The Role of Glass Compounds in Autoclaved Bricks
Abstract
1. Introduction
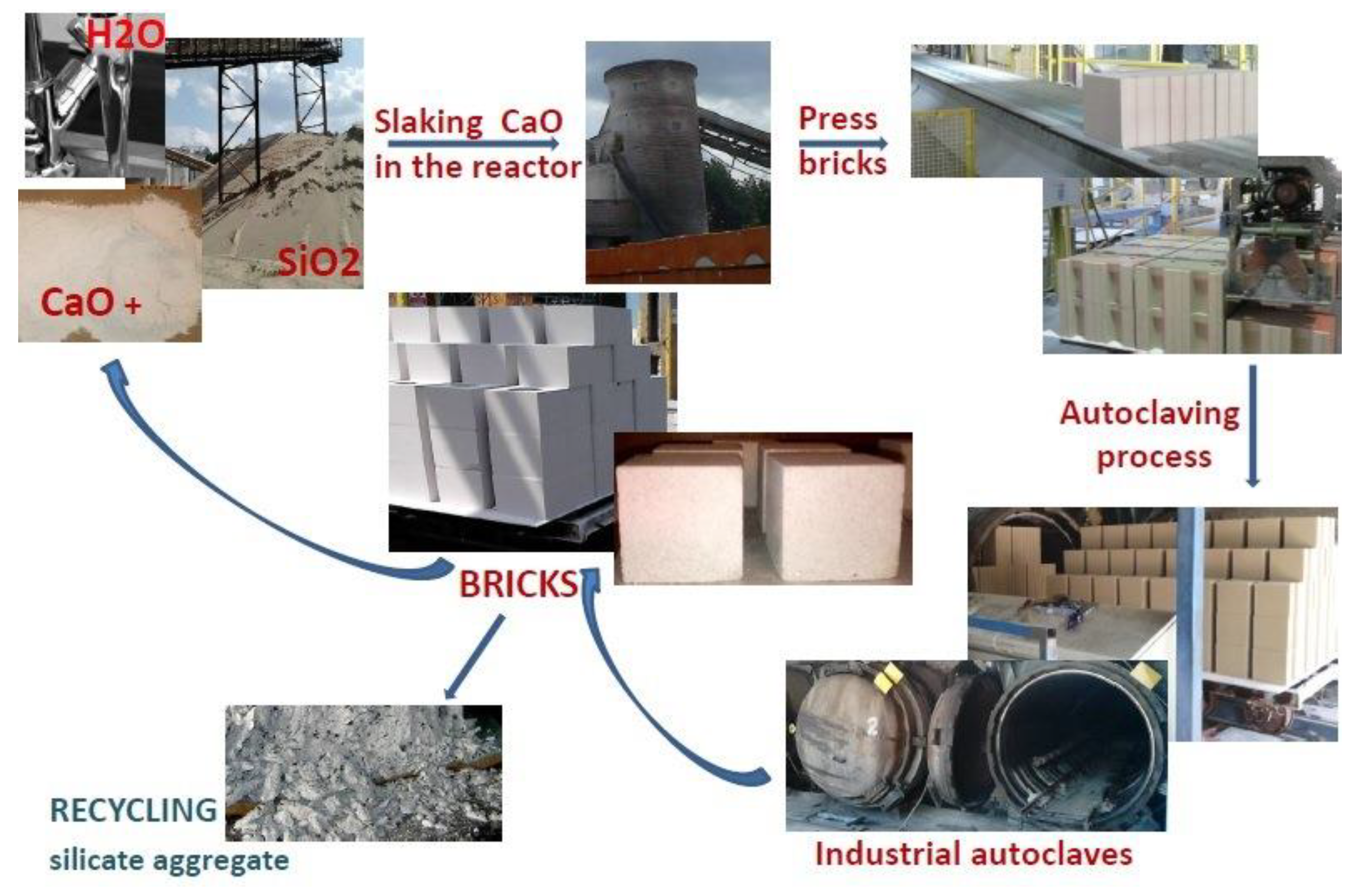
2. Autoclaving Process during the Industrial Production of Traditional Sand-Lime Bricks
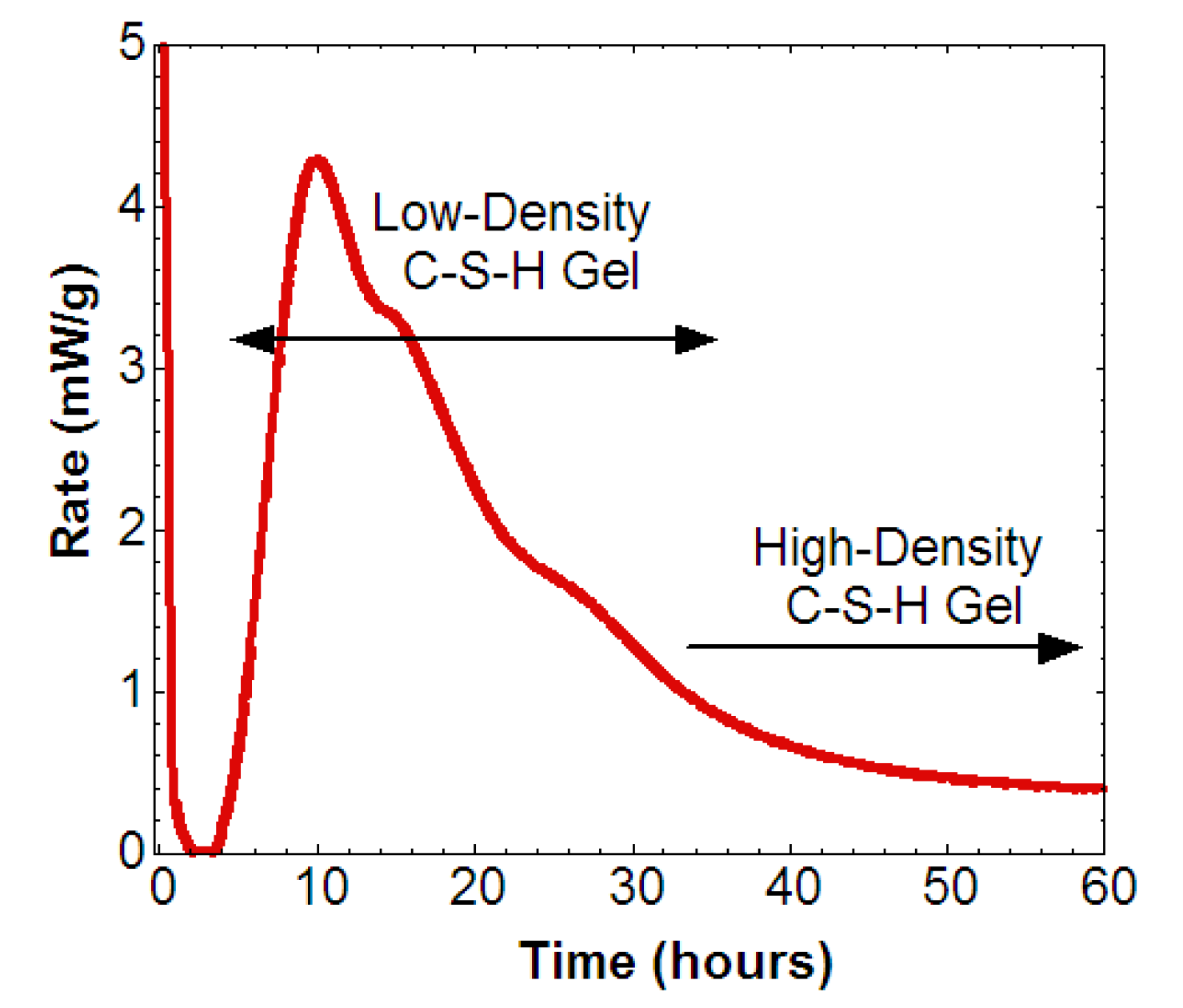
Calcium Silicates Hydrates Formation under Hydrothermal Conditions
3. Methods and Materials
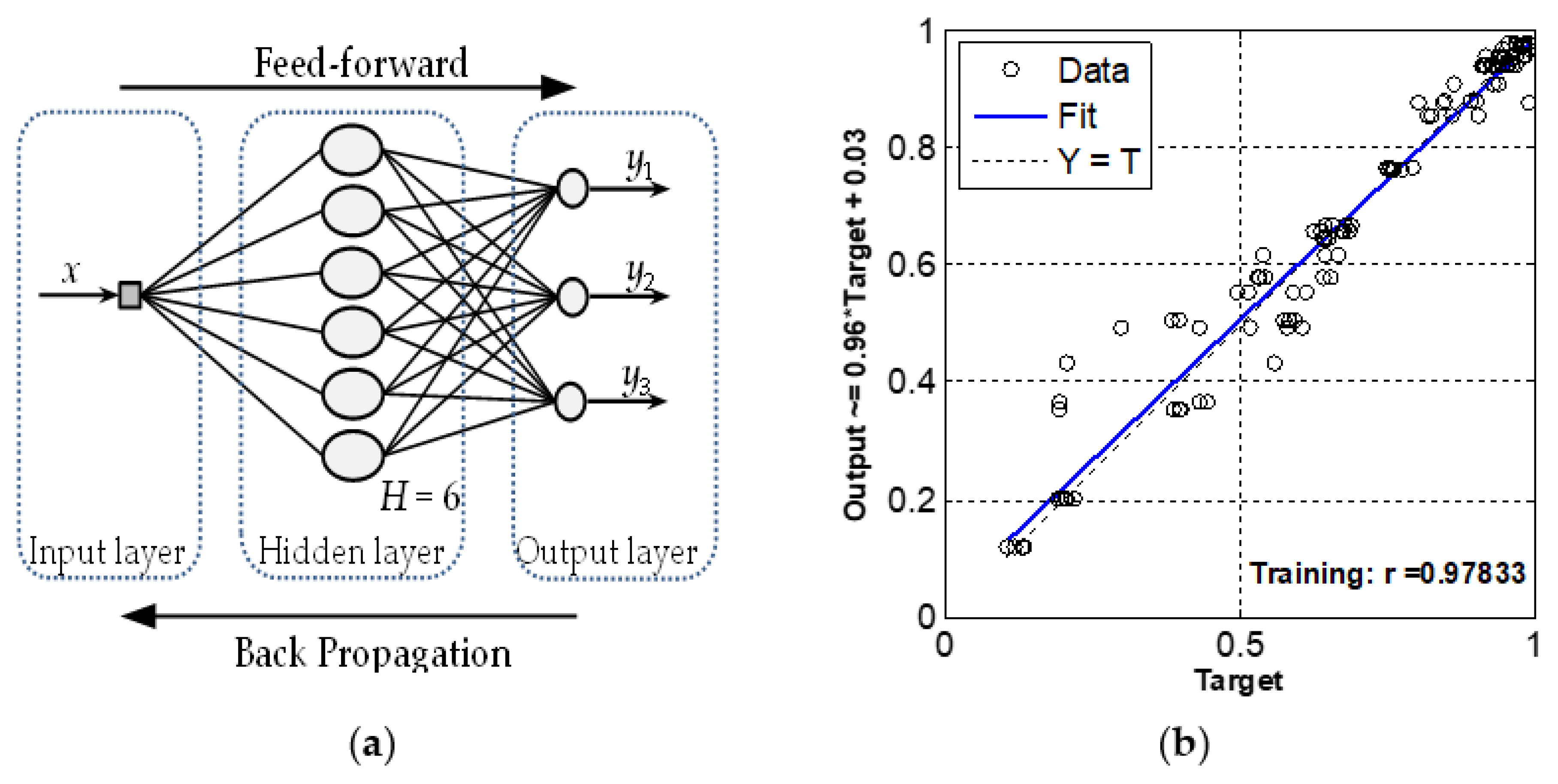
3.1. Preparation of the Artificial Neural Network (ANN)
3.2. X-ray Diffraction (XRD) Analysis
3.3. Thermodynamic Equilibrium-Based Modelling
3.4. Scanning Electron Microscope (SEM)
3.5. Quartz Sand ‘QS’ and Glass Sand ‘GS’
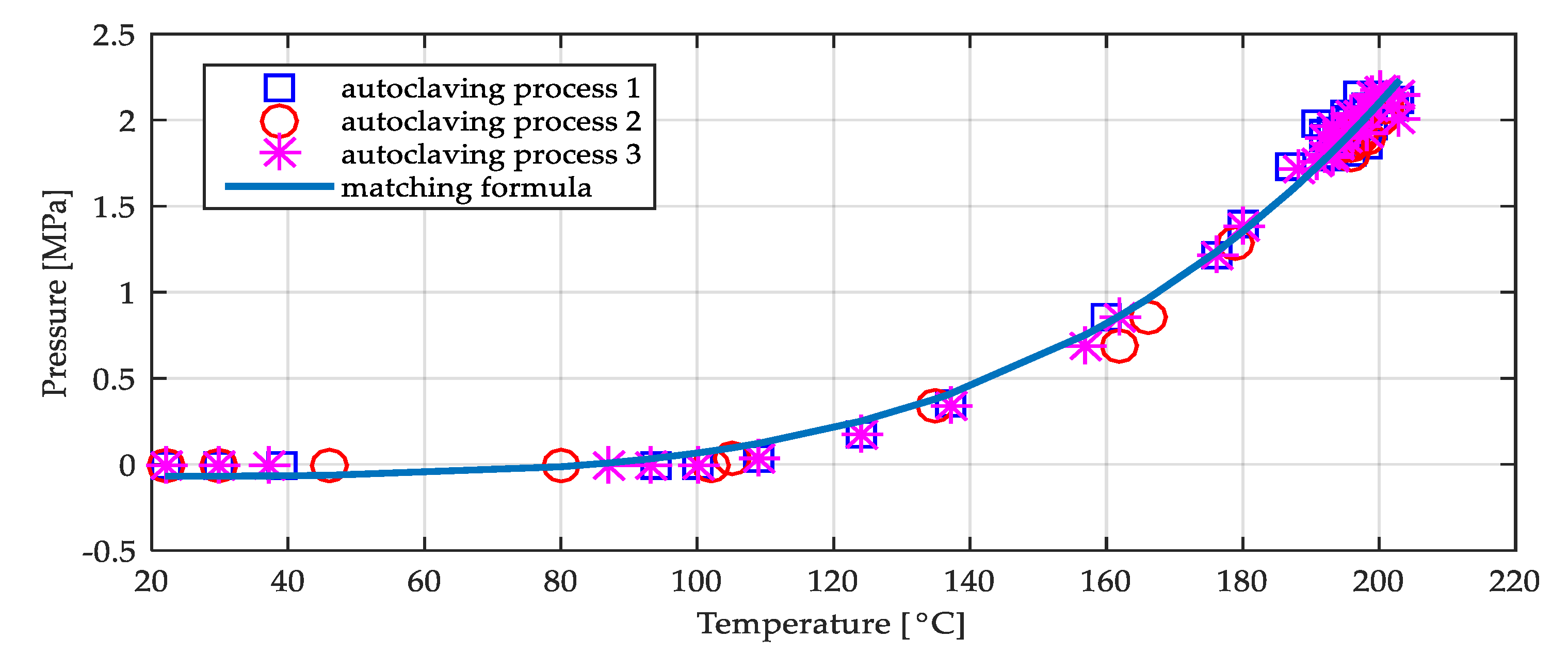
3.6. Hydrothermal Conditions and Laboratory Conducted Autoclaving Process
4. Results
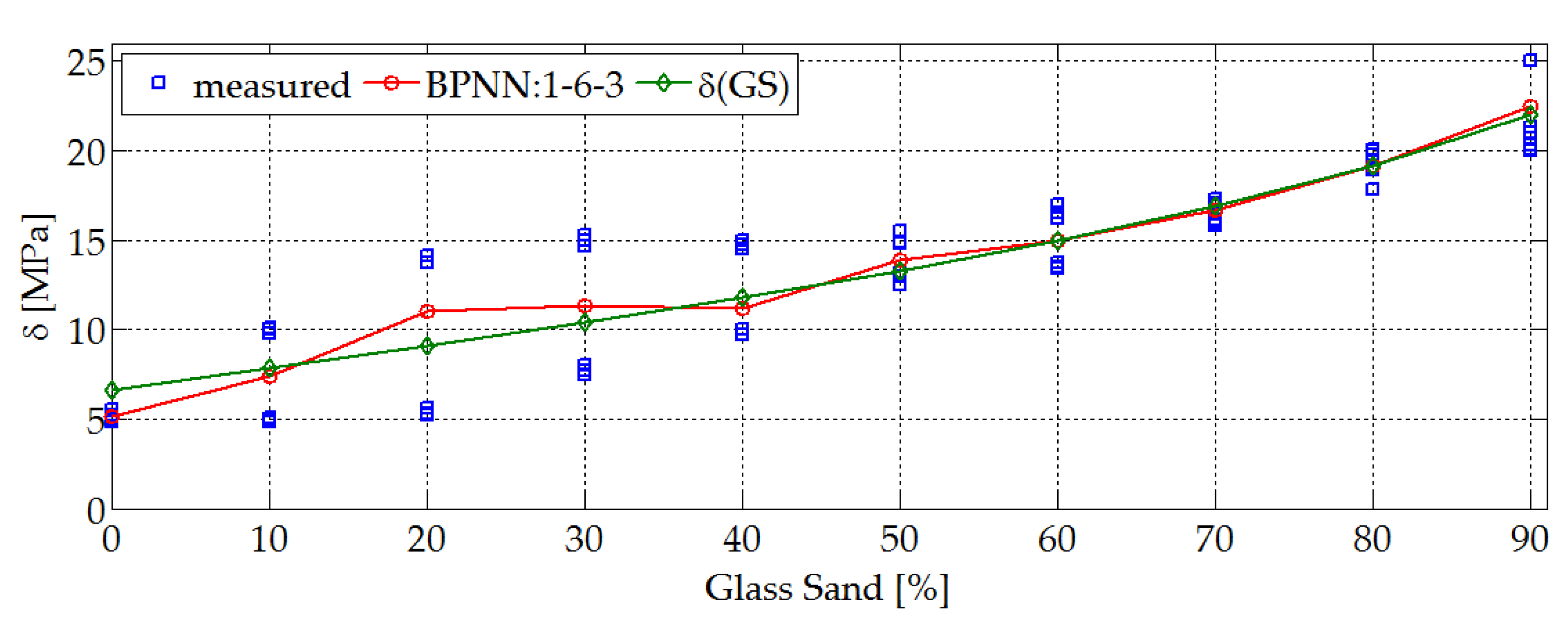
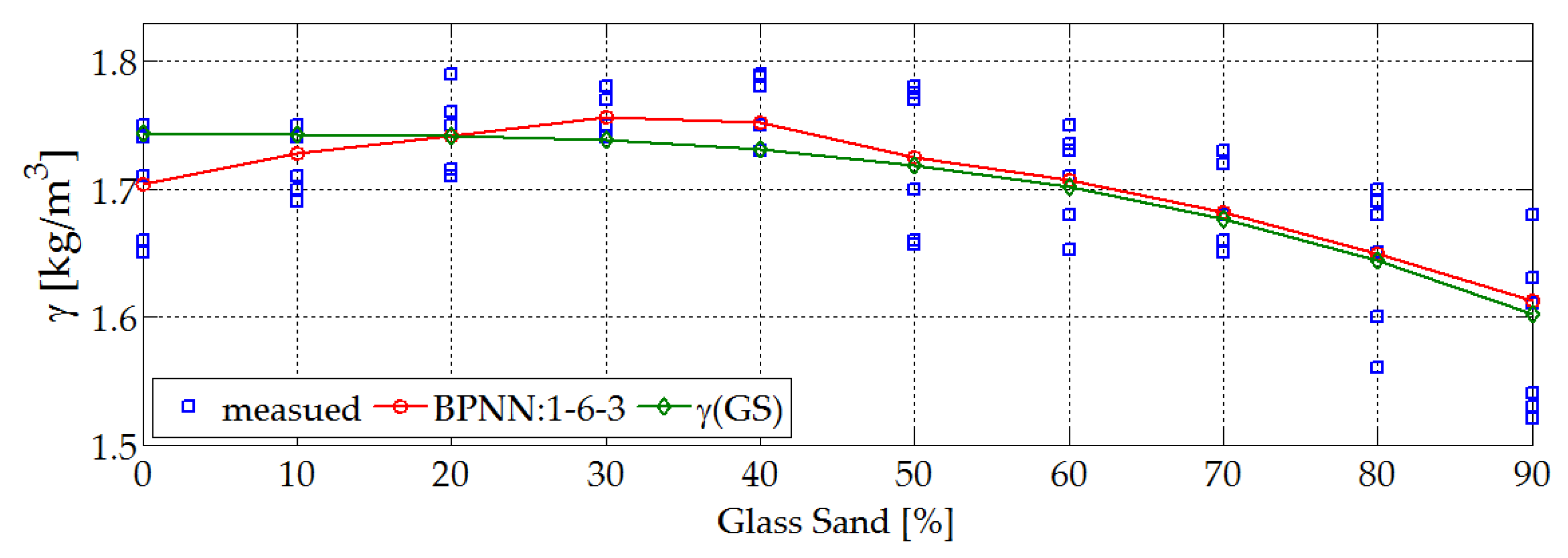
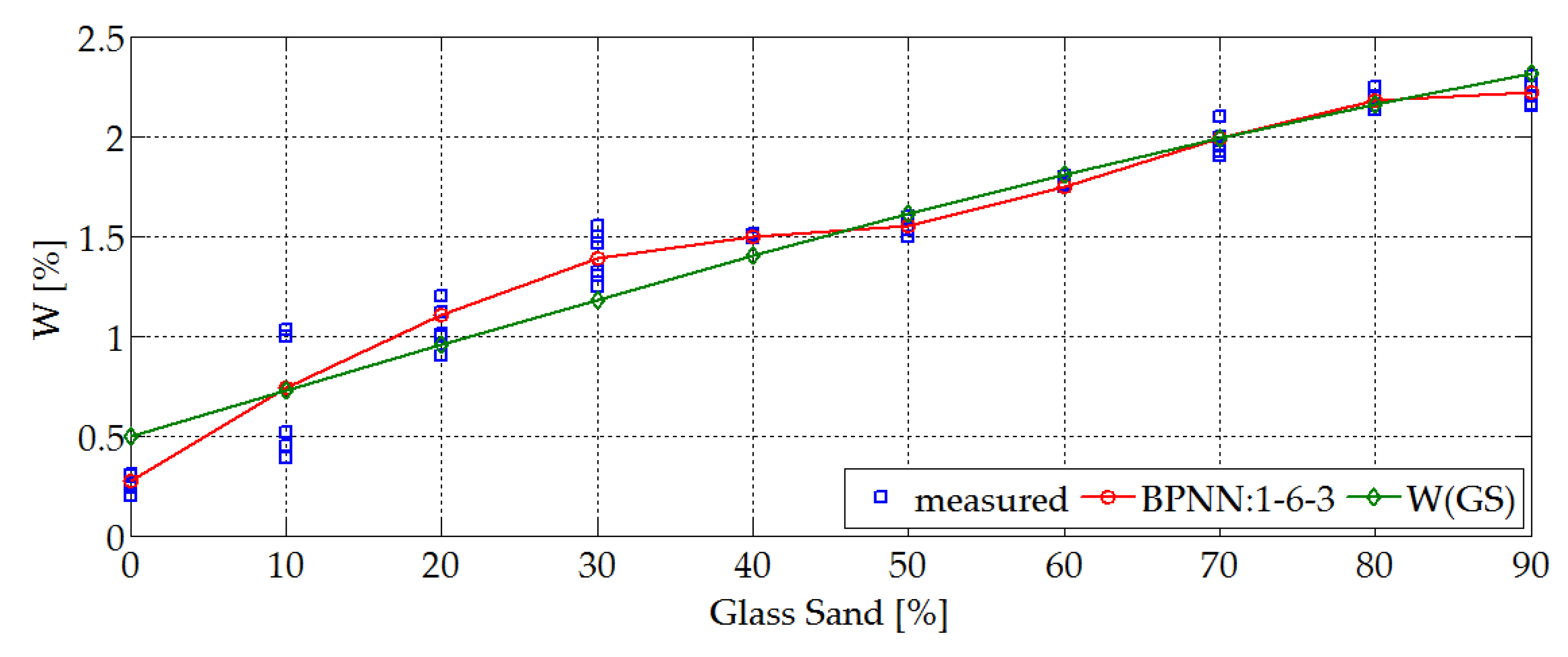
4.1. Physical and Mechanical Properties. Research and Prediction of Characteristics of Sand-Lime Bricks Using Neural Networks
- compressive strength,
- bulk density,
- moisture content,
4.2. Structure and Microstructure
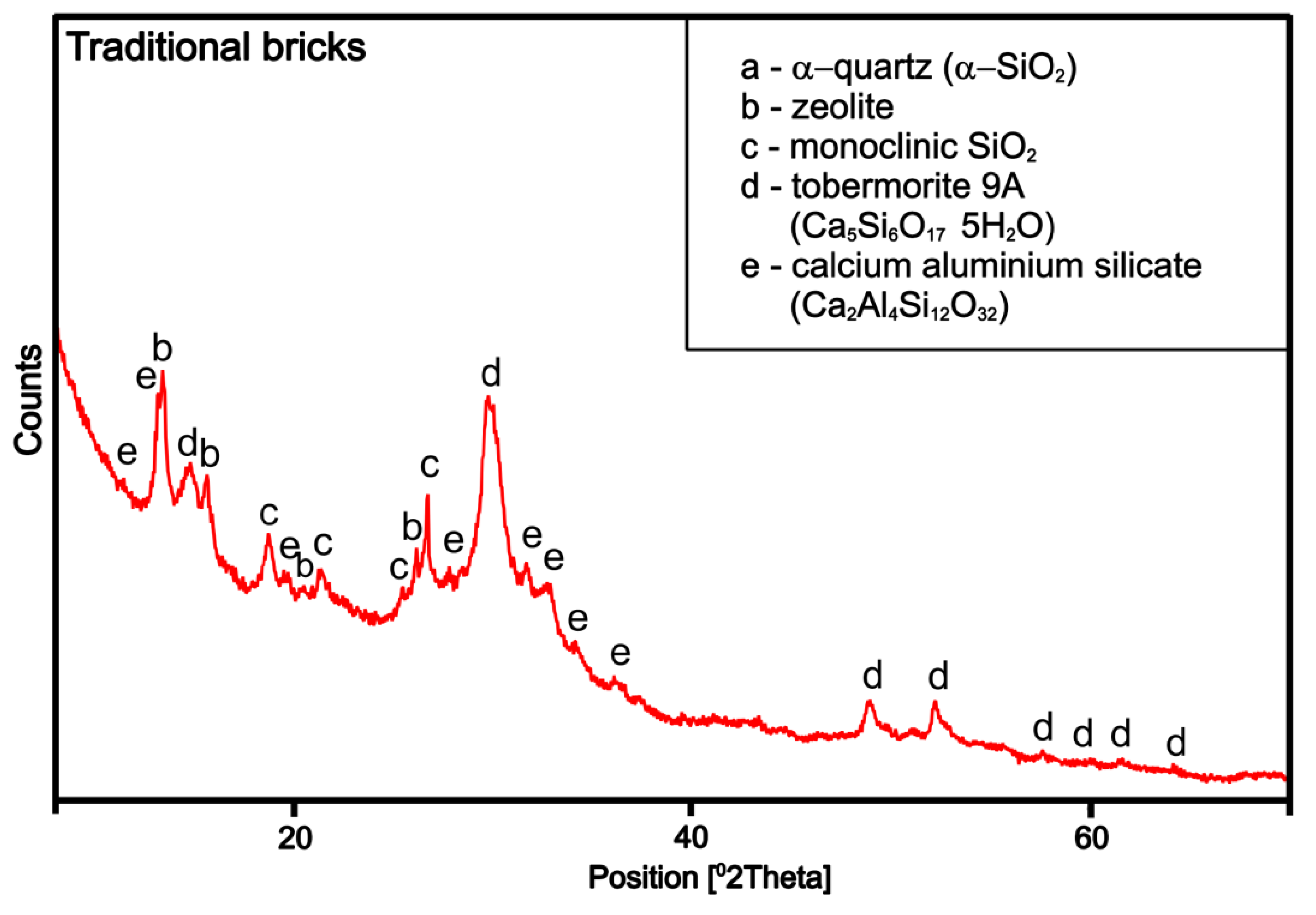
4.2.1. XRD of Traditional Sand-Lime Laboratory Sample
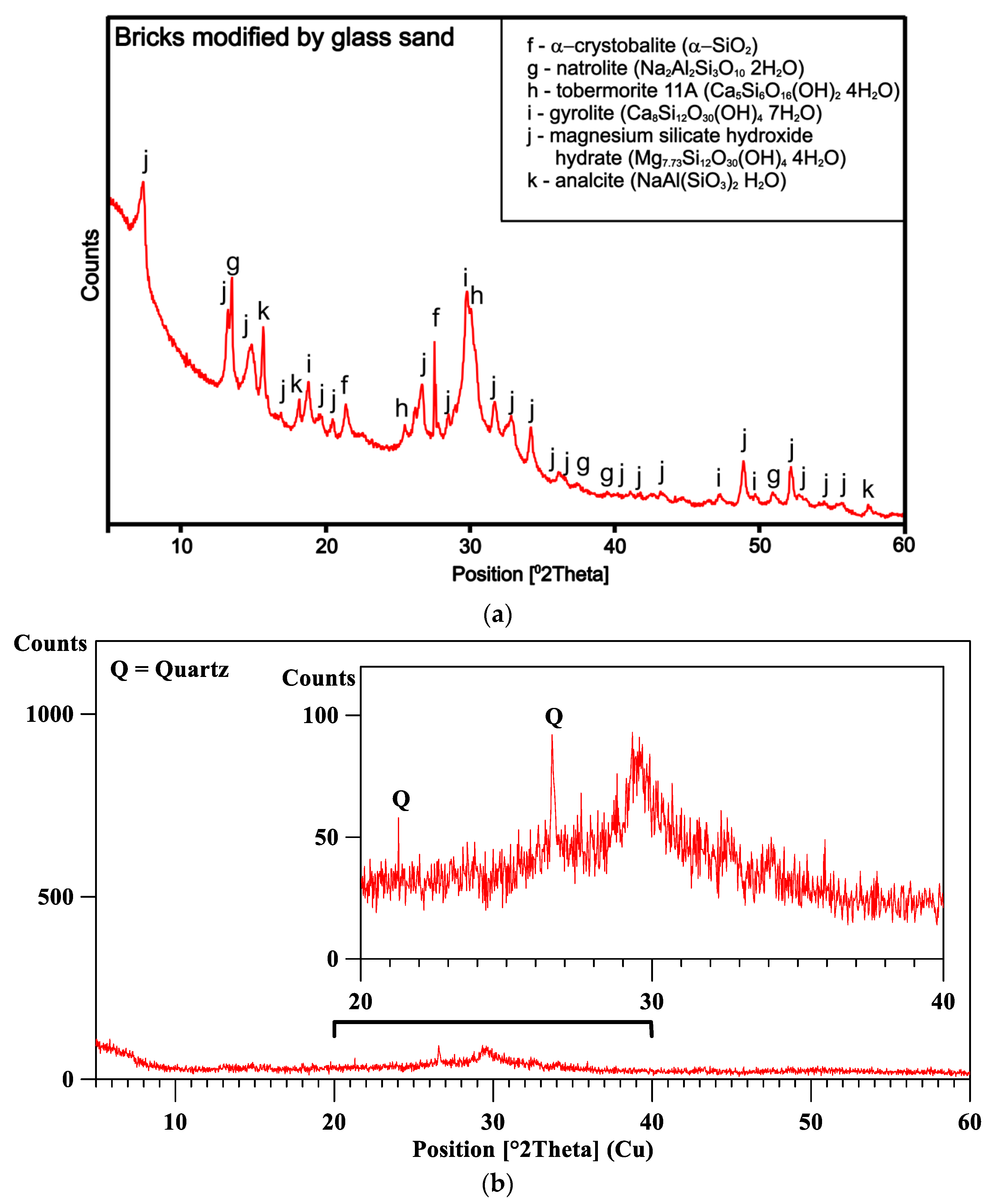
4.2.2. XRD for Laboratory Brick Sample Modified by Glass Sand (90% GS)
4.2.3. Scanning Electron Microscopy (SEM) of Traditional Sand-Lime Bricks
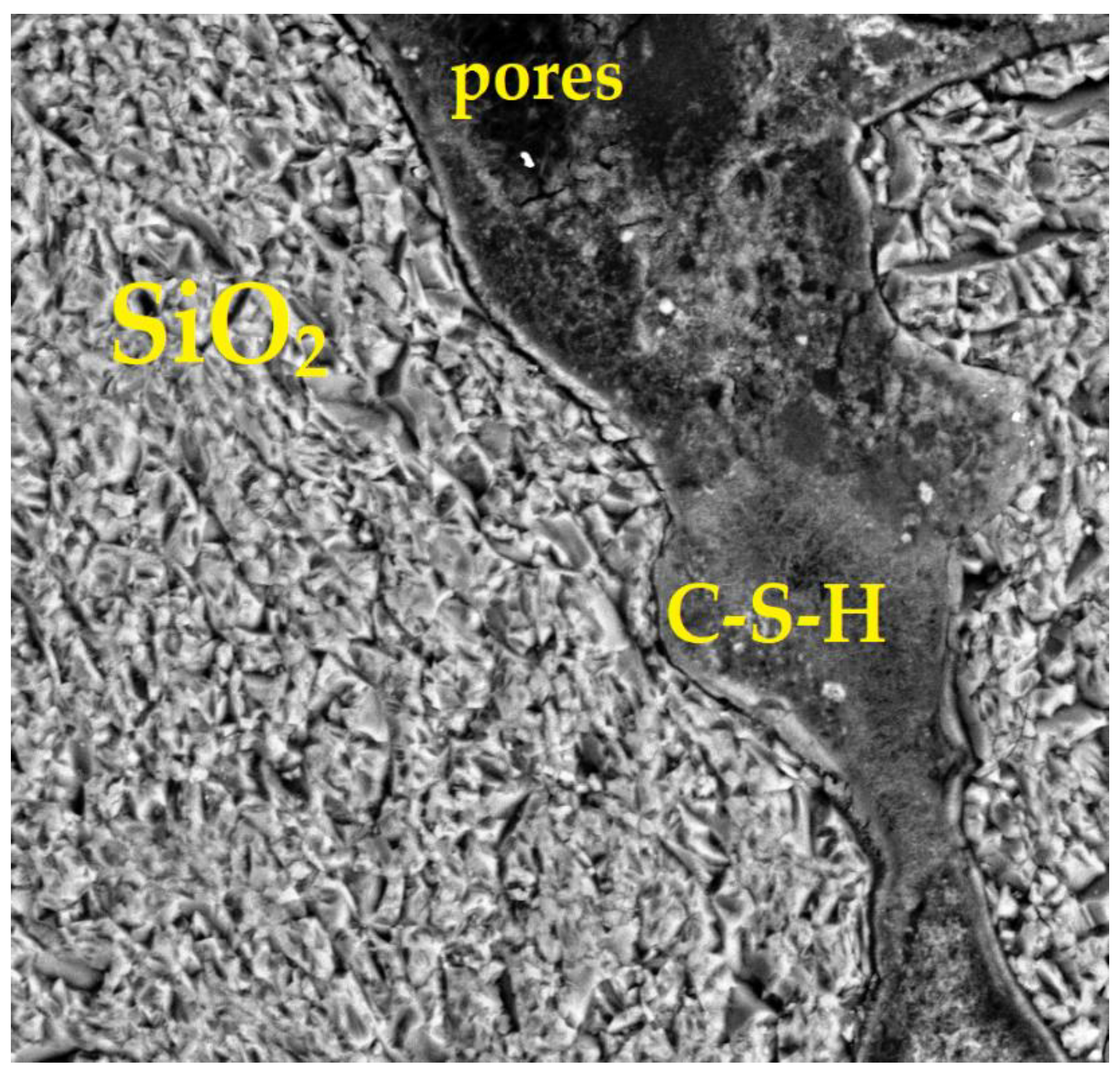
4.2.4. SEM of the Sample with 90% GS
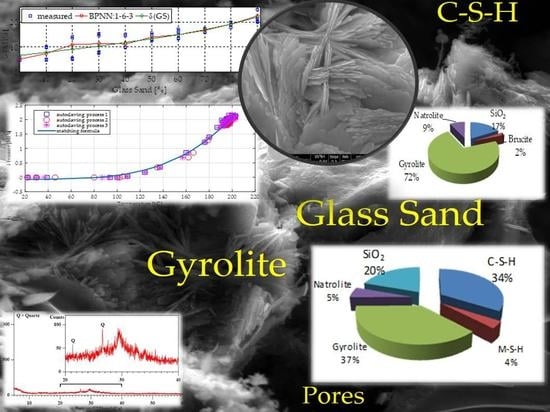
4.3. Thermodynamic Modelling
