Compound Effects of Sodium Chloride and Gypsum on the Compressive Strength and Sulfate Resistance of Slag-Based Geopolymer Concrete
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiment Methods
- —dynamic elastic modulus ()
- —relative dynamic elastic modulus
- V—ultrasonic velocity ()
- —density of concrete ()
- —Poisson ratio
- —ultrasonic velocity of specimen before erosion ()
- —ultrasonic velocity of specimen after n days immersion ()
- —sound time before erosion ()
- —sound time after n days of erosion ()
- Wb—mass before immersion
- Wh—mass after immersion
- R2—compressive strength in sodium sulfate solution
- R1—compressive strength in water
3. Results and Analysis
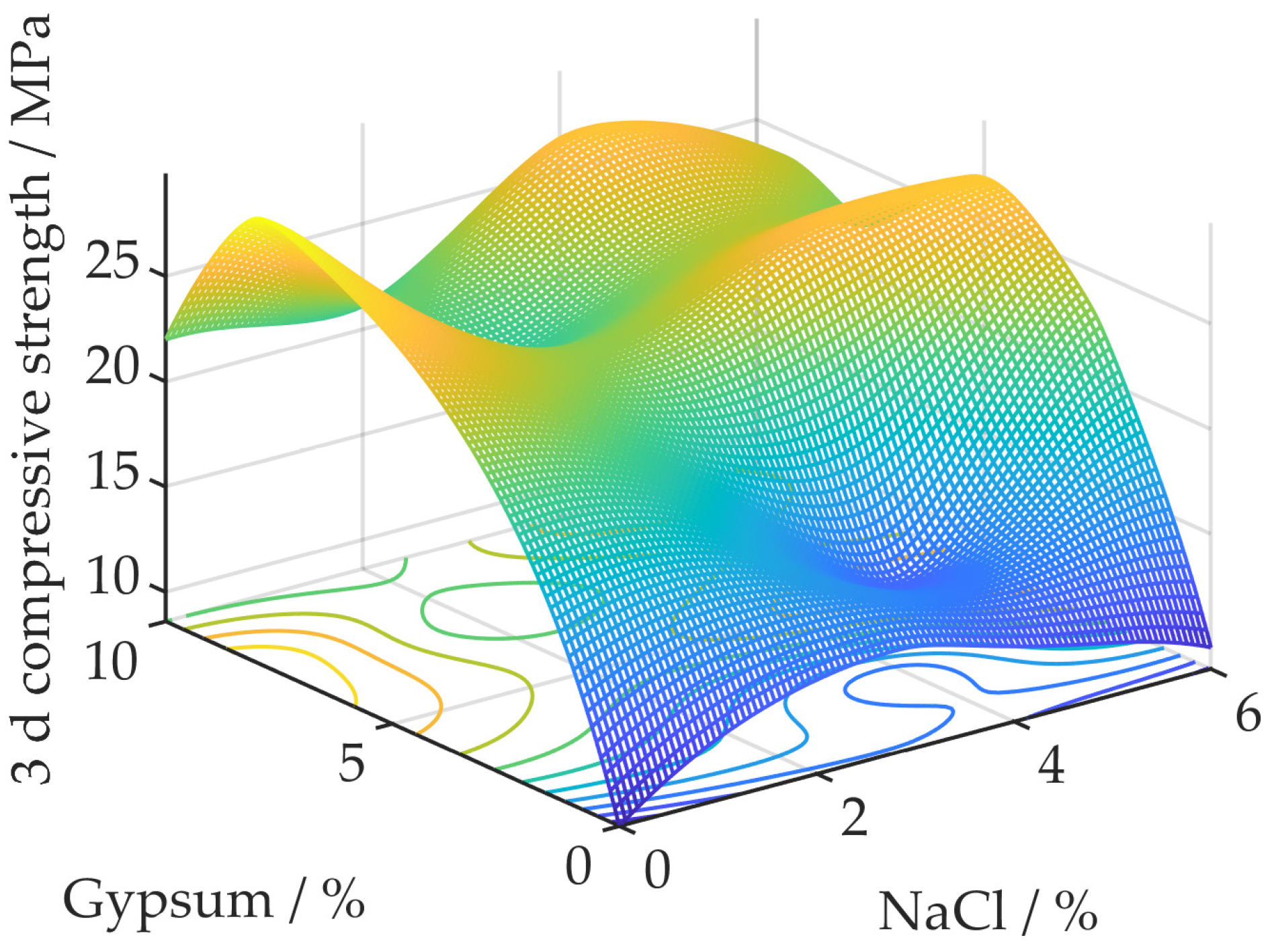
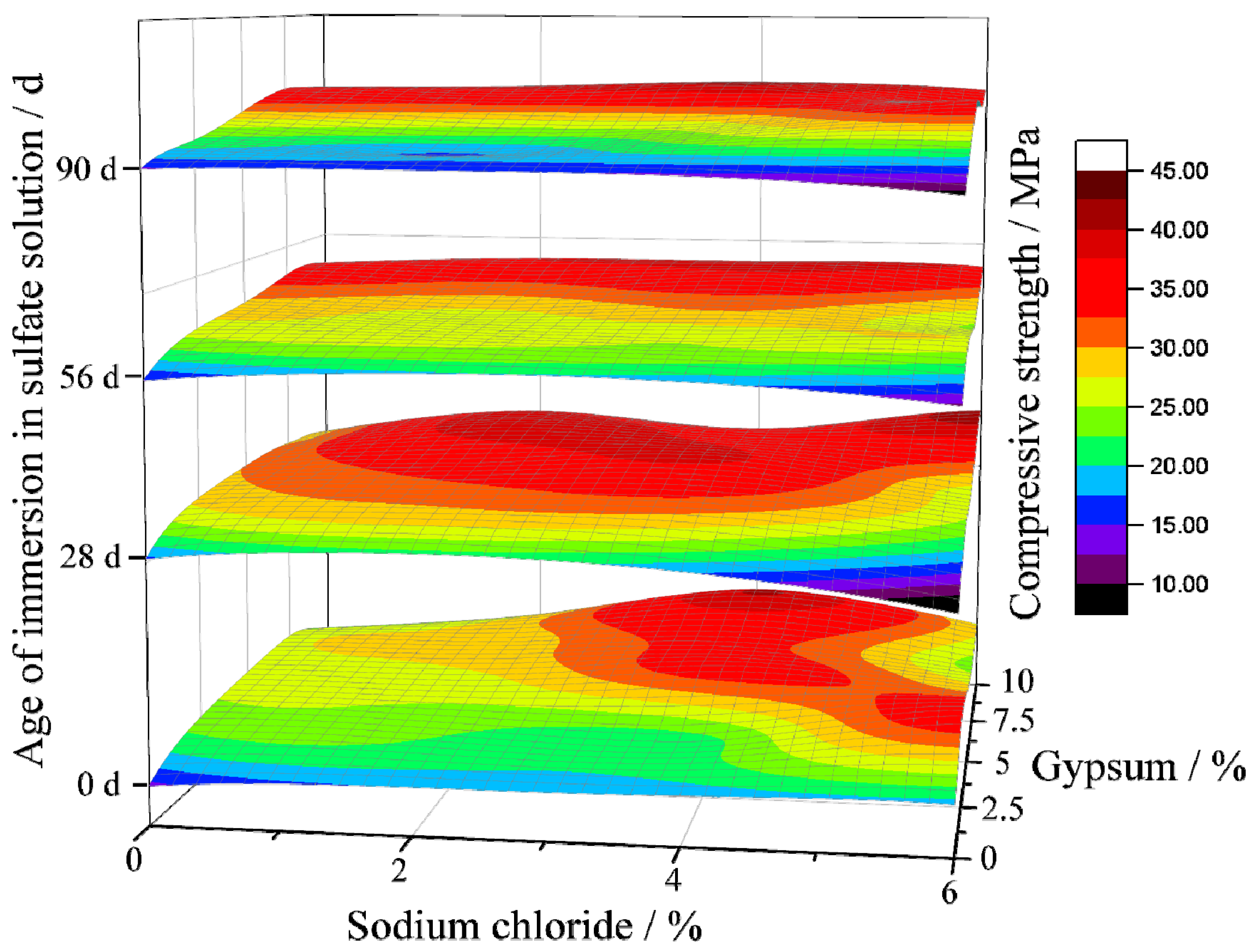
