Microstructure Properties of Popular Alkali-Activated Pastes Cured in Ambient Temperature
Abstract
1. Introduction
2. Materials and Experimental Program
2.1. Raw Materials
Aluminosilicate Binders
2.2. Water Glass Solution
2.3. Aluminosilicate Paste
2.4. Alkali-Activated-Aluminosilicate Mortar
2.5. Experimental Program
2.5.1. Fresh Properties of Paste
2.5.2. Mechanical Properties of Mortar
2.5.3. Micro-Level Properties of Paste
3. Results and Discussion
3.1. Setting Properties of Alkali-Activated FA, GGBS, and MK Pastes
3.2. Mechanical Properties
3.2.1. Alkali-Activated-Mortar-Compressive-Strength
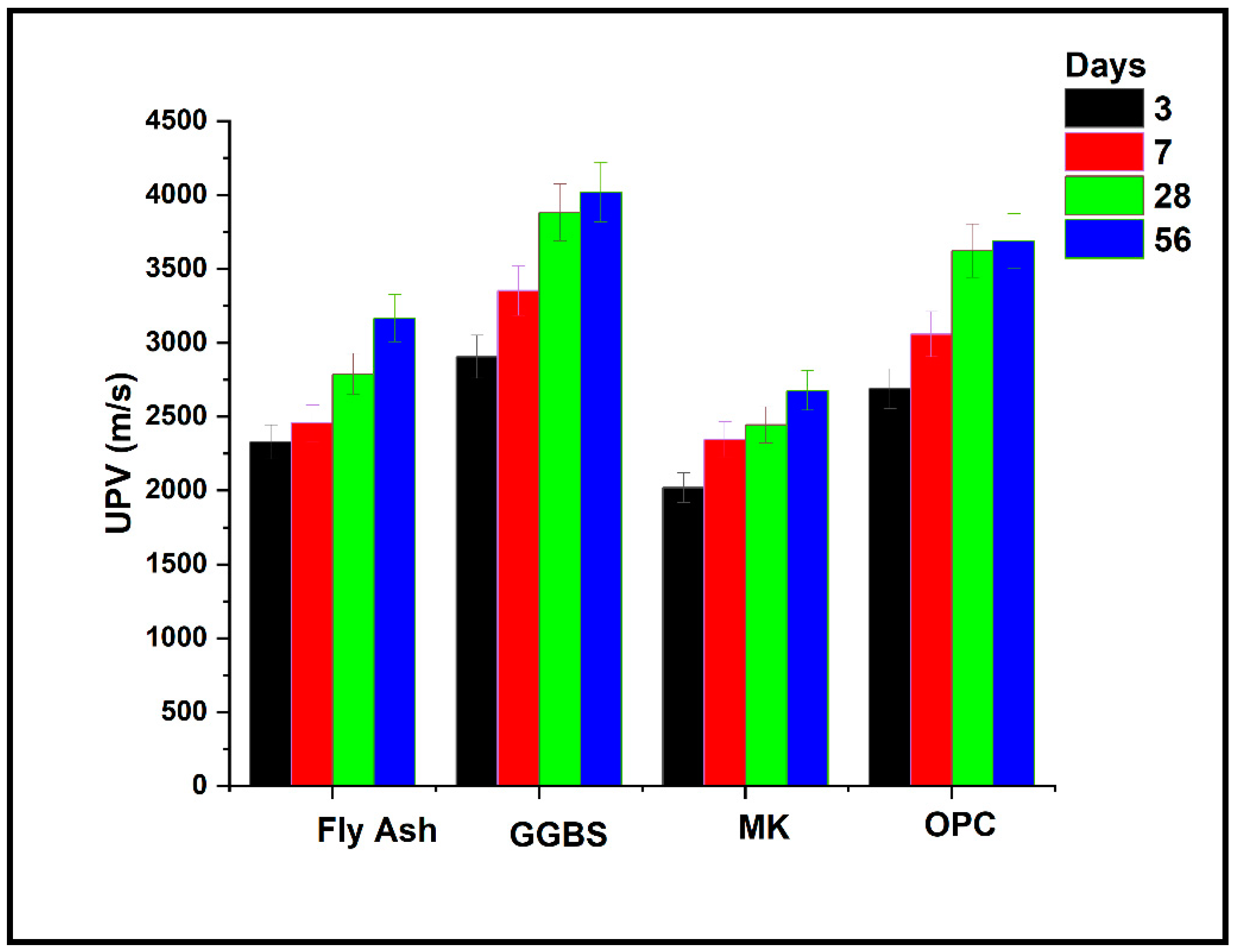
3.2.2. Alkali-Activated Mortar UPV Results
3.3. Microstructure of Alkali-Activated FA, GGBS, and MK
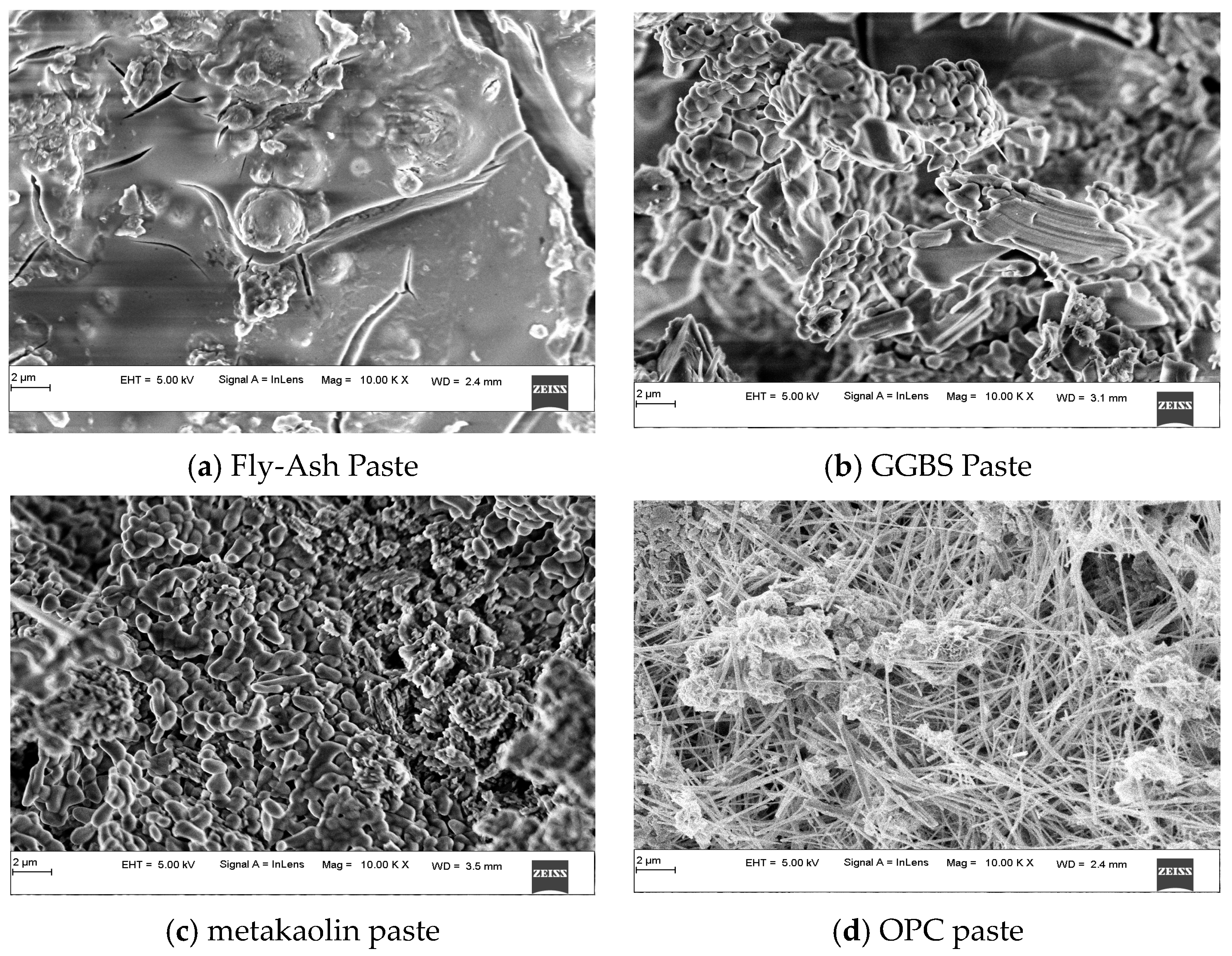
3.3.1. Alkali-Activated FA, GGBS, and MK Studied Using FE-SEM
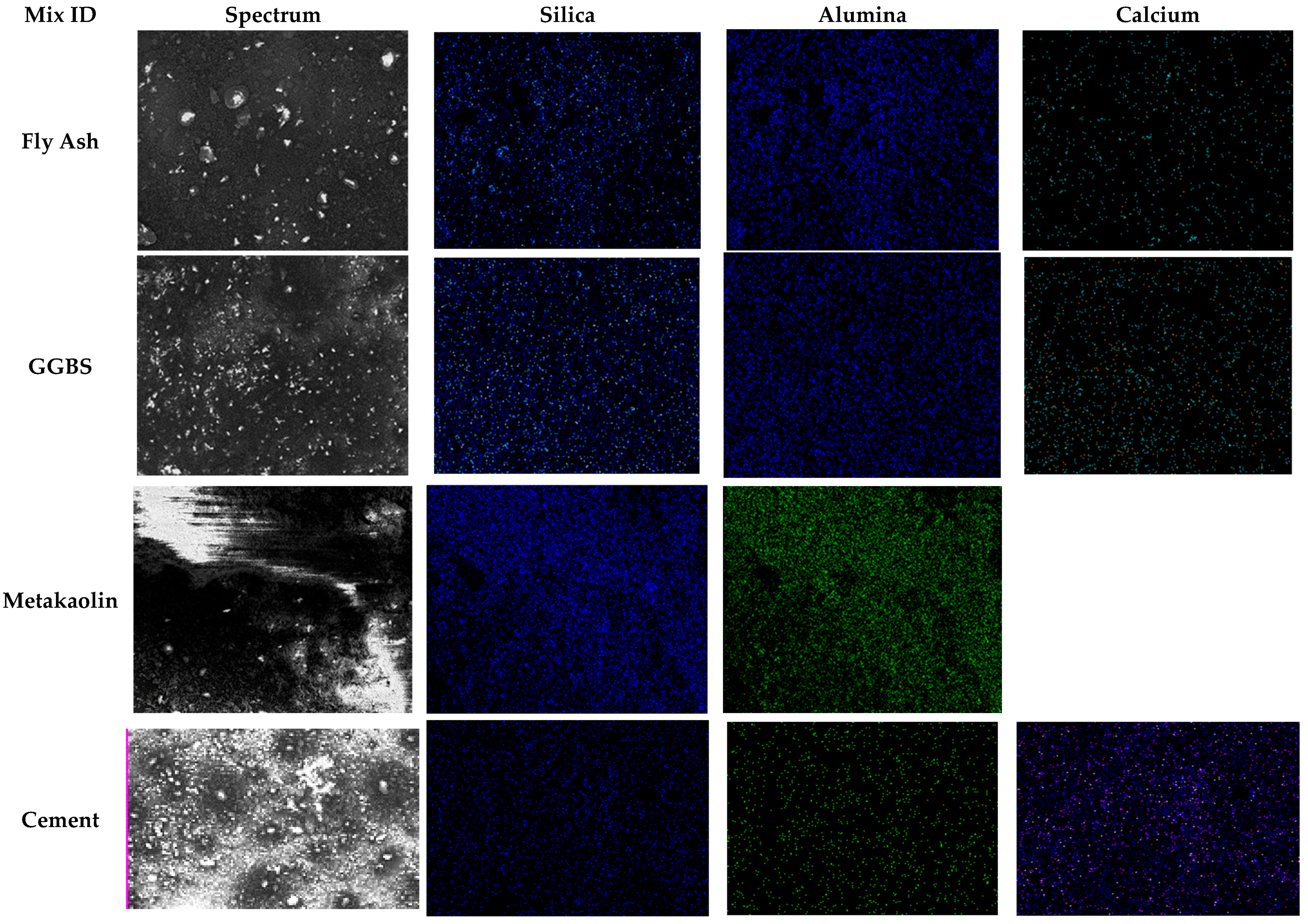
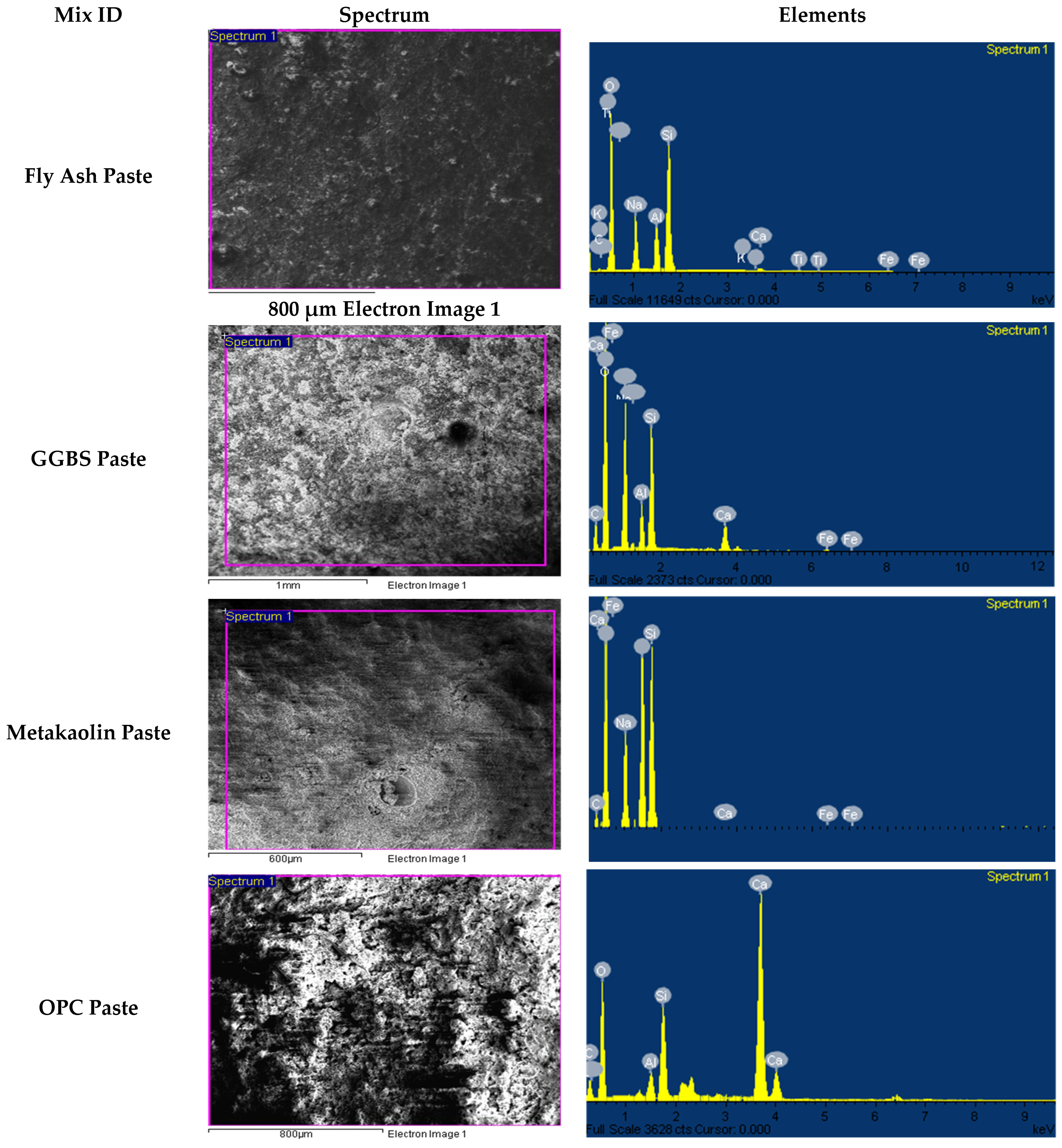
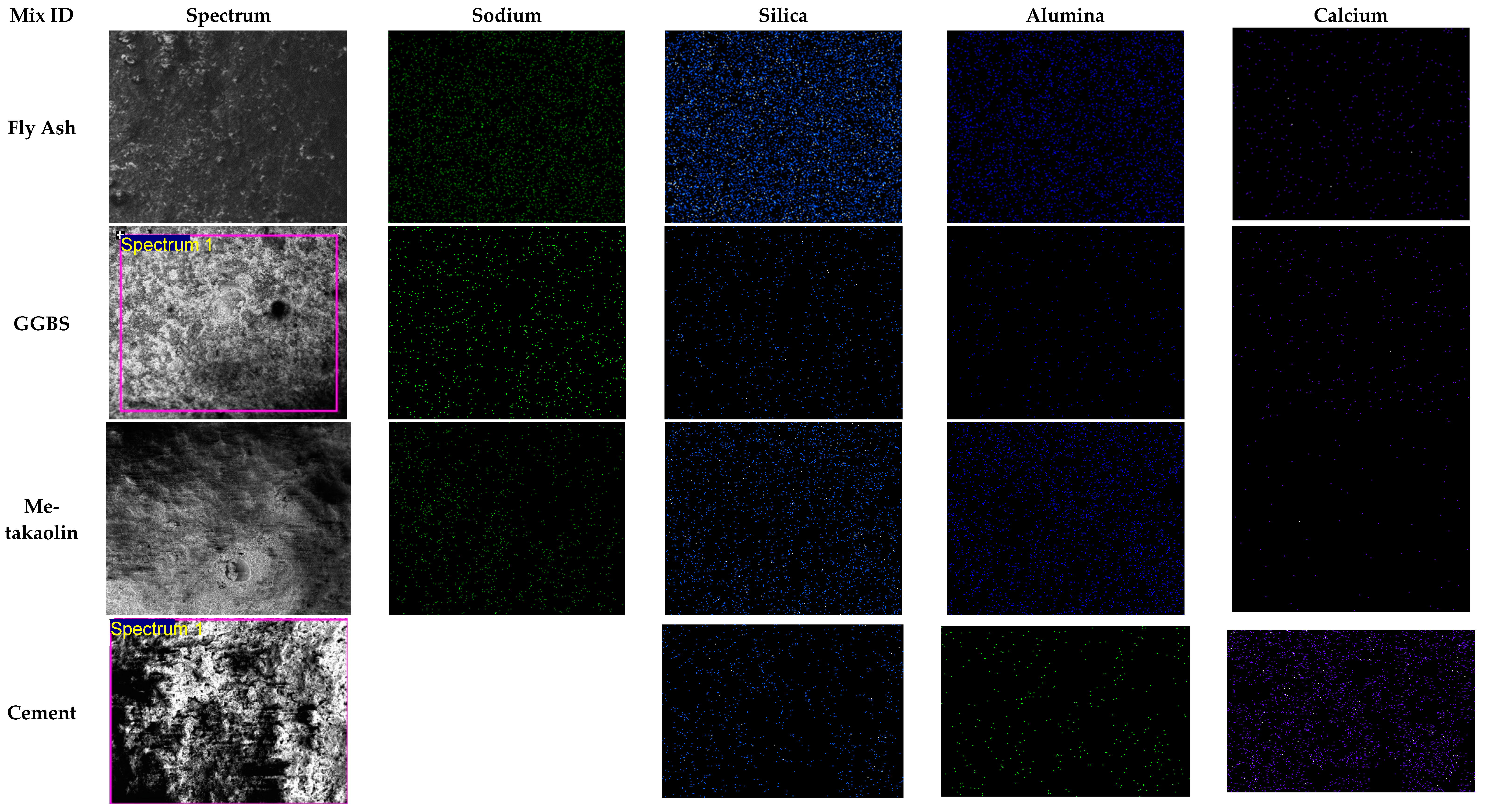
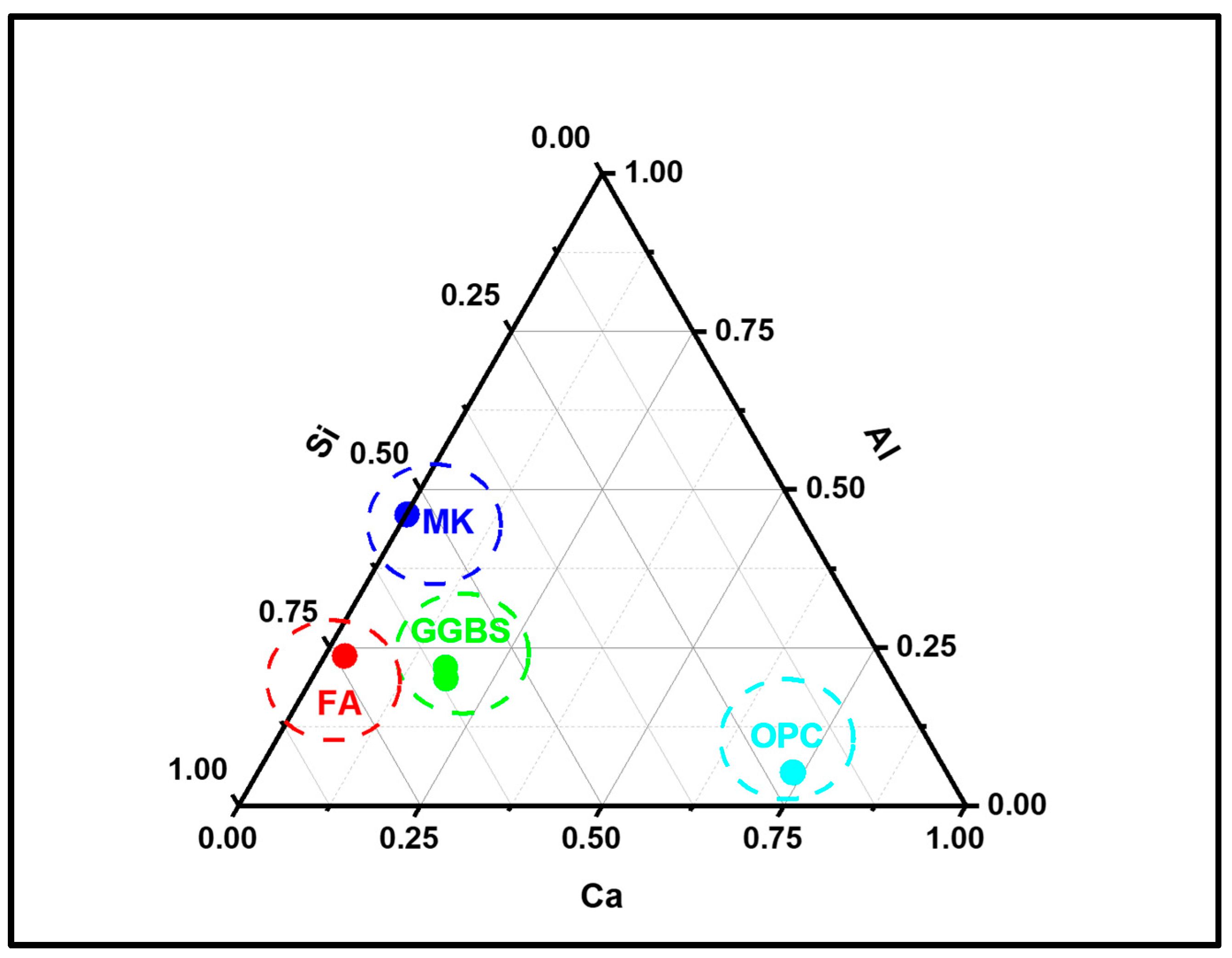
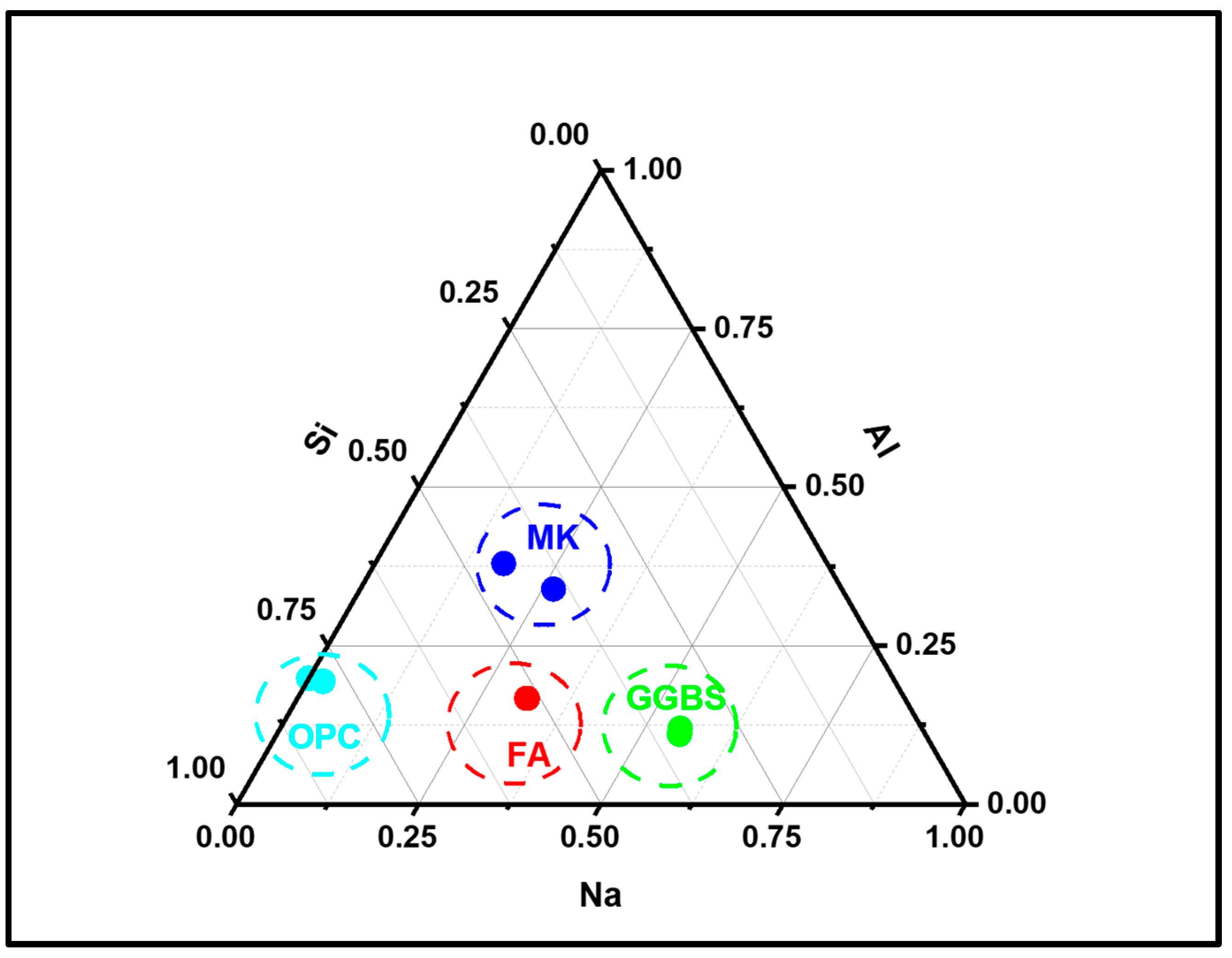
3.3.2. Alkali-Activated FA, GGBS, and MK Studied Using EDS
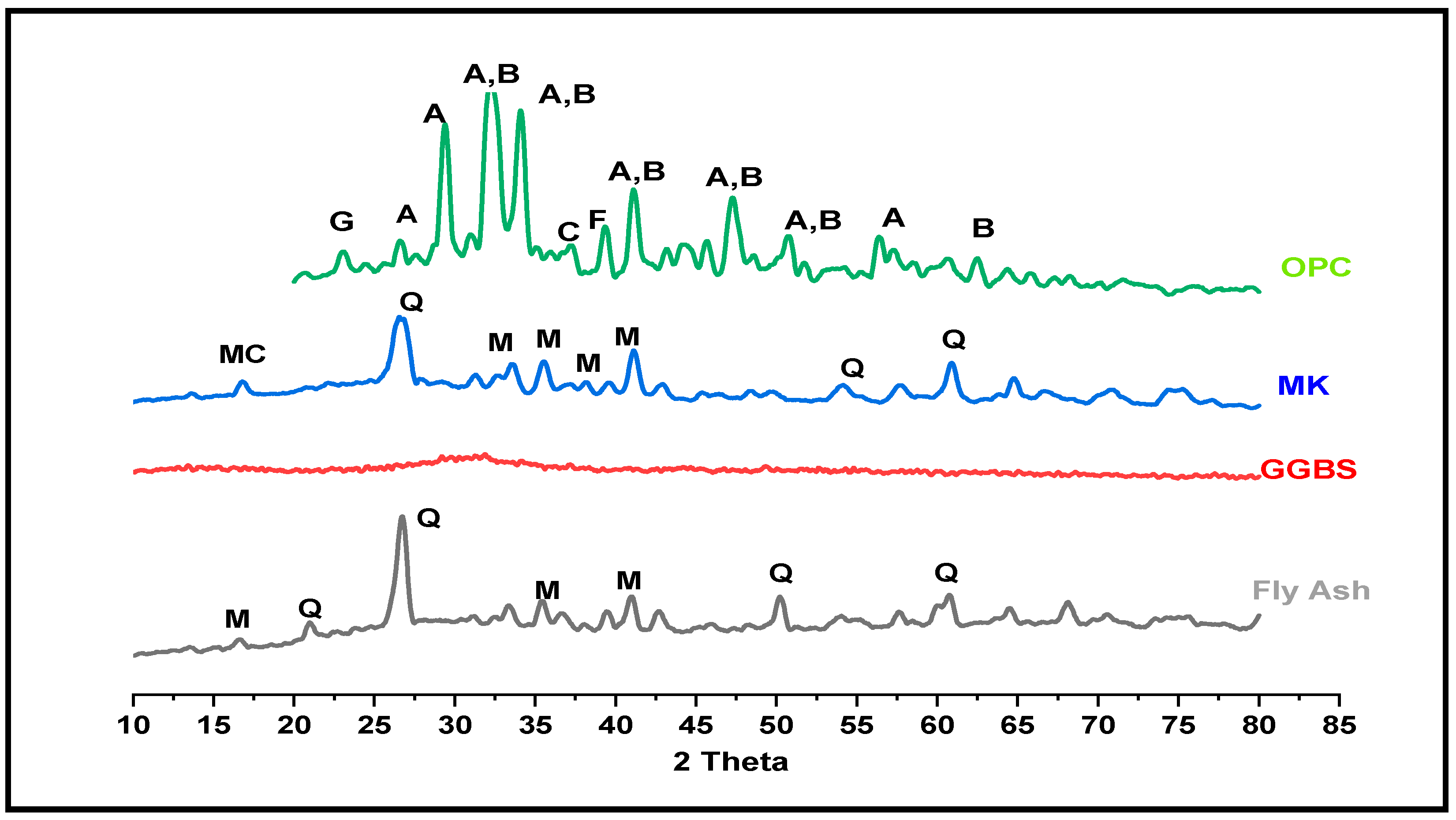
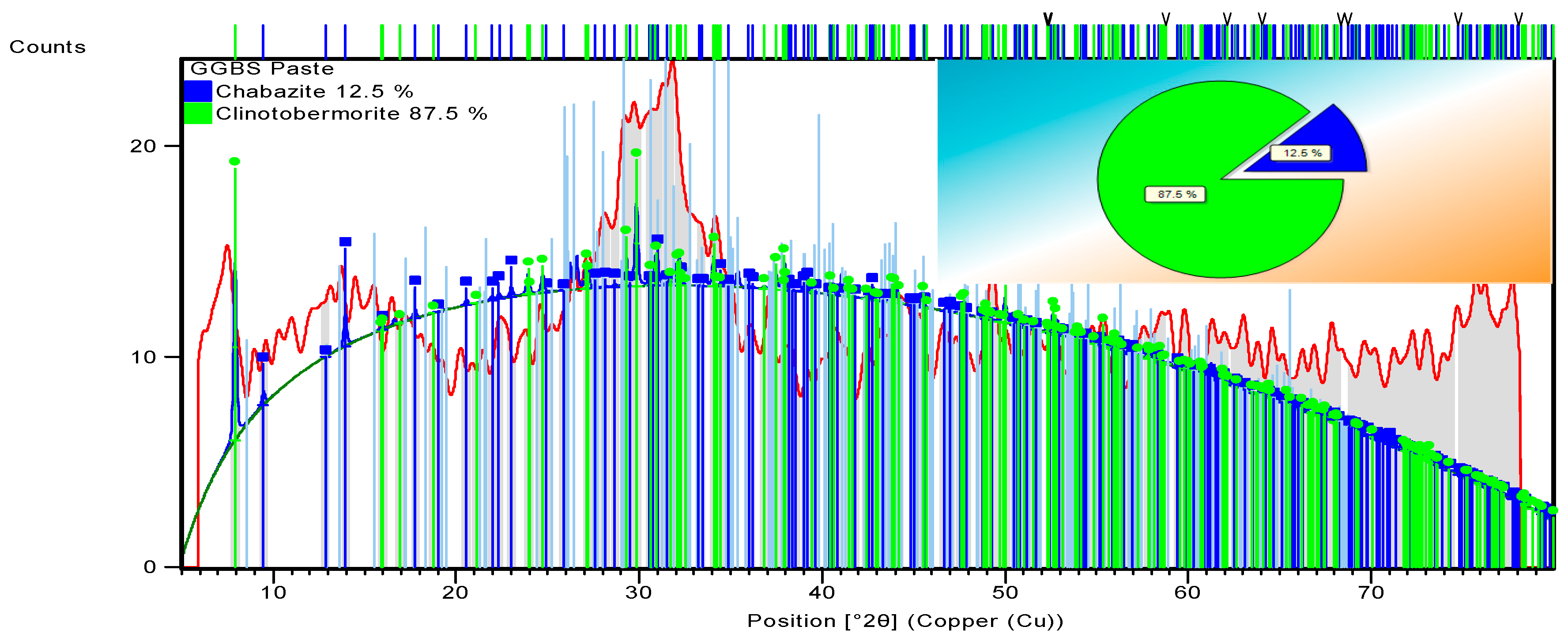
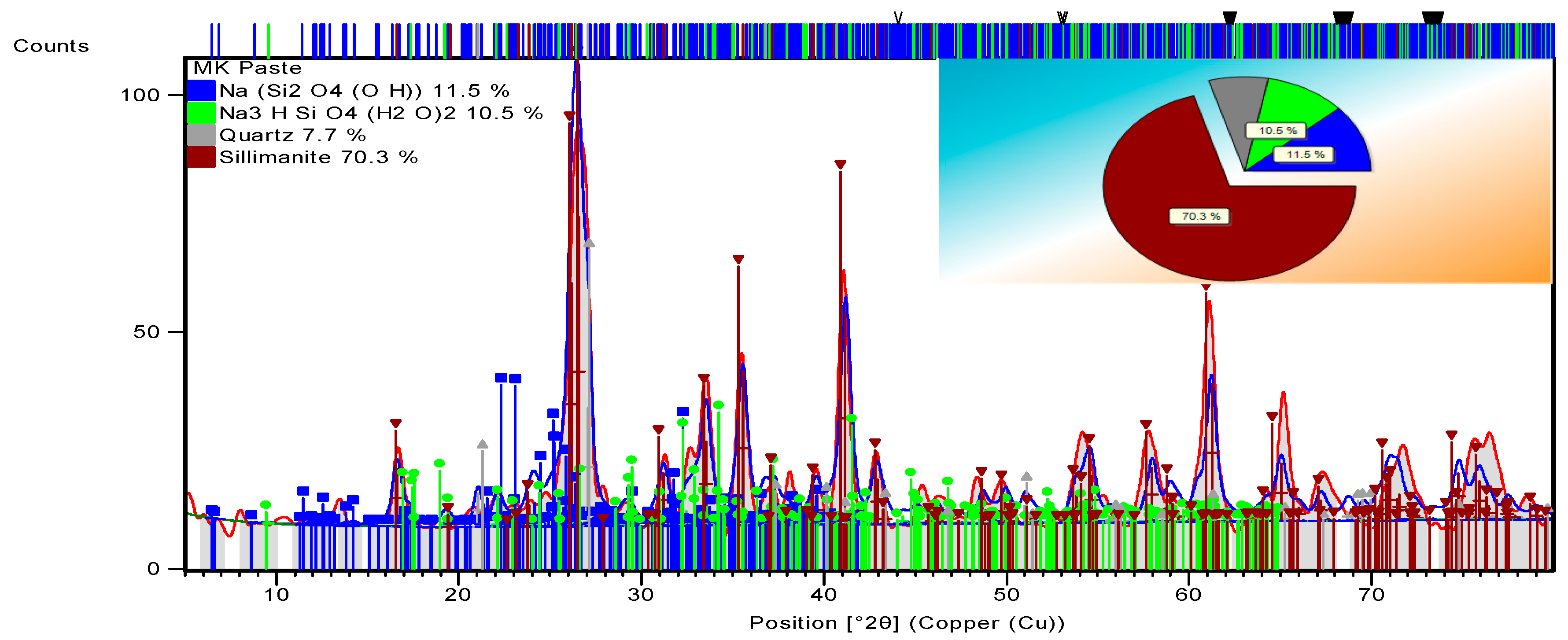
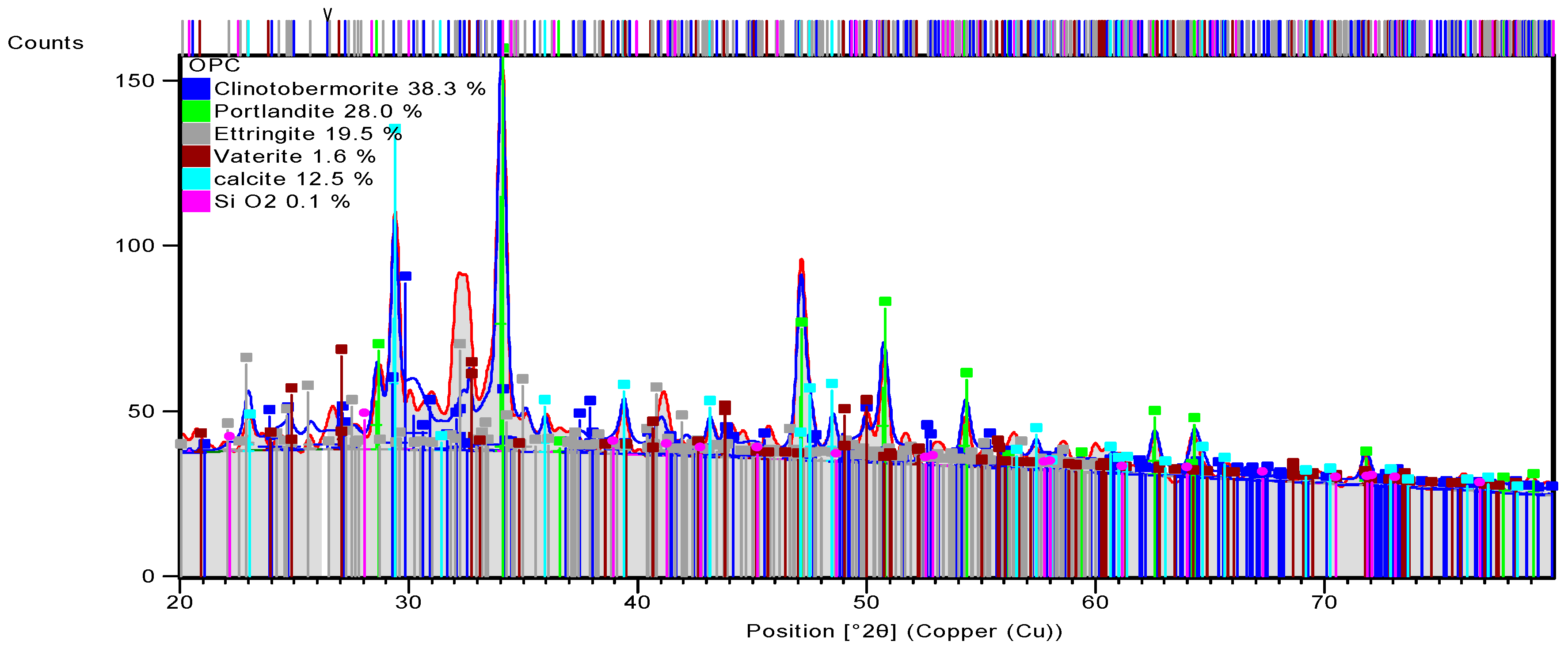
3.3.3. Alkali-Activated FA, GGBS, and MK Studied Using XRD
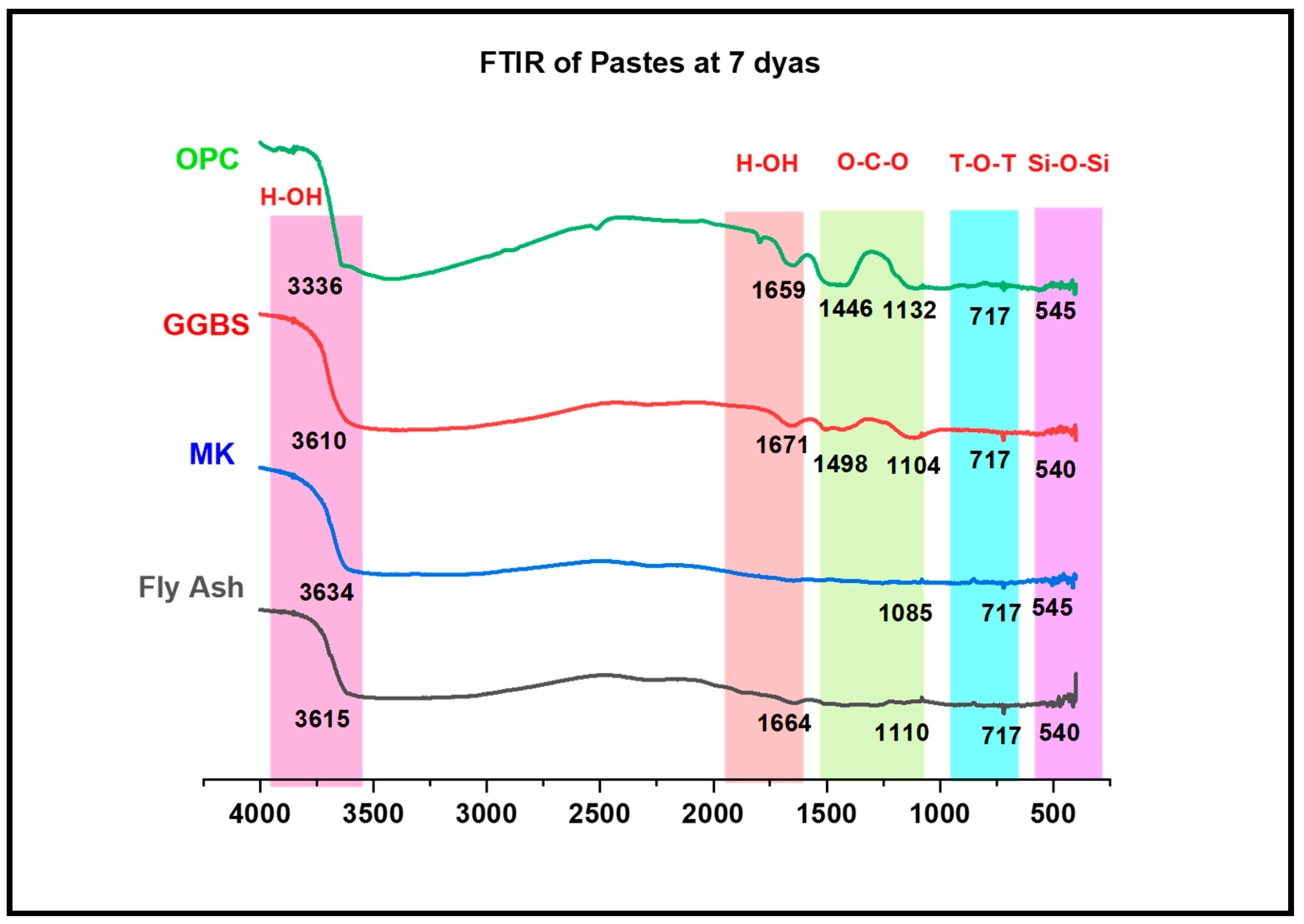
3.3.4. Alkali-Activated FA, GGBS and MK Studied Using FTIR
4. Conclusions
- Alkali-activated FA and MK pastes have a prolonged setting time, whereas the alkali-activated GGBS paste possesses quick-setting characteristics;
- Mortars made of alkali-activated FA and MK binder systems showed lower compressive strength, while slag and OPC-based mortars showed higher compressive strengths. The compressive strength showed that a binder system containing more calcium exhibits superior compressive strength;
- UPV values of mortar prepared with alkali-activated FA and MK based binders showed lower values, which means the matrix is less dense. The alkali-activated GGBS mortar-prepared binder had values of UPV slightly higher than that of the OPC-based mortar;
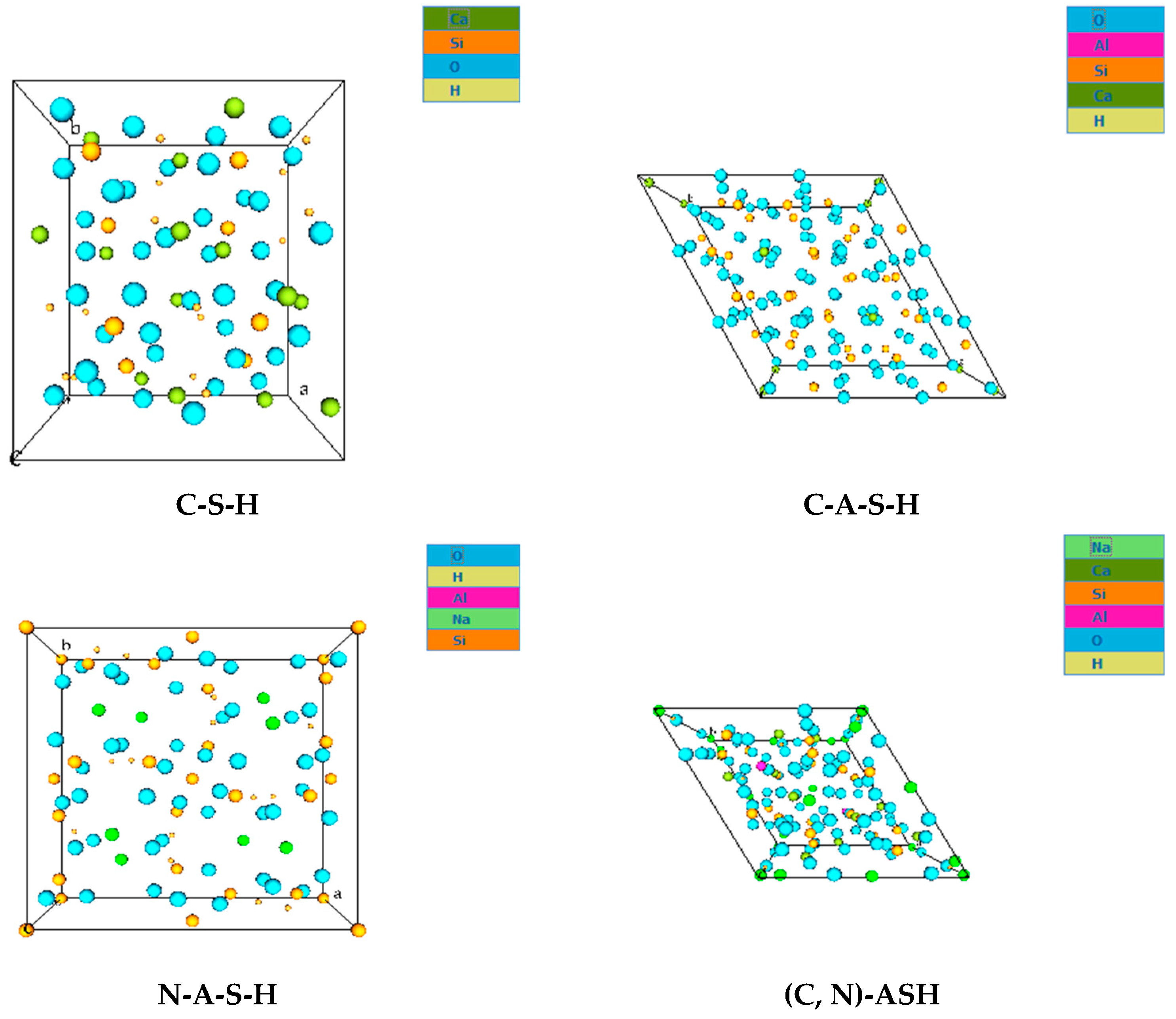
- FESEM images indicated the presence of NASH gel in the low calcium pastes such as FA and MK, and the CASH gel dominated in the slag-based high-calcium system. The CASH gel was characterized by a compact morphology, while the NASH gel displayed a fragmented morphology and contains unreacted particles;
- EDX elemental analysis revealed the Ca/(Si + Al), Na/(Si + Al), Ca/Si, Na/Al, Na/Si, and Ca/Al ratios that define the binder paste’s efficacy in comparison to OPC. The presence of NASH gel was observed in fly ash and MK, whereas multiple gel types in the GGBS such as CASH, NASH, C, NASH, and CSH were noted. EDX mapping of the paste also showed a similar pattern;
