. Concr. Res. 2014, 57, 1–12. [Google Scholar] [CrossRef]
- Huang, J.; Li, W.; Huang, D.; Wang, L.; Chen, E.; Wu, C.; Wang, B.; Deng, H.; Tang, S.; Shi, Y.; et al. Fractal analysis on pore structure and hydration of magnesium oxysulfate cements by first principle, thermodynamic and microstructure-based methods. Fractal Fract. 2021, 5, 164. [Google Scholar] [CrossRef]
- Kayali, O.; Khan, M.; Ahmed, M. The role of hydrotalcite in chloride binding and corrosion protection in concretes with ground granulated blast furnace slag. Cem. Concr. Compos. 2012, 34, 936–945. [Google Scholar] [CrossRef]
- Wang, Y.; Suraneni, P. Experimental methods to determine the feasibility of steel slags as supplementary cementitious materials. Constr. Build. Mater. 2019, 204, 458–467. [Google Scholar] [CrossRef]
- Adesanya, E.; Sreenivasan, H.; Kantola, A.M.; Telkki, V.V.; Ohenoja, K.; Kinnunen, P.; Illikainen, M. Ladle slag cement—Characterization of hydration and conversion. Constr. Build. Mater. 2018, 193, 128–134. [Google Scholar] [CrossRef]
- Ríos, C.; Williams, C.; Fullen, M. Hydrothermal synthesis of hydrogarnet and tobermorite at 175 °C from kaolinite and metakaolinite in the CaO–Al2O3–SiO2–H2O system: A comparative study. Appl. Clay Sci. 2009, 43, 228–237. [Google Scholar] [CrossRef]
- Wei, T.; Cheng, X.; Gu, T.; Huang, S.; Zhang, C.; Zhuang, J.; Zheng, Y. The change and influence mechanism of the mechanical properties of tricalcium silicate hardening at high temperature. Constr. Build. Mater. 2021, 308, 125065. [Google Scholar] [CrossRef]
- Seifan, M.; Berenjian, A. Application of microbially induced calcium carbonate precipitation in designing bio self-healing concrete. World J. Microbiol. Biotechnol. 2018, 34, 168. [Google Scholar] [CrossRef]
- Poon, C.; Lam, L.; Kou, S.; Wong, Y.; Wong, R. Rate of pozzolanic reaction of metakaolin in high-performance cement pastes. Cem. Concr. Res. 2001, 31, 1301–1306. [Google Scholar] [CrossRef]
- Tironi, A.; Trezza, M.; Scian, A.; Irassar, E. Assessment of pozzolanic activity of different calcined clays. Cem. Concr. Compos. 2013, 37, 319–327. [Google Scholar] [CrossRef]
- Vance, K.; Aguayo, M.; Oey, T.; Sant, G.; Neithalath, N. Hydration and strength development in ternary portland cement blends containing limestone and fly ash or metakaolin. Cem. Concr. Compos. 2013, 39, 93–103. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, D.; Ueda, T.; Fan, Y.; Li, C.; Li, J. Beneficial effect of nanomaterials on the interfacial transition zone (ITZ) of non-dispersible underwater concrete. Constr. Build. Mater. 2021, 293, 123472. [Google Scholar] [CrossRef]
