Real-Time Measurement on the Heat Release Property of Titanium Blended with Different Carbon Allotropes, under Externally Constant Heat Flux
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Characterizations
3. Results
3.1. HRR of Ti Blended with Different Carbon Allotropes
3.2. XRD of Samples after Reactions in CC
3.3. TG/DSC of Samples
3.4. Morphologies of Samples after Reaction in CC
3.4.1. Macro-Appearances of Samples after Reactions
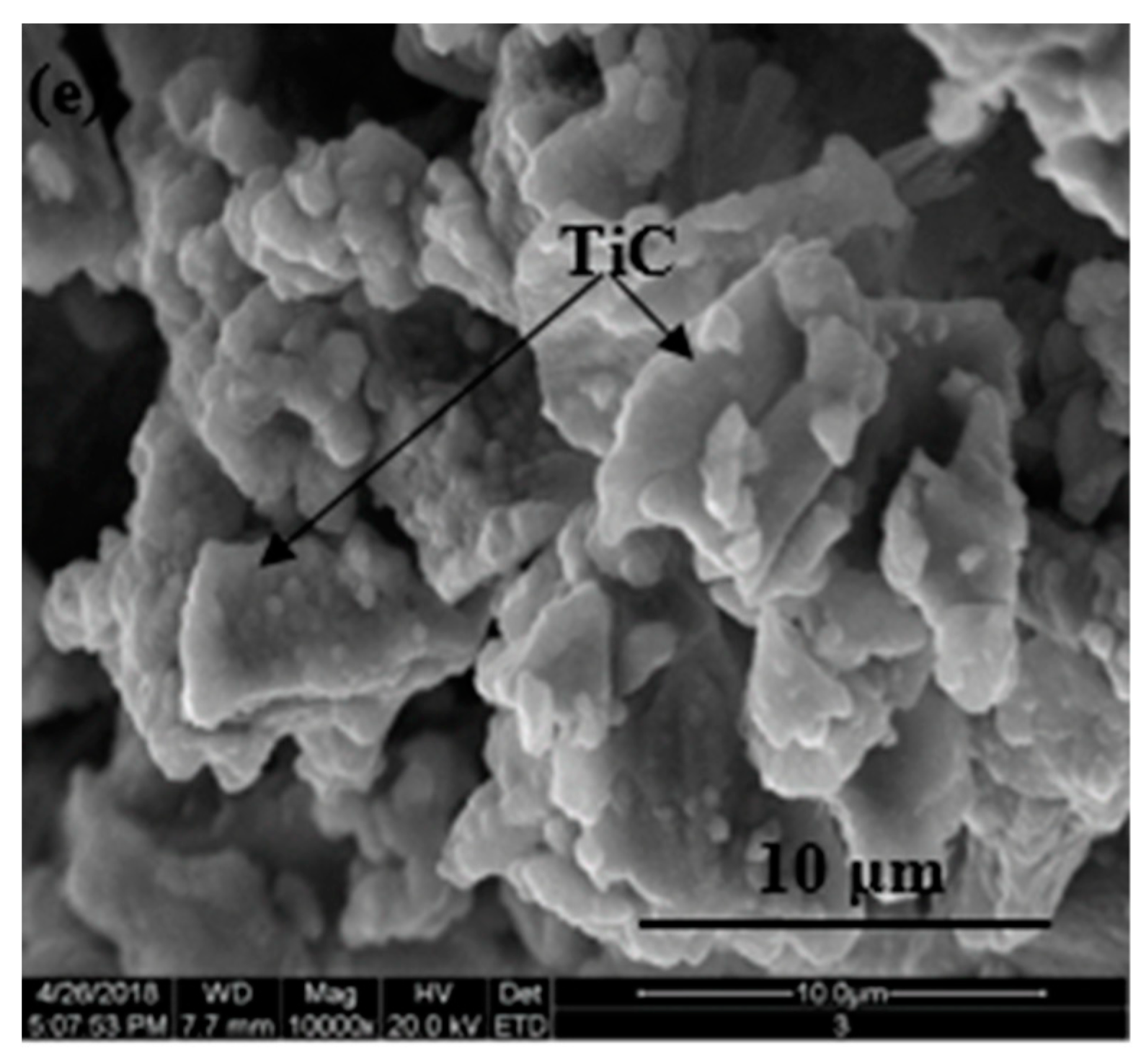
3.4.2. Micro-Morphologies of Samples after Reaction
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huczkoa, A.; Kurcza, M.; Dąbrowskaa, A.; Baranowskia, P.; Bhattaraib, A.; Gierlotkac, S. Self-propagating high-temperature synthesis (SHS) of crystalline nanomaterials. J. Cryst. Growth 2014, 401, 469–473. [Google Scholar] [CrossRef]
- Gaggero, L.; Ferretti, M. The self-sustained high temperature synthesis (SHS) technology as novel approach in the management of asbestos waste. J. Environ. Manag. 2018, 216, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Lagos, M.A.; Agote, I.; Atxaga, G.; Adarraga, O.; Pambaguian, L. Fabrication and characterisation of titanium matrix composites obtained using a combination of self propagating high temperature synthesis and spark plasma sintering. Mater. Sci. Eng. A 2016, 655, 44–49. [Google Scholar] [CrossRef]
- Hajalilou, A.; Hashim, M.; Ebrahimi-Kahizsangi, R.; Ismail, I.; Sarami, N. Synthesis of titanium carbide and TiC–SiO2 nanocomposite powder using rutile and Si by mechanically activated sintering. Adv. Powder Technol. 2014, 25, 1094–1102. [Google Scholar] [CrossRef]
- Yang, S.; Xiao, G.; Ding, D.; Ren, Y.; Lv, L.; Yang, P. Dissolution-precipitation mechanism of combustion synthesis of calcium aluminate. Ceram. Int. 2017, 43, 15918–15926. [Google Scholar] [CrossRef]
- Yang, S.; Xiao, G.; Ding, D.; Ren, Y.; Lv, L.; Yang, P.; Gao, J. Solid-phase combustion synthesis of calcium aluminate with CaAl2O4, nanofiber structures. Ceram. Int. 2018, 44, 6186–6191. [Google Scholar] [CrossRef]
