Effect of Iron Phase on the Formation of Barium Calcium Sulphoaluminate Clinker
Abstract
:1. Introduction
2. Raw Materials and Experimental Methods
2.1. Raw Materials
2.2. Experimental Methods
3. Results
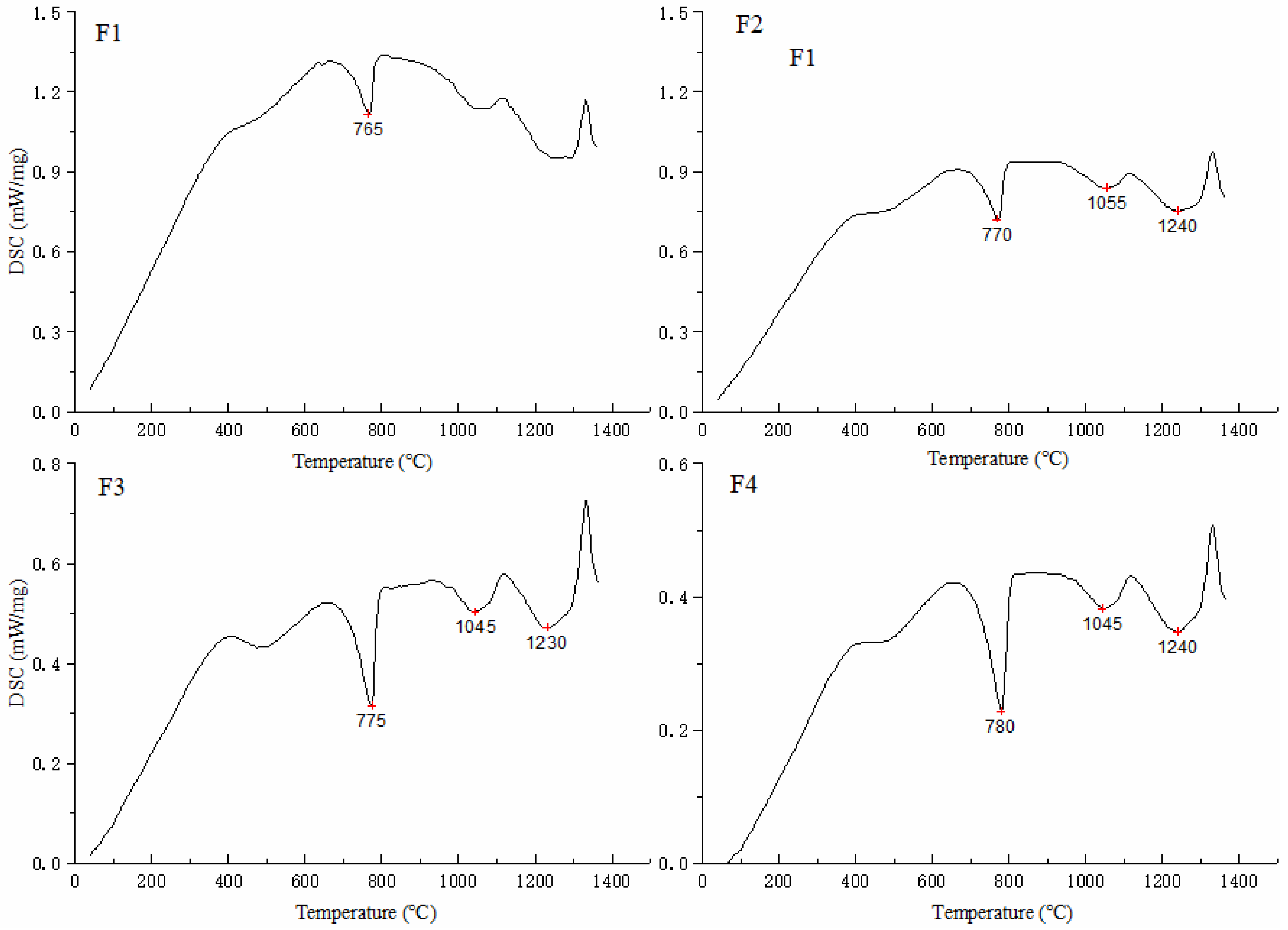
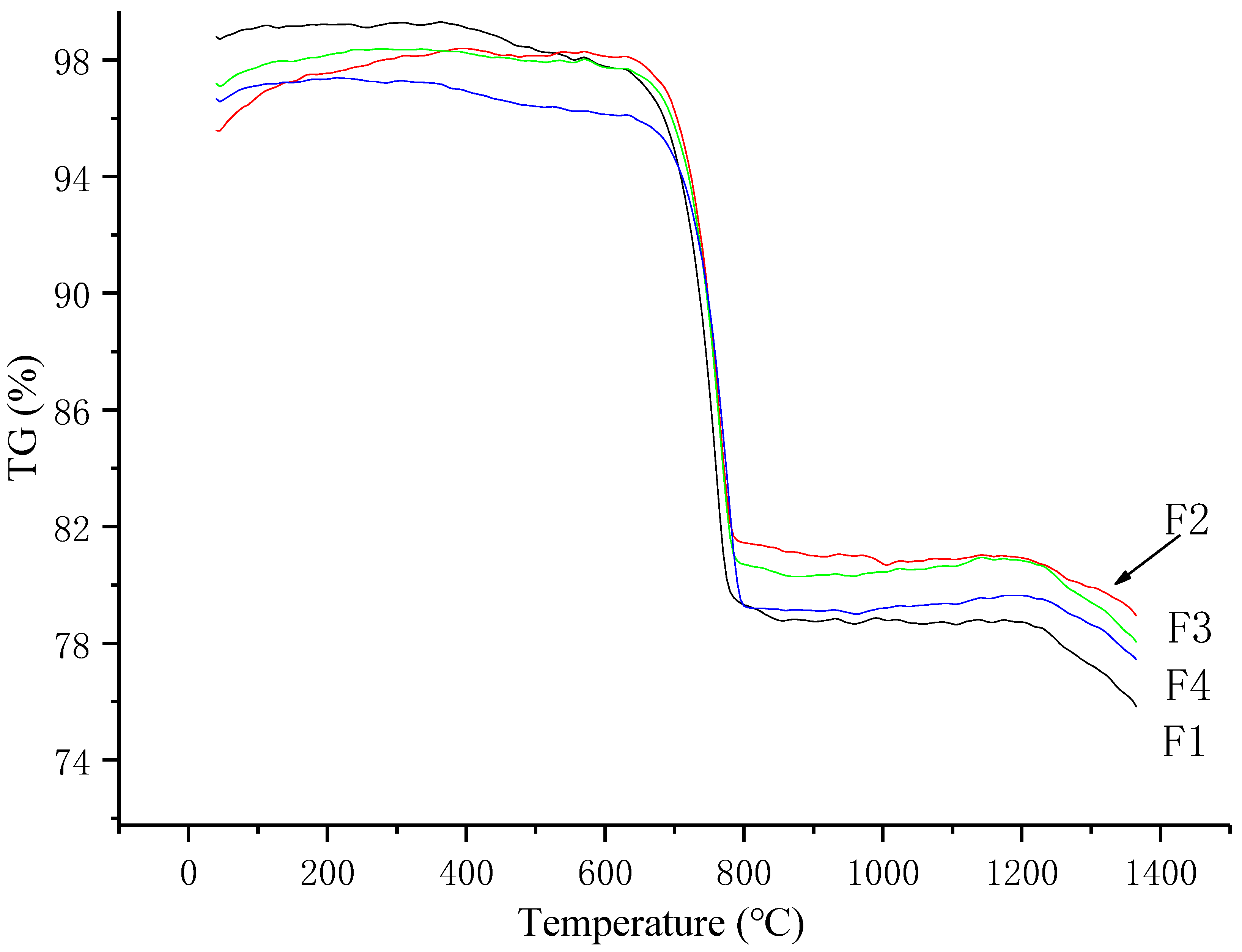
