Comprehensive Properties of a Novel Quaternary Sn-Bi-Sb-Ag Solder: Wettability, Interfacial Structure and Mechanical Properties
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
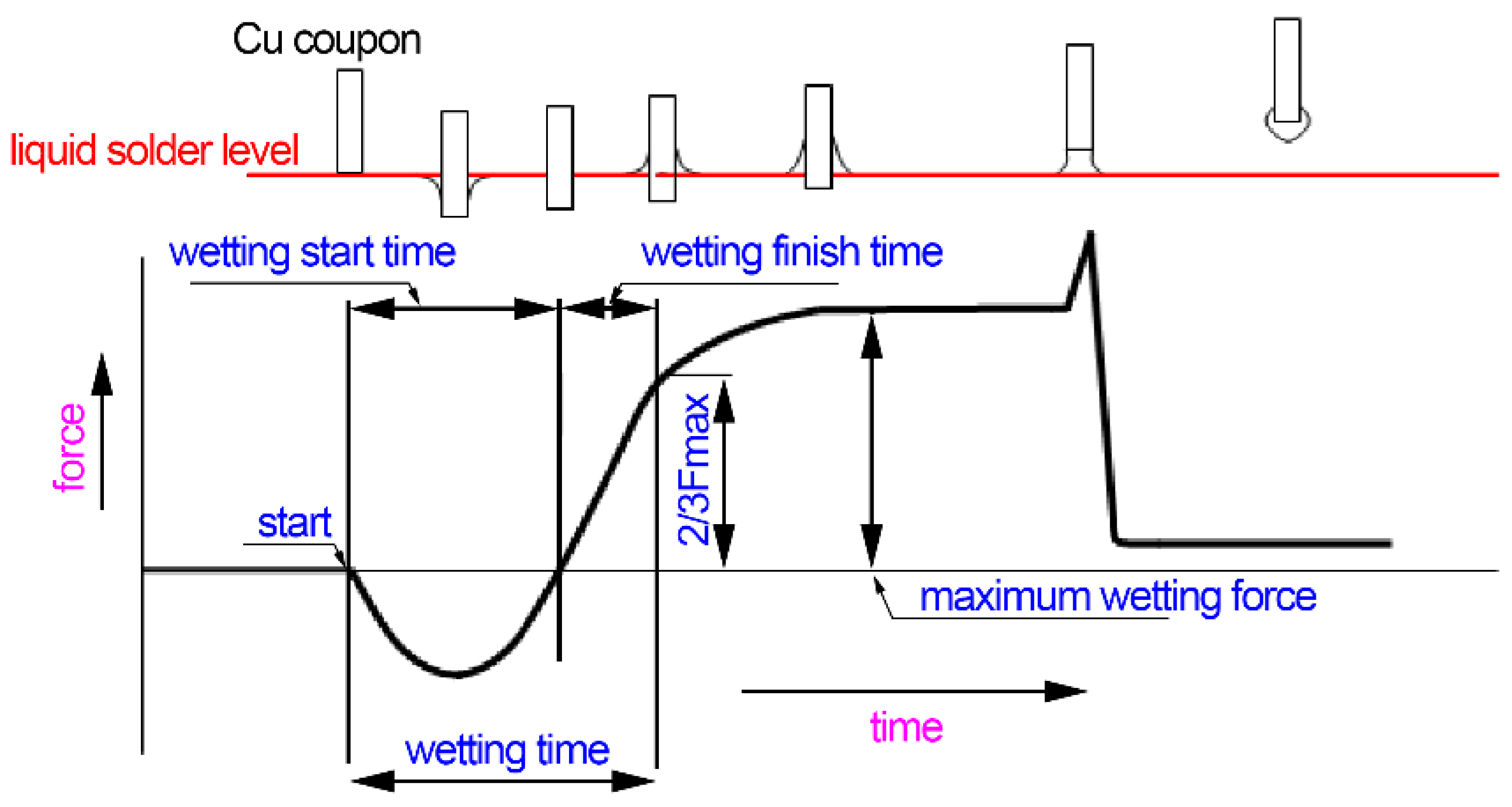
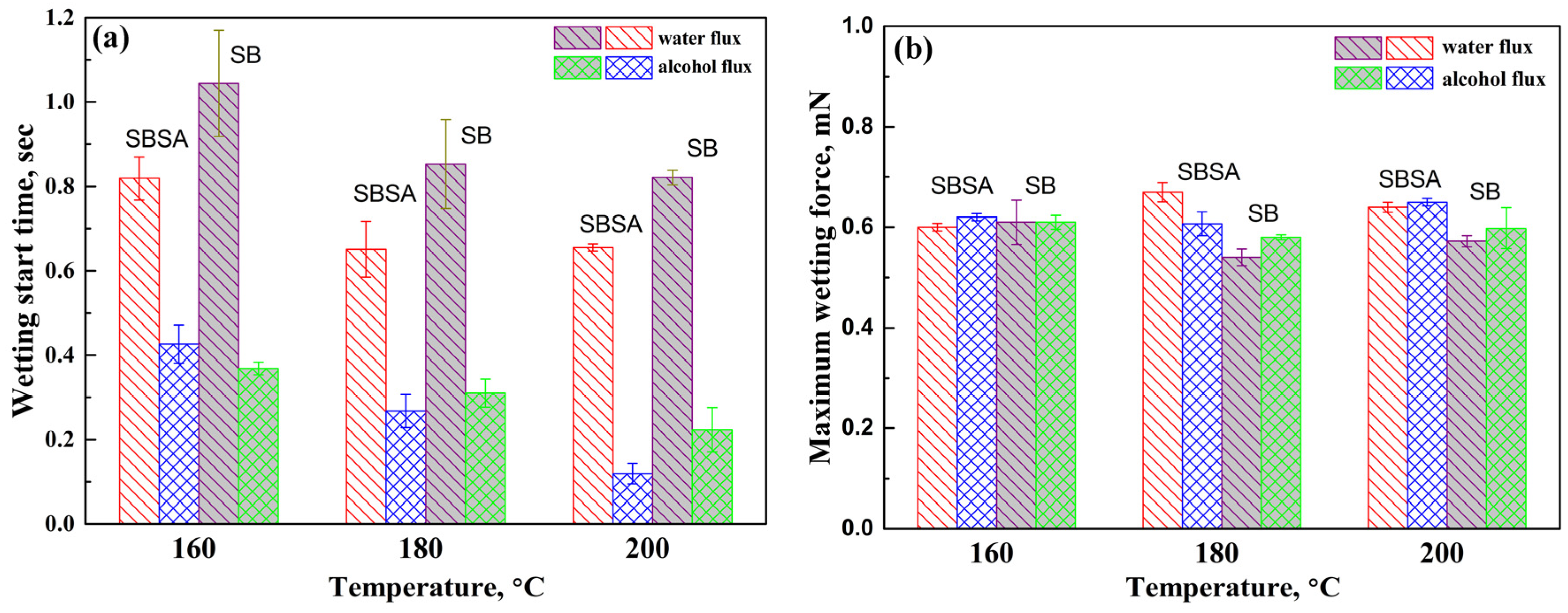
3.1. Wettability