- Oghenevweta, J.E.; Wexler, D.; Calka, A. Study of reaction sequences during MSR synthesis of TiC by controlled ball milling of titanium and graphite. Mater. Charact. 2018, 140, 299–311. [Google Scholar] [CrossRef]
- Vasanthakumar, K.; Bakshi, S.R. Effect of C/Ti ratio on densification, microstructure and mechanical properties of TiCx prepared by reactive spark plasma sintering. Ceram. Int. 2018, 44, 484–494. [Google Scholar] [CrossRef]
- Akhlaghi, M.; Tayebifard, S.A.; Salahi, E.; Asl, M.S.; Schmidt, G. Self-propagating high-temperature synthesis of Ti3AlC2 max phase from mechanically-activated Ti/Al/graphite powder mixture. Ceram. Int. 2018, 44, 9671–9678. [Google Scholar] [CrossRef]
- Martínez-Martínez, D.; Sánchez-López, J.C. Determination of the thickness of the embedding phase in 0d nanocomposites. Appl. Surf. Sci. 2017, 421, 179–184. [Google Scholar] [CrossRef]
- Thomas, T.; Bowen, C.R. Effect of particle size on the formation of Ti2AlC using combustion synthesis. Ceram. Int. 2016, 42, 4150–4157. [Google Scholar] [CrossRef]
- Sadeghi, N.; Aghajani, H.; Akbarpour, M.R. Microstructure and tribological properties of in-situ TiC-C/Cu nanocomposites synthesized using different carbon sources (graphite, carbon nanotube and graphene) in the Cu-Ti-C system. Ceram. Int. 2018, 44, 22059–22067. [Google Scholar] [CrossRef]
- Ramos, J.P.; Stora, T.; Senos, A.M.R.; Bowen, P. Thermal stability of nanometric TiC-carbon composites: effects of carbon. J. Eur. Ceram. Soc. 2018, 38, 4882–4891. [Google Scholar] [CrossRef]
- Wang, Y.C.; Zhao, J.P. Benign design and the evaluation of pyrolysis kinetics of polyester resin based intumescent system comprising of alkali-activated silica fume. Prog. Org. Coat. 2018, 122, 30–37. [Google Scholar] [CrossRef]
- Wang, Y.C.; Zhao, J.P. Comparative study on flame retardancy of silica fume-based geopolymer activated by different activators. J. Alloys Compd. 2018, 743, 108–114. [Google Scholar] [CrossRef]
