Optimized Preparation of Porous Coal Gangue-Based Geopolymer and Quantitative Analysis of Pore Structure
Abstract
1. Introduction
2. Experiment and Method
2.1. Materials
2.2. Experiment Design
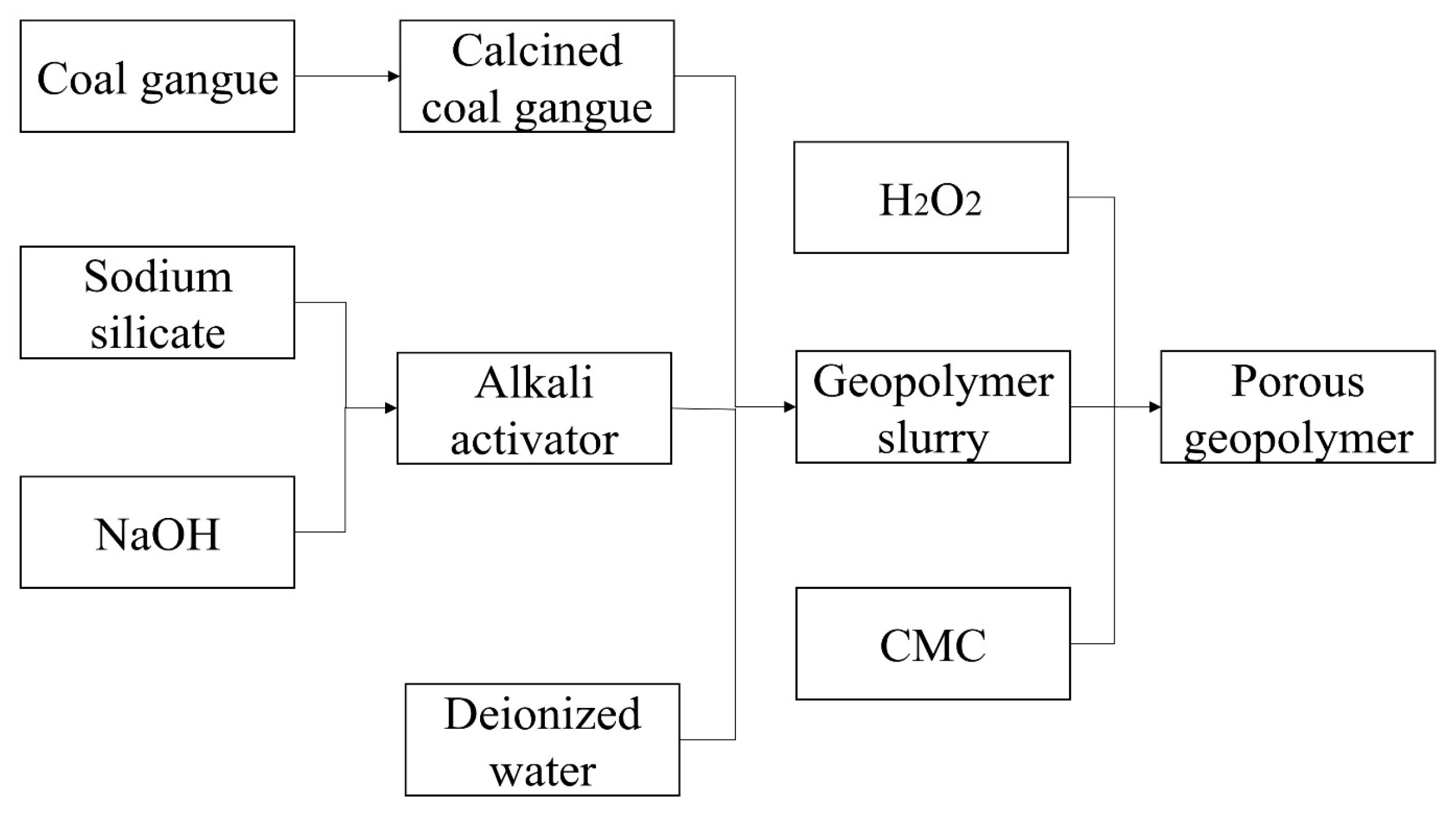
2.3. Preparation and Characterization of Porous Coal Gangue-Based Geopolymer
2.4. Automatic Image Analysis Methodology for Quantitative Pore Structure Analysis
3. Results and Discussions
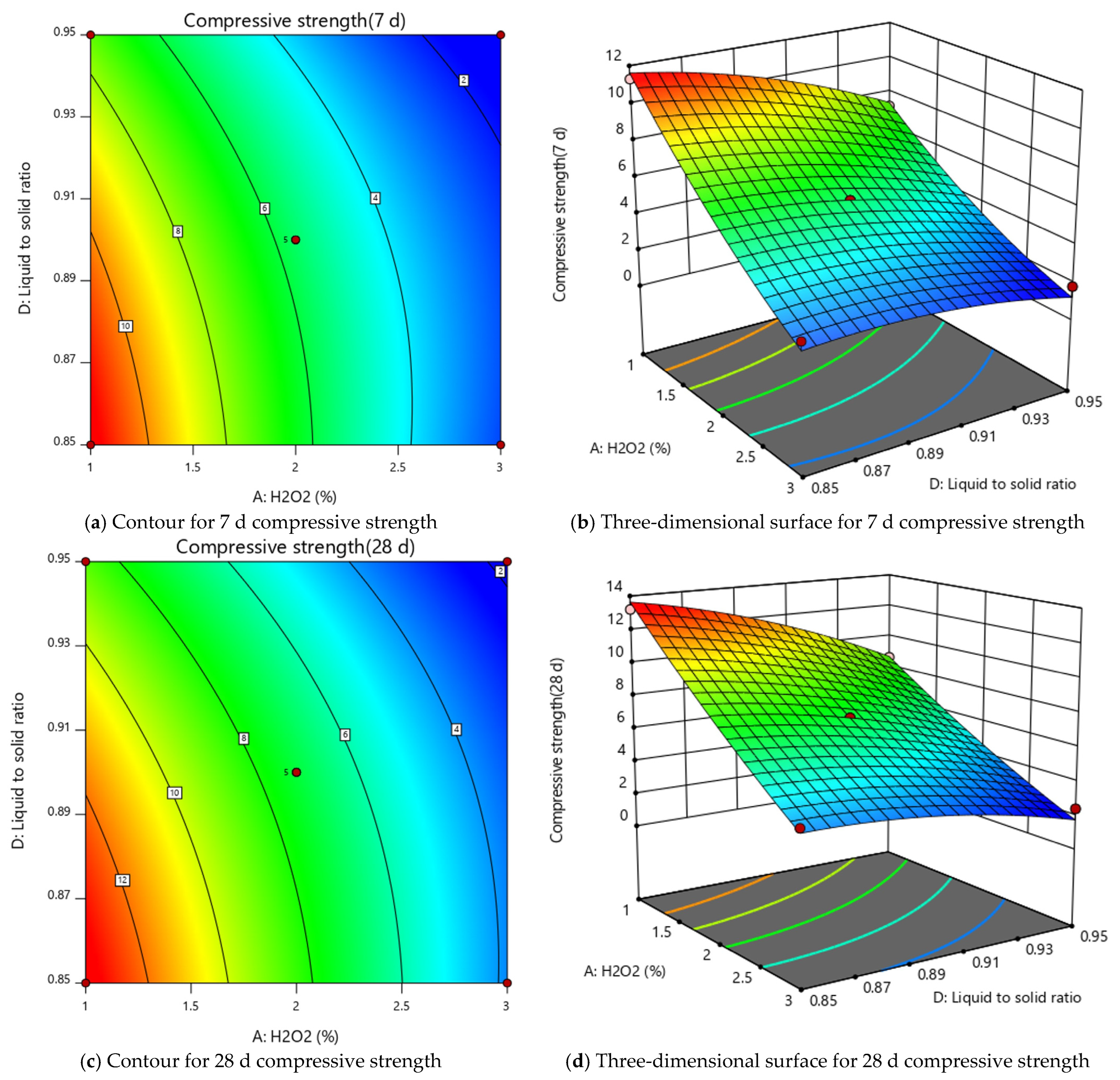
3.1. RSM Experiment Results and Analysis
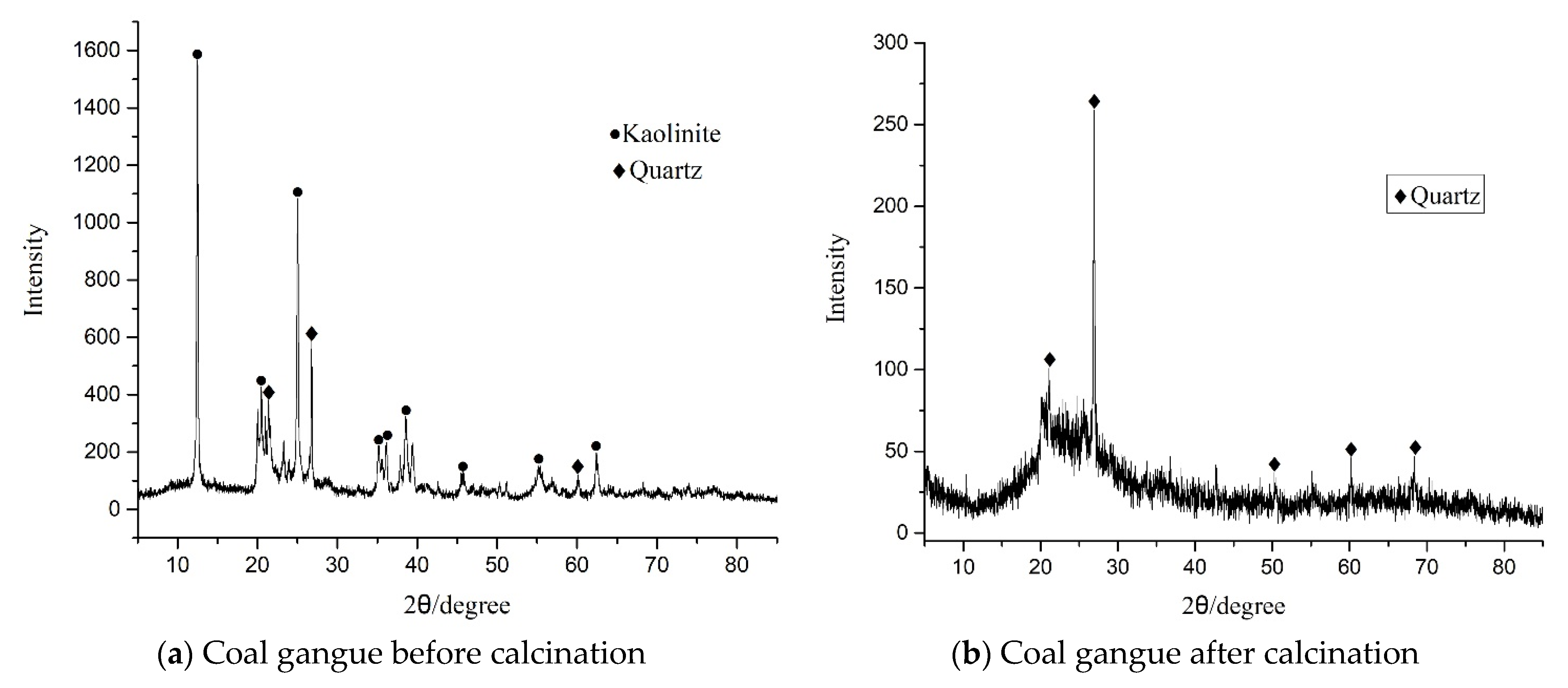
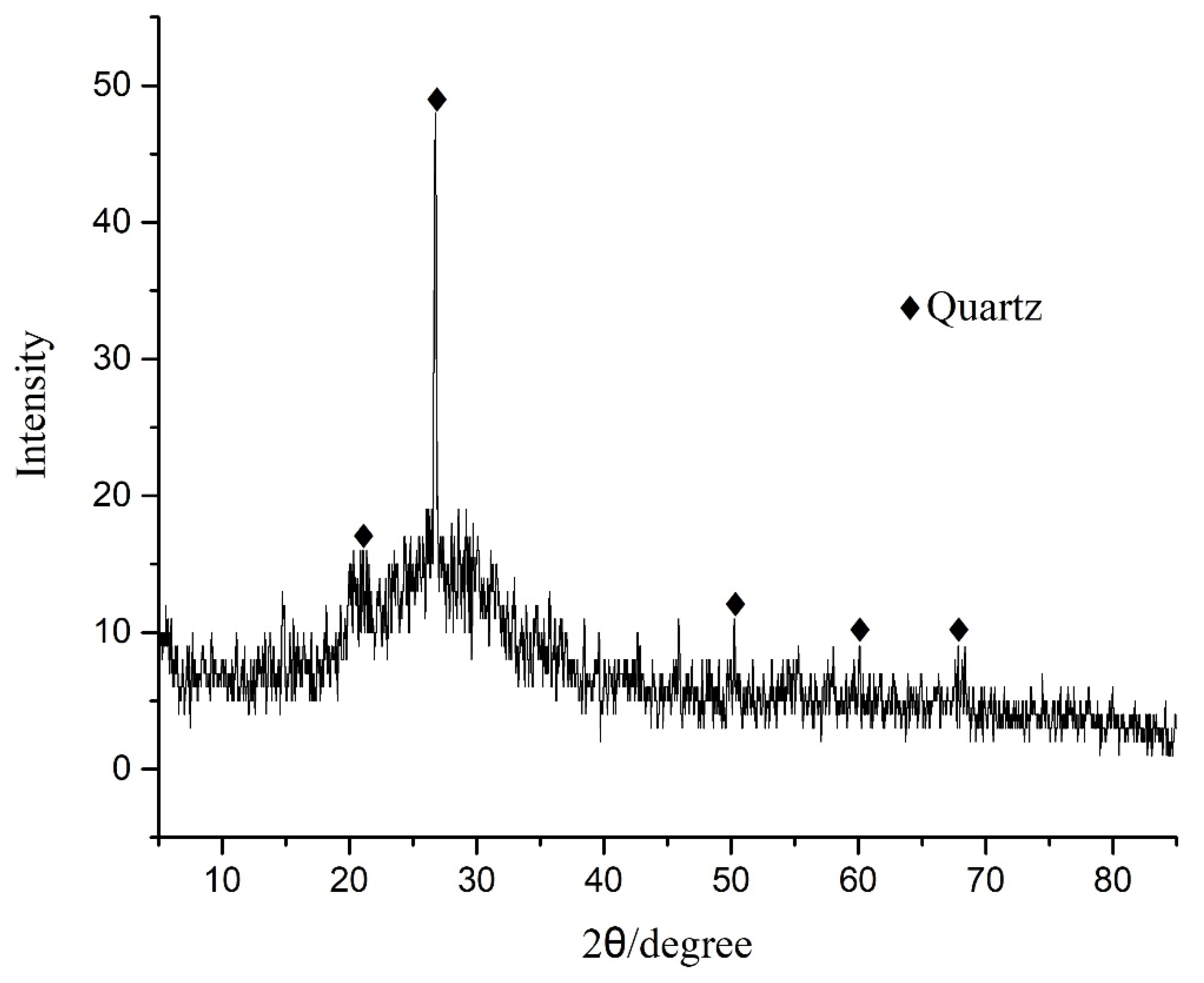
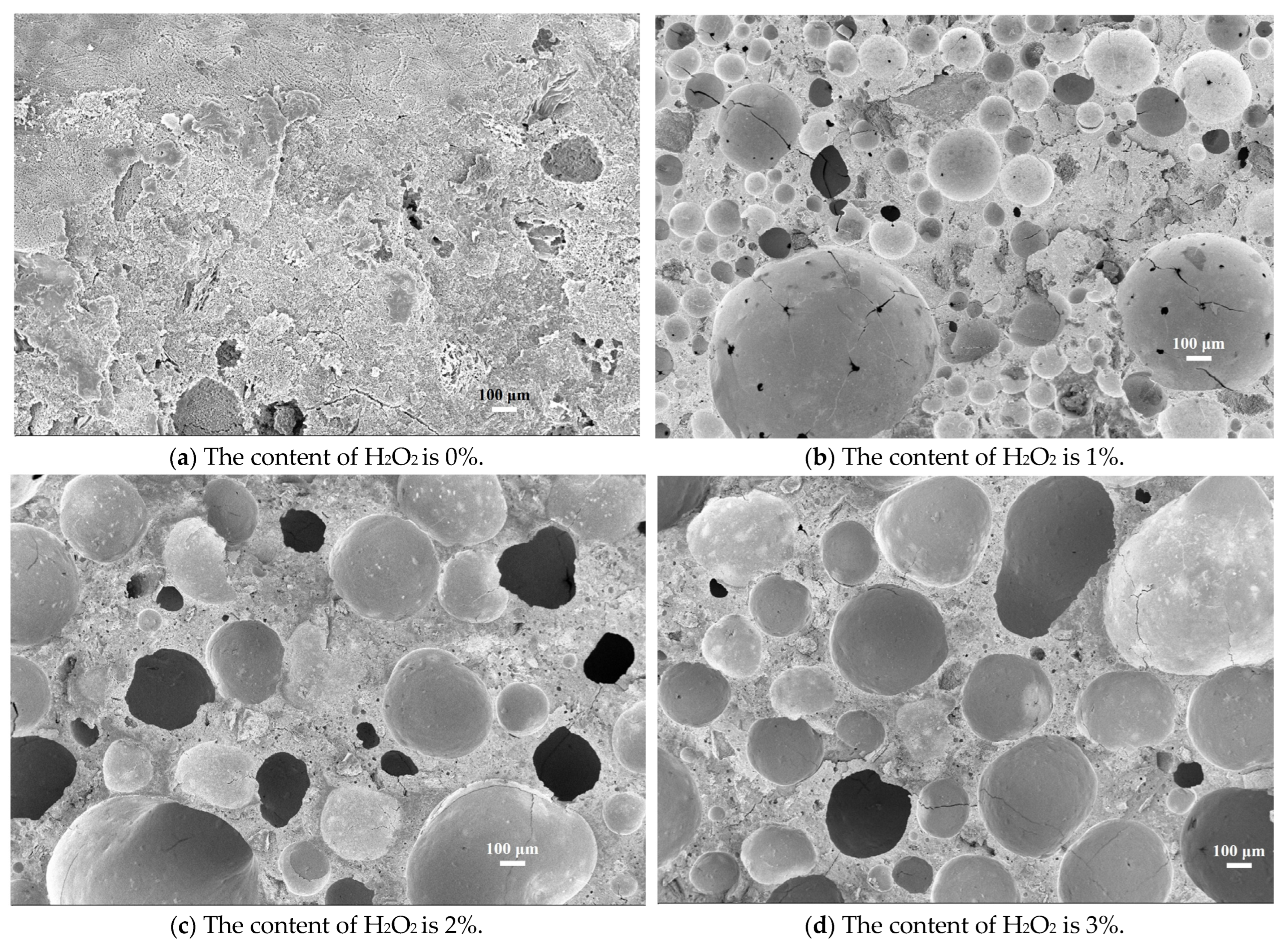
3.2. XRD and SEM Analysis
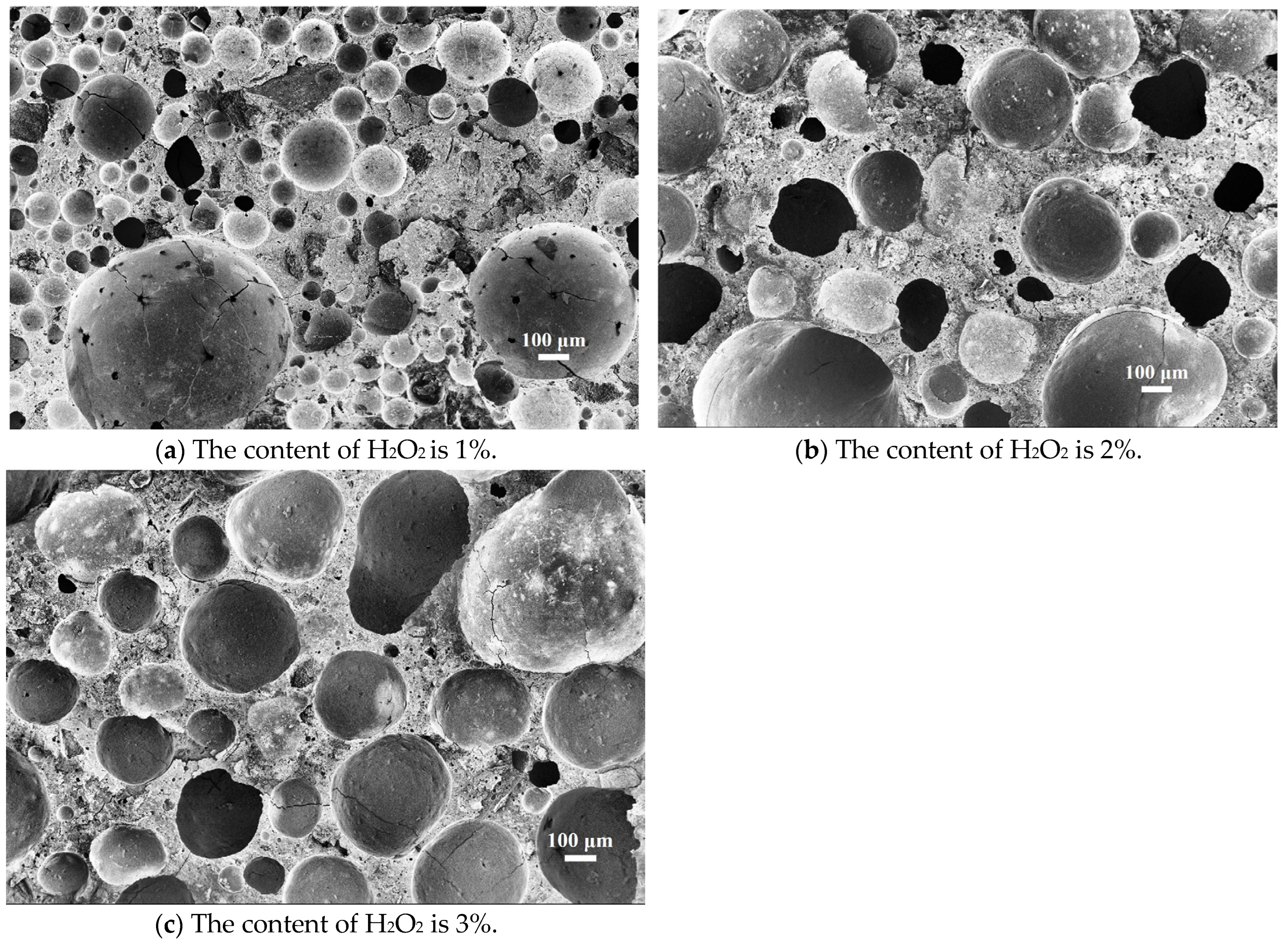
3.3. Quantitative Pore Structure Analysis by Automatic Image Analysis Method
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Geopolymer foam concrete: An emerging material for sustainable construction. Constr. Build. Mater. 2014, 56, 113–127. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Liu, M.; Hu, X. Novel sustainable geopolymer based syntactic foams: An eco-friendly alternative to polymer based syntactic foams. Chem. Eng. J. 2017, 313, 74–82. [Google Scholar] [CrossRef]
- Colombo, P.; Formia, A.; Antonaci, P.; Brini, S.; Tulliani, J.-M. Geopolymer technology for application-oriented dense and lightened materials. Elaboration and characterization. Ceram. Int. 2015, 41, 12967–12979. [Google Scholar]
- Davidovits, J. Geopolymers:Inorganic polymeric new materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Wan, Q.; Rao, F.; Song, S.; García, R.E.; Estrella, R.M.; Patiño, C.L.; Zhang, Y. Geopolymerization reaction, microstructure and simulation of metakaolin-based geopolymers at extended Si/Al ratios. Cem. Concr. Compos. 2017, 79, 45–52. [Google Scholar] [CrossRef]