3.1. Compound Effect of NaCl and Gypsum on the Compressive Strength of Geopolymer Concrete
3.2. Effect of NaCl and Gypsum on Sulfate Resistance of Geopolymer Concrete
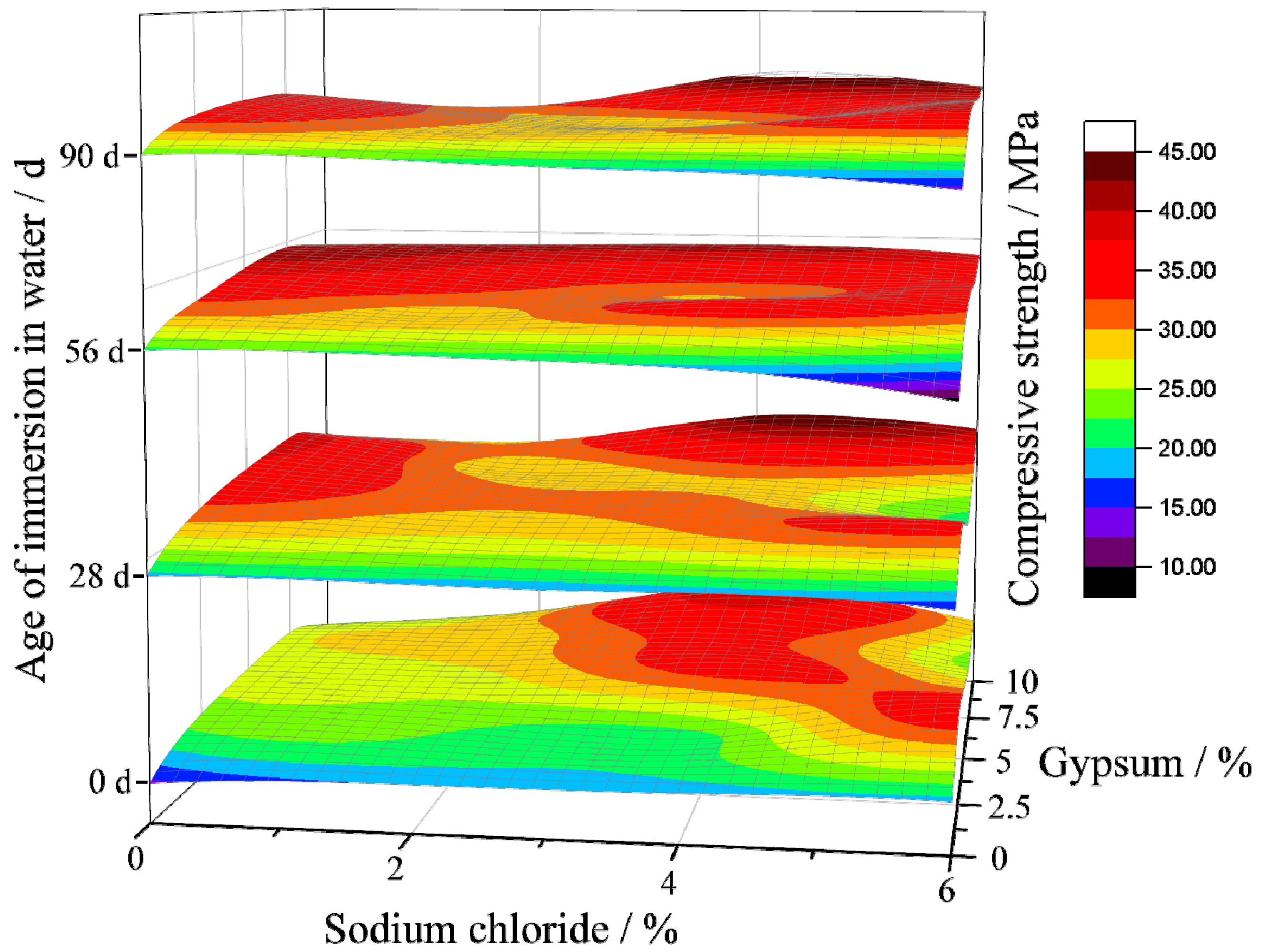
3.2.1. Strength under Water and Sulfate Immersion Environment
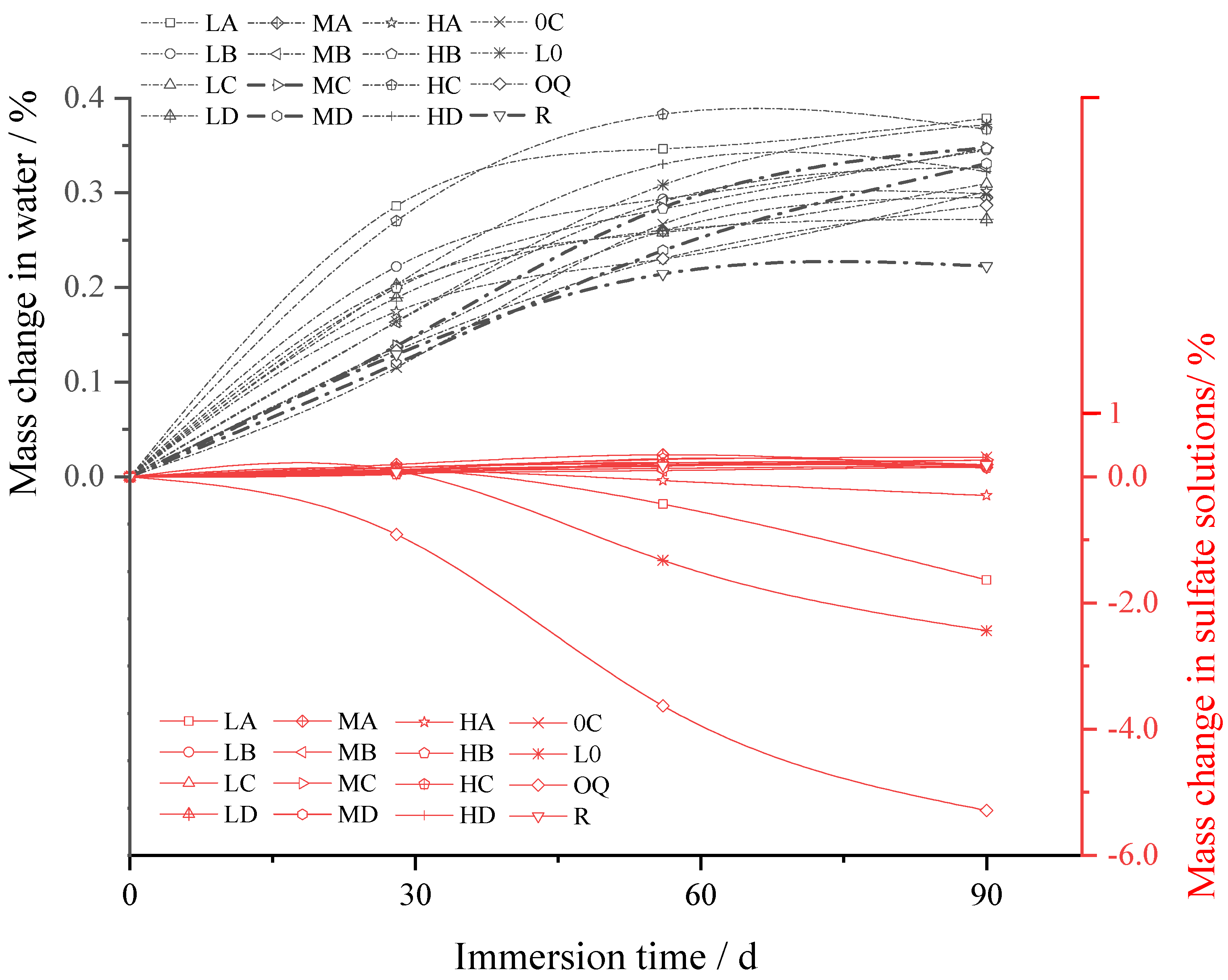
3.2.2. Mass Change
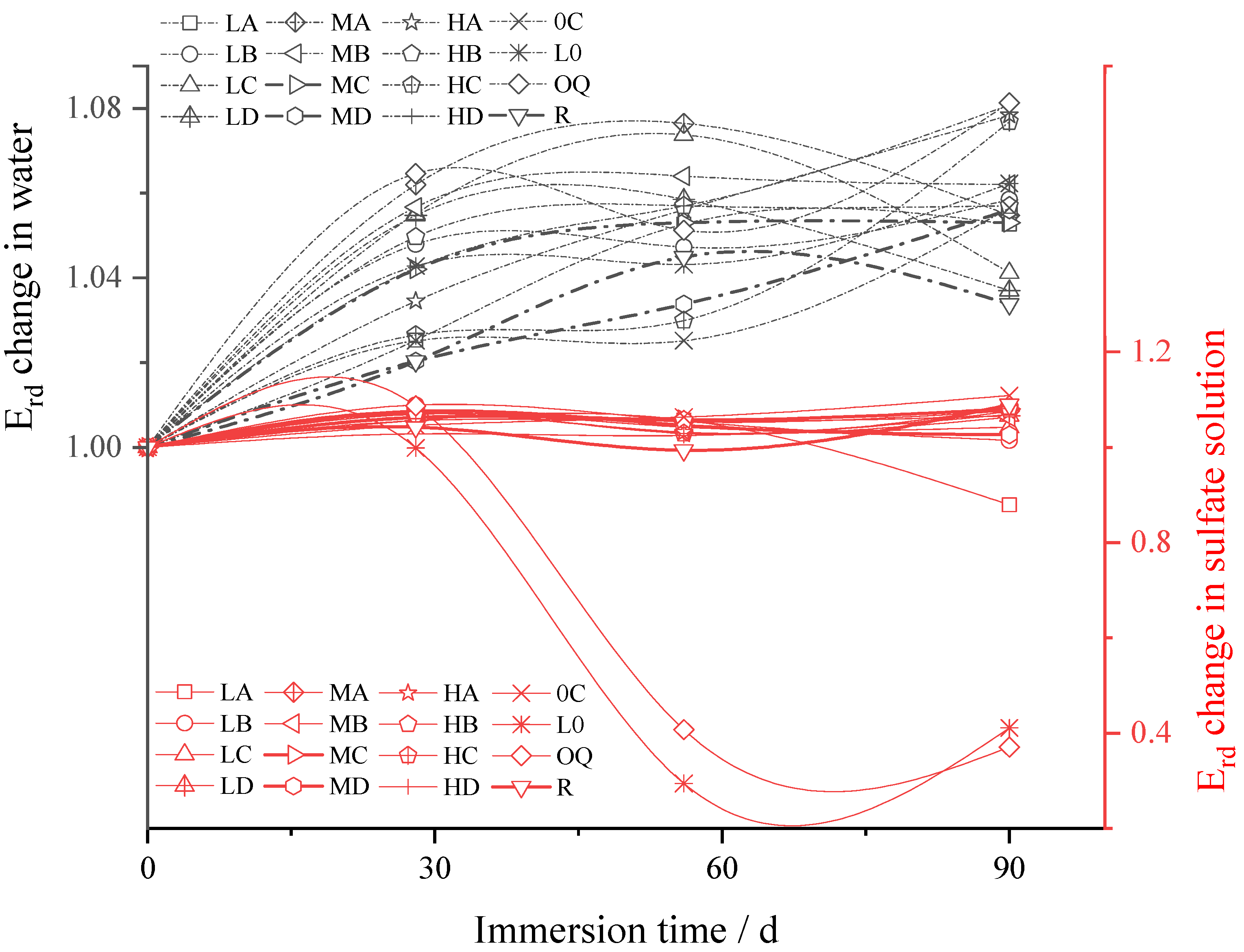
3.2.3. Change of Relative Dynamic Elastic Modulus
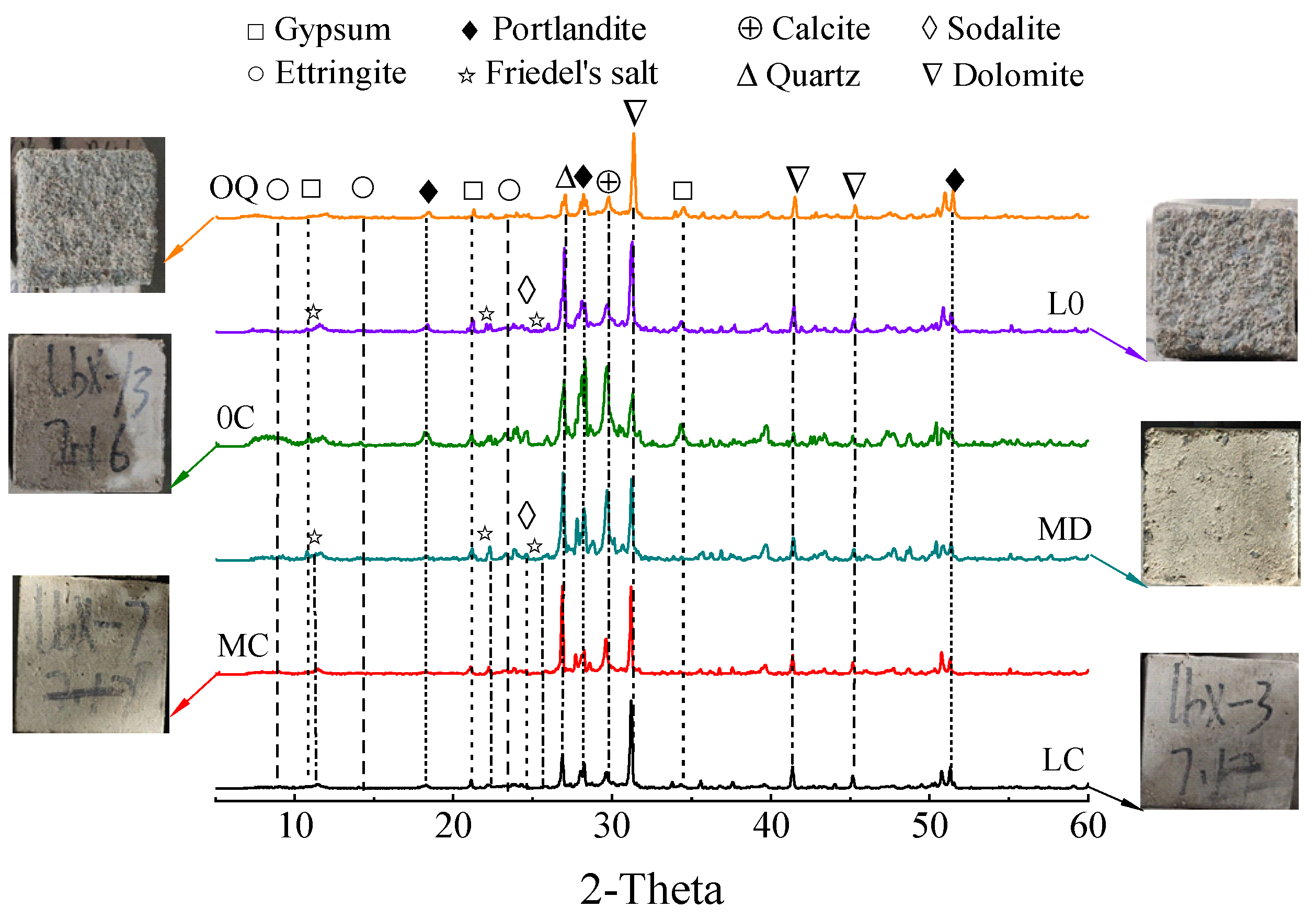
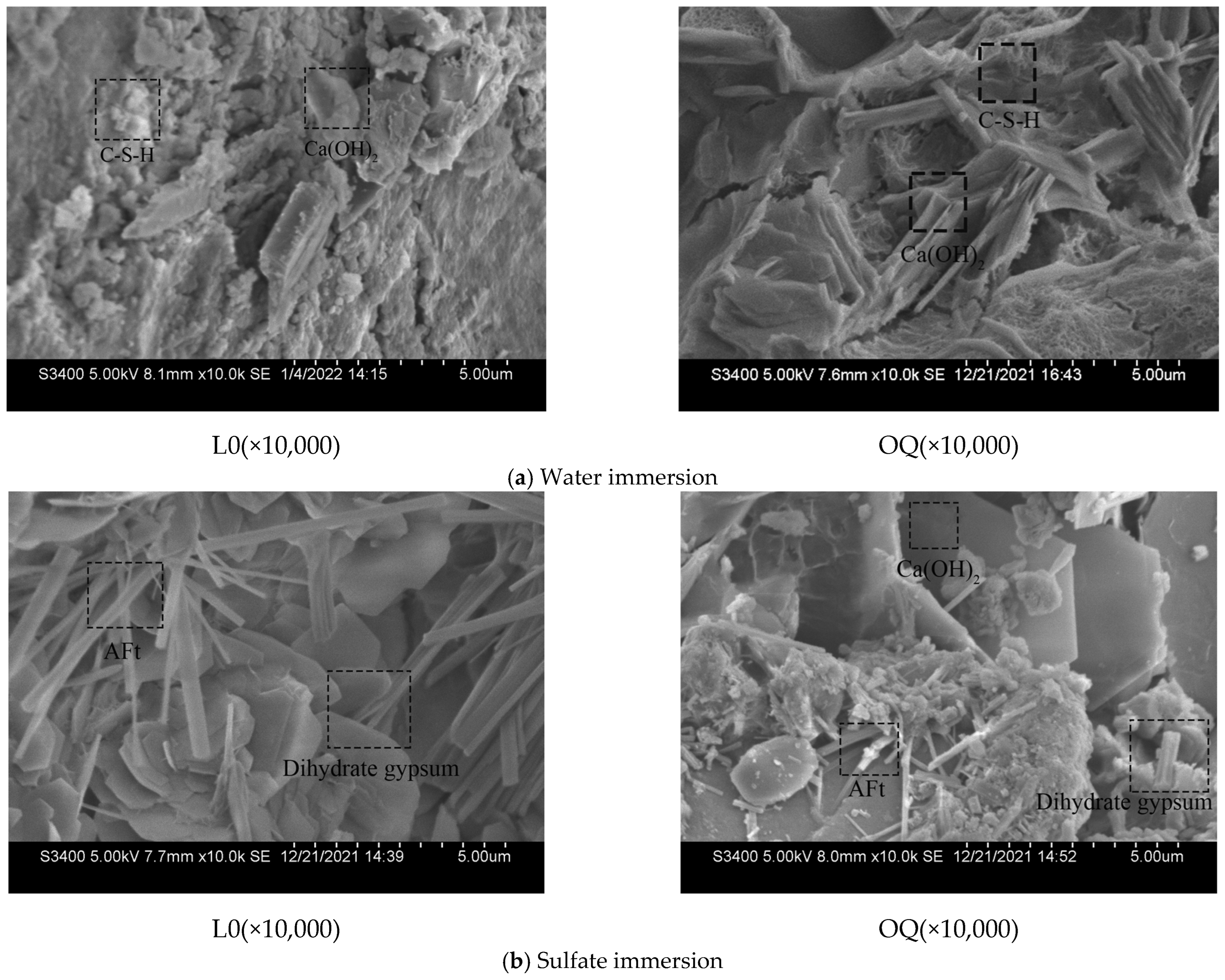
3.2.4. Sulfate Attack Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di, D.; Liu, Y.; Li, J. Analysis and Revision Opinions on the Norm of Energy Consumption per Unit Product of Cement. Cem. Technol. 2021, 3, 15–21. (In Chinese) [Google Scholar] [CrossRef]
