3.1. Synthesis of Calcium Silicate Hydrates
In developing the technology for the production of the fillers, the following factors were considered: a density of the liquid glass, an amount of addition of the precipitant and the rate of its introduction, and a mode of drying the precipitate and the time of its storage.
Table 3 shows that with an increase in the precipitate additive content during the filler synthesis, the lime compositions’ compressive strengths with fillers increases.
It was found that the output of the filler synthesized from the water glass in the presence of CaCl2 in the form of a 15% solution in an amount of 30 or 50% of the weight of liquid glass was 85%. This refers to the first set of specimens (liquid glass modulus 2.9). The output of the filler synthesized from the liquid glass in the presence of CaCl2 in the form of 7.5% solution in the amount of 30 or 50% of the weight of liquid glass was 100%. After drying at a temperature of 105 °C, the filler’s real density was 2200 kg/m3, and the bulk density was 1935 kg/m3.
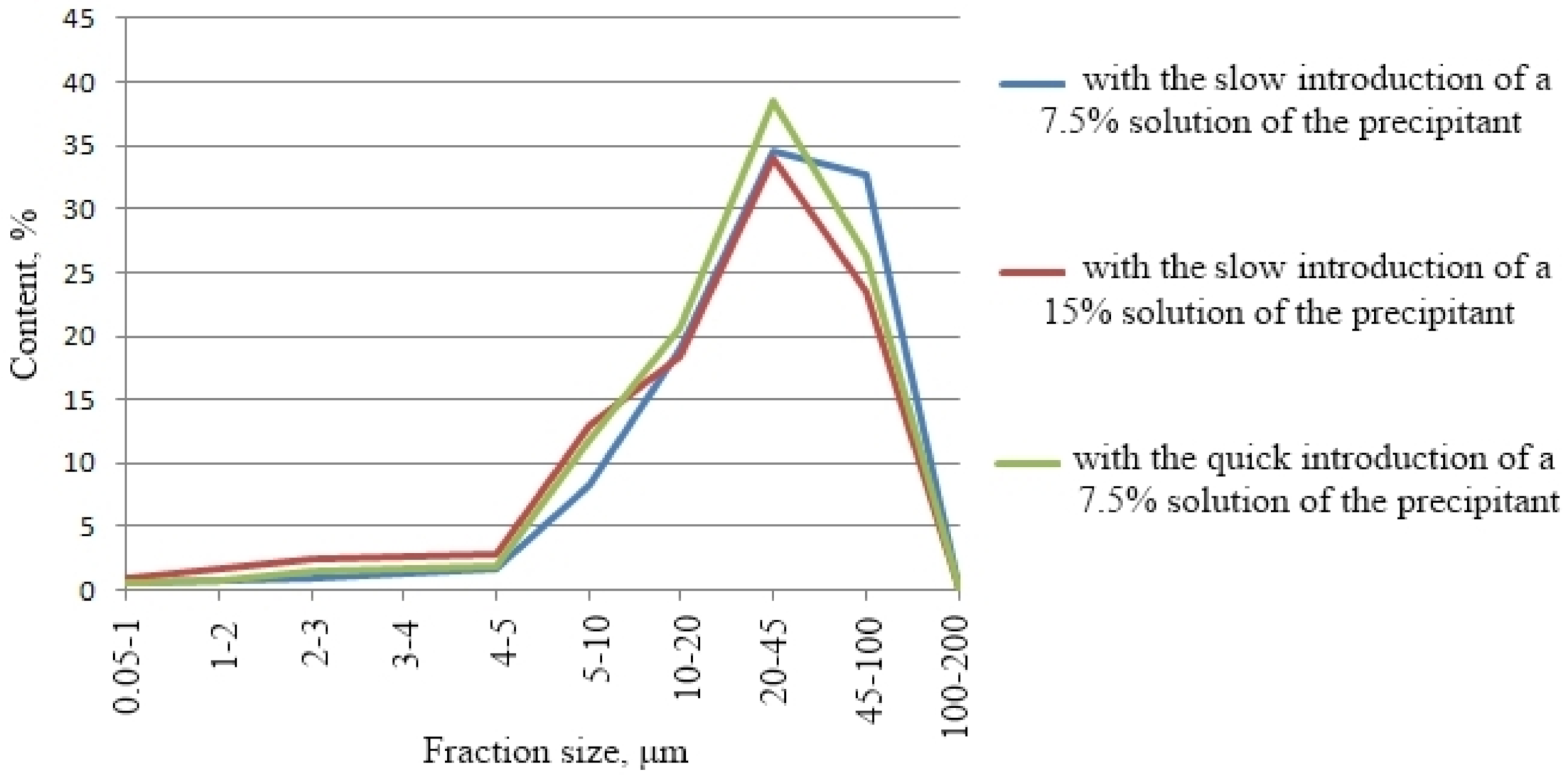
It was revealed that the particle size distribution of the silicate-containing filler has a similar polyfractional nature; the size distribution of the filler particles is bimodal (
Figure 2).
The slow introduction of the precipitant solution was carried out within 10 min, and the fast one was within 1 min. With the slow introduction of the CaCl2 precipitating additive, a small number of large particles were formed in the sediment. The granulometric data indicate that particles with sizes of 20–45 μm prevail, the contents of which was in the range 34–39%. The arithmetic mean value of the filler’s particle size synthesized with the slow introduction of the CaCl2 precipitating additive was 29.35 μm.
The use of more dilute solutions (7.5%) led to a slowdown in precipitation and the appearance of larger crystals. The content of particles with a size of 45–100 μm was 32.62%, crystals with a particle size of 100–200 μm appeared, and their content was 0.04%.
