Abstract
The addition of ruthenium to tungsten carbide-cobalt hard metals improves their mechanical properties. Since ruthenium is a platinum group metal, the recovery of ruthenium together with cobalt from the scrap of hard metals is of great importance. In order to develop a recovery process of ruthenium and cobalt, separation experiments were performed from the synthetic HCl leaching solution of the scrap of hard metals. In this work, solvent extraction and ion exchange were employed to investigate the separation behavior of the two metal ions as a function of HCl concentration. Ru(III) was selectively extracted over Co(II) by Aliquat 336 (trioctyl methylammonium chloride) and Alamine 300 (tri-n-octyl amine) when HCl concentration was lower than 5 M. The highest separation factor between Ru(III) and Co(II) was obtained at 3 M HCl. The loaded Ru(III) was stripped from Aliquat 336 by dilute HCl solution. Only Ru(III) was loaded into the anion exchange resins employed in this work in the HCl concentration range from 1 to 9 M. The highest loading percentage of Ru(III) was obtained from 3 M HCl solution. The loading of Ru(III) into anion exchange resins followed Freundlich isotherm and the loading capacity of the resins were determined. The loaded Ru(III) was eluted by the mixture of HCl and thiourea. Compared to solvent extraction, ion exchange was found to be more efficient to separate Ru(III) and Co(II) from the HCl solution in terms of separation factor and the ease of operation.
1. Introduction
Tungsten carbide (WC) has not only high hardness and wear resistance at high temperatures but also good toughness. In manufa cturing hard metals, WC is surrounded by a ductile metal, such as cobalt, nickel or iron. In particular, Co is superior to Ni and Fe in improving the wettability of WC. Therefore, WC-Co hard metals with the hexagonal structure are widely used as cutting and abrasion tools.
It has been found that the addition of platinum group metals (PGMs) can improve the mechanical and chemical properties of the WC-Co hard metals. Among PGMs, many studies have been done on the effect of the addition of ruthenium (Ru) [1,2,3,4,5]. Ruthenium reduces the stacking fault energy of the cobalt phase and thus promotes the transformation of the cobalt structure from cubic to hexagonal, resulting in precipitation hardening of the binder phase [4,6]. Therefore, the addition of Ru to WC-Co improves its mechanical properties without the loss of toughness [3].
Ruthenium, tungsten, and cobalt are valuable metals. Therefore, it is necessary to recover these metals from WC-Co scrap and spent WC-Co. Many works have been reported on the recovery of cobalt and tungsten from the WC-Co scrap without ruthenium. However, no work has been reported on the recovery of ruthenium and cobalt from the WC-Co scrap containing ruthenium within our knowledge. In the hydrometallurgical treatment, the WC-Co hard metals can be dissolved by employing either an acid or alkaline solution [7,8]. In acid leaching of WC-Co, tungsten can be separated by precipitation as tungstic acid. Cobalt would be dissolved in the alkaline solution in the presence of ammonia and the supply of oxygen gas. Moreover, it is necessary to employ alkaline molten salts treatment to dissolve ruthenium in the alkaline solution. Therefore, acid leaching is considered to be more efficient than alkaline leaching in recovering valuable metals from the scrap of hard metals containing ruthenium.
In this work, solvent extraction and ion exchange experiments were done to investigate the separation of Ru(III) and Co(II) from the hydrochloric acid solution. For this purpose, amine extractants (Alamine 300 and Aliquat 336) and anion exchange resins (Amberlite XAD-7HP, AG 1-X8, Amberlite IRA 402, Bonite BA 304, and Lewatit MP-64) were employed. The extraction and loading behavior of the two metal ions were investigated by varying the concentration of HCl, extractants and resins. Ion exchange was found to be more efficient than solvent extraction in separating Co(II) and Ru(III) from hydrochloric acid solutions in terms of separation factor and the ease of operation. Moreover, 3 M HCl concentration was the optimum condition to selectively separate Ru(III) by solvent extraction and ion exchange.
2. Materials and Methods
2.1. Reagents and Chemicals
The synthetic solution was prepared by dissolving RuCl3 (99.9%, Sigma-Aldrich) and CoCl2·6H2O (99%, Junsei, Tokyo, Japan) in hydrochloric acid solution (35%, Daejung Chemical & Metals Co., Ltd., Siheung-si, Korea). Doubly distilled water was used in the experiments. In the synthetic solution, the concentration of Co(II) was fixed at 100 mg/L, while that of Ru(III) was varied from 10 to 100 mg/L. 10 v/v% of H2O2 (30%, Daejung Chemical & Metals Co., Ltd., Siheung-si, Korea). Thiourea (96%, Daejung Chemical & Metals Co., Ltd., Siheung-si, Korea), acetone (99.8%, Daejung Chemical & Metals Co., Ltd., Siheung-si, Korea), NaOH (97%, Daejung Chemical & Metals Co., Ltd., Siheung-si, Korea) and nitric acid (60%, Daejung Chemical & Metals Co., Ltd., Siheung-si, Korea) were used for stripping experiments.
