Roles of MgO and Al2O3 on the Viscous and Structural Behavior of Blast Furnace Primary Slag, Part 1: C/S = 1.3 Containing TiO2
Abstract
:1. Introduction
2. Experimental Samples and Procedure
2.1. Starting Materials
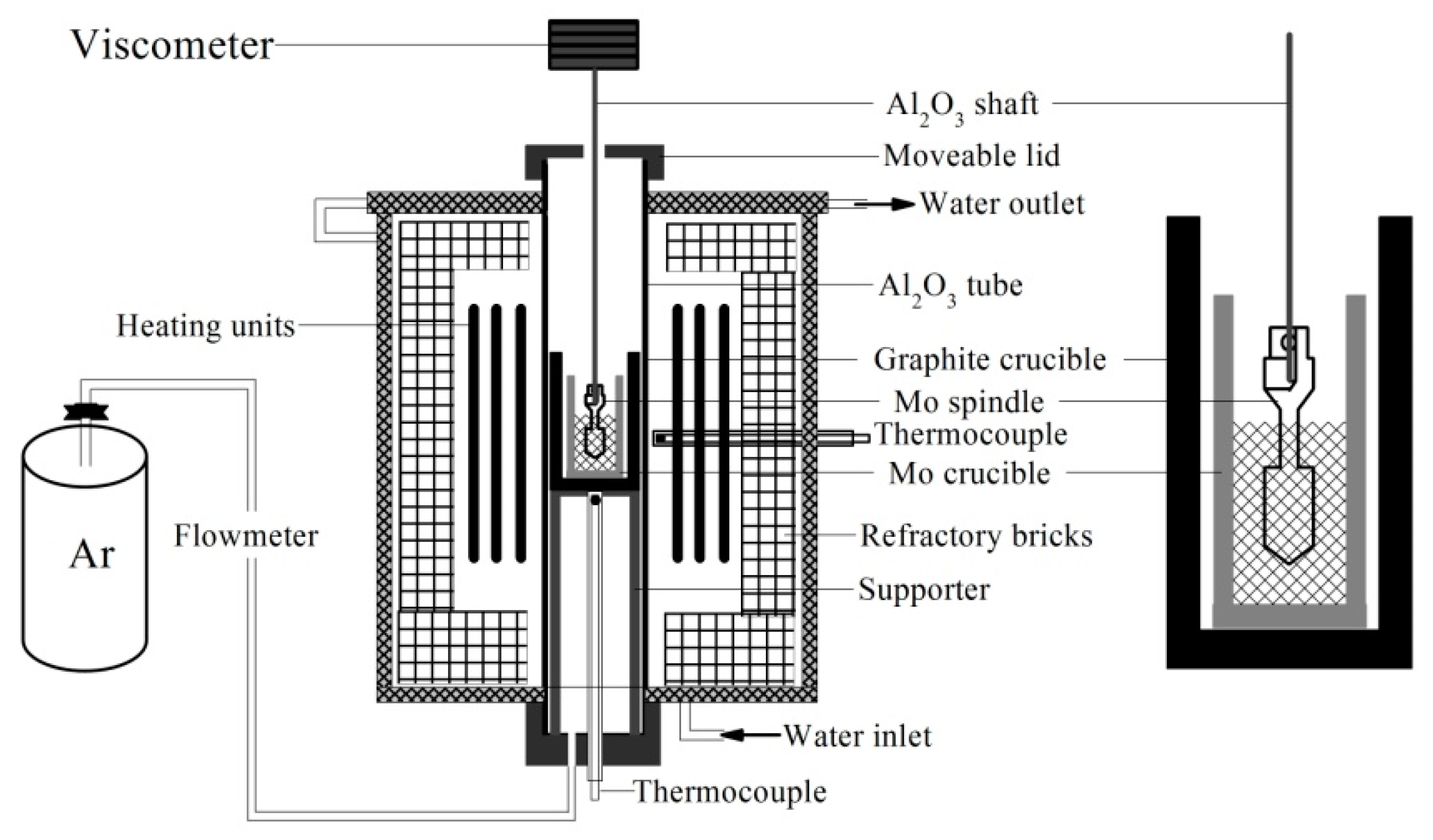
2.2. Experimental Methods
2.3. Characterization of the Slag’s Network Structure
3. Results
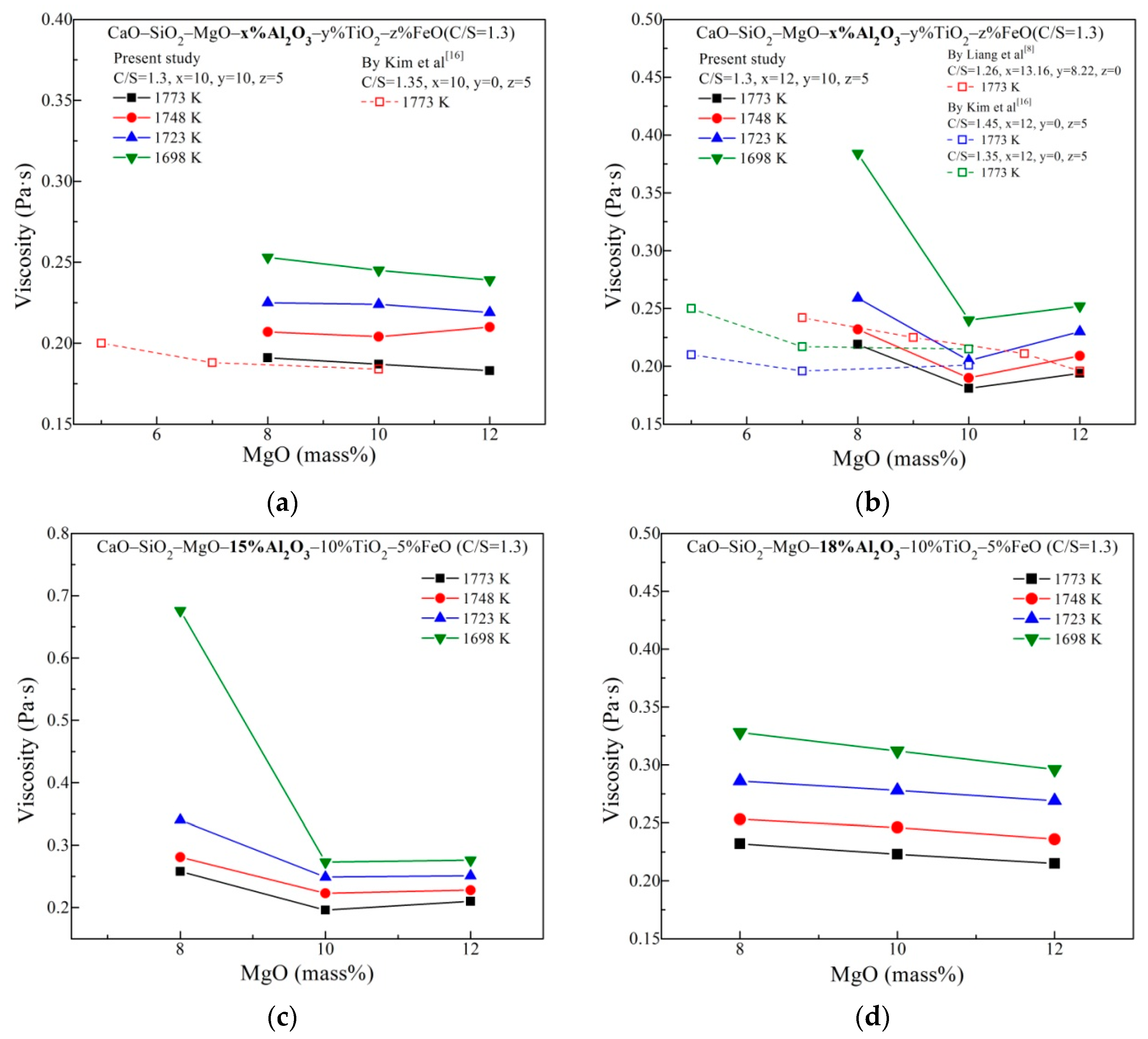
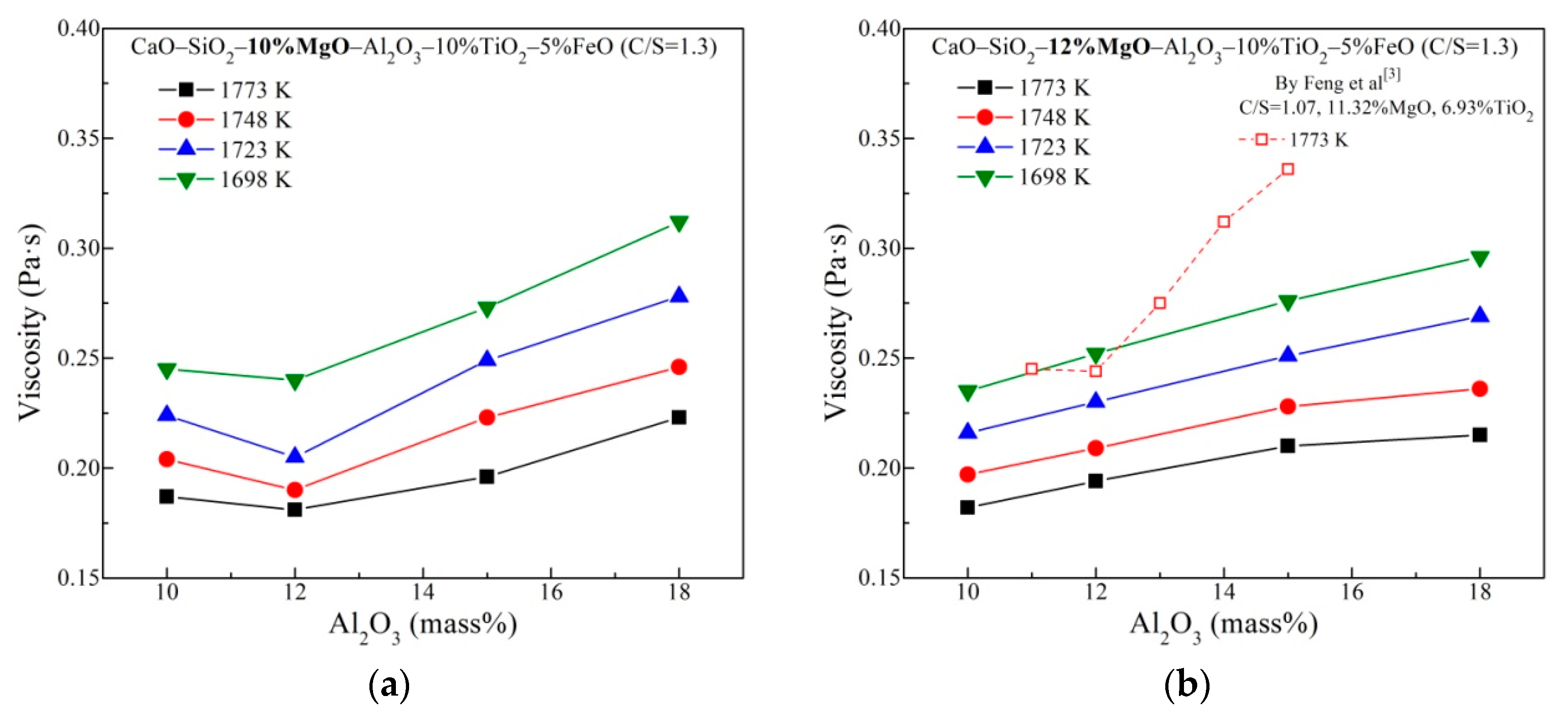
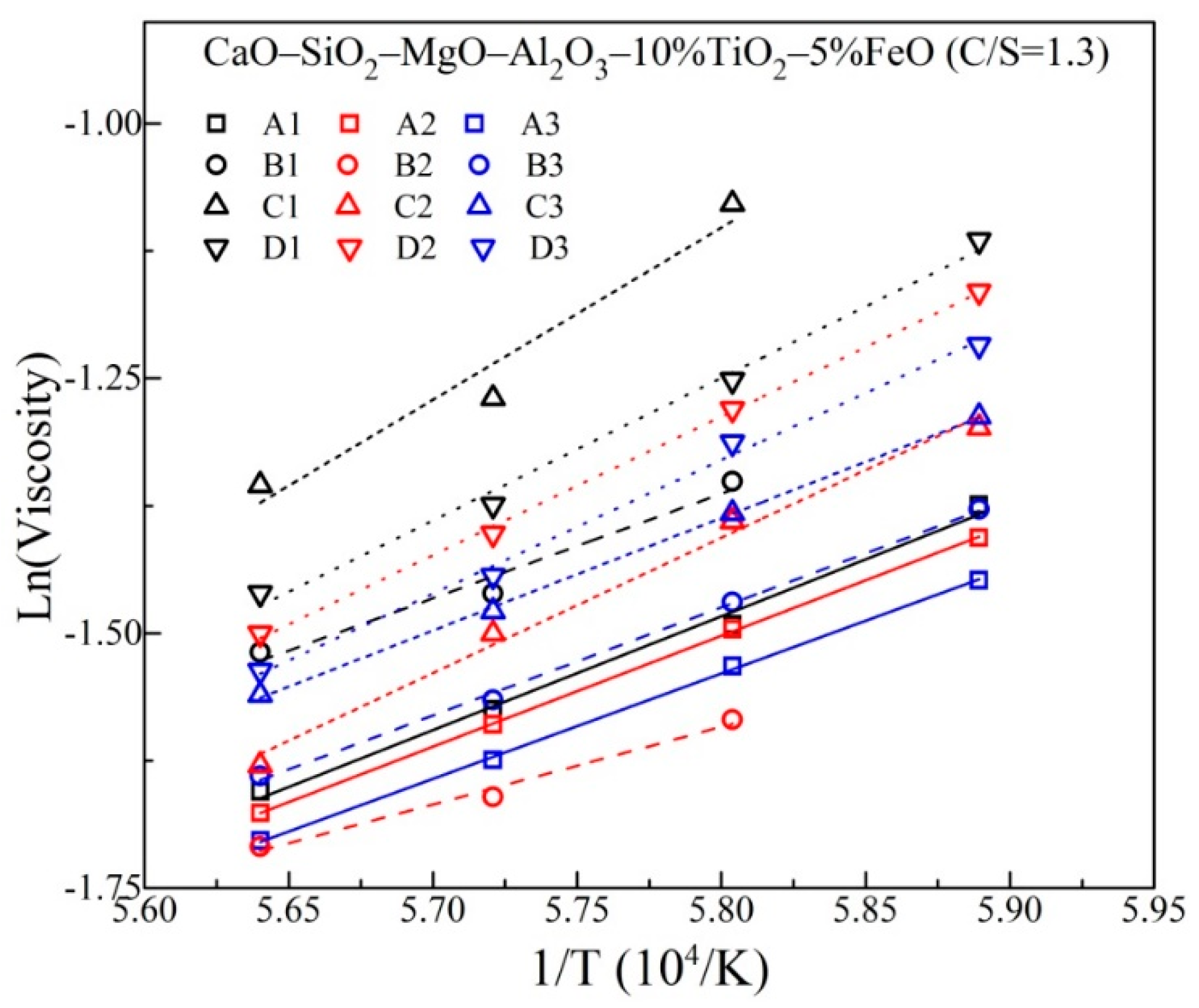
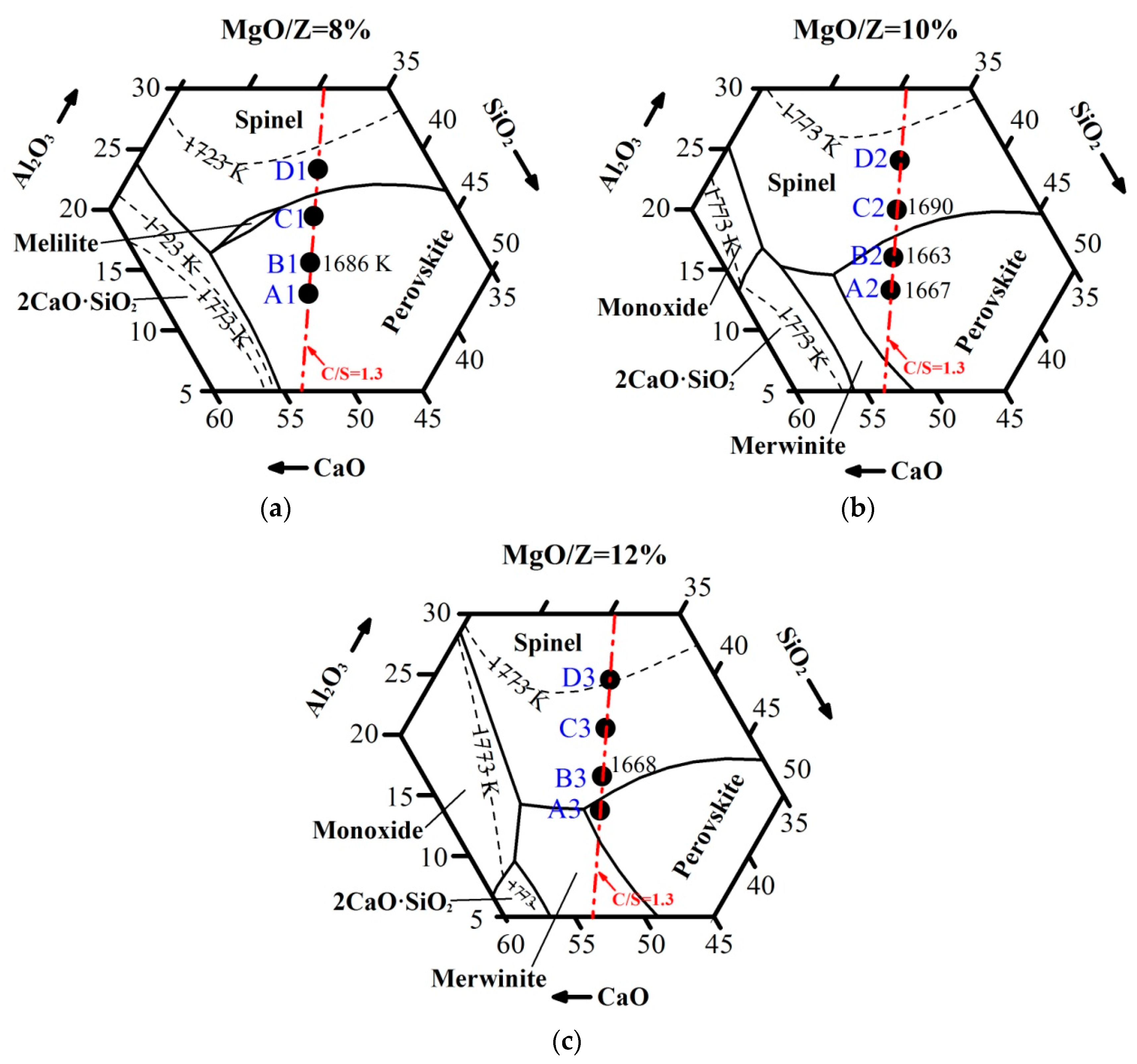
3.1. Viscous Behavior with Varying MgO and Al2O3
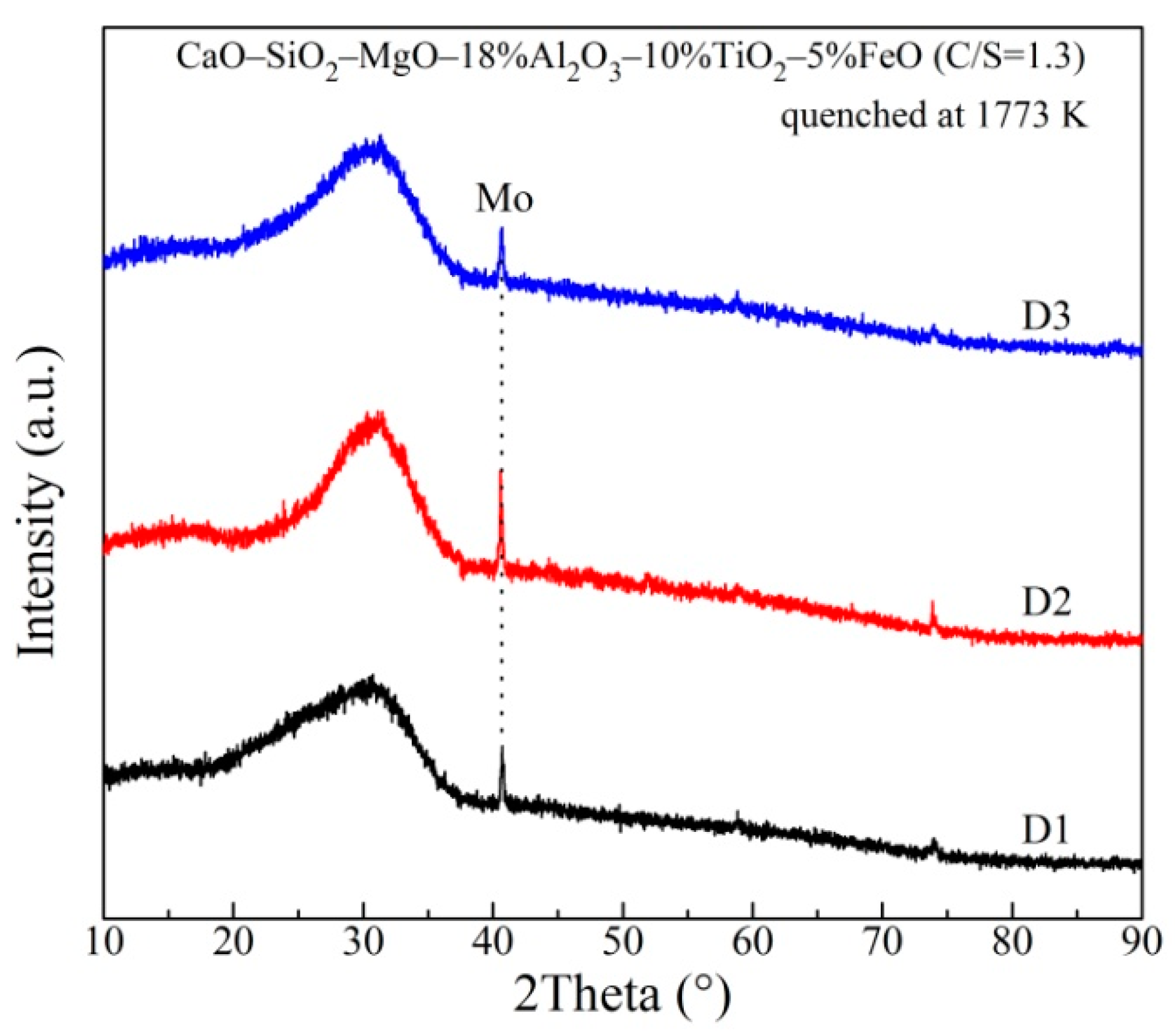
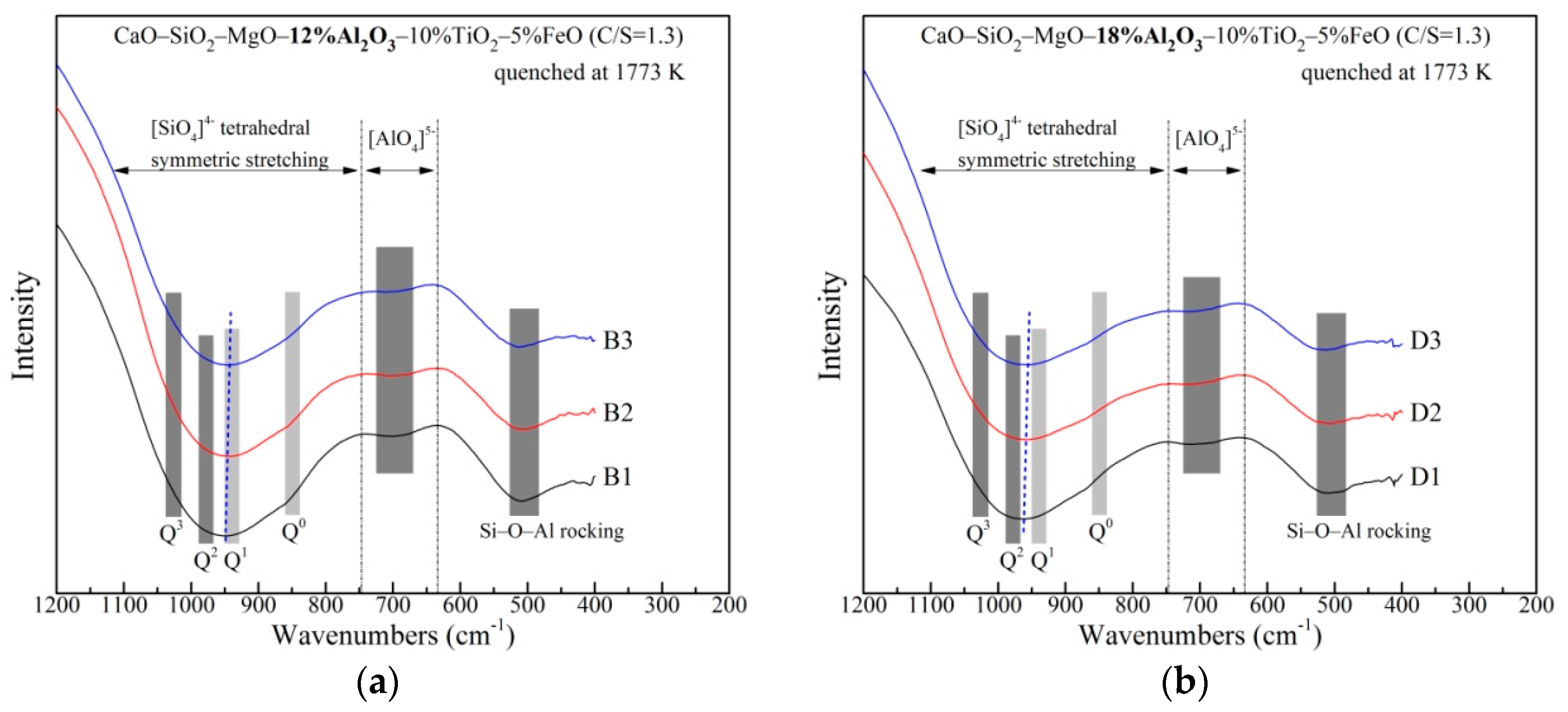
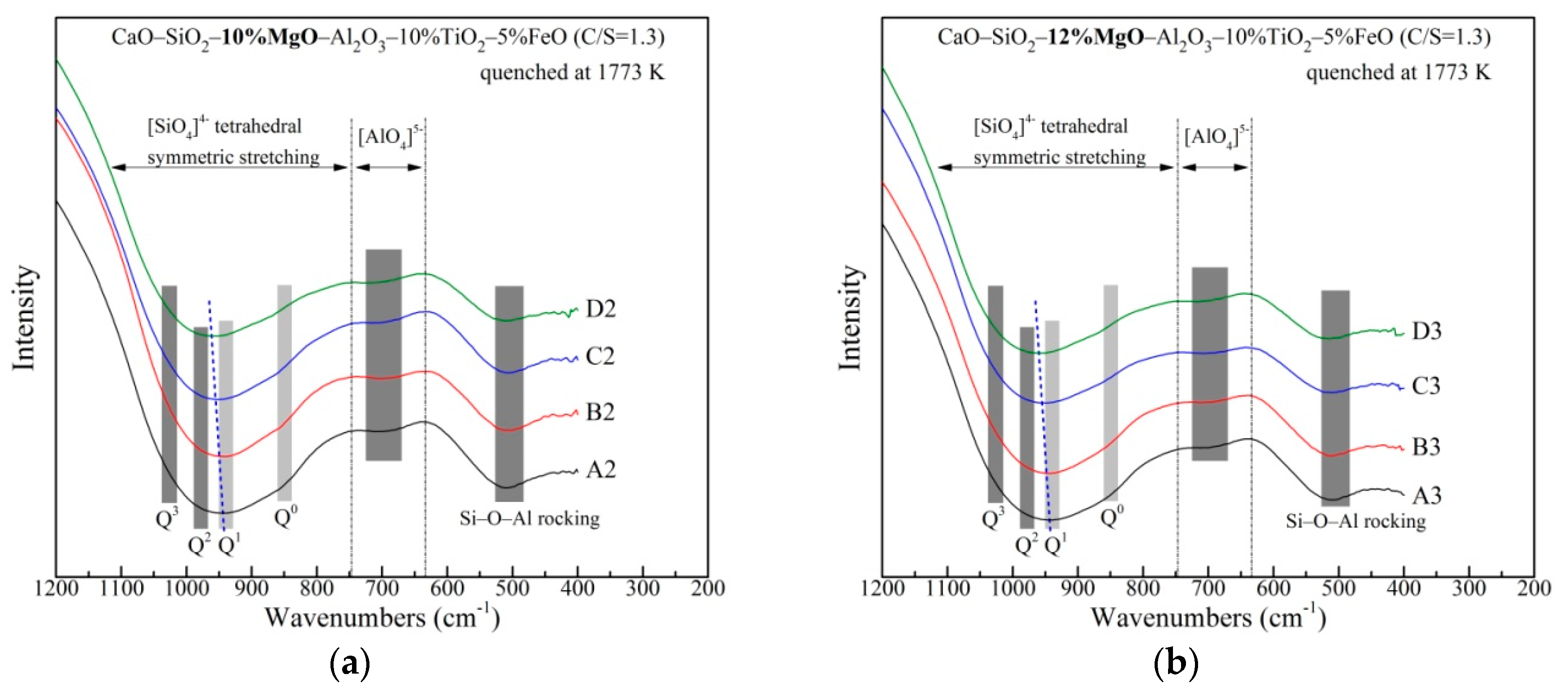
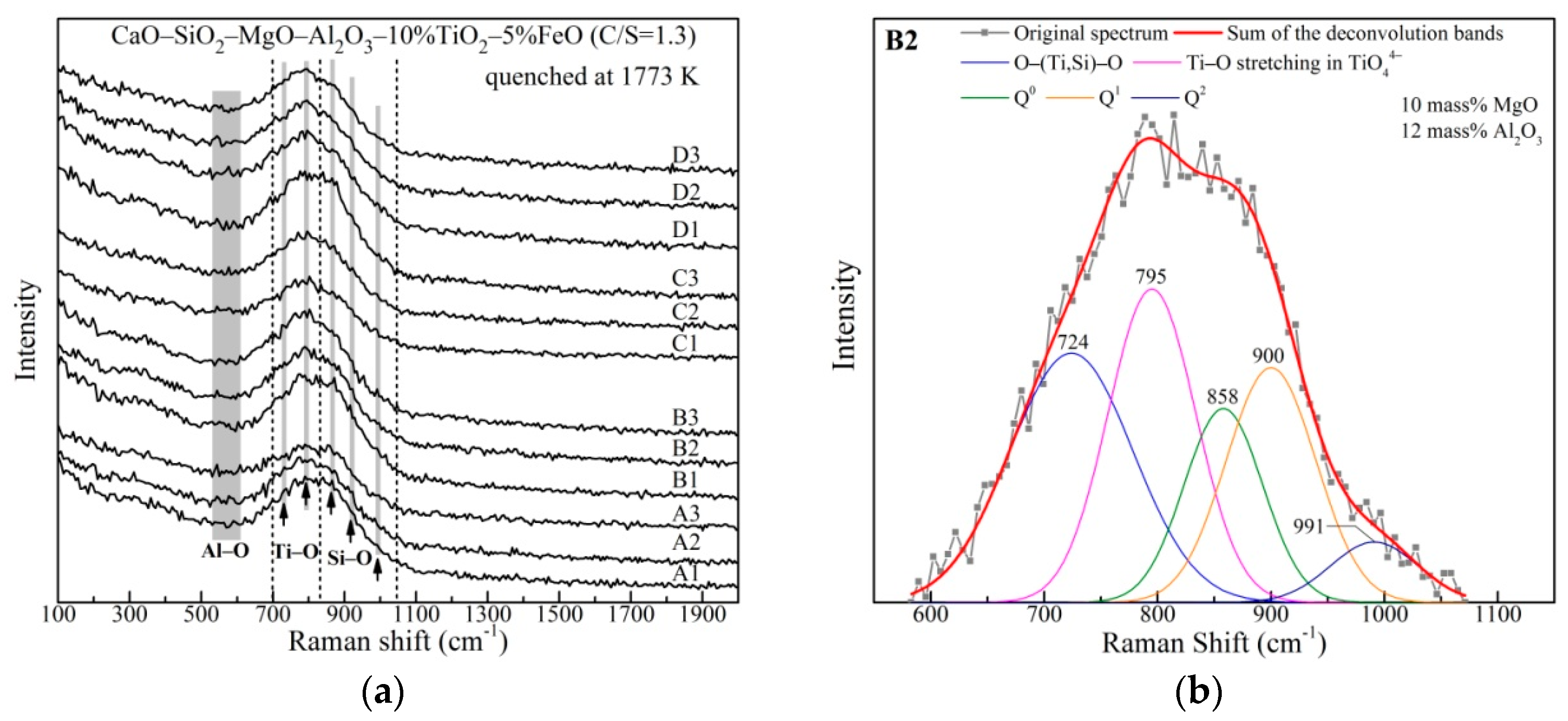
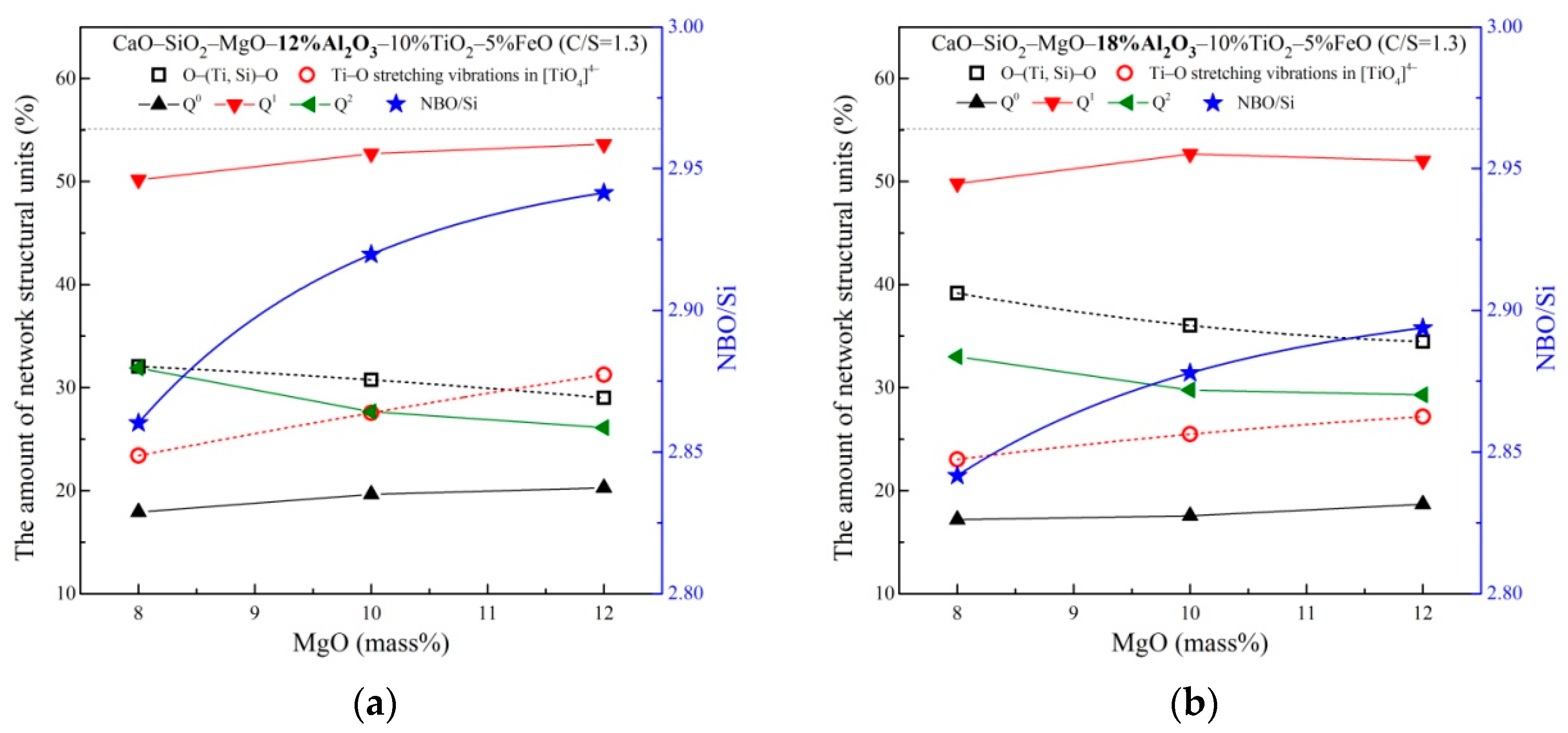
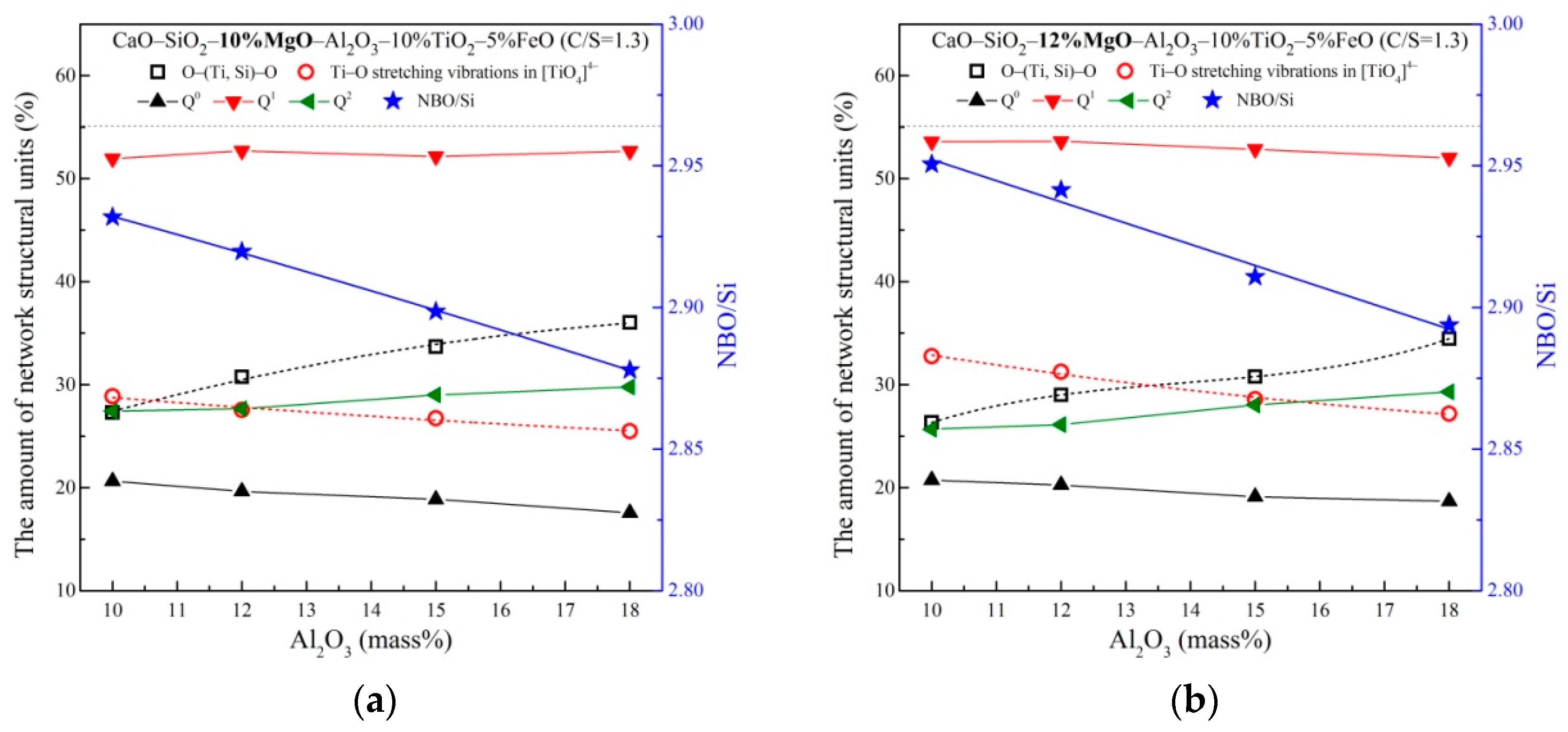
3.2. The Network Structure
4. Discussion
4.1. Correlation Between Viscosity and Network Structure
4.2. Interpretation of the Unusual Viscosity
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Z.S.; Davis, C. Ironmaking and Steelmaking. Metals 2019, 9, 525. [Google Scholar] [CrossRef]
- Zhou, X.L.; Luo, Y.H.; Chen, T.J.; Zhu, D.Q. Enhancing the reduction of high-aluminum iron ore by synergistic reducing with high-manganese iron ore. Metals 2019, 9, 15. [Google Scholar] [CrossRef]
- Feng, C.; Chu, M.S.; Tang, J.; Tang, Y.T.; Liu, Z.G. Effect of CaO/SiO2 and Al2O3 on viscous behaviors of the titanium–bearing blast furnace slag. Steel Res. Int. 2016, 87, 1274–1283. [Google Scholar] [CrossRef]