- Oikawa, K.; Toyota, K.; Sakatani, S.; Hayashi, Y.; Takizawa, H. Facile synthesis and thermal properties of waterglass-based silica xerogel. Ceram. Int. 2019, 45, 4201–4207. [Google Scholar] [CrossRef]
- Ahmed, Y.M.Z.; Zaki, Z.I.; Besisa, D.H.A.; Amin, A.M.M.; Bordia, R.K. Effect of zirconia and iron on the mechanical properties of Al2O3/TiC composites processed using combined self-propagating synthesis and direct consolidation technique. Mater. Sci. Eng. A 2017, 696, 182–189. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, B129, 695–716. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, Y.; Hu, Z. Preparation and characterization of foamed concrete with Ti-extracted residues and red gypsum. Constr. Build. Mater. 2018, 171, 109–119. [Google Scholar] [CrossRef]
- Dyjak, S.; Norek, M.; Polański, M.; Cudziło, S.; Bystrzycki, J. A simple method of synthesis and surface purification of titanium carbide powder. Int. J. Refract. Met. Hard Mater 2013, 38, 87–91. [Google Scholar] [CrossRef]
- Li, M.; Mu, B. Effect of different dimensional carbon materials on the properties and application of phase change materials: A review. Appl. Energy 2019, 242, 695–715. [Google Scholar] [CrossRef]
- Rubin, A.E.; Ma, C. Meteoritic minerals and their origins. Chem. Erde 2017, 77, 325–385. [Google Scholar] [CrossRef]
- Boutefnouchet, H.; Curfs, C.; Triki, A.; Boutefnouchet, A.; Vrel, D. Self-propagating high-temperature synthesis mechanisms within the Ti–C–Ni system: A time resolved x-ray diffraction study. Powder Technol. 2012, 217, 443–450. [Google Scholar] [CrossRef]
- Liu, G.; Yang, Z.; Lia, J.; He, G.; Chen, Y.; Chen, K.; Fan, D. Reaction path in formation of Ti1-xWxC solid solution by combustion synthesis. Ceram. Int. 2018, 44, 6127–6136. [Google Scholar] [CrossRef]