Alamine 300 (97%, Samchun Pure Chemical Co., Pyeongtaek-si, Korea) and Aliquat 336 (99%, BASF Co., Ludwigshafen, Germany) were employed in solvent extraction experiments. Kerosene (99.9%, Daejung Chemical & Metals Co., Ltd., Siheung-si, Korea) was used as a diluent. Anionic exchange resins were employed in the experiments, such as Amberlite XAD-7HP (Sigma-Aldrich, St. Louis, MO, USA), AG®1-X8 (Bio-Rad, Hercules, CA, USA), Amberlite® IRA402 (Dow Chemical Company, Midland, MI, USA), Bonlite®BA304 (Born Chemical Co., Ltd.) and Lewatit® MP-64 (Lanxess Energizing Chemistry). The properties of these resins are shown in Table 1 [9]. The above extractants and ion exchange resins were used without any pretreatment.

Table 1.
Physical properties of the resins used in this study.
2.2. Solvent Extraction and Ion Exchange Experimental Procedures
The synthetic aqueous solution was prepared by dissolving the reagent grade RuCl3 and CoCl2 in dilute HCl solution. The concentration of the extractants was controlled by diluting them with kerosene. The extraction and stripping experiments were carried out by contacting equal volume (10 mL) of the aqueous and organic phases at unity phase ratio for 40 min using a wrist action shaker (Burrel, Burrel Scientific, PA, USA) at ambient temperature. After the disengagement of the two phases, the concentration of metals in the aqueous phase was measured by ICP-OES (Spectro Arcos model) and metal concentration in the organic phase was calculated by mass balance [10].
In ion exchange experiments, the synthetic solution and ion exchange resins were put in a 50 mL bottle, and the mixture was stirred at room temperature for 6 h. In these experiments, an incubator (HB-201SF, Hanbaek Scientific Co., Bucheon, Korea) was employed to control the ambient temperature and stirring speed. The stirring speed was fixed at 200 rpm. After the adsorption and elution experiments, the ion exchange resins were separated by filter paper (Advantec No. 2: 110 mm).
3. Results and Discussion
3.1. Leaching of W, Co, Ru with HCl Solution
There are some reports on the HCl leaching of WC-Co hard metals without ruthenium. Therefore, the reported optimum condition for the leaching of these WC-Co hard metals was employed to investigate the leaching percentage of WC-Co hard metals containing ruthenium [11,12,13]. The leaching conditions are as follows: 5 M HCl, reaction temperature of 70 °C, stirring speed of 200 rpm, pulp density of 3 g/L and reaction time of 5 h. In order to enhance the leaching of ruthenium and cobalt, 10 vol% of H2O2 was added to the leaching solution as an oxidizing agent. The leaching percentage of Co(II) and Ru(III) was 98 and 95% at the above leaching conditions. The concentration of Co(II) and Ru(III) was 98 and 16 mg/L, while that of W(VI) was only 3 mg/L. Therefore, it can be said that most of W present in WC-Co alloy can be separated by leaching with HCl solution. Since the concentration of W(VI) in the leaching solution was very small, the synthetic leaching solution was prepared by dissolving only Co(II) and Ru(III) and this solution was employed in all of the experiments.
3.2. Separation of Ru(III) and Co(II) by Solvent Extraction
3.2.1. Extraction of Ru(III) and Co(II) by Amine Extractants
Ru(III) and Co(II) have a strong tendency to form complexes with chloride ion. Therefore, HCl concentration affects the nature of the predominant species of Ru(III) and Co(II). Most of Ru(III) exists as anionic complexes when HCl concentration is higher than 2 M, while anionic species of Co(II) can form when HCl concentration is higher than 5 M [14,15]. Therefore, the effect of HCl concentration on the extraction of the two metal ions was first investigated by varying HCl concentration from 1 to 9 M. In these experiments, the concentration of Ru(III) and Co(II) was fixed at 10 and 100 mg/L. Figure 1 shows that the extraction behavior of Ru(III) and Co(II) were different from each other. Moreover, the extraction percentage of Ru(III) and Co(II) by Aliquat 336 was higher than that by Alamine 300 in our experimental range. In the case of Ru(III), maximum extraction percentage occurred at 3 M HCl by Aliquat 336 and Alamine 300. The extraction percentage of Ru(III) by both extractants decreased rapidly as HCl concentration increased from 3 M to 9 M. By contrast, the extraction percentage of Co(II) increased rapidly as HCl concentration increased from 3 M to 9 M. Since HCl concentration affects the extraction percentage of both metal ions, the separation factor between Ru(III) and Co(II) also depends on HCl concentration.