- XRD and FTIR confirmed the existence of NASH in the FA and MK and (C, N)-ASH in the GGBS paste was present only in traces.
Future Scope
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okoye, F.N.; Prakash, S.; Singh, N.B. Durability of Fly Ash Based Geopolymer Concrete in the Presence of Silica Fume. J. Clean. Prod. 2017, 149, 1062–1067. [Google Scholar] [CrossRef]
- Zannerni, G.M.; Fattah, K.P.; Al-Tamimi, A.K. Ambient-Cured Geopolymer Concrete with Single Alkali Activator. Sustain. Mater. Technol. 2020, 23, e00131. [Google Scholar] [CrossRef]
- Mendes, B.C.; Pedroti, L.G.; Vieira, C.M.F.; Marvila, M.; Azevedo, A.R.G.; Franco de Carvalho, J.M.; Ribeiro, J.C.L. Application of Eco-Friendly Alternative Activators in Alkali-Activated Materials: A Review. J. Build. Eng. 2021, 35, 102010. [Google Scholar] [CrossRef]
- König, K.; Traven, K.; Pavlin, M.; Ducman, V. Evaluation of Locally Available Amorphous Waste Materials as a Source for Alternative Alkali Activators. Ceram. Int. 2021, 47, 4864–4873. [Google Scholar] [CrossRef]
- Gavali, H.R.; Bras, A.; Faria, P.; Ralegaonkar, R.V. Development of Sustainable Alkali-Activated Bricks Using Industrial Wastes. Constr. Build. Mater. 2019, 215, 180–191. [Google Scholar] [CrossRef]
- Dong, M.; Elchalakani, M.; Karrech, A. Development of High Strength One-Part Geopolymer Mortar Using Sodium Metasilicate. Constr. Build. Mater. 2020, 236, 117611. [Google Scholar] [CrossRef]
- Vo, D.H.; Hwang, C.L.; Yehualaw, M.D.; Liao, M.C. The Influence of MgO Addition on the Performance of Alkali-Activated Materials with Slag−rice Husk Ash Blending. J. Build. Eng. 2021, 33, 101605. [Google Scholar] [CrossRef]
- Habert, G. Assessing the Environmental Impact of Conventional and “Green” Cement Production; Woodhead Publishing: Sawston, UK, 2013; ISBN 9780857097675. [Google Scholar]
- Zhang, J.; Tan, H.; Bao, M.; Liu, X.; Luo, Z.; Wang, P. Low Carbon Cementitious Materials: Sodium Sulfate Activated Ultra-Fine Slag/Fly Ash Blends at Ambient Temperature. J. Clean. Prod. 2021, 280, 124363. [Google Scholar] [CrossRef]
- Adesanya, E.; Perumal, P.; Luukkonen, T.; Yliniemi, J.; Ohenoja, K.; Kinnunen, P.; Illikainen, M. Opportunities to Improve Sustainability of Alkali-Activated Materials: A Review of Side-Stream Based Activators. J. Clean. Prod. 2021, 286, 125558. [Google Scholar] [CrossRef]
- Shetty, P.P.; Rao, A.U.; Pai, B.H.V.; Kamath, M. V Performance of High-Strength Concrete with the Effects of Seashell Powder as Binder Replacement and Waste Glass Powder as Fine Aggregate. J. Compos. Sci. 2023, 7, 92. [Google Scholar] [CrossRef]
- Madurwar, M.V.; Ralegaonkar, R.V.; Mandavgane, S.A. Application of Agro-Waste for Sustainable Construction Materials: A Review. Constr. Build. Mater. 2013, 38, 872–878. [Google Scholar] [CrossRef]
