High-Temperature Reduction and Melting Mechanism of Sinter with Different MgO Content
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization
2.2. Procedures
2.3. Analysis Methods
3. Results
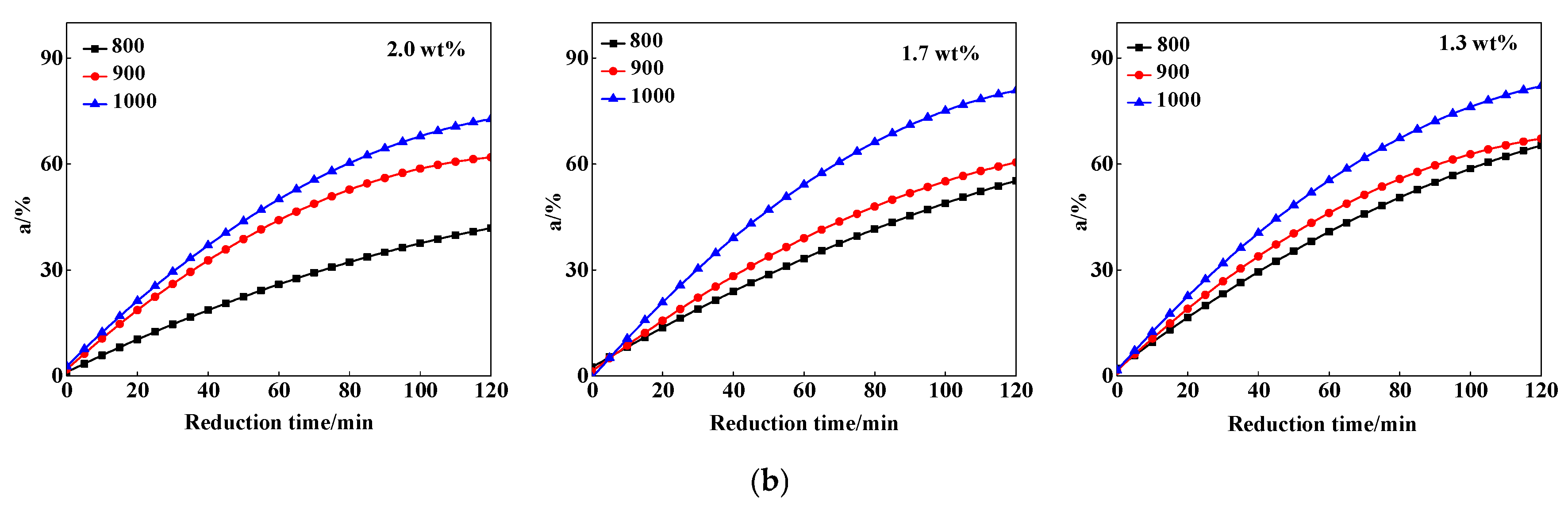
3.1. The Reduction Behavior of Different MgO Sinter
