Metallic Glasses: A New Approach to the Understanding of the Defect Structure and Physical Properties
Abstract
1. Introduction
2. The Interstitialcy Theory
3. Verification of the Main Starting Hypotheses of the Interstitialcy Theory
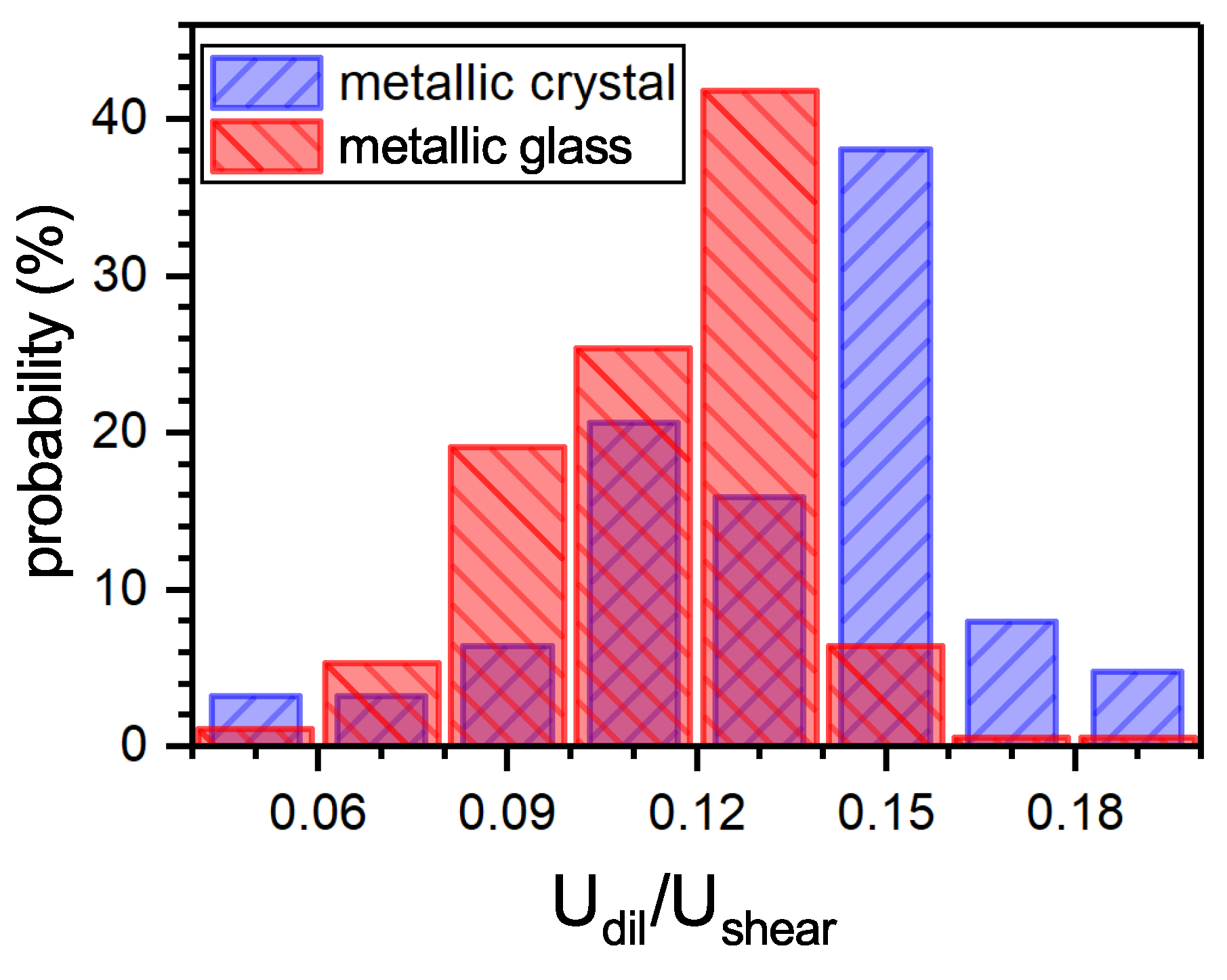
3.1. Shear and Dilatation Contributions into the Defect Elastic Energy
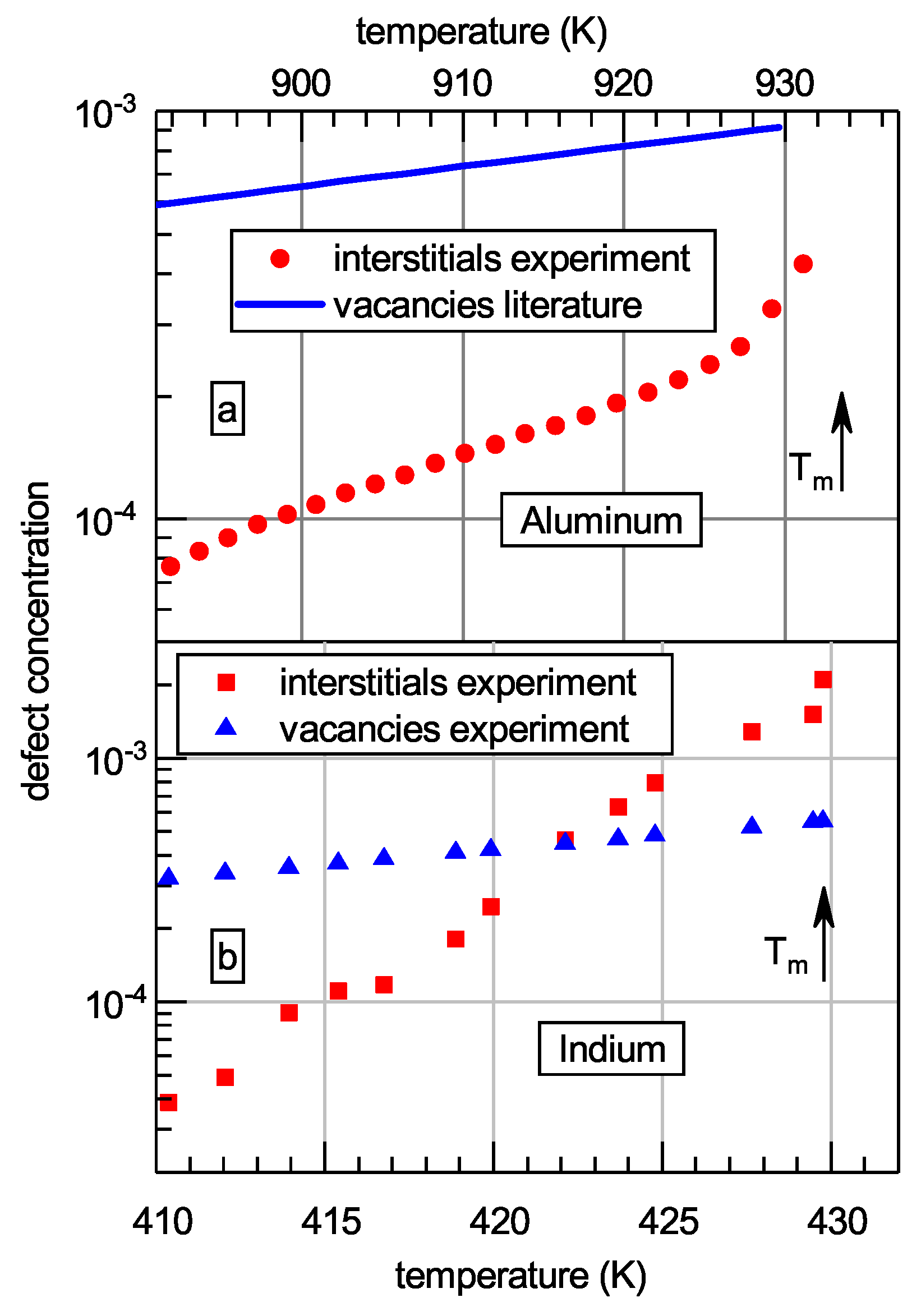
3.2. Increase of the Interstitial Concentration before Melting
3.3. Identification of Interstitial-Type Defects in the Glassy State
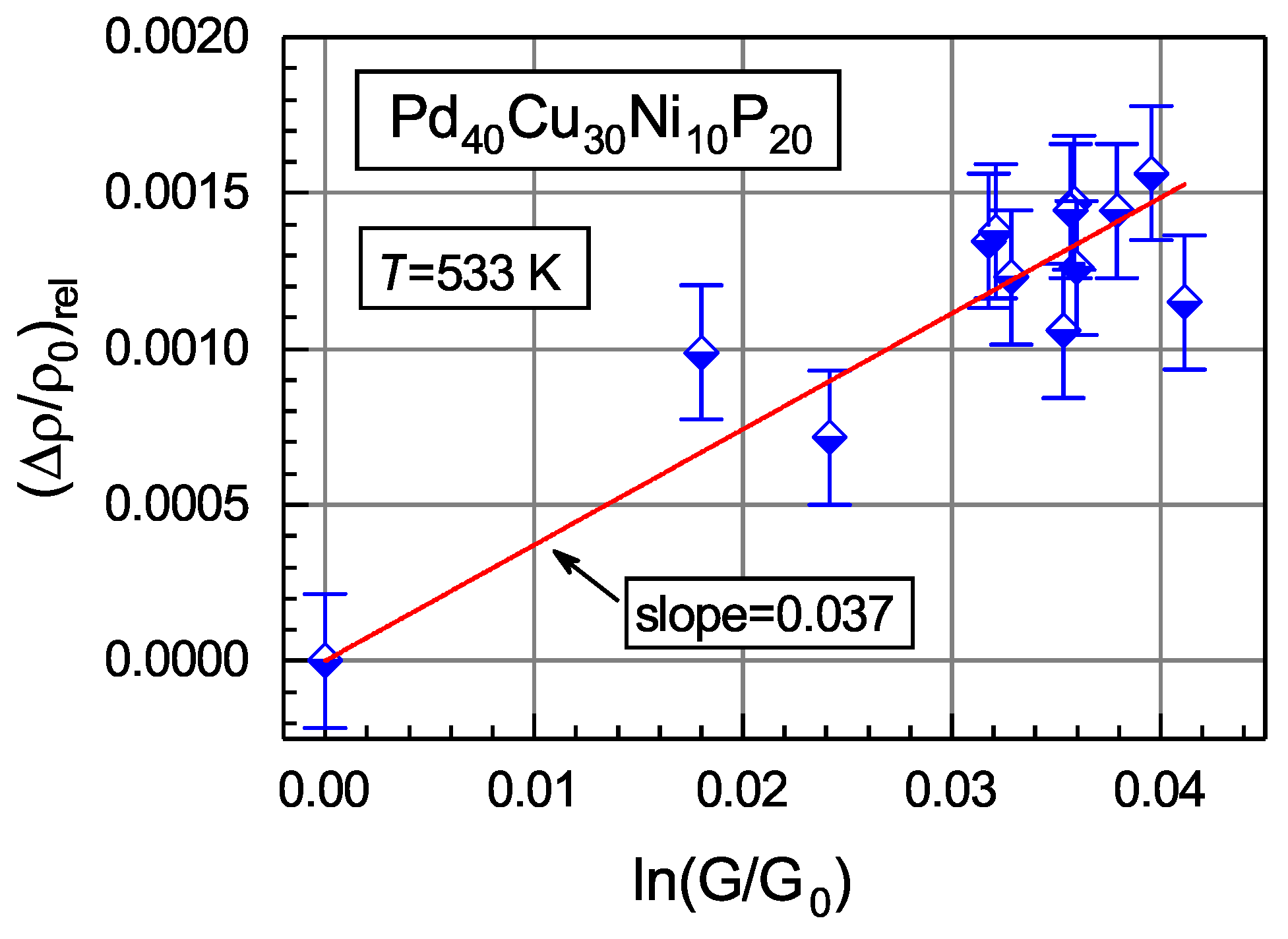
3.4. Shear Susceptibility
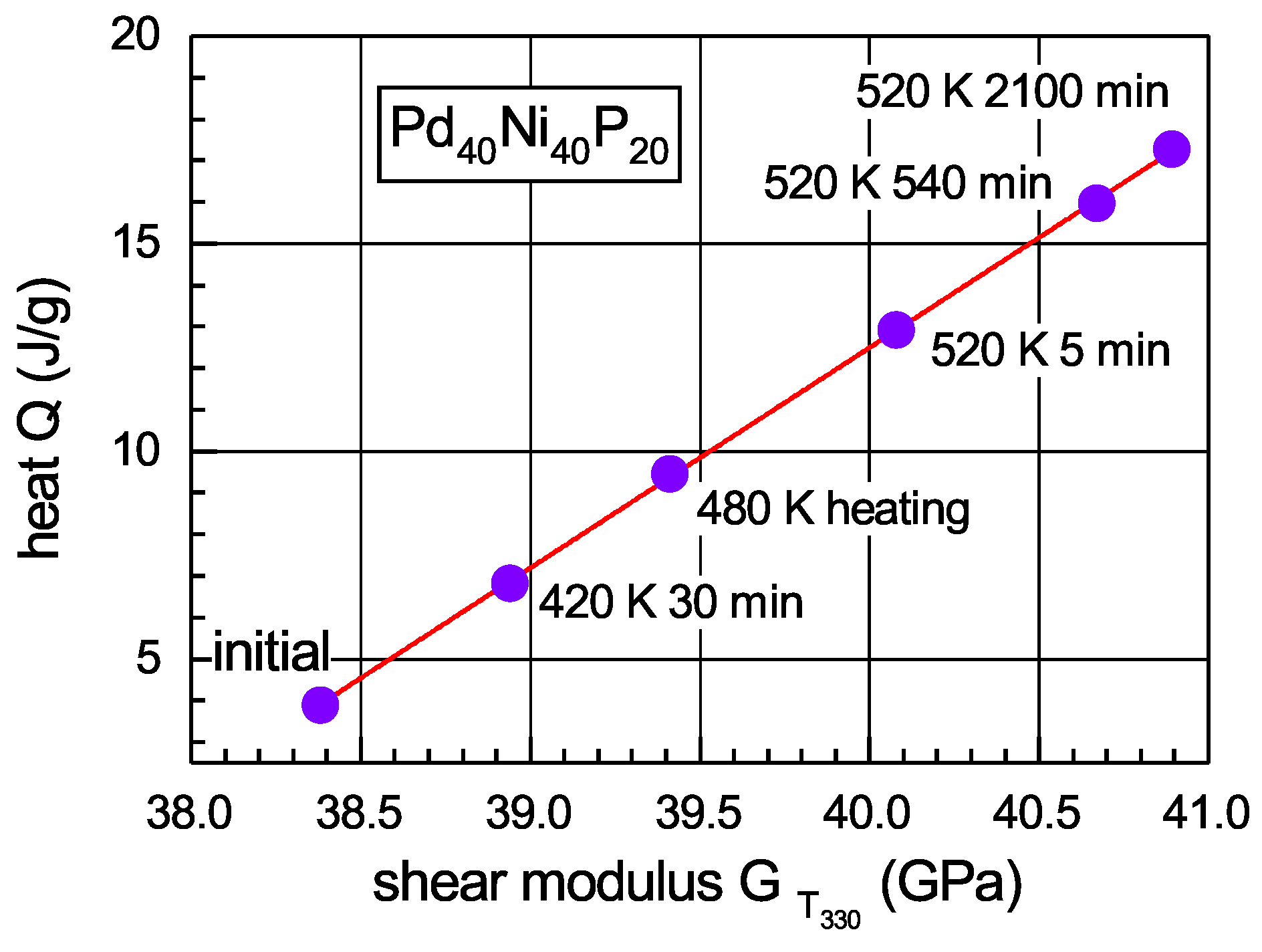
3.5. Relation between the Shear Modulus and Heat Effects
4. Refinement of the Parameters of the Interstitialcy Theory
5. Recent Experiments
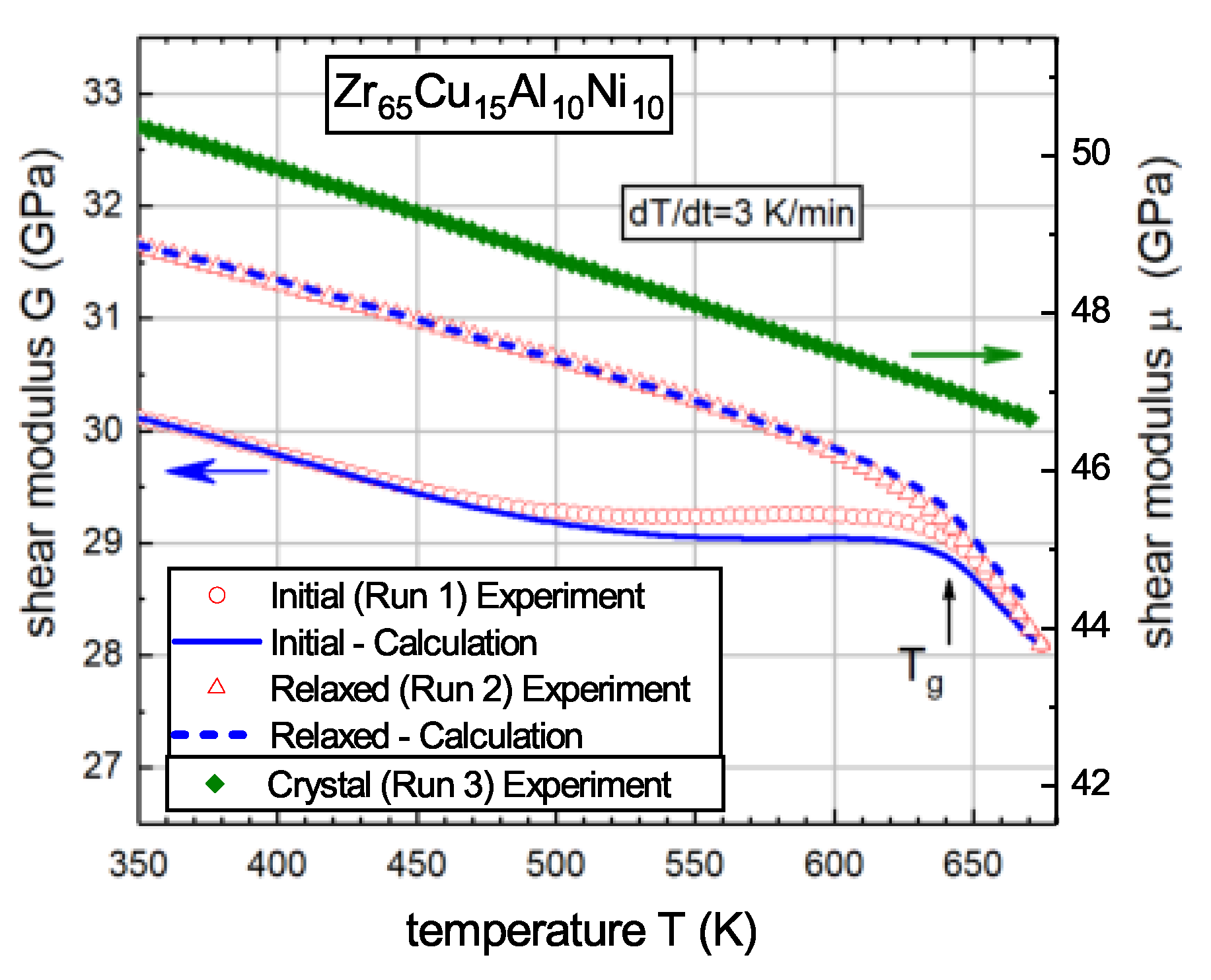
5.1. Reconstruction of Temperature Dependence of the Shear Modulus Using Calorimetric Data
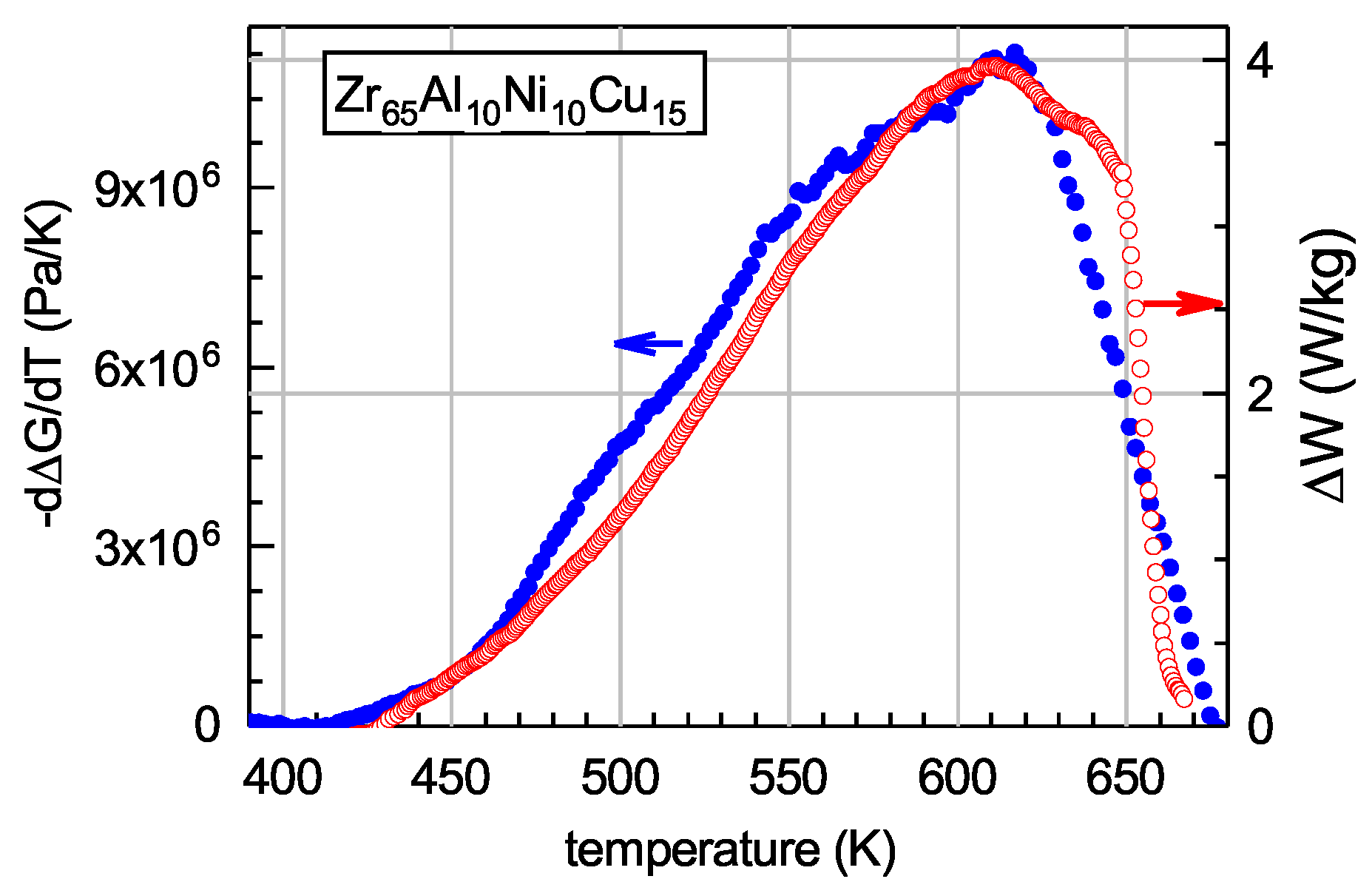
5.2. Heat Absorption Occurring upon Heating of Relaxed Glass
5.3. Density Changes upon Structural Relaxation and Crystallization
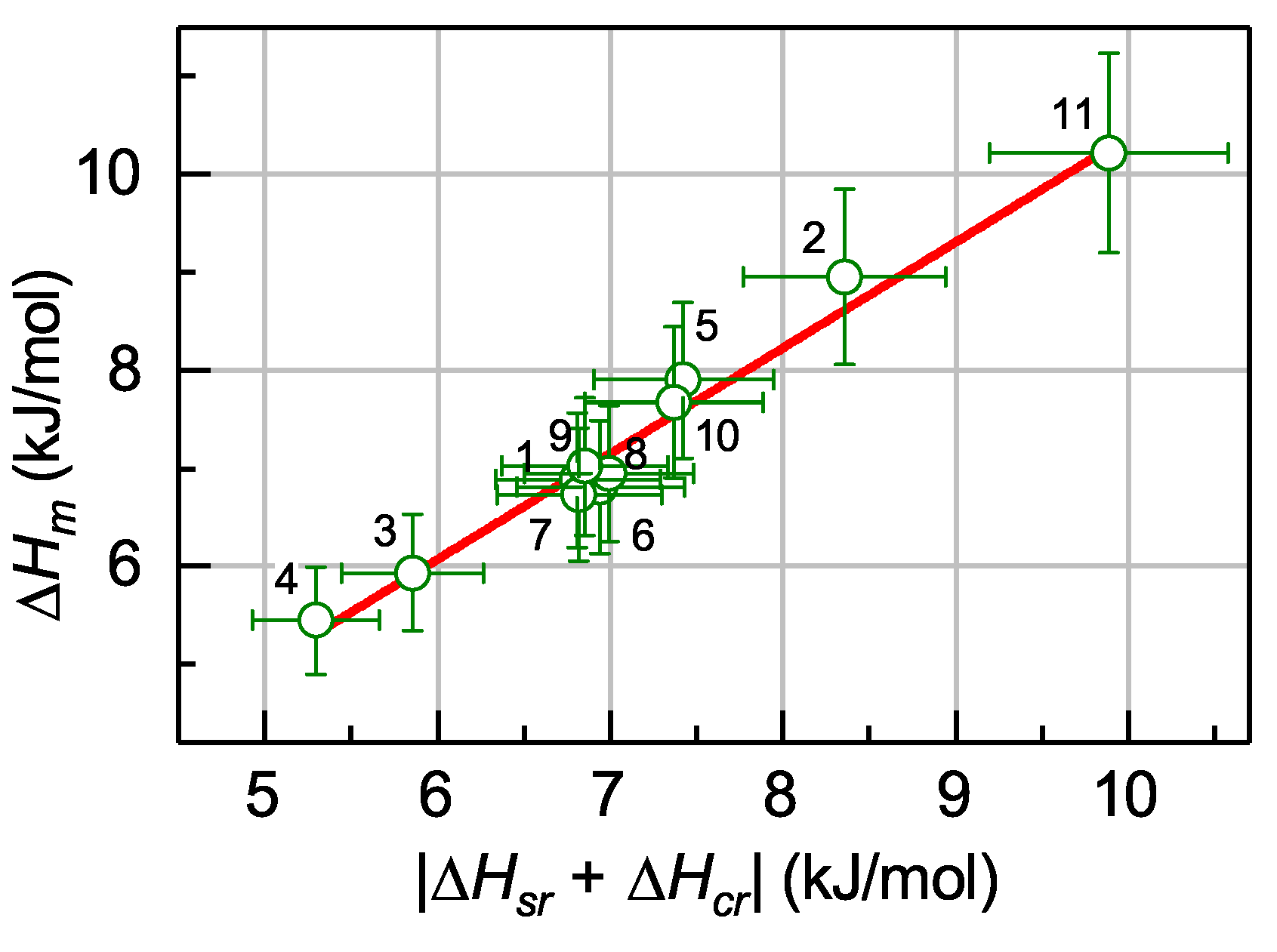
5.4. Relation between the Enthalpies of Relaxation, Crystallization and Melting
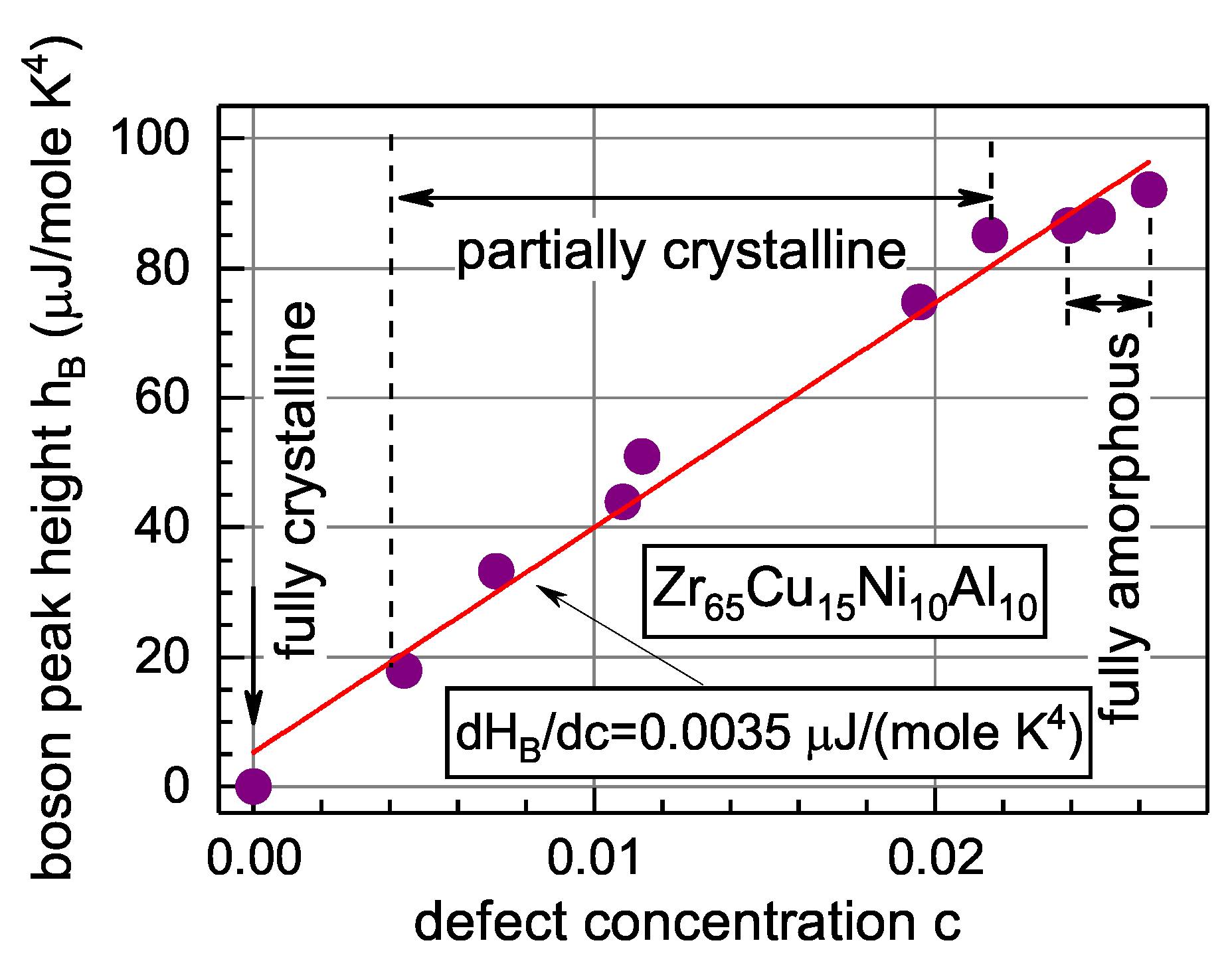
5.5. Relation of the Boson Heat Capacity Peak to the Defect Structure
5.6. Relation between the Properties of Glass and Maternal Crystal
6. Development of the Interstitialcy Theory
7. Comparison with Other Models
8. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spaepen, F. A microscopic mechanism for steady state inhomogeneous flow in metallic glasses. Acta Metall. 1977, 25, 407–415. [Google Scholar] [CrossRef]
- Argon, A.S. Plastic deformation in metallic glasses. Acta Metall. 1979, 27, 47–58. [Google Scholar] [CrossRef]
- Falk, M.L.; Langer, J.S. Dynamics of viscoplastic deformation in amorphous solids. Phys. Rev. E 1998, 57, 7192–7205. [Google Scholar] [CrossRef]
- Miracle, D.B. The efficient cluster packing model—An atomic structural model for metallic glasses. Acta Mater. 2006, 54, 4317–4336. [Google Scholar] [CrossRef]
- Miracle, D.B.; Egami, T.; Flores, K.M.; Kelton, K.F. Structural aspects of metallic glasses. MRS Bull. 2007, 32, 629–634. [Google Scholar] [CrossRef]
- Miracle, D.B.; Greer, A.L.; Kelton, K.F. Icosahedral and dense random cluster packing in metallic glass structures. J. Non-Cryst. Sol. 2008, 354, 4049–4055. [Google Scholar] [CrossRef]
- Cheng, Y.Q.; Ma, E. Atomic-level structure and structure–property relationship in metallic glasses. Prog. Mater. Sci. 2011, 56, 379–473. [Google Scholar] [CrossRef]
- Egami, T. Atomic level stresses. Prog. Mater. Sci. 2011, 56, 637–653. [Google Scholar] [CrossRef]
