Austenite Reversion Tempering-Annealing of 4 wt.% Manganese Steels for Automotive Forging Application
Abstract
1. Introduction
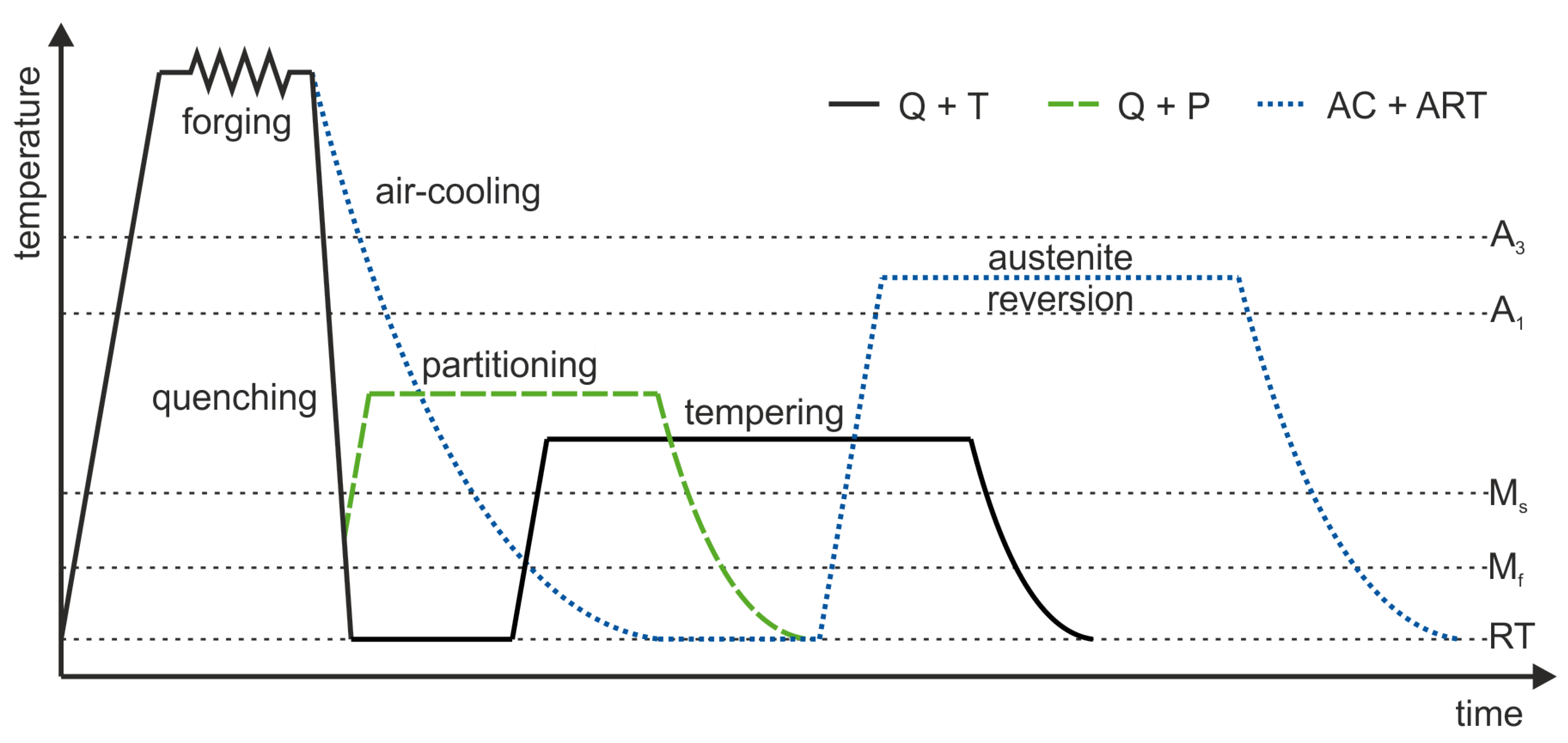