3.1.1. Effect of MgO Content and Reduction Temperature
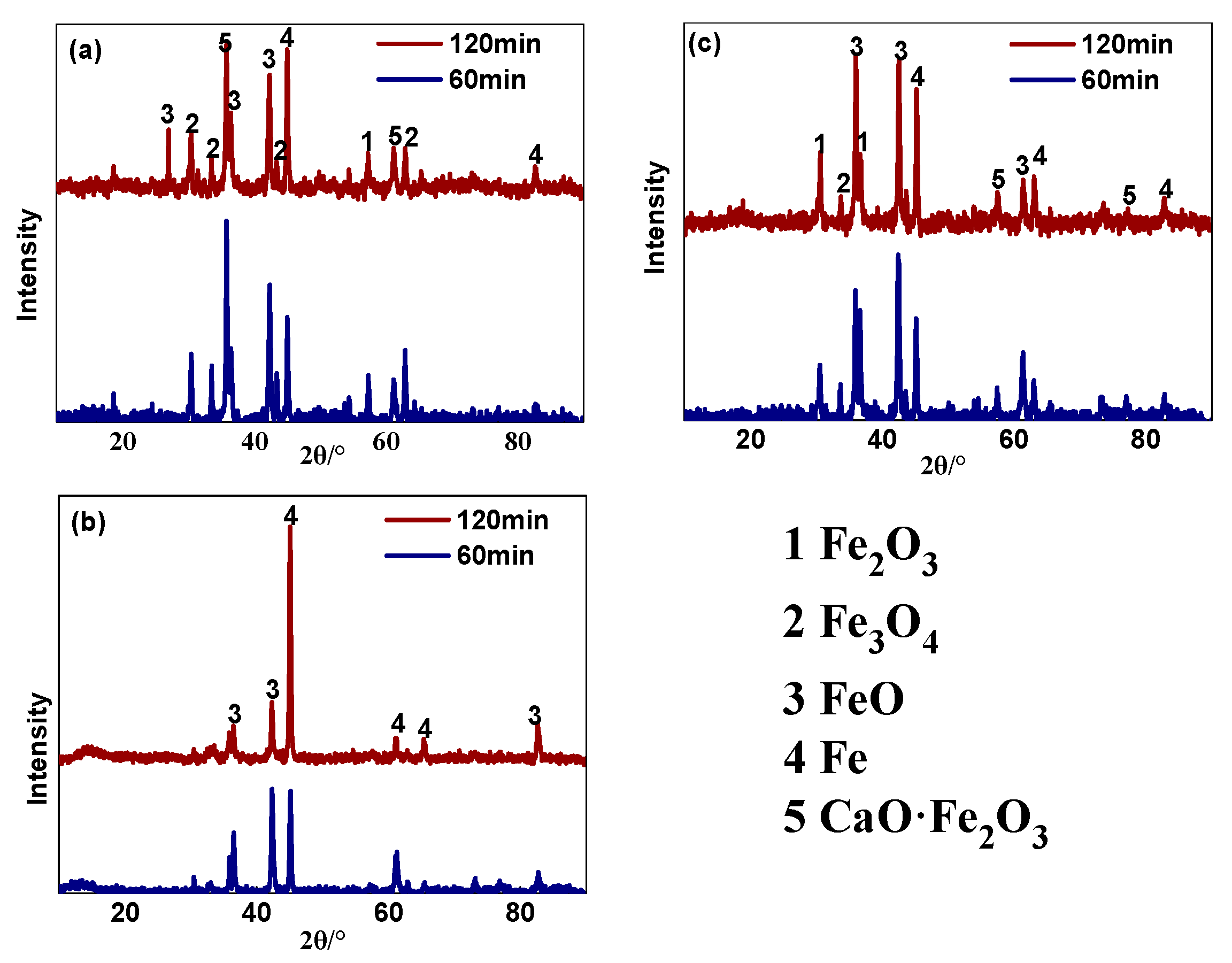
