Development of Photocatalytic 3D-Printed Cementitious Mortars: Influence of the Curing, Spraying Time Gaps and TiO2 Coating Rates
Abstract
:1. Introduction
2. Research Significance
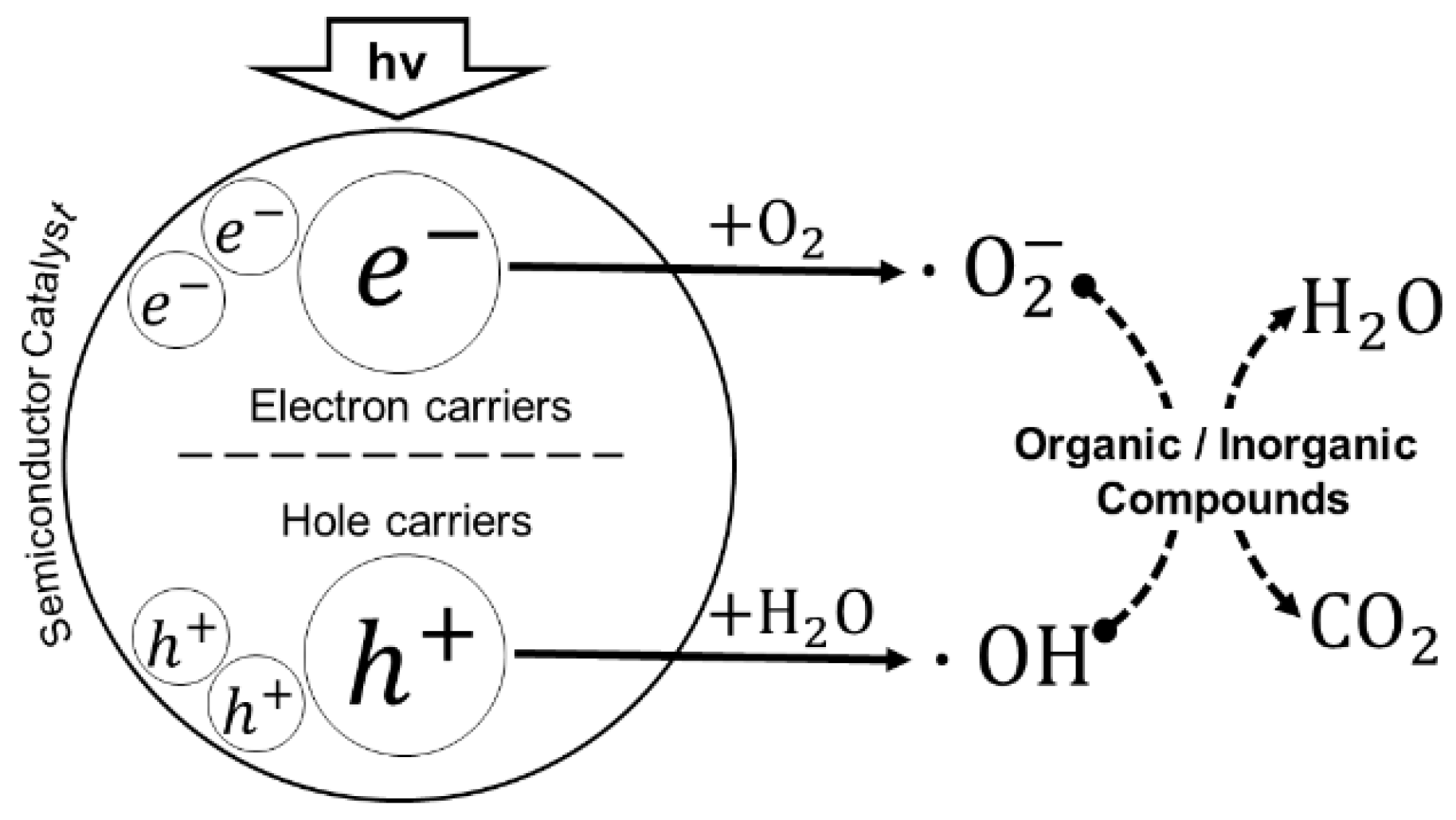
3. Photocatalytic Process
4. Materials and Experimental Methods
4.1. Materials
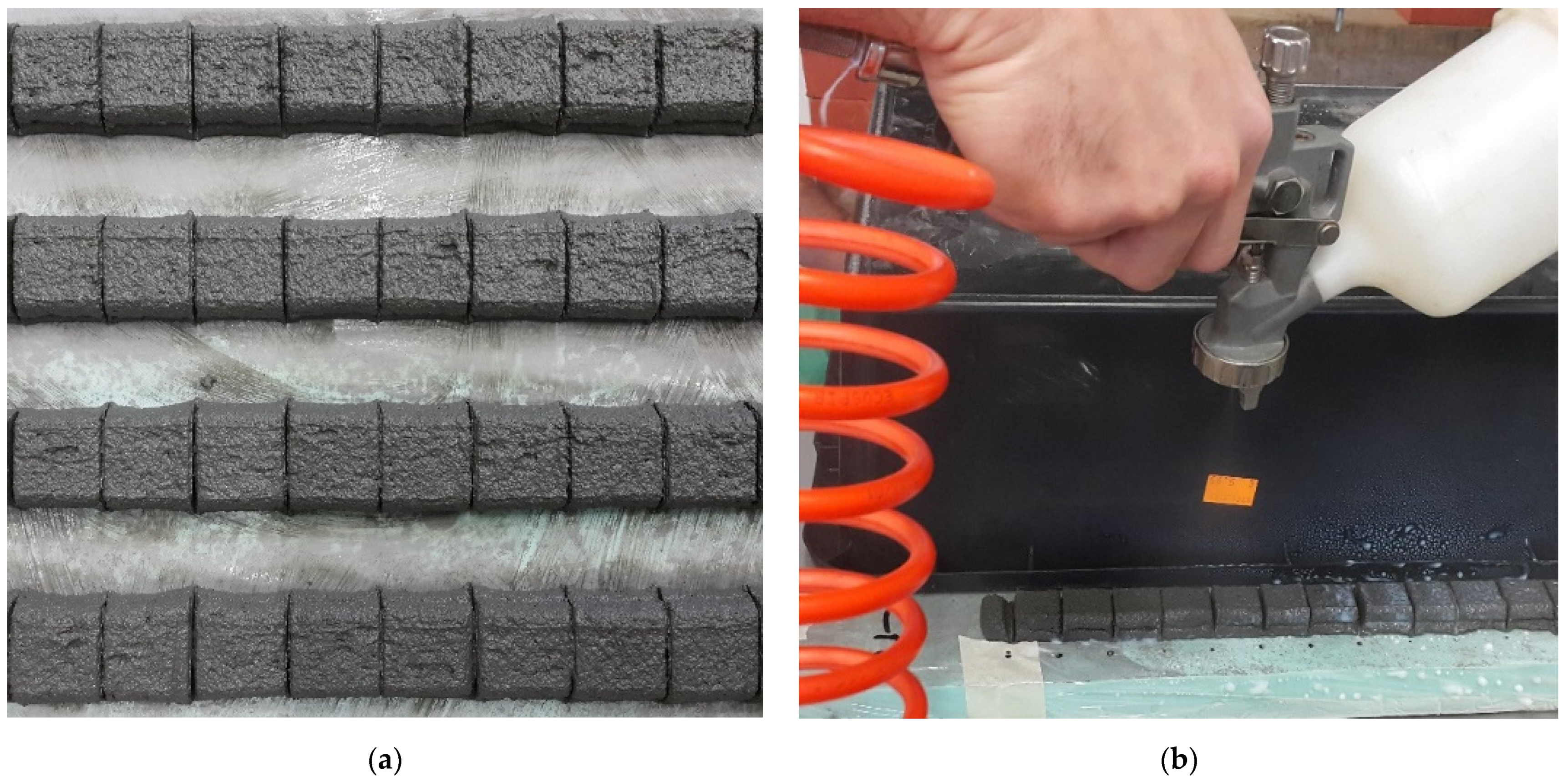
4.2. Sample Preparation
4.3. Methodology for Evaluating the Photocatalytic Efficiency
5. Results and Discussions
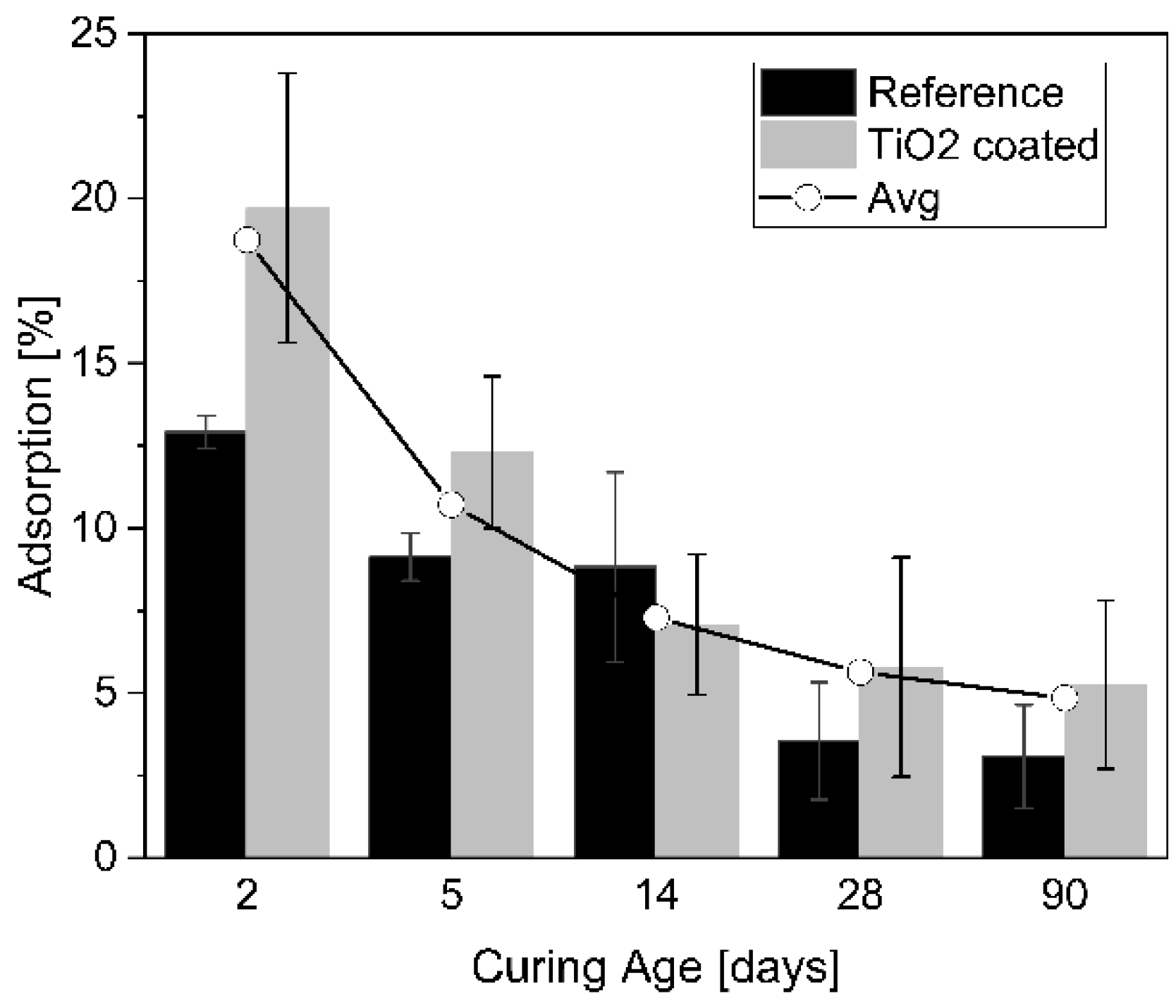
5.1. Influence of Curing Age on Adsorption
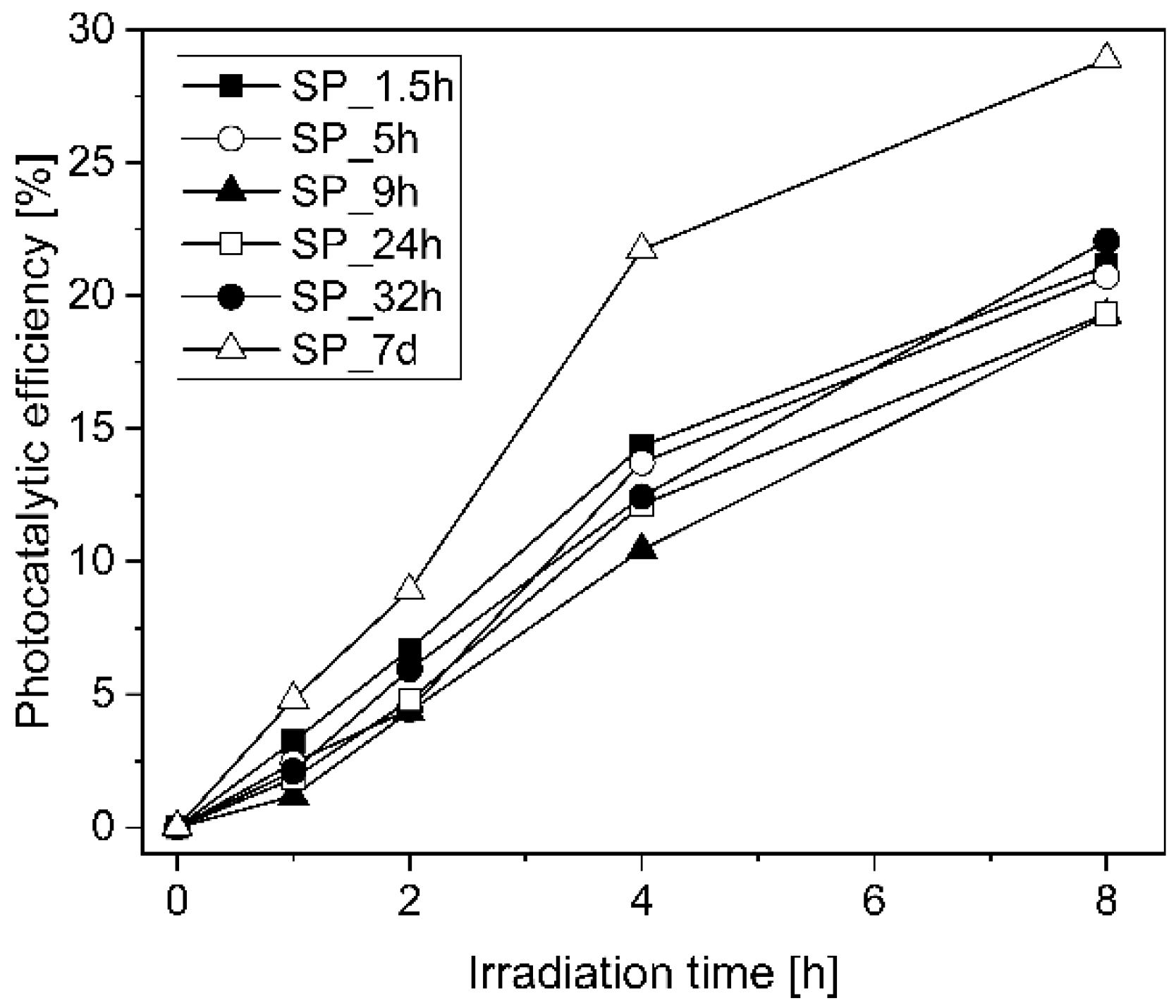
5.2. Influence of the Spraying Time Gap on the Photocatalytic Efficiency
5.3. Influence of the TiO2 Coating Rates on the Photocataytic Efficiency
6. Conclusions
- Adsorption in cementitious materials decreases with increasing curing age. This can be ascribed to the change in the matrix microstructure while the hydration process is ongoing. However, the adsorption of the functionalized specimens coated with TiO2 nanoparticles was higher when compared to the reference specimens, regardless of the curing ages that have been analysed. The high specific surface area of TiO2 nanoparticles could be the main reason for higher adsorption on the functionalized specimens.
- The coating time measured from the beginning of hydration process did not have a significant influence on the photocatalytic efficiency. The only exception occurred for the specimens SP_7d, i.e., coated for 7 days, which showed 47% higher photocatalytic efficiency when compared to the other specimens. Nonetheless, the coating at the beginning of the hydration process when the mixture is still fresh can improve the bonding of TiO2 particles to the surface of the cementitious mortar specimens. Therefore, using the advantages of the 3D printing technology as a free-form construction methodology, the TiO2 aqueous suspension can be sprayed immediately after printing the elements. On the other hand, adequate