- Peng, H.L.; Li, M.Z.; Wang, W.H. Structural signature of plastic deformation in metallic glasses. Phys. Rev. Lett. 2011, 106, 135503. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.P.; Zhu, Z.G.; Xue, R.J.; Ding, D.W.; Bai, H.Y.; Wang, W.H. Structural perspectives on the elastic and mechanical properties of metallic glasses. J. Appl. Phys. 2013, 114, 173505. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, B.A.; Bai, H.Y.; Wang, W.H. Evolution of hidden localized flow during glass-to-liquid transition in metallic glass. Nat. Commun. 2014, 5, 5823. [Google Scholar] [CrossRef]
- Zhang, H.; Zhong, C.; Douglas, J.F.; Wang, X.; Cao, Q.; Zhang, D.; Jiang, J.-Z. Role of string-like collective atomic motion on diffusion and structural relaxation in glass forming Cu-Zr alloys. J. Chem. Phys. 2015, 142, 164506. [Google Scholar] [CrossRef]
- Granato, A.V. Interstitialcy model for condensed matter states of face-centered-cubic metals. Phys. Rev. Lett. 1992, 68, 974–977. [Google Scholar] [CrossRef]
- Granato, A.V. Interstitialcy theory of simple condensed matter. Eur. J. Phys. 2014, 87, 18. [Google Scholar] [CrossRef]
- Khonik, V.A. Understanding of the structural relaxation of metallic glasses within the framework of the interstitialcy theory. Metals 2015, 5, 504–529. [Google Scholar] [CrossRef]
- Khonik, V.A. Interstitialcy theory of condensed matter states and its application to non-crystalline metallic materials. Chin. Phys. B 2017, 26, 016401. [Google Scholar] [CrossRef]
- Holder, J.; Rehn, L.E.; Granato, A.V. Effect of self-interstitials on the elastic constants of copper. Phys. Rev. B 1974, 10, 363–375. [Google Scholar] [CrossRef]
- Holder, J.; Granato, A.V.; Rehn, L.E. Experimental evidence for split interstitials in copper. Phys. Rev. Lett. 1974, 32, 1054–1057. [Google Scholar] [CrossRef]
- Robrock, K.-H.; Schilling, W. Diaelastic modulus change of aluminum after low temperature electron irradiation. J. Phys. F Metal Phys. 1976, 6, 303–314. [Google Scholar] [CrossRef]
- Seitz, F. On the theory of diffusion in metals. Acta Cryst. 1950, 3, 355–363. [Google Scholar] [CrossRef]
- Nowick, A.S.; Berry, B.S. Anelastic Relaxation in Crystalline Solids; Academic Press: New York, NY, USA; London, UK, 1972. [Google Scholar]
- Schilling, W. Self-interstitial atoms in metals. J. Nucl. Mater. 1978, 69–70, 465–489. [Google Scholar] [CrossRef]
- Robrock, K.H. Mechanical Relaxation of Interstitials in Irradiated Metals; Springer: Berlin, Germany, 1990. [Google Scholar]
- Wolfer, W.G. Fundamental properties of defects in metals. In Comprehensive Nuclear Materials; Konings, R.J.M., Ed.; Elsevier: New York, NY, USA, 2012. [Google Scholar]