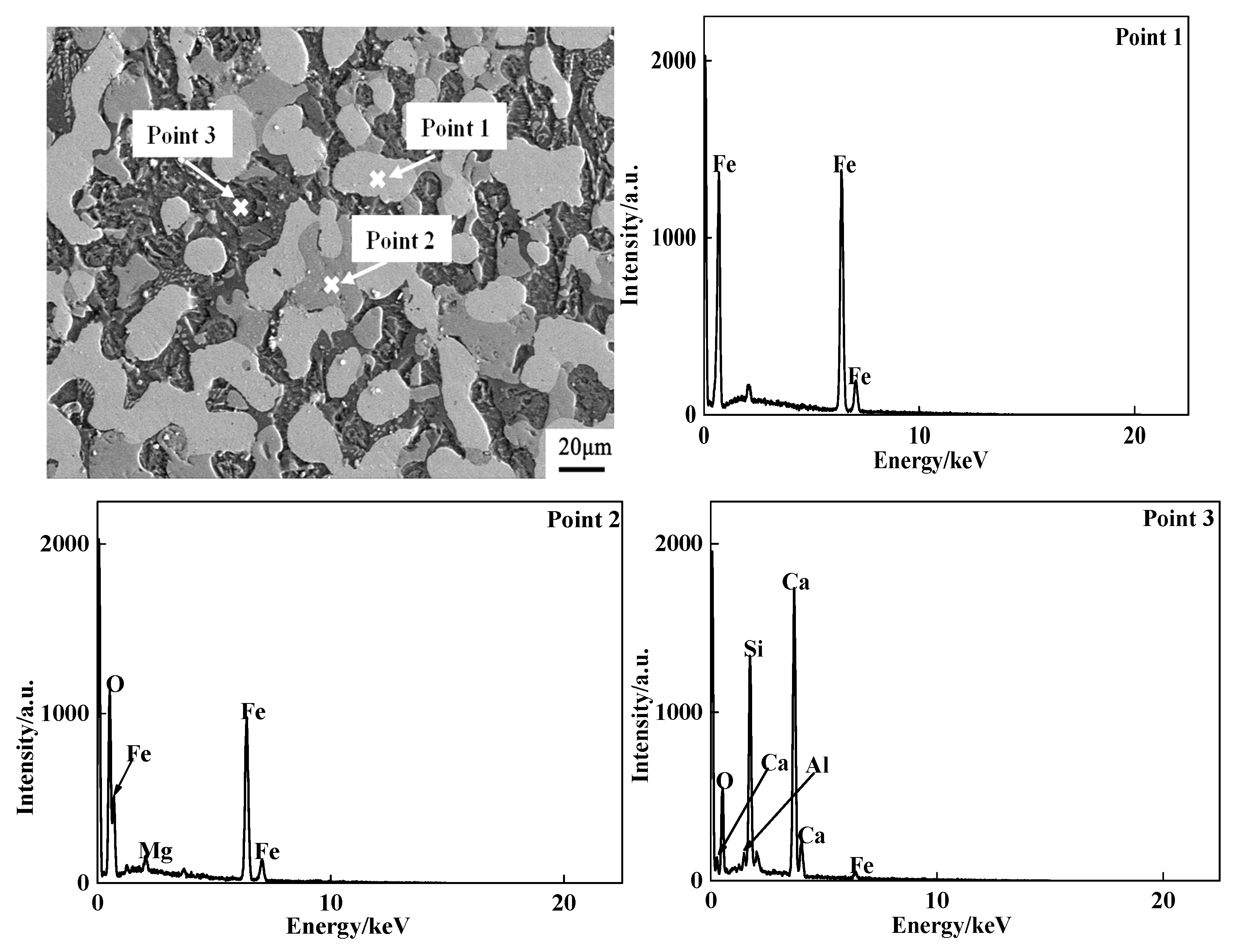
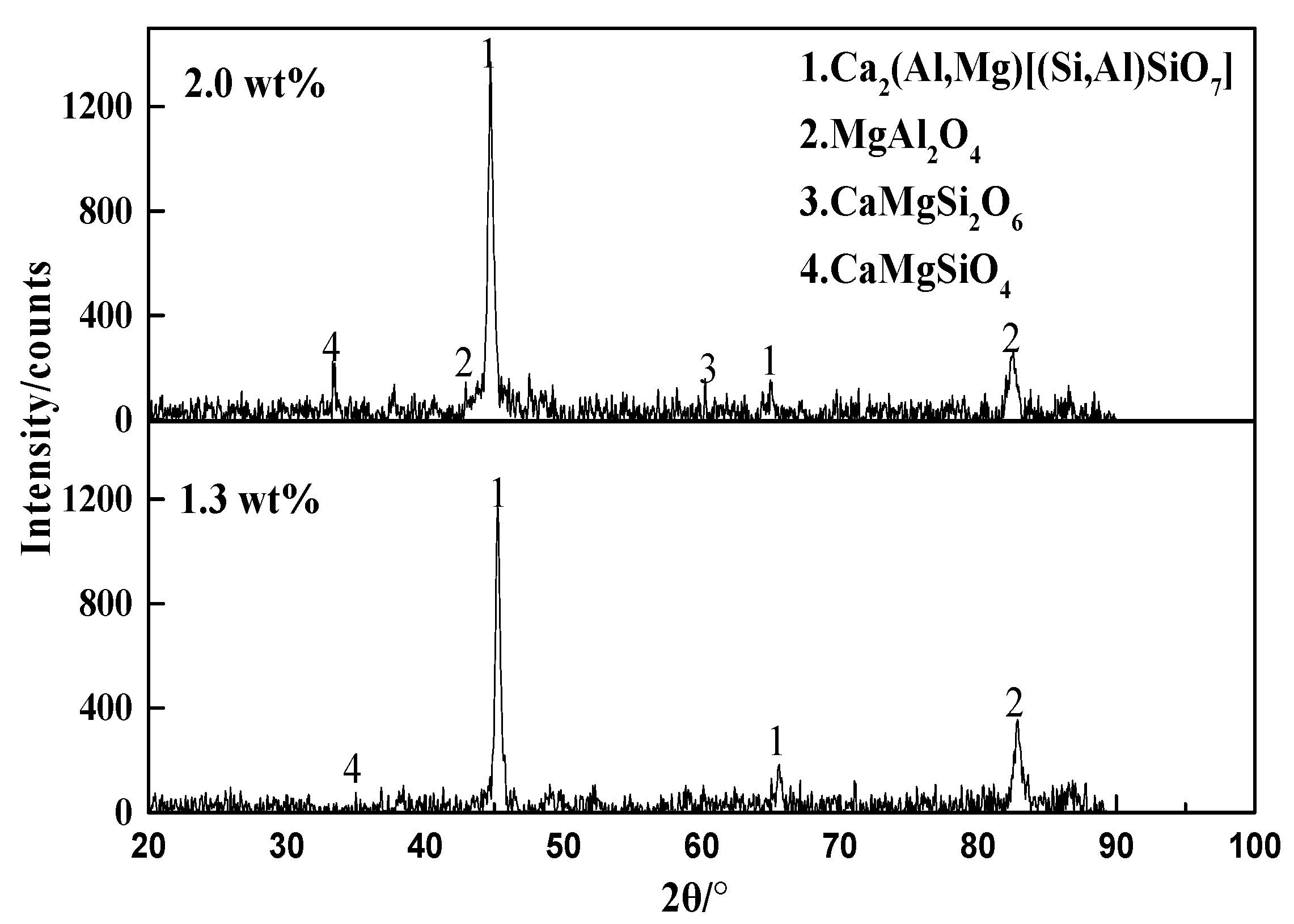