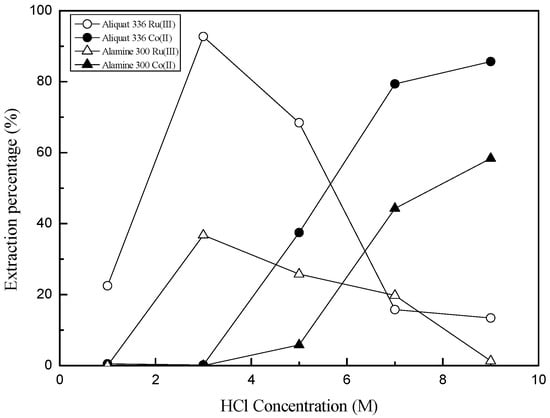
Figure 1.
Effect of HCl concentration on the extraction of Co(II) and Ru(III) by Alamine 300 and Aliquat 336. ([Extractant] = 0.1 M, [Co(II)] = 100 mg/L and [Ru(III)] = 10 mg/L).
In HCl solution, Ru(III) forms several kinds of anionic complexes with chloride ion. These anionic complexes of Ru(III) can be extracted by amines. The fact that the highest extraction percentage of Ru(III) by Aliquat 336 and Alamine 300 occurred at 3 M HCl might be ascribed to the distribution of Ru(III)-chloro complexes with HCl concentration [15]. Ru(III) forms octahedral complexes with H2O and Cl− coordinated. As HCl concentration increases, the H2O in coordination sites of the complexes is replaced with chloride ion. The mole fraction of [RuCl4(H2O)2]− is highest at 3 M HCl solution and the predominant species of Ru(III) becomes RuCl63− when HCl concentration is higher than 6 M [15]. Our extraction data on Ru(III) by the two amines indicate that [RuCl4(H2O)2]− takes part in the solvent extraction with Aliquat 336 and Alamine 300. Co(II) begins to form CoCl42− when HCl concentration is higher than 5 M, and most of the Co(II) exists as CoCl42− at 8 M HCl [14]. The extraction data of Co(II) shown in Figure 1 indicate that CoCl42− is extracted by Aliquat 336 and Alamine 300 [15,16,17,18].
The different dependence of the extraction of Ru(III) and Co(II) on HCl concentration indicates that control of HCl concentration is very important for the separation of the two metal ions by solvent extraction. When HCl concentration was 3 M, only Ru(III) was extracted by Aliquat 336 and Alamine 300. By contrast, a small amount of Ru(III) was extracted together with Co(II) when HCl concentration was higher than 7 M.
The effect of Ru(III) concentration was investigated by varying Ru(III) concentration from 10 to 100 mg/L. In these experiments, the concentration of HCl and Co(II) was fixed at 3 M and 100 mg/L, respectively. Figure 2 shows that Ru(III) was selectively extracted over Co(II) from 3 M HCl solution as long as the concentration ratio of Ru(III) to Co(II) was less than one.
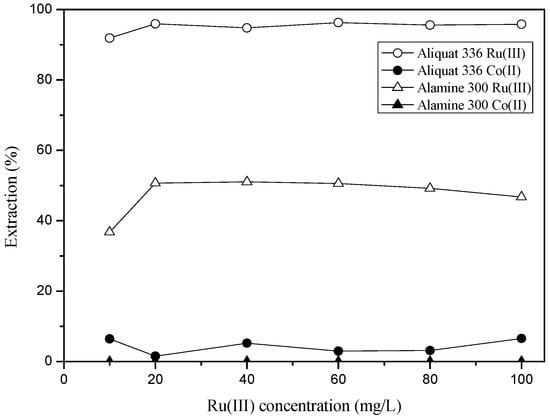
Figure 2.
Effect of Ru(III) concentration on the separation of Co(II) from 3 M HCl solution. ([Extractant] = 0.1 M and [Co(II)] = 100 mg/L).