- Ma, P.-W.; Dudarev, S.L. Universality of point defect structure in body-centered cubic metals. Phys. Rev. Mater. 2019, 3, 013605. [Google Scholar] [CrossRef]
- Ma, P.-W.; Dudarev, S.L. Symmetry-broken self-interstitial defects in chromium, molybdenum, and tungsten. Phys. Rev. Mater. 2019, 3, 043606. [Google Scholar] [CrossRef]
- Konchakov, R.A.; Makarov, A.S.; Afonin, G.A.; Kretova, M.A.; Kovelev, N.P.; Khonik, V.A. Relation between the shear and dilatation energy of interstitial defects in metallic crystals. J. Exp. Theor. Phys. Lett. 2019, 109, 473–478. [Google Scholar]
- Dederichs, P.H.; Lehman, C.; Schober, H.R.; Scholz, A.; Zeller, R. Lattice theory of point defects. J. Nucl. Mater. 1978, 69–70, 176–199. [Google Scholar] [CrossRef]
- Born, M. Thermodynamics of crystals and melting. J. Chem. Phys. 1939, 7, 591–603. [Google Scholar] [CrossRef]
- Forsblom, M.; Grimvall, G. How superheated crystals melt. Nat. Mater 2005, 4, 388–390. [Google Scholar] [CrossRef] [PubMed]
- Nemilov, S.V. Kinetics of elementary processes in the condensed state. II. Shear relaxation and the equation of state for solids. Russ. J. Phys. Chem. 1968, 42, 726–729. [Google Scholar]
- Dyre, J.C.; Olsen, N.B.; Christensen, T. Local elastic expansion model for viscous-flow activation energies of glass-forming molecular liquids. Phys. Rev. B 1996, 53, 2171–2174. [Google Scholar] [CrossRef]
- Nordlund, K.; Ashkenazy, Y.; Averback, R.S.; Granato, A.V. Strings and interstitials in liquids, glasses and crystals. Europhys. Lett. 2005, 71, 625–631. [Google Scholar] [CrossRef]
- Dyre, J.C. Dominance of shear elastic energy far from a point defect in a solid. Phys. Rev. B 2007, 75, 092102. [Google Scholar] [CrossRef]
- Safonova, E.V.; Mitrofanov, Y.P.; Konchakov, R.A.; Vinogradov, A.Y.; Kobelev, N.P.; Khonik, V.A. Experimental evidence for thermal generation of interstitials in a metallic crystal near the melting temperature. J. Phys. Condens. Matter 2016, 28, 215401. [Google Scholar] [CrossRef]
- Goncharova, E.V.; Makarov, A.S.; Konchakov, R.A.; Kobelev, N.P.; Khonik, V.A. Premelting generation of interstitial defects in polycrystalline indium. J. Exp. Theor. Phys. Lett 2017, 106, 35–39. [Google Scholar] [CrossRef]
- Gottstein, G. Physical Foundations of Materials Science; Springer: Berlin, Germany, 2004. [Google Scholar]
- Safonova, E.V.; Konchakov, R.A.; Mitrofanov, Y.P.; Kobelev, N.P.; Vinogradov, A.Yu.; Khonik, V.A. Contribution of interstitial defects and anharmonicity to the premelting increase in the heat capacity of single-crystal aluminum. J. Exp. Theor. Lett. 2016, 103, 765–768. [Google Scholar] [CrossRef]
- Kobelev, N.P.; Khonik, V.A. On the enthalpy and entropy of point defect formation in crystals. J. Exp. Theor. Phys. 2018, 126, 340–346. [Google Scholar] [CrossRef]