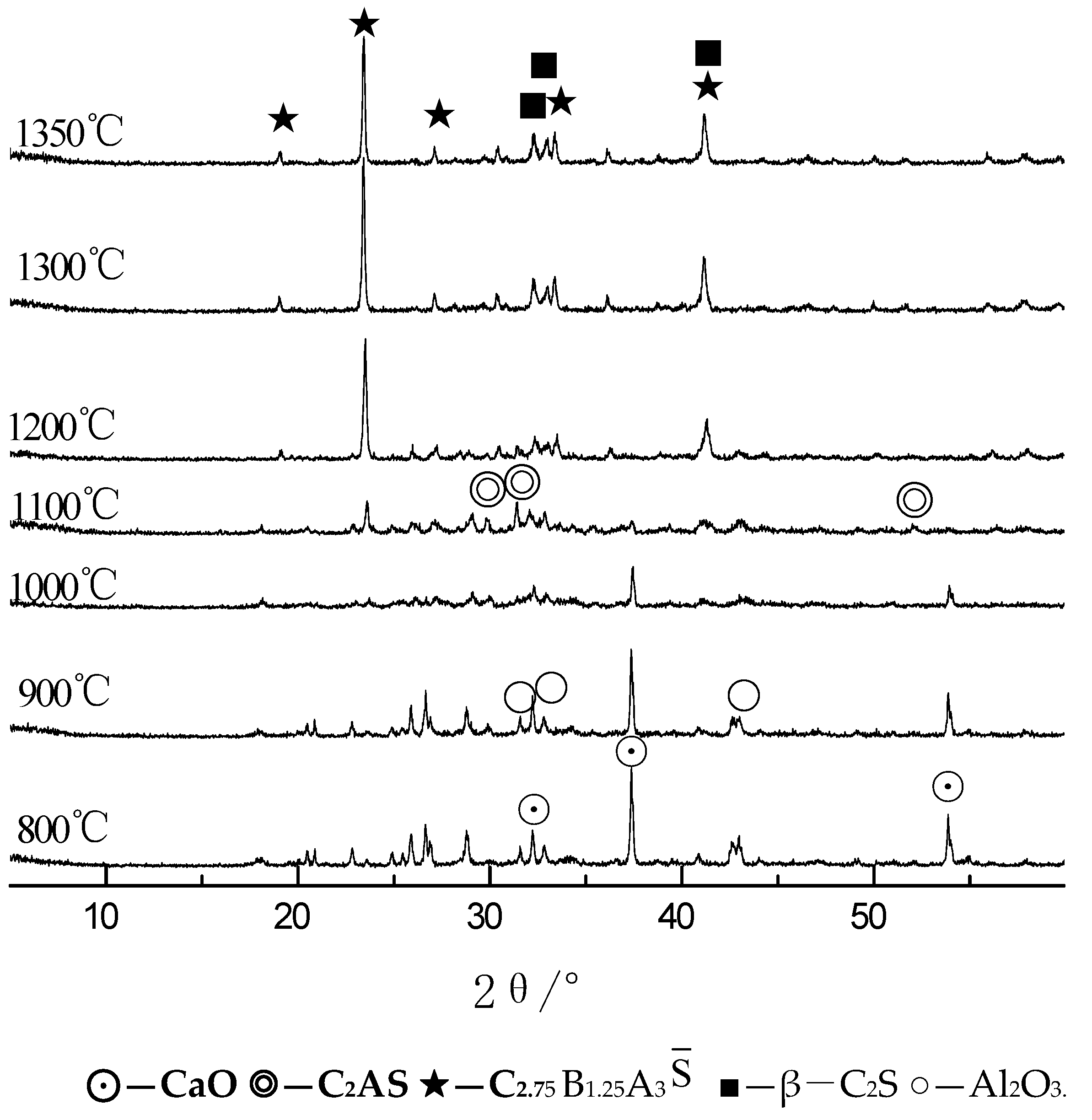
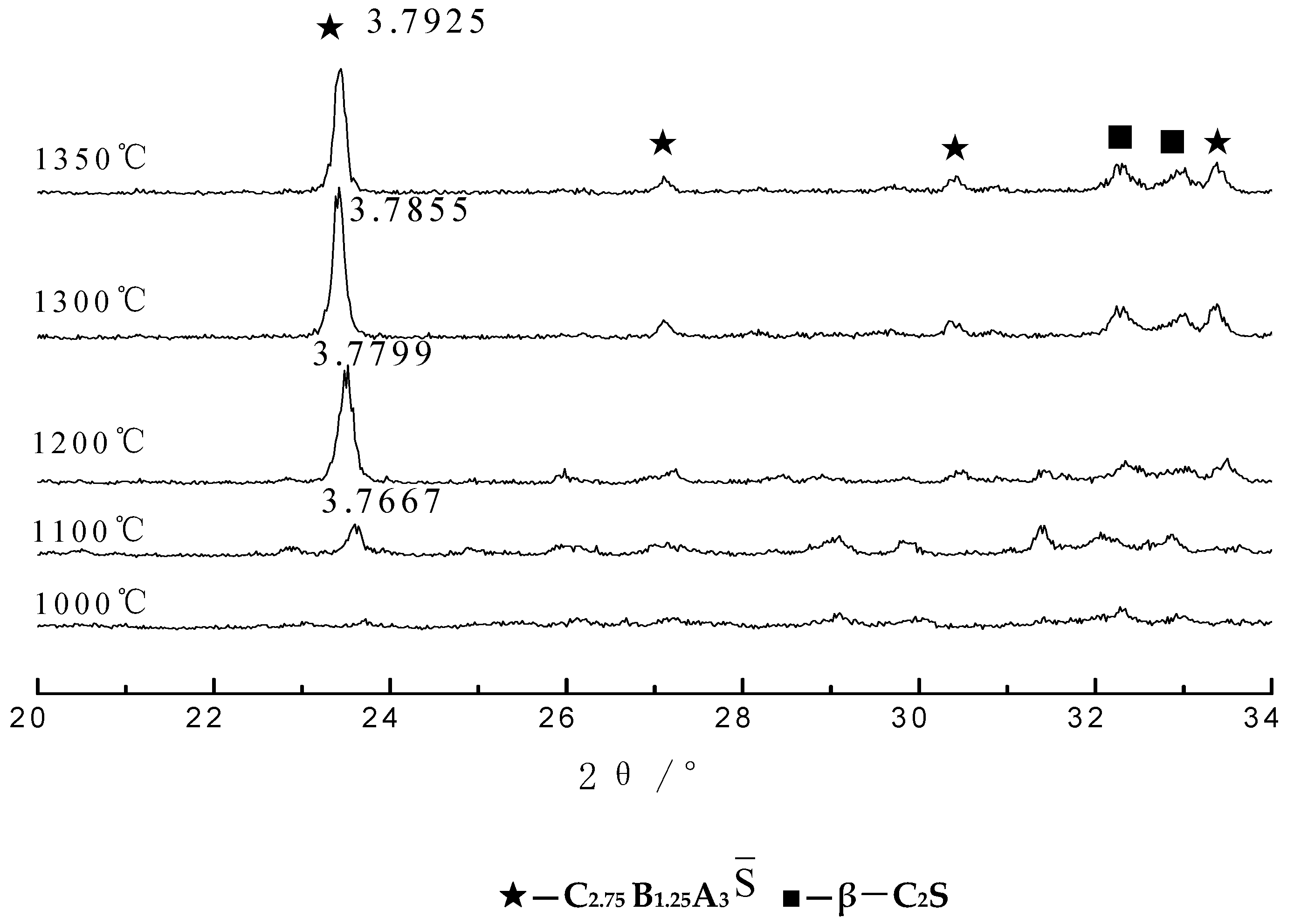
3.1. Analysis of the Clinker Calcination Process
3.1.1. TG Analysis
3.1.2. f-CaO Analysis
3.1.3. XRD Analysis
3.2. Analysis of the Cement Clinker
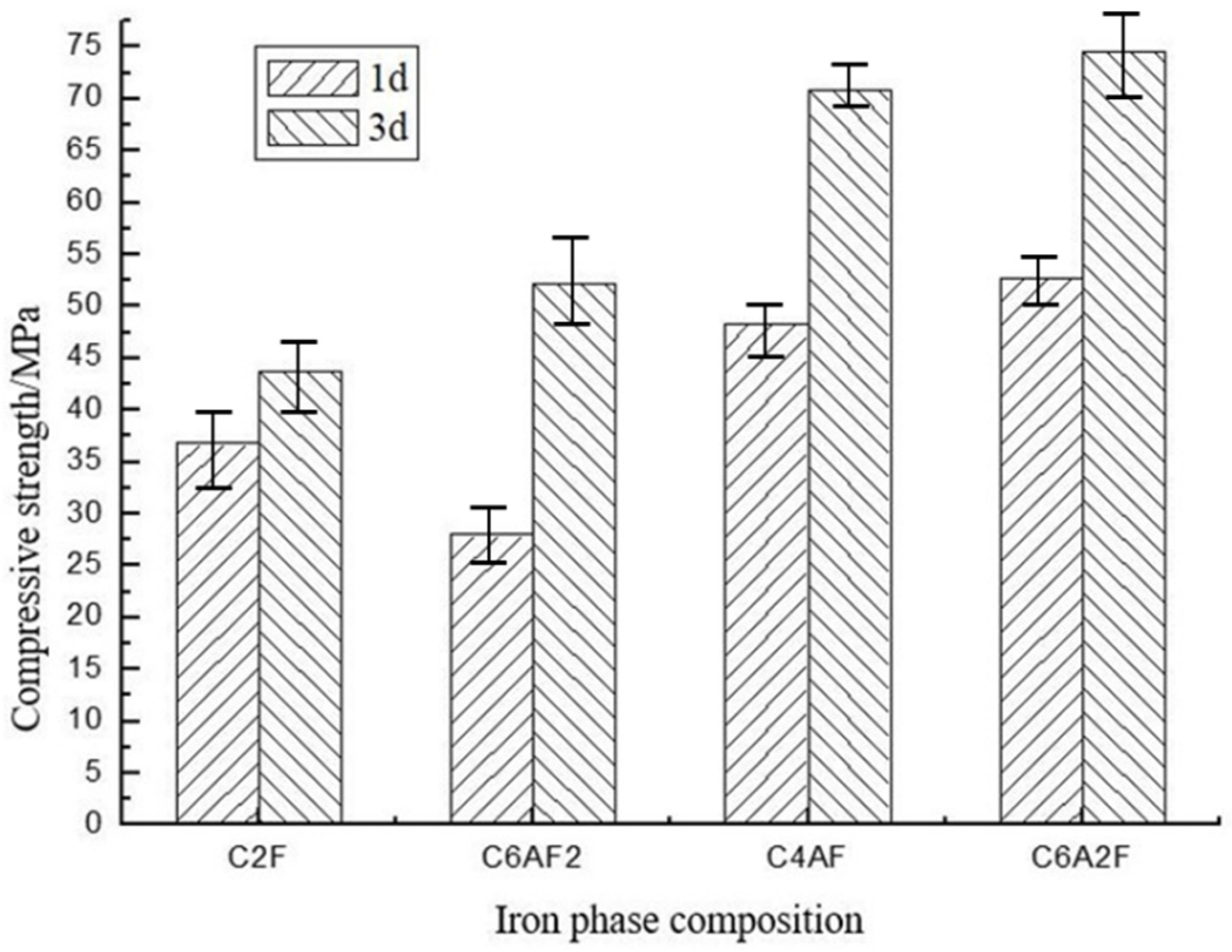