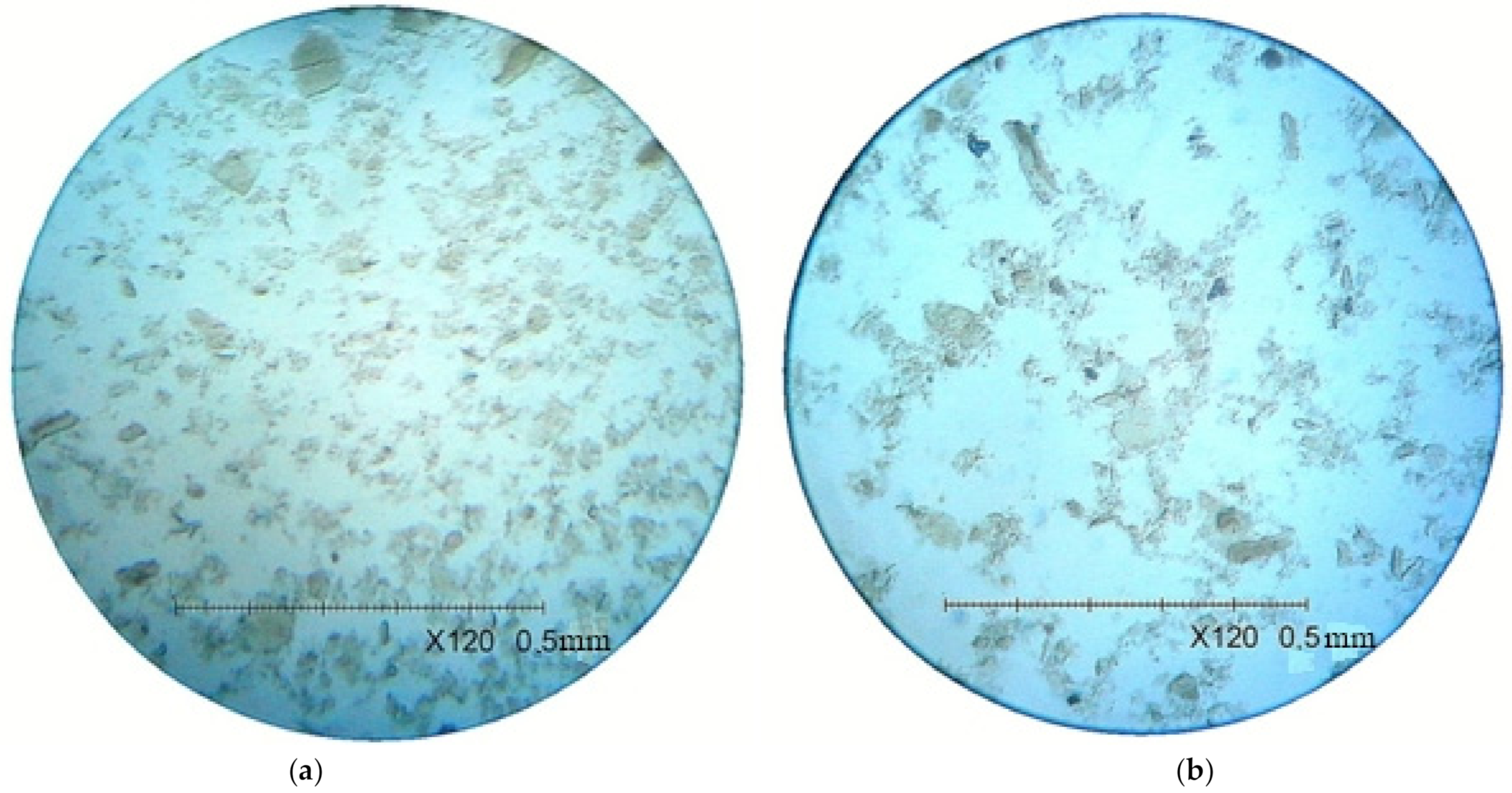
With an increase in the sediment’s maturation time, crystal growth was observed (
Figure 3). The content of particles with a size of 45–100 µm increased to 33.21%. The content of particles with a size of 100–200 µm increased by 1.02%.