- GB 16780-2012; The Norm of Energy Consumption per Unit Products of Cement. Standards Press of China: Beijing, China, 2013. (In Chinese)
- Hoenig, V.; Hoppe, H.; Emberger, B. Carbon Capture Technology-Options and Potentials for the Cement Industry; European Cement Research Academy: Duesseldorf, Germany, 2007. [Google Scholar]
- Prabahar, J.; Vafaei, B.; Ghahremaninezhad, A. The Effect of Hydrogels with Different Chemical Compositions on the Behavior of Alkali-Activated Slag Pastes. Gels 2022, 8, 731. [Google Scholar] [CrossRef] [PubMed]
- Cormos, C.C. Decarbonization options for cement production process: A techno-economic and environmental evaluation. Fuel 2022, 320, 123907. [Google Scholar] [CrossRef]
- Yasin, O.; Mohammad, J.; Hassan, K.M.; Alireza, Z.A.; Kazem, S.; Afsaneh, L.S.; Rozhin, D. Numerical and experimental investigation of natural gas injection effects on NOx reburning at the rotary cement kiln exhaust. Process Saf. Environ. Prot. 2021, 151, 290–298. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, C.; Li, B.; Wang, J.; Ravat, R.; Chen, X.; Wie, J.; Yu, Q. Linking the SO2 emission of cement plants to the sulfur characteristics of their limestones: A study of 80 NSP cement lines in China. J. Clean. Prod. 2019, 220, 200–211. [Google Scholar] [CrossRef]
- Statistical Communique of 2021 National Economic and Social Development of the People’s Republic of China. Available online: http://www.stats.gov.cn/xxgk/sjfb/zxfb2020/202202/t20220228_1827971.html (accessed on 28 February 2022).