- Tantri, A.; Nayak, G.; Kamath, M.; Shenoy, A.; Shetty, K.K. Utilization of Cashew Nut-Shell Ash as a Cementitious Material for the Development of Reclaimed Asphalt Pavement Incorporated Self Compacting Concrete. Constr. Build. Mater. 2021, 301, 124197. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications, 5th, ed.; Geopolymer Publishing: Hauppauge, NY, USA, 2020. [Google Scholar]
- Li, C.; Sun, H.; Li, L. A Review: The Comparison between Alkali-Activated Slag (Si + Ca) and Metakaolin (Si + Al) Cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Flatt, R.J.; Roussel, N.; Cheeseman, C.R. Concrete: An Eco Material That Needs to Be Improved. J. Eur. Ceram. Soc. 2012, 32, 2787–2798. [Google Scholar] [CrossRef]
- Alnahhal, M.F.; Kim, T.; Hajimohammadi, A. Waste-Derived Activators for Alkali-Activated Materials: A Review. Cem. Concr. Compos. 2021, 118, 103980. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Zhu, H.J.; Zhou, C.H.; Wang, H. Geopolymer from Kaolin in China: An Overview. Appl. Clay Sci. 2016, 119, 31–41. [Google Scholar] [CrossRef]
- Bhagath Singh, G.V.P.; Subramaniam, K.V.L. Quantitative XRD Study of Amorphous Phase in Alkali Activated Low Calcium Siliceous Fly Ash. Constr. Build. Mater. 2016, 124, 139–147. [Google Scholar] [CrossRef]
- Jagadisha, A.; Rao, K.B.; Nayak, G.; Kamath, M. Influence of Nano-Silica on the Microstructural and Mechanical Properties of High-Performance Concrete of Containing EAF Aggregate and Processed Quarry Dust. Constr. Build. Mater. 2021, 304, 124392. [Google Scholar] [CrossRef]
- Alventosa, K.M.L.; White, C.E. The Effects of Calcium Hydroxide and Activator Chemistry on Alkali-Activated Metakaolin Pastes. Cem. Concr. Res. 2021, 145, 106453. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Ceramic-like Inorganic Polymers. J. Ceram. Sci. Technol. 2017, 8, 335–350. [Google Scholar] [CrossRef]
- Ruiz-Santaquiteria, C.; Fernández-Jiménez, A.; Palomo, A. Alternative Prime Materials for Developing New Cements: Alkaline Activation of Alkali Aluminosilicate Glasses. Ceram. Int. 2016, 42, 9333–9340. [Google Scholar] [CrossRef]
- Gavali, H.R.; Ralegaonkar, R.V. Design Development of Sustainable Alkali-Activated Bricks. J. Build. Eng. 2020, 30, 101302. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Zhang, W.; Li, Z.; Zhang, Y.; Li, Y.; Ren, Y. Effects of Si/Al Ratio on the Efflorescence and Properties of Fly Ash Based Geopolymer. J. Clean. Prod. 2020, 244, 118852. [Google Scholar] [CrossRef]
- Khan, K.A.; Raut, A.; Chandrudu, C.R.; Sashidhar, C. Design and Development of Sustainable Geopolymer Using Industrial Copper Byproduct. J. Clean. Prod. 2021, 278, 123565. [Google Scholar] [CrossRef]
- Puligilla, S.; Mondal, P. Co-Existence of Aluminosilicate and Calcium Silicate Gel Characterized through Selective Dissolution and FTIR Spectral Subtraction. Cem. Concr. Res. 2015, 70, 39–49. [Google Scholar] [CrossRef]