5. Discussion
6. Conclusions
- (1)
- The replacement of crystalline quartz sand (90 wt% QS) with amorphous glass sand during the brick production process reduced the lime hydration temperature. During the stirring process of sand-lime mass, the temperature of the reaction between lime and water in the presence of quartz sand was determined to be 83 °C. In the presence of glass sand, measured temperature was only 42 °C. The temperature of lime hydration depended on the number of QS and GS in the sand-lime mass and decreased as the share of GS in the test increased.
- (2)
- For 5 h of autoclaving process in a laboratory autoclave, the compressive strength for the material with QS was around 6.5 MPa, and for material containing GS around 20 MPa. The test was carried out after 30 days from the date of production. The compressive strength for bricks manufactured on an industrial scale is 15–20 MPa. This aspect was met in laboratory conditions for bricks modified with GS.
- (3)
- With the increase in the proportion of a ‘new material’ (GS), the bulk density of this type of bricks slightly decreases. For the traditional laboratory made samples bulk density was 1.7 kg/dm3, and for those modified with glass sand was 1.65 kg/dm3. Standards for these types of materials have, therefore, been met.
- (4)
- Humidity is higher for GS modified materials (this property increases with the increase of GS content in the silicate mass and increase occurs from the level of 0.5% to 2.1%).
