A Comparative Study of the Diffuse-Interface Model and Sharp-Interface Model in the Soldering Related Wetting Spreading Systems
Abstract
:1. Introduction
2. Experimental Procedure and Wetting Kinetics
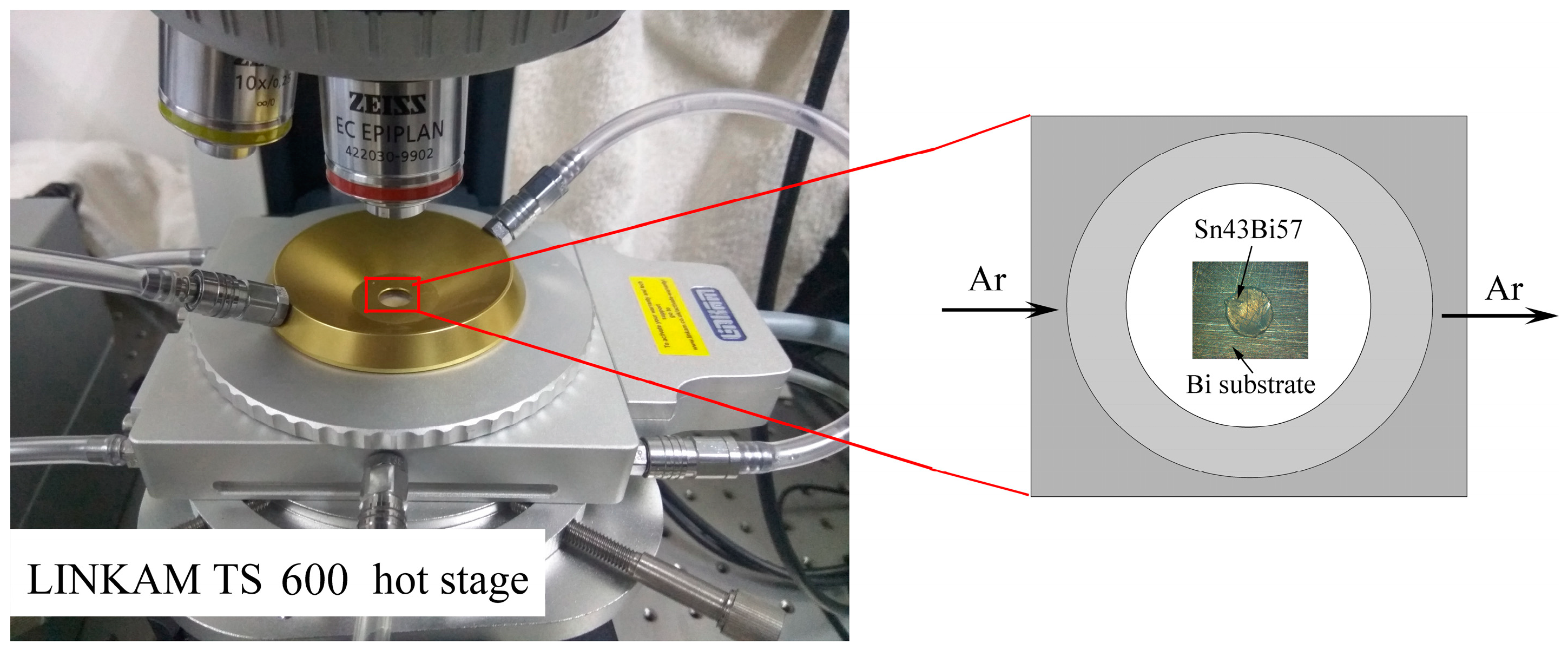
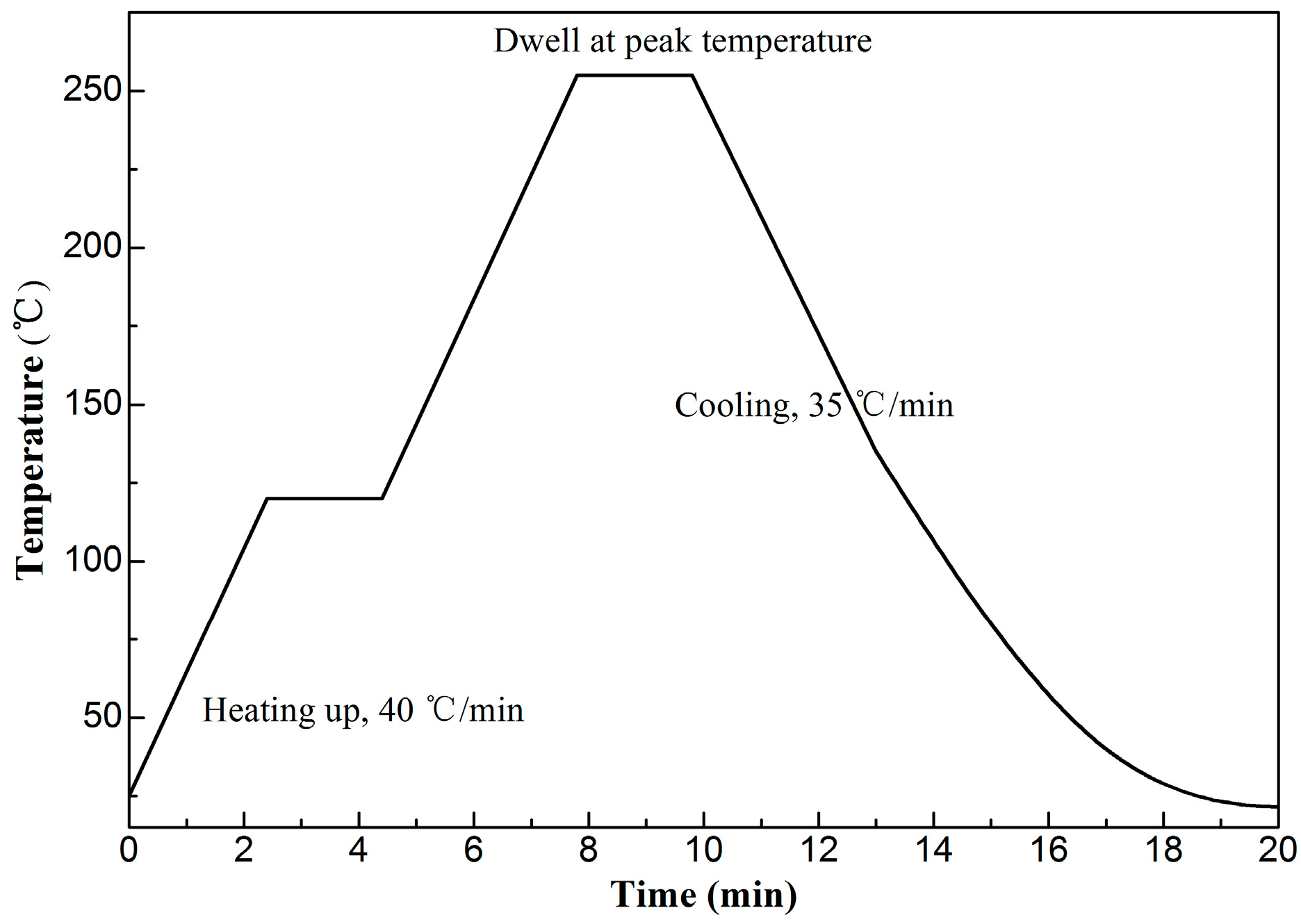
2.1. Experimental Procedure
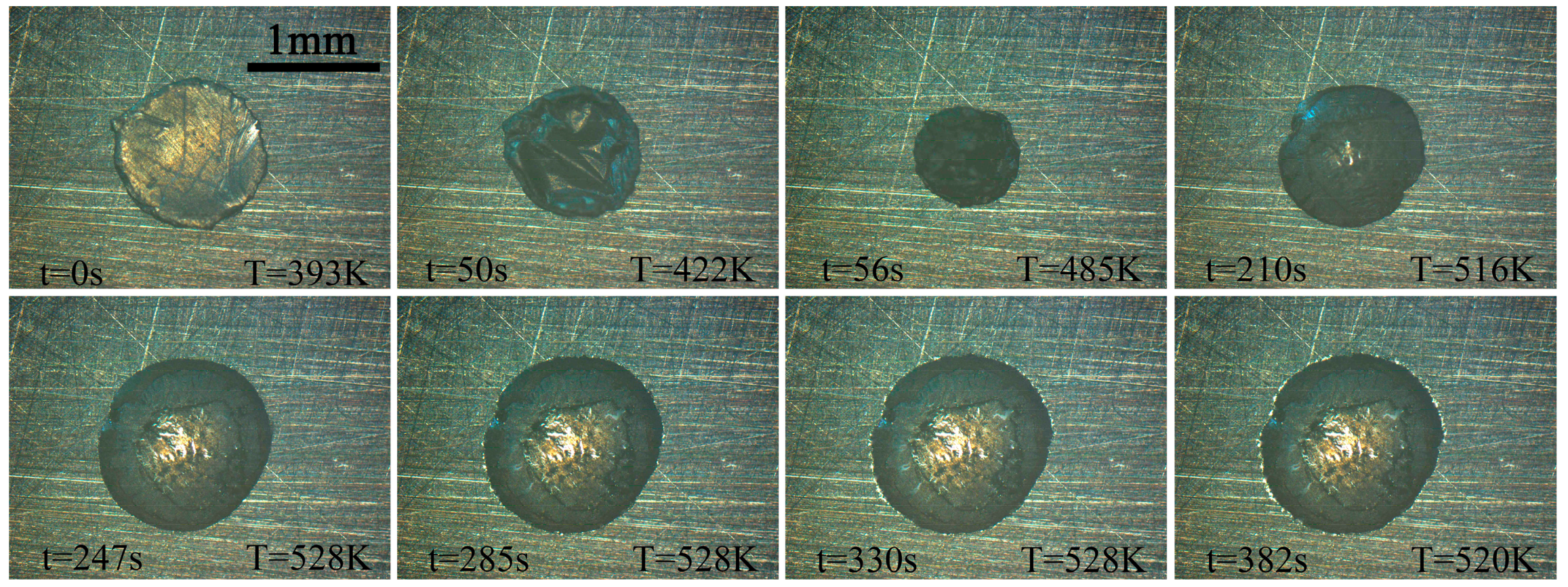
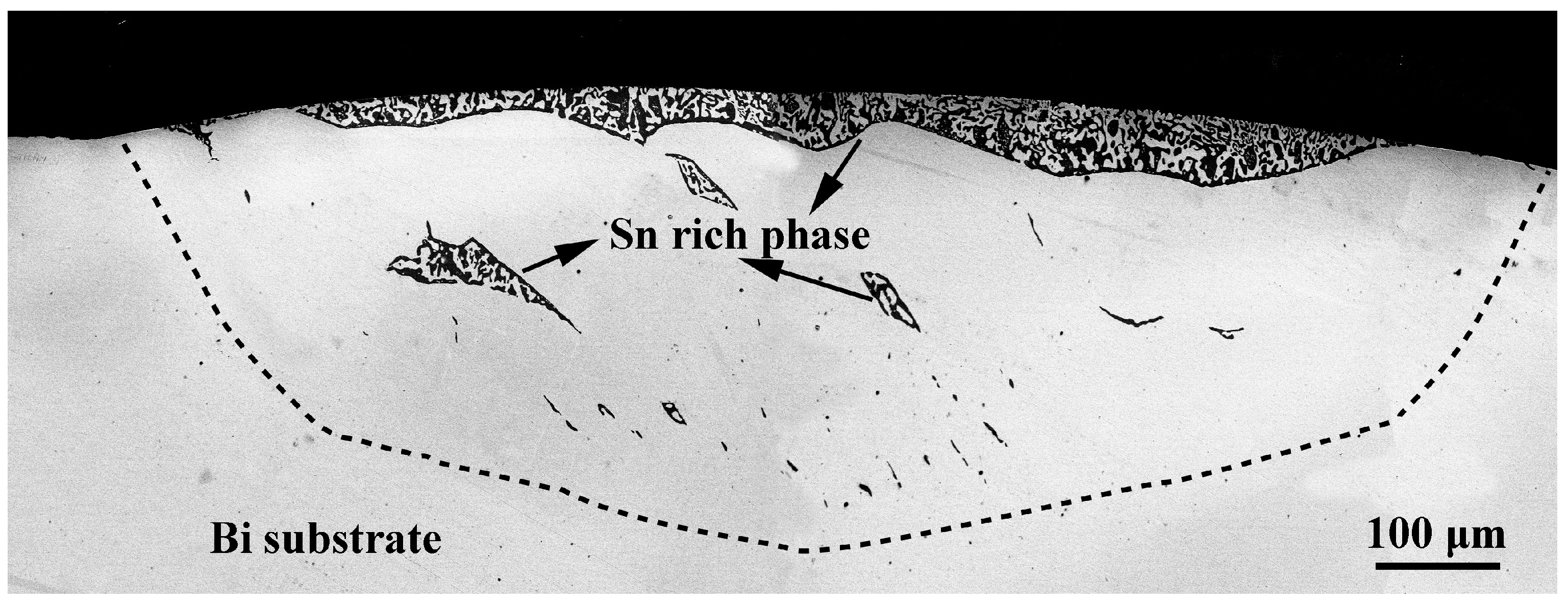
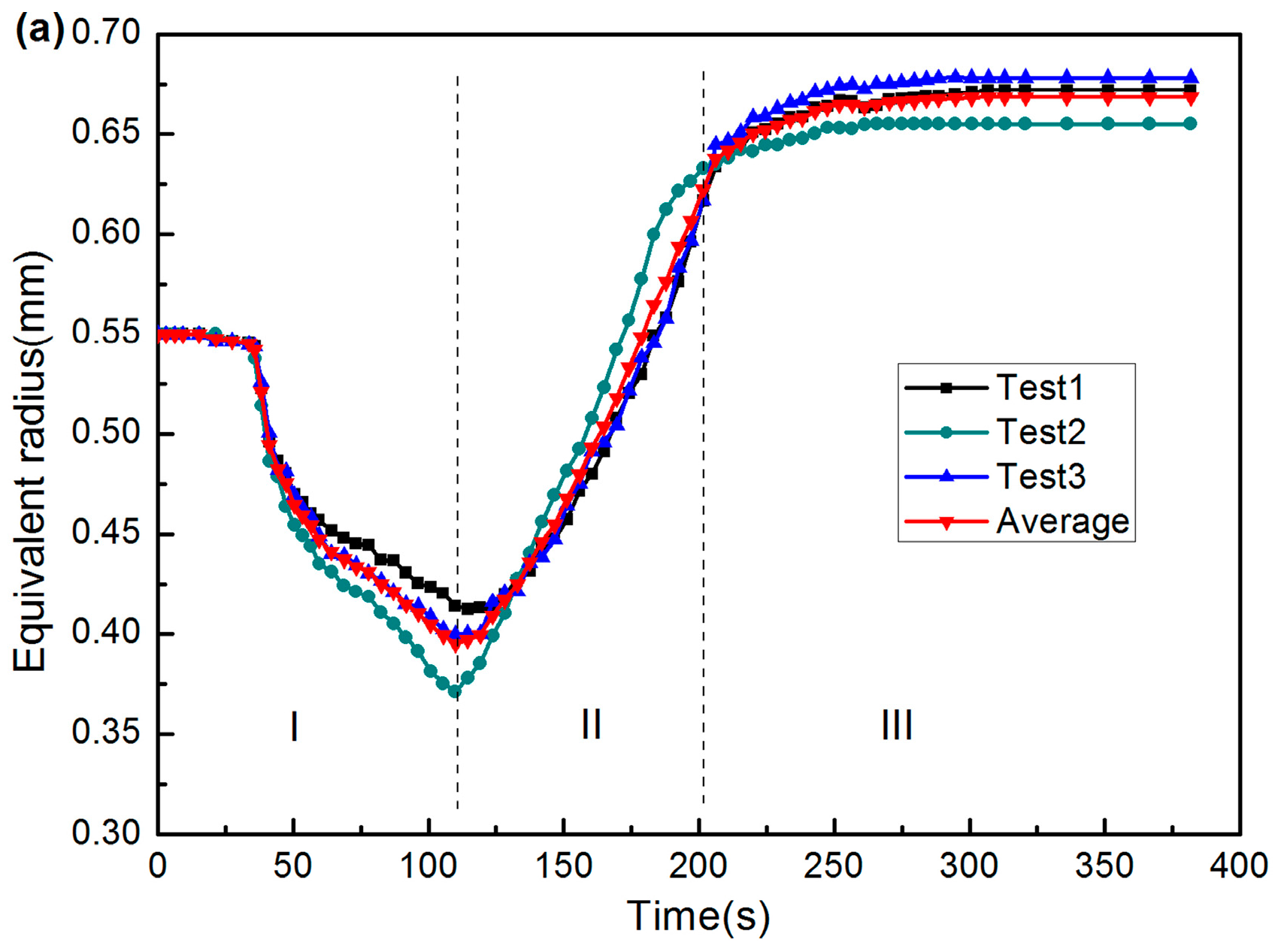
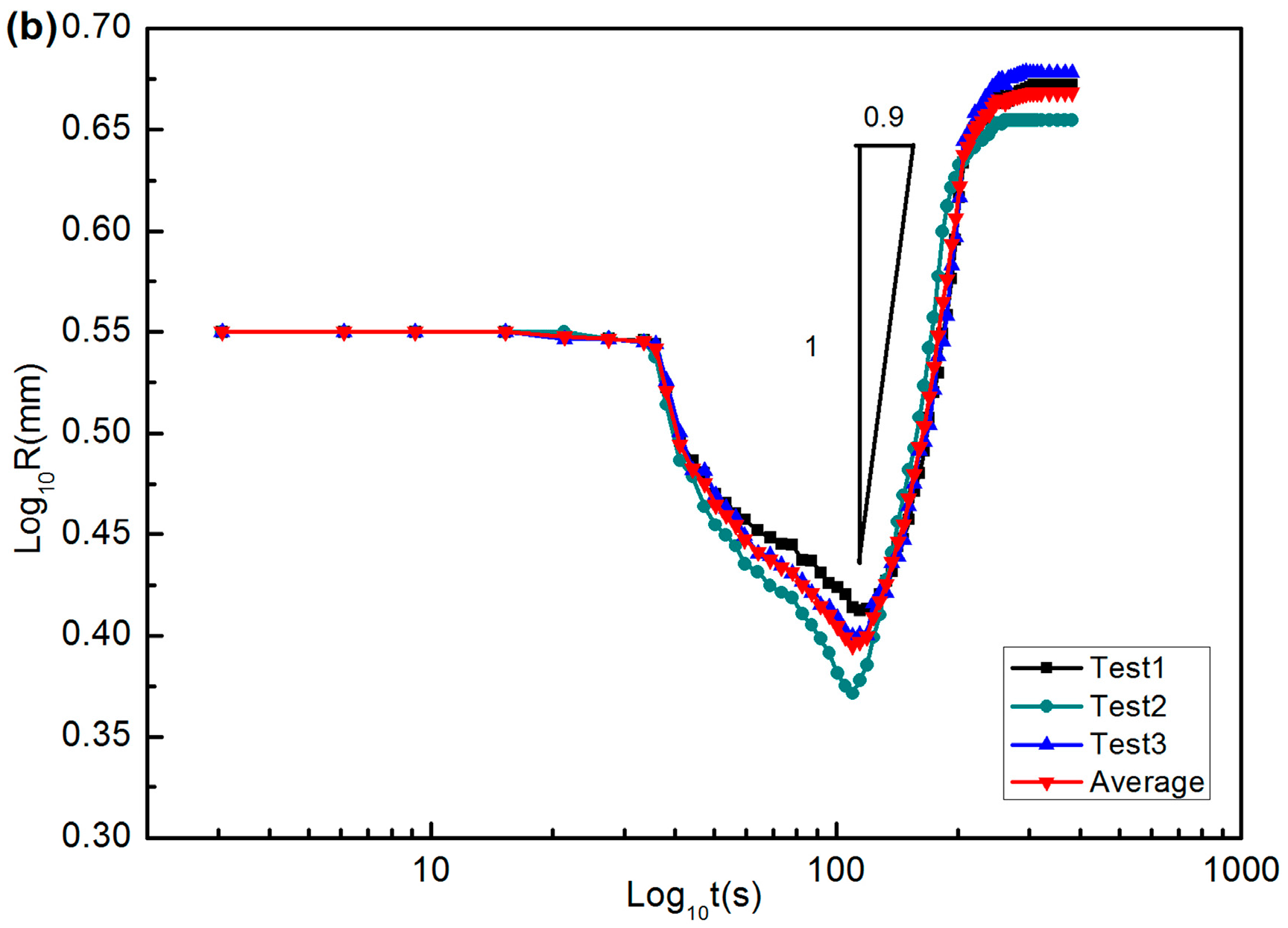
2.2. Results and Wetting Kinetics
3. The Differences and Relations between the Sharp-Interface and Diffuse-Interface
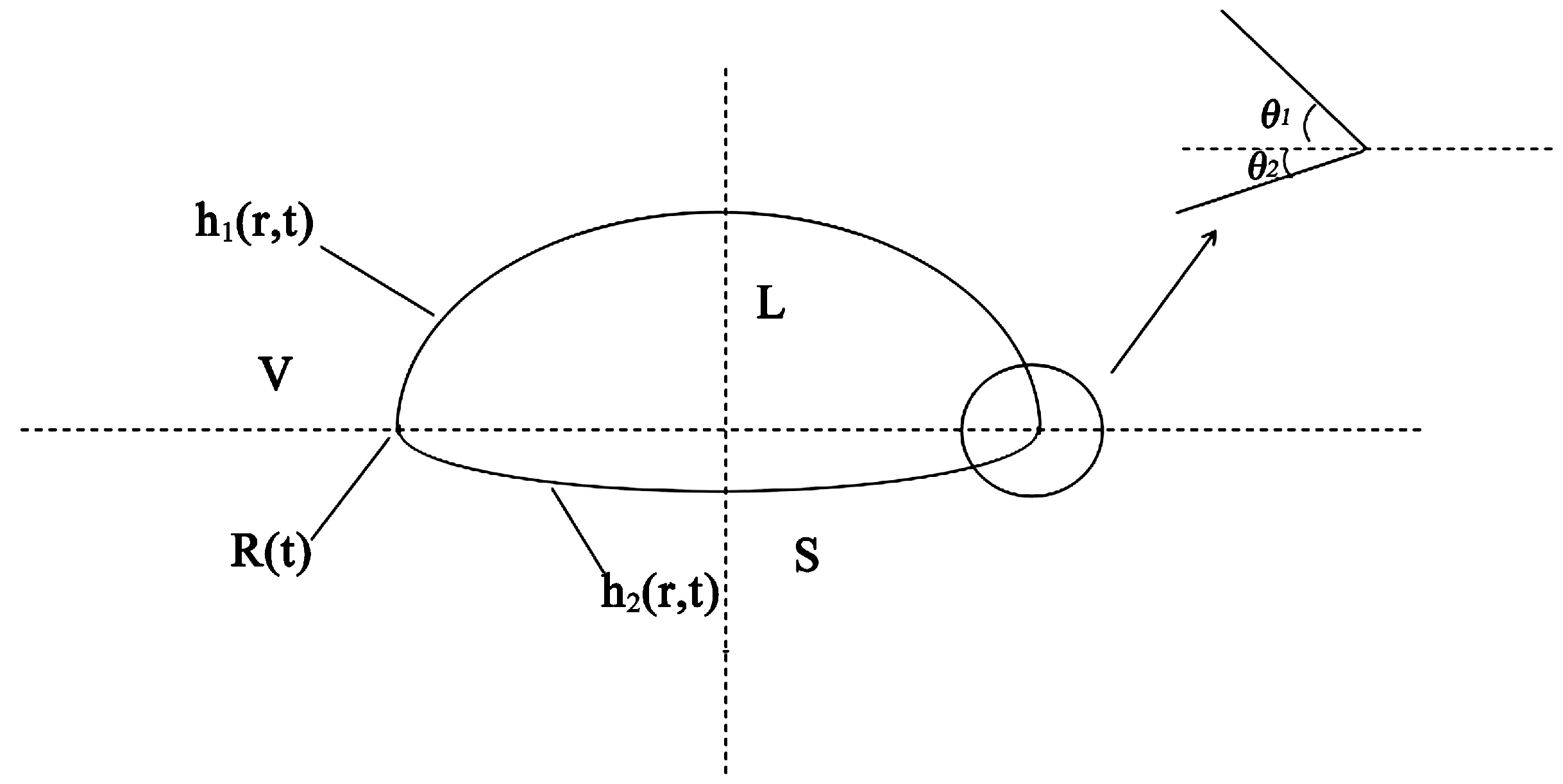
3.1. The Differences between the Basic Theories
3.2. The Relations between the Basic Theories
4. The Sharp-Interface Model and Diffuse-Interface Model in the Practical Applications
4.1. Practical Application of Sharp-Interface Model
4.2. Practical Application of Diffuse-Interface Model
5. Comparison of the Simulation Results Based on the Two Interface Models