- Yan, Z.M.; Lv, X.W.; Zhang, J.; Qin, Y.; Bai, C.G. Influence of MgO, Al2O3 and CaO/SiO2 on the viscosity of blast furnace type slag with high Al2O3 and 5 wt% TiO2. Can. Metall. Quart. 2016, 55, 186–194. [Google Scholar] [CrossRef]
- Bian, L.T.; Gao, Y.H. Influence of Al2O3, CaO/SiO2, and B2O3 on viscous behavior of high alumina and medium titania blast furnace slag. J. Chem. 2017, 2017, 6895928. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, D.W.; Wang, H.F.; Xu, H.X. Research of influence of Al2O3 on metallurgical properties for the medium titanium slag. J. Mater. Metall. 2014, 13, 6–10, In Chinese. [Google Scholar]
- Feng, C.; Chu, M.S.; Tang, J.; Qin, J.; Li, F.; Liu, Z.G. Effects of MgO and TiO2 on the viscous behaviors and phase compositions of titanium–bearing slag. Int. J. Miner. Metall. Mater. 2016, 23, 868–880. [Google Scholar] [CrossRef]
- Liang, H.L.; Chu, M.S.; Feng, C.; Tang, J.; Liu, Z.G.; Wang, W.P. Optimisation study and affecting mechanism of CaO/SiO2 and MgO on viscous behaviours of titanium–bearing blast furnace slag. Ironmak. Steelmak. 2018, 45. [Google Scholar] [CrossRef]
- Tang, Z.H.; Ding, X.Y.; Dong, Y.; Liu, C.H.; Wei, G. Influence of w(MgO) on viscous flow property of high Ti-containing blast furnace slag. Chin. J. Mater. Res. 2016, 30, 443–447. (In Chinese) [Google Scholar] [CrossRef]
- Brawer, S.A.; White, W.B. Raman spectroscopic investigation of the structure of silicate glasses (II). soda-alkaline earth-alumina ternary and quaternary glasses. J. Non–Cryst. Solids. 1977, 23, 261–278. [Google Scholar] [CrossRef]