- Rashidian-Dezfouli, H.; Rangaraju, P.R. Comparison of strength and durability characteristics of a geopolymer produced from fly ash, ground glass fiber and glass powder. Mater. Constr. 2017, 67, e136. [Google Scholar] [CrossRef]
- Ye, N.; Yang, J.; Liang, S.; Hu, Y.; Hu, J.; Xiao, B.; Huang, Q. Synthesis and strength optimization of one-part geopolymer based on red mud. Constr. Build. Mater. 2016, 111, 317–325. [Google Scholar] [CrossRef]
- Hassan, H.S.; Abdel-Gawwad, H.A.; Vásquez García, S.R.; Israde-Alcántara, I. Fabrication and characterization of thermally-insulating coconut ash-based geopolymer foam. Waste Manag. 2018, 80, 235–240. [Google Scholar] [CrossRef]
- Panda, B.; Paul, S.C.; Hui, L.; Tay, Y.W.D.; Tan, M.J. Additive manufacturing of geopolymer for sustainable built environment. J. Clean. Prod. 2017, 167, 281–288. [Google Scholar] [CrossRef]
- Graytee, A.; Sanjayan, J.G.; Nazari, A. Development of a high strength fly ash-based geopolymer in short time by using microwave curing. Ceram. Int. 2018, 44, 8216–8222. [Google Scholar] [CrossRef]
- Hlaváček, P.; Šmilauer, V.; Škvára, F.; Kopecký, L.; Šulc, R. Inorganic foams made from alkali-activated fly ash: Mechanical, chemical and physical properties. J. Eur. Ceram. Soc. 2015, 35, 703–709. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications; Institut Géopolymère: Saint-Quentin, France, 2008. [Google Scholar]
- Chindaprasirt, P.; Silva, P.; Sagoe-Crentsil, K.; Hanjitsuwan, S. Effect of SiO2 and Al2O3 on the setting and hardening of high calcium fly ash-based geopolymer systems. J. Mater. Sci. 2012, 47, 4876–4883. [Google Scholar] [CrossRef]
- Bai, C.; Colombo, P. High-porosity geopolymer membrane supports by peroxide route with the addition of egg white as surfactant. Ceram. Int. 2017, 43, 2267–2273. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Ngo, T.; Mendis, P.; Sanjayan, J. Regulating the chemical foaming reaction to control the porosity of geopolymer foams. Mater. Des. 2017, 120, 255–265. [Google Scholar] [CrossRef]