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclatures
| QS | Quartz sand |
| GS | Glass sand |
| XRF | X-ray Powder Diffraction |
| SEM | Scanning Electron Microscope |
| XRD | X-ray Powder Diffraction [keV] |
| γ | Bulk density [kg/dm3] |
| p | Pressure [MPa] |
| δ | Compressive strength [MPa] |
| V | Molar volume [cm3·mol−1] |
| M | Molar mass [g·mol−1] |
| T | Temperature [°C or K] |
| Log10 K | Solubility product at T = 298 K (25 °C)(this information is the basis for determining the pH of the material) |
| ∆fG° | Standard Gibbs free energy of formation [J·mol−1] |
| ∆fH° | Standard enthalpy of formation, is the change in enthalpy when one mole of a substance is formed from its elements under a standard pressure of 1 atm/1 bar [J·mol−1] |
| Cp° | Specific heat [J·mol−1·K−1] |
| S° | Standard entropy of formation [J·mol−1·K−1] |
| BPNN | Backpropagation Neural Network |
| MSE | Mean Square Error |
| X | Network input |
| Y | Output from the network |
| H | Number of neurons in hidden layer |
| l | Number of learning and validating patterns |
| t | Number of testing patterns |
| r | Linear regression coefficient |
References
- Nijland, T.G.; Adan, O.C.G.; van Hees, R.P.J.; van Etten, B.D. Evaluation of the Effects of Expected Climate Change on the Durability of Building Materials with Suggestions for Adaptation. Heron 2009, 54, 37–48. Available online: https://www.researchgate.net/publication/280016232 (accessed on 12 December 2019).