- Mysen, B.O.; Virgo, D.; Kushiro, I. The structural role of aluminium in silicate melts–A Raman spectroscopic study at 1 atmosphere. Am. Mineral. 1981, 66, 678–701. [Google Scholar]
- Li, T.L.; Sun, C.Y.; Lan, D.; Song, J.; Song, S.; Wang, Q. Effect of mineral elements migration on softening–melting properties of Ti–bearing high basicity sinter. ISIJ Int. 2019, 59, 245–252. [Google Scholar] [CrossRef]
- Li, T.L.; Sun, C.Y.; Song, S.; Wang, Q. Influences of Al2O3 and TiO2 content on viscosity and structure of CaO–8% MgO–Al2O3–SiO2–TiO2–5% FeO blast furnace primary slag. Metals 2019, 9, 743. [Google Scholar] [CrossRef]
- Park, J.H.; Min, D.J.; Song, H.S. FT-IR spectroscopic study on structure of CaO–SiO2 and CaO–SiO2–CaF2 slags. ISIJ Int. 2002, 42, 344–351. [Google Scholar] [CrossRef]
- Park, J.H.; Min, D.J.; Song, H.S. Structural investigation of CaO–Al2O3 and CaO–Al2O3–CaF2 slags via Fourier Transform Infrared Spectra. ISIJ Int. 2002, 42, 38–43. [Google Scholar] [CrossRef]
- Kim, J.R.; Lee, Y.S.; Min, D.J.; Jung, S.M.; Yi, S.H. Influence of MgO and Al2O3 contents on viscosity of blast furnace type slags containing FeO. ISIJ Int. 2004, 44, 1291–1297. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, C.S.; Sohn, I. Viscous behavior of alumina rich calcium–silicate based mold fluxes and its correlation to the melt structure. ISIJ Int. 2013, 53, 170–176. [Google Scholar] [CrossRef]
- Song, M.; Shu, Q.F.; Du, S.C. Viscosities of the quaternary Al2O3–CaO–MgO–SiO2 slags. Steel Res. Int. 2011, 82, 260–268. [Google Scholar] [CrossRef]