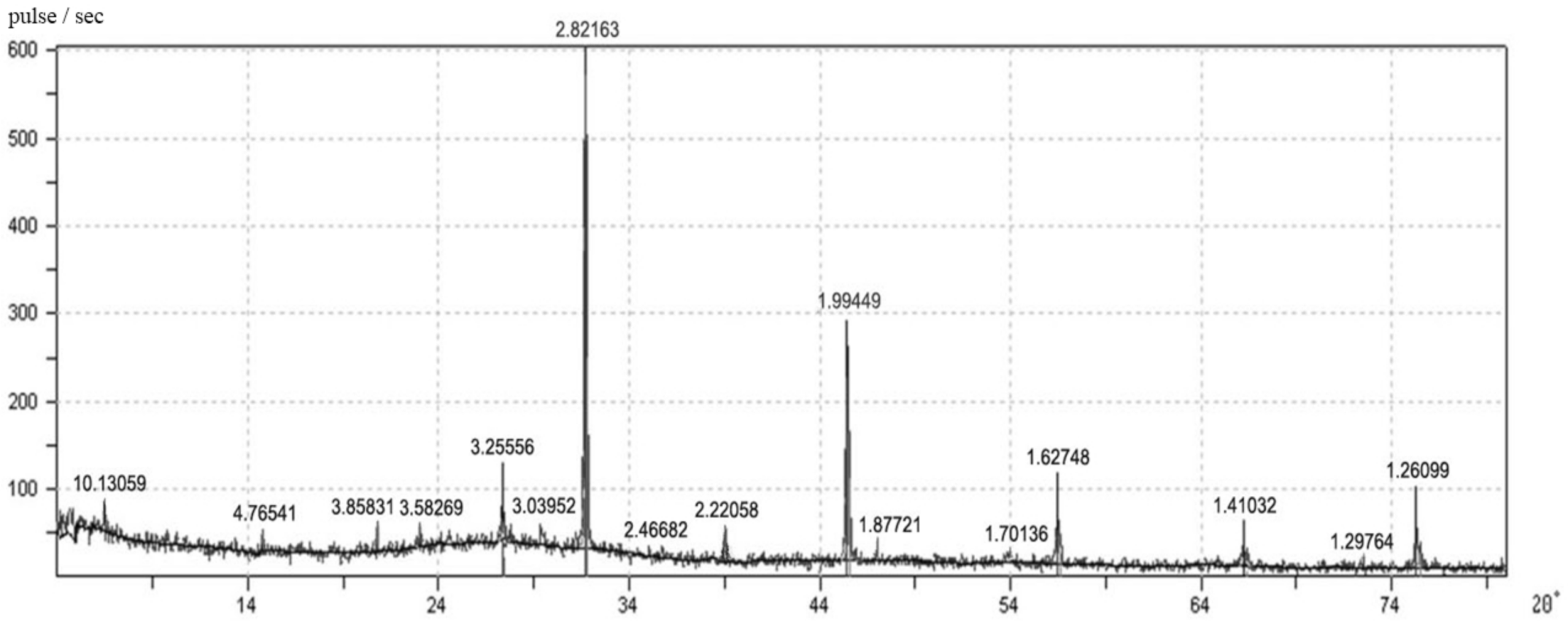
In studying the new growth’s qualitative composition, it was found that the samples’ degree of crystallization is low. The X-ray diffraction pattern (
Figure 4) of the filler samples contains diffraction lines (Å) of the following compounds: calcium silicate hydrates of the tobermorite group (10.13059; 3.58269; 3.25556; 3.2579; 2.82015; 2.4662; 1.29764; 1.2618); solid solution CSH (B) in the form of slightly crystallized gel (4.76541; 3.03952; 2.82163) solid solution C–S–H (II): (2.22058; 1.87721; 1.41032); hydrohalites: (3.85831; 1.99449; 1.62748).
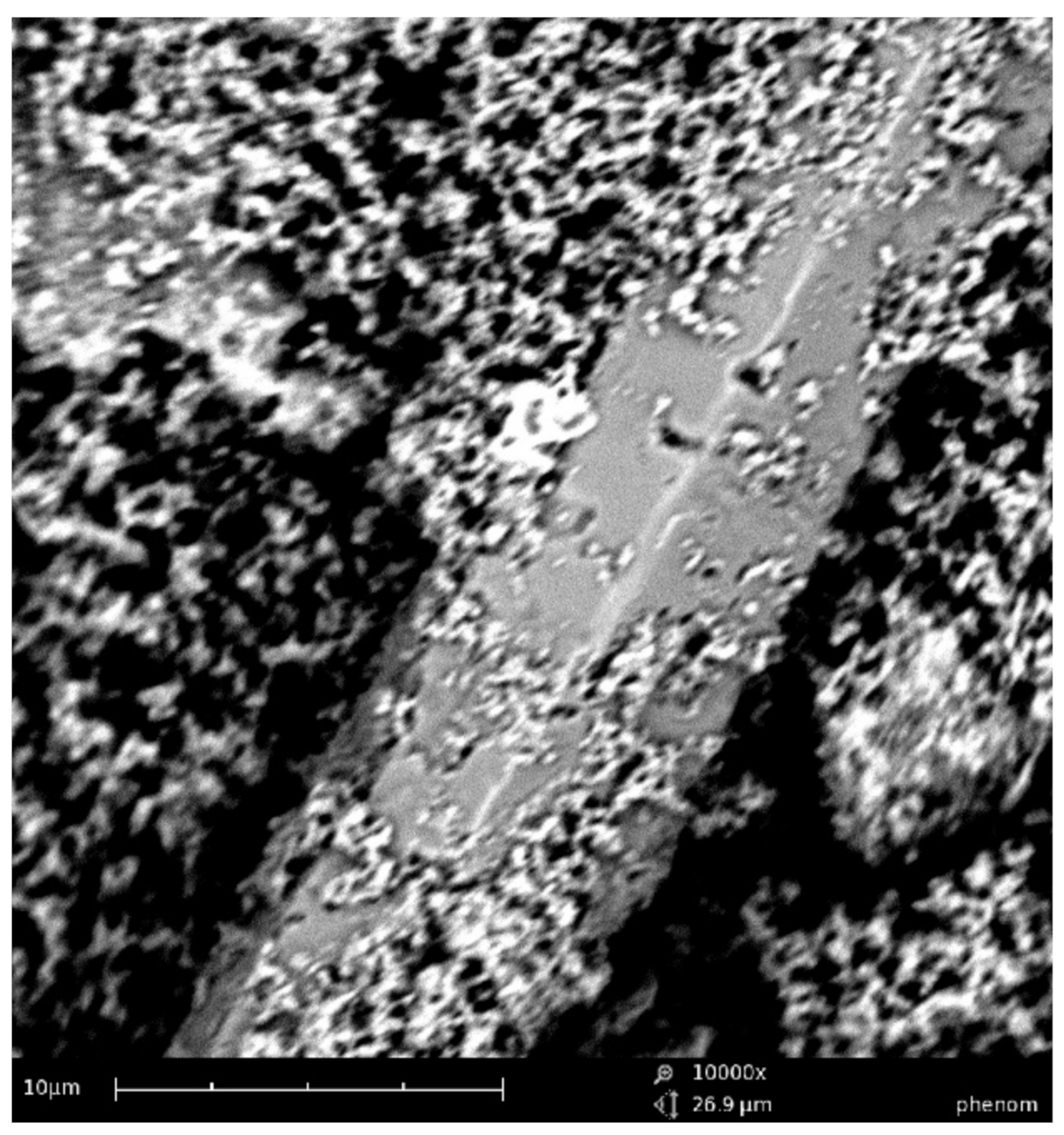
The filler structure is represented by lamellar and acicular formations corresponding to calcium silicate hydrates (
Figure 5).