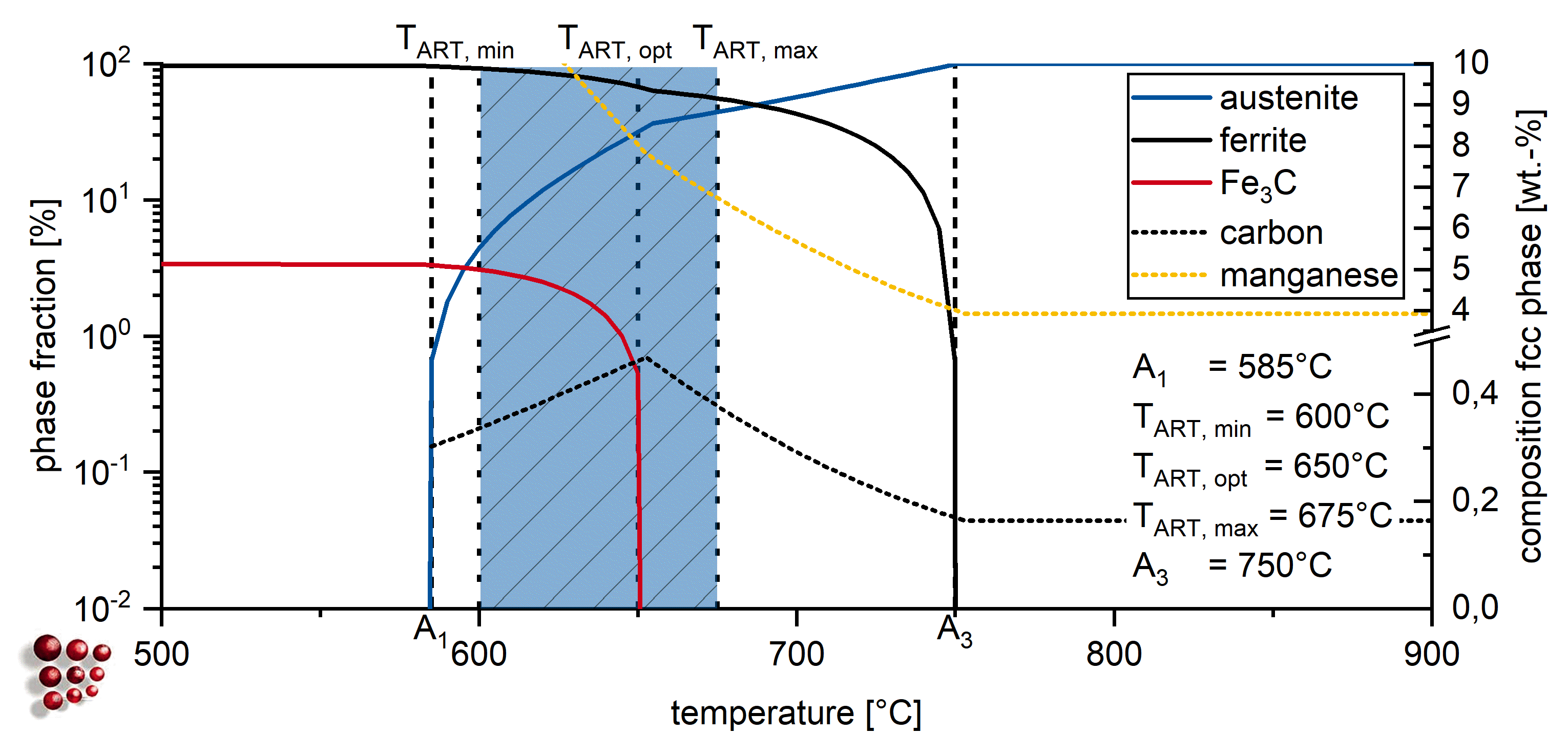
2. Materials and Methods
3. Results
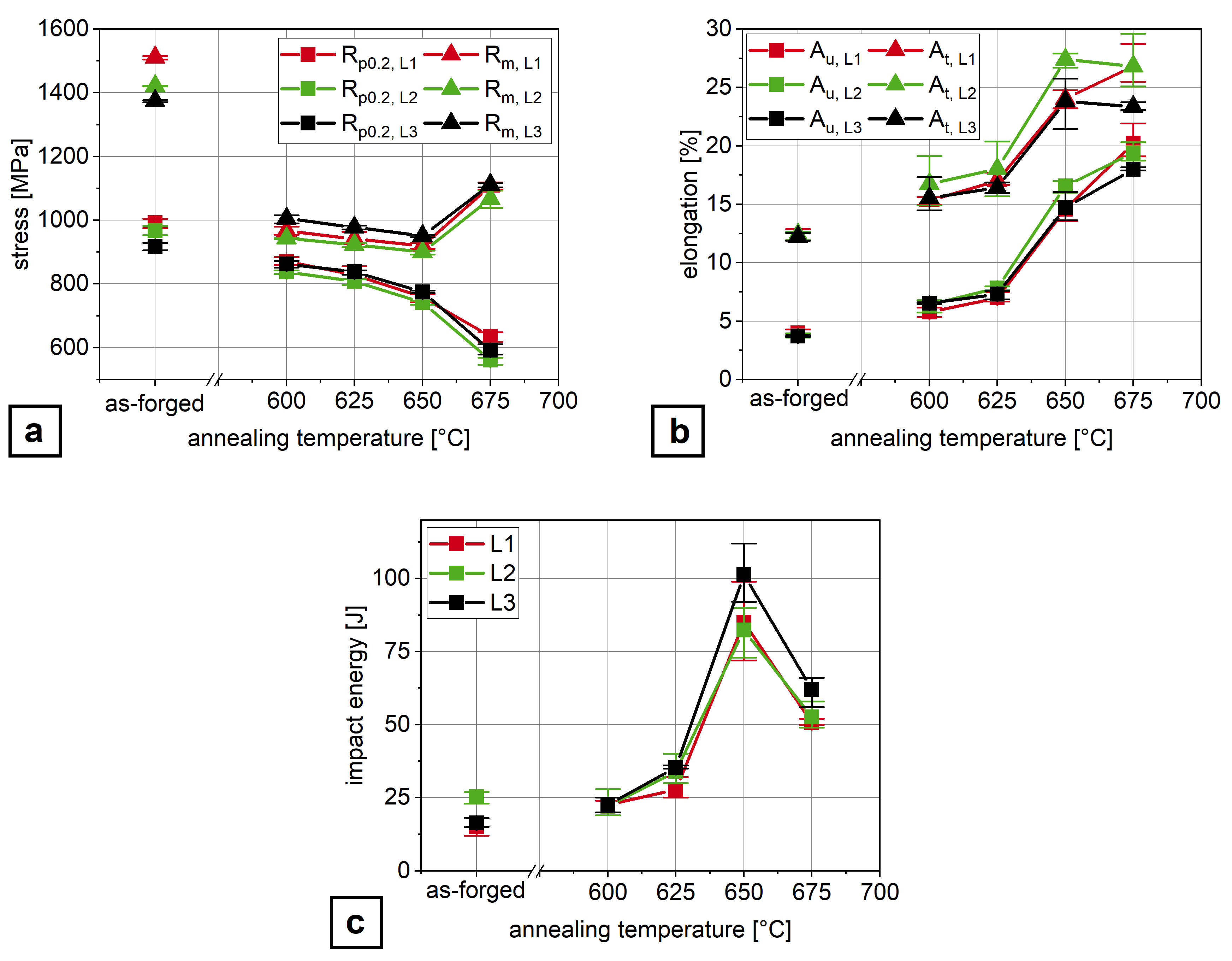