- Li, Z.R.; You, X.C.; Li, M.; Wang, Q.; He, S.P.; Wang, Q.Q. Effect of substituting CaO with BaO and CaO/Al2O3 ratio on the viscosity of CaO–BaO–Al2O3–CaF2–Li2O mold flux system. Metals 2019, 9, 142. [Google Scholar] [CrossRef]
- Hu, K.; Lv, X.W.; Li, S.P.; Lv, W.; Song, B.; Han, K.X. Viscosity of TiO2–FeO–Ti2O3–SiO2–MgO–CaO–Al2O3 for high–titania slag smelting process. Metall. Mater. Trans. B 2018, 49, 1963–1973. [Google Scholar] [CrossRef]
- Stevels, J.M. Glass as a high polymer. Verres Refract. 1953, 7, 91–104. [Google Scholar]
- Stevels, J.M. Networks in glasses and other polymers. Glass Ind. 1954, 35, 657–662. [Google Scholar]
- Min, D.J.; Tsukihashi, F. Recent advances in understanding physical properties of metallurgical slags. Met. Mater. Int. 2017, 23, 1–19. [Google Scholar] [CrossRef]
- Shen, X.; Chen, M.; Wang, N.; Wang, D. Viscosity property and melt structure of CaO–MgO–SiO2–Al2O3–FeO slag system. ISIJ Int. 2019, 59, 9–15. [Google Scholar] [CrossRef]
- Kim, J.B.; Sohn, I. Effect of SiO2/Al2O3 and TiO2/SiO2 ratios on the viscosity and structure of the TiO2–MnO–SiO2–Al2O3 welding flux system. ISIJ Int. 2014, 54, 2050–2058. [Google Scholar] [CrossRef]
- Park, H.; Park, J.Y.; Kim, G.H.; Sohn, I. Effect of TiO2 on the viscosity and slag structure in blast furnace type slags. Steel Res. Int. 2012, 83, 150–156. [Google Scholar] [CrossRef]
- Jiao, K.X.; Zhang, J.L.; Wang, Z.Y.; Chen, C.L.; Liu, Y.X. Effect of TiO2 and FeO on the viscosity and structure of blast furnace primary slags. Steel Res. Int. 2017, 88, 1600296. [Google Scholar] [CrossRef]