results obtained for the specimens coated and aged for 7 days could also demonstrate the efficiency of this type of functionalizing method for hardened cementitious matrices. However, TiO2 particles sprayed onto the dried cementitious surfaces may be more prone to be separated from the specimens, which may lead to a decrease in photocatalytic efficiency of samples exposed to real environmental conditions.
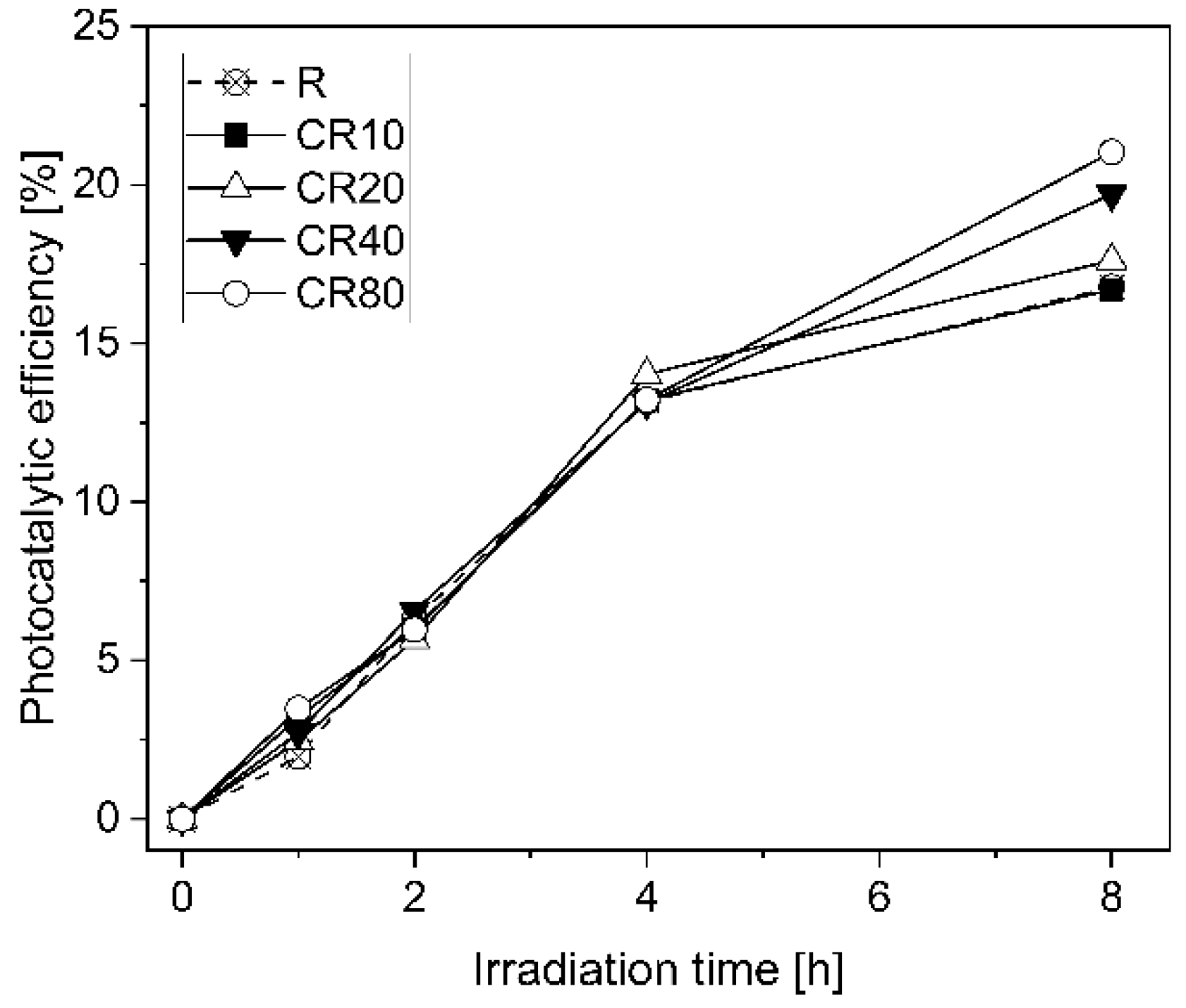
- The photocatalytic efficiency could be enhanced by increasing the coating rates of the TiO2 nanoparticles onto the surface of the specimens. After 8 h of light irradiation, photocatalytic efficiencies of 17% and 21% were obtained for the lowest and the highest coating rates of 10 and 80 mg/cm2, respectively. Similar photocatalytic behaviour for all the series up to 4 h of irradiation was observed. This behaviour may be ascribed to the accumulation of adsorbed pollutants and the produced dye decomposition products onto the specimens’ surface, which could partially inactivate the reaction sites. Nonetheless, by increasing the light exposure period up to 8 h, the reaction products created from the degradation mechanism have enough time to be removed from the surface of the specimen and the TiO2 nanoparticles could be reactivated again in order to degrade a higher quantity of pollutants.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smits, M.; Chan, C.K.; Tytgat, T.; Craeye, B.; Costarramone, N.; Lacombe, S.; Lenaerts, S. Photocatalytic Degradation of Soot Deposition: Self-Cleaning Effect on Titanium Dioxide Coated Cementitious Materials. Chem. Eng. J. 2013, 222, 411–418. [Google Scholar] [CrossRef]
- Rocha Segundo, I.; Freitas, E.; Landi, S., Jr.; Costa, M.F.M.; Carneiro, J.O. Smart, Photocatalytic and Self-Cleaning Asphalt Mixtures: A Literature Review. Coatings 2019, 9, 696. [Google Scholar] [CrossRef] [Green Version]