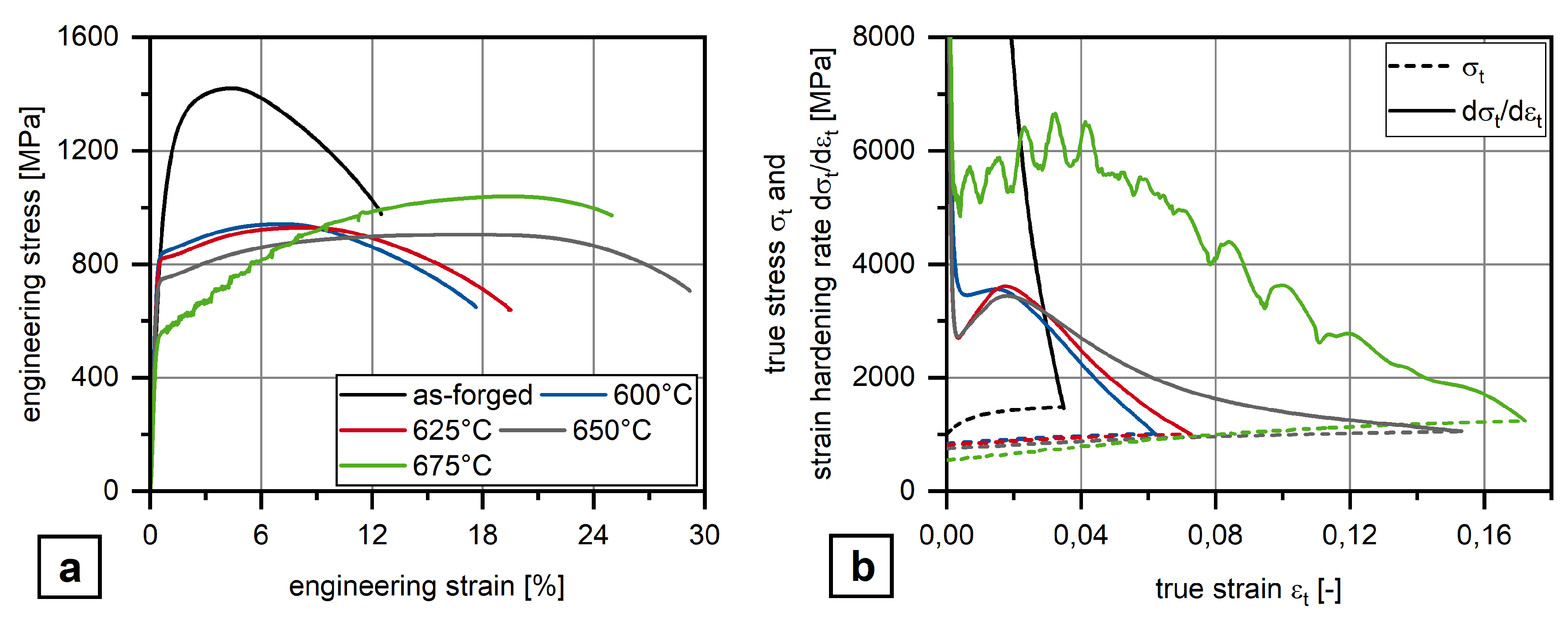
3.1. Mechanical Properties
3.2. Microstructure
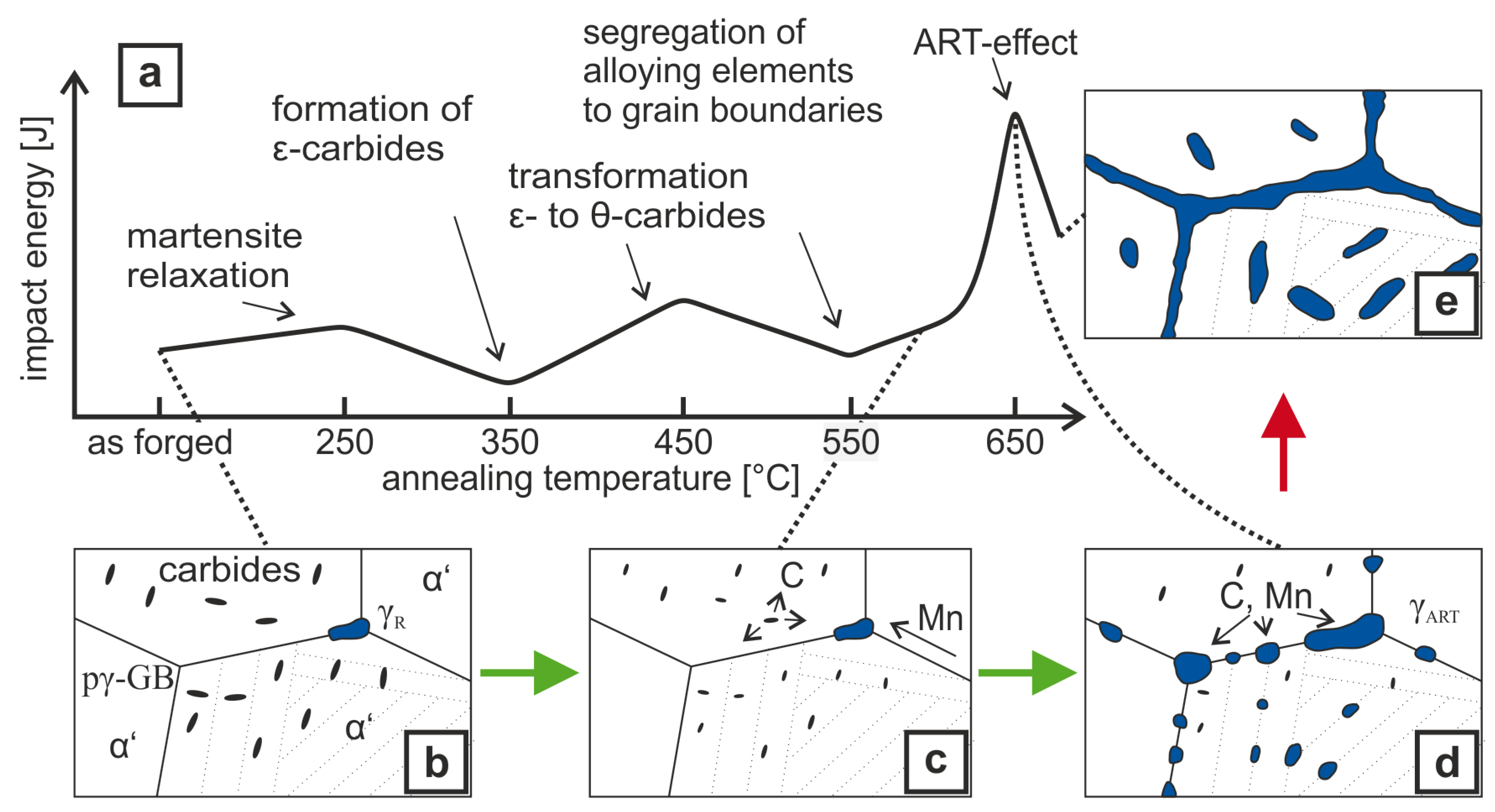
4. Discussion
5. Conclusions
- Air-hardening martensitic forging steels develop high ultimate tensile strengths from 1370 –1510 but low room temperature Charpy impact energy of below 20 .