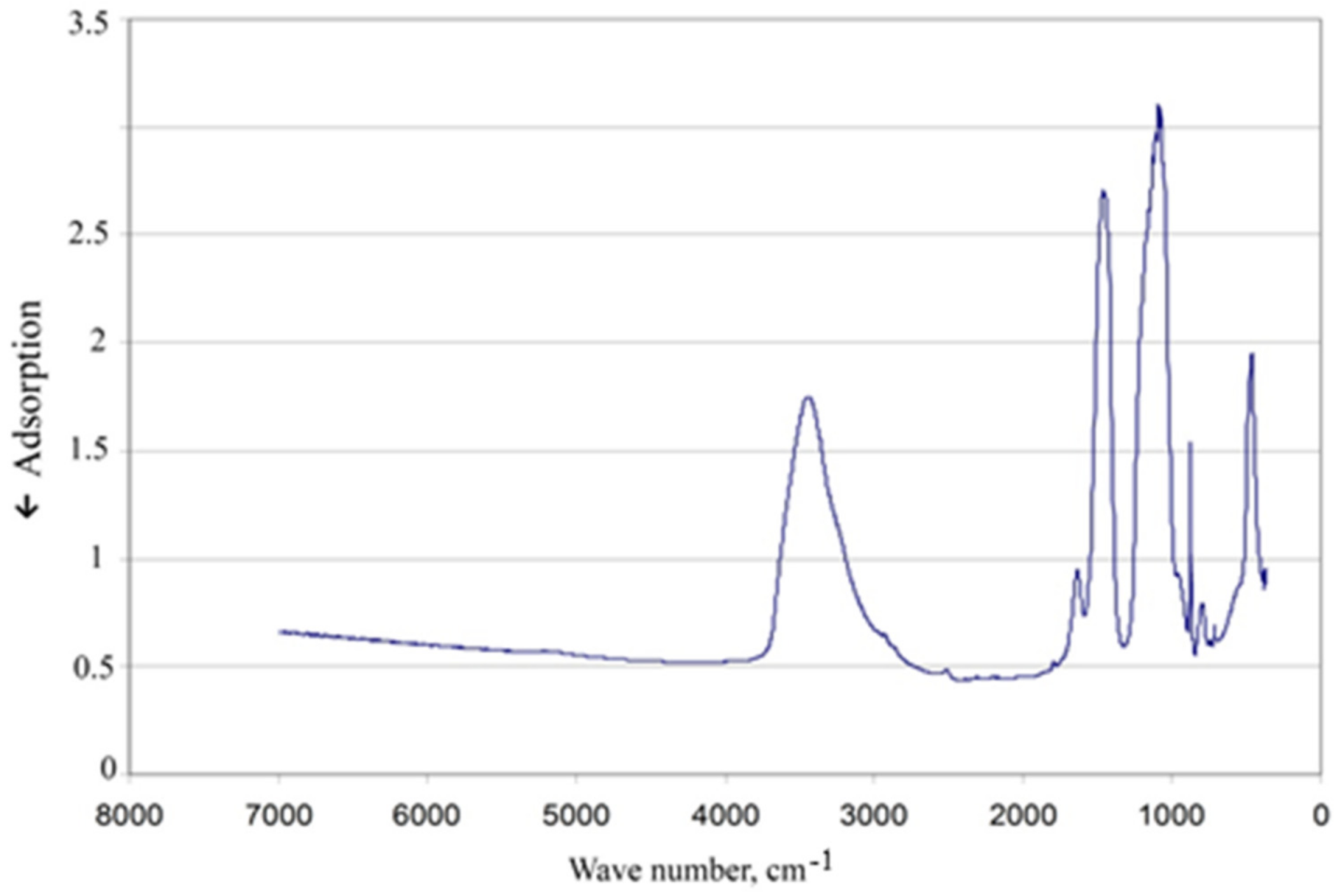
Analysis of the IR spectrum of the obtained filler’s sample was performed for additional assessment.
Figure 6 shows clearly distinguished absorption bands in the regions of 850–1100 cm
–1, 550–750 cm
–1, 400–550 cm
–1, confirming the presence of calcium silicate hydrates in the synthesized material.
The study of the hygroscopic properties of the fillers showed that they have a high sorption capacity. At a relative humidity of 72%, the sorption humidification after 10 days was 20%, and at a relative humidity of 100% it was 95%.
It was found that storage of the filler under conditions that exclude moisture access does not change its activity. After 20 days of storage under conditions excluding moisture access, the decrease in the activity of the filler was 5%, and after storage for 30–40 days it was 9–20%.
3.2. Development of the Modified Lime Compositions
The influence of the water–lime ratio (w/L), the content of modifying additives, the CSH filler, and the technology of its production on the properties of the finishing compositions was evaluated. It was revealed that the introduction of filler based on CSH into the lime composition changes the character of structure formation. A thermodynamic analysis of possible reactions was carried out during the interaction of the mix components (lime, filler based on calcium silicate hydrates, and mixing water) in accordance with the second law of thermodynamics (
Table 4).
The calculation results indicate the probability of all considered reactions proceeding in the forward direction (negative values of ΔН0298). The large numerical value ΔG0298 found for the formation reaction makes it possible with sufficient probability to speak about the possibility of the reaction proceeding at standard temperature (25 °C) and at other temperatures.