The extraction percentage of Ru(III) by Aliquat 336 was higher than that by Alamine 300. Therefore, the effect of Aliquat 336 concentration was investigated by varying its concentration from 0.05 M to 0.7 M (see Figure 3). In these experiments, the concentration of Ru(III) and Co(II) in 3 M HCl solution was 10 and 100 mg/L, respectively. Within Aliquat 336 concentration from 0.05 M to 0.7 M, the plot of log [Aliquat 336] vs. log D of Ru(III) and Co(II) results in linear lines whose slope values were close to unity. The slope values of Figure 3 indicate that one mole of Aliquat 336 takes part in the solvent extraction of one mole of Ru(III) and Co(II) from 3 M HCl solution. Since the distribution coefficient of Co(II) increased linearly with Aliquat 336 concentration, it is better to reduce Aliquat 336 concentration and increase the number of counter-current stages in terms of the separation feasibility.
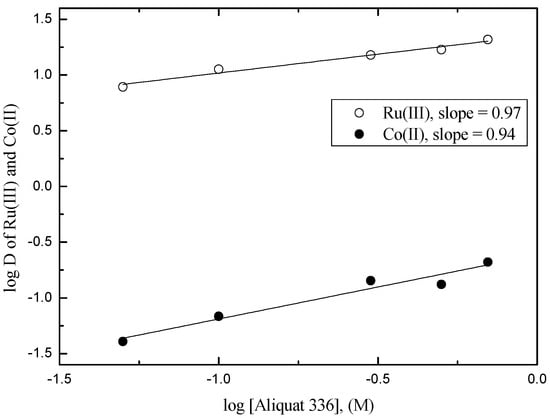
Figure 3.
Plot of log [Aliquat 336] vs. log D of Ru(III) and Co(II) from 3 M HCl solution. ([Ru(III)] = 10 mg/L and [Co(II)] =100 mg/L).
The extraction behavior of Ru(III) by the two amines was investigated to identify the existence of polymerized Ru(III) species in the organic phase. For this purpose, the concentration of HCl was fixed at 3 M and then that of Ru(III) was varied from 10 to 100 mg/L. The log vs. log plot of the concentration of Ru(III) in the aqueous and organic phase is shown in Figure 4. The slopes of the straight lines were around one, indicating that the Ru(III) in the loaded organic exists as a monomer. Based on these results together with the data in Figure 3, the solvent extraction reaction of Ru(III) by Alamine 300 and Aliquat 336 can be represented as Equations (1) and (2), respectively [19,20,21].
R3NH+Cl−(org) + [RuCl4(H2O)2]− = R3NH+[RuCl4(H2O)2]−(org) + Cl−,
R3NCH3+Cl−(org) + [RuCl4(H2O)2]− = R3NCH3+[RuCl4(H2O)2]−(org) + Cl−,
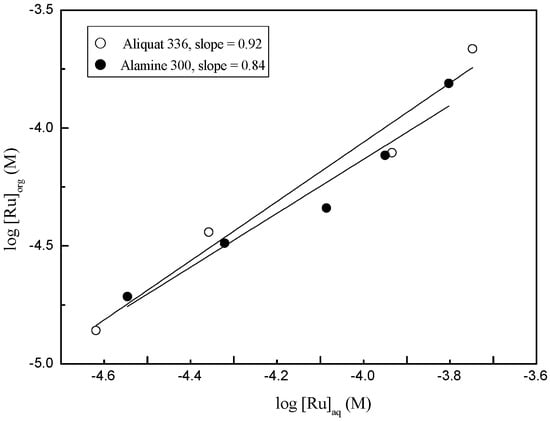
Figure 4.
Identification of the extracted species of Ru(III) into Alamine 300 and Aliquat 336 from 3 M HCl solution. ([Extractant] = 0.01 M and [Ru(III)] =10–100 mg/L).
3.2.2. Stripping of Ru(III) from the Loaded Aliquat 336
Extraction results showed that Ru(III) could be selectively extracted over Co(II) from 3 M HCl solution by Aliquat 336. Therefore, stripping experiments were done from the loaded Aliquat 336. The loaded Aliquat 336 was prepared by extracting the aqueous solution with 10 mg/L Ru(III) and 100 mg/L Co(II). Aliquat 336 concentration was 0.1 M and 10 v/v% TBP (Tributyl phosphate) was added to prevent the formation of a third phase. Figure 1 showed that the extraction of Ru(III) was the highest at 3 M HCl concentration. This indicates that weak HCl solution can strip Ru(III) from the loaded Aliquat 336. Therefore, HCl concentration was varied from 0.01 M to 3 M. As expected, the stripping percentage of Ru(III) from the loaded Aliquat 336 decreased rapidly to zero as HCl concentration increased from 0.01 M to 3 M (see Figure 5).