- The Effects of Climate Change. Available online: https://climate.nasa.gov/effects/ (accessed on 18 November 2019).
- Piotrowski, J.Z.; Stroy, A.; Olenets, M. Mathematical modelling of the steady state heat transfer processes in the convectional elements of passive solar heating systems. Arch. Civ. Mech. Eng. 2013, 13, 394–400. [Google Scholar] [CrossRef]
- Bomberg, M.; Wojcik, R.; Piotrowski, J.Z. A concept of integrated environmental approach, Part 2: Integrated approach to rehabilitation. J. Build. Phys. 2016, 39, 482–502. [Google Scholar] [CrossRef]
- Piotrowski, J.; Stroy, A.; Olenets, M. Mathematical Description of Heat Transfer and Air Movement Processes in Convectional Elements of a Building’s Passive Solar Heating Systems. Energy Procedia 2014, 57, 2070–2079. [Google Scholar] [CrossRef][Green Version]
- H+H Partner w Budowaniu Ścian (H+H Partner in Building Walls). Available online: https://www.hplush.pl/zaklad-ludynia (accessed on 23 August 2019).
- Noszczyk, P.; Nowak, H. Inverse contrast in non-destructive materials research by using active thermography. Materials 2019, 12, 835. [Google Scholar] [CrossRef] [PubMed]
- Kurdowski, W. Poradnik Technologa Przemysłu Cementowego/Cement Industry Technologist’s Guide; Wyd. ARKADY: Warszawa, Poland, 1981. [Google Scholar]
- Orłowski, Z. Podstawy Technologii Betonowego Budownictwa Monolitycznego/Fundamentals of Monolithic Concrete Construction Technology; Wyd. Naukowe PWN SA: Warszawa, Poland, 2010. [Google Scholar]
- Cement Production Globally and in the U.S. from 2010 to 2019. Available online: https://www.statista.com/statistics/219343/cement-production-worldwide (accessed on 6 December 2019).
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Oey, T.; Timmons, J.; Stutzman, P.; Bullard, W.J.; Balonis, M.; Mauchy, M.; Sant, G. An improved basis for characterizing suitability of fly ash as a cement replacement agent. J. Am. Ceram. Soc. 2017, 100. Available online: http://matse1.matse.illinois.edu/concrete/hist.html (accessed on 23 November 2019). [CrossRef]
- Stepien, A.; Potrzeszcz-Sut, B.; Kostrzewa, P. Influence and Application of Glass Cullet in Autoclaved Materials. IOP Conf. Ser. Mater. Sci. Eng. 2019, 471, 032065. [Google Scholar] [CrossRef]
- Kostrzewa, P.; Stępień, A.; Dziadek, K.; Szmidt, A. Technological aspect of brick production using the method of autoclaving. Struct. Environ. 2018, 11, 249–257. [Google Scholar] [CrossRef]
- Stepien, A.; Kostrzewa, P.; Dachowski, R. Influence of barium and lithium compounds onsilica autoclaved materials properties and on the microstructure. J. Clean. Prod. 2019, 236, 117507. [Google Scholar] [CrossRef]
- Stepien, A. Wpływ Modyfikacji Składu Wyrobów Silikatowych na ich Mikrostrukturę i Właściwości Użytkowe/Impact of Modification of Silicate Products Composition on Their Microstructure and Functional Properties. Ph.D. Thesis, Civil Engineering and Architecture Department, Kielce University of Technology, Kielce, Poland, 3 July 2013; pp. 7–163. [Google Scholar]
- SILKA, Historia Silikatów. Available online: http://silikaty.blogspot.com/2012/09/historia-silikatow.html (accessed on 23 August 2019).
- Olenets, M.O.; Piotrowski, J.Z.; Stroy, A.F. Heat transfer and air movement in the ventilated air gap of passive solar heating systems with regulation of the heat supply. Energy Build. 2015, 103, 198–205. [Google Scholar] [CrossRef]
- Witthohn, M.; Wittenborn, D.E.; Klemm, R.; Chemnitz, D.E. Proces Zwiększania Pojemności Cieplnej Cegieł Wapienno-Silikatowych Oraz Cegła z Materiału Wapienno-Silikatowego/The Process of Increasing the Heat Capacity of Lime-Silicate Bricks and Brick of Lime-Silicate Material. Europejski Builetyn Patentowy PL/EP 1752509 T3, 8 August 2007. Available online: https://tech.money.pl/przemysl/patenty/ep-1752509-117739.html (accessed on 13 December 2019).
- Khomchenko, Y.V.; Semeykin, A.Y. Improving the Efficiency and Safety in the Technology of Lime and Silicate Materials. Mater. Sci. Forum 2019, 974, 243–248. [Google Scholar] [CrossRef]
- Nocuń-Wczelik, W. Structure and the most important properties of selected hydrated calcium silicates. Ceramics 1999, 59, 17–32. [Google Scholar]
- Paradisoa, P.; Santosc, R.L.; Horta, R.B.; Lopesa, J.N.C.; Ferreira, P.J.; Colaço, R. Formation of nanocrystalline tobermorite in calcium silicate binders with low C/S ratio. Acta Mater. 2018, 152, 7–15. [Google Scholar] [CrossRef]
- Labus, M.; Such, P. Microstructural characteristics of wellbore cement and formation rocks under sequestration conditions. J. Pet. Sci. Eng. 2016, 138, 77–87. [Google Scholar] [CrossRef]
- Lothenbach, B.; Kulik, D.A.; Matschei, T.; Balonis, M.; Baquerizo, L.; Dilnes, B.; Miron, J.D.; Myers, R.J. Cemdata18: A chemical thermodynamic database for hydrated Portland cements and alkali-activated materials. Cem. Concr. Res. 2019, 115, 472–506. [Google Scholar] [CrossRef]
- Matschei, T.; Lothenbach, B.; Glasser, F.P. The role of calcium carbonate in cement hydration. Cem. Concr. Res. 2007, 37, 551–558. [Google Scholar] [CrossRef]
- Richardson, I. Tobermorite/jennite- and tobermorite/calcium hydroxide-based models for the structure of C-S-H: Applicability to hardened pastes of tricalcium silicate, beta-dicalcium silicate, Portland cement, and blends of Portland cement with blast-fumace slag, metakaolin, or silica fume. Cem. Concr. Res. 2004, 34, 1733–1777. [Google Scholar]
- Kittipong, K.; Suwimol, A.; Kwannate, S. Effect of Fine Al-Containing Waste in Autoclaved-Aerated; Concrete Incorporating Rice-Husk Ash 10.1061/(ASCE), MT.1943-5533.0001149. ©; American Society of Civil Engineers: Reston, VA, USA, 2014. [Google Scholar]
- Pytel, Z. The Influence of Ground Limestone on the Properties of Sand-Lime Bricks; Ceramic Science, Polish Ceramic Bulletin Polish Academy of Science-Krakow Division, Polish Ceramic Society: Krakow, Poland, 2005. [Google Scholar]
- Taha, B.; Nounu, G. Utilizing Waste Recycled Glass as Sand/Cement Replacement in Concrete; Cement and Concrete Composities; Elsevier: Amsterdam, The Netherlands, 2008; pp. 497–505. Available online: https://www.researchgate.net/publication/248399903 (accessed on 17 November 2019).
- Schmidt, A.; Saia, W.H.F. Alkali-Aggregate Reaction Tests on Glass Used for Exposed Aggregate Wall Panel Work. J. Am. Concr. Inst. Aci Mater. J. 1963, 60, 1235–1236. [Google Scholar]
- Pattengill, M.; Shutt, T.C. Use of Ground glass as a pozzolan. In Symposium on Utilisation of Waste Glass in Secondary Products; The University of New Mexico: Albuquerque, NM, USA, 1973. [Google Scholar]
- Diamond, S. ASR Another Look at Mechanisms. In Proceedings of the 8th International Conference on Alkali-Aggregate Reaction in Concrete, Kyoto, Japan, 17–20 July 1989; Elsevier Science: New York, NY, USA, 1989; pp. 83–94. [Google Scholar]
- Chatterji, S. The role of Ca(OH)2 in the breakdown of Portland cement concrete due to alkali-silica reaction. Cem. Concr. Res. 1979, 9, 185–188. [Google Scholar] [CrossRef]
- Helmuth, R. Alkali-Silica Reactivity: An Overview of Research; Strategic Highway Research Program SHRP-C-432; National Research Council: Washington, DC, USA, 1993; pp. 7–103. [Google Scholar]
- Niang, A.; Roy, N.; Tagnit-Hamou, A. Structural Behavior of Concrete Incorporating Glass Powder Used in Reinforced Concrete Columns; American Society of Civil Engineers: New York, NY, USA, 2015; Volume 141, Issue 3–March. [Google Scholar]
- Shilpa, R.; Kumar, P.R. Effect of Using Glass Powder in Concrete. In Proceedings of the International Conference on Innovations & Advances in Science, Engineering And Technology, IC-IASET 2014, Kerala, India, 16–18 July 2014. [Google Scholar]
- H + H Silikaty Produkty Podstawowe. Available online: https://www.hplush.pl/h-h-silikaty-produkty-podstawowe (accessed on 11 November 2019).
- Murator-Budowa. Available online: https://miesiecznik.murator.pl/budowa/cegly-licowe_1521 (accessed on 11 November 2019).
- Rayment, D.L.; Majumdar, A.J. The composition of the C-S-H phases in portland cement pastes. Cem. Concr. Res. 1982, 12, 753–764. [Google Scholar] [CrossRef]
- Nonat, A. Thermodynamic Modelling of C-S-H Composition in Neat and Blended Cement Pastes. In Proceedings of the 19th Ibausil ConferenceAt, Weimar, Germany, 16–18 September 2015. [Google Scholar]
- Du, T.; Li, H.; Zhou, Q.; Wang, Z.; Sant, G.; Ryan, J.V.; Bauchy, M. Chemical composition of calcium-silicate-hydrate gels: Competition between kinetics and thermodynamics. Phys. Rev. Mater. 2019, 3, 065603. [Google Scholar] [CrossRef]
- Flint, E.P.; Wells, L.S. Study of the system CaO-SiO2-H2O at 30 °C and of the reaction of water on the anhydrous calcium silicates. Bur. Stand. J. Res. 1934, 12, 751–783. [Google Scholar] [CrossRef]
- Constantinides, G.; Franz-Josef, U. The nanogranular nature of C–S–H. J. Mech. Phys. Solids Pergamon 2007, 55, 64–90. [Google Scholar] [CrossRef]
- Dissertation: Jeff Thomas, Hamlin Jennings (Northwestern University, Evanston, IL). Available online: http://iti.northwestern.edu/cement/monograph/Monograph5_4_2.html/ (accessed on 21 August 2019).
- Arabi, N.; Jauberthie, R. Calcium Silicate Materials: Substitution of Hydrated Lime by Ground Granulated Blast Furnace Slag in Autoclaving Conditions. J. Mater. Civ. Eng. Mater. Civ. Eng. 2012, 24, 1230–1236. [Google Scholar] [CrossRef]
- Hong, S.Y.; Glasser, F.P. Phase relations in the CaO–SiO2–H2O system to 200 °C at saturated steam pressure. Cem. Concr. Res. 2004, 34, 1529–1534. [Google Scholar] [CrossRef]
- Baltušnikas, A.; Lukošiūtė, I.; Baltakys, K. XRD Characterization of Organically Modified Gyrolite. Mater. Sci. (Medžiagotyra) 2009, 15, 325. [Google Scholar]
- Galvánková, L.; Másilko, J.; Solný, T.; Štepánková, E. Tobermorite synthesis under hydrothermal conditions. Procedia Eng. 2016, 151, 100–107. [Google Scholar] [CrossRef]
- Siauciunas, R.; Baltakys, K. Formation of gyrolite during hydrothermal synthesis in the mixtures of CaO and amorphous SiO2 or quartz. Cem. Concr. Res. 2004, 34, 2029–2036. [Google Scholar] [CrossRef]
- Maschio, S.; Tonello, G.; Furlani, E. Recycling Glass Cullet from Waste CRTs for the Production of High Strength Mortars. Hindawi Publ. Corp. J. Waste Manag. 2013, 2013. [Google Scholar] [CrossRef]
- CEN. PN-EN 772-13: 2001 Methods of Test for Masonry Units-Part 13: Determination of the Density of the Net and Gross Density of Masonry in the Dry State (Except for Natural Stone); CEN: Brussels, Belgium, 2001. [Google Scholar]
- CEN. PN-EN 1996-2: 2010 Eurocode 6-Design of Masonry Structures-Part 2: Design, Selection of Materials and Execution of Masonry; CEN: Brussels, Belgium, 2010. [Google Scholar]
- CEN. PN-EN 771-2: Specification for Masonry Units. Part 2: Calcium Silicate Masonry Units; CEN: Brussels, Belgium, 2010. [Google Scholar]
- Potrzeszcz-Sut, B.; Pabisek, E. ANN constitutive material model in the shakedown analysis of an aluminum structure. Comput. Assist. Methods Eng. Sci. 2014, 21, 49–58. [Google Scholar]
- Young, B.A.; Hall, A.; Pilon, L.; Gupta, P.; Sant, G. Can the compressive strength of concrete be estimated from knowledge of the mixture proportions? New insights from statistical analysis and machine learning methods. Cem. Concr. Res. 2019, 115, 379–388. [Google Scholar] [CrossRef]
- Fausett, L. Fundamentals of Neural Networks: Architectures, Algorithms and Applications; Prentice Hall: Englewood Cliffs, NJ, USA, 1994. [Google Scholar]
- Gallant, S. Neural Network Learning and Expert Systems; MIT Press: Cambridge, MA, USA, 1993. [Google Scholar]
- Haykin, S. Neural Networks-A Comprehensive Foundation; Prentice Hall: New York, NY, USA, 1999. [Google Scholar]
- Hornik, K. Approximation capabilities of multilayer feedforward networks. Neural Netw. 1991, 4, 251–257. [Google Scholar] [CrossRef]
- Hudson, B.M.; Hagan, M.; Demuth, H. Neural Network Toolbox for Use with MATLAB; User’s Guide; The MathWorks. Inc.: Natick, MA, USA, 2011. [Google Scholar]
- GEM Software (GEMS). Available online: http://gems.web.psi.ch (accessed on 29 August 2019).