- Mysen, B.O.; Ryerson, F.J.; Virgo, D. The influence of TiO2 on the structure and derivative properties of silicate melts. Am. Mineral. 1980, 65, 1150–1165. [Google Scholar]
- Bihuniak, P.P.; Condrate, R.A. Structures, spectra and related properties of group IVB–doped vitreous silica. J. Non–Cryst. Solids. 1981, 44, 331–343. [Google Scholar] [CrossRef]
- Zhen, Y.L.; Zhang, G.H.; Chou, K.C. Influence of Al2O3/TiO2 ratio on viscosities and structure of CaO–MgO–Al2O3–SiO2–TiO2 melts. ISIJ Int. 2014, 54, 985–989. [Google Scholar] [CrossRef]
- Qiu, G.B.; Chen, L.; Zhu, J.Y.; Lv, X.W.; Bai, C.G. Effect of Cr2O3 addition on viscosity and structure of Ti-bearing blast furnace slag. ISIJ Int. 2015, 53, 1367–1376. [Google Scholar] [CrossRef]
- Mysen, B.O.; Virgo, D.; Seifert, F.A. Relationships between properties and structure of aluminosilicate melts. Am. Mineral. 1985, 70, 88–105. [Google Scholar]
- Mysen, B.O.; Virgo, D.; Scarfe, C.M.; Cronin, D.J. Viscosity and structure of iron-and aluminum-bearing calcium silicate melts at 1 atm. Am. Mineral. 1985, 70, 487–498. [Google Scholar]
- Sun, Y.Q.; Zhang, Z.T.; Liu, L.L.; Wang, X.D. FTIR, Raman and NMR investigation of CaO–SiO2–P2O5 and CaO–SiO2–TiO2–P2O5 glasses. J. Non–Cryst. Solids. 2015, 420, 26–33. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Wang, H.; Zhang, Z.T. Understanding the relationship between structure and thermophysical properties of CaO–SiO2–MgO–Al2O3molten slags. Metall. Mater. Trans. B 2018, 49, 677–687. [Google Scholar] [CrossRef]