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dezellus, O.; Eustathopoulos, N. Fundamental issues of reactive wetting by liquid metals. J. Mater. Sci. 2010, 45, 4256–4264. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.L.; Wang, Z.L.; Long, W.F.; Lei, M.; Hu, X.W. Wetting kinetics and spreading phenomena of Sn-35Bi-1Ag solder on different substrates. J. Mater. Sci. Mater. Electron. 2018, 29, 13914–13924. [Google Scholar] [CrossRef]
- Liu, W.; Sekulic, D.P. Capillary driven molten metal flow over topographically complex substrates. Langmuir 2011, 27, 6720–6730. [Google Scholar] [CrossRef] [PubMed]
- Tanner, L.H. The spreading of silicone oil drops on horizontal surfaces. J. Phys. D Appl. Phys. 1979, 12, 1473. [Google Scholar] [CrossRef]
- Eustathopoulos, N.; Voytovych, R. The role of reactivity in wetting by liquid metals: A review. J. Mater. Sci. 2016, 51, 425–437. [Google Scholar] [CrossRef]
- Landry, K.; Rado, C.; Voitovich, R.; Eustathopoulos, N. Mechanisms of reactive wetting: The question of triple line configuration. Acta Mater. 1997, 45, 3079–3085. [Google Scholar] [CrossRef]