- Microalloying with boron and molybdenum only slightly increases the Charpy impact energy after air cooling.
- Through ART-annealing, finely distributed retained austenite islands are obtained that can provide Charpy impact energy values above 80 .
- Obviously, there exist an optimum austenite content as by austenite fractions above about 10 vol.% Charpy impact energy decreases again together with the development of serrated plastic flow behavior in the tensile test.
- The Charpy impact energy improvement is due to the occurrence of a continuous TRIP effect and less Mn segregation at grain boundaries.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AC + ART | Air cooling and austenite reversion tempering |
| Q + T | Quenching and tempering |
| Q + P | Quenching and partitioning |
| ART | Austenite reverted transformation |
| EDS | Energy dispersive X-ray spectroscopy |
| PHFP | Precipitation hardening ferritic pearlitic |
| EBSD | Electron backscattering diffraction |
| SE | Secondary electron |
| SEM | Scanning electron microscopy |
| LOM | Light optical microscopy |
| HDB | High ductile bainite |
| LHD | Air hardening ductile (German abbreviation) |
References
- Bleck, W.; Bambach, M.; Wirths, V.; Stieben, A. Microalloyed Engineering Steels with Improved Performance—An Overview. HTM J. Heat Treatm. Mat. 2017, 72, 346–354. [Google Scholar] [CrossRef]
- Bleck, W.; Keul, C.; Zeislmair, B. Entwicklung eines höherfesten mikro legierten ausscheidungshärtenden ferritisch/perlitischen Schmiedestahls AFP-M. [The development of a high-strength, microalloyed, precipitation hardening, ferritic-perlitic formed steel AFP-M]. Schmiede J. 2010, 3, 42–44. [Google Scholar]
- Keul, C.; Wirths, V.; Bleck, W. New bainitic steels for forging. Arch. Civ. Mech. Eng. 2012, 12, 119–125. [Google Scholar] [CrossRef]
- Keul, C.; Urban, M.; Back, A.; Hirt, G.; Bleck, W. Entwicklung eines hochfesten duktilen bainitischen (HDB) Stahls für hochbeanspruchte Schmiedebauteile. [The Development of a High-Strength, Ductile, Bainitic (HDB) Steel for Heavy Duty Forged Components]. Schmiede J. 2010, 9, 28–31. [Google Scholar]