- Ahmed, Y.M.Z.; Zaki, Z.I.; Bordia, R.K.; Besisa, D.H.A.; Amin, A.M.M. Simultaneous synthesis and sintering of TiC/Al2O3, composite via self-propagating synthesis with direct consolidation technique. Ceram. Int. 2016, 42, 16589–16597. [Google Scholar] [CrossRef]
- Zhu, X.; He, Q.; Hu, Y.; Huang, R.; Shao, N.; Gao, Y. A comparative study of structure, thermal degradation, and combustion behavior of starch from different plant sources. J. Therm. Anal. Calorim. 2018, 132, 1–9. [Google Scholar] [CrossRef]
- Yang, Y.F.; Mu, D.K.; Jiang, Q.C. A simple route to fabricate TiC–TiB2/Ni composite via thermal explosion reaction assisted with external pressure in air. Mater. Chem. Phys. 2014, 143, 480–485. [Google Scholar] [CrossRef]
- Sharifitabar, M.; Khaki, J.V.; Sabzevar, M.H. Formation mechanism of TiC–Al2O3–Fe3Al composites during self-propagating high-temperature synthesis of TiO2–Al–C–Fe system. Ceram. Int. 2016, 42, 12361–12370. [Google Scholar] [CrossRef]
- Rahimi-Vahedi, A.; Adeli, M.; Saghafian, H. Formation of Fe-TiC composite clad layers on steel using the combustion synthesis process. Surf. Coat. Technol. 2018, 347, 217–224. [Google Scholar] [CrossRef]
- Zarezadeh-Mehrizi, M.; Beygi, R.; Eisaabadi, G.; Velashjerdi, M.; Nematzadeh, F. Mechanically activated combustion synthesis of Ti3AlC2/Al2O3 nanocomposite from TiO2/Al/C powder mixtures. Adv. Powder Technol. 2019, 30, 311–316. [Google Scholar] [CrossRef]
- Jam, A.; Nikzad, L.; Razavi, M. TiC-based cermet prepared by high-energy ball-milling and reactive spark plasma sintering. Ceram. Int. 2017, 43, 2448–2455. [Google Scholar] [CrossRef]
- Sundaram, D.; Yang, V.; Yetter, R.A. Metal-based nanoenergetic materials: Synthesis, properties, and applications. Prog. Energy Combust. Sci. 2017, 61, 293–365. [Google Scholar] [CrossRef]
- Liu, Z.; Rakita, M.; Xu, W.; Wang, X.; Han, Q. Ultrasound assisted combustion synthesis of TiC in Al–Ti–C system. Ultrason. Sonochem. 2015, 27, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Findenig, G.; Buchegger, C.; Lengauer, W.; Veitsch, C.; Demoly, A. Investigation of the main influencing parameters on the degassing behavior of titanium carbonitrides using mass spectrometry. Int. J. Refract. Met. Hard Mater. 2017, 63, 38–46. [Google Scholar] [CrossRef]