- Liu, Z. Perspective Cement Production Changes. China Build. Mater. 2022, 4, 114–118. (In Chinese) [Google Scholar] [CrossRef]
- Deep Understanding and Grasp Carbon Peak Carbon Neutrality. Available online: https://m.gmw.cn/baijia/2022-09/01/35994488.html (accessed on 1 September 2022).
- Ding, M. Carbon emissions in cement industry: Status analysis and discussion on the key path of emission reduction. China Cem. 2021, 230, 46–49. [Google Scholar] [CrossRef]
- Shan, Y.; Huang, Q.; Guan, D.; Hubacek, K. China CO2 emission accounts 2016–2017. Sci. Data 2020, 7, 465–474. [Google Scholar] [CrossRef]
- Prabahar, J.; Vafaei, B.; Baffoe, E.; Ghahremaninezhad, A. The Effect of Biochar on the Properties of Alkali-Activated Slag Pastes. Constr. Mater. 2022, 2, 1–14. [Google Scholar] [CrossRef]
- Wang, L.; Luo, R.; Zhang, W.; Jin, M.; Tang, S. Effects of fineness and content of phosphorus slag on cement hydration, permeability, pore structure and fractal dimension of concrete. Fractals 2021, 29, 2140004. [Google Scholar] [CrossRef]
- Ibrahim, Y.E.; Adamu, M.; Marouf, M.L.; Ahmed, O.S.; Drmosh, Q.A.; Malik, M.A. Mechanical Performance of Date-Palm-Fiber-Reinforced Concrete Containing Silica Fume. Buildings 2022, 12, 1642. [Google Scholar] [CrossRef]
- Živica, V. Effects of type and dosage of alkaline activator and temperature on the properties of alkali-activated slag mixtures. Constr. Build. Mater. 2007, 21, 1463–1469. [Google Scholar] [CrossRef]
- Roy, A.; Schilling, P.J.; Eaton, H.C.; Malone, P.G.; Brabston, W.N.; Wakeley, L.D. Activation of Ground Blast-Furnace Slag by Alkali-Metal and Alkaline-Earth Hydroxides. J. Am. Ceram. Soc. 1992, 75, 3233–3240. [Google Scholar] [CrossRef]
- Provis, J.L. Activating solution chemistry for geopolymers. In Geopolymers; Provis, J.L., Deventer, J.S.J., Eds.; Woodhead Publishing: Sawston, UK, 2009; Volume 1, pp. 50–71. [Google Scholar] [CrossRef]
- Dai, X.; Aydin, S.; Yardimci, M.Y.; Schutter, G.D. Rheology and structural build-up of sodium silicate- and sodium hydroxide-activated GGBFS mixtures. Cem. Concr. Compos. 2022, 131, 104570. [Google Scholar] [CrossRef]
- Wang, S.; Scrivener, K.L.; Pratt, P.L. Factors affecting the strength of alkali-activated slag. Cem. Concr. Res. 1994, 24, 1033–1043. [Google Scholar] [CrossRef]
- Qiao, Y. Comparative analysis of caustic soda production by diaphragm method and ion membrane method. Qilu Petrochem. Technol. 2010, 38, 108–110. (In Chinese) [Google Scholar] [CrossRef]
- Chen, Z. Caustic potash production status in domestic and abroad. China Chlor-Alkali 2003, 7, 5–8. (In Chinese) [Google Scholar] [CrossRef]
- He, W.; Liu, C.; Zhang, L. Effects of sodium chloride on the mechanical properties of slag composite matrix geopolymer. Adv. Cem. Res. 2019, 31, 389–398. [Google Scholar] [CrossRef]
- Liu, C.; Ji, H.; Liu, J. Experimental study on slag composite cementitious material for solidifying coastal saline soil. J. Build. Mater. 2015, 18, 82–87. (In Chinese) [Google Scholar] [CrossRef]
- DB12 046.47-2008; Calculation Method and Stipulation of Comprehensive Energy Consumption Norm for per Unit Product of Calces. Tianjin Bureau of Quality and Technical Supervision: Tianjin, China, 2008. (In Chinese)
- GB 33654-2017; The Norm of Energy Consumption per Unit Throughput of Gypsum Plaster. Standards Press of China: Beijing, China, 2017. (In Chinese)
- DB13-T 5324-2020; Energy Consumption Limit per Unit Product of Granulated Blast Furnace slag Powder Used in Cement, Mortar and Concrete. Market Supervision Administration of Hebei Province: Tianjin, China, 2020. (In Chinese)
- Chen, E.; Li, T. The ecological and environmental protection effect of high-quality granulated blast furnace slag powder. China Concrete 2011, 23, 24–28. [Google Scholar]
- Wang, Y.; Zhong, X. CO2 Emissions and Influencing Factors in China’s Lime Industry. J. Subtrop. Resour. Environ. 2018, 13, 7–12. (In Chinese) [Google Scholar] [CrossRef]
- Wang, Y. Construction of industrial by-product gypsum deep processing and storage and transportation base to promote industrial by-product gypsum resource utilization. China Build. Mater. News 2021, 3, 10. (In Chinese) [Google Scholar] [CrossRef]
- Cai, G.; Zhou, Y.; Pan, Z. Physical and mechanical performance of quicklime-activated GGBS stabilized Hong Kong marine sediment at high water content. Rock Soil Mech. 2022, 43, 327–336. (In Chinese) [Google Scholar] [CrossRef]
- Liu, S.; Liu, G.; Li, G. Mechanism of Early Hydration of Alkali-activated Cementitious Materials Controlled by Quicklime. Min. Res. Dev. 2021, 41, 48–52. (In Chinese) [Google Scholar] [CrossRef]
- Wang, Q.; Sun, S.; Yao, G.; Wang, Z.; Lyu, X. Preparation and characterization of an alkali-activated cementitious material with blast-furnace slag, soda sludge, and industrial gypsum. Constr. Build. Mater. 2022, 340, 127735. [Google Scholar] [CrossRef]