- Rao, A.U.; Bhandary, R.; Maddodi, B.; Tantri, A.; Shenoy, S.; Kamath, M.; Shetty, R.S. Performance Evaluation of Hybrid Glass Wastes Incorporated Concrete. Eng. Sci. 2022, 20, 188–198. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, K.; Li, Q.; Wang, J.; Ling, Y. Fabrication and Engineering Properties of Concretes Based on Geopolymers/Alkali-Activated Binders—A Review. J. Clean. Prod. 2020, 258, 120896. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J.S.J. The Geopolymerisation of Alumino-Silicate Minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef]
- Zhao, D.; Khoshnazar, R. Microstructure of Cement Paste Incorporating High Volume of Low-Grade Metakaolin. Cem. Concr. Compos. 2020, 106, 103453. [Google Scholar] [CrossRef]
- Wardhono, A.; Gunasekara, C.; Law, D.W.; Setunge, S. Comparison of Long Term Performance between Alkali Activated Slag and Fly Ash Geopolymer Concretes. Constr. Build. Mater. 2017, 143, 272–279. [Google Scholar] [CrossRef]
- Kumar, M.; Shreelaxmi, P.; Kamath, M. Review on Characteristics of Sewage Sludge Ash and Its Partial Replacement as Binder Material in Concrete; Springer: Berlin/Heidelberg, Germany, 2021; Volume 105, ISBN 9789811582929. [Google Scholar]
- Kamath, M.V.; Prashanth, S.; Kumar, M. Review of Low to High Strength Alkali-Activated and Geopolymer Concrete. In Lecture Notes in Civil Engineering; Springer: Singapore, 2021; pp. 105–113. ISBN 9789811582929. [Google Scholar]
- Alnahhal, M.F.; Kim, T.; Hajimohammadi, A. Distinctive Rheological and Temporal Viscoelastic Behaviour of Alkali-Activated Fly Ash/Slag Pastes: A Comparative Study with Cement Paste. Cem. Concr. Res. 2021, 144, 106441. [Google Scholar] [CrossRef]
- Li, Z.; Wyrzykowski, M.; Dong, H.; Granja, J.; Azenha, M.; Lura, P.; Ye, G. Internal Curing by Superabsorbent Polymers in Alkali-Activated Slag. Cem. Concr. Res. 2020, 135, 106123. [Google Scholar] [CrossRef]
- Xu, C.; Ni, W.; Li, K.; Zhang, S.; Xu, D. Activation Mechanisms of Three Types of Industrial By-Product Gypsums on Steel Slag–Granulated Blast Furnace Slag-Based Binders. Constr. Build. Mater. 2021, 288, 123111. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.L.; Brouwers, H.J.H. Properties of Alkali Activated Slag-Fly Ash Blends with Limestone Addition. Cem. Concr. Compos. 2015, 59, 119–128. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.L.; Brouwers, H.J.H. Characterization of Alkali Activated Slag-Fly Ash Blends Containing Nano-Silica. Constr. Build. Mater. 2015, 98, 397–406. [Google Scholar] [CrossRef]
- Li, Z.; Liang, X.; Chen, Y.; Ye, G. Effect of Metakaolin on the Autogenous Shrinkage of Alkali-Activated Slag-Fly Ash Paste. Constr. Build. Mater. 2021, 278, 122397. [Google Scholar] [CrossRef]
- Kamath, M.V.; Prashanth, S.; Kumar, M.; Tantri, A. Machine-Learning-Algorithm to Predict the High-Performance Concrete Compressive Strength Using Multiple Data. J. Eng. Des. Technol. 2021. [Google Scholar] [CrossRef]