- Diamanti, M.V.; Ormellese, M.; Pedeferri, M. Characterization of Photocatalytic and Superhydrophilic Properties of Mortars Containing Titanium Dioxide. Cem. Concr. Res. 2008, 38, 1349–1353. [Google Scholar] [CrossRef]
- Sikora, P.; Cendrowski, K.; Markowska-Szczupak, A.; Horszczaruk, E.; Mijowska, E. The Effects of Silica/Titania Nanocomposite on the Mechanical and Bactericidal Properties of Cement Mortars. Constr. Build. Mater. 2017, 150, 738–746. [Google Scholar] [CrossRef]
- Chen, C.; Tang, B.; Cao, X.; Gu, F.; Huang, W. Enhanced Photocatalytic Decomposition of NO on Portland Cement Concrete Pavement Using Nano-TiO2 Suspension. Constr. Build. Mater. 2021, 275, 122135. [Google Scholar] [CrossRef]
- Cassar, L. Photocatalysis of Cementitious Materials: Clean Buildings and Clean Air. MRS Bull. 2004, 29, 328–331. [Google Scholar] [CrossRef]
- Chen, J.; Poon, C.-S. Photocatalytic Activity of Titanium Dioxide Modified Concrete Materials—Influence of Utilizing Recycled Glass Cullets as Aggregates. J. Environ. Manag. 2009, 90, 3436–3442. [Google Scholar] [CrossRef]
- Carneiro, J.O.; Azevedo, S.; Teixeira, V.; Fernandes, F.; Freitas, E.; Silva, H.; Oliveira, J. Development of Photocatalytic Asphalt Mixtures by the Deposition and Volumetric Incorporation of TiO2 Nanoparticles. Constr. Build. Mater. 2013, 38, 594–601. [Google Scholar] [CrossRef]
- Jimenez-Relinque, E.; Rodriguez-Garcia, J.R.; Castillo, A.; Castellote, M. Characteristics and Efficiency of Photocatalytic Cementitious Materials: Type of Binder, Roughness and Microstructure. Cem. Concr. Res. 2015, 71, 124–131. [Google Scholar] [CrossRef]
- Ramirez, A.M.; De Belie, N. Application of TiO2 Photocatalysis to Cementitious Materials for Self-Cleaning Purposes. In Applications of Titanium Dioxide Photocatalysis to Construction Materials; Ohama, Y., Van Gemert, D., Eds.; Springer: Dordrecht, The Netherlands, 2011; ISBN 978-94-007-1296-6. [Google Scholar]