- Mortensen, A.; Drevet, B.; Eustathopoulos, N. Kinetics of diffusion-limited spreading of sessile drops in reactive wetting. Scr. Mater. 1997, 36, 645–651. [Google Scholar] [CrossRef] [Green Version]
- Ding, H.; Spelt, P.D.M. Wetting condition in diffuse interface simulations of contact line motion. Phys. Rev. E 2007, 75, 046708. [Google Scholar] [CrossRef]
- Liu, H.R.; Gao, P.; Ding, H. Fluid–structure interaction involving dynamic wetting: 2D modeling and simulations. J. Comput. Phys. 2017, 348, 45–65. [Google Scholar] [CrossRef]
- Khatavkar, V.V.; Anderson, P.D.; Meijer, H.E.H. Capillary spreading of a droplet in the partially wetting regime using a diffuse-interface model. J. Fluid Mech. 2007, 572, 367–387. [Google Scholar] [CrossRef] [Green Version]
- Jacqmin, D. Contact-line dynamics of a diffuse fluid interface. J. Fluid Mech. 2000, 402, 57–88. [Google Scholar] [CrossRef]
- Leo, P.H.; Lowengrub, J.S.; Jou, H.J. A diffuse interface model for microstructural evolution in elastically stressed solids. Acta Mater. 1998, 46, 2113–2130. [Google Scholar] [CrossRef]