- Shi, C.B.; Zheng, D.L.; Shin, S.H.; Li, J.; Cho, J.W. Effect of TiO2 on the viscosity and structure of low-fluoride slag used for electroslag remelting of Ti-containing steels. Int. J. Miner. Metall. Mater. 2017, 24, 18–24. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Chen, M.; Zhao, B.J. Modification of the microstructures of CaO–SiO2–Al2O3–MgO slags using various minor elements. J. Non–Cryst. Solids. 2019, 515, 50–57. [Google Scholar] [CrossRef]
- Frantza, J.D.; Mysen, B.O. Raman spectra and structure of BaO–SiO2, SrO–SiO2 and CaO–SiO2 melts to 1600 °C. Chem. Geol. 1995, 121, 155–176. [Google Scholar] [CrossRef]
- Feng, C.; Tang, J.; Gao, L.H.; Liu, Z.G.; Chu, M.S. Effects of CaO/SiO2 on viscous behaviors and structure of CaO–SiO2–11.00wt%MgO–11.00wt%Al2O3–43.00wt%TiO2 slag systems. ISIJ Int. 2019, 59, 31–38. [Google Scholar] [CrossRef]
- Mysen, B.O.; Richet, P. Silicate Glasses and Melts: Properties and Structure; Elsevier Science & Technology: Amsterdam, The Netherlands, 2005; pp. 372–381. [Google Scholar]
- Mudersbach, D.; Drissen, P.M.; Kühn, M.; Geiseler, J. Viscosity of slags. Steel Res. Int. 2001, 72, 86–90. [Google Scholar] [CrossRef]
- Kim, H.; Kim, W.H.; Sohn, I.; Min, D.J. The effect of MgO on the viscosity of the CaO–SiO2–20wt%Al2O3–MgO slag system. Steel Res. Int. 2010, 81, 261–264. [Google Scholar] [CrossRef]
- Sun, C.Y.; Liu, X.H.; Li, J.; Yin, X.T.; Song, S.; Wang, Q. Influence of Al2O3 and MgO on the viscosity and stability of CaO–MgO–SiO2–Al2O3 slags with CaO/SiO2=1.0. ISIJ Int. 2017, 57, 978–982. [Google Scholar] [CrossRef]