3.2.1. Compressive Strength
3.2.2. XRD Analysis of Clinker
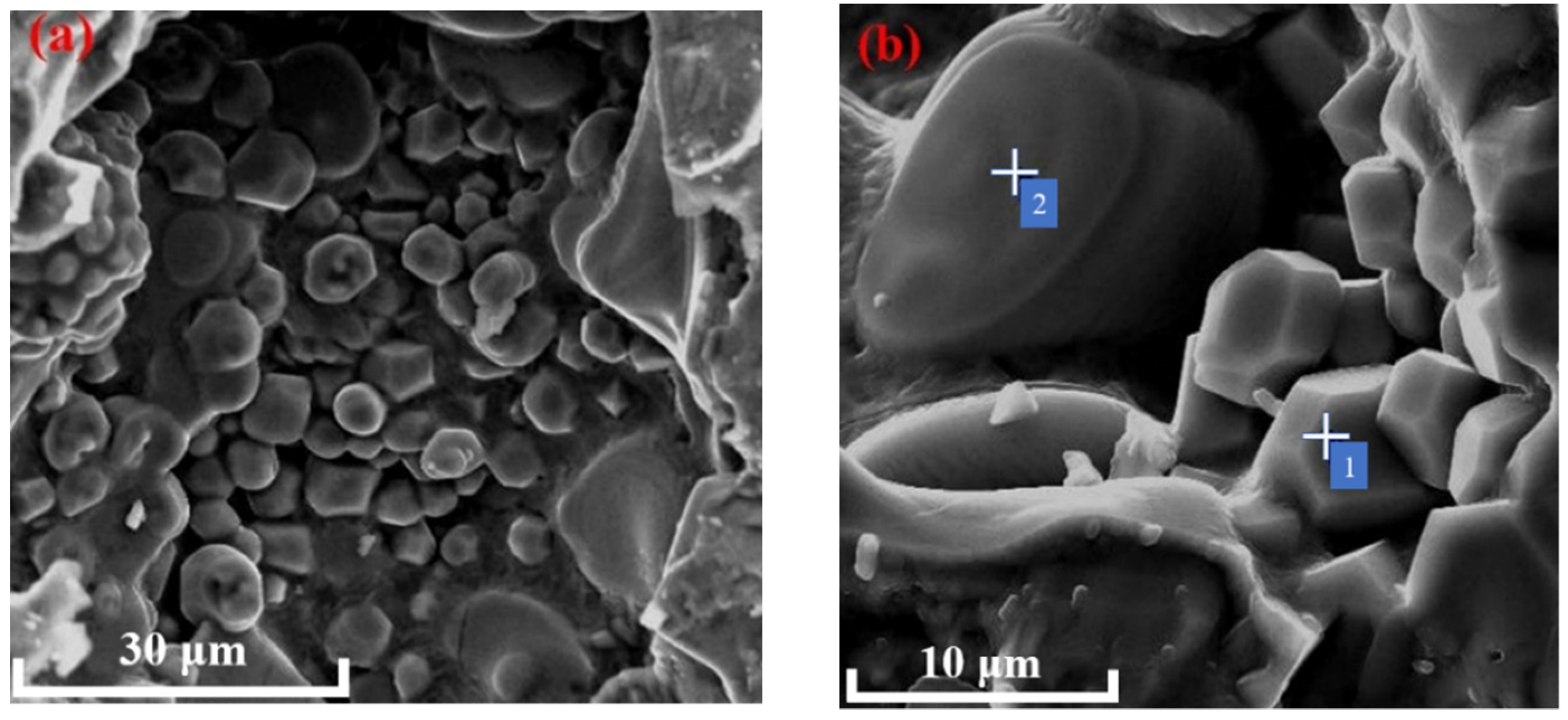
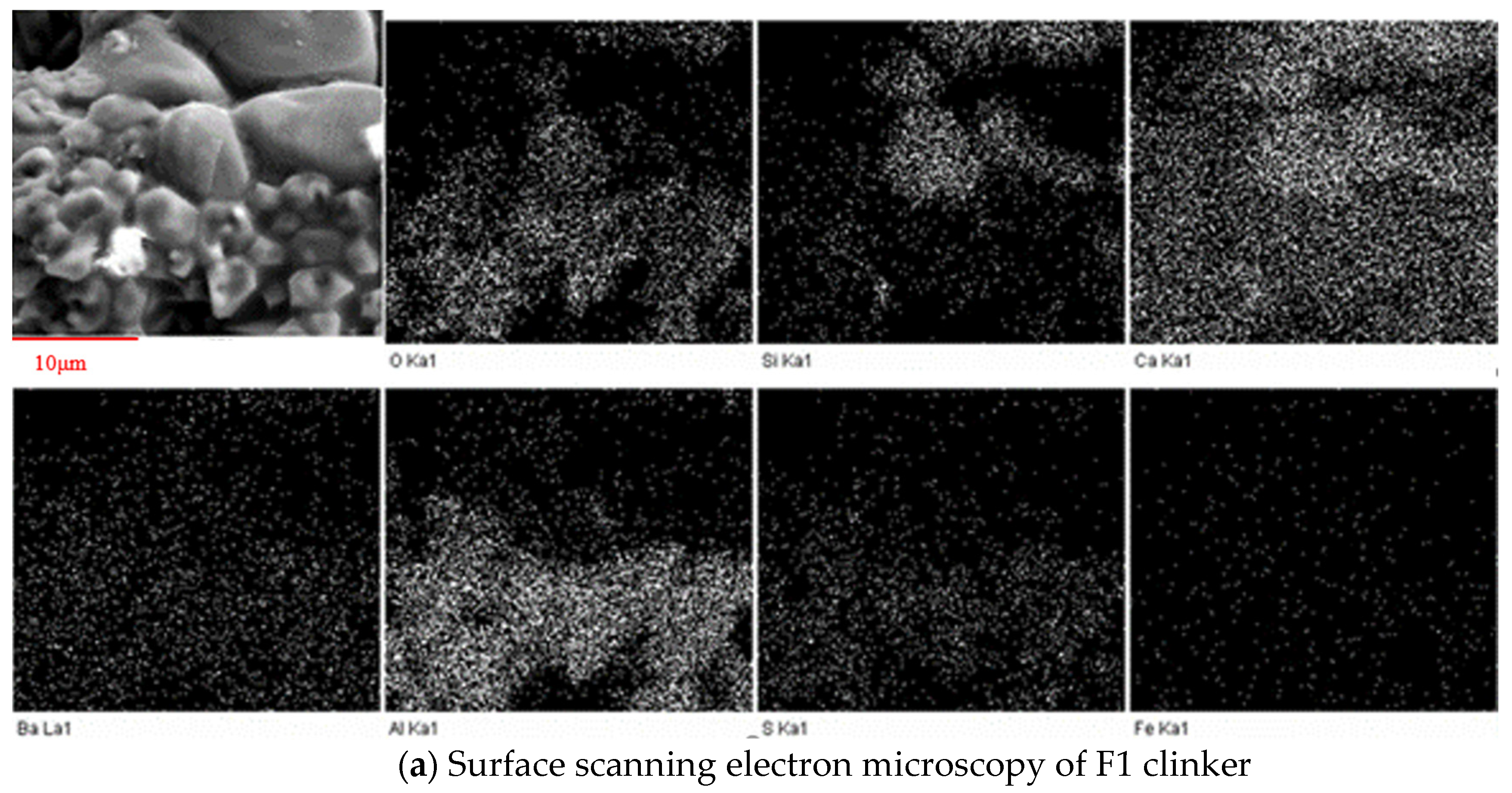
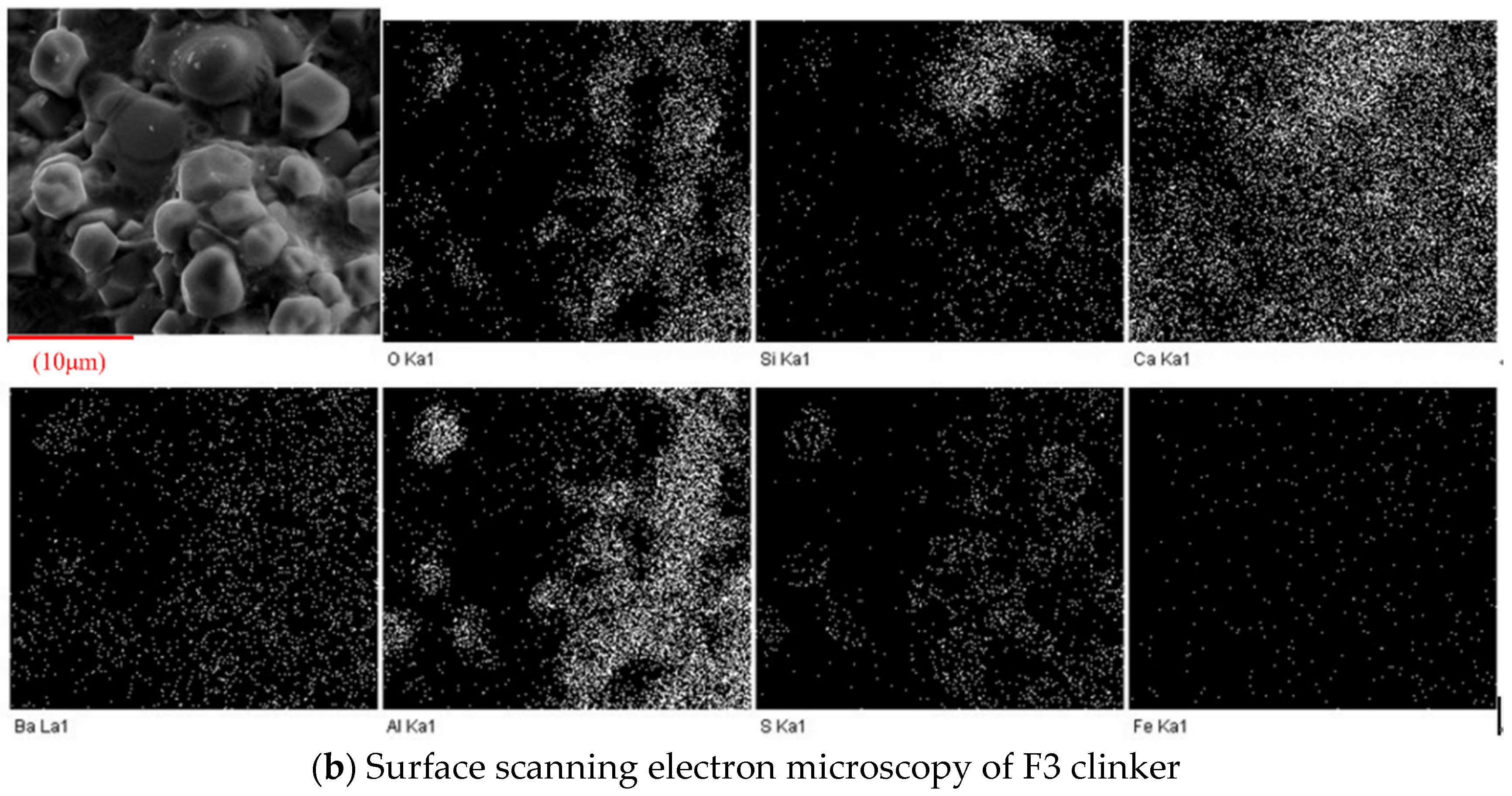
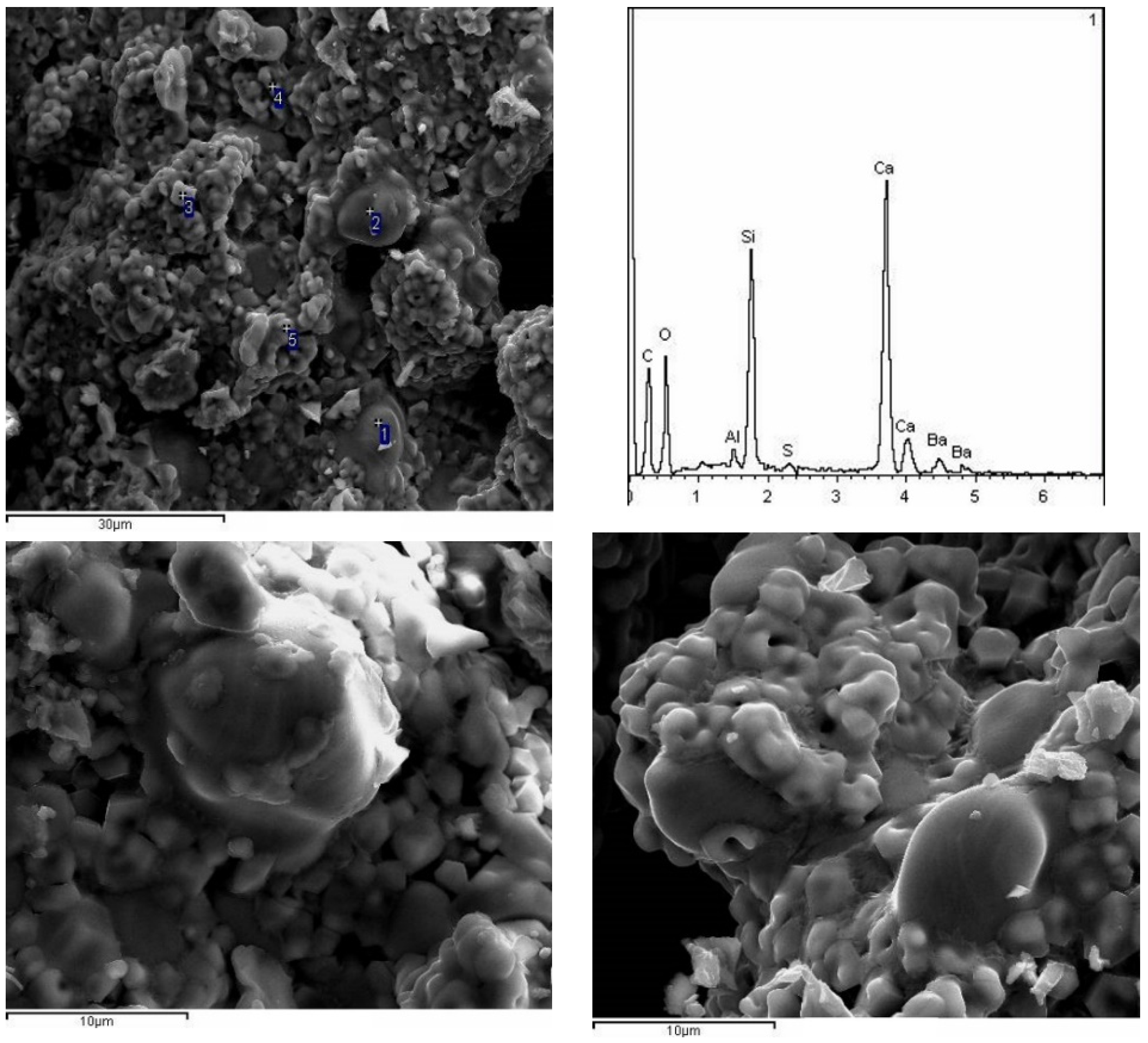
3.2.3. SEM Observation