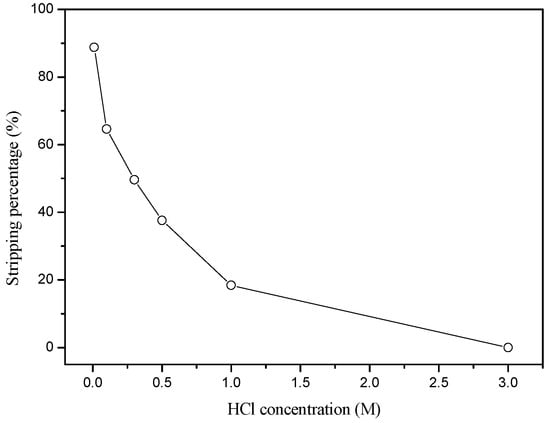
Figure 5.
Effect of HCl concentration on the stripping of Ru(III) from the loaded Aliquat 336.
When HCl concentration is too low, there might be some change in the predominant species of Ru(III). Therefore, 0.1 M HCl was selected as an optimum stripping solution and a McCabe-Thiele stripping diagram was constructed. The volume ratio of aqueous to organic was varied from 10:1 to 1:3 and the corresponding McCabe-Thiele diagram is shown in Figure 6. Three stages of counter-current stripping are necessary to strip the Ru(III) completely from the loaded Aliquat 336 at an O/A ratio of two.
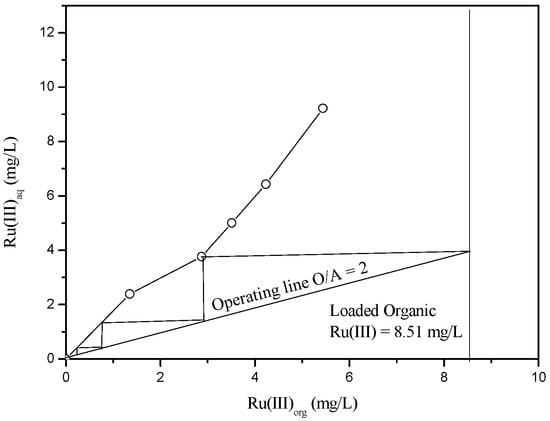
Figure 6.
McCabe-Thiele diagram for the stripping of Ru(III) from the loaded Aliquat 336 by 0.1 M HCl.
3.3. Ru(III) and Co(II) Separation by Ion Exchange
3.3.1. Adsorption of Ru(III) and Co(II) into Anionic Exchange Resins
In order to investigate the possibility of separating Ru(III) and Co(II) by ion exchange, the HCl concentration was varied from 1 M to 9 M. In these experiments, the concentration of Ru(III) and Co(II) was fixed at 10 and 100 mg/L, respectively, while the concentration of the anion exchange resins (AG 1-X8, Amberlite IRA 402, Amberlie XAD 7HP, Bonite BA 304, and Lewatit MP-64) was fixed at 0.5 g/L. The loading percentage of Ru(III) by the anion resins was the highest when HCl concentration was 3 M HCl, which is the same as the solvent extraction results of Ru(III) (see Figure 7). Unlike solvent extraction results, Co(II) was not loaded at all into the above resins in our experimental ranges. Even though HCl concentration was higher than 6 M, Co(II) was not loaded into the resins. The loading percentage of Ru(III) by AG 1X-8 and Lewatit MP-64 from 3 M HCl solution was the same and the ion exchange reaction can be represented as Equation (3) [9,22].
RCH2N+(CH3)Cl−(resin) + [RuCl4(H2O)]− = RCH2N+(CH3)[RuCl4(H2O)]−(resin) + Cl−,
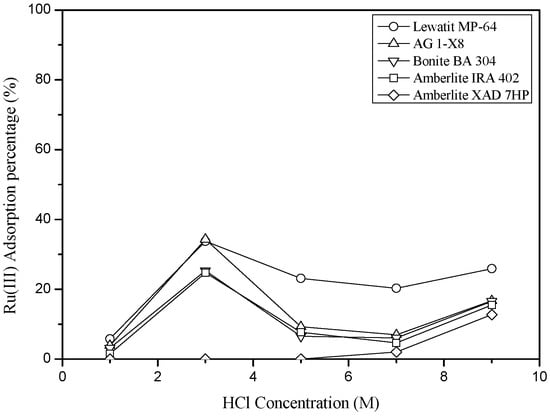
Figure 7.
Effect of HCl concentration on the adsorption of Ru(III) and Co(II) onto the anion exchange resins. ([Time] = 6 h, [Resin] = 0.5 g/L, [Co(II)] = 100 mg/L and [Ru(III)] = 10 mg/L).