- Zhang, L.; Jahanshahi, S. Review and modeling of viscosity of silicate melts: Part II. viscosity of melts containing iron oxide in the CaO–MgO–MnO–FeO–Fe2O3–SiO2 system. Metall. Mater. Trans. B 1998, 29, 187–195. [Google Scholar] [CrossRef]
- Lee, S.; Min, D.J. Viscous behavior of FeO-bearing slag melts considering structure of slag. Steel Res. Int. 2018, 89, 1–6. [Google Scholar] [CrossRef]
- FactSage. Available online: http://www.factsage.com (accessed on 15 February 2018).
- Park, H.S.; Park, S.S.; Sohn, I. The viscous behavior of FeOt–Al2O3–SiO2 copper smelting slags. Metall. Mater. Trans. B 2011, 42, 692–699. [Google Scholar] [CrossRef]
| Slags | Designed | Analyzed | |||||
|---|---|---|---|---|---|---|---|
| CaO | SiO2 | MgO | Al2O3 | TiO2 | FeO | FeO | |
| A1 a | 37.87 | 29.13 | 8.00 | 10.00 | 10.00 | 5.00 | 4.62 |
| A2 | 36.74 | 28.26 | 10.00 | 10.00 | 10.00 | 5.00 | 4.86 |
| A3 | 35.61 | 27.39 | 12.00 | 10.00 | 10.00 | 5.00 | 4.69 |
| B1 a | 36.74 | 28.26 | 8.00 | 12.00 | 10.00 | 5.00 | 4.43 |
| B2 | 35.61 | 27.39 | 10.00 | 12.00 | 10.00 | 5.00 | 4.71 |
| B3 | 34.48 | 26.52 | 12.00 | 12.00 | 10.00 | 5.00 | 4.68 |
| C1 a | 35.04 | 26.96 | 8.00 | 15.00 | 10.00 | 5.00 | 4.71 |
| C2 | 33.91 | 26.09 | 10.00 | 15.00 | 10.00 | 5.00 | 4.54 |
| C3 | 32.78 | 25.22 | 12.00 | 15.00 | 10.00 | 5.00 | 4.60 |
| D1 a | 33.35 | 25.65 | 8.00 | 18.00 | 10.00 | 5.00 | 4.57 |
| D2 | 32.22 | 24.78 | 10.00 | 18.00 | 10.00 | 5.00 | 4.66 |
| D3 | 31.09 | 23.91 | 12.00 | 18.00 | 10.00 | 5.00 | 4.55 |
| Slag | MgO (mass%) | Al2O3 (mass%) | Eη (kJ/mol) |
|---|---|---|---|
| A1 | 8 | 10 | 92.9 |