- Essawy, A.A.; El. Aleem, S.A. Physico-Mechanical Properties, Potent Adsorptive and Photocatalytic Efficacies of Sulfate Resisting Cement Blends Containing Micro Silica and Nano-TiO2. Constr. Build. Mater. 2014, 52, 1–8. [Google Scholar] [CrossRef]
- Banerjee, S.; Gopal, J.; Muraleedharan, P.; Tyagi, A.; Raj, B. Physics and Chemistry of Photocatalytic Titanium Dioxide: Visualization of Bactericidal Activity Using Atomic Force Microscopy. Curr. Sci. 2006, 90, 1378–1383. [Google Scholar]
- Aïssa, A.H.; Puzenat, E.; Plassais, A.; Herrmann, J.-M.; Haehnel, C.; Guillard, C. Characterization and Photocatalytic Performance in Air of Cementitious Materials Containing TiO2. Case Study of Formaldehyde Removal. Appl. Catal. B Environ. 2011, 107, 1–8. [Google Scholar] [CrossRef]
- Carneiro, J.O.; Teixeira, V.; Portinha, A.; Dupák, L.; Magalhães, A.; Coutinho, P. Study of the Deposition Parameters and Fe-Dopant Effect in the Photocatalytic Activity of TiO2 Films Prepared by Dc Reactive Magnetron Sputtering. Vacuum 2005, 78, 37–46. [Google Scholar] [CrossRef]
- Kamaruddin, S.; Stephan, D. Sol–Gel Mediated Coating and Characterization of Photocatalytic Sand and Fumed Silica for Environmental Remediation. Water. Air. Soil Pollut. 2014, 225, 1948. [Google Scholar] [CrossRef]
- Hamidi, F.; Aslani, F. TiO2-Based Photocatalytic Cementitious Composites: Materials, Properties, Influential Parameters, and Assessment Techniques. Nanomaterials 2019, 9, 1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, S.; Song, J.; Liu, F.; Fu, X.; Guo, H.; Zhu, J.; Zeng, Q.; Peng, X.; Wang, X.; Ouyang, Y.; et al. Photocatalytic Properties, Mechanical Strength and Durability of TiO2/Cement Composites Prepared by a Spraying Method for Removal of Organic Pollutants. Chemosphere 2020, 254, 126813. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, F.; Germinario, S.; Basile, R.; Montagno, R.; Kapetanaki, K.; Gobakis, K.; Kolokotsa, D.; Lagou, A.M.; Dania, P.; Enna, M.T.; et al. Development of Eco-Friendly and Self-Cleaning Lime-Pozzolan Plasters for Bio-Construction and Cultural Heritage. Buildings 2020, 10, 172. [Google Scholar] [CrossRef]