- Thermoddem. Thermochemical and Mineralogical Tables for Geochemical Modeling. Available online: http://thermoddem.brgm.fr (accessed on 19 August 2019).
- Taylor, H.F.W. Cement Chemistry; Thomas Telford Publishing: London, UK, 1997. [Google Scholar]
- Stepien, A.; Leśniak, M.; Sitarz, M. A Sustainable Autoclaved Material Made of Glass Sand. Buildings 2019, 9, 232. [Google Scholar] [CrossRef]
- Brew, D.R.M.; Glasser, F.P. Synthesis and characterisation of magnesium silicate hydrate gels. Cem. Concr. Res. 2005, 35, 85–98. [Google Scholar] [CrossRef]
- Wawrzeńczyk, J.; Kozak, W. Protected Paste Volume (PPV) as a parameter linking the air-pore structure in concrete with the frost resistance results. Constr. Build. Mater. 2016, 112, 360–365. [Google Scholar] [CrossRef]
- Pereira de Oliveira, L.A.; Castro-Gomes, J.P.; Santos, P. Mechanical and Durability Properties of Concrete with Ground Waste Glass Sand. Conf. 11dbmc Int. Conf. Durab. Build. Mater. Compon. 2008, 1, 1–8. [Google Scholar]


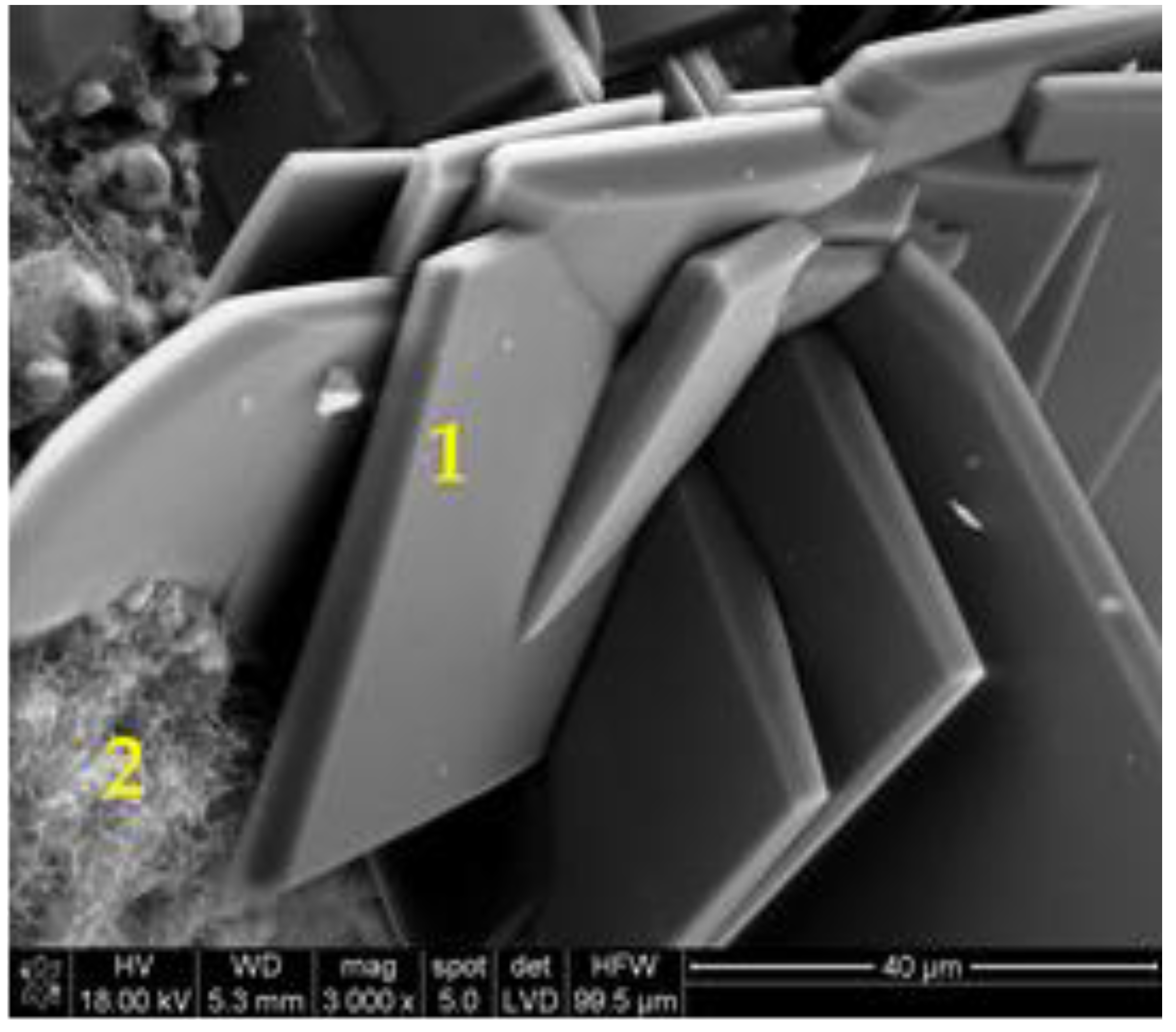
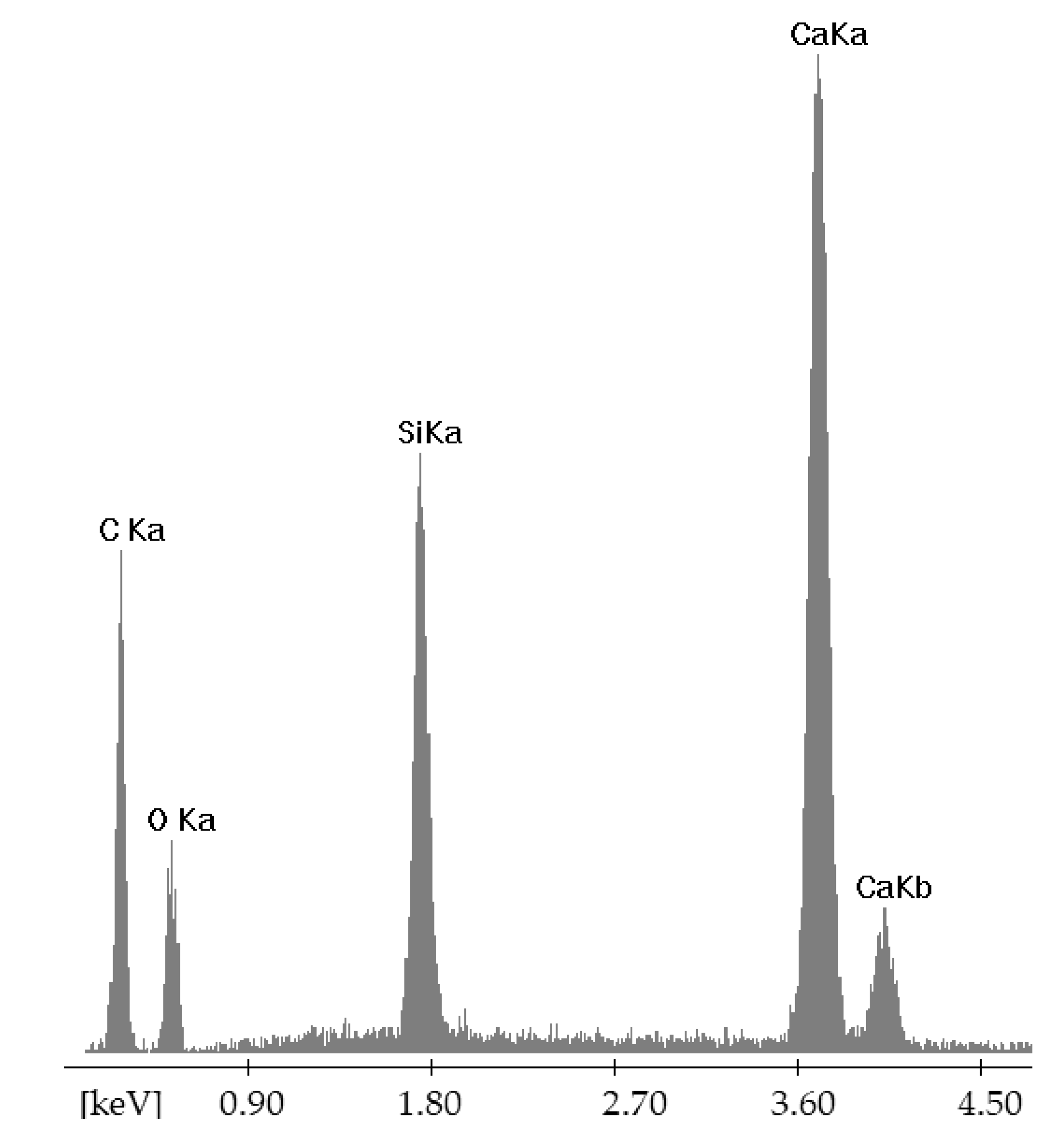
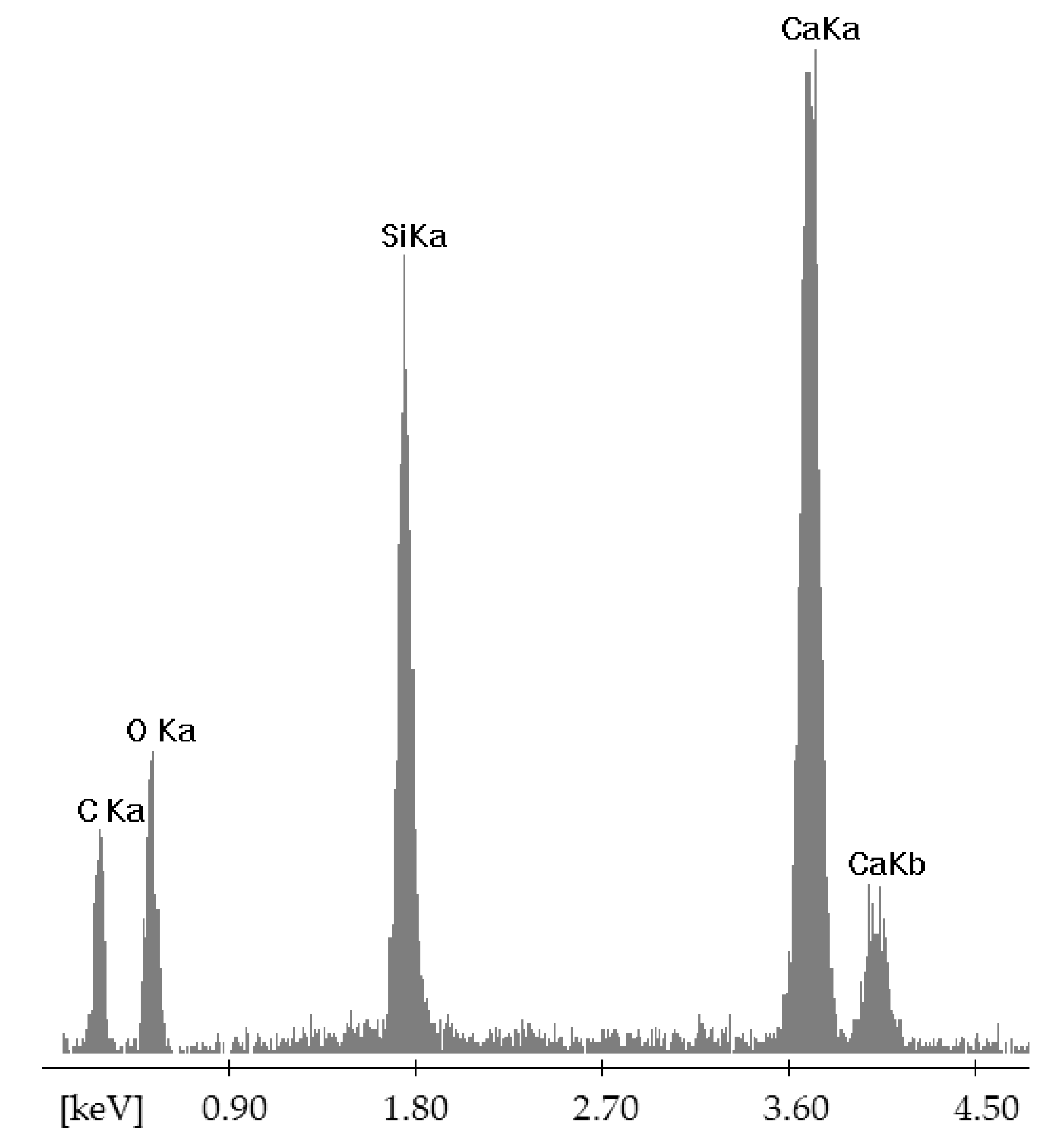
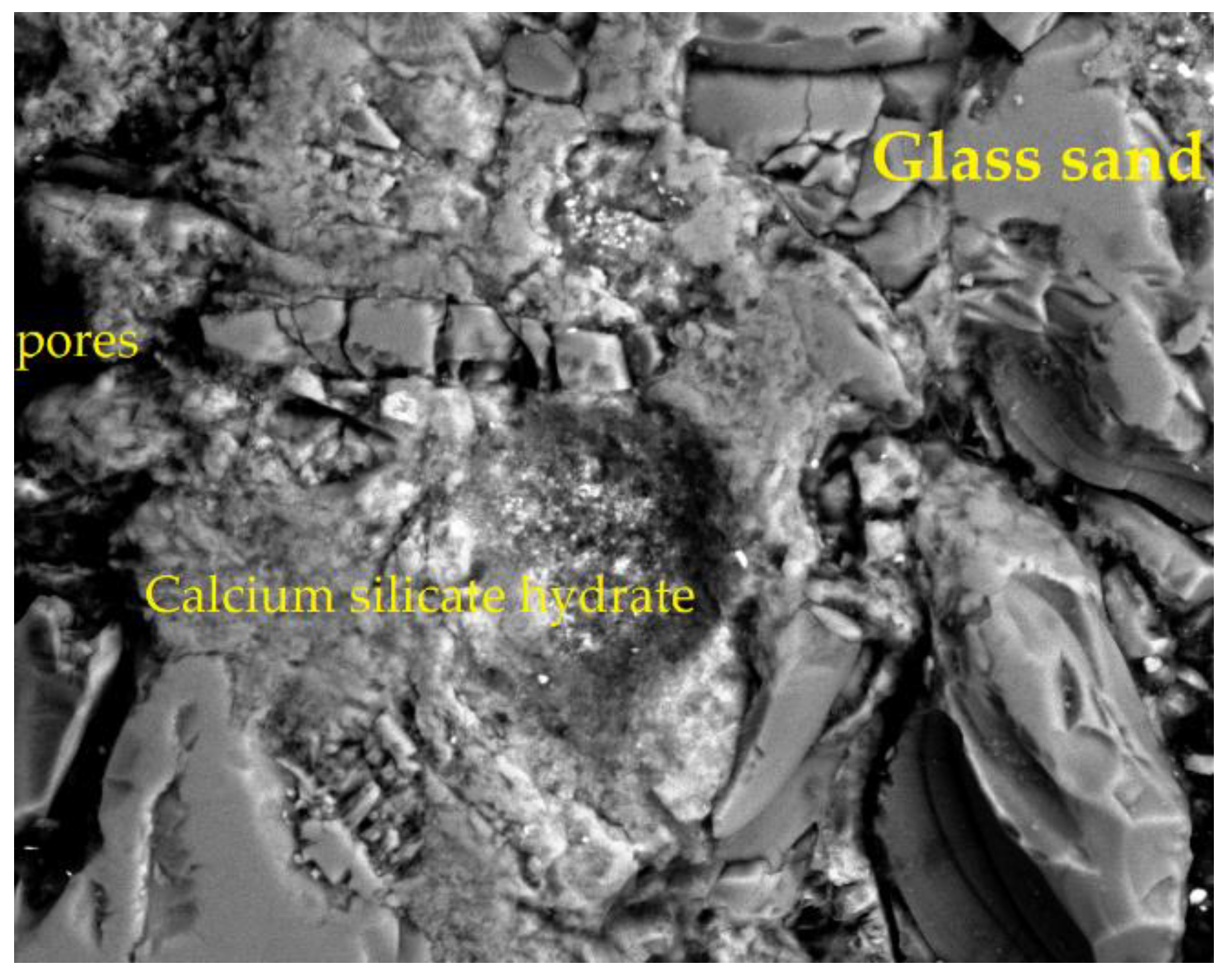


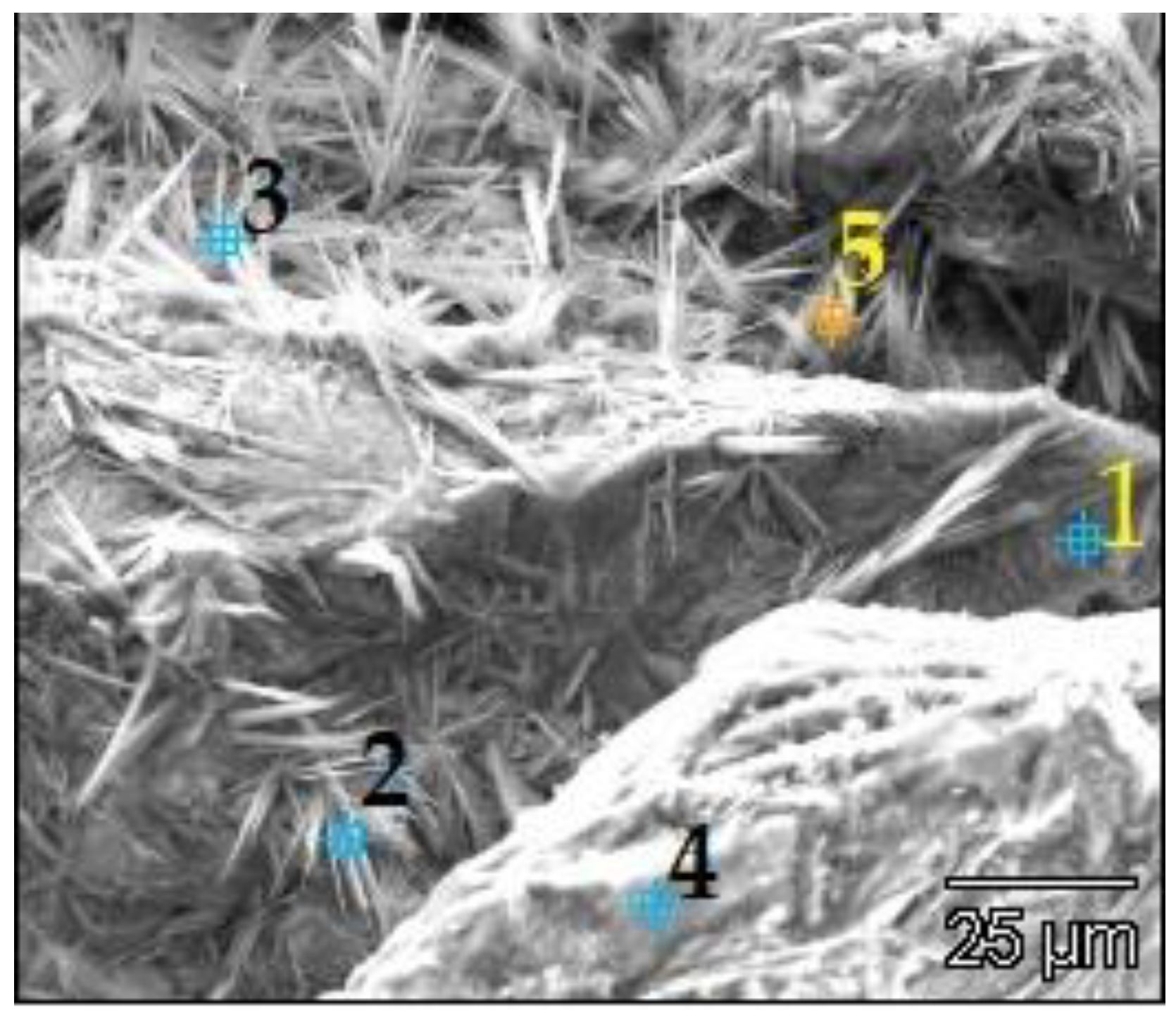