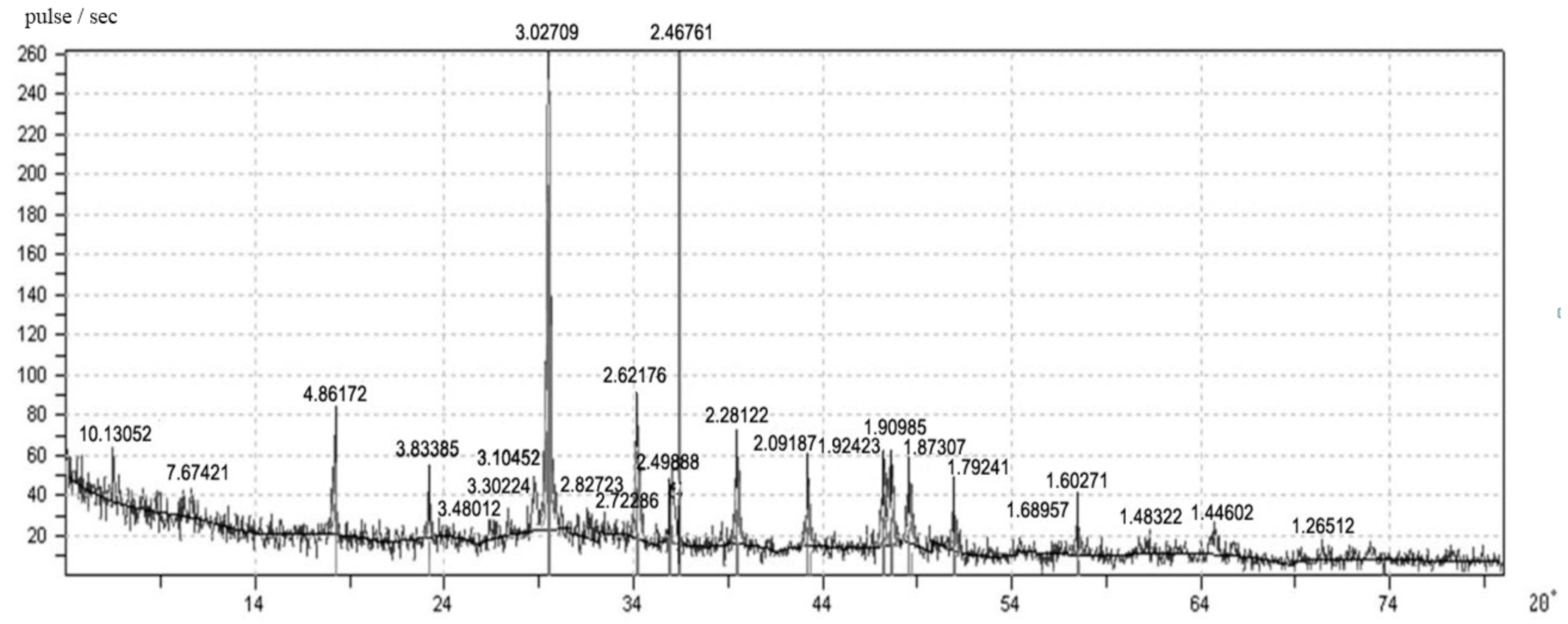
In the study of solid-phase reactions occurring in the process of structure formation of lime compositions (
Figure 7), it was revealed that in the samples of the lime composite with the synthesized filler there were diffraction lines (Å) of the following compounds: afwillites: 3.48012; 3.30452; 2.72286; 1.79241; 1.68957; 1.6027; portlandites: 2.62176; 1.48322; 1.44602; calcites: 2.28122; 1.9098; 2.49888; hillebrandites: 10.13052; 2.28122; 1.9242; 1.8730; okenites: 7.67421; 3.10452; 3.02709; tobermorites: 4.86172; 2.82723; 2.46761; calcium silicate hydroxides: 3.83385; 2.09187.
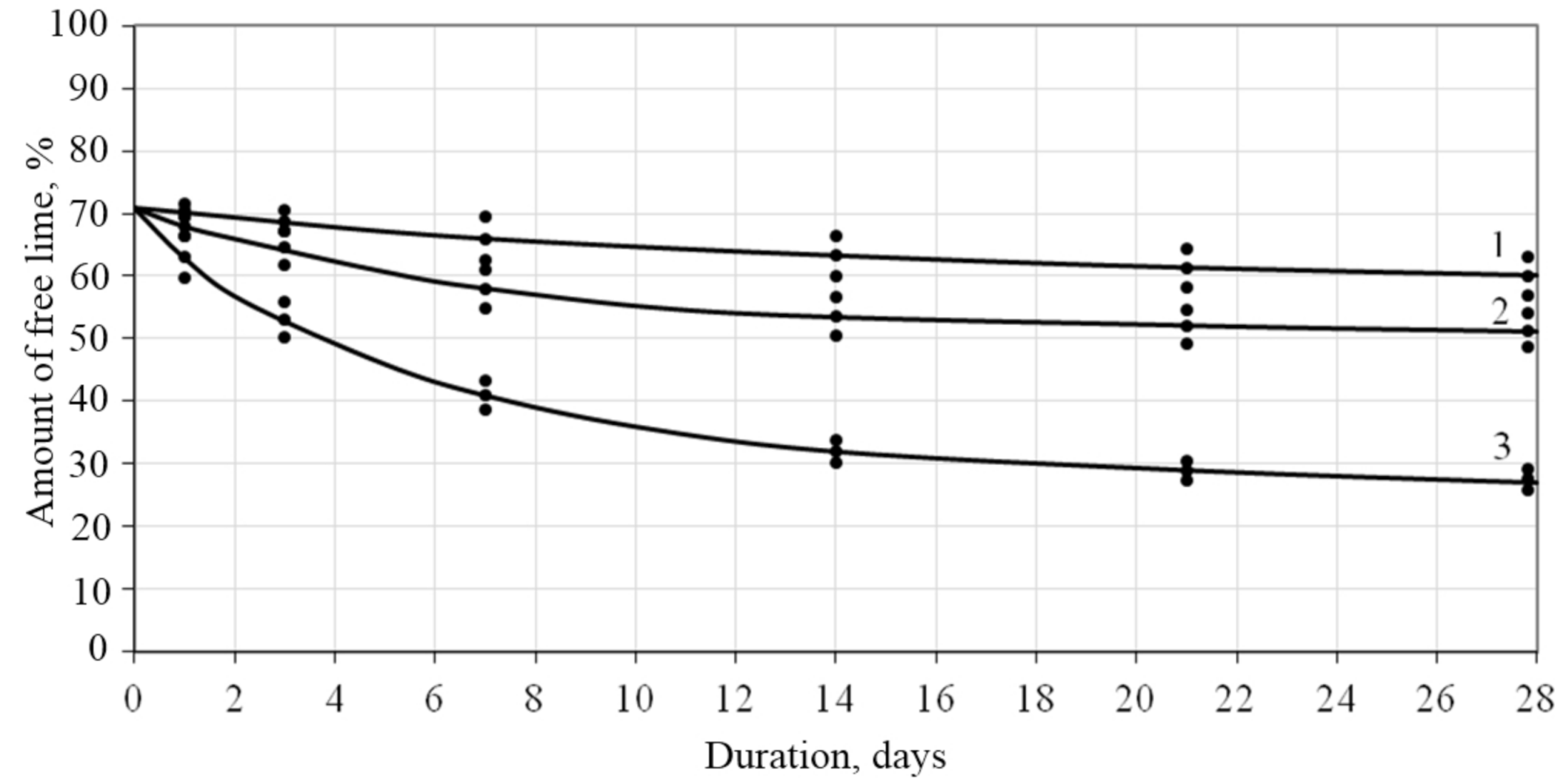