- Ao, C.H.; Lee, S.C. Indoor Air Purification by Photocatalyst TiO2 Immobilized on an Activated Carbon Filter Installed in an Air Cleaner. Chem. Eng. Sci. 2005, 60, 103–109. [Google Scholar] [CrossRef]
- Anderson, A.-L.; Chen, S.; Romero, L.; Top, I.; Binions, R. Thin Films for Advanced Glazing Applications. Buildings 2016, 6, 37. [Google Scholar] [CrossRef]
- Hakki, A.; Yang, L.; Wang, F.; Elhoweris, A.; Alhorr, Y.; Macphee, D. Photocatalytic Functionalized Aggregate: Enhanced Concrete Performance in Environmental Remediation. Buildings 2019, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Folli, A.; Pade, C.; Hansen, T.B.; De Marco, T.; Macphee, D.E. TiO2 Photocatalysis in Cementitious Systems: Insights into Self-Cleaning and Depollution Chemistry. Cem. Concr. Res. 2012, 42, 539–548. [Google Scholar] [CrossRef]
- Bao, Y.; Xu, M.; Soltan, D.; Xia, T.; Shih, A.; Clack, H.L.; Li, V.C. Three-Dimensional Printing Multifunctional Engineered Cementitious Composites (ECC) for Structural Elements. In First RILEM International Conference on Concrete and Digital Fabrication—Digital Concrete 2018; Wangler, T., Flatt, R.J., Eds.; Springer International Publishing: Cham, Switzerland, 2019; Volume 19, pp. 115–128. ISBN 978-3-319-99518-2. [Google Scholar]
- Han, B.; Zhang, L.; Ou, J. Smart and Multifunctional Concrete toward Sustainable Infrastructures; Springer: Singapore, 2017; ISBN 978-981-10-4348-2. [Google Scholar]
- Han, B.; Ding, S.; Wang, J.; Ou, J. Nano-Engineered Cementitious Composites: Principles and Practices; Springer: Singapore, 2019; ISBN 9789811370779. [Google Scholar]
- Ruot, B.; Plassais, A.; Olive, F.; Guillot, L.; Bonafous, L. TiO2-Containing Cement Pastes and Mortars: Measurements of the Photocatalytic Efficiency Using a Rhodamine B-Based Colourimetric Test. Sol. Energy 2009, 83, 1794–1801. [Google Scholar] [CrossRef]