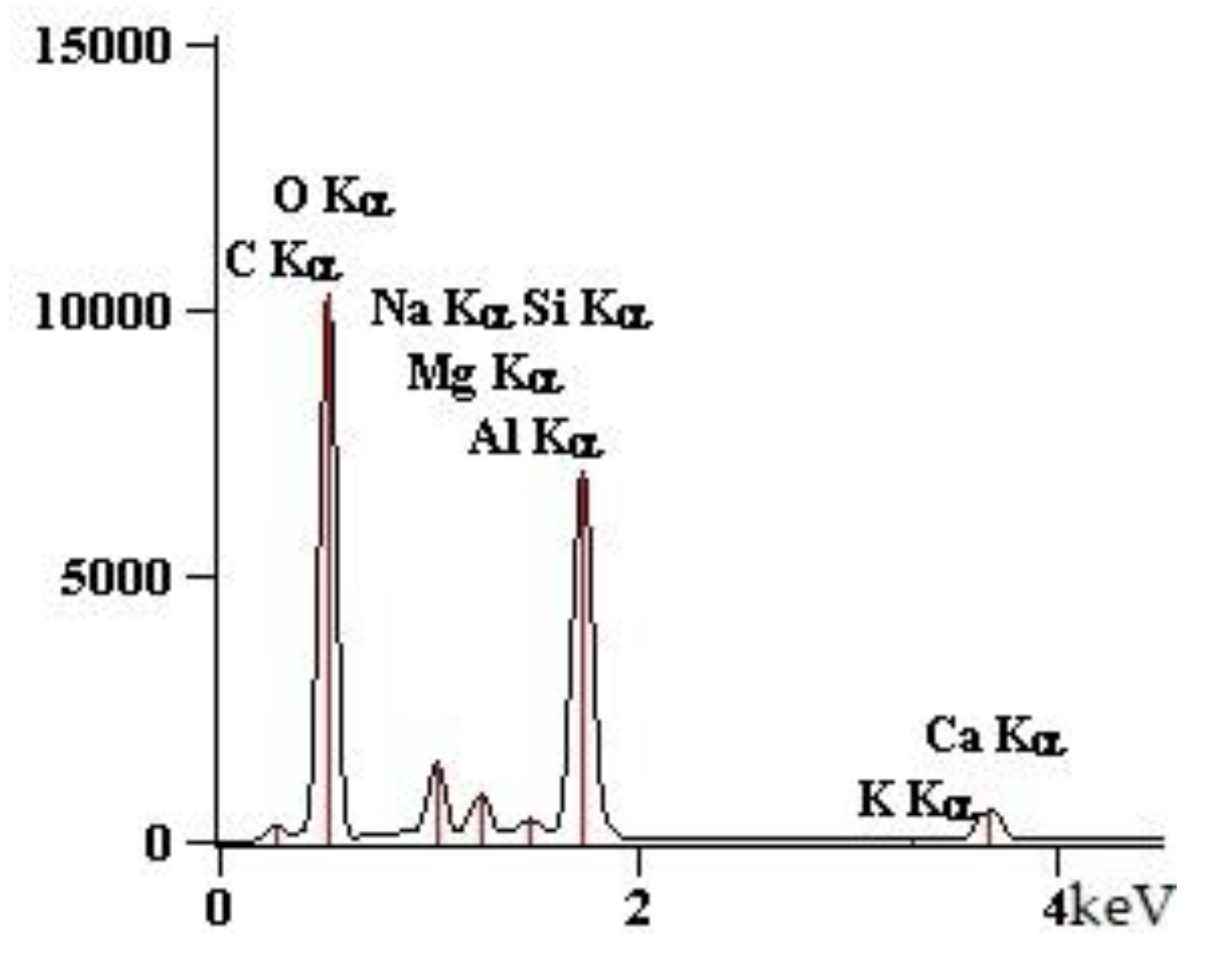
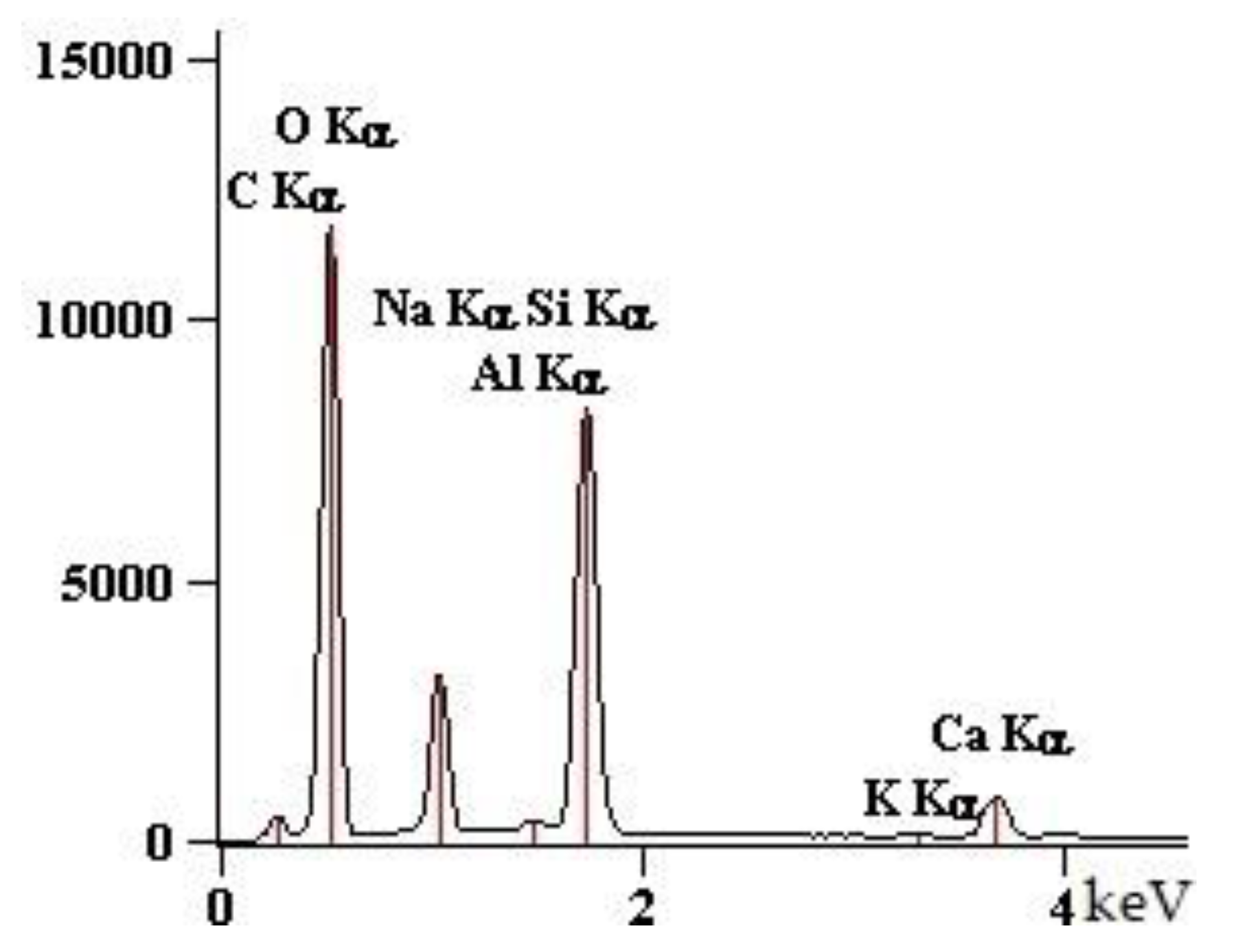
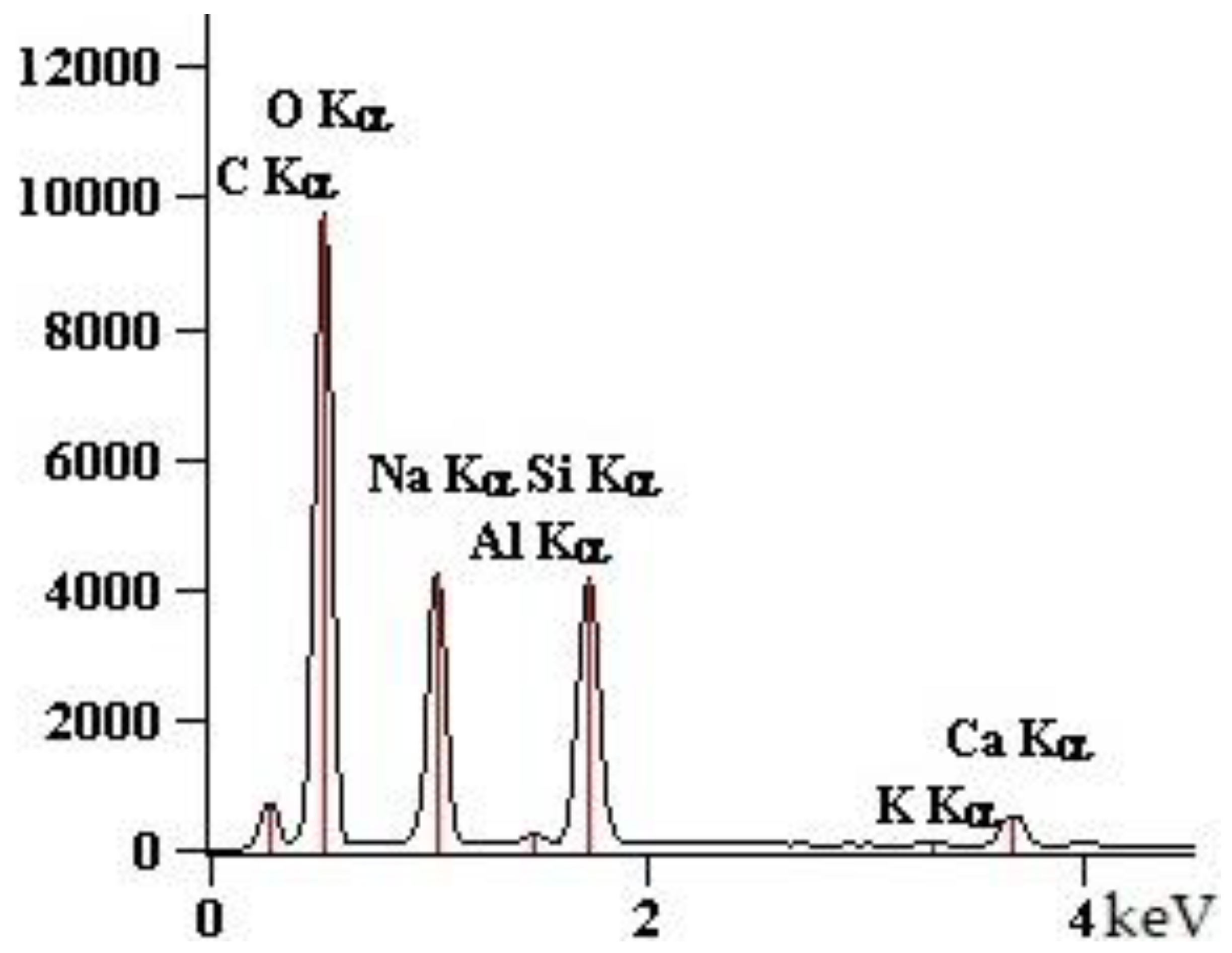
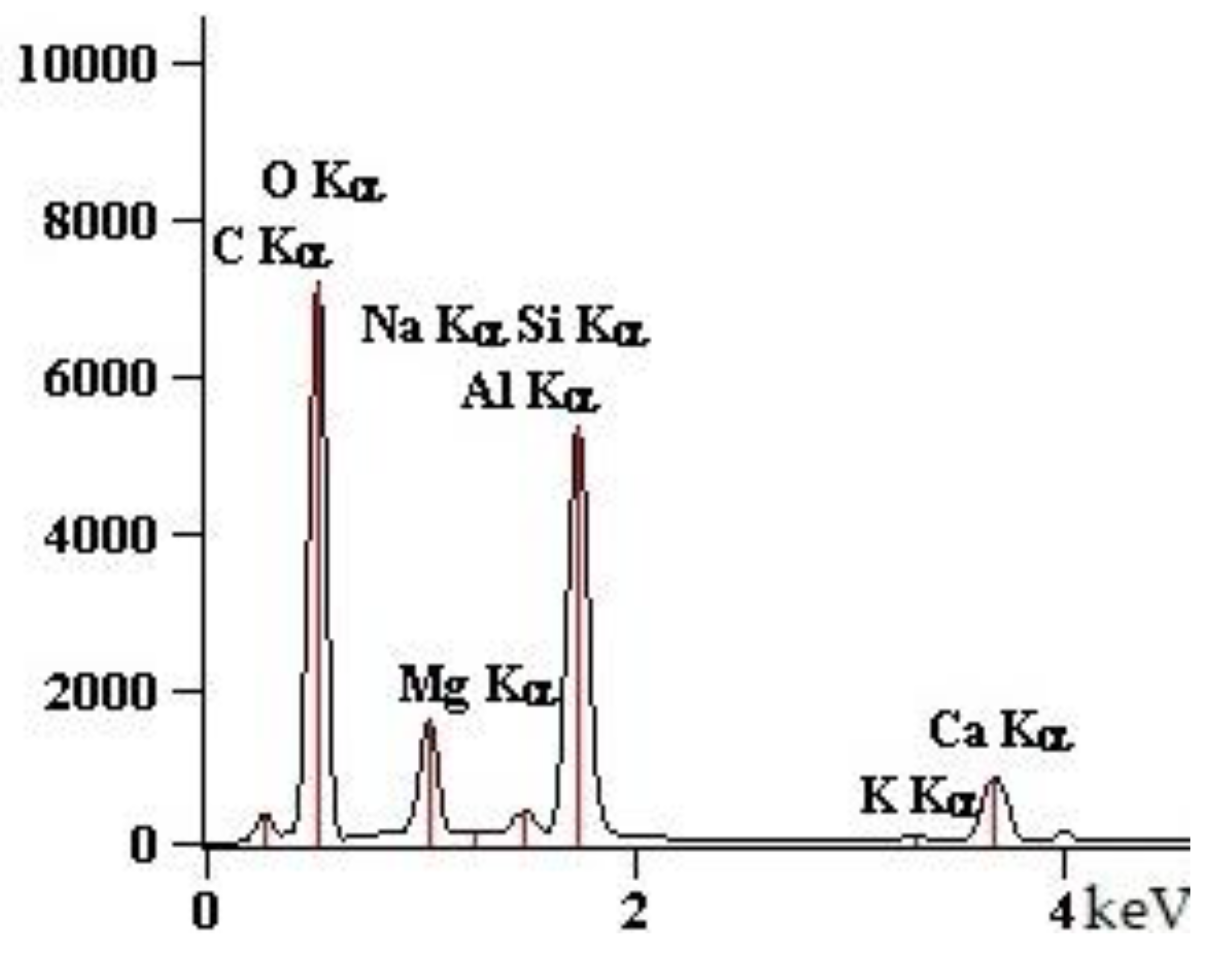
| Point | C-K | O-K | Na-K | Mg-K | Al-K | Si-K | K-K | Ca-K |
|---|---|---|---|---|---|---|---|---|
| 90GS(2)_pt1 | 1547 | 76,166 | 10,448 | 5786 | 1895 | 67,173 | 712 | 7650 |
| 90GS(2)_pt2 | 2703 | 87,880 | 24,477 | 1411 | 79,796 | 993 | 10,422 | |
| 90GS(2)_pt3 | 4759 | 73,284 | 34,184 | 849 | 40,133 | 684 | 6528 | |
| 90GS(2)_pt4 | 2199 | 53,040 | 11,628 | 13 | 2256 | 51,578 | 959 | 10,255 |
| 90GS(2)_pt5 | 3686 | 79,056 | 29,717 | 938 | 44,821 | 561 | 5868 |
| Mineral Name | Formula | ΔfG° [kJ/mol] | ΔfH° [kJ/mol] | S° [J/K·mol] | C°p [J/K·mol] | V° [cm3/mol] | M [g/mol] |
|---|---|---|---|---|---|---|---|
| C-S-H | Ca0.8SiO2.8:1.54H2O | −1769.0 | −1945.13 | 107.850 | 138.3 | 59.2 | 132.6 |
| Tobermorite 11A | Ca5Si6O16(OH)2 | −9889.3 | −10,680.0 | 692.5 | 764.9 | 286.1 | 739.9 |
| Grossular | Ca3Al2Si3O12 | −6279.6 | −6640. | 260.1 | 327.8 | 125.3 | 450.4 |
| α-SiO2 | −856.2 | −910.7 | 41.4 | 44.5 | 22.6 | 60.0 | |
| β-SiO2 | −854.9 | −908.6 | 43.7 | 44.7 | - | 60.0 |
| Mineral Name | Formula | ΔfG° [kJ/mol] | ΔfH° [kJ/mol] | S° [J/K·mol] | C°p [J/K·mol] | V° [cm3/mol] | M [g/mol] |