- Medpelli, D.; Seo, J.M.; Seo, D.K. Geopolymer with hierarchically meso-/macroporous structures from reactive emulsion templating. J. Am. Ceram. Soc. 2014, 97, 70–73. [Google Scholar] [CrossRef]
- Papa, E.; Medri, V.; Benito, P.; Vaccari, A.; Bugani, S.; Jaroszewicz, J.; Landi, E. Insights into the macroporosity of freeze-cast hierarchical geopolymers. RSC Adv. 2016, 6, 24635–24644. [Google Scholar] [CrossRef]
- Liew, Y.M.; Heah, C.Y.; Al Bakri, M.M.; Hussin, K. Structure and properties of clay-based geopolymer cements: A review. Prog. Mater. Sci. 2016, 83, 595–629. [Google Scholar] [CrossRef]
- Aguilar, R.A.; Díaz, O.B.; García, J.I.E. Lightweight concretes of activated metakaolin-fly ash binders, with blast furnace slag aggregates. Constr. Build. Mater. 2010, 24, 1166–1175. [Google Scholar] [CrossRef]
- Tang, Q.; Ge, Y.; Wang, K.; He, Y.; Cui, X.-M. Preparation and characterization of porous metakaolin-based inorganic polymer spheres as an adsorbent. Mater. Des. 2015, 88, 1244–1249. [Google Scholar] [CrossRef]
- Ge, Y.; Cui, X.; Kong, Y.; Li, Z.; He, Y.; Zhou, Q. Porous geopolymeric spheres for removal of Cu(II) from aqueous solution: Synthesis and evaluation. J. Hazard. Mater. 2015, 283, 244–251. [Google Scholar] [CrossRef]
- Siyal, A.; Shamsuddin, M.; Khan, M.; Rabat, N.E.; Zulfiqar, M.; Man, Z.; Siame, J.; Azizli, K.A. A review on geopolymers as emerging materials for the adsorption of heavy metals and dyes. J. Environ. Manag. 2018, 224, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Ying, H.; Guo, M.; Zhao, P.; Liu, Q.; Liu, C.; Song, C.; Ji, N.; Lu, X.; Ma, D.; et al. Synthesis of zeolite SSZ-13 from coal gangue via ultrasonic pretreatment combined with hydrothermal growth method. Ultrason. Sonochem. 2019, 59, 104703. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Dou, Y.; Zhang, Y.; Zhang, Y.; Zhang, B. Effects of the variety and content of coal gangue coarse aggregate on the mechanical properties of concrete. Constr. Build. Mater. 2019, 220, 386–395. [Google Scholar] [CrossRef]