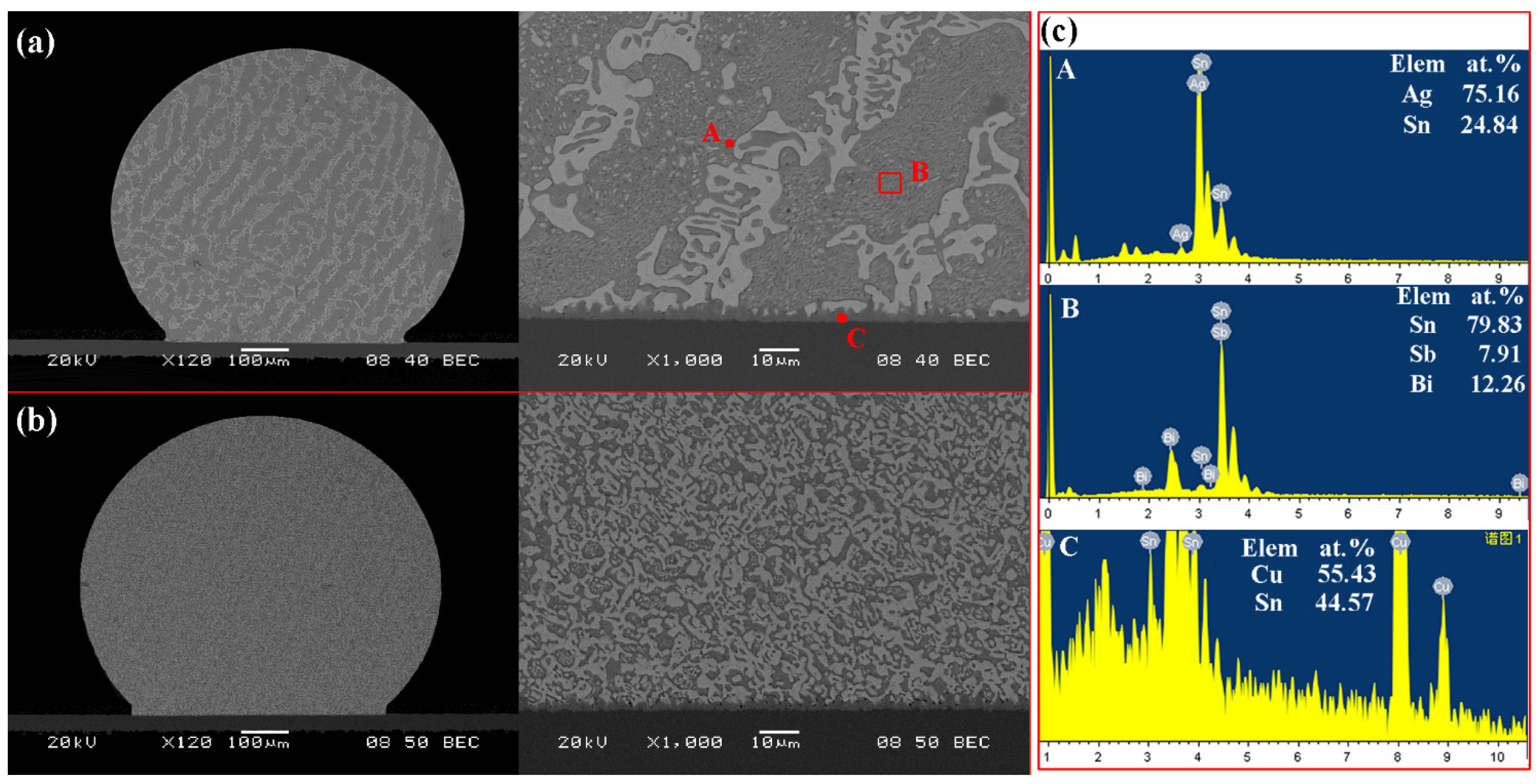
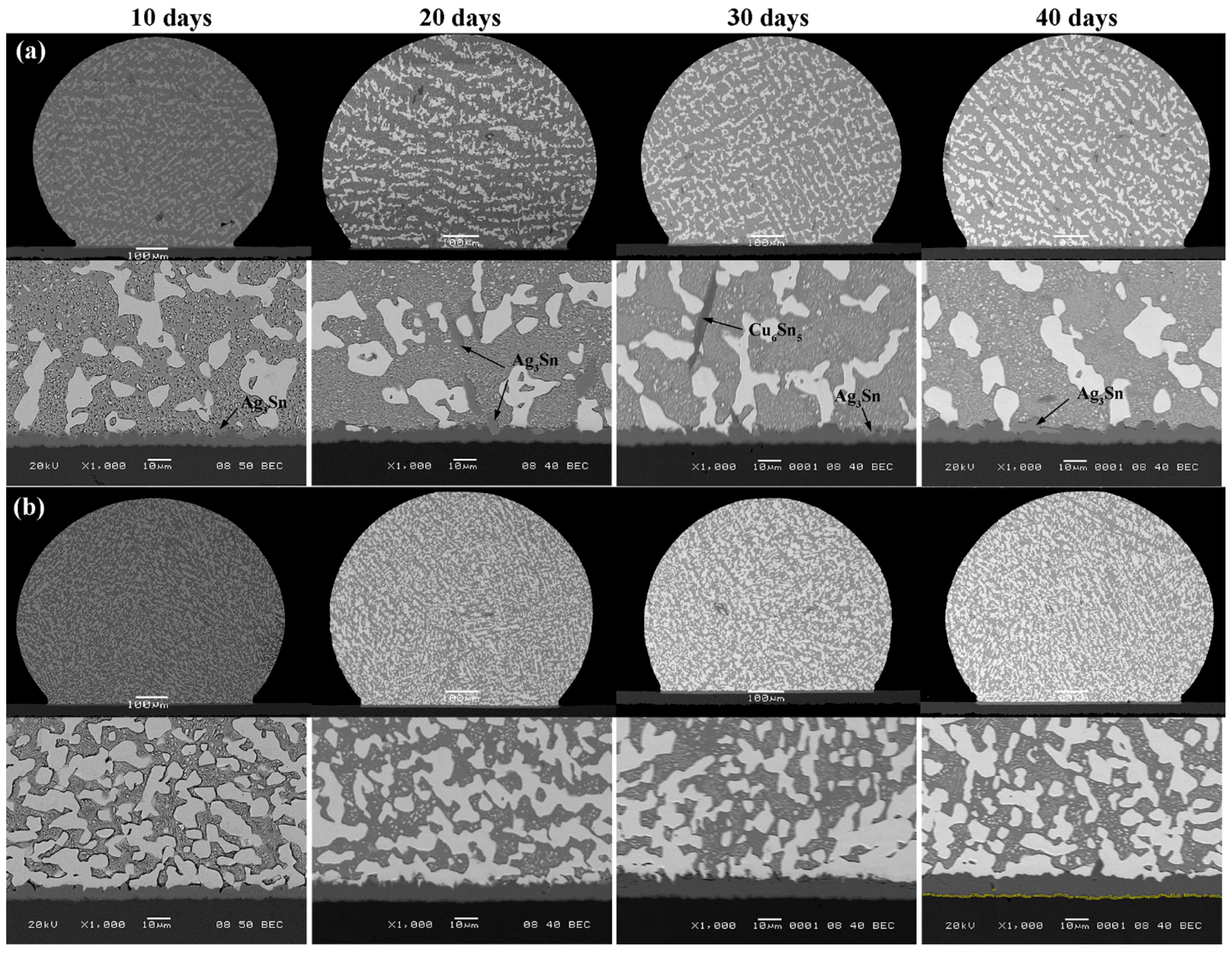
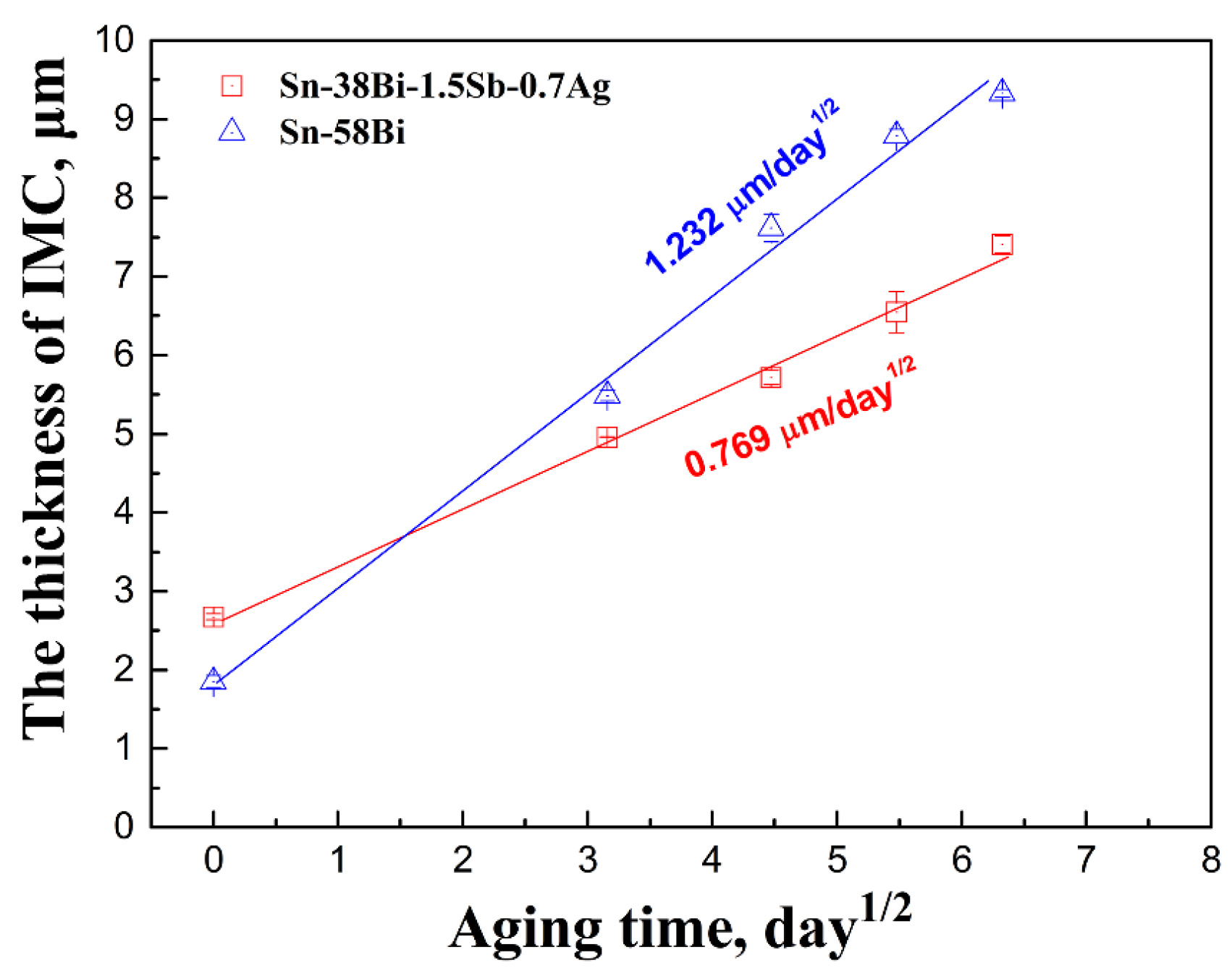
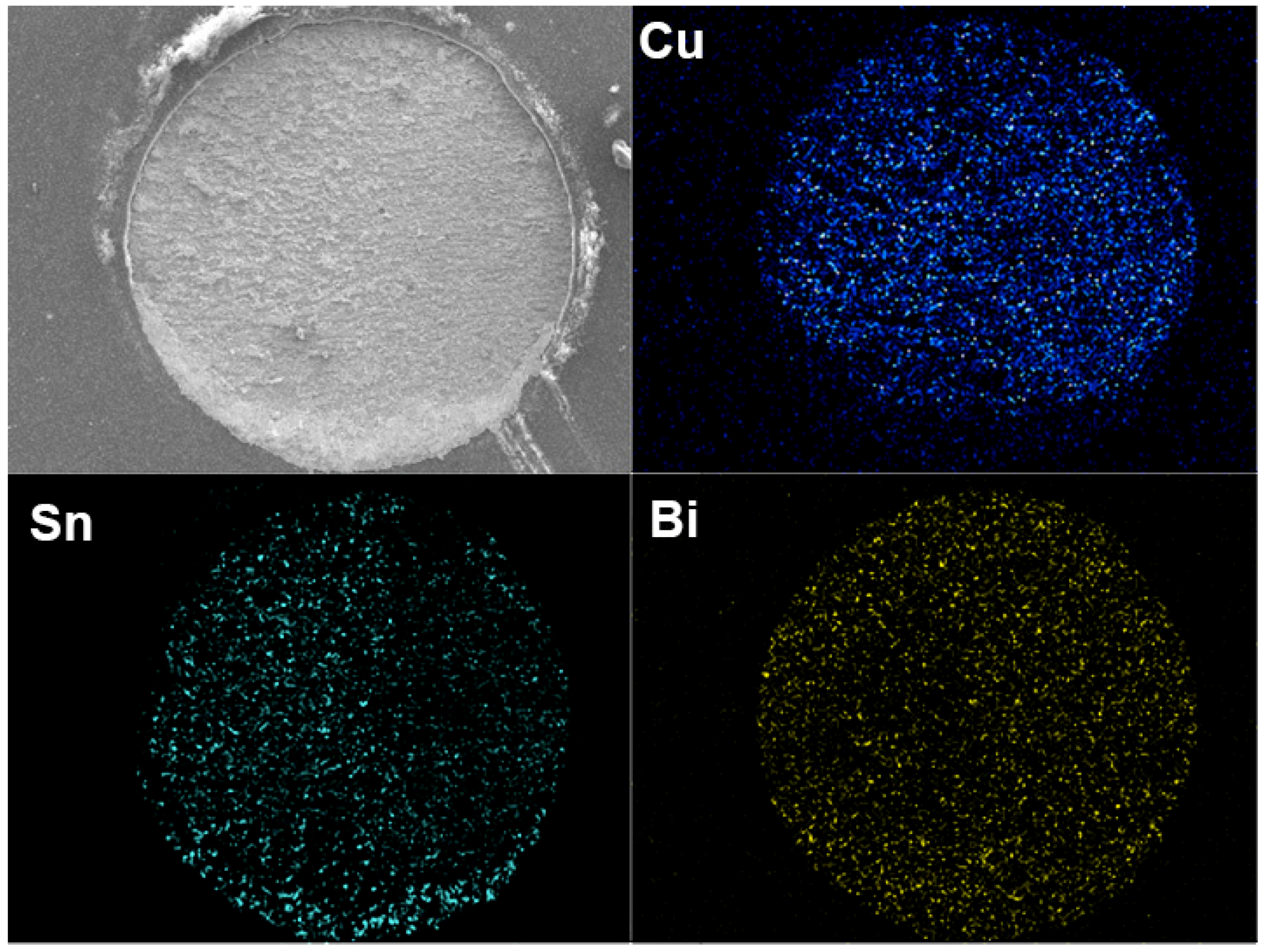