- Wirths, W.; Wagener, R.; Bleck, W.; Melz, T. Bainitic Forging Steels for Cyclic Loading. Adv. Mat. Res. 2014, 922, 813–818. [Google Scholar] [CrossRef]
- Edmonds, D.V.; He, K.; Rizzo, F.C.; De Cooman, B.C.; Matlock, D.K.; Speer, J.G. Quenching and partitioning martensite—A novel steel heat treatment. Mater. Sci. Eng. A 2006, 438–440, 25–34. [Google Scholar] [CrossRef]
- Stieben, A.; Bleck, W.; Schönborn, S. Lufthärtender duktiler Stahl mit mittlerem Mangangehalt für die Massivumformung. [Air hardening ductile steel with medium manganese content for forging]. massivUmformung 2016, 9, 50–55. [Google Scholar]
- Stieben, A. Eigenschaften lufthärtender martensitischer Schmiedestähle mit Mangangehalten von 3–10 Gew.-%. [Properties of Air-hardening Martensitic Forging Steels with Manganese Concentrations of 3–10 wt.-%]. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 16 March 2018. [Google Scholar]
- Hwang, S.K.; Morris, J.W. The use of a boron addition to prevent intergranular embrittlement in Fe-12Mn. Metall. Trans. A 1980, 11, 1197–1206. [Google Scholar] [CrossRef]
- Kuzmina, M.; Ponge, D.; Raabe, D. Grain boundary segregation engineering and austenite reversion turn embrittlement into toughness: Example of a 9 wt.% medium Mn steel. Acta Mater. 2015, 86, 182–192. [Google Scholar] [CrossRef]
- Song, S.; Faulkner, R.G.; Flewitt, P.E.J. Quenching and tempering-induced molybdenum segregation to grain boundaries in a 2.25Cr–1Mo steel. Mater. Sci. Eng. A 2000, 281, 23–27. [Google Scholar] [CrossRef]
- Bolton, J.D.; Petty, E.R.; Allen, G.B. The mechanical properties of α-phase low carbon Fe-Mn alloys. Metall. Trans. 1971, 2, 2915–2923. [Google Scholar] [CrossRef]
- Suh, D.W.; Ryu, J.H.; Joo, M.S.; Yang, H.S.; Lee, K.; Bhadeshia, H.K.D.K. Medium-Alloy Manganese-Rich Transformation-Induced Plasticity Steels. Metall. Mater. Trans. A 2013, 44A, 286–293. [Google Scholar] [CrossRef]