- Long, J.; Zhang, S.; Luo, K. Selenium in Chinese coal gangue: Distribution, availability, and recommendations. Resour. Conserv. Recycl. 2019, 149, 140–150. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, H.; Tang, W.; Liu, X.; Yang, X.; Zhang, J. Preparation and characterization of a novel porous silicate material from coal gangue. Microporous Mesoporous Mater. 2015, 217, 210–218. [Google Scholar] [CrossRef]
- Moghadam, M.J.; Ajalloeian, R.; Hajiannia, A. Preparation and application of alkali-activated materials based on waste glass and coal gangue: A review. Constr. Build. Mater. 2019, 221, 84–98. [Google Scholar] [CrossRef]
- Heah, C.Y.; Kamarudin, H.; Bakri, A.; Bnhussain, M.; Luqman, M.; Nizar, I.K.; Ruzaidi, C.M.; Liew, Y.M. Study on solids-to-liquid and alkaline activator ratios on kaolin-based geopolymers. Constr. Build. Mater. 2012, 35, 912–922. [Google Scholar] [CrossRef]
- Xu, F.; Deng, X.; Peng, C.; Zhu, J.; Chen, J. Mix design and flexural toughness of PVA fiber reinforced fly ash-geopolymer composites. Constr. Build. Mater. 2017, 150, 179–189. [Google Scholar] [CrossRef]
- Belmokhtar, N.; El Ayadi, H.; Ammari, M.; Allal, L.B. Effect of structural and textural properties of a ceramic industrial sludge and kaolin on the hardened geopolymer properties. Appl. Clay Sci. 2018, 162, 1–9. [Google Scholar] [CrossRef]
- Borges, P.H.R.; Banthia, N.; Alcamand, H.; Vasconcelos, W.L.; Nunes, E.H.M. Performance of blended metakaolin/blastfurnace slag alkali-activated mortars. Cem. Concr. Compos. 2016, 71, 42–52. [Google Scholar] [CrossRef]