3.2. Interfacial Evolution in Solder Joints
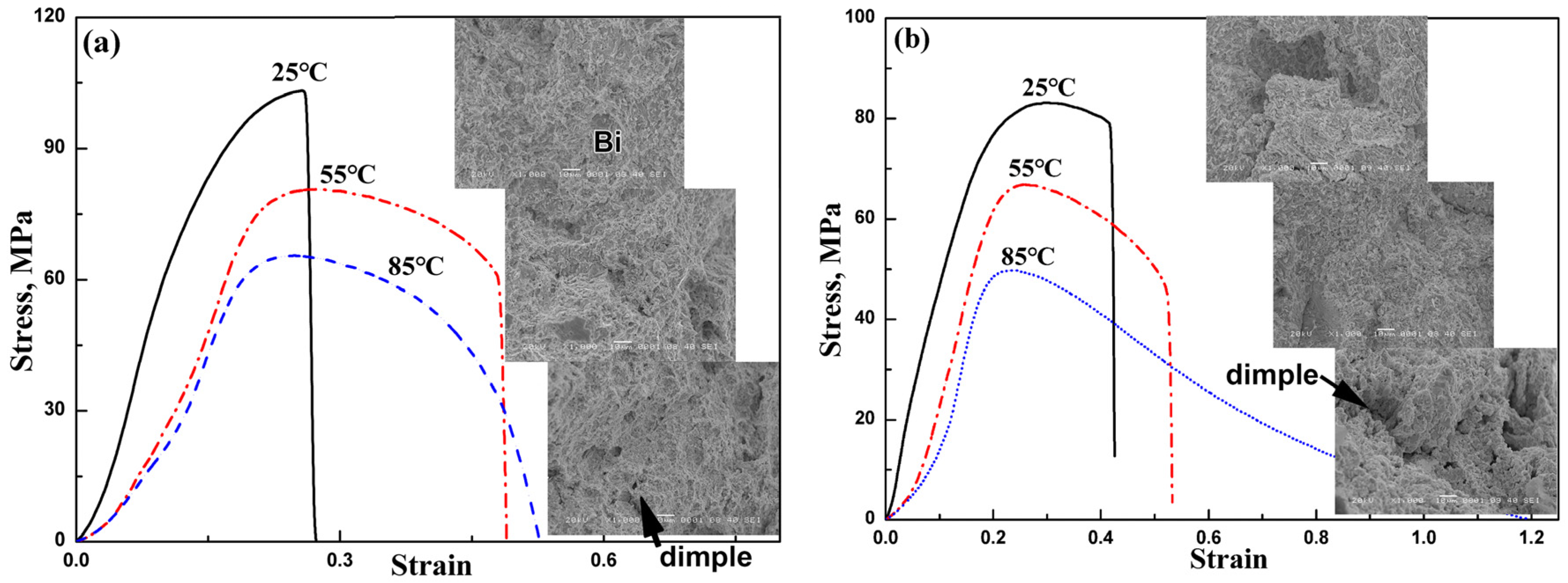
3.3. Tensile Properties of Bulk Solder Alloy