- Konchakov, R.A.; Kobelev, N.P.; Khonik, V.A.; Makarov, A.S. Elastic dipoles in the model of single-crystal and amorphous copper. Phys. Sol. State 2016, 58, 215–222. [Google Scholar] [CrossRef]
- Goncharova, E.V.; Konchakov, R.A.; Makarov, A.S.; Kobelev, N.P.; Khonik, V.A. Identification of interstitial-like defects in a computer model of glassy aluminum. J. Phys. Condens. Matter 2017, 29, 305701. [Google Scholar] [CrossRef]
- Kobelev, N.P.; Kolyvanov, E.L.; Khonik, V.A. Higher order elastic moduli of the bulk metallic glass Zr52.5Ti5Cu17.9Ni14.6Al10. Phys. Sol. State 2007, 49, 1209–1215. [Google Scholar] [CrossRef]
- Kobelev, N.P.; Kolyvanov, E.L.; Khonik, V.A. Higher-order elastic moduli of the metallic glass Pd40Cu30Ni10P20. Phys. Sol. State 2015, 57, 1483–1487. [Google Scholar] [CrossRef]
- Konchakov, R.A.; Makarov, A.S.; Afonin, G.V.; Mitrofanov, Y.P.; Kobelev, N.P.; Khonik, V.A. Estimate of the fourth-rank shear modulus in metallic glasses. J. Alloys Compd. 2017, 714, 168–171. [Google Scholar] [CrossRef]
- Mitrofanov, Y.P.; Wang, D.P.; Makarov, A.S.; Wang, W.H.; Khonik, V.A. Towards understanding of heat effects in metallic glasses on the basis of macroscopic shear elasticity. Sci. Rep. 2016, 6, 23026. [Google Scholar] [CrossRef]
- Makarov, A.S.; Afonin, G.V.; Mitrofanov, Y.P.; Kobelev, N.P.; Khonik, V.A. Heat effects occurring in the supercooled liquid state and upon crystallization of metallic glasses as a result of thermally activated evolution of their defect systems. Phys. Stat. Sol. (A) 2019, submitted. [Google Scholar]
- Kobelev, N.P.; Khonik, V.A. Theoretical analysis of the interconnection between the shear elasticity and heat effects in metallic glasses. J. Non-Cryst. Sol. 2015, 427, 184–190. [Google Scholar] [CrossRef]
- Afonin, G.V.; Mitrofanov, Y.P.; Makarov, A.S.; Kobelev, N.P.; Wang, W.H.; Khonik, V.A. Universal relationship between crystallization-induced changes of the shear modulus and heat release in metallic glasses. Acta Mater. 2016, 115, 204–209. [Google Scholar] [CrossRef]
- Makarov, A.S.; Mitrofanov, Y.P.; Afonin, G.V.; Kobelev, N.P.; Khonik, V.A. Shear susceptibility—A universal integral parameter relating the shear softening, heat effects, anharmonicity of interatomic interaction and “defect” structure of metallic glasses. Intermetallics 2017, 87, 1–5. [Google Scholar] [CrossRef]
- Kobelev, N.P.; Khonik, V.A.; Makarov, A.S.; Afonin, G.V.; Mitrofanov, Yu.P. On the nature of heat effects and shear modulus softening in metallic glasses: A generalized approach. J. Appl. Phys. 2014, 115, 033513. [Google Scholar] [CrossRef]
- Makarov, A.S.; Mitrofanov, Y.P.; Afonin, G.V.; Kobelev, N.P.; Khonik, V.A. Predicting temperature dependence of the shear modulus of metallic glasses using calorimetric data. Scr. Mater. 2019, 168, 10–13. [Google Scholar] [CrossRef]