- Li, H.; Yang, X.; Wen, W. Optimization Test of the Proportion of Phosphogypsum-slag Composite Cementitious Material. Met. Mine 2021, 3, 28. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, L.; Song, S.; Liang, Q. Effect of gypsum content on properties of alkali-activated slag cement mortar. Appl. Chem. Ind. 2020, 49, 2168–2172+2177. (In Chinese) [Google Scholar] [CrossRef]
- Joshi, N.R.; Matsumoto, A.; Asamoto, S.; Miura, T.; Kawabata, K. Investigation of the mechanical behaviour of concrete with severe delayed ettringite formation expansion focusing on internal damage propagation under various compressive loading patterns. Cem. Concr. Compos. 2022, 128, 104433. [Google Scholar] [CrossRef]
- Althoey, F. Compressive strength reduction of cement pastes exposed to sodium chloride solutions: Secondary ettringite formation. Constr. Build. Mater. 2021, 299, 123965. [Google Scholar] [CrossRef]
- Yan, P.; Qin, X. The effect of expansive agent and possibility of delayed ettringite formation in shrinkage-compensating massive concrete. Cem. Concr. Res. 2001, 31, 335–337. (In Chinese) [Google Scholar] [CrossRef]
- Lee, H.; Cody, R.D.; Cody, A.M.; Spry, P.G. The formation and role of ettringite in Iowa highway concrete deterioration. Cem. Concr. Res. 2005, 35, 332–343. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, X. Experiment on effect of chlorine salt on strength of alkali—Activated slag paste. J. Beijing Univ. Aeronaut. Astronaut. 2015, 41, 693–700. (In Chinese) [Google Scholar] [CrossRef]
- Liu, C.; Fan, J.; Gao, X.; Zhou, Y.; Chen, X. Compressive flexing resistance and microstructure of NaCl-mixed slag cementitious agent. J. Lanzhou Univ. Technol. 2014, 40, 130–134. (In Chinese) [Google Scholar] [CrossRef]
- Shi, Z.; Shui, Z.; Li, Q.; Geng, H. Combined effect of metakaolin and sea water on performance and microstructures of concrete. Constr. Build. Mater. 2015, 74, 57–64. [Google Scholar] [CrossRef]
- Barberon, F.; Baroghel-Bouny, V.; Zanni, H.; Bresson, B.; Caillerie, J.B.E.; Malosse, L.; Gan, Z. Interactions between chloride and cement-paste materials. Magn. Reson. Imaging 2005, 23, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Pel, L.; Zhang, X.; Sun, Z. Cl− and Na+ ions binding in slag and fly ash cement paste during early hydration as studied by 1H, 35Cl and 23Na NMR. Constr. Build. Mater. 2021, 266, 121606. [Google Scholar] [CrossRef]
- Suryavanshi, A.K.; Scantlebury, J.D.; Lyon, S.B. Pore size distribution of OPC & SRPC mortars in presence of chlorides. Cem. Concr. Res. 1995, 25, 980–988. [Google Scholar] [CrossRef]
- Wang, S.; Huang, Y.; Wang, Z. Concrete resistance to chloride ingress: Effect of cement composition. J. Chin. Ceram. Soc. 2000, 6, 570–574. (In Chinese) [Google Scholar] [CrossRef]
- Balonis, M.; Lothenbach, B.; Saout, G.L.; Glasser, F.P. Impact of chloride on the mineralogy of hydrated Portland cement systems. Cem. Concr. Res. 2010, 40, 1009–1022. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, X.; Li, Y.; Yang, H.; Tang, S. Influences of MgO and PVA Fiber on the Abrasion and Cracking Resistance, Pore Structure and Fractal Features of Hydraulic Concrete. Fractal. Fract. 2022, 6, 674. [Google Scholar] [CrossRef]
- Chen, Z. Durability design of concrete structures. Archit. Technol. 2003, 34, 328–332. (In Chinese) [Google Scholar] [CrossRef]
- Sun, W. Improving durability and service life is the top priority of major concrete engineering construction. Jiangsu Sci. Technol. Inf. 2006, 7, 1–4. (In Chinese) [Google Scholar]
- Yu, H. Study on high performance concrete in salt lake: Durability, mechanism and service life prediction. Ph.D. Thesis, Southeast University, Nanjing, China, 2004. (In Chinese). [Google Scholar]
- Mehta, P.K.; Monteiro, P. Concrete: Microstructure, Properties, and Materials, 4th ed.; McGraw-Hill Education: New York, NY, USA, 2014; pp. 148–154. [Google Scholar]
- Liu, S.; Li, Q.; Zhang, J. Investigation of the effect of fly ash content on the bonding performance of CFRP-concrete interface in sulfate environment. Sci. Rep. 2022, 12, 17468. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Yuan, Y. Mechanism of concrete destruction under sodium sulfate and magnesium sulfate solution. J. Chin. Ceram. Soc. 2007, 4, 504–508. (In Chinese) [Google Scholar] [CrossRef]
- Liu, K.; Wan, A.; Sun, D. Recent Progress of Ettringite Formation and Its Expansion Mechanisms during Sulfate Attack. Bull. Chin. Ceram. Soc. 2016, 35, 4014–4019. (In Chinese) [Google Scholar] [CrossRef]
- Mesbah, A.; François, M.; Cau-dit-Coumes, C.; Frizon, F.; Filinchuk, Y.; Leroux, F.; Ravaux, J.; Renaudin, G. Crystal structure of Kuzel’s salt 3CaO·Al2O3·1/2CaSO4·1/2CaCl2·11H2O determined by synchrotron powder diffraction. Cem. Concr. Res. 2011, 41, 504–509. [Google Scholar] [CrossRef]
- Cao, Y.; Guo, L.; Chen, B.; Fei, X. Modeling early age hydration kinetics and the hydrated phase of cement paste blended with chloride and sulfate. Constr. Build. Mater. 2020, 261, 120537. [Google Scholar] [CrossRef]
- Weerdt, K.D.; Justnes, H.; Geiker, M.R. Changes in the phase assemblage of concrete exposed to sea water. Cem. Concr. Compos. 2014, 47, 53–63. [Google Scholar] [CrossRef]