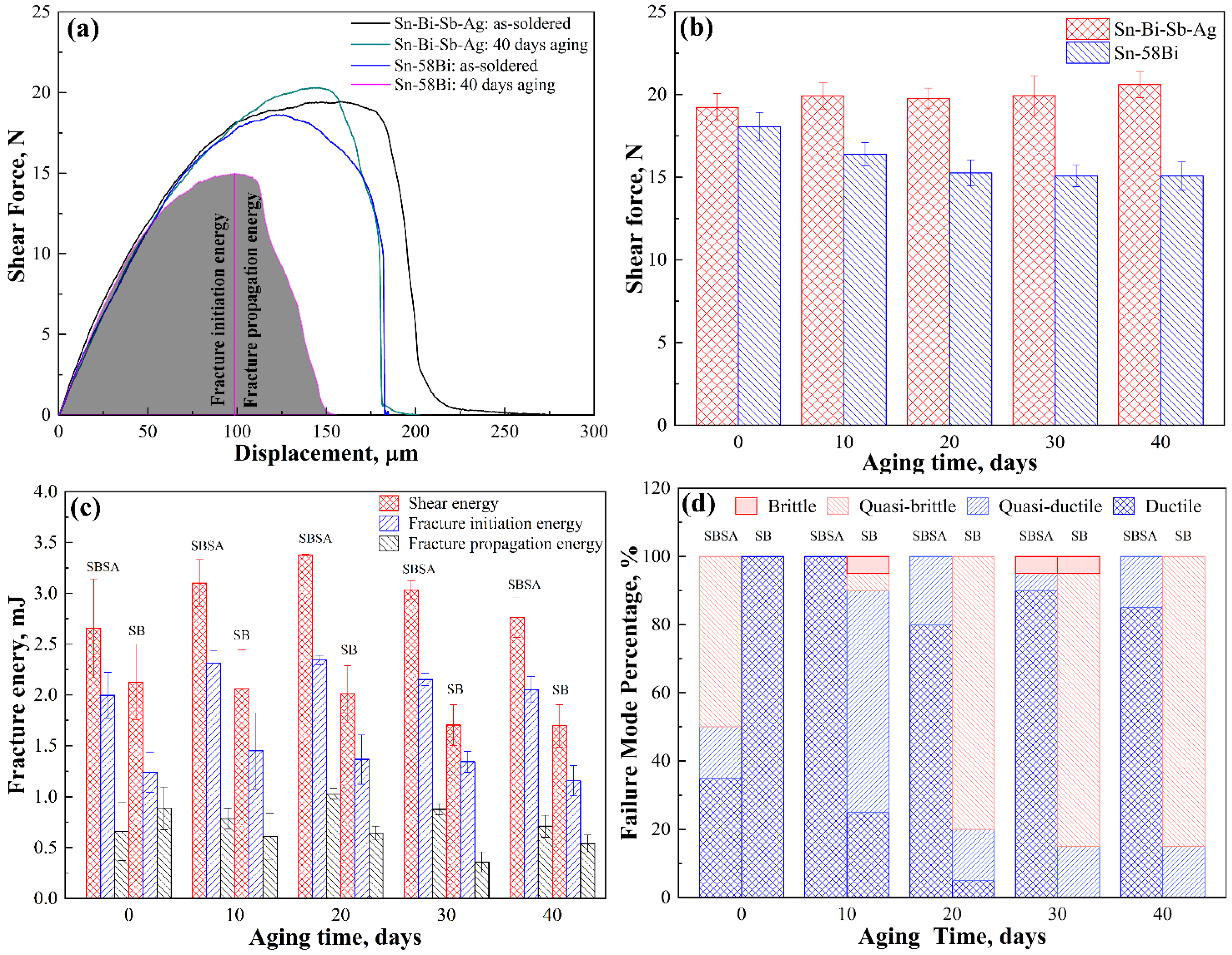
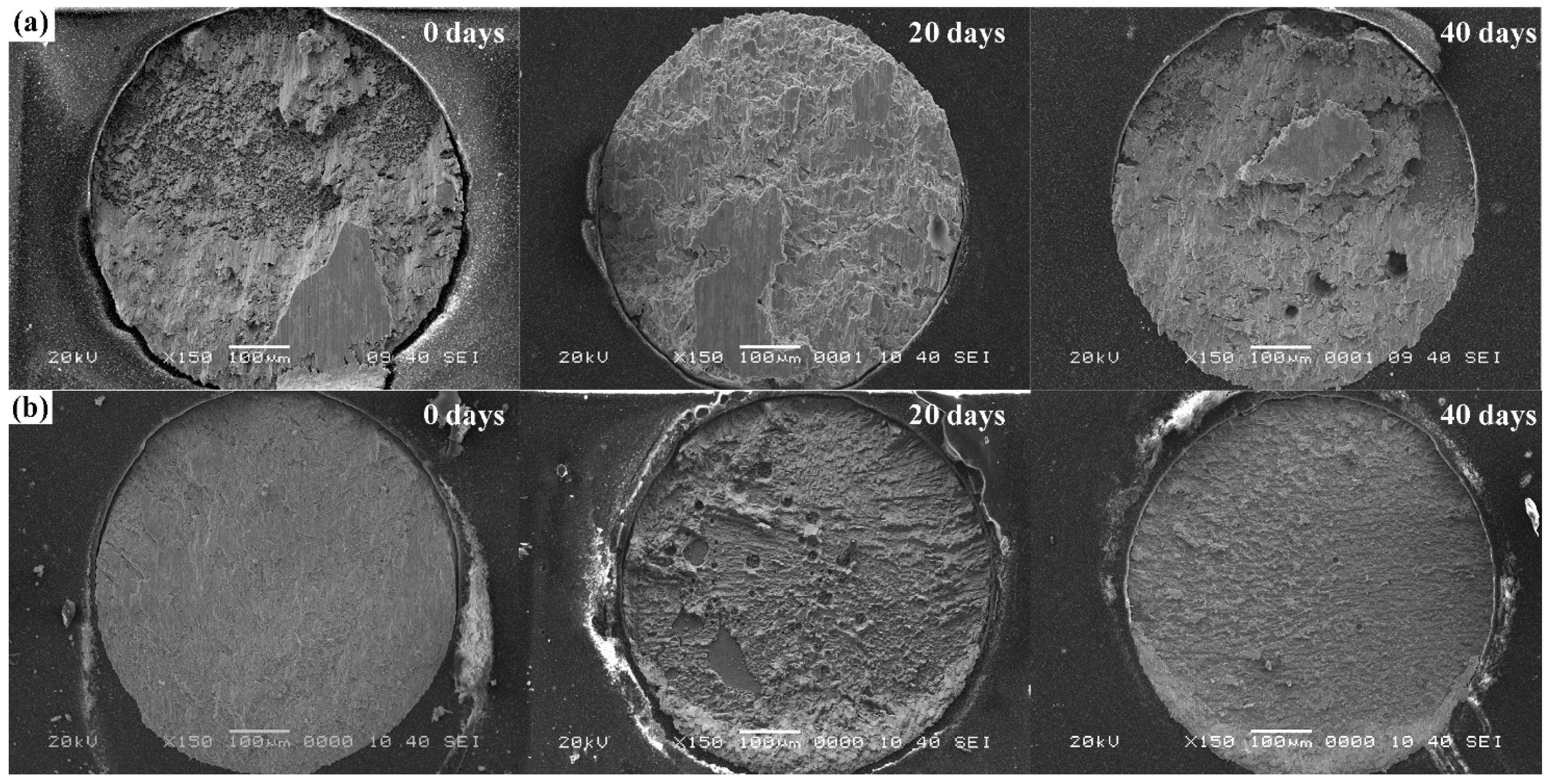
3.4. Mechanical Properties of Solder Joints
4. Conclusions
- (1)
- Wetting balance tests illustrated that Sn-38Bi-1.5Sb-0.7Ag solder had a better wettability on the wetting time than Sn-58Bi solder.