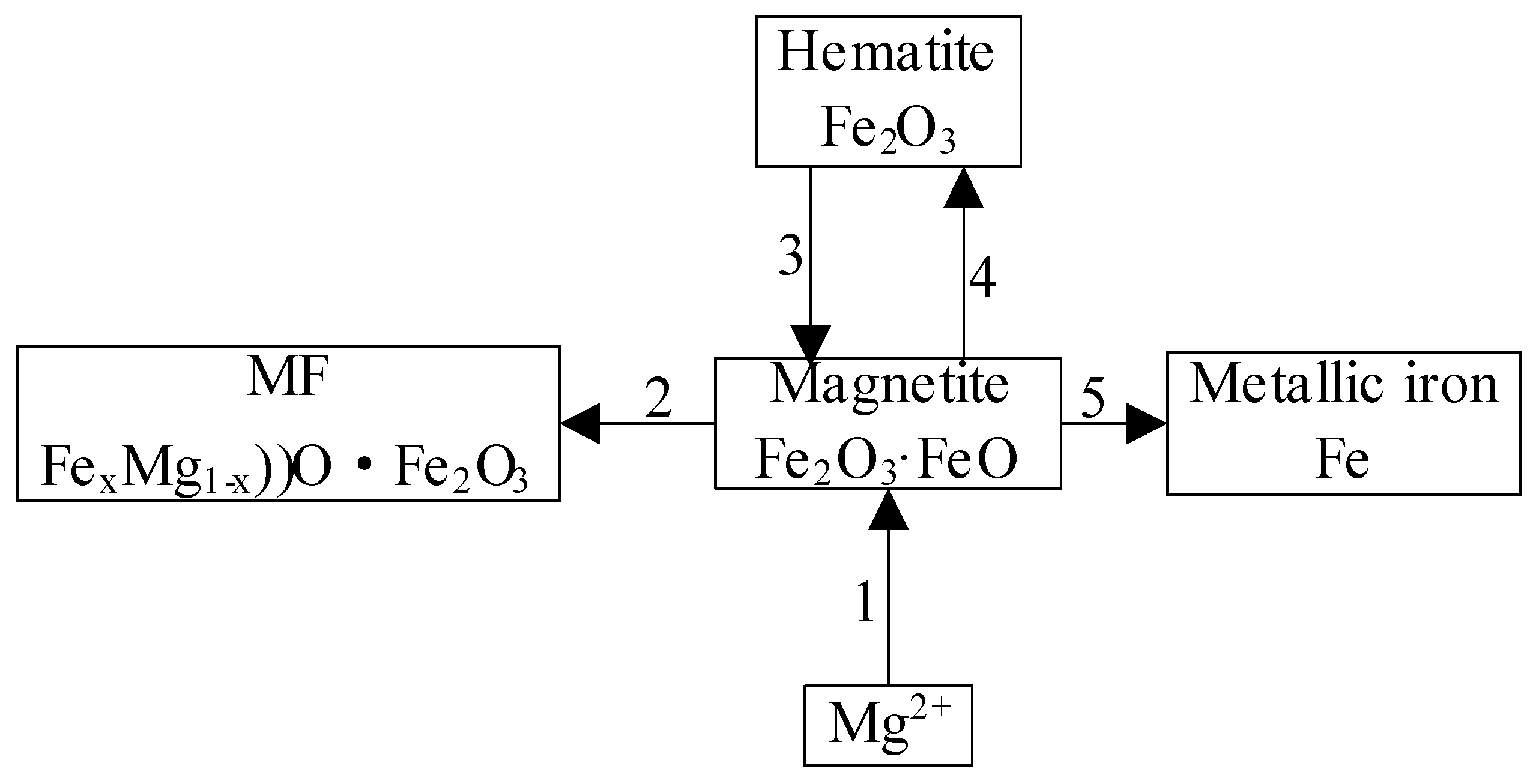
3.1.2. Phase Composition
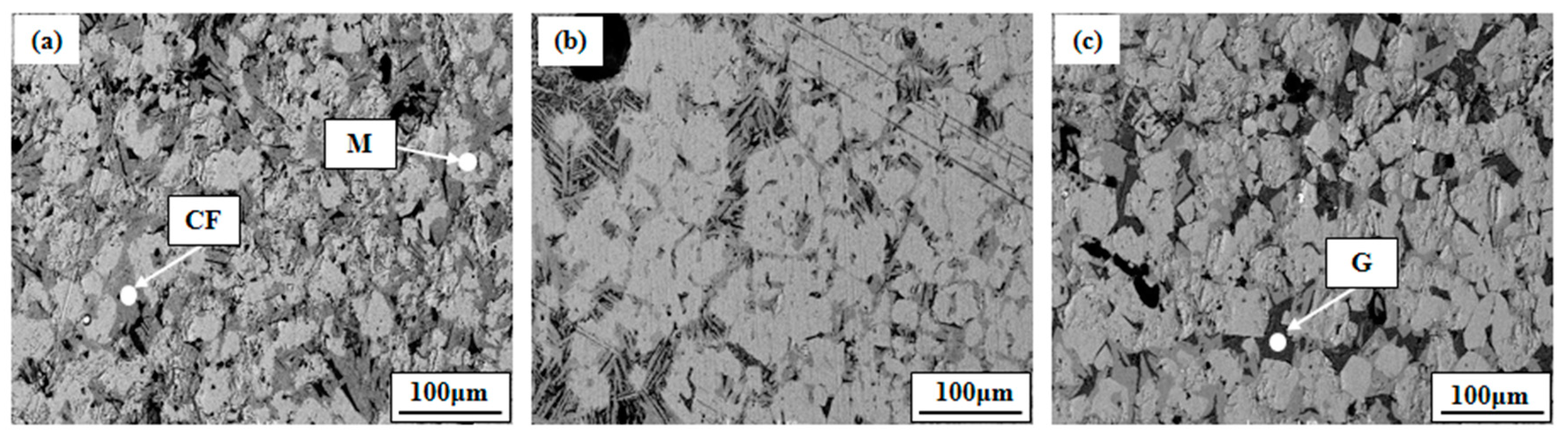