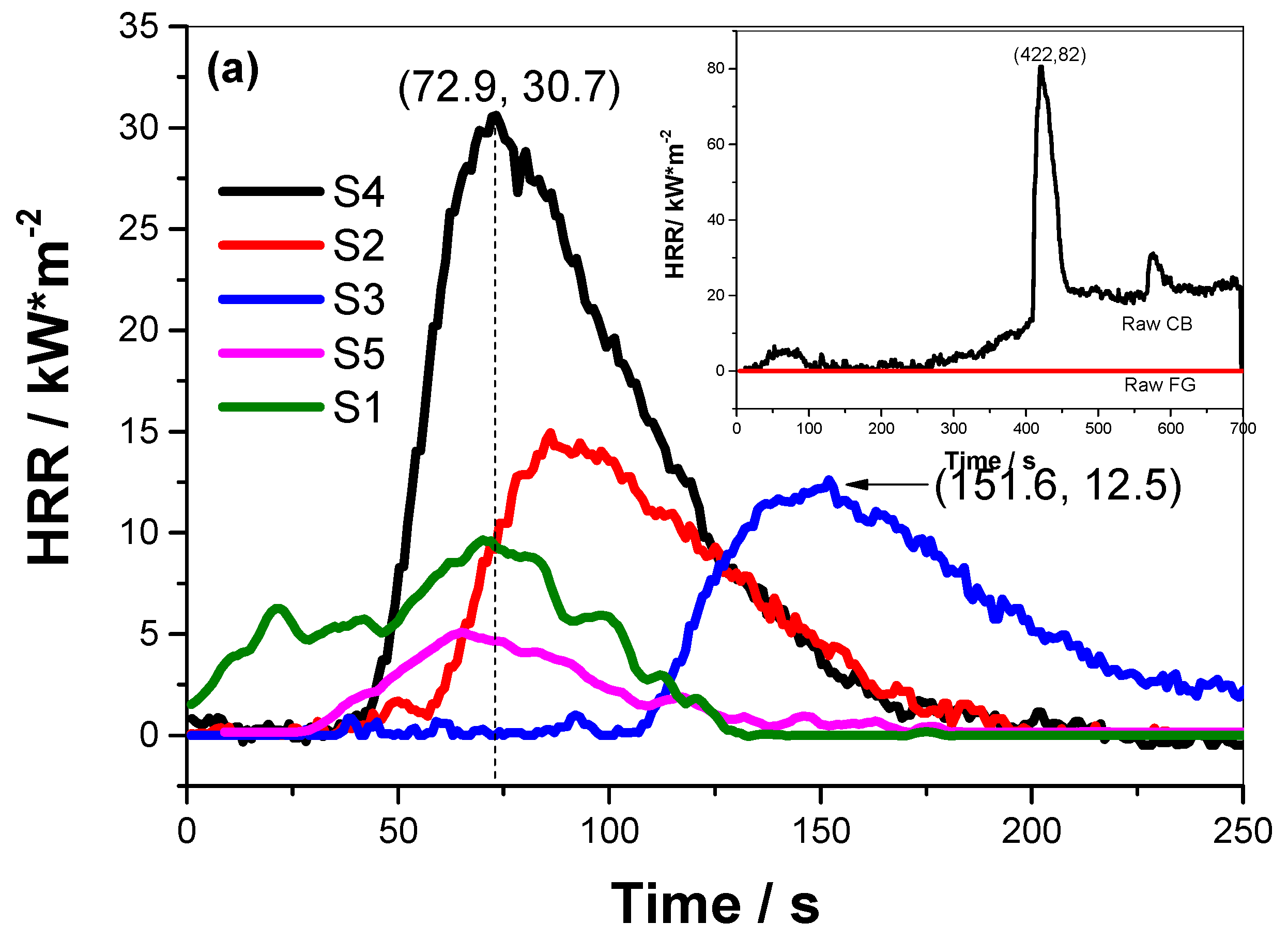











| Samples | Ingredient | TTI/s | THR/kW | pHRR/kW·m−2 | Tp/s | FPI/s·m2·kW−1 | FGI/kW·m−2·s−1 |
|---|---|---|---|---|---|---|---|
| S1 | Pure Ti | 3 | 2.51 | 9.6 | 71.2 | 0.31 | 0.13 |
| S2 | Ti + LP | 16 | 8.77 | 14.9 | 85.5 | 1.07 | 0.17 |
| S3 | Ti + CB | 25 | 8.46 | 12.5 | 151.6 | 2.00 | 0.08 |
| S4 | Ti + EG | 2 | 17.19 | 30.7 | 72.9 | 0.07 | 0.42 |
| S5 | Ti + VG | 30 | 6.08 | 5.2 | 64.3 | 5.77 | 0.08 |
| S6 | Ti + LP-NaOH | 28 | 8.11 | 16.4 | 110.1 | 1.71 | 0.15 |
| S7 | Ti + CB-NaOH | 16 | 5.43 | 8.5 | 71.2 | 1.88 | 0.12 |
| S8 | Ti + EG-NaOH | 2 | 7.01 | 23.8 | 44.5 | 0.08 | 0.53 |
| S9 | Ti + VG-NaOH | 35 | 2.78 | 7.9 | 45.1 | 4.43 | 0.18 |
| T/K | Equation (1)/kJ × mol−1 | Equation (2)/kJ × mol−1 | Equation (3)/kJ × mol−1 | Equation (4)/kJ × mol−1 | ||||
| ∆H | ∆G | ∆H | ∆G | ∆H | ∆G | ∆H | ∆G | |
| 273 | −1154.3 | −1107.7 | −184.6 | −181.2 | 551.5 | 500.0 | 366.9 | 318.8 |
| 373 | −1154.3 | −1090.7 | −184.4 | −180.0 | 551.2 | 481.2 | 366.8 | 301.1 |
| 473 | −1153.9 | −1073.7 | −184.1 | −178.9 | 550.4 | 462.5 | 366.3 | 283.6 |
| 573 | −1153.4 | −1056.8 | −183.8 | −177.8 | 549.4 | 444.0 | 365.5 | 266.2 |
| 673 | −1152.8 | −1039.9 | −183.7 | −176.8 | 548.2 | 425.7 | 364.6 | 248.9 |
| 773 | −1152.1 | −1023.2 | −183.7 | −175.8 | 547.1 | 407.6 | 363.5 | 231.8 |
| 873 | −1151.3 | −1006.6 | −183.8 | −174.7 | 546.1 | 389.6 | 362.3 | 214.9 |
| 973 | −1150.6 | −990.1 | −183.9 | −173.7 | 545.0 | 371.8 | 361.1 | 198.1 |
| 1073 | −1149.8 | −973.6 | −184.3 | −172.6 | 544.2 | 354.0 | 359.9 | 181.4 |
| 1173 | −1148.9 | −957.3 | −189.4 | −171.4 | 548.0 | 336.2 | 358.6 | 164.8 |
| 1273 | −1148.1 | −941.0 | −189.2 | −169.9 | 546.6 | 318.3 | 357.4 | 148.3 |