- Wang, L.; Huang, Y.; Zhao, F.; Huo, T.; Chen, E.; Tang, S. Comparison between the influence of finely ground phosphorous slag and fly ash on frost resistance, pore structures and fractal features of hydraulic concrete. Fractal Fract. 2022, 6, 598. [Google Scholar] [CrossRef]
- Huang, J.; Li, W.; Huang, D.; Wang, L.; Chen, E.; Wu, C.; Wang, B.; Deng, H.; Tang, S.; Shi, Y.; et al. Fractal analysis on pore structure and hydration of magnesium oxysulfate cements by First Principle, thermodynamic and microstructure-based methods. Fractal Fract. 2021, 5, 164. [Google Scholar] [CrossRef]
- Peng, Y.; Tang, S.; Huang, J.; Tang, C.; Wang, L.; Liu, Y. Fractal analysis on pore structure and modeling of hydration of magnesium phosphate cement paste. Fractal Fract. 2022, 6, 337. [Google Scholar] [CrossRef]
- Xu, F.; Gu, G.; Zhang, W.; Wang, H.; Huang, X.; Zhu, J. Pore structure analysis and properties evaluations of fly ash-based geopolymer foams by chemical foaming method. Ceram. Int. 2018, 44, 19989–19997. [Google Scholar] [CrossRef]
- Shao, N.; Zhang, Y.; Liu, Z.; Wang, D.-M.; Zhang, Z.-T. Fabrication of hollow microspheres filled fly ash based foam geopolymers with ultra-low thermal conductivity and relative high strength. Constr. Build. Mater. 2018, 185, 567–573. [Google Scholar] [CrossRef]
- Cai, L.; Wang, H.; Fu, Y. Freeze-thaw resistance of alkali-slag concrete based on response surface methodology. Constr. Build. Mater. 2013, 49, 70–76. [Google Scholar] [CrossRef]
- Zahid, M.; Shafiq, N.; Isa, M.H.; Gil, L. Statistical modeling and mix design optimization of fly ash based engineered geopolymer composite using response surface methodology. J. Clean. Prod. 2018, 194, 483–498. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, J.; Luo, X.; Zhu, J.; Nie, L. Experiment research on mix design and early mechanical performance of alkali-activated slag using response surface methodology (RSM). Ceram. Int. 2016, 42, 11666–11673. [Google Scholar] [CrossRef]