- Subhedar, A.; Steinbach, I.; Varnik, F. Modeling the flow in diffuse interface methods of solidification. Phys. Rev. E 2015, 92, 023303. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, F.; Herlach, D.M. Kinetics of triple-junctions in eutectic solidification: A sharp interface model. J. Mater. Sci. 2015, 50, 176–188. [Google Scholar] [CrossRef]
- Theillard, M.; Gibou, F.; Pollock, T. A sharp computational method for the simulation of the solidification of binary alloys. J. Sci. Comput. 2015, 63, 330–354. [Google Scholar] [CrossRef]
- Anderson, D.M.; McFadden, G.B.; Wheeler, A.A. Diffuse-interface methods in fluid mechanics. Annu. Rev. Fluid. Mech. 1998, 30, 139–165. [Google Scholar] [CrossRef]
- Collins, J.B.; Levine, H. Diffuse interface model of diffusion-limited crystal growth. Phys. Rev. B 1985, 31, 6119. [Google Scholar] [CrossRef] [PubMed]
- Galenko, P.; Jou, D. Diffuse-interface model for rapid phase transformations in nonequilibrium systems. Phys. Rev. E 2005, 71, 046125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Liu, F.; Zhai, H.; Wang, K. Application of the maximal entropy production principle to rapid solidification: A sharp interface model. Acta Mater. 2012, 60, 1444–1454. [Google Scholar] [CrossRef]