- Kumar, M.; Prashant, S.; Kamath, M.V. Enhancing the Sustainability of High Strength Concrete in Terms of Embodied Energy and Carbon Emission by Incorporating Sewage Sludge and Fly Ash. Innov. Infrastruct. Solut. 2022, 7, 240. [Google Scholar] [CrossRef]
- Kamath, M.; Prashant, S.; Kumar, M. Micro-Characterisation of Alkali Activated Paste with Fly Ash-GGBS-Metakaolin Binder System with Ambient Setting Characteristics. Constr. Build. Mater. 2021, 277, 122323. [Google Scholar] [CrossRef]
- ASTM C618-12; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use. ASTM: West Conshohocken, PA, USA, 2010; pp. 3–6. [CrossRef]
- IS 383:2016; IS383 Coarse and Fine Aggregate for Concrete, 3rd ed. BIS: Delhi, India, 2016; pp. 1–17.
- ASTM Standards C191-04; Time of Setting of Hydraulic Cement by Vicat Needle. ASTM: West Conshohocken, PA, USA, 2008; pp. 1–10. [CrossRef]
- ASTM C109/C109M-16a; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens). ASTM: West Conshohocken, PA, USA, 2016.
- ASTM C597; Standard Test Method for Pulse Velocity Through Concrete. ASTM: West Conshohocken, PA, USA, 2010; pp. 3–6. [CrossRef]
- Dai, X.; Aydin, S.; Yardimci, M.Y.; Lesage, K.; de Schutter, G. Influence of Water to Binder Ratio on the Rheology and Structural Build-up of Alkali-Activated Slag/Fly Ash Mixtures. Constr. Build. Mater. 2020, 264, 120253. [Google Scholar] [CrossRef]
- Almakhadmeh, M.; Soliman, A.M. Effects of Mixing Water Temperatures on Properties of One-Part Alkali-Activated Slag Paste. Constr. Build. Mater. 2021, 266, 121030. [Google Scholar] [CrossRef]
- Pham, T.M. Enhanced Properties of High-Silica Rice Husk Ash-Based Geopolymer Paste by Incorporating Basalt Fibers. Constr. Build. Mater. 2020, 245, 118422. [Google Scholar] [CrossRef]
- Abhishek, H.S.; Prashant, S.; Kamath, M.V.; Kumar, M. Fresh Mechanical and Durability Properties of Alkali-Activated Fly Ash-Slag Concrete: A Review. Innov. Infrastruct. Solut. 2022, 7, 116. [Google Scholar] [CrossRef]
- Ng, C.; Alengaram, U.J.; Wong, L.S.; Mo, K.H.; Jumaat, M.Z.; Ramesh, S. A Review on Microstructural Study and Compressive Strength of Geopolymer Mortar, Paste and Concrete. Constr. Build. Mater. 2018, 186, 550–576. [Google Scholar] [CrossRef]
- Naghizadeh, A.; Ekolu, S.O. Method for Comprehensive Mix Design of Fly Ash Geopolymer Mortars. Constr. Build. Mater. 2019, 202, 704–717. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Liu, J.C.; Wu, B. Mechanical Properties and Reaction Mechanism of One-Part Geopolymer Mortars. Constr. Build. Mater. 2021, 273, 121973. [Google Scholar] [CrossRef]