- Li, Z.; Lu, D.; Gao, X. Optimization of mixture proportions by statistical experimental design using response surface method—A review. J. Build. Eng. 2020, 36, 102101. [Google Scholar] [CrossRef]
- Subasi, A.; Sahin, B.; Kaymaz, I. Multi-objective optimization of a honeycomb heat sink using Response Surface Method. Int. J. Heat Mass Transf. 2016, 101, 295–302. [Google Scholar] [CrossRef]
- He, P.; Zhang, Y.; Chen, H.; Liu, L.C. Development of an eco-efficient CaMoO4/electroconductive geopolymer composite for recycling silicomanganese slag and degradation of dye wastewater. J. Clean. Prod. 2019, 208, 1476–1487. [Google Scholar] [CrossRef]
- Wang, R.; Wang, J.; Song, Q. The effect of Na+ and H2O on structural and mechanical properties of coal gangue-based geopolymer: Molecular dynamics simulation and experimental study. Constr. Build. Mater. 2020, 268, 121081. [Google Scholar] [CrossRef]
- Bourret, J.; Prud’homme, E.; Rossignol, S.; Smith, D.S. Thermal conductivity of geomaterial foams based on silica fume. J. Mater. Sci. 2012, 47, 391–396. [Google Scholar] [CrossRef]
- Nambiar, E.; Ramamurthy, K. Air-void characterisation of foam concrete. Cem. Concr. Res. 2007, 37, 221–230. [Google Scholar] [CrossRef]

| Factor | Code | Level of Code | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| H2O2 content | A | 1% | 2% | 3% |
| Curing temperature | B | 20 | 30 | 40 |
| CMC content | C | 1% | 1.5% | 2% |
| Liquid-to-solid ratio | D | 0.85 | 0.9 | 0.95 |
| Composition | SiO2 | Al2O3 | Na2O | MgO | P2O5 | K2O | SO3 | TiO2 | CaO | Fe2O3 |
|---|---|---|---|---|---|---|---|---|---|---|
| Calcined gangue | 53.415% | 42.292% | 0.163% | 0.214% | 0.049% | 0.893% | 0.229% | 1.328% | 0.343% | 1.067% |
| Experiment Number | Factor | Compressive Strength/MPa | ||||
|---|---|---|---|---|---|---|
| H2O2 Content | Curing Temperature | CMC Content | Liquid-to-Solid Ratio | 7 d | 28 d | |