- Warren, J.A.; Boettinger, W.J.; Roosen, A.R. Modeling reactive wetting. Acta Mater. 1998, 46, 3247–3264. [Google Scholar] [CrossRef]
- Yin, L.; Murray, B.T.; Singler, T.J. Dissolutive wetting in the Bi–Sn system. Acta Mater. 2006, 54, 3561–3574. [Google Scholar] [CrossRef]
- Su, S.; Yin, L.; Sun, Y.; Murray, B.T.; Singler, T.J. Modeling dissolution and spreading of Bi–Sn alloy drops on a Bi substrate. Acta Mater. 2009, 57, 3110–3122. [Google Scholar] [CrossRef]
- Chen, L.Q. Phase-field models for microstructure evolution. Annu. Rev. Mater. Res. 2002, 32, 113–140. [Google Scholar] [CrossRef]
- Drolet, F.; Elder, K.R.; Grant, M.; Kosterlitz, J.M. Phase-field modeling of eutectic growth. Phys. Rev. E 2000, 61, 6705. [Google Scholar] [CrossRef] [PubMed]
- Loginova, I.; Odqvist, J.; Amberg, G.; Ågren, J. The phase-field approach and solute drag modeling of the transition to massive γ→α transformation in binary Fe-C alloys. Acta Mater. 2003, 51, 1327–1339. [Google Scholar] [CrossRef]