An increase in the number of peaks in the X-ray diffraction pattern of samples of a lime composite with filler based on CSH suggests that this filler was highly active and indicates a chemical interaction with lime. The studies carried out show that the CSH filler has a hydraulic activity of 178–289 mg/g depending on the synthesis mode. It was found that the amount of free lime in the control samples after 28 days of air-dry hardening was 60%, and in the samples with the CSH filler of the composition lime: CSH = 1:0.3, w/L = 0.9–27%, which indicates about the chemical interaction of calcium silicate hydrates with lime (
Figure 8).
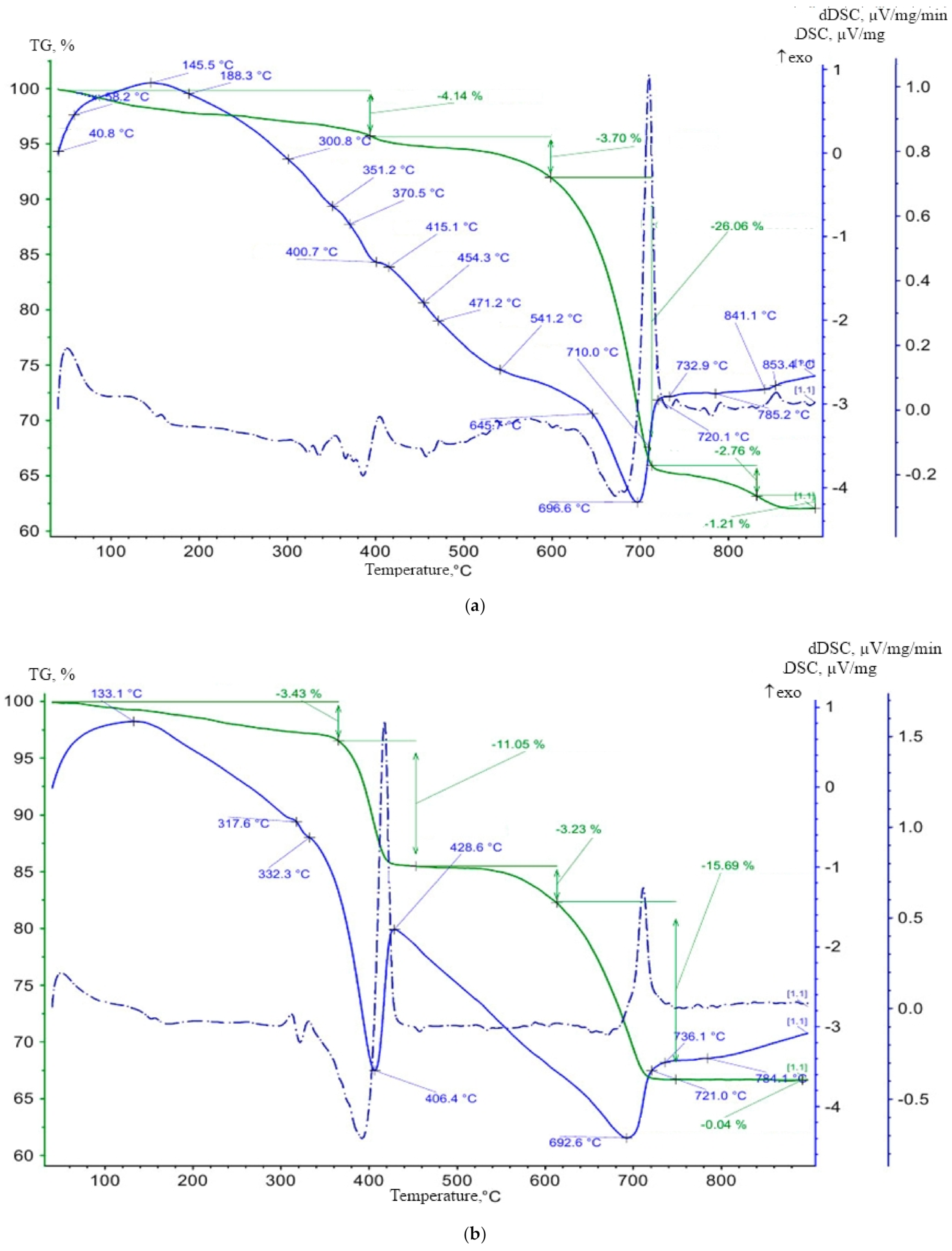
The data obtained were further confirmed during the differential thermal analysis (
Figure 9).