| 1 | 0 | −1 | 1 | 0 | 5.8 | 7.0 |
| 2 | −1 | 1 | 0 | 0 | 10.7 | 12.1 |
| 3 | 0 | 0 | 0 | 0 | 5.6 | 7.2 |
| 4 | 1 | 0 | 0 | −1 | 2.9 | 4.1 |
| 5 | 1 | −1 | 0 | 0 | 2.1 | 2.9 |
| 6 | 1 | 1 | 0 | 0 | 2.6 | 3.2 |
| 7 | 0 | 1 | 0 | −1 | 7.0 | 8.2 |
| 8 | −1 | 0 | 0 | −1 | 11.3 | 13.2 |
| 9 | 1 | 0 | 0 | 1 | 1.8 | 2.4 |
| 10 | 0 | −1 | 0 | −1 | 6.3 | 8.2 |
| 11 | 0 | 0 | 1 | −1 | 7.2 | 8.8 |
| 12 | 0 | 0 | 0 | 0 | 5.6 | 7.2 |
| 13 | 0 | −1 | −1 | 0 | 5.4 | 6.9 |
| 14 | 0 | 0 | 0 | 0 | 5.6 | 7.2 |
| 15 | −1 | 0 | 1 | 0 | 11.0 | 12.3 |
| 16 | 0 | 1 | 0 | 1 | 4.1 | 4.8 |
| 17 | 1 | 0 | −1 | 0 | 2.4 | 3.0 |
| 18 | 0 | 0 | −1 | −1 | 6.2 | 8.5 |
| 19 | 0 | 0 | −1 | 1 | 3.8 | 4.5 |
| 20 | 0 | −1 | 0 | 1 | 4.0 | 4.3 |
| 21 | −1 | −1 | 0 | 0 | 10.3 | 11.2 |
| 22 | 0 | 1 | 1 | 0 | 7.5 | 7.9 |
| 23 | −1 | 0 | −1 | 0 | 10.2 | 11.9 |
| 24 | 0 | 1 | −1 | 0 | 6.9 | 7.5 |
| 25 | 0 | 0 | 0 | 0 | 5.6 | 7.2 |
| 26 | 0 | 0 | 1 | 1 | 3.7 | 5.2 |
| 27 | −1 | 0 | 0 | 1 | 7.2 | 8.6 |
| 28 | 1 | 0 | 1 | 0 | 2.8 | 3.5 |
| 29 | 0 | 0 | 0 | 0 | 5.6 | 7.2 |
| Model | Equation | R2 | Adjusted R2 | F Value | p Value |
|---|---|---|---|---|---|
| Compressive strength (7 d) | 5.60 − 3.84A + 0.4083B + 0.2583C − 1.36D + 0.0250AB − 0.1000AC + 0.7500AD + 0.0500BC − 0.1500BD − 0.2750CD + 0.6458A2 + 0.3208B2 + 0.3458C2 − 0.5792D2 | 0.9895 | 0.9790 | 94.23 | <0.0001 |
| Compressive strength (28 d) | 7.20 − 4.18A + 0.2667B + 0.2000C − 1.77D − 0.1500AB + 0.0250AC + 0.7250AD + 0.0750BC + 0.1250BD + 0.1000CD + 0.3583A2 − 0.1667B2 + 0.1833C2 − 0.5917D2 | 0.9948 | 0.9895 | 190.11 | <0.0001 |
| Factor | Compressive Strength (7 d) | Compressive Strength (28 d) | ||
|---|---|---|---|---|
| F Value | p Value | F Value | p Value | |
| A | 1101.96 | <0.0001 | 2188.62 | <0.0001 |
| B | 12.45 | 0.0033 | 8.89 | 0.0099 |
| C | 4.98 | 0.0424 | 5.00 | 0.0421 |