- (2)
- Compared with Sn-58Bi solder, Sn-38Bi-1.5Sb-0.7Ag bulk solder had a higher tensile strength and a similar elongation. The tensile strength increased while the elongation of solder decreased with testing temperature.
- (3)
- In solder/Cu joints, the IMC growth rate in Sn-38Bi-1.5Sb-0.7Ag solder joints was slower than that in Sn-58Bi eutectic joints during isothermal aging.
- (4)
- Ball shear tests illustrated that Sn-38Bi-1.5Sb-0.7Ag solder joints had a higher shear strength than Sn-58Bi solder joints after isothermal aging.
Author Contributions
Funding
Conflicts of Interest
References
- Shnawah, D.A.; Sabri, M.F.M.; Badruddin, I.A. A review on thermal cycling and drop impact reliability of SAC solder joint in portable electronic products. Microelectron. Reliab. 2012, 52, 90–99. [Google Scholar] [CrossRef]
- Ma, H.; Suhling, J.C. A review of mechanical properties of lead-free solders for electronic packaging. J. Mater. Sci. 2009, 44, 1141–1158. [Google Scholar] [CrossRef]
- Sidhu, R.S.; Aspandiar, R.; Vandervoort, S.; Amir, D.; Murtagian, G. Impact of processing conditions and solder materials on surface mount assembly defects. JOM 2011, 63, 47–51. [Google Scholar] [CrossRef]
- Kotadia, H.R.; Howes, P.D.; Mannan, S.H. A review: On the development of low melting temperature Pb-free solders. Microelectron. Reliab. 2014, 54, 1253–1273. [Google Scholar] [CrossRef]
- Wang, F.; Chen, H.; Huang, Y.; Liu, L.; Zhang, Z. Recent progress on the development of Sn–Bi based low-temperature Pb-free solders. J. Mater. Sci.: Mater. Electron. 2019, 30, 3222–3243. [Google Scholar] [CrossRef]
- Zou, H.F.; Zhang, Q.K.; Zhang, Z.F. Transition of Bi embrittlement of SnBi/Cu joint couples with reflow temperature. J. Mater. Res. 2011, 26, 449–454. [Google Scholar] [CrossRef]
- Chi, C.C.; Tsao, C.W.; Chuang, T.H. Intermetallic reactions in reflowed and aged Sn-58Bi BGA packages with Au/Ni/Cu pads. J. Mater. Eng. Perform. 2007, 17, 134–140. [Google Scholar] [CrossRef]
- Li, J.F.; Mannan, S.H.; Clode, M.P.; Chen, K.; Whalley, D.C.; Liu, C.; Hutt, D.A. Comparison of interfacial reactions of Ni and Ni-P in extended contact with liquid Sn-Bi-based solders. Acta Mater. 2007, 55, 737–752. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, L.; Wang, X.; He, P. Microstructural evolution and joint strength of Sn-58Bi/Cu joints through minor Zn alloying substrate during isothermal aging. J. Alloys Compd. 2016, 688, 639–648. [Google Scholar] [CrossRef]
- Myung, W.-R.; Kim, Y.; Kim, K.-Y.; Jung, S.-B. Drop reliability of Epoxy-contained Sn-58 wt.%Bi solder joint with ENIG and ENEPIG surface finish under temperature and humidity test. J. Electron. Mater. 2016, 45, 3651–3658. [Google Scholar] [CrossRef]
- Yu, X.; Hu, X.; Li, Y.; Liu, T.; Zhang, R.; Min, Z. Tensile properties of Cu/Sn–58Bi/Cu soldered joints subjected to isothermal aging. J. Mater. Sci.: Mater. Electron. 2014, 25, 2416–2425. [Google Scholar] [CrossRef]
- Lai, Z.; Ye, D. Microstructure and fracture behavior of non eutectic Sn–Bi solder alloys. J. Mater. Sci.: Mater. Electron. 2016, 27, 3182–3192. [Google Scholar] [CrossRef]
- Wang, F.; Huang, Y.; Zhang, Z.; Yan, C. Interfacial reaction and mechanical properties of Sn-Bi solder joints. Materials 2017, 10, 920. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Septiwerdani, P.; Chen, Z. Elastic modulus, hardness and creep performance of SnBi alloys using nanoindentation. Mater. Sci. Eng. A 2012, 558, 253–258. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Long, W.; Lei, M.; Hu, X. Wetting kinetics and spreading phenomena of Sn-35Bi-1Ag solder on different substrates. J. Mater. Sci.: Mater. Electron. 2018, 29, 13914–13924. [Google Scholar] [CrossRef]
- Gain, A.K.; Zhang, L. Effect of thin gold/nickel coating on the microstructure, wettability and hardness of lead-free tin–bismuth–silver solder. J. Mater. Sci.: Mater. Electron. 2017, 28, 4885–4896. [Google Scholar] [CrossRef]
- Fouzder, T.; Gain, A.K.; Chan, D.K. Microstructure, wetting characteristics and hardness of tin-bismuth-silver (Sn–Bi–Ag) solders on silver (Ag)-surface finished copper (Cu) substrates. J. Mater. Sci.: Mater. Electron. 2017, 28, 16921–16931. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, L.; Guo, Y.-h. Microstructures and properties of Sn58Bi, Sn35Bi0.3Ag, Sn35Bi1.0Ag solder and solder joints. J. Mater. Sci.: Mater. Electron. 2015, 26, 7629–7634. [Google Scholar] [CrossRef]