| A2 | 10 | 10 | 90.5 |
| A3 | 12 | 10 | 86.0 |
| B1 | 8 | 12 | 85.3 |
| B2 | 10 | 12 | 63.3 |
| B3 | 12 | 12 | 88.2 |
| C1 | 8 | 15 | 140.4 |
| C2 | 10 | 15 | 110.4 |
| C3 | 12 | 15 | 91.7 |
| D1 | 8 | 18 | 116.4 |
| D2 | 10 | 18 | 113.1 |
| D3 | 12 | 18 | 109.1 |
| Raman Shift (cm−1) | Raman Assignments | References |
|---|---|---|
| 710~730 | O−(Ti, Si)−O deformation vibrations in sheet units | [28,29] |
| 790~810 | Ti−O stretching vibrations in [TiO4]4− monomers | [28,30,31] |
| 900~920 | Q0 | [32,33] |
| 840~860 | Q1 | [32,33] |
| 980~1000 | Q2 | [32,33,34] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Sun, C.; Song, S.; Wang, Q. Roles of MgO and Al2O3 on the Viscous and Structural Behavior of Blast Furnace Primary Slag, Part 1: C/S = 1.3 Containing TiO2. Metals 2019, 9, 866. https://doi.org/10.3390/met9080866
Li T, Sun C, Song S, Wang Q. Roles of MgO and Al2O3 on the Viscous and Structural Behavior of Blast Furnace Primary Slag, Part 1: C/S = 1.3 Containing TiO2. Metals. 2019; 9(8):866. https://doi.org/10.3390/met9080866
Chicago/Turabian StyleLi, Tingle, Changyu Sun, Sunny Song, and Qi Wang. 2019. "Roles of MgO and Al2O3 on the Viscous and Structural Behavior of Blast Furnace Primary Slag, Part 1: C/S = 1.3 Containing TiO2" Metals 9, no. 8: 866. https://doi.org/10.3390/met9080866
APA StyleLi, T., Sun, C., Song, S., & Wang, Q. (2019). Roles of MgO and Al2O3 on the Viscous and Structural Behavior of Blast Furnace Primary Slag, Part 1: C/S = 1.3 Containing TiO2. Metals, 9(8), 866. https://doi.org/10.3390/met9080866