3.3. Influence of Calcination Temperature on the Clinker
3.3.1. Compressive Strength
3.3.2. XRD Analysis
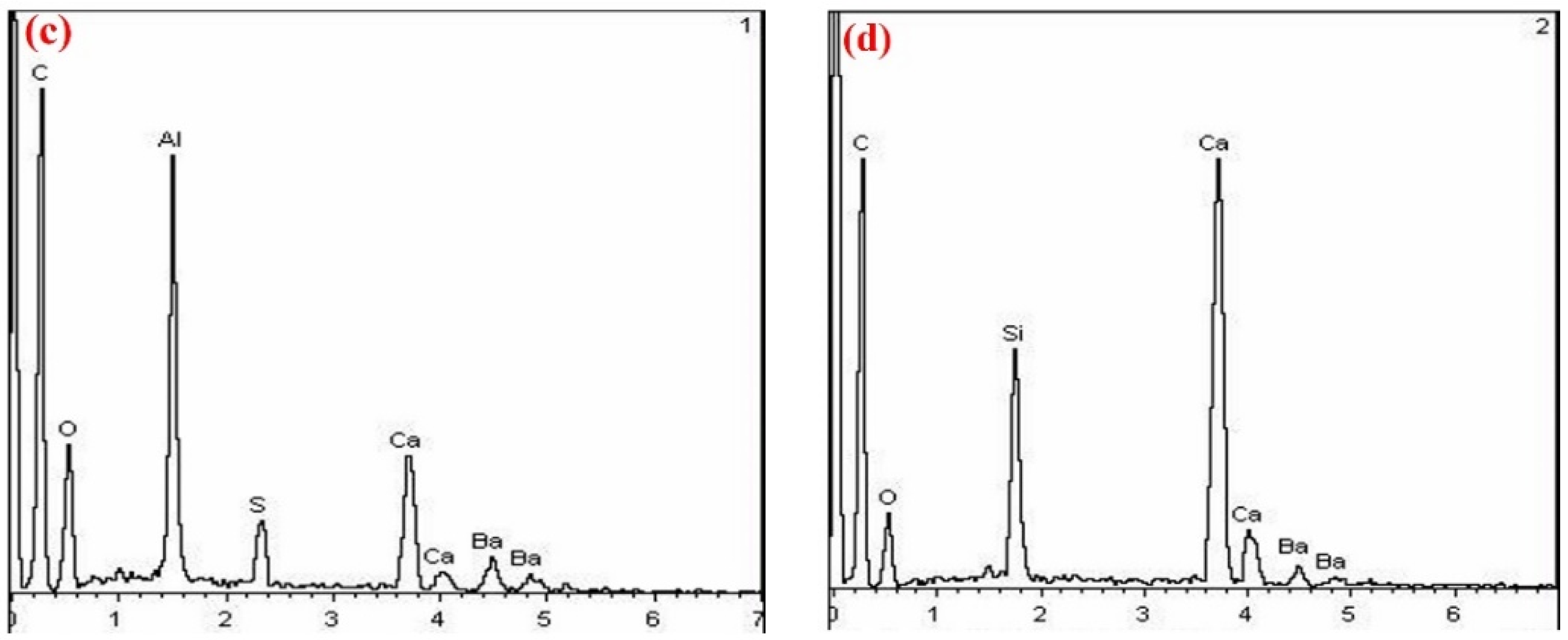
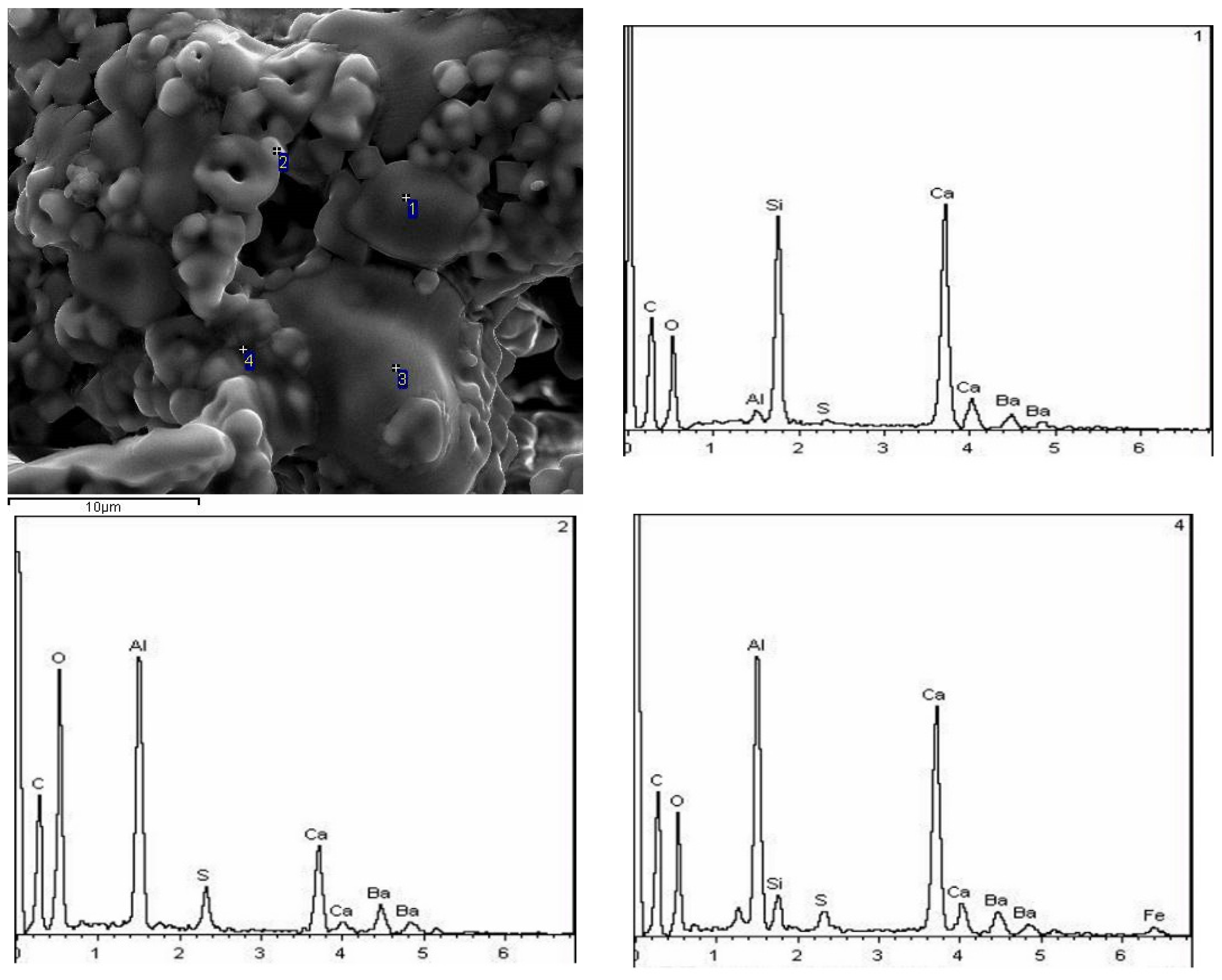
3.3.3. SEM-EDS Analysis
4. Conclusions
- (1)
- The iron phase reduces the decomposition temperature of calcium carbonate. As the ratio of Fe to Al in the iron phase decreases, the decomposition temperature of calcium carbonate increases, but the decomposition temperature range does not change significantly. With an increase in temperature, the content of f-CaO in the sample first increased and then decreased. At 800 °C, the content of f-CaO reached a maximum of 34.7%. When the temperature reached 1200 °C, the free calcium in each group was almost zero; the CaO produced by the decomposition reaction was almost entirely involved in the reaction.