- Campos Teixeira, A.H.; Soares Junior, P.R.R.; Silva, T.H.; Barreto, R.R.; da Silva Bezerra, A.C. Low-Carbon Concrete Based on Binary Biomass Ash—Silica Fume Binder to Produce Eco-Friendly Paving Blocks. Materials 2020, 13, 1534. [Google Scholar] [CrossRef] [Green Version]
- Zahabizadeh, B.; Pereira, J.; Gonçalves, C.; Pereira, E.N.B.; Cunha, V.M.C.F. Influence of the Printing Direction and Age on the Mechanical Properties of 3D Printed Concrete. Mater. Struct. 2021, 54, 73. [Google Scholar] [CrossRef]
- Chen, J.; Poon, C. Photocatalytic Construction and Building Materials: From Fundamentals to Applications. Build. Environ. 2009, 44, 1899–1906. [Google Scholar] [CrossRef]
- Liu, T.; Wang, L.; Lu, X.; Fan, J.; Cai, X.; Gao, B.; Miao, R.; Wang, J.; Lv, Y. Comparative Study of the Photocatalytic Performance for the Degradation of Different Dyes by ZnIn2S4: Adsorption, Active Species, and Pathways. RSC Adv. 2017, 7, 12292–12300. [Google Scholar] [CrossRef] [Green Version]
- Kang, X.; Liu, S.; Dai, Z.; He, Y.; Song, X.; Tan, Z. Titanium Dioxide: From Engineering to Applications. Catalysts 2019, 9, 191. [Google Scholar] [CrossRef] [Green Version]
- Fujishima, A.; Zhang, X.; Tryk, D. TiO2 Photocatalysis and Related Surface Phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, K.; Yu, H.; Zhao, L.; Zhu, X.; Zhang, J. Laboratory Experiment on the Nano-TiO2 Photocatalytic Degradation Effect of Road Surface Oil Pollution. Nanotechnol. Rev. 2020, 9, 922–933. [Google Scholar] [CrossRef]
- Bogutyn, S.; Arboleda, C.; Bordelon, A.; Tikalsky, P. Rejuvenation Techniques for Mortar Containing Photocatalytic TiO2 Material. Constr. Build. Mater. 2015, 96, 96–101. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and Mechanisms of Photocatalytic Dye Degradation on TiO2 Based Photocatalysts: A Comparative Overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Zahabizadeh, B.; Pereira, J.; Gonçalves, C.; Cunha, V.M.C.F. Development of Cement-Based Mortars for 3D Printing through Wet Extrusion. In Proceedings of the IABSE Symposium 2019 Guimarães: Towards a Resilient Built Environment—Risk and Asset Management, Guimarães, Portugal, 27–29 March 2019. [Google Scholar]