|---|---|---|---|---|---|---|---|
| Gyrolite | Ca2Si3O7.5(OH)·2H2O | −4550 | −4917 | 309 | 325 | 137 | 337.4 |
| Xonotlite | Ca6Si6O17(OH)2 | −9465 | −10,022 | 573 | 628 | 256.9 | 714.9 |
| Natrolite | Na2(Al2Si3)O10:2H2O | −5316.6 | −5718.6 | 359 | 359.2 | 169.2 | 380.2 |
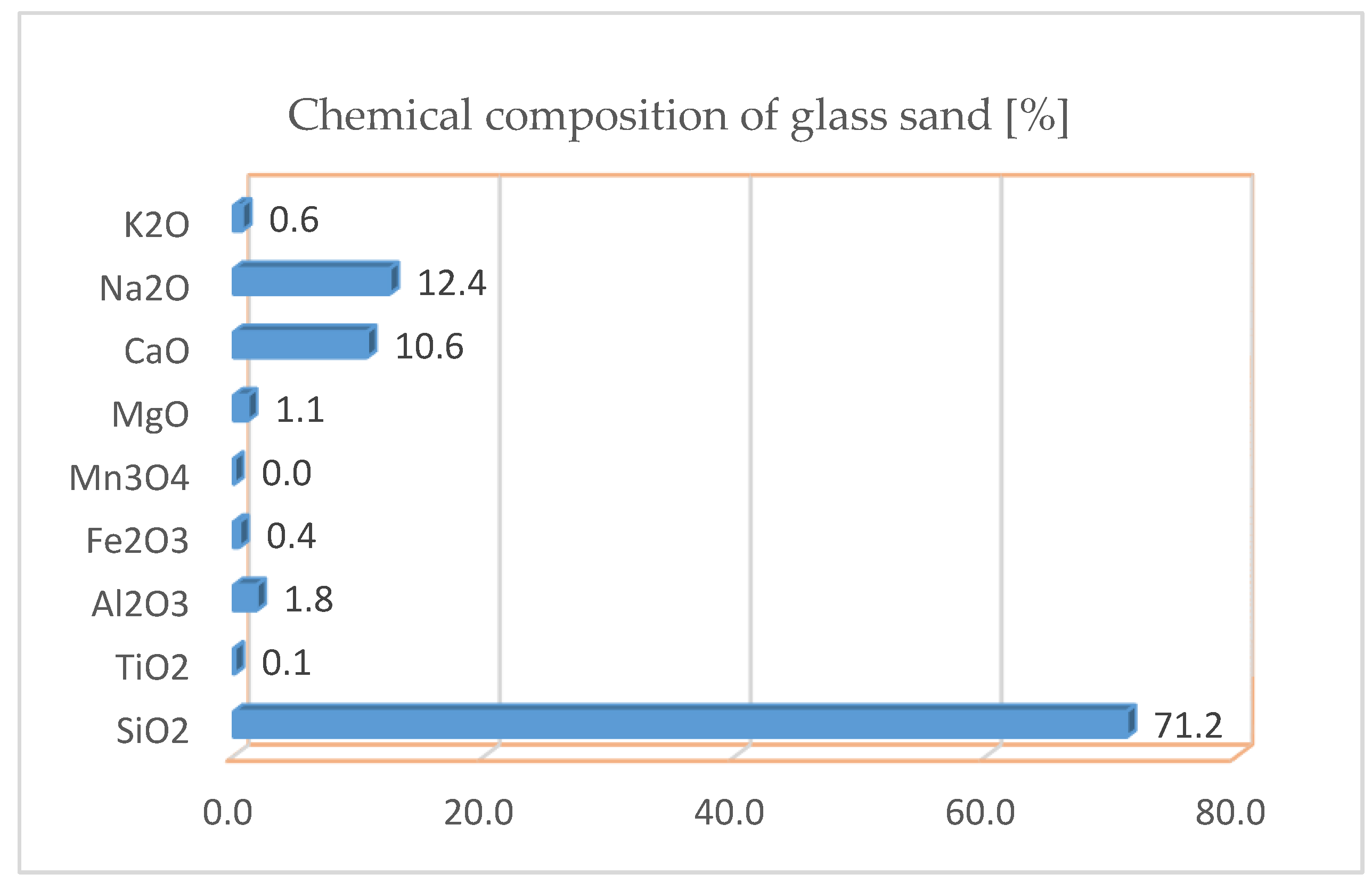
| XRF-CaO | XRF-GS | ||||
|---|---|---|---|---|---|
| Final Weight CaO LOI (%): | 7.7113 g 0.018 | Final weight GS LOI (%): | 7.7046 g 1.304 | ||
| Compound | Value | Unit | Compound | Value | Unit |
| SiO2 | 1.691 | % | SiO2 | 71.2 | % |
| TiO2 | 0.026 | % | TiO2 | 0.1 | % |
| Al2O3 | 0.342 | % | Al2O3 | 1.8 | % |
| Fe2O3 | 0.182 | % | Fe2O3 | 0.4 | % |
| Mn3O4 | 0.024 | % | MgO | 1.1 | % |
| MgO | 0.921 | % | CaO | 10.6 | % |
| CaO | 96.034 | % | Na2O | 12.4 | % |
| K2O | 0.6 | % | |||
| Elements | Glass Sand (90%) 903 (g) | Quartz Sand (0%) 0 (g) | CaO (7%) 73.5 (g) | H2O (3 + 4 = 7%) 73.5 (g) |
|---|---|---|---|---|
| SiO2 | 642.94 | - | 1.24 | |
| Al2O3 | 16.25 | - | 0.25 | |
| MgO | 9.93 | - | 0.13 | |
| CaO | 95.72 | - | 0.02 | |
| Na2O | 111.97 | - | 0.68 | |
| K2O | 5.42 | - | 70.59 |
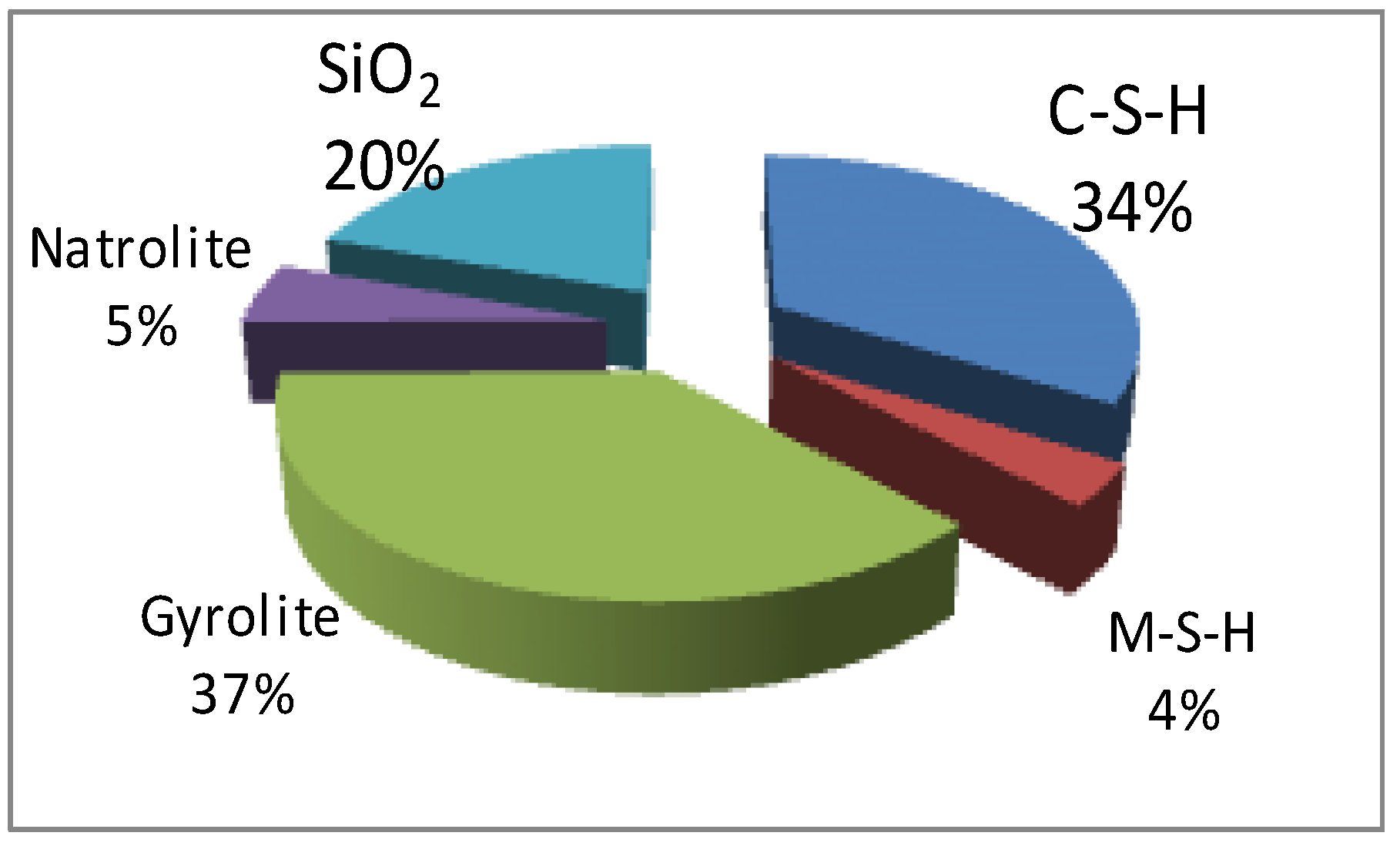
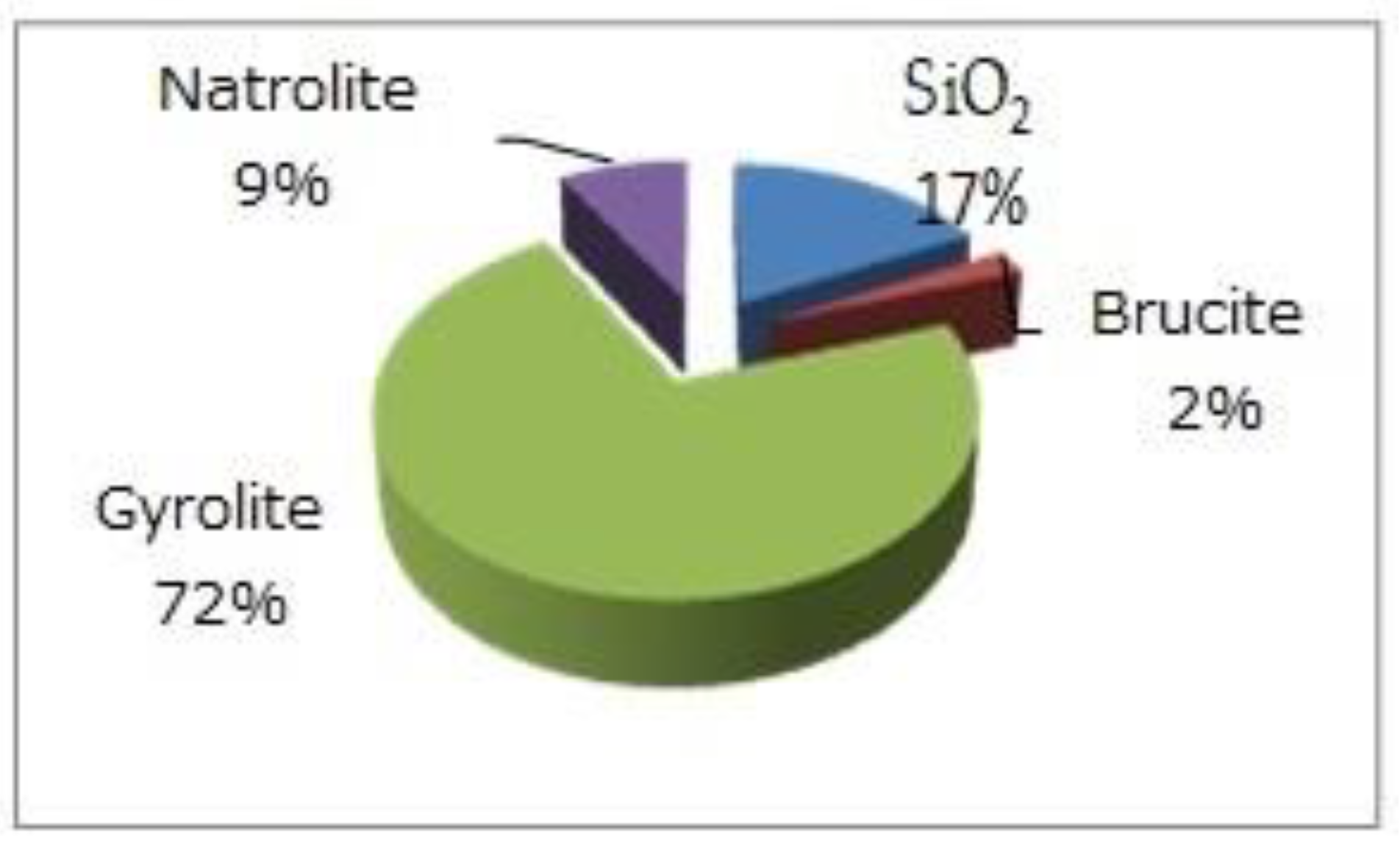