- (2)
- With the increase in temperature, it was favorable for Ba2+ to replace Ca2+, and the substitution reaction occurred, to generate barium calcium sulfoaluminate minerals. From the SEM-EDS analysis, it can be seen that the egg-shaped clinker minerals are β-C2S and the hexagonal flakes are C2.75B1.25A3 minerals. The Ba element was mainly distributed in the area of barium and calcium sulfoaluminate and was also partially dissolved in C2S; The Fe element was distributed between C2.75B1.25A3 and C2S grains in the form of an iron phase solid solution, which acts as a solvent, and its content increased with the increase in the content of iron phase.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cui, K.; Chang, J.; Feo, L.; Chow, C.L.; Lau, D. Developments and Applications of Carbon Nanotube Reinforced Cement-Based Composites as Functional Building Materials. Front. Mater. 2022, 9, 861646. [Google Scholar] [CrossRef]
- Mehdizadeh, H.; Mo, K.H.; Ling, T. CO2-fixing and recovery of high-purity vaterite CaCO3 from recycled concrete fines. Resour. Conserv. Recycl. 2023, 188, 106695. [Google Scholar] [CrossRef]
- Higuchi, T.; Morioka, M.; Yoshioka, I.; Yokozeki, K. Development of a new ecological concrete with CO2 emissions below zero. Constr. Build. Mater. 2014, 67, 338–343. [Google Scholar] [CrossRef]
- Di Filippo, J.; Karpman, J.; DeShazo, J.R. The impacts of policies to reduce CO2 emissions within the concrete supply chain. Cem. Concr. Compos. 2019, 101, 67–82. [Google Scholar] [CrossRef]
- Alsalman, A.; Assi, L.N.; Kareem, R.S.; Carter, K.; Ziehl, P. Energy and CO2 emission assessments of alkali-activated concrete and Ordinary Portland Cement concrete: A comparative analysis of different grades of concrete. Clean. Environ. Syst. 2021, 3, 100047. [Google Scholar] [CrossRef]
- Sinkhonde, D. Generating response surface models for optimisation of CO2 emission and properties of concrete modified with waste materials. Clean. Mater. 2022, 6, 100146. [Google Scholar] [CrossRef]
- Cui, K.; Lau, D.; Zhang, Y.; Chang, J. Mechanical properties and mechanism of nano-CaCO3 enhanced sulphoaluminate cement-based reactive powder concrete. Constr. Build. Mater. 2021, 309, 125099. [Google Scholar] [CrossRef]
- Chang, J.; Jiang, T.; Cui, K. Influence on compressive strength and CO2 capture after accelerated carbonation of combination β-C2S with γ-C2S. Constr. Build. Mater. 2021, 312, 125359. [Google Scholar] [CrossRef]
- Chang, J.; Cui, K.; Zhang, Y. Effect of hybrid steel fibers on the mechanical performances and microstructure of sulphoaluminate cement-based reactive powder concrete. Constr. Build. Mater. 2020, 261, 120502. [Google Scholar] [CrossRef]
- Zhang, R.; Arrigoni, A.; Panesar, D.K. Could reactive MgO cement be a green solution? The effect of CO2 mineralization and manufacturing route on the potential global warming impact. Cem. Concr. Compos. 2021, 124, 104263. [Google Scholar] [CrossRef]
- Wang, X.; Lee, H. Effect of global warming on the proportional design of low CO2 slag-blended concrete. Constr. Build. Mater. 2019, 225, 1140–1151. [Google Scholar] [CrossRef]
- Ozturk, E.; Ince, C.; Derogar, S.; Ball, R. Factors affecting the CO2 emissions, cost efficiency and eco-strength efficiency of concrete containing rice husk ash: A database study. Constr. Build. Mater. 2022, 326, 126905. [Google Scholar] [CrossRef]
- Bennett, B.; Visintin, P.; Xie, T. Global warming potential of recycled aggregate concrete with supplementary cementitious materials. J. Build. Eng. 2022, 52, 104394. [Google Scholar] [CrossRef]
- Ekolu, S.O. Implications of global CO2 emissions on natural carbonation and service lifespan of concrete infrastructures—Reliability analysis. Cem. Concr. Compos. 2020, 114, 103744. [Google Scholar] [CrossRef]
- Moro, C.; Francioso, V.; Lopez-Arias, M.; Velay-Lizancos, M. The impact of CO2 uptake rate on the environmental performance of cementitious composites: A new dynamic Global Warming Potential analysis. J. Clean Prod. 2022, 375, 134155. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Kang, F.; Zhang, J. Monitoring and evaluation of the repair quality of concrete cracks using piezoelectric smart aggregates. Constr. Build. Mater. 2022, 317, 125775. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Kang, F. Real-time monitoring of humidity inside concrete structures utilizing embedded smart aggregates. Constr. Build Mater. 2022, 331, 127317. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Kang, F.; Zhang, J. Monitoring depth and width of cracks in underwater concrete structures using embedded smart aggregates. Measurement 2022, 204, 112078. [Google Scholar] [CrossRef]
- Cau Dit Coumes, C.; Dhoury, M.; Champenois, J.; Mercier, C.; Damidot, D. Combined effects of lithium and borate ions on the hydration of calcium sulfoaluminate cement. Cem. Concr. Res. 2017, 97, 50–60. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, J.; Zhao, J.; Fang, Y. Nanostructural characterization of Al(OH)3 formed during the hydration of calcium sulfoaluminate cement. J. Am. Ceram. Soc. 2018, 101, 4262–4274. [Google Scholar] [CrossRef]