It was found that in the samples of a lime composite with CSH filler, along with the decomposition of calcium oxide and calcium carbonate, dehydration of tobermorite (454.2 °C), afwilite (471.2 °C), hillebrandite (541.2 °C), okenite (645.7 °C) was observed, whose products are calcium silicates and modifications, wollastonite and crystobalite (
Figure 9a). The total weight loss in the lime composite samples with the filler in the temperature range 400–700 °C was 26%, and in the control sample was only 5% (
Figure 9b). A significant loss of water by weight in the samples of the lime composite with the filler suggests that a large number of highly basic calcium silicate hydrates were formed as a result of the chemical reaction of the filler and the lime.
The obtained XRD and DTA data indicate the formation of a stronger structure of the lime composite with CSH filler.
Table 5 shows the compressive strength data of the lime composite depending on the filler content.
The highest values of strength indicators for modified compositions were ratios of lime:filler = 1:0.3 at w/L = 0.7 and lime:filler = 1:0.5 at w/L = 0.9.
An increase in the dispersion of the filler leads to an increase in its chemical activity and a regular increase in the values of the compressive strength (
Table 6).
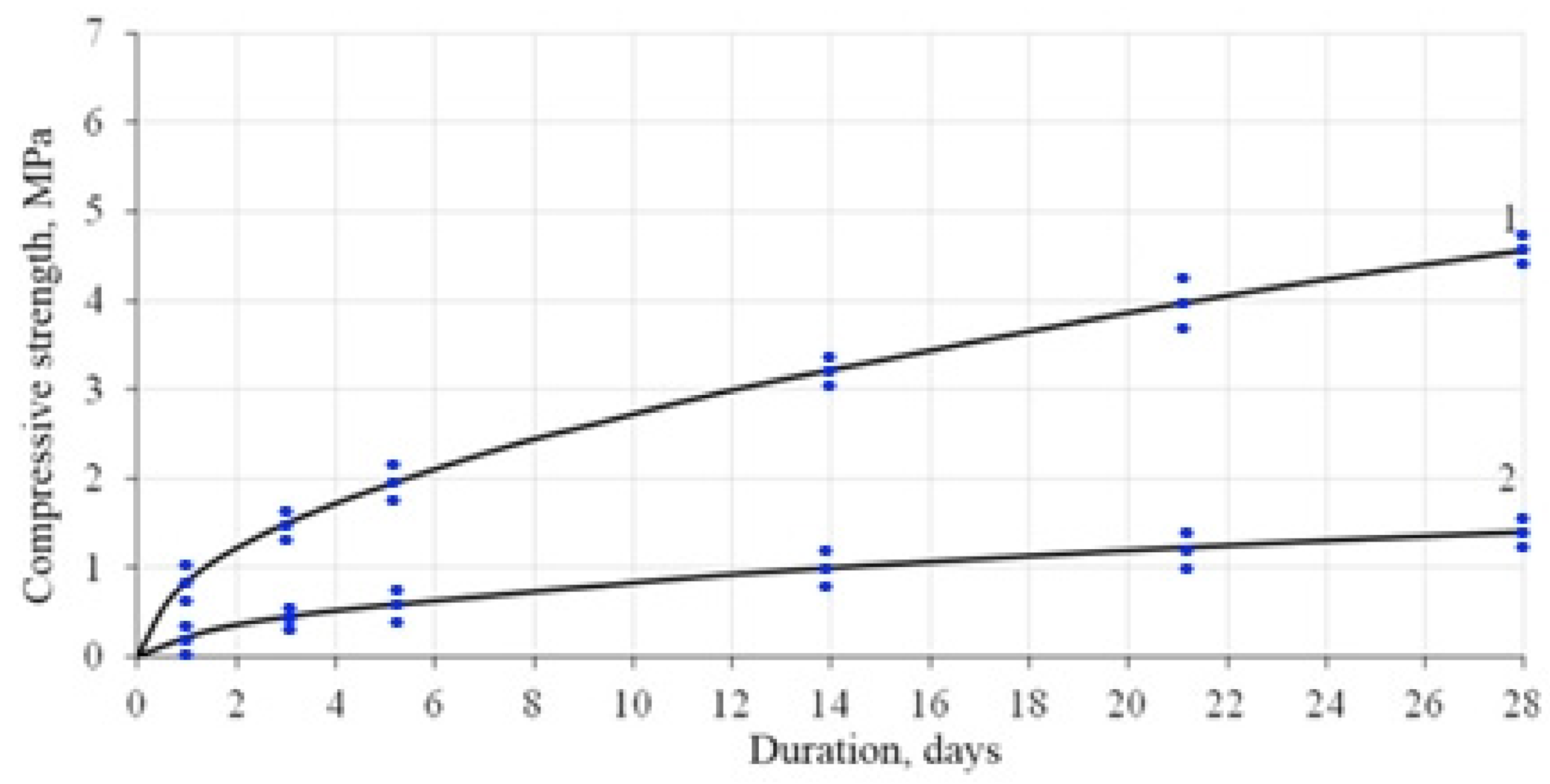
Figure 9 shows the compressive strength values of lime paste during hardening. So, in 2 days, the compressive strength of lime paste with the CSH addition was 1.22 MPa, and for the reference composition was 0.36 MPa.