- Xu, L.Y.; Alrefaei, Y.; Wang, Y.S.; Dai, J.G. Recent Advances in Molecular Dynamics Simulation of the N-A-S-H Geopolymer System: Modeling, Structural Analysis, and Dynamics. Constr. Build. Mater. 2021, 276, 122196. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; MacPhee, D.E. Compatibility Studies between N-A-S-H and C-A-S-H Gels. Study in the Ternary Diagram Na2O-CaO-Al2O3-SiO 2-H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Cercel, J.; Adesina, A.; Das, S. Performance of Eco-Friendly Mortars Made with Alkali-Activated Slag and Glass Powder as a Binder. Constr. Build. Mater. 2021, 270, 121457. [Google Scholar] [CrossRef]
- Farhan, N.A.; Sheikh, M.N.; Hadi, M.N.S. Investigation of Engineering Properties of Normal and High Strength Fly Ash Based Geopolymer and Alkali-Activated Slag Concrete Compared to Ordinary Portland Cement Concrete. Constr. Build. Mater. 2019, 196, 26–42. [Google Scholar] [CrossRef]
- Sitarz, M.; Hager, I.; Choińska, M. Evolution of Mechanical Properties with Time of Fly-ash-based Geopolymer Mortars under the Effect of Granulated Ground Blast Furnace Slag Addition. Energies 2020, 13, 1135. [Google Scholar] [CrossRef]
- Abdalqader, A.F.; Jin, F.; Al-Tabbaa, A. Development of Greener Alkali-Activated Cement: Utilisation of Sodium Carbonate for Activating Slag and Fly Ash Mixtures. J. Clean. Prod. 2016, 113, 66–75. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Z.; Ghiassi, B.; Yin, S.; Ye, G. Fracture Properties and Microstructure Formation of Hardened Alkali-Activated Slag/Fly Ash Pastes. Cem. Concr. Res. 2021, 144, 106447. [Google Scholar] [CrossRef]
- Cao, Y.F.; Tao, Z.; Pan, Z.; Wuhrer, R. Effect of Calcium Aluminate Cement on Geopolymer Concrete Cured at Ambient Temperature. Constr. Build. Mater. 2018, 191, 242–252. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, X.; Hou, D.; Zhao, T.; Cui, Y. The Effect of Mechanical Load on Transport Property and Pore Structure of Alkali-Activated Slag Concrete. Constr. Build. Mater. 2018, 189, 397–408. [Google Scholar] [CrossRef]
- Li, Z.; Li, S. Effects of Wetting and Drying on Alkalinity and Strength of Fly Ash/Slag-Activated Materials. Constr. Build. Mater. 2020, 254, 119069. [Google Scholar] [CrossRef]
- Lothenbach, B.; Kulik, D.A.; Matschei, T.; Balonis, M.; Baquerizo, L.; Dilnesa, B.; Miron, G.D.; Myers, R.J. Cemdata18: A Chemical Thermodynamic Database for Hydrated Portland Cements and Alkali-Activated Materials. Cem. Concr. Res. 2019, 115, 472–506. [Google Scholar] [CrossRef]
- Marathe, S.; Mithanthaya, I.R.; Shenoy, R.Y. Durability and Microstructure Studies on Slag-Fly Ash-Glass Powder Based Alkali Activated Pavement Quality Concrete Mixes. Constr. Build. Mater. 2021, 287, 123047. [Google Scholar] [CrossRef]
- Sun, R.; Fang, C.; Zhang, H.; Ling, Y.; Feng, J.; Qi, H.; Ge, Z. Chemo-Mechanical Properties of Alkali-Activated Slag/Fly Ash Paste Incorporating White Mud. Constr. Build. Mater. 2021, 291, 123312. [Google Scholar] [CrossRef]
- Salih, M.A.; Abang Ali, A.A.; Farzadnia, N. Characterization of Mechanical and Microstructural Properties of Palm Oil Fuel Ash Geopolymer Cement Paste. Constr. Build. Mater. 2014, 65, 592–603. [Google Scholar] [CrossRef]