- Zhang, J.; Li, G.; Ye, W.; Chang, Y.; Liu, Q.; Song, Z. Effects of ordinary Portland cement on the early properties and hydration of calcium sulfoaluminate cement. Constr. Build. Mater. 2018, 186, 1144–1153. [Google Scholar] [CrossRef]
- Gastaldi, D.; Paul, G.; Marchese, L.; Irico, S.; Boccaleri, E.; Mutke, S.; Buzzi, L.; Canonico, F. Hydration products in sulfoaluminate cements: Evaluation of amorphous phases by XRD/solid-state NMR. Cem. Concr. Res. 2016, 90, 162–173. [Google Scholar] [CrossRef]
- Xie, H.; Ren, C.; Zhao, B.; Liu, S.; Zhu, D.; Guan, H.; Xu, D.; Wang, J.; Yang, H. Characterization and use of biomass power plant ash in sulfoaluminate cementitious materials. Constr. Build. Mater. 2022, 325, 126667. [Google Scholar] [CrossRef]
- Cui, K.; Chang, J. Hydration, reinforcing mechanism, and macro performance of multi-layer graphene-modified cement composites. J. Build. Eng. 2022, 57, 104880. [Google Scholar] [CrossRef]
- Cui, K.; Liang, K.; Chang, J.; Lau, D. Investigation of the macro performance, mechanism, and durability of multiscale steel fiber reinforced low-carbon ecological UHPC. Constr. Build. Mater. 2022, 327, 126921. [Google Scholar] [CrossRef]
- Wu, S.; Yao, X.; Yao, Y.; Ren, C.; Wu, C.; Zhang, C.; Wang, W. Recycling phosphogypsum as the sole calcium oxide source in calcium sulfoaluminate cement production and solidification of phosphorus. Sci. Total Environ. 2022, 808, 152118. [Google Scholar] [CrossRef] [PubMed]
- Cau Dit Coumes, C.; Courtois, S.; Peysson, S.; Ambroise, J.; Pera, J. Calcium sulfoaluminate cement blended with OPC: A potential binder to encapsulate low-level radioactive slurries of complex chemistry. Cem. Concr. Res. 2009, 39, 740–747. [Google Scholar] [CrossRef]
- Liu, X.; Tan, H.; Ma, B.; Luo, Z.; Lv, Z.; Chen, P.; Zhang, T. Effect of the prepared barium@hydrogel capsule on chloride ion binding of Portland cement paste. Compos. Part B: Eng. 2022, 247, 110314. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, C.; Zhang, P.; Zhao, K.; Wu, Z. Degradation behaviour of non-sintered graphene/barium titanate/magnesium phosphate cement bio-piezoelectric composites. Ceram. Int. 2020, 46, 12626–12636. [Google Scholar] [CrossRef]
- Wang, S.; Lu, L.; Chen, C. Effects of fly ash and slag on the hydration process of alite- barium calcium sulphoaluminate cement. Procedia Eng. 2012, 27, 261–268. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, Y.; Zhang, L.; Guo, B.; Fan, G.; Guan, X.; Zhao, R. Revealing the doping mechanism of barium in sulfoaluminate cement clinker phases. J. Clean Prod. 2021, 295, 126405. [Google Scholar] [CrossRef]
- Xin, C.; Jun, C.; Lingchao, L.; Futian, L.; Bing, T. Study on the hydration of Ba-bearing calcium sulphoaluminate in the presence of gypsum. Cem. Concr. Res. 2004, 34, 2009–2013. [Google Scholar] [CrossRef]
- Chang, J.; Cheng, X.; Liu, F.; Lu, L.; Teng, B. Influence of fluorite on the Ba-bearing sulphoaluminate cement. Cem. Concr. Res. 2001, 31, 213–216. [Google Scholar] [CrossRef]
- Xin, C.; Jun, C.; Lingchao, L.; Futian, L.; Bing, T. Study of Ba-bearing calcium sulphoaluminate minerals and cement. Cem. Concr. Res. 2000, 30, 77–81. [Google Scholar] [CrossRef]
- Chang, J.; Zhang, J.; Yuan, Y.; Cui, K. Effect of Iron Phase on Calcination and Properties of Barium Calcium Sulfoaluminate Cement. Materials 2021, 14, 5813. [Google Scholar] [CrossRef] [PubMed]




| Sample NO. | Iron Phase | Chemical Composition w/% | |||||
|---|---|---|---|---|---|---|---|
| CaO | Al2O3 | Fe2O3 | SiO2 | BaO | SO3 | ||
| F1 | C2F | 37.50 | 25.08 | 2.94 | 12.21 | 15.71 | 6.56 |
| F2 | C6AF2 | 37.65 | 25.75 | 2.11 | 12.21 | 15.71 | 6.56 |
| F3 | C4AF | 37.74 | 26.13 | 1.64 | 12.21 | 15.71 | 6.56 |
| F4 | C6A2F | 37.84 | 26.53 | 1.14 | 12.21 | 15.71 | 6.56 |
| Temperature (°C) | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| 700 | 29.1 | 29.6 | 28.6 | 28.6 |
| 800 | 32.2 | 28.6 | 31.8 | 34.7 |
| 900 | 25.0 | 26.1 | 25.6 | 25.6 |
| 1000 | 16.9 | 13.8 | 15.2 | 16.6 |
| 1100 | 4.1 | 5.0 | 4.5 | 5.5 |
| 1200 | 0.1 | 0.1 | 0.1 | 0.1 |
| 1300 | 0.0 | 0.0 | 0.0 | 0.0 |
| 1350 | 0.0 | 0.0 | 0.0 | 0.0 |
| Number | Iron Phase Composition | Compressive Strength/MPa | ||
|---|---|---|---|---|
| 1 d | 3 d | 28 d | ||
| F1 | C2F | 64.0 | 78.4 | 107.9 |
| F2 | C6AF2 | 69.9 | 77.5 | 108.7 |
| F3 | C4AF | 73.2 | 97.9 | 106.9 |
| F4 | C6A2F | 72.1 | 74.4 | 111.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Zhang, P.; Chang, J.; Li, L. Effect of Iron Phase on the Formation of Barium Calcium Sulphoaluminate Clinker. Buildings 2022, 12, 2075. https://doi.org/10.3390/buildings12122075
Zhang B, Zhang P, Chang J, Li L. Effect of Iron Phase on the Formation of Barium Calcium Sulphoaluminate Clinker. Buildings. 2022; 12(12):2075. https://doi.org/10.3390/buildings12122075
Chicago/Turabian StyleZhang, Bingxin, Ping Zhang, Jun Chang, and Li Li. 2022. "Effect of Iron Phase on the Formation of Barium Calcium Sulphoaluminate Clinker" Buildings 12, no. 12: 2075. https://doi.org/10.3390/buildings12122075
APA StyleZhang, B., Zhang, P., Chang, J., & Li, L. (2022). Effect of Iron Phase on the Formation of Barium Calcium Sulphoaluminate Clinker. Buildings, 12(12), 2075. https://doi.org/10.3390/buildings12122075