- Gramlich, A.; Emmrich, R.; Bleck, W. Annealing of 4 wt.-% Mn forging steels for automotive application. In Proceedings of the 4th International Conference on Medium and High Manganese Steels, Aachen, Germany, 1–3 April 2019; pp. 283–286. [Google Scholar]
- Miller, R.L. Ultrafine-grained microstructures and mechanical properties of alloy steels. Metall. Mater. Trans. B 1972, 3, 905–912. [Google Scholar] [CrossRef]
- Ma, Y. Medium-manganese steels processed by austenite-reverted-transformation annealing for automotive applications. Mater. Sci. Tech. 2017, 33, 1713–1727. [Google Scholar] [CrossRef]
- Kaar, S.; Schneider, R.; Krizan, D.; Béal, D.; Sommitsch, C. Influence of the Quenching and Partitioning Process on the Transformation Kinetics and Hardness in a Lean Medium Manganese TRIP Steel. Metals 2019, 9, 353. [Google Scholar] [CrossRef]
- Grajcar, A.; Kilarski, A.; Kozlowska, A. Microstructure–Property Relationships in Thermomechanically Processed Medium-Mn Steels with High Al Content. Metals 2018, 8, 929. [Google Scholar] [CrossRef]
- Liu, C.; Peng, Q.; Xue, Z.; Deng, M.; Wang, S.; Yang, Y. Microstructure-Tensile Properties Relationship and Austenite Stability of a Nb-Mo Micro-Alloyed Medium-Mn TRIP Steel. Metals 2018, 8, 615. [Google Scholar] [CrossRef]
- Sevsek, S.; Haase, C.; Bleck, W. Strain-Rate-Dependent Deformation Behavior and Mechanical Properties of a Multi-Phase Medium-Manganese Steel. Metals 2019, 9, 344. [Google Scholar] [CrossRef]
- Luo, H.; Shi, J.; Wang, C.; Cao, W.; Sun, X.; Dong, H. Experimental and numerical analysis on formation of stable austenite during the intercritical annealing of 5Mn steel. Acta Mater. 2011, 59, 4002–4014. [Google Scholar] [CrossRef]
- Chen, J.; Lv, M.; Liu, Z.; Wang, G. Combination of ductility and toughness by the design of fine ferrite/tempered martensite-austenite microstructure in a low carbon medium manganese alloyed steel plate. Mater. Sci. Eng. A 2015, 648, 51–56. [Google Scholar] [CrossRef]
- Twardowski, R.; Prahl, U. Microstructure based flow curve modeling of high-Mn steels with TWIP and TRIP effect. Comput. Methods Mater. Sci. 2012, 12, 130–136. [Google Scholar]