- Carneiro, J.O.; Azevedo, S.; Fernandes, F.; Freitas, E.; Pereira, M.; Tavares, C.J.; Lanceros-Méndez, S.; Teixeira, V. Synthesis of Iron-Doped TiO2 Nanoparticles by Ball-Milling Process: The Influence of Process Parameters on the Structural, Optical, Magnetic, and Photocatalytic Properties. J. Mater. Sci. 2014, 49, 7476–7488. [Google Scholar] [CrossRef]
- Hernández-Alonso, M.D.; Fresno, F.; Suárez, S.; Coronado, J.M. Development of Alternative Photocatalysts to TiO2: Challenges and Opportunities. Energy Environ. Sci. 2009, 2, 1231. [Google Scholar] [CrossRef]
- Khan, A.F.; Mehmood, M.; Durrani, S.K.; Ali, M.L.; Rahim, N.A. Structural and Optoelectronic Properties of Nanostructured TiO2 Thin Films with Annealing. Mater. Sci. Semicond. Process. 2015, 29, 161–169. [Google Scholar] [CrossRef]
- Rocha Segundo, I.; Landi, S., Jr.; Oliveira, S.M.B.; Freitas, E.F.; Carneiro, J.A.O. Photocatalytic Asphalt Mixtures: Mechanical Performance and Impacts of Traffic and Weathering Abrasion on Photocatalytic Efficiency. Catal. Today 2019, 326, 94–100. [Google Scholar] [CrossRef]
- Rocha Segundo, I.; Landi, S., Jr.; Oliveira, S.; Freitas, E.; Costa, M.F.; Carneiro, J. Photocatalytic Asphalt Mixtures: Semiconductors’ Impact in Skid Resistance and Texture. Road Mater. Pavement Des. 2019, 20, S578–S589. [Google Scholar] [CrossRef]
- Espinosa, R.M.; Franke, L. Influence of the Age and Drying Process on Pore Structure and Sorption Isotherms of Hardened Cement Paste. Cem. Concr. Res. 2006, 36, 1969–1984. [Google Scholar] [CrossRef]
- Folli, A.; Jakobsen, U.H.; Guerrini, G.L.; Macphee, D.E. Rhodamine B Discolouration on TiO2 in the Cement Environment: A Look at Fundamental Aspects of the Self-Cleaning Effect in Concretes. J. Adv. Oxid. Technol. 2009, 12, 126–133. [Google Scholar] [CrossRef]