- Warren, J.A.; Boettinger, W.J. Prediction of dendritic growth and microsegregation patterns in a binary alloy using the phase-field method. Acta Mater. 1995, 43, 689–703. [Google Scholar] [CrossRef]
- Huh, J.Y.; Hong, K.K.; Kim, Y.B.; Kim, K.T. Phase field simulations of intermetallic compound growth during soldering reactions. J. Electron. Mater. 2004, 33, 1161–1170. [Google Scholar] [CrossRef]
- Hong, K.K.; Huh, J.Y. Phase field simulations of morphological evolution and growth kinetics of solder reaction products. J. Electron. Mater. 2006, 35, 56–64. [Google Scholar] [CrossRef]
- Villanueva, W.; Granhagen, K.; Amberg, G.; Ågren, J. Multicomponent and multiphase modeling and simulation of reactive wetting. Phys. Rev. E 2008, 77, 056313. [Google Scholar] [CrossRef]
- Villanueva, W.; Boettinger, W.J.; Warren, J.A.; Amberg, G. Effect of phase change and solute diffusion on spreading on a dissolving substrate. Acta Mater. 2009, 57, 6022–6036. [Google Scholar] [CrossRef]
- Eustathopoulos, N. Dynamics of wetting in reactive metal/ceramic systems. Acta Mater. 1998, 46, 2319–2327. [Google Scholar]