3.3.2. Loading Behavior of Ru(III) and Co(II) into the Resins at 3M HCl
Effect of resin concentration on the loading of Ru(III) and separation of the two metal ions was investigated from 3 M HCl solution. The concentration of Ru(III) and Co(II) was 10 and 100 mg/L, respectively. Figure 8 shows the variation in the loading percentage of Ru(III) and Co(II) as the concentration of ion exchange resins increased from 0.5 g/L to 10 g/L. In these experiments, no Co(II) was loaded but the loading percentage of Ru(III) increased rapidly until 4 g/L and then remained constant as the resin concentration increased to 10 g/L.
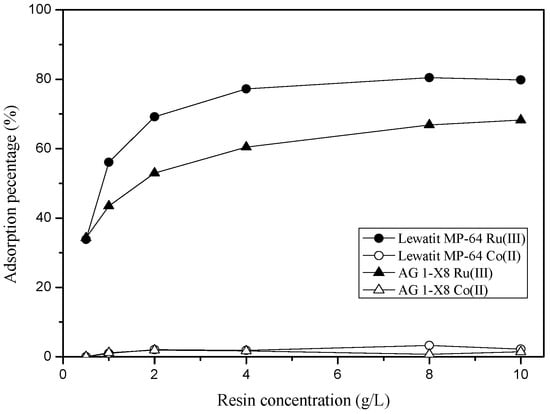
Figure 8.
Effect of resin concentration on the adsorption of Co(II) and Ru(III) onto AG 1-X8 and Lewatit MP-64. ([Time] = 6 h, [Co(II)] = 100 mg/L and [Ru(III)] = 10 mg/L).
Loading capacity of AG 1-X8 and Lewatit MP-64 for Ru(III) was measured by consecutively contacting fresh aqueous solutions. In these experiments, the concentration of the resins was fixed at 0.5 g/L. Figure 9 shows the cumulative mass of Ru(III) loaded into the two resins. The loading capacity of AG 1-X8 and Lewatit MP-64 was determined to be 27.1 and 28.7 mg of Ru(III) by 1 g of resin, respectively.
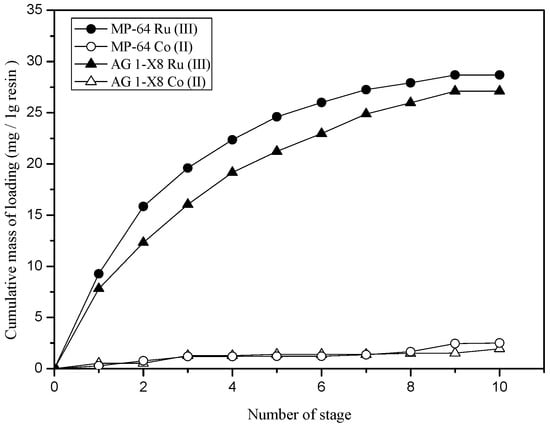
Figure 9.
Determination of loading capacity of AG 1-X8 and Lewatit MP-64 resins for Ru(III). ([Time] = 6 h, [Resin] = 0.5 g/L, [Co(II)] = 100 mg/L and [Ru(III)] = 10 mg/L).
In order to identify the loading isotherm of Ru(III) into the AG 1-X8 and Lewatit MP-64 resins, Freundlich isotherm was plotted from the loading data obtained from 3 M HCl. Figure 10 indicates that the loading of Ru(III) into the two resins follow the Freundlich isotherm, which can be represented as Equation (4) [23].
where q is the mass of Ru(III) loaded on the resin, CA is the concentration of Ru(III) in solution after adsorption, K and n are constants. From the values of the slope in Figure 10, the values of n for AG 1-X8 and Lewatit MP-64 was determined to be 0.86 and 1.02, respectively.
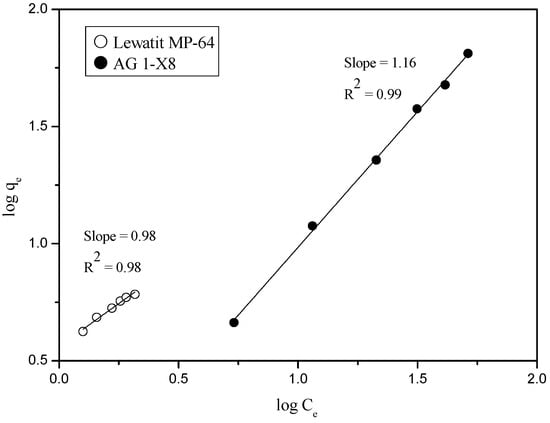
Figure 10.
Verification of Freundlich isotherm for the adsorption of Ru(III) onto AG 1-X8 and Lewatit MP-64.