- Makarov, A.S.; Afonin, G.V.; Mitrofanov, Y.P.; Konchakov, R.A.; Kobelev, N.P.; Qiao, J.C.; Khonik, V.A. Relationship between the heat effects and shear modulus changes occurring upon heating of a metallic glass into the supercooled liquid state. J. Non-Cryst. Sol. 2018, 500, 129–132. [Google Scholar] [CrossRef]
- Mitrofanov, Y.P.; Afonin, G.V.; Makarov, A.S.; Kobelev, N.P.; Khonik, V.A. A new understanding of the sub-Tg enthalpy relaxation in metallic glasses. Intermetallics 2018, 101, 116–122. [Google Scholar] [CrossRef]
- Chen, H.S. On mechanisms of structural relaxation in a Pd48Ni32P20 glass. J. Non-Cryst. Sol. 1981, 46, 289–305. [Google Scholar] [CrossRef]
- Busch, R.; Johnson, W.L. The kinetic glass transition of the Zr46.75Ti8.25Cu7.5Ni10Be27.5 bulk metallic glass former-supercooled liquids on a long time scale. Appl. Phys. Lett. 1998, 72, 2695–2697. [Google Scholar] [CrossRef]
- Gordon, C.A.; Granato, A.V.; Simmons, R.O. Evidence for the self-interstitial model of liquid and amorphous states from lattice parameter measurements in krypton. J. Non-Cryst. Sol. 1996, 205–207, 216–220. [Google Scholar] [CrossRef]
- Goncharova, E.V.; Konchakov, R.A.; Makarov, A.S.; Kobelev, N.P.; Khonik, V.A. On the nature of density changes upon structural relaxation and crystallization of metallic glasses. J. Non-Cryst. Sol. 2017, 471, 396–399. [Google Scholar] [CrossRef]
- Harms, U.; Jin, O.; Schwarz, R.B. Effects of plastic deformation on the elastic modulus and density of bulk amorphous Pd40Cu30Ni10P20. Non-Cryst. Sol. 2003, 317, 200–205. [Google Scholar] [CrossRef]
- Shen, T.D.; Harms, U.; Schwarz, R.B. Correlation between the volume change during crystallization and the thermal stability of supercooled liquids. Appl. Phys. Lett. 2003, 83, 4512–4514. [Google Scholar] [CrossRef]
- Safarik, D.J.; Schwarz, R.B. Elastic constants of amorphous and single-crystal Pd40Cu40P20. Acta Mater. 2007, 55, 5736–5746. [Google Scholar] [CrossRef]
- Panova, G.K.; Chernoplekov, N.A.; Shikov, A.A.; Savel’ev, B.I.; Khlopkin, M.N. Effects of amorphization on the vibrational specific heat of metallic glasses. Sov. Phys. JETP 1985, 61, 595–598. [Google Scholar]
- Zhao, Y.; Zhang, B.; Sato, K. Unusual volume change associated with crystallization in Ce-Ga-Cu bulk metallic glass. Intermetallics 2017, 88, 1–5. [Google Scholar] [CrossRef]
- Makarov, A.S.; Mitrofanova, Y.P.; Konchakov, R.A.; Kobelev, N.P.; Csach, K.; Qiao, J.C.; Khonik, V.A. Density and shear modulus changes occurring upon structural relaxation and crystallization of Zr-based bulk metallic glasses: In situ measurements and their interpretation. J. Non-Cryst. Sol. 2019. submitted. [Google Scholar]