| D | 137.77 | <0.0001 | 390.33 | <0.0001 |
| AB | 0.0156 | 0.9025 | 0.9380 | 0.3492 |
| AC | 0.2489 | 0.6256 | 0.0261 | 0.8741 |
| AD | 14 | 0.0022 | 21.91 | 0.0004 |
| BC | 0.0622 | 0.8066 | 0.2345 | 0.6357 |
| BD | 0.5600 | 0.4666 | 0.6514 | 0.4331 |
| CD | 1.88 | 0.1917 | 0.4169 | 0.5289 |
| A2 | 16.83 | 0.0011 | 8.68 | 0.0106 |
| B2 | 4.15 | 0.0609 | 1.88 | 0.1922 |
| C2 | 4.83 | 0.0453 | 2.27 | 0.1539 |
| D2 | 13.54 | 0.0025 | 23.67 | 0.0003 |
| H2O2 | 0% | 1% | 2% | 3% |
|---|---|---|---|---|
| Roundness | - | 0.81 | 0.87 | 1.09 |
| Average perimeter (μm) | - | 1200.37 | 1470.66 | 1710.18 |
| Average diameter (μm) | - | 320.83 | 410.78 | 540.62 |
| Diameter distribution(μm) | - | 60–680 | 80–610 | 120–600 |
| Porosity (image analysis) | - | 30.16% | 51.36% | 67.23% |
| Porosity (experimental result) | - | 41.12% | 60.46% | 75.97% |
| 7 d compressive strength (MPa) | 56.6 | 11.3 | 4.5 | 2.9 |
| 28 d compressive strength (MPa) | 57.4 | 13.2 | 6.8 | 4.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Wang, J.; Song, Q. Optimized Preparation of Porous Coal Gangue-Based Geopolymer and Quantitative Analysis of Pore Structure. Buildings 2022, 12, 2079. https://doi.org/10.3390/buildings12122079
Wang R, Wang J, Song Q. Optimized Preparation of Porous Coal Gangue-Based Geopolymer and Quantitative Analysis of Pore Structure. Buildings. 2022; 12(12):2079. https://doi.org/10.3390/buildings12122079
Chicago/Turabian StyleWang, Rui, Jingsong Wang, and Qingchun Song. 2022. "Optimized Preparation of Porous Coal Gangue-Based Geopolymer and Quantitative Analysis of Pore Structure" Buildings 12, no. 12: 2079. https://doi.org/10.3390/buildings12122079
APA StyleWang, R., Wang, J., & Song, Q. (2022). Optimized Preparation of Porous Coal Gangue-Based Geopolymer and Quantitative Analysis of Pore Structure. Buildings, 12(12), 2079. https://doi.org/10.3390/buildings12122079