Based on the results shown in
Figure 10, the models of the kinetics of hardening of the lime composites are proposed:
where
R is a compressive strength of the lime composite;
t is a hardening duration.
In order to regulate the rheological, technological, and functional properties of the finishing compositions, various superplasticizers were introduced into the mix formulation. The amount of the additive was 0.7–2% by weight of the binder. The use of superplasticizers S-3 or SP-3 gives the maximum plasticizing effect of the finishing lime composition. In this case, the water-reducing effect is 1.6 (
Table 7). The introduction of the CSH filler to the lime increases the water-reducing effect to 1.8. The water-reducing effect is a reduction in water consumption while maintaining equal flowability.
The research results showed that the plasticizing effect, depending on the type of additive, persists for 1–2 h. The plasticizing effect was not observed at low dosages of the additives F15G (0.2–0.4%) and 1641F (0.2%). At the maximum dosage recommended by additive manufacturers, the water-reducing coefficient in CSH formulations was 1.7–1.8.
The filler’s introduction into the lime composition increased the water retention capacity up to 96%. The superplasticizer’s introduction into the lime composition with the CSH filler increased the water retention capacity to 99% (
Table 8).
When evaluating the structure, it was found that lime compositions form coatings characterized by high porosity and a significant volume of open pores (
Table 9).
The introduction of the CSH filler into the lime formulation leads to a decrease in porosity and an increase in the volume of closed pores; as a result of this, water absorption of the lime paste decreases.
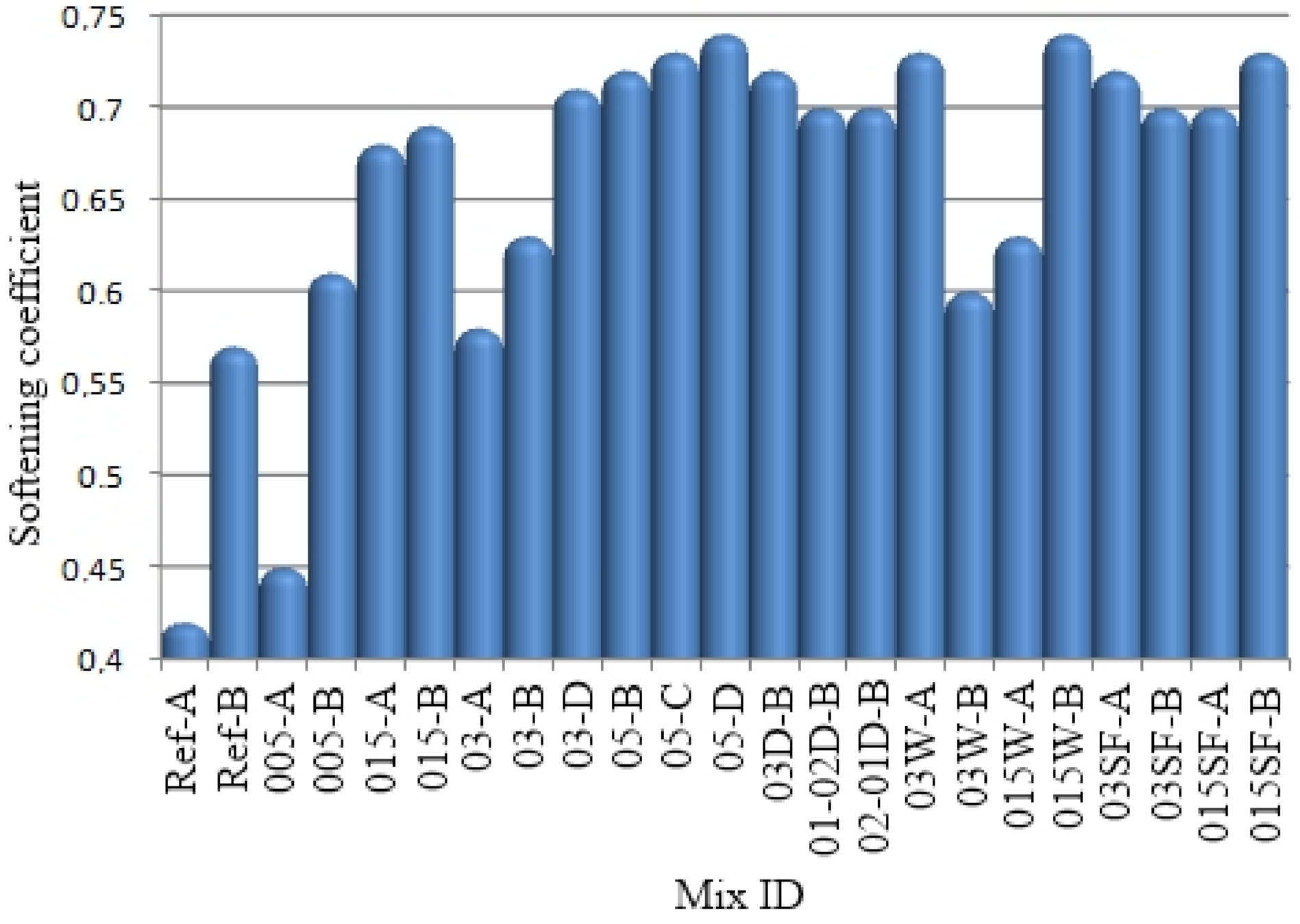
Low values of open porosity and high values of strength of the developed composites made it possible to achieve high softening coefficients (
Figure 11).