- Silva, B.L.; Xavier, M.G.C.; Garcia, A.; Spinelli, J.E. Cu and Ag additions affecting the solidification microstructure and tensile properties of Sn-Bi lead-free solder alloys. Mater. Sci. Eng. A 2017, 705, 325–334. [Google Scholar] [CrossRef]
- Mokhtari, O.; Nishikawa, H. Correlation between microstructure and mechanical properties of Sn–Bi–X solders. Mater. Sci. Eng. A 2016, 651, 831–839. [Google Scholar] [CrossRef]
- Sakuyama, S.; Akamatsu, T.; Uenishi, K.; Sato, T. Effects of a third element on microstructure and mechanical properties of eutectic Sn-Bi solder. Trans. Jpn. Inst. Electron. Packag. 2009, 2, 98–103. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.-d.; Qian, G.-t.; Zhou, J.; Xue, F. Effect of Sb content on properties of Sn-Bi solders. Trans. Nonferrous Met. Soc. China 2014, 24, 184–191. [Google Scholar] [CrossRef]
- r Kattner, U.; Boettinger, W.J. On the Sn-Bi-Ag ternary phase diagram. J. Electron. Mater. 1994, 23, 603–610. [Google Scholar] [CrossRef]
- Yan, J.K.; Xu, F.X.; Chen, D.D.; Teng, Y.; Gan, Y.W. A study on microstructure and properties of quaternary SnBiAgSb solder alloy. J. Kunming Univ. Sci. Technol. 2018, 43, 22–27. [Google Scholar]
- Hasnine, M. Effects of In and Sb addition on the thermal and wetting behavior of Sn-3.5Ag solder. Solder. Surf. Mt. Technol. 2018, 30, 233–240. [Google Scholar] [CrossRef]
- Jung, D.-H.; Jung, J.-P. Review of the wettability of solder with a wetting balance test for recent advanced microelectronic packaging. Crit. Rev. Solid State Mater. Sci. 2018, 43, 1–20. [Google Scholar] [CrossRef]
- Noor, E.E.M.; Nasir, N.F.M.; Idris, S.R.A. A review: Lead free solder and its wettability properties. Solder. Surf. Mt. Technol. 2016, 28, 125–132. [Google Scholar] [CrossRef]
- Martorano, K.M.; Martorano, M.A.; Brandi, S.D. Optimal conditions for the wetting balance test. J. Mater. Process. Technol. 2009, 209, 3089–3095. [Google Scholar] [CrossRef]
- Chen, B.L.; Li, G.Y. Influence of Sb on IMC growth in Sn–Ag–Cu–Sb Pb-free solder joints in reflow process. Thin Solid Films 2004, 462, 395–401. [Google Scholar] [CrossRef]
- Che, F.X.; Zhu, W.H.; Poh, E.S.W.; Zhang, X.W.; Zhang, X.R. The study of mechanical properties of Sn–Ag–Cu lead-free solders with different Ag contents and Ni doping under different strain rates and temperatures. J. Alloys Compd. 2010, 507, 215–224. [Google Scholar] [CrossRef]
- Alden, T.H. The origin of superplasticity in the Sn-5%Bi alloy. Acta Metall. 1967, 15, 469–480. [Google Scholar] [CrossRef]
- Salleh, M.A.A.M.; McDonald, S.D.; Nogita, K. Effects of Ni and TiO2 additions in as-reflowed and annealed Sn0.7Cu solders on Cu substrates. J. Mater. Process. Technol. 2017, 242, 235–245. [Google Scholar] [CrossRef]
- Wang, F.J.; Chen, H.; Huang, Y.; Yan, C. Interfacial behavior and joint strength of Sn-Bi solder with solid solution compositions. J. Mater. Sci.: Mater. Electron. 2018, 29, 11409–11420. [Google Scholar] [CrossRef]
- Wang, F.; Li, D.; Zhang, Z.; Wu, M.; Yan, C. Improvement on interfacial structure and properties of Sn–58Bi/Cu joint using Sn–3.0Ag–0.5Cu solder as barrier. J. Mater. Sci.: Mater. Electron. 2017, 28, 19051–19060. [Google Scholar] [CrossRef]
- Zhang, Q.K.; Zou, H.F.; Zhang, Z.F. Influences of substrate alloying and reflow temperature on Bi segregation behaviors at Sn-Bi/Cu interface. J. Electron. Mater. 2011, 40, 2320–2328. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Wang, F.; Huang, Y.; Qi, K. Comprehensive Properties of a Novel Quaternary Sn-Bi-Sb-Ag Solder: Wettability, Interfacial Structure and Mechanical Properties. Metals 2019, 9, 791. https://doi.org/10.3390/met9070791
Wang K, Wang F, Huang Y, Qi K. Comprehensive Properties of a Novel Quaternary Sn-Bi-Sb-Ag Solder: Wettability, Interfacial Structure and Mechanical Properties. Metals. 2019; 9(7):791. https://doi.org/10.3390/met9070791
Chicago/Turabian StyleWang, Kaipeng, Fengjiang Wang, Ying Huang, and Kai Qi. 2019. "Comprehensive Properties of a Novel Quaternary Sn-Bi-Sb-Ag Solder: Wettability, Interfacial Structure and Mechanical Properties" Metals 9, no. 7: 791. https://doi.org/10.3390/met9070791
APA StyleWang, K., Wang, F., Huang, Y., & Qi, K. (2019). Comprehensive Properties of a Novel Quaternary Sn-Bi-Sb-Ag Solder: Wettability, Interfacial Structure and Mechanical Properties. Metals, 9(7), 791. https://doi.org/10.3390/met9070791