- Afonin, G.V.; Mitrofanov, Y.P.; Kobelev, N.P.; da Silva Pinto, M.W.; Wilde, G.; Khonik, V.A. Relationship between the enthalpies of structural relaxation, crystallization and melting in metallic glass-forming systems. Scr. Mater. 2019, 166, 6–9. [Google Scholar] [CrossRef]
- Philips, W.A. Amorphous Solids: Low Temperature Properties; Springer: Berlin, Germany, 1981. [Google Scholar]
- Gil, L.; Ramos, M.A.; Bringer, A.; Buchenau, U. Low-temperature specific heat and thermal conductivity of glasses. Phys. Rev. Lett. 1993, 70, 182–185. [Google Scholar] [CrossRef]
- Li, Y.; Bai, H.Y.; Wang, W.H.; Samwer, K. Low-temperature specific-heat anomalies associated with the boson peak in CuZr-based bulk metallic glasses. Phys. Rev. B 2006, 74, 052201. [Google Scholar] [CrossRef]
- Zorn, R. The boson peak demystified? Physics 2011, 4, 44. [Google Scholar] [CrossRef]
- Shintani, H.; Tanaka, H. Universal link between the boson peak and transverse phonons in glass. Nat. Mater. 2008, 7, 870–877. [Google Scholar] [CrossRef]
- Sheng, H.; Ma, E.; Kramer, M. Relating dynamic properties to atomic structure in metallic glasses. JOM 2012, 64, 856–881. [Google Scholar] [CrossRef]
- Jakse, N.; Nassour, A.; Pasturel1, A. Structural and dynamic origin of the boson peak in a Cu-Zr metallic glass. Phys. Rev. B 2012, 85, 174201. [Google Scholar] [CrossRef]
- Granato, A.V. Interstitial resonance modes as a source of the boson peak in glasses and liquids. Physica B 1996, 219–220, 270–272. [Google Scholar] [CrossRef]
- Khonik, V.A.; Kobelev, N.P.; Mitrofanov, Y.P.; Zakharov, K.V.; Vasiliev, A.N. Boson heat capacity peak in metallic glasses: Evidence of the same defect-induced heat absorption mechanism in structurally relaxed and partially crystallized states. Phys. Stat. Sol. RRL 2018, 12, 1700412. [Google Scholar] [CrossRef]
- Mitrofanov, Y.P.; Makarov, A.S.; Afonin, G.V.; Zakharov, K.V.; Vasiliev, A.N.; Kobelev, N.P.; Wilde, G.; Khonik, V.A. Relationship between the boson heat capacity peak and the excess enthalpy of a metallic glass. Phys. Stat. Sol. RRL 2019, 1900046. [Google Scholar] [CrossRef]
- Brink, T.; Koch, L.; Albe, K. Structural origins of the boson peak in metals: From high-entropy alloys to metallic glasses. Phys. Rev. B 2016, 94, 224203. [Google Scholar] [CrossRef]
- Ding, J.; Patinet, S.; Falk, M.L.; Cheng, Y.; Ma, E. Soft spots and their structural signature in a metallic glass. Proc. Natl. Acad. Sci. USA 2014, 111, 14052. [Google Scholar] [CrossRef]
- Mitrofanov, Y.P.; Kobelev, N.P.; Khonik, V.A. On the relationship of the properties of metallic glasses and their maternal crystals. Phys. Sol. State 2019, 61, 962–968. [Google Scholar]