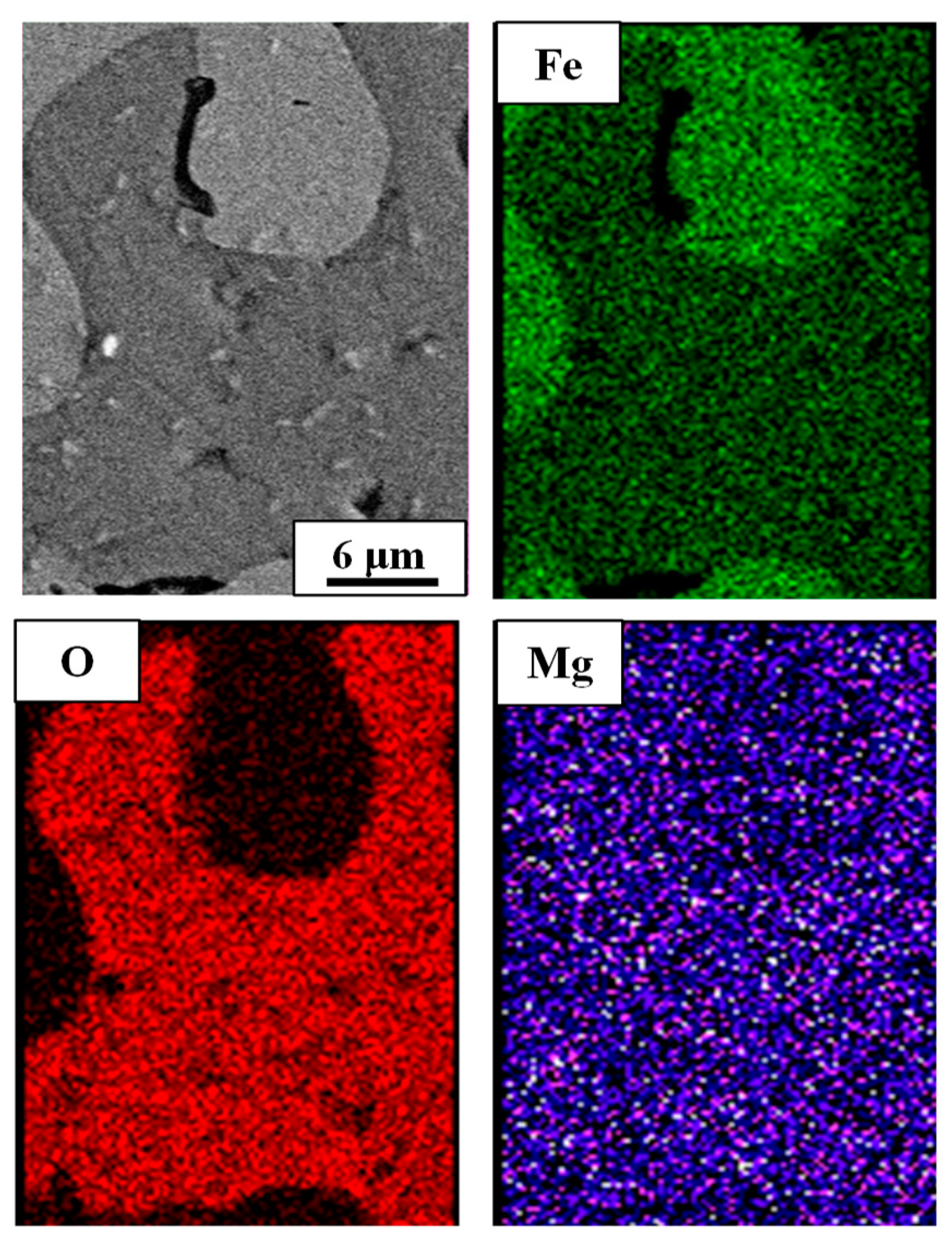
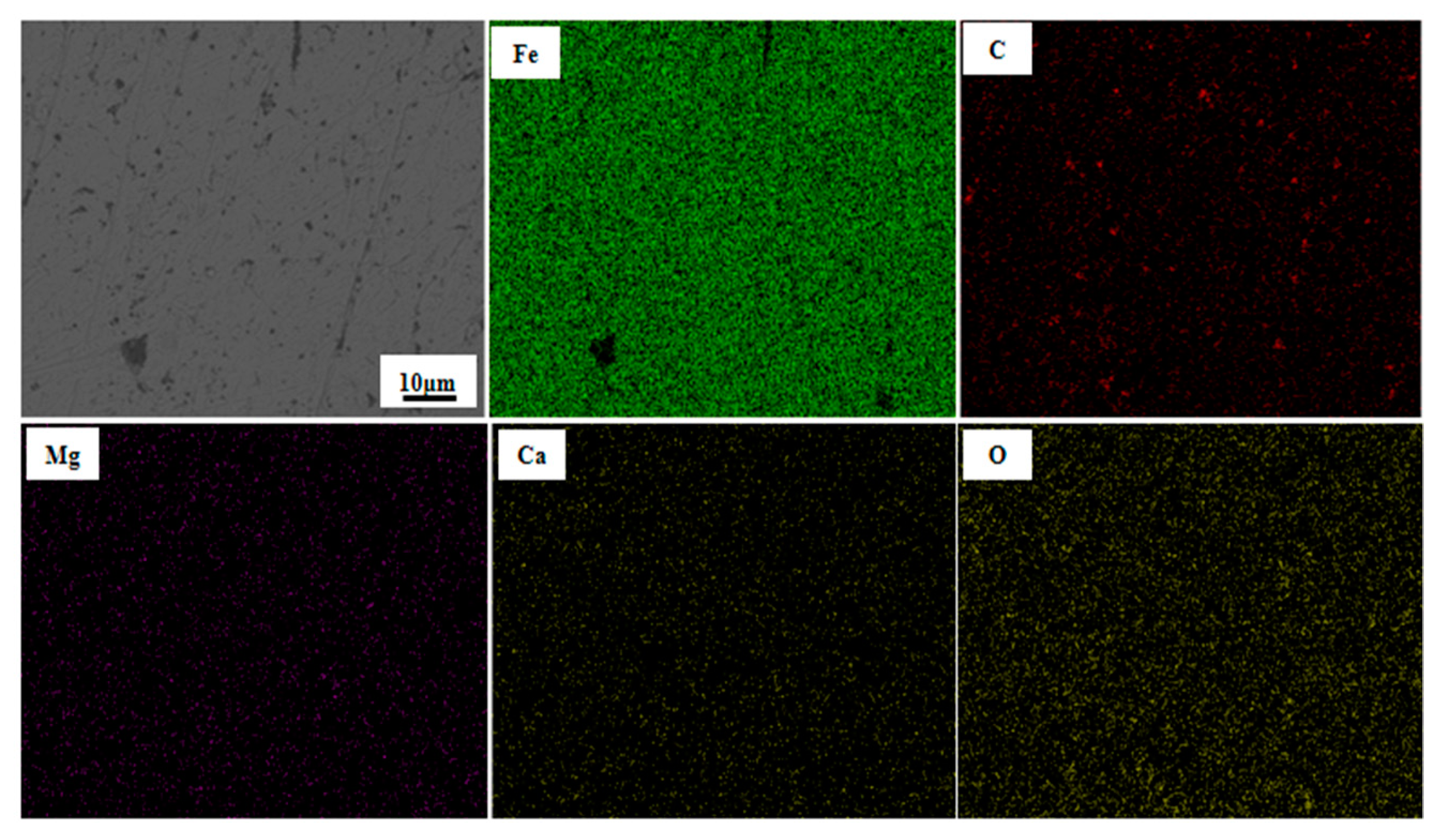
3.1.3. Morphology Change