| T/K | Equation (5)/kJ × mol−1 | Equation (6)/kJ × mol−1 | Equation (7)/kJ × mol−1 | Equation (8)/kJ × mol−1 | ||||
| ∆H | ∆G | ∆H | ∆G | ∆H | ∆G | ∆H | ∆G | |
| 273 | 539.1 | 443.2 | −364.3 | −329.0 | −1154.3 | −1107.7 | −871.4 | −848.3 |
| 373 | 540.0 | 407.9 | −364.4 | −316.0 | −1154.3 | −1090.7 | −870.8 | −839.9 |
| 473 | 539.9 | 372.5 | −364.1 | −303.1 | −1153.9 | −1073.7 | −870.2 | −831.7 |
| 573 | 539.1 | 337.2 | −363.5 | −290.2 | −1153.4 | −1056.8 | −869.6 | −823.6 |
| 673 | 537.8 | 302.1 | −363.0 | −277.5 | −1152.8 | −1039.9 | −869.1 | −815.6 |
| 773 | 536.1 | 267.2 | −362.4 | −264.8 | −1152.1 | −1023.2 | −868.6 | −807.7 |
| 873 | 534.1 | 232.5 | −361.8 | −252.2 | −1151.3 | −1006.6 | −868.2 | −799.9 |
| 973 | 532.0 | 198.1 | −361.3 | −239.7 | −1150.6 | −990.1 | −867.7 | −792.1 |
| 1073 | 529.8 | 163.8 | −361.0 | −227.3 | −1149.8 | −973.6 | −867.3 | −784.3 |
| 1173 | 527.6 | 129.8 | −365.2 | −214.7 | −1148.9 | −957.3 | −866.9 | −776.6 |
| 1273 | 525.2 | 96.0 | −364.3 | −201.9 | −1148.1 | −941.0 | −866.5 | −769.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhao, J. Real-Time Measurement on the Heat Release Property of Titanium Blended with Different Carbon Allotropes, under Externally Constant Heat Flux. Metals 2019, 9, 981. https://doi.org/10.3390/met9090981
Wang Y, Zhao J. Real-Time Measurement on the Heat Release Property of Titanium Blended with Different Carbon Allotropes, under Externally Constant Heat Flux. Metals. 2019; 9(9):981. https://doi.org/10.3390/met9090981
Chicago/Turabian StyleWang, Yachao, and Jiangping Zhao. 2019. "Real-Time Measurement on the Heat Release Property of Titanium Blended with Different Carbon Allotropes, under Externally Constant Heat Flux" Metals 9, no. 9: 981. https://doi.org/10.3390/met9090981
APA StyleWang, Y., & Zhao, J. (2019). Real-Time Measurement on the Heat Release Property of Titanium Blended with Different Carbon Allotropes, under Externally Constant Heat Flux. Metals, 9(9), 981. https://doi.org/10.3390/met9090981