- Chen, P.; Ma, B.; Tan, H.; Liu, X.; Zhang, T.; Qi, H.; Peng, Y.; Yang, Q.; Wang, J. Effects of amorphous aluminum hydroxide on chloride immobilization in cement-based materials. Constr. Build. Mater. 2019, 231, 117171. [Google Scholar] [CrossRef]
- Ariyachandra, E.; Peethamparan, S.; Patel, S.; Orlov, A. Chloride diffusion and binding in concrete containing NO2 sequestered recycled concrete aggregates (NRCAs). Constr. Build. Mater. 2021, 291, 123328. [Google Scholar] [CrossRef]
- Yu, H.; Hua, P.; Qu, W.; Sun, W.; Yan, L. The field exposure experiment of corrosion resistant concrete pole in the north-west salt lake area. China Concr. Cem. Prod. 2003, 6, 23–26. (In Chinese) [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, Q.; Yu, R. China Saline Soil; Science Press: Beijing, China, 1993; pp. 9–12. (In Chinese) [Google Scholar]
- Tang, X.; Xie, Y.; Long, G. Application of dynamic elastic modulus in evaluation of corrosion resistance of concrete. J. Hunan Univ. Sci. Technol. 2007, 1, 55–58. (In Chinese) [Google Scholar] [CrossRef]
- Zhu, B.; Qiao, H.; Feng, Q.; Lu, C. Accelerated life testing of concrete based on stochastic approach and assessment. Comput. Concr. 2017, 19, 111–120. [Google Scholar] [CrossRef]
- Qiao, X.; Hakuzweyezu, T.; Yang, B.; Li, K. Experimental study on sulfate erosion resistance of nano-CaCO3 modified concrete. Can. J. Civ. Eng. 2021, 49, 590–596. [Google Scholar] [CrossRef]
- Luo, Q.; Bung, J.H. Measurement of surface wave velocity and dynamic elastic modulus of concrete by longitudinal wave ultrasonic transducer. Hydro-Sci. Eng. 1996, 3, 264–270. (In Chinese) [Google Scholar] [CrossRef]
- Akhras, N. Detecting freezing and thawing damage in concrete using signal energy. Cem. Concr. Res. 1998, 28, 1275–1280. [Google Scholar] [CrossRef]
- Ke, G.; Yang, X.; Peng, H. Progress of research on chemical activating mechanisms of fly ash. J. China Coal Sci. 2005, 3, 366–370. (In Chinese) [Google Scholar]
- Shi, H.; Xia, M.; Guo, X. Research Development on Mechanism of Fly Ash-based Geopolymer and Effect of Each Component. J. Chin. Ceram. Soc. 2013, 41, 972–980. [Google Scholar]
- Yao, Z.; Xia, M.; Ye, Y. Effects of sodium carbonate and sodium chloride additives on alkaline fusion of coal fly ash. J. Cent. South Univ. 2011, 42, 1220–1225. (in Chinese). [Google Scholar]
- Panias, D.; Giannopoulou, I.P.; Perraki, T. Effect of synthesis parameters on the mechanical properties of fly ash-based geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2007, 301, 246–254. [Google Scholar] [CrossRef]
- Lv, Q.; Wang, Z.; Chen, Y.; Shan, X.; Gu, L. Study on the influence mechanism of sodium chloride on the strength of alkali-activated geopolymer. J. Funct. Mater. 2020, 51, 2067–2071+2092. (In Chinese) [Google Scholar] [CrossRef]
- Chen, Z.W.; Wu, S.P.; Xiao, Y.; Zeng, W.B.; Yi, M.W.; Wan, J.M. Effect of hydration and silicone resin on Basic Oxygen Furnace slag and its asphalt mixture. J. Clean. Prod. 2016, 112, 392–400. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.; Cheng, Y.-B. Effect of admixtures on properties of alkali-activated slag concrete. Cem. Concr. Res. 2000, 30, 1367–1374. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, J.; Xiao, Y.; Chen, J.; Wu, S. Characteristics of bonding behavior between basic oxygen furnace slag and asphalt binder. Constr. Build. Mater. 2014, 64, 60–66. [Google Scholar] [CrossRef]
- Lian, H.; Sun, X.; Yu, Z.; Yang, T.; Zhang, J.; Li, G.; Guan, Z.; Diao, M. Research on the fracture mechanical performance of basalt fiber nano-CaCO3 concrete based on DIC technology. Constr. Build. Mater. 2022, 329, 127193. [Google Scholar] [CrossRef]
- Peng, J.; Lin, F. Study on water resistance mechanism of gypsum-based exterior wall paint material. J. Chongqing Jianzhu Univ. 1995, 17, 9–14. (In Chinese) [Google Scholar]
- Ruan, C.; Huang, X.; Liu, L.; Huang, Y.P. Research state and progress on waterproofing performance of gypsum. Chem. Bioeng. 2014, 31, 14–18+37. (In Chinese) [Google Scholar]
- Carde, C.; François, R.; Torrenti, J.-M. Leaching of both calcium hydroxide and C-S-H from cement paste: Modeling the mechanical behavior. Cem. Concr. Res. 1996, 26, 1257–1268. [Google Scholar] [CrossRef]
- Carde, C.; François, R. Effect of the leaching of calcium hydroxide from cement paste on mechanical and physical properties. Cem. Concr. Res. 1997, 27, 539–550. [Google Scholar] [CrossRef]
- Xiang, L.; Peiyu, Y. Microstructural variation of hardened cement-fly ash pastes leached by soft water. Sci. China Tech. Sci. 2010, 53, 3033–3038. [Google Scholar] [CrossRef]
- Zang, W.; Guo, L.; Cao, Y. The interaction between chloride ion and sulfate ion doped in cement paste. Mater. Rep. 2019, 33, 1317–1321. (In Chinese) [Google Scholar] [CrossRef]
- Enevoldsen, J.N.; Hansson, C.M.; Hope, B.B. Binding of chloride in mortar containing admixed or penetrated chlorides. Cem. Concr. Res. 1994, 24, 1525–1533. [Google Scholar] [CrossRef]