- Granato, A.V. Self-interstitials as basic structural units if liquids and glasses. J. Phys. Chem. Sol. 1994, 55, 931–939. [Google Scholar] [CrossRef]
- Wakeda, M.; Saida, J.; Li, J.; Ogata, S. Controlled rejuvenation of amorphous metals with thermal processing. Sci. Rep. 2015, 5, 10545. [Google Scholar] [CrossRef]
- Guo, W.; Yamada, R.; Saida, J.; Lü, S.; Wu, S. Thermal rejuvenation of a heterogeneous metallic glass. J. Non-Cryst. Sol. 2018, 498, 8–13. [Google Scholar] [CrossRef]
- Ingle, W.; Perrin, R.C.; Schober, H.R. Interstitial cluster in FCC metals. J. Phys. F Met. Phys. 1981, 11, 1161–1173. [Google Scholar] [CrossRef]
- Greer, A.L. Metallic glasses. In Physical Metallurgy; Laughlin, D.E., Hono, K., Eds.; Elsevier: Oxford, UK, 2014; Volume I, pp. 305–385. [Google Scholar]
- Cheng, Y.Q.; Cao, A.J.; Ma, E. Correlation between the elastic modulus and the intrinsic plastic behavior of metallic glasses: The roles of atomic configuration and alloy composition. Acta Mater. 2009, 57, 3253–3267. [Google Scholar] [CrossRef]
- Ding, J.; Cheng, Y.Q.; Ma, E. Full icosahedra dominate local order in Cu64Zr34 metallic glass and supercooled liquid. Acta Mater. 2014, 69, 343–354. [Google Scholar] [CrossRef]
- Mitrofanov, Y.P.; Kobelev, N.P.; Khonik, V.A. Different metastable equilibrium states in metallic glasses occurring far below and near the glass transition. J. Non-Cryst. Sol. 2018, 497, 48–55. [Google Scholar] [CrossRef]
- Makarov, A.S.; Afonin, G.V.; Mitrofanov, Y.P.; Konchakov, R.A.; Kobelev, N.P.; Qiao, J.C.; Khonik, V.A. Evolution of the activation energy spectrum and defect concentration upon structural relaxation of a metallic glass determined using calorimetry and shear modulus data. J. Alloys Comp. 2018, 745, 378–384. [Google Scholar] [CrossRef]
- Donati, C.; Glotzer, S.C.; Poole, P.H.; Kob, W.; Plimpton, S.J. Spatial correlations of mobility and immobility in a glass-forming Lennard-Jones liquid. Phys. Rev. E 1999, 60, 3107–3119. [Google Scholar] [CrossRef]
- Oligschleger, C.; Schober, H.R. Collective jumps in a soft-sphere glass. Phys. Rev. B 1999, 59, 811–821. [Google Scholar] [CrossRef]
- Schober, H.R. Collectivity of motion in undercooled liquids and amorphous solids. J. Non-Cryst. Sol. 2002, 307–310, 40–49. [Google Scholar] [CrossRef]
- Betancourt, B.A.P.; Douglas, J.F.; Starr, F.W. String model for the dynamics of glass-forming liquids. J. Chem. Phys. 2014, 140, 204509. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Du, J.-P.; Shinzato, S.; Dai, L.-H.; Ogata, S. A free energy landscape perspective on the nature of collective diffusion in amorphous solids. Acta Mater. 2018, 157, 165–173. [Google Scholar] [CrossRef]