- Enesca, A.; Isac, L. The Influence of Light Irradiation on the Photocatalytic Degradation of Organic Pollutants. Materials 2020, 13, 2494. [Google Scholar] [CrossRef]
| Material | Quantity [kg/m3] | Specific Weight [g/cm3] |
|---|---|---|
| Sand | 1183 | 2.66 |
| Cement (CEM I 42.5 R) | 286 | 3.11 |
| Fly ash (type F) | 423 | 2.35 |
| Silica fume | 79 | 2.25 |
| Water | 248 | 1 |
| Superplasticizer | 10.2 [1.3%] | 1.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahabizadeh, B.; Segundo, I.R.; Pereira, J.; Freitas, E.; Camões, A.; Tavares, C.J.; Teixeira, V.; Cunha, V.M.C.F.; Costa, M.F.M.; Carneiro, J.O. Development of Photocatalytic 3D-Printed Cementitious Mortars: Influence of the Curing, Spraying Time Gaps and TiO2 Coating Rates. Buildings 2021, 11, 381. https://doi.org/10.3390/buildings11090381
Zahabizadeh B, Segundo IR, Pereira J, Freitas E, Camões A, Tavares CJ, Teixeira V, Cunha VMCF, Costa MFM, Carneiro JO. Development of Photocatalytic 3D-Printed Cementitious Mortars: Influence of the Curing, Spraying Time Gaps and TiO2 Coating Rates. Buildings. 2021; 11(9):381. https://doi.org/10.3390/buildings11090381
Chicago/Turabian StyleZahabizadeh, Behzad, Iran Rocha Segundo, João Pereira, Elisabete Freitas, Aires Camões, Carlos J. Tavares, Vasco Teixeira, Vítor M. C. F. Cunha, Manuel F. M. Costa, and Joaquim O. Carneiro. 2021. "Development of Photocatalytic 3D-Printed Cementitious Mortars: Influence of the Curing, Spraying Time Gaps and TiO2 Coating Rates" Buildings 11, no. 9: 381. https://doi.org/10.3390/buildings11090381
APA StyleZahabizadeh, B., Segundo, I. R., Pereira, J., Freitas, E., Camões, A., Tavares, C. J., Teixeira, V., Cunha, V. M. C. F., Costa, M. F. M., & Carneiro, J. O. (2021). Development of Photocatalytic 3D-Printed Cementitious Mortars: Influence of the Curing, Spraying Time Gaps and TiO2 Coating Rates. Buildings, 11(9), 381. https://doi.org/10.3390/buildings11090381