- Jagadisha; Balakrishna Rao, K.; Nayak, G.; Adithya Shenoy, B. A Review on Properties of Sustainable Concrete Using Iron and Steel Slag Aggregate as Replacement for Natural Aggregate; Springer: Singapore, 2021; Volume 105, ISBN 9789811582929. [Google Scholar]
- Wang, R.; Wang, J.; Dong, T.; Ouyang, G. Structural and Mechanical Properties of Geopolymers Made of Aluminosilicate Powder with Different SiO2/Al2O3 Ratio: Molecular Dynamics Simulation and Microstructural Experimental Study. Constr. Build. Mater. 2020, 240, 117935. [Google Scholar] [CrossRef]
- Zerzouri, M.; Bouchenafa, O.; Hamzaoui, R.; Ziyani, L.; Alehyen, S. Physico-Chemical and Mechanical Properties of Fly Ash Based-Geopolymer Pastes Produced from Pre-Geopolymer Powders Obtained by Mechanosynthesis. Constr. Build. Mater. 2021, 288, 123135. [Google Scholar] [CrossRef]
- Lin, H.; Liu, H.; Li, Y.; Kong, X. Properties and Reaction Mechanism of Phosphoric Acid Activated Metakaolin Geopolymer at Varied Curing Temperatures. Cem. Concr. Res. 2021, 144, 106425. [Google Scholar] [CrossRef]
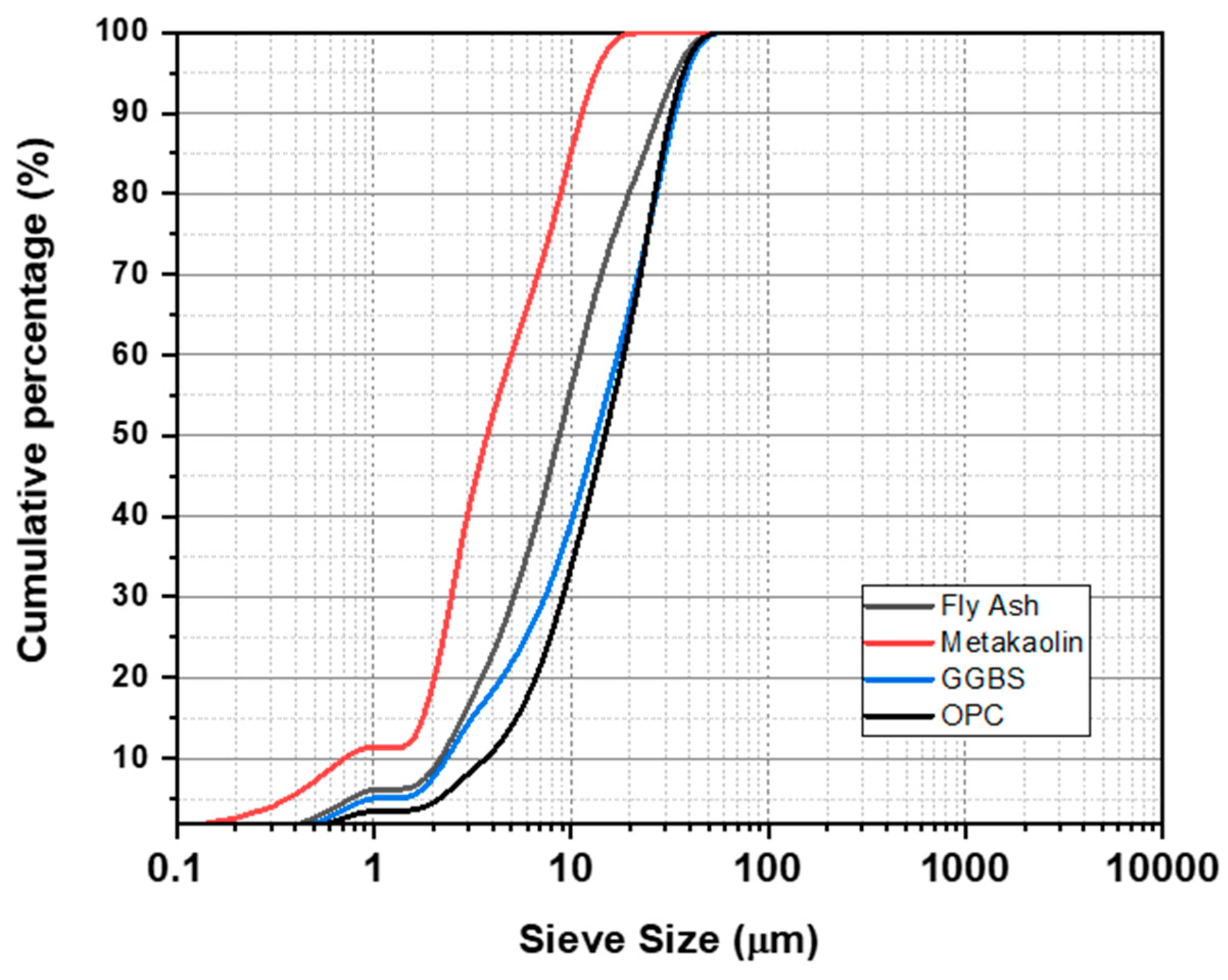
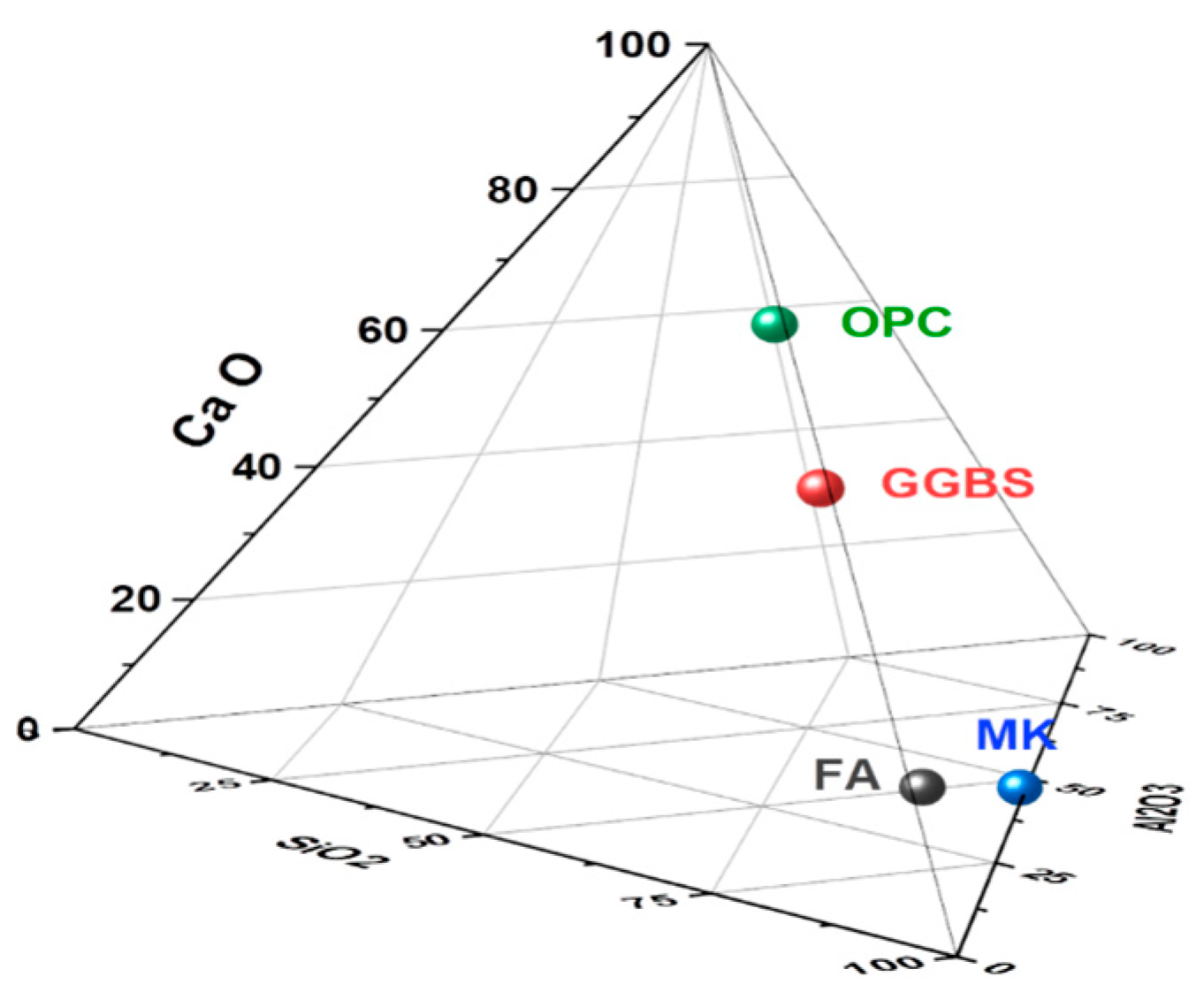
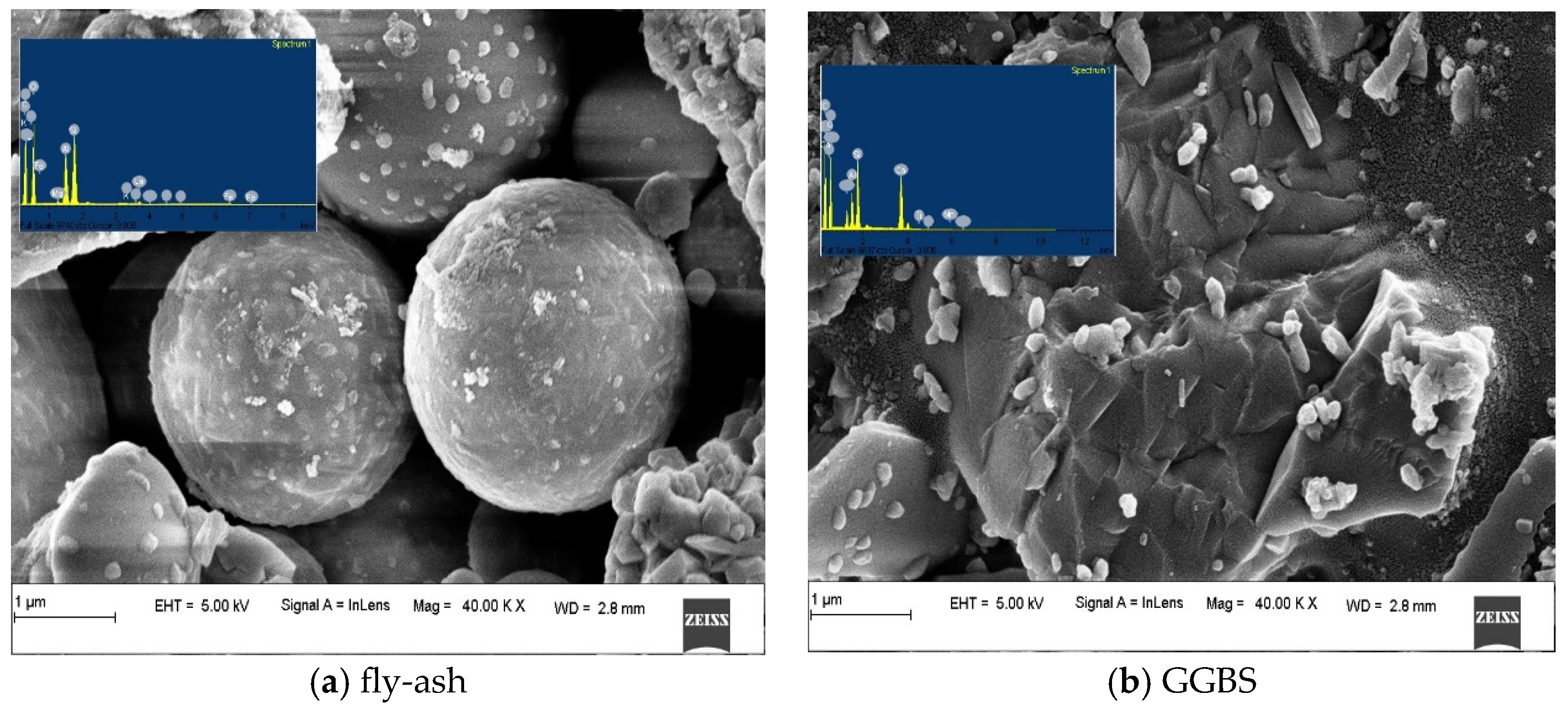
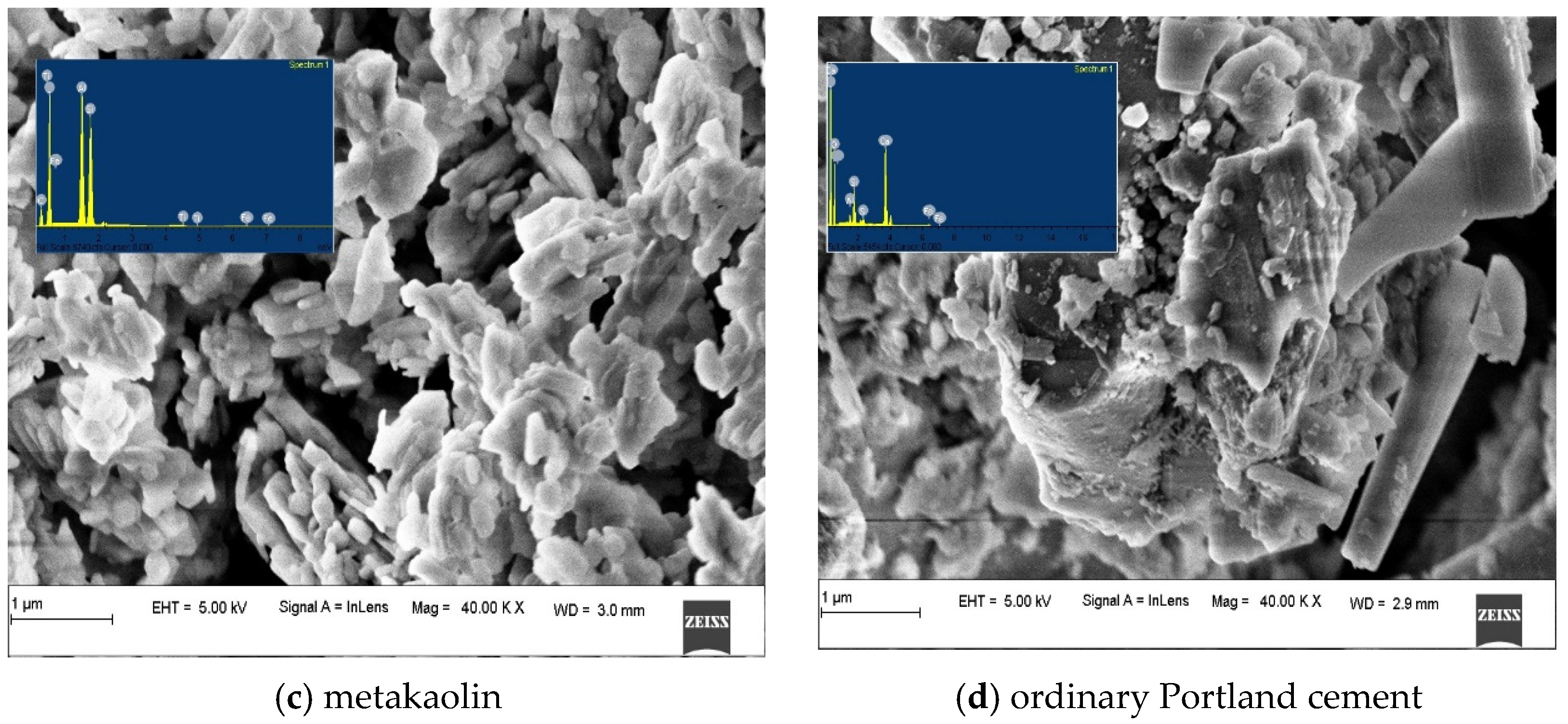




| Materials | Fly Ash | GGBS | Metakaolin | OPC | ||
|---|---|---|---|---|---|---|
| Physical-Properties | Specific-surface area (m2/g) | 0.454 | 0.5 | 19–20 | 0.33 | |
| Specific Gravity | 2.24 | 2.67 | 2.6 | 3.15 | ||
| Particle Size(µm) | d10 | 2.17 | 2.33 | 0.71 | 3.70 | |
| d50 | 8.72 | 13.37 | 3.75 | 14.83 | ||
| d90 | 27.99 | 33.36 | 11.37 | 32.20 | ||
| Chemical-Properties (wt.%) | SiO2 | 54.11 | 40 | 52 | 20.21 | |
| Al2O3 | 26.51 | 4.1 | 46 | 9.08 | ||
| Fe2O3 | 6.4 | 2 | 0.6 | 3.64 | ||
| Ca O | 4.7 | 42 | 0.09 | 59.67 | ||
| Mg O | 1.04 | 6.2 | 0.03 | 2.02 | ||
| SO3 | 1.29 | 0.1 | - | 2.41 | ||
| Na2O | 2.22 | - | 0.1 | - | ||
| K2O | 0.87 | - | 0.03 | - | ||
| TiO2 | - | - | 0.65 | 0.45 | ||
| LOI | 2.85 | 0.25 | - | 1.45 | ||
| Binders | Chemical Element (% by wt.) and, Their Ratio | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mg | Al | Si | Ca | Ti | Fe | S | Mn | K | Si/Al | Ca/Si | Ca/Al | |
| OPC | - | 0.84 | 3.57 | 12.62 | - | 0.86 | 0.51 | - | - | 4.25 | 3.54 | 15.02 |
| GGBS | 1.88 | 3.41 | 6.91 | 7.91 | 0.14 | - | - | 0.18 | - | 2.03 | 1.14 | 2.31 |
| FA | 0.28 | 6.07 | 9.53 | 0.6 | 0.25 | 0.78 | - | - | 0.28 | 1.57 | 0.06 | 0.098 |
| MK | - | 9.63 | 9.35 | - | 0.23 | 0.22 | - | - | - | 0.97 | 0.00 | 0 |
| Mix ID | Designation | Content (%) | Alkali(M) | SH/SS | S/B | T (°C) |
|---|---|---|---|---|---|---|
| 1 | FA | 100 | 12 | 2.5 | 0.35 | Ambient |
| 2 | GGBS | 100 | 12 | 2.5 | 0.35 | Ambient |
| 3 | MK | 100 | 12 | 2.5 | 0.35 | Ambient |
| 4 | OPC | 100 | - | - | 0.35 | Moist |
| Sl. No. | Parameter Studied | Standards/Instrument |
|---|---|---|