- Li, W.; Gao, Y.; Bei, H. On the correlation between microscopic structural heterogeneity and embrittlement behavior in metallic glasses. Sci. Rep. 2015, 5, 14786. [Google Scholar] [CrossRef]
- Liu, S.T.; Li, F.X.; Li, M.Z.; Wang, W.H. Structural and dynamical characteristics of flow units in metallic glasses. Sci. Rep. 2017, 7, 11558. [Google Scholar] [CrossRef]
- Ma, E. Tuning order in disorder. Nat. Mater. 2015, 14, 547–552. [Google Scholar] [CrossRef]
- van den Beukel, A.; Radelaar, S. On the kinetics of structural relaxation in metallic glasses. Acta Metall. 1983, 31, 419–427. [Google Scholar] [CrossRef]
- van den Beukel, A.; Sietsma, J. The glass transition as a free volume related kinetic phenomenon. Acta Met. Mater. 1990, 38, 383–389. [Google Scholar] [CrossRef]
- Spaepen, F. Homogeneous flow of metallic glasses: A free volume perspective. Scr. Mater. 2006, 54, 363–367. [Google Scholar] [CrossRef]
- Wang, W.H. The elastic properties, elastic models and elastic perspectives of metallic glasses. Prog. Mater. Sci. 2012, 57, 487–656. [Google Scholar] [CrossRef]
- Cheng, Y.Q.; Ma, E. Indicators of internal structural states for metallic glasses: Local order, free volume, and configurational potential energy. Appl. Phys. Lett. 2008, 93, 051910. [Google Scholar] [CrossRef]
- Khonik, V.A.; Kobelev, N.P. Alternative understanding for the enthalpy vs. volume change upon structural relaxation of metallic glasses. J. Appl. Phys. 2014, 115, 093510. [Google Scholar] [CrossRef]

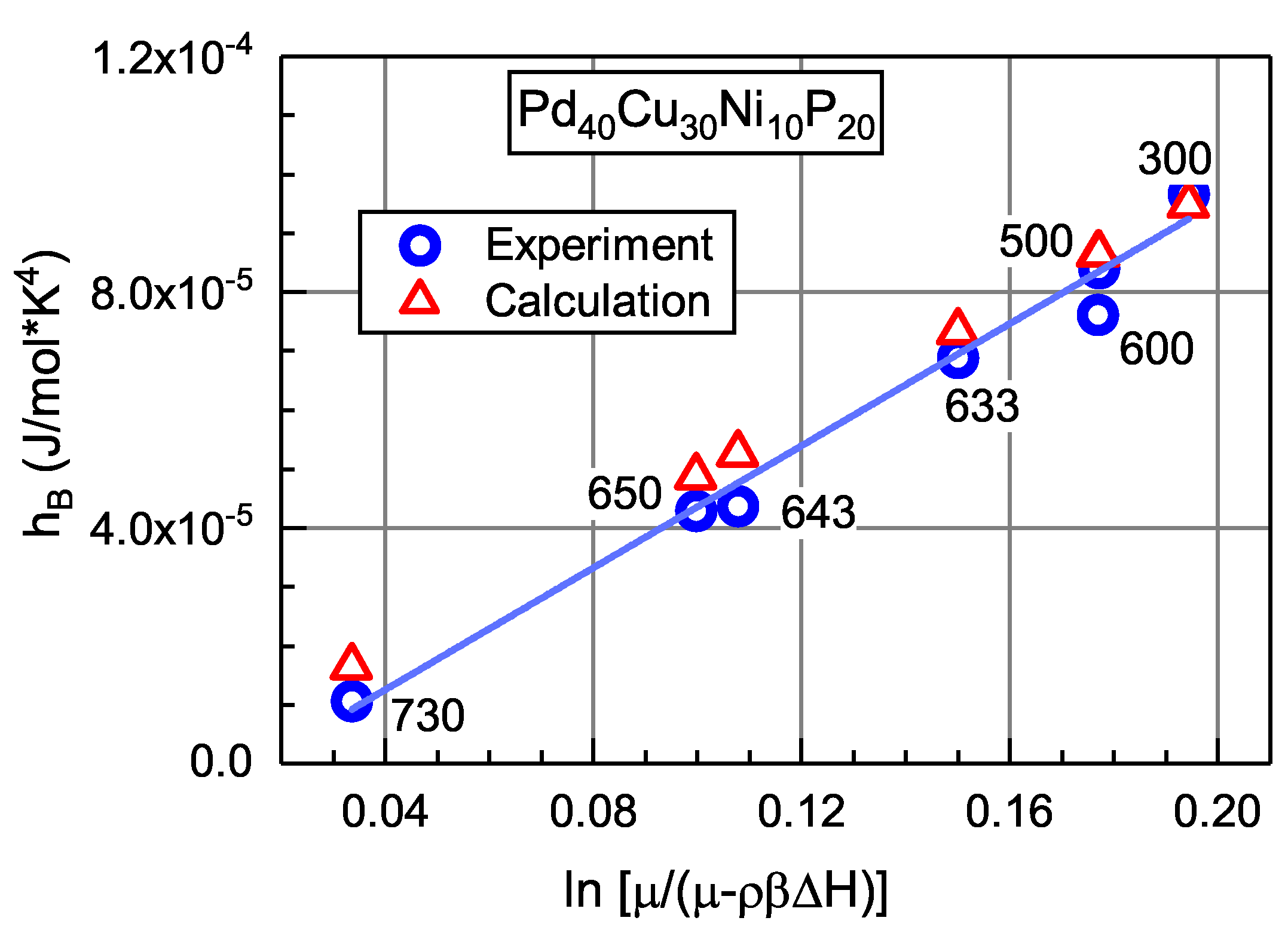
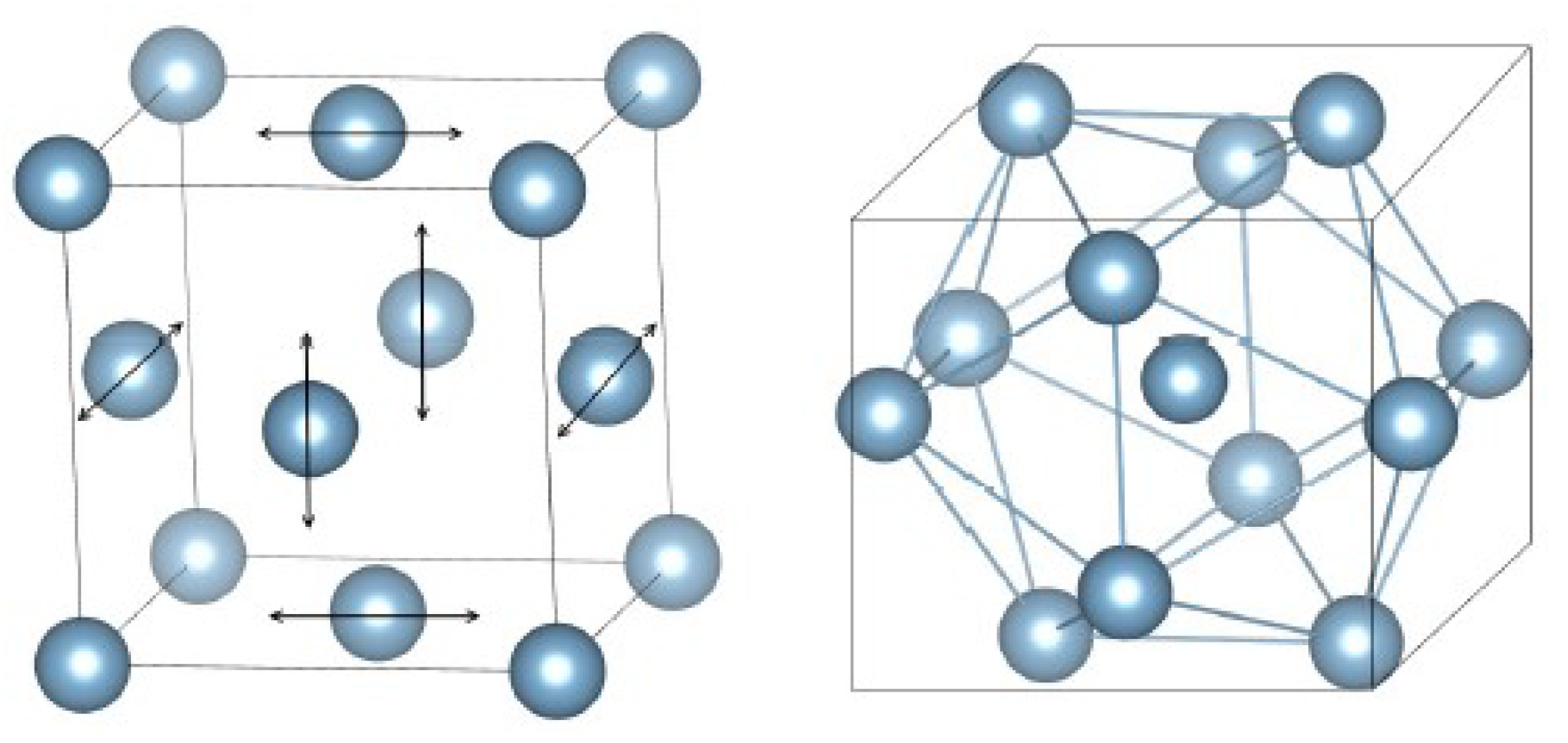
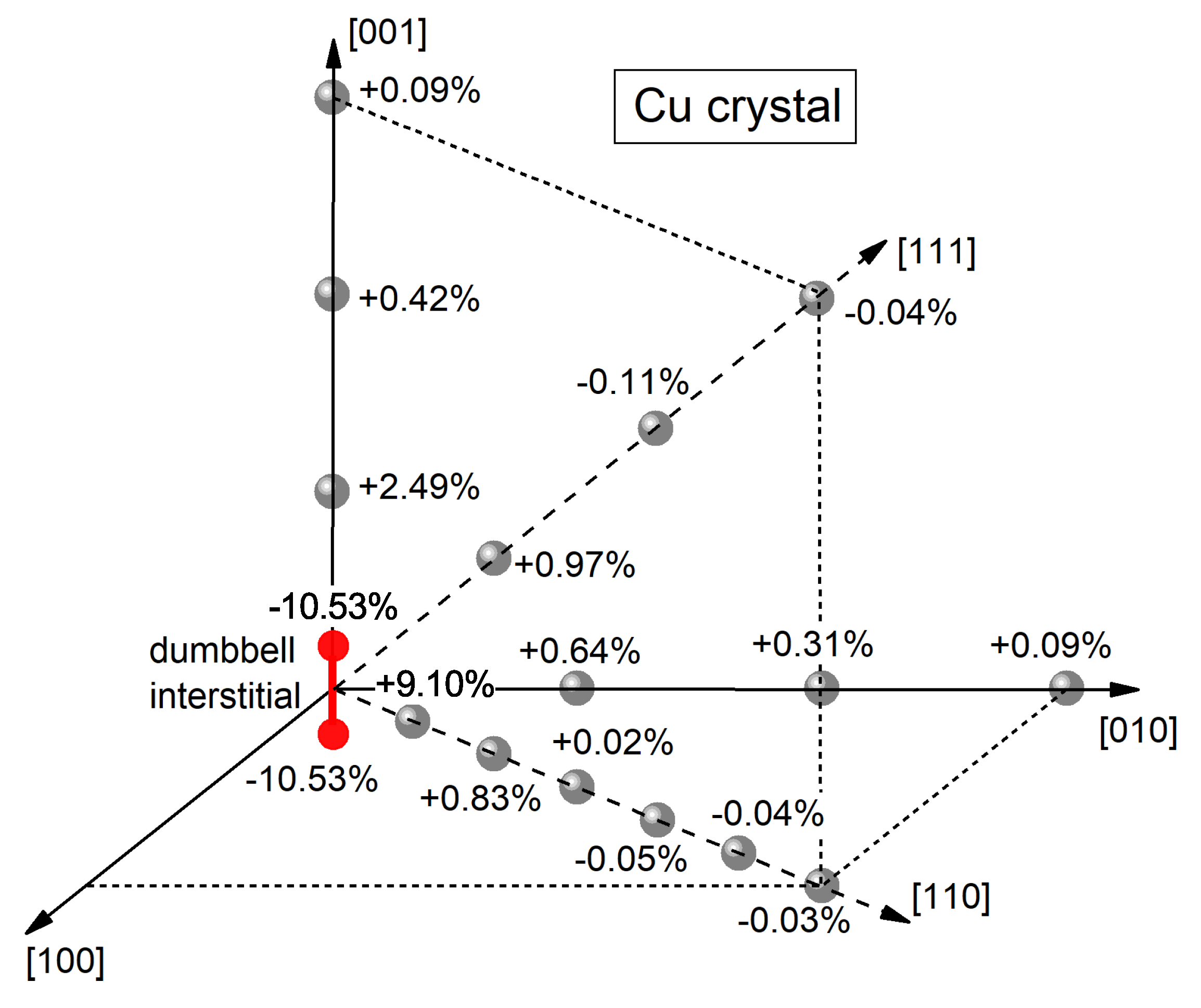
| No | Metallic Glass | |
|---|---|---|
| 1 | La55Al25Co20 | 21 |
| 2 | Zr46Cu46Al8 | 19 |
| 3 | Zr46Cu45Al7Ti2 | 21 |
| 4 | Zr56Co28Al16 | 17 |
| 5 | Zr65Al10Ni10Cu15 | 22 |
| 6 | Pd40Ni40P20 | 15 |
| 7 | Pd41.25Cu41.25P17.5 | 21 |
| 8 | Pd40Cu30Ni10P20 | 19 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khonik, V.; Kobelev, N. Metallic Glasses: A New Approach to the Understanding of the Defect Structure and Physical Properties. Metals 2019, 9, 605. https://doi.org/10.3390/met9050605
Khonik V, Kobelev N. Metallic Glasses: A New Approach to the Understanding of the Defect Structure and Physical Properties. Metals. 2019; 9(5):605. https://doi.org/10.3390/met9050605
Chicago/Turabian StyleKhonik, Vitaly, and Nikolai Kobelev. 2019. "Metallic Glasses: A New Approach to the Understanding of the Defect Structure and Physical Properties" Metals 9, no. 5: 605. https://doi.org/10.3390/met9050605
APA StyleKhonik, V., & Kobelev, N. (2019). Metallic Glasses: A New Approach to the Understanding of the Defect Structure and Physical Properties. Metals, 9(5), 605. https://doi.org/10.3390/met9050605