3.3.3. Elution of Ru(III) from the Loaded Resin
Table 2 lists the elution results of Ru(III) from the loaded AG 1-X8 and Lewatit MP-64 resins by several eluants employed in this study. Since no Co(II) was loaded into these two resins, only the elution percentage of Ru(III) was reported. The elution behavior of Ru(III) from the two resins by the eluants was similar to each other. The highest elution percentage of Ru(III) was obtained from the mixture of HCl and thiourea. According to the HSAB (Hard and Soft Acids and Bases) principle, Ru(III) is a soft acid and thus has a strong tendency to react with thiourea, a soft base [24]. The elution percentage of Ru(III) by single thiourea was lower than that by the mixture of thiourea and HCl. This can be ascribed to the formation of a complex between the eluted Ru(III) and chloride ion, which enhances the elution reaction.

Table 2.
Elution percentage of Ru(III) from the adsorbed AG 1-X8 and Lewatit MP-64.
4. Conclusions
The addition of ruthenium to WC-Co metals improves their mechanical properties, such as hardness, wear resistance and toughness. In order to develop a process for the recovery of ruthenium and cobalt from the scrap, solvent extraction and ion exchange experiments were done. For this purpose, a synthetic solution containing Ru(III) and Co(II) was employed in the experiments. In solvent extraction with Aliquat 336 and Alamine 300, Ru(III) was selectively extracted over Co(II) when HCl concentration was less than 5 M, while Co(II) was selectively extracted at higher HCl concentration. The extraction percentage of the metals by Aliquat 336 was higher than that by Alamine 300. 3 M HCl solution was found to be the optimum condition to separate Ru(III) and Co(II). In separating these two metal ions by Aliquat 336 from 3 M HCl solution, control of Aliquat 336 concentration was very important to suppress the extraction of Co(II). The loaded Ru(III) in Aliquat 336 was successfully stripped by dilute HCl solution. The McCabe-Thiele diagram for the stripping of Ru(III) from Aliquat 336 was constructed.
In the HCl concentration range of 1 to 9 M, only Ru(III) was loaded into the anion exchange resins employed in this work (AG 1-X8, Amberlite IRA 402, Amberlie XAD 7HP, Bonite BA 304, and Lewatit MP-64). Since Co(II) remained in the effluent, it was possible to completely separate the metal ions. Highest loading percentage of Ru(III) was obtained from 3 M HCl. The loading capacity of AG 1-X8 and Lewatit MP-64 for Ru(III) was measured to be 27.1 and 28.7 mg/g. The loading of Ru(III) into AG 1-X8 and Lewatit MP-64 followed Freundlich adsorption isotherm. The Ru(III) loaded into AG 1-X8 and Lewatit MP-64 was eluted by the mixture of HCl and thiourea.
Our results showed that ion exchange was better than solvent extraction in separating Ru(III) and Co(II) from the HCl solution. A process can be developed to recover Co(II), Ru(III) and W(VI) from the WC-Co hard metals containing ruthenium by leaching with HCl solution followed by ion exchange.
Author Contributions
M.S.L. designed the research and helped to analyze the data. H.H.A. performed the experiments.
Acknowledgments
This Research was funded by National Research Foundation of Korea, grant number 2018R1D1A1B07044951. We gratefully thank the Gwangju branch of the Korea Basic Science Institute (KBSI) for ICP data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lisovskii, A.F. Cemented carbides alloyed with ruthenium, osmium and rhenuim. Powder Metall. Met. Ceram. 2000, 39, 9–10. [Google Scholar] [CrossRef]
- Luyckx, S. High temperature hardness of WC-Co-Ru. J. Mater. Sci. Lett. 2002, 21, 1681–1682. [Google Scholar] [CrossRef]
- García, J.; Collado Ciprés, V.; Blomqvist, A.; Kaplan, B. Cemented carbide microstructures: A review. Int. J. Refract. Met. Hard Mat. 2019, 80, 40–68. [Google Scholar] [CrossRef]
- Shing, T.L.; Luyckx, S.; Northrop, I.T.; Wol, I. The effect of ruthenium additions on the hardness, toughness and grain size of Wc-Co. Int. J. Refract. Met. Hard Mat. 2001, 19, 41–44. [Google Scholar] [CrossRef]