- Geng, J.; Yang, H.; Mo, L. Effect of attack of sodium sulfate solution on the stability of bounded chloride ions. J. Build. Mater. 2015, 18, 919–925. (In Chinese) [Google Scholar] [CrossRef]
- Golewski, G.L. Combined Effect of Coal Fly Ash (CFA) and Nanosilica (nS) on the Strength Parameters and Microstructural Properties of Eco-Friendly Concrete. Energies 2023, 16, 452. [Google Scholar] [CrossRef]
- Elvis, B.; Ali, G. On the interaction between proteins and cracked cementitious surface. Constr. Build. Mater. 2022, 352, 128982. [Google Scholar] [CrossRef]
- He, H. Durability analysis of cement- based permeable crystalline waterproof material. Adhesion 2022, 49, 91–94+98. [Google Scholar]
- Wang, A.; Zheng, Y.; Zhang, Z.; Liu, K.; Li, Y.; Shi, L.; Sun, D. The Durability of Alkali-Activated Materials in Comparison with Ordinary Portland Cements and Concretes: A Review. Engineering 2020, 6, 695–706. [Google Scholar] [CrossRef]
- Yan, X.; Jiang, L.; Guo, M.; Chen, Y.; Song, Z.; Bian, R. Evaluation of sulfate resistance of slag contained concrete under steam curing. Constr. Build. Mater. 2019, 195, 231–237. [Google Scholar] [CrossRef]
- Alcamand, H.A.; Borges, P.H.R.; Silva, F.A.; Trindade, A.C.C. The effect of matrix composition and calcium content on the sulfate durability of metakaolin and metakaolin/slag alkali-activated mortars. Ceram. Int. 2018, 44, 5037–5044. [Google Scholar] [CrossRef]
- Thokchom, S.; Ghosh, P.; Ghosh, S. Performance of fly ash based geopolymer mortars in sulphate solution. J. Eng. Sci. Technol. Rev. 2010, 3, 36–40. [Google Scholar] [CrossRef]
- Friedel, P.M. Sur un chloro-aluminate de calcium hydraté se maclant par compression. Bull. Société Fr. Minéralogie 1987, 19, 122–136. [Google Scholar] [CrossRef]
- Wang, X.; Shi, C.; He, F. Chloride binding and its effects on microstructure of cement-based materials. J. Chin. Ceram. Soc. 2013, 41, 12. [Google Scholar] [CrossRef]
- Thomas, M.D.A.; Hooton, R.D.; Scott, A.; Zibara, H. The effect of supplementary cementitious materials on chloride binding in hardened cement paste. Cem. Concr. Res. 2012, 42, 1–7. [Google Scholar] [CrossRef]
- Kuzel, H.-J. Röntegnuntersuchung im System 3CaO·Al2O3·CaSO4·nH2O-3CaO·Al2O3·CaCl2·nH2O-H2O. Neues Jahrb. Min. Mh 1966, 193–200. [Google Scholar]
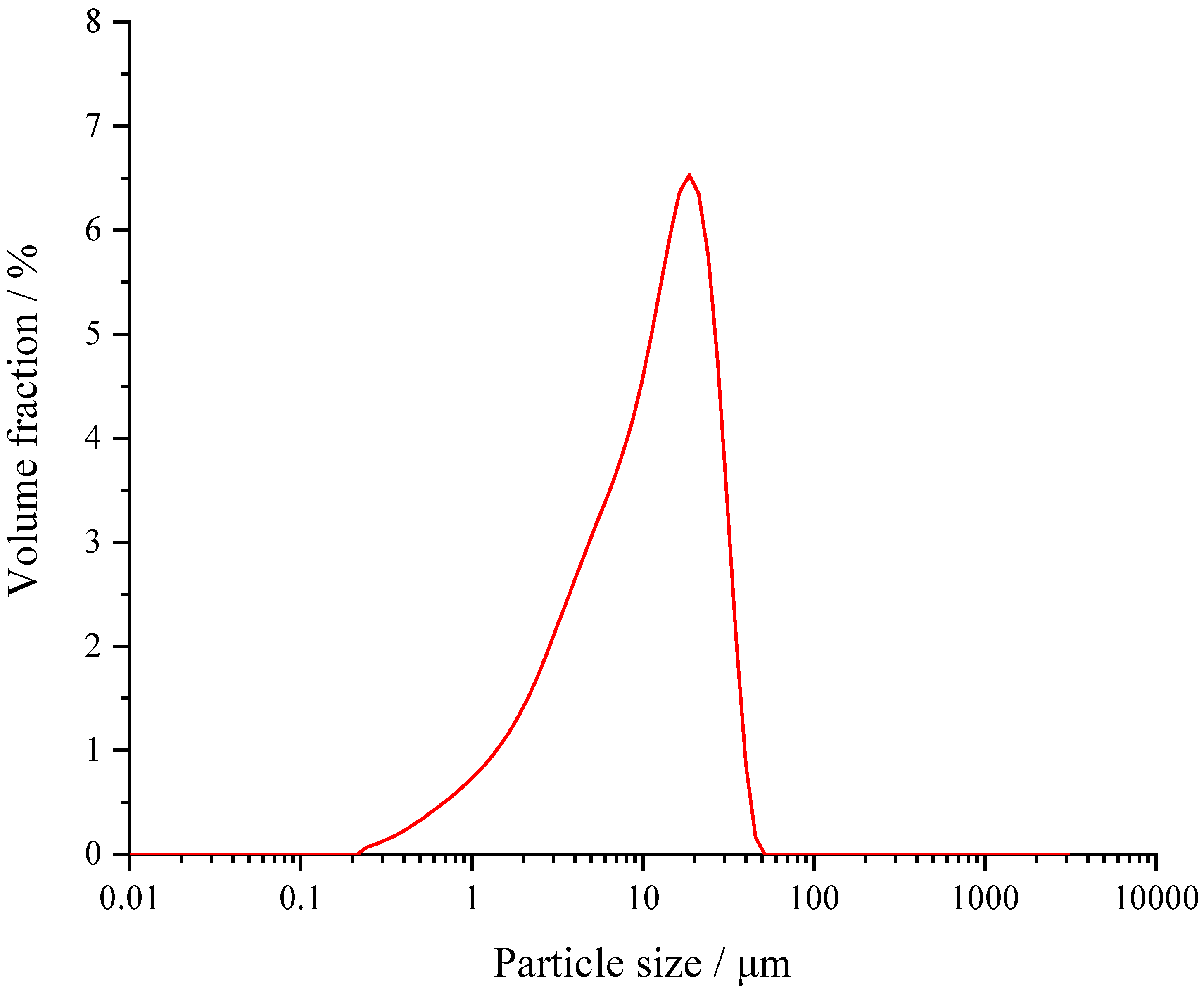








| SiO2 | Al2O3 | SO3 | MgO | CaO | Fe2O3 | Na2O | K2O |
| 28.65 | 16.49 | 2.20 | 9.04 | 40.86 | 0.23 | 0.46 | 0.48 |
| Cl | P2O5 | TiO2 | MnO | SrO2 | Y2O3 | ZrO2 | Others |
| 0.04 | 0.05 | 0.78 | 0.52 | 0.13 | 0.01 | 0.05 | 0.01 |
| Specific Surface Area | Dx (10) | Dx (50) | Dx (90) |
|---|---|---|---|
| 432.9 m²/kg | 2.23 | 12.0 | 28.3 |
| No. | Sample | NaCl | Building Gypsum | Quicklime | Slag |
|---|---|---|---|---|---|
| 1 | LA | 2 | 2.5 | 20.5 | 75 |
| 2 | LB | 5 | 18 | 75 | |
| 3 | LC | 7.5 | 15.5 | 75 | |
| 4 | LD | 10 | 13 | 75 | |
| 5 | MA | 4 | 2.5 | 18.5 | 75 |
| 6 | MB | 5 | 16 | 75 | |
| 7 | MC | 7.5 | 13.5 | 75 | |
| 8 | MD | 10 | 11 | 75 | |
| 9 | HA | 6 | 2.5 | 16.5 | 75 |
| 10 | HB | 5 | 14 | 75 | |
| 11 | HC | 7.5 | 11.5 | 75 | |
| 12 | HD | 10 | 9 | 75 | |
| 13 | 0C | 0 | 8 | 17 | 75 |
| 14 | L0 | 3 | 0 | 22 | 75 |
| 15 | OQ | 0 | 0 | 25 | 75 |
| 16 | R | 32.5 slag Portland cement | |||
| No. | Sample | Standard Curing | Water/Sulfate Attack Test | |||||
|---|---|---|---|---|---|---|---|---|
| Compressive Strength | Compressive Strength after Immersion | Mass Change | Relative Dynamic Elastic Modulus | Erosion Products Analysis of 90 d | ||||
| Immersion Age | ||||||||
| 3 d | 28 d | 0 d | 28 d | 56 d | 90 d | |||
| 1 | LA | √ | √ | √ | √ | √ | √ | / |
| 2 | LB | / | ||||||
| 3 | LC | √ | ||||||
| 4 | LD | / | ||||||
| 5 | MA | / | ||||||
| 6 | MB | / | ||||||
| 7 | MC | √ | ||||||
| 8 | MD | √ | ||||||
| 9 | HA | / | ||||||
| 10 | HB | / | ||||||
| 11 | HC | / | ||||||
| 12 | HD | / | ||||||
| 13 | 0C | √ | ||||||
| 14 | L0 | √ | ||||||
| 15 | OQ | √ | ||||||
| 16 | Reference | / | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Li, B.; Meng, X.; Shen, Q. Compound Effects of Sodium Chloride and Gypsum on the Compressive Strength and Sulfate Resistance of Slag-Based Geopolymer Concrete. Buildings 2023, 13, 675. https://doi.org/10.3390/buildings13030675
He W, Li B, Meng X, Shen Q. Compound Effects of Sodium Chloride and Gypsum on the Compressive Strength and Sulfate Resistance of Slag-Based Geopolymer Concrete. Buildings. 2023; 13(3):675. https://doi.org/10.3390/buildings13030675
Chicago/Turabian StyleHe, Wei, Benxiao Li, Xia Meng, and Quan Shen. 2023. "Compound Effects of Sodium Chloride and Gypsum on the Compressive Strength and Sulfate Resistance of Slag-Based Geopolymer Concrete" Buildings 13, no. 3: 675. https://doi.org/10.3390/buildings13030675
APA StyleHe, W., Li, B., Meng, X., & Shen, Q. (2023). Compound Effects of Sodium Chloride and Gypsum on the Compressive Strength and Sulfate Resistance of Slag-Based Geopolymer Concrete. Buildings, 13(3), 675. https://doi.org/10.3390/buildings13030675