- Zhao, H.; Nalagatla, D.R.; Sekulic, D.P. Wetting kinetics of eutectic lead and lead-free solders: Spreading over the Cu Surface. J. Electron. Mater. 2009, 38, 284–291. [Google Scholar] [CrossRef]
- De Gennes, P.G. Wetting: Statics and dynamics. Rev. Mod. Phys. 1985, 57, 827. [Google Scholar] [CrossRef]
- Asthana, R.; Sobczak, N. Wettability, spreading, and interfacial phenomena in high-temperature coatings. JOM-e 2000, 52, 1–19. [Google Scholar]
- Yu, X.; Yang, J.; Yan, M.; Hu, X.W.; Li, Y.L. Kinetics of wetting and spreading of AgCu filler metal over Ti–6Al–4V substrates. J. Mater. Sci. 2016, 51, 10960–10969. [Google Scholar] [CrossRef]
- Qin, R.S.; Bhadeshia, H.K. Phase field method. Mater. Sci. Technol. 2010, 26, 803–811. [Google Scholar] [CrossRef]
- Elder, K.R.; Grant, M.; Provatas, N.; Kosterlitz, J.M. Sharp interface limits of phase-field models. Phys. Rev. E 2001, 64, 021604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caginalp, G.; Xie, W. Phase-field and sharp-interface alloy models. Phys. Rev. E 1993, 48, 1897. [Google Scholar] [CrossRef]