- Chen, J.; Lv, M.; Liu, Z.; Wang, G. Influence of Heat Treatments on the Microstructural Evolution and Resultant Mechanical Properties in a Low Carbon Medium Mn Heavy Steel Plate. Metall. Mater. Trans. A 2016, 47A, 2300–2312. [Google Scholar] [CrossRef]
- Nasim, M.; Edwards, B.C.; Wilson, E.A. A study of grain boundary embrittlement in an Fe-8%Mn alloy. Mater. Sci. Eng. A 2000, 281, 56–67. [Google Scholar] [CrossRef]
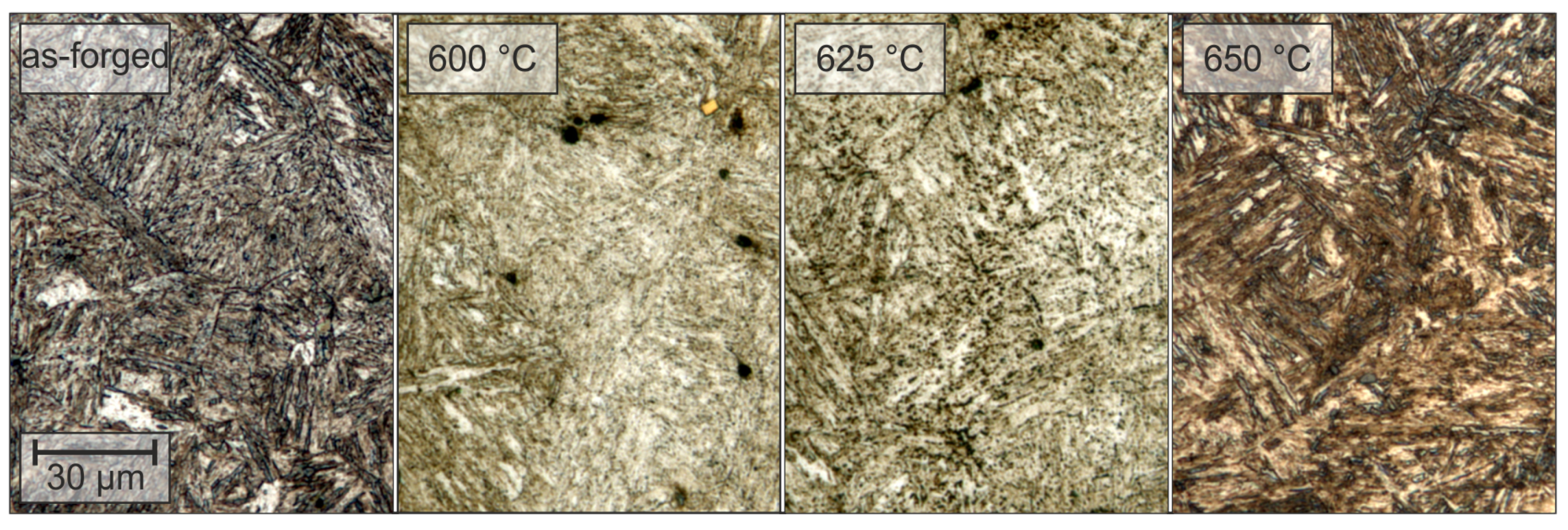
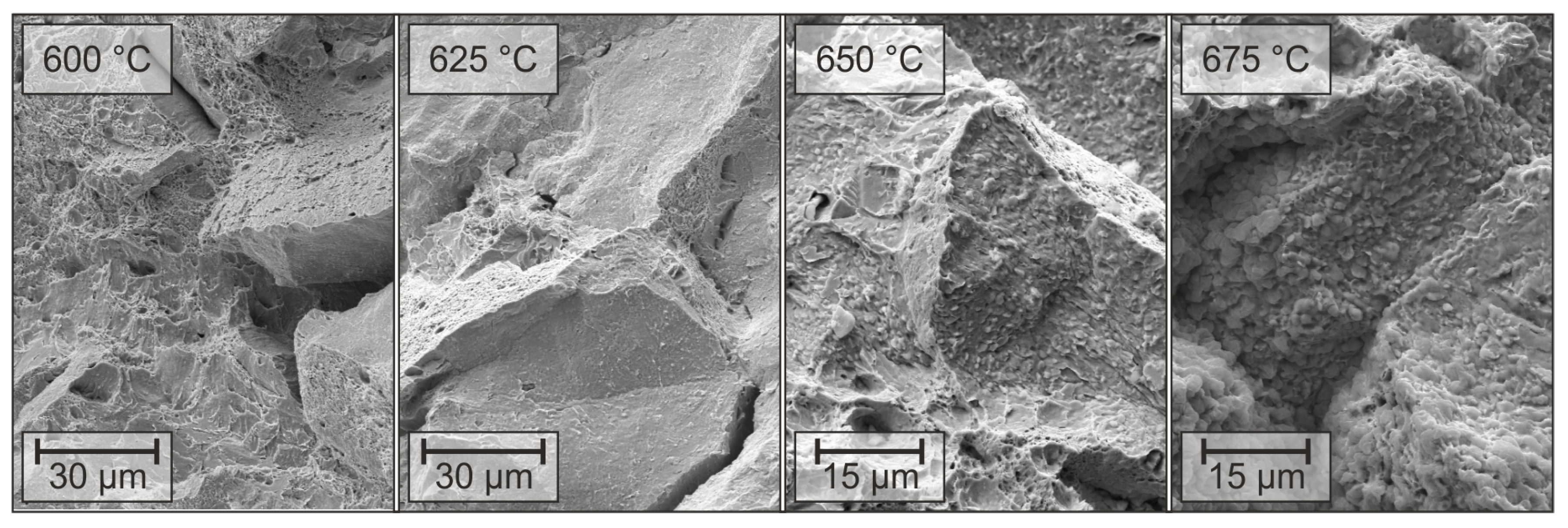
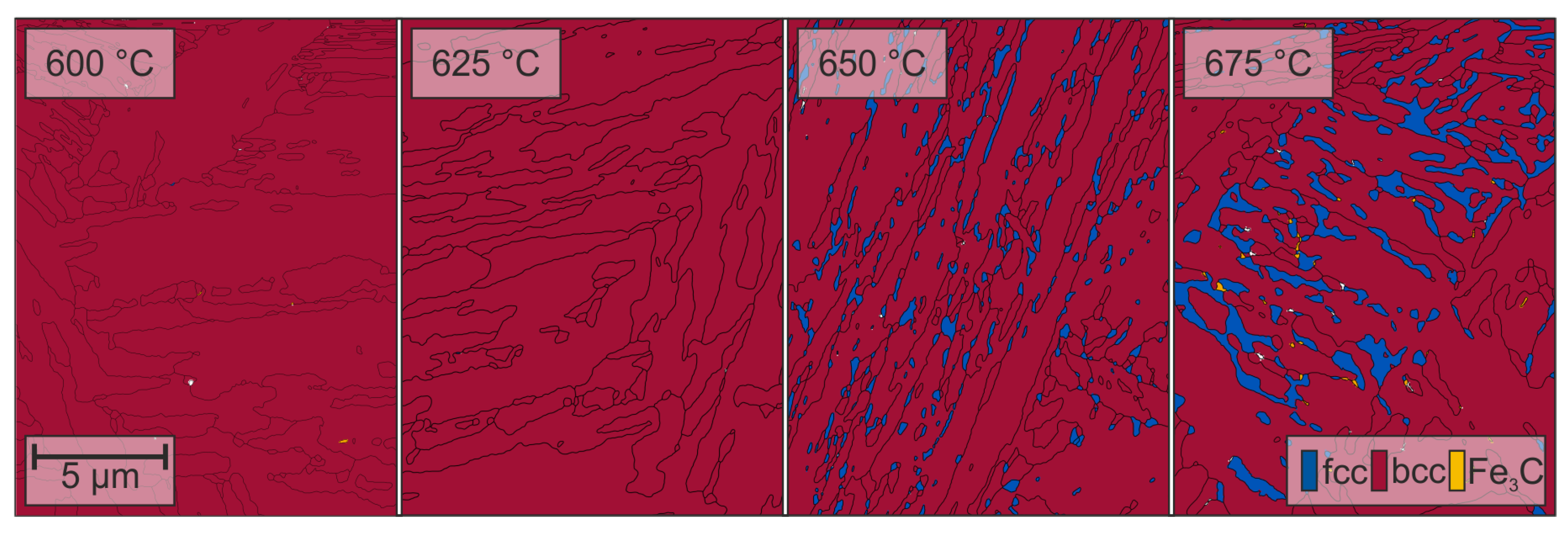
| Alloy | C * | Si | Mn | P | S * | Al | Mo | Ti | Nb | B | N |
|---|---|---|---|---|---|---|---|---|---|---|---|
| L1 | 0.19 | 0 50 | 4.02 | 0.008 | 0.011 | 0.031 | 0.02 | 0.020 | 0.035 | 0.0016 | 0.011 |
| L2 | 0.17 | 0.50 | 3.99 | 0.010 | 0.009 | 0.025 | 0.02 | 0.020 | 0.033 | 0.0057 | 0.010 |
| L3 | 0.15 | 0.49 | 4.02 | 0.011 | 0.009 | 0.027 | 0.20 | <0.003 | 0.035 | <0.0005 | 0.010 |
| Alloy | As-Forged | 600 C | 625 C | 650 C | 675 C |
|---|---|---|---|---|---|
| L1 | 15 | 23 | 28 | 85 | 51 |
| L2 | 25 | 22 | 34 | 82 | 53 |
| L3 | 16 | 22 | 35 | 101 | 62 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gramlich, A.; Emmrich, R.; Bleck, W. Austenite Reversion Tempering-Annealing of 4 wt.% Manganese Steels for Automotive Forging Application. Metals 2019, 9, 575. https://doi.org/10.3390/met9050575
Gramlich A, Emmrich R, Bleck W. Austenite Reversion Tempering-Annealing of 4 wt.% Manganese Steels for Automotive Forging Application. Metals. 2019; 9(5):575. https://doi.org/10.3390/met9050575
Chicago/Turabian StyleGramlich, Alexander, Robin Emmrich, and Wolfgang Bleck. 2019. "Austenite Reversion Tempering-Annealing of 4 wt.% Manganese Steels for Automotive Forging Application" Metals 9, no. 5: 575. https://doi.org/10.3390/met9050575
APA StyleGramlich, A., Emmrich, R., & Bleck, W. (2019). Austenite Reversion Tempering-Annealing of 4 wt.% Manganese Steels for Automotive Forging Application. Metals, 9(5), 575. https://doi.org/10.3390/met9050575