| 1 | Setting time | ASTM C191 [46] |
| 2 | Compressive strength | ASTM C109 [47] |
| 3 | UPV | ASTM C597 [48] |
| 4 | FESEM | Carl Zeiss FESEM, Oxford Instrument |
| 5 | EDS | Carl Zeiss FESEM, Oxford Instrument |
| 6 | XRD | Rigaku Mini flex |
| 7 | FTIR | JASCO FT/IR-6300 |
| Mixes | Spectrum | Elements (% by wt.) and Their Ratio | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na | Al | Si | Ca | Si + Al | Si/Al | Ca/ (Si + Al) | Na/ (Si + Al) | Ca/Si | Na/Al | Na/Si | Ca/Al | ||
| FA | 1 | 5.84 | 3.08 | 9.57 | 0.36 | 12.65 | 3.11 | 0.03 | 0.46 | 0.04 | 1.90 | 0.61 | 0.12 |
| 2 | 6.04 | 3.2 | 9.97 | 0.37 | 13.17 | 3.12 | 0.03 | 0.46 | 0.04 | 1.89 | 0.61 | 0.12 | |
| GGBS | 1 | 8.88 | 1.77 | 5.42 | 1.63 | 7.19 | 3.06 | 0.23 | 1.24 | 0.30 | 5.02 | 1.64 | 0.92 |
| 2 | 9.06 | 1.97 | 5.47 | 1.58 | 7.44 | 2.78 | 0.21 | 1.22 | 0.29 | 4.60 | 1.66 | 0.80 | |
| MK | 1 | 2.97 | 6.37 | 7.46 | 0.02 | 13.83 | 1.17 | 0.00 | 0.21 | 0.00 | 0.47 | 0.40 | 0.00 |
| 2 | 4.84 | 6.18 | 7.21 | 0.02 | 13.39 | 1.17 | 0.00 | 0.36 | 0.00 | 0.78 | 0.67 | 0.00 | |
| OPC | 1 | 0.12 | 1.07 | 4.34 | 15.14 | 5.41 | 4.06 | 2.80 | 0.02 | 3.49 | 0.11 | 0.03 | 14.15 |
| 2 | 0 | 1.09 | 4.41 | 15.31 | 5.50 | 4.05 | 2.78 | 0.00 | 3.47 | 0.00 | 0.00 | 14.05 | |
| Samples | PDF Card No. | Compound Name | Chem. Formula |
|---|---|---|---|
| Fly Ash | |||
| 96-900-9667 | Quartz | Si3O6 | |
| 96-900-9471 | Reyerite | Na1.6Ca14Si22Al2O72H8 | |
| 96-900-5048 | Natrolite | Na16Al16Si24O96H32 | |
| GGBS | |||
| 96-101-1270 | Chabazite | Ca6Al12Si24O108H0 | |
| 96-100-0047 | Clinotobermorite | Ca5Si6O18H0 | |
| MK | |||
| 96-153-3379 | NASH | Na32Si64H32O160 | |
| 96-210-7160 | NASH | Na24Si8H40O48 | |
| 96-901-2602 | Quartz | Si3O6 | |
| 96-900-1409 | Sillimanite | Al8Si4O20 | |
| OPC | 96-100-0047 | Clinotobermorite | Ca5Si6O18H0 |
| 96-100-8782 | Portlandite | Ca1O2H2 | |
| 96-901-5085 | Ettringite | Ca12Al4S6O100 H128 | |
| 96-901-6216 | Vaterite | Ca4.00 C4.00 O12.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamath, M.; Prashant, S.; Ralegaonkar, R. Microstructure Properties of Popular Alkali-Activated Pastes Cured in Ambient Temperature. Buildings 2023, 13, 858. https://doi.org/10.3390/buildings13040858
Kamath M, Prashant S, Ralegaonkar R. Microstructure Properties of Popular Alkali-Activated Pastes Cured in Ambient Temperature. Buildings. 2023; 13(4):858. https://doi.org/10.3390/buildings13040858
Chicago/Turabian StyleKamath, Muralidhar, Shreelaxmi Prashant, and Rahul Ralegaonkar. 2023. "Microstructure Properties of Popular Alkali-Activated Pastes Cured in Ambient Temperature" Buildings 13, no. 4: 858. https://doi.org/10.3390/buildings13040858
APA StyleKamath, M., Prashant, S., & Ralegaonkar, R. (2023). Microstructure Properties of Popular Alkali-Activated Pastes Cured in Ambient Temperature. Buildings, 13(4), 858. https://doi.org/10.3390/buildings13040858