- Potgieter, J.H.; Thanjekwayo, N.; Olubambi, P.; Maledi, N.; Potgieter-Vermaak, S.S. Influence of Ru additions on the corrosion behaviour of WC–Co cemented carbide alloys in sulphuric acid. Int. J. Refract. Met. Hard Mat. 2011, 29, 478–487. [Google Scholar] [CrossRef]
- Potgieter, J.H.; Olubambi, P.; Potgieter-Vermaak, S.S. The corrosion behaviour of WC-Co-Ru alloys in aggressive chloride media. Int. J. Electrochem. 2014, 2014, 1–11. [Google Scholar] [CrossRef][Green Version]
- Shemi, A.; Magumisea, A.; Ndlovua, S.; Sacks, N. Recycling of tungsten carbide scrap metal: A review of recycling methods and future prospects. Miner. Eng. 2018, 122, 195–205. [Google Scholar] [CrossRef]
- Martins, J.I. Leaching systems of wolframite and scheelite: A thermodynamic approach. Miner. Process Extr. Metall. Rev. 2013, 35, 23–43. [Google Scholar] [CrossRef]
- Xing, W.D.; Lee, M.S. Recovery of gold(iii) from the stripping solution containing palladium(ii) by ion exchange and synthesis of gold particles. J. Ind. Eng. Chem. 2019, 69, 255–262. [Google Scholar] [CrossRef]
- Wang, L.Y.; Lee, M.S. Separation of Co(II) and Ni(II) from chloride leach solution of nickel laterite ore by solvent extraction with cyanex 301. Int. J. Miner. Process. 2017, 166, 45–52. [Google Scholar] [CrossRef]
- Katiyara, P.K.; Randhawa, N.S.; Hait, J.; Jana, R.K.; Singh, K.K.; Mankhand, T.R. An overview on different processes for recovery of valuable metals from tungsten carbide scrap. In Proceedings of the 18th International Conference on Nonferrous Minerals and Metals, Nagpur, India, 11–12 July 2014. [Google Scholar]
- Farrell, G.; Anderson, D.M.; Walton, M.E. Tungsten Recovery from Carbides. US Patent 4533527, 6 August 1985. [Google Scholar]
- Reilly, K.T. Recovery of Refractory Metal Values from Scrap Cemented Carbide. US Patent 4406866, 27 September 1983. [Google Scholar]
- Wellens, S.; Thijs, B.; Binnemans, K. An environmentally friendlier approach to hydrometallurgy: Highly selective separation of cobalt from nickel by solvent extraction with undiluted phosphonium ionic liquids. Green Chem. 2012, 14, 1657–1665. [Google Scholar] [CrossRef]
- Viljoen, K. The Interconversion of the Hexachlororuthenate(iii) and Aquapentachlororuthenate(iii) Species. Master’s Thesis, University of Stellenbosch, Stellenbosch, South Africa, 2003. [Google Scholar]
- Filiz, M.; Sayar, A.A.; Sayar, A.A. Extraction of cobalt(ii) from aqueous hydrochloric acid solutions into alamine 336–m-xylene mixtures. Hydrometallurgy 2006, 81, 167–173. [Google Scholar] [CrossRef]
- Banda, R.; Sohn, S.H.; Lee, M.S. Solvent extraction separation of Mo and Co from chloride solution containing Al. Mater. Trans. 2013, 54, 61–65. [Google Scholar] [CrossRef]
- Shen, Y.F.; Xue, W.Y.; Niu, W.Y. Recovery of Co(II) and Ni(II) from hydrochloric acid solution of alloy scrap. Trans. Nonferrous Met. Soc. 2008, 18, 1262–1268. [Google Scholar] [CrossRef]
- Goralska, E.; Coll, M.T.; Fortuny, A.; Kedari, C.S.; Sastre, A.M. Studies on the selective separation of Ir(IV), Ru(III) and Rh(III) from chloride solutions using alamine 336 in kerosene. Solvent Extr. Ion Exch. 2007, 25, 65–77. [Google Scholar] [CrossRef]
- Panigrahi, S.; Dash, T.; Nathsarma, K.C.; Sarangi, K. Extraction of ruthenium using both tertiary and quaternary amine from chloride media. Sep. Sci. Technol. 2014, 49, 545–552. [Google Scholar] [CrossRef]
- Kedari, S.; Col, M.T.; Fortuny, A.; Goralska, E.; Sastre, A.M. Liquid-liquid extraction of ir, ru and rh from chloride solutions and their separation using different commercially available solvent extraction reagents. Sep. Sci. Technol. 2005, 40, 1927–1946. [Google Scholar] [CrossRef]
- Lee, S.H.; Yoo, J.H.; Kim, J.H. Ion exchange characteristics of rhodium and ruthenium from a simulated radioactive liquid waste. Korean J. Chem. Eng. 2004, 5, 1038–1043. [Google Scholar] [CrossRef]
- Gao, N.; Liu, D.; Zhang, G.; Zhang, J.; Jia, S.; Yu, C.; Jiang, K.; Gao, N. The role of lactic acid adsorption by ion exchange chromatography. PLoS ONE 2010, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Miessler, G.L.; Fischer, P.J.; Tarr, D.A. Inorganic Chemistry; Pearson Education: London, UK, 2014; pp. 205–214. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).