- Song, X.G.; Cao, J.; Wang, Y.F.; Feng, J.C. Effect of Si3N4-particles addition in Ag-Cu-Ti filler alloy on Si3N4/TiAl brazed joint. Mater. Sci. Eng. A 2011, 528, 5135–5140. [Google Scholar] [CrossRef]
- Yue, P.; Zhou, C.; Feng, J.J. Sharp-interface limit of the Cahn-Hilliard model for moving contact lines. J. Fluid Mech. 2010, 645, 279–294. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Zhang, J.; Lei, M.; Li, Y.; Li, X. A Comparative Study of the Diffuse-Interface Model and Sharp-Interface Model in the Soldering Related Wetting Spreading Systems. Metals 2019, 9, 944. https://doi.org/10.3390/met9090944
Liu G, Zhang J, Lei M, Li Y, Li X. A Comparative Study of the Diffuse-Interface Model and Sharp-Interface Model in the Soldering Related Wetting Spreading Systems. Metals. 2019; 9(9):944. https://doi.org/10.3390/met9090944
Chicago/Turabian StyleLiu, Guanpeng, Jianyang Zhang, Min Lei, Yulong Li, and Xuewen Li. 2019. "A Comparative Study of the Diffuse-Interface Model and Sharp-Interface Model in the Soldering Related Wetting Spreading Systems" Metals 9, no. 9: 944. https://doi.org/10.3390/met9090944