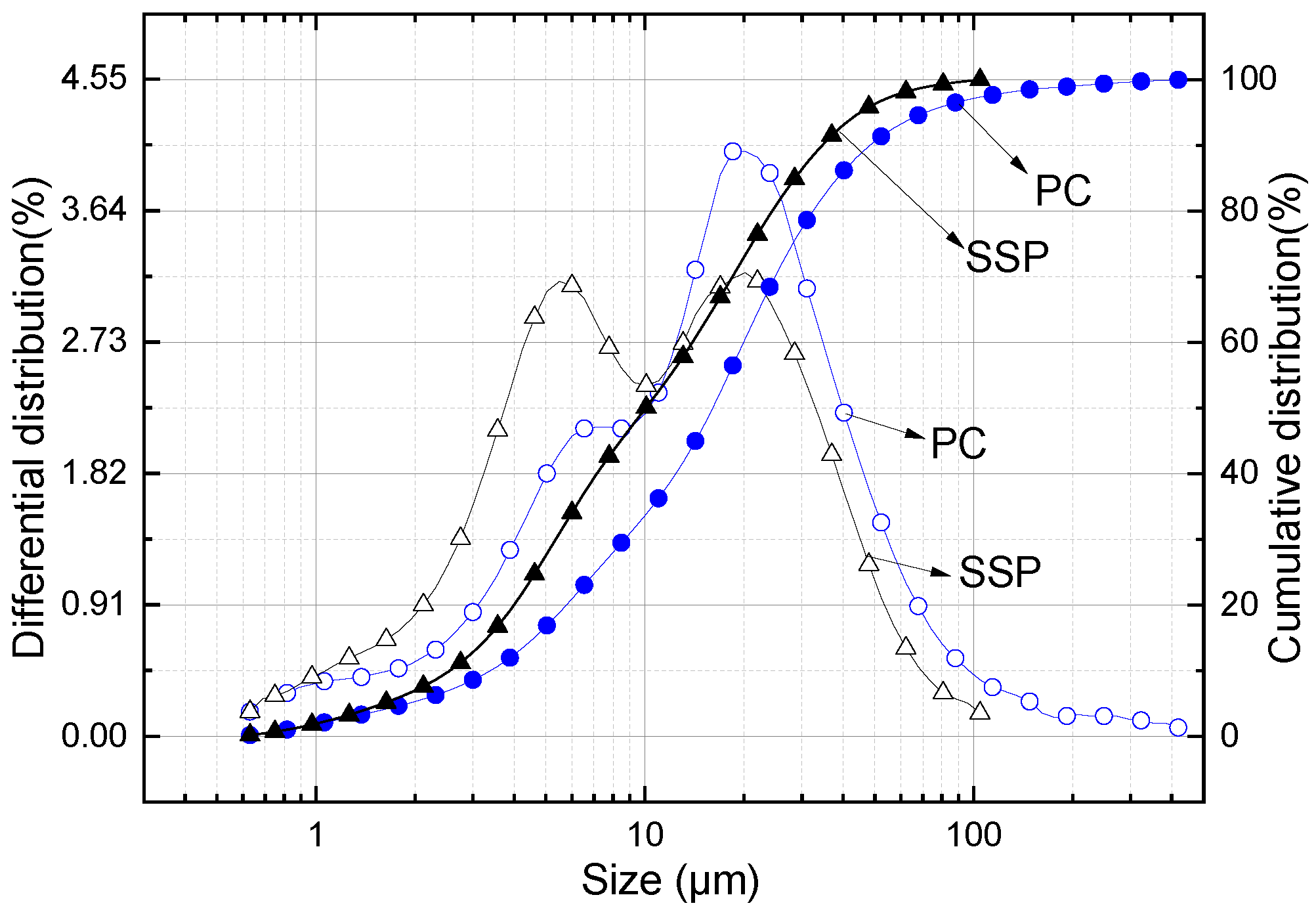






| Composition | CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | MgO | K2O | MnO | P2O5 | Na2O | MnO | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PC | 64.47 | 20.87 | 4.87 | 3.59 | 2.52 | 2.13 | 0.65 | 0.09 | 0.29 | 0.11 | 0.09 | 2.4 |
| SSP | 38.62 | 18.46 | 7.12 | 22.5 | 1.08 | 6.60 | 0.15 | 2.11 | 1.25 | 0.21 | 2.11 | 6.2 |
| Sample | PC | SSP | Water | Curing Condition |
|---|---|---|---|---|
| Ref | 100 | 0 | 30 | S7h: cured at 80 °C steam for 7 h |
| 100 | 0 | 30 | S7d: cured at 80 °C steam for 7 days | |
| 100 | 0 | 30 | N28d: cured at 20 ± 2 °C, R.H. ≥ 95% for 28 days | |
| S20 | 80 | 20 | 30 | S7h |
| 80 | 20 | 30 | S7d | |
| 80 | 20 | 30 | N28d |
| Group | Water Requirement (wt. %) | Soundness | Setting Time (min) | Fluidity (mm) | ||
|---|---|---|---|---|---|---|
| Le-Chatelier Expansion (mm) | Autoclave Expansion (%) | Initial | Final | |||
| Ref | 27.4 | 0.5 | 0.08 | 172 | 237 | 210 |
| S20 | 27.2 | 2.5 | 0.15 | 230 | 327 | 220 |
| Specimens | Curing Condition | 60–950 °C Mass Loss | 60–600 °C Mass Loss | CH Content |
|---|---|---|---|---|
| Ref | S7h | 18.33 | 14.83 | 14.71 |
| S7d | 18.15 | 15.63 | 18.61 | |
| N28d | 17.80 | 15.63 | 15.21 | |
| S20 | S7h | 16.80 | 13.41 | 13.03 |
| S7d | 15.85 | 14.18 | 14.84 | |
| N28d | 16.80 | 14.85 | 14.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Lu, X.; Huo, B.; Du, Y.; Liu, Y.; Cheng, Y.; Liu, Z. The Early Age Hydration Products and Mechanical Properties of Cement Paste with Steel Slag Powder as Additive under Steam Curing Conditions. Buildings 2023, 13, 2192. https://doi.org/10.3390/buildings13092192
Li B, Lu X, Huo B, Du Y, Liu Y, Cheng Y, Liu Z. The Early Age Hydration Products and Mechanical Properties of Cement Paste with Steel Slag Powder as Additive under Steam Curing Conditions. Buildings. 2023; 13(9):2192. https://doi.org/10.3390/buildings13092192
Chicago/Turabian StyleLi, Baoliang, Xue Lu, Binbin Huo, Yuheng Du, Yuyi Liu, Yongzhen Cheng, and Zejun Liu. 2023. "The Early Age Hydration Products and Mechanical Properties of Cement Paste with Steel Slag Powder as Additive under Steam Curing Conditions" Buildings 13, no. 9: 2192. https://doi.org/10.3390/buildings13092192
APA StyleLi, B., Lu, X., Huo, B., Du, Y., Liu, Y., Cheng, Y., & Liu, Z. (2023). The Early Age Hydration Products and Mechanical Properties of Cement Paste with Steel Slag Powder as Additive under Steam Curing Conditions. Buildings, 13(9), 2192. https://doi.org/10.3390/buildings13092192