| Solid Phases Predicted to Form | ||
|---|---|---|
| Type of Phase | Mass of Solid Phases (g) | Volume of Solid Phases (cm3) |
| C-S-H (ECSH-I) about the structure of tobermorite low Ca/Si ratio: | 373.29 | 132.84 |
| MSH (magnesium silicate hydrate) | 40.63 | 17.11 |
| gyrolie | 414.16 | 97.28 |
| natrolite | 59.66 | 26.55 |
| SiO2 | 218.37 | 82.45 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepien, A.; Potrzeszcz-Sut, B.; Prentice, D.P.; Oey, T.J.; Balonis, M. The Role of Glass Compounds in Autoclaved Bricks. Buildings 2020, 10, 41. https://doi.org/10.3390/buildings10030041
Stepien A, Potrzeszcz-Sut B, Prentice DP, Oey TJ, Balonis M. The Role of Glass Compounds in Autoclaved Bricks. Buildings. 2020; 10(3):41. https://doi.org/10.3390/buildings10030041
Chicago/Turabian StyleStepien, Anna, Beata Potrzeszcz-Sut, Dale P. Prentice, Tandre J. Oey, and Magdalena Balonis. 2020. "The Role of Glass Compounds in Autoclaved Bricks" Buildings 10, no. 3: 41. https://doi.org/10.3390/buildings10030041
APA StyleStepien, A., Potrzeszcz-Sut, B., Prentice, D. P., Oey, T. J., & Balonis, M. (2020). The Role of Glass Compounds in Autoclaved Bricks. Buildings, 10(3), 41. https://doi.org/10.3390/buildings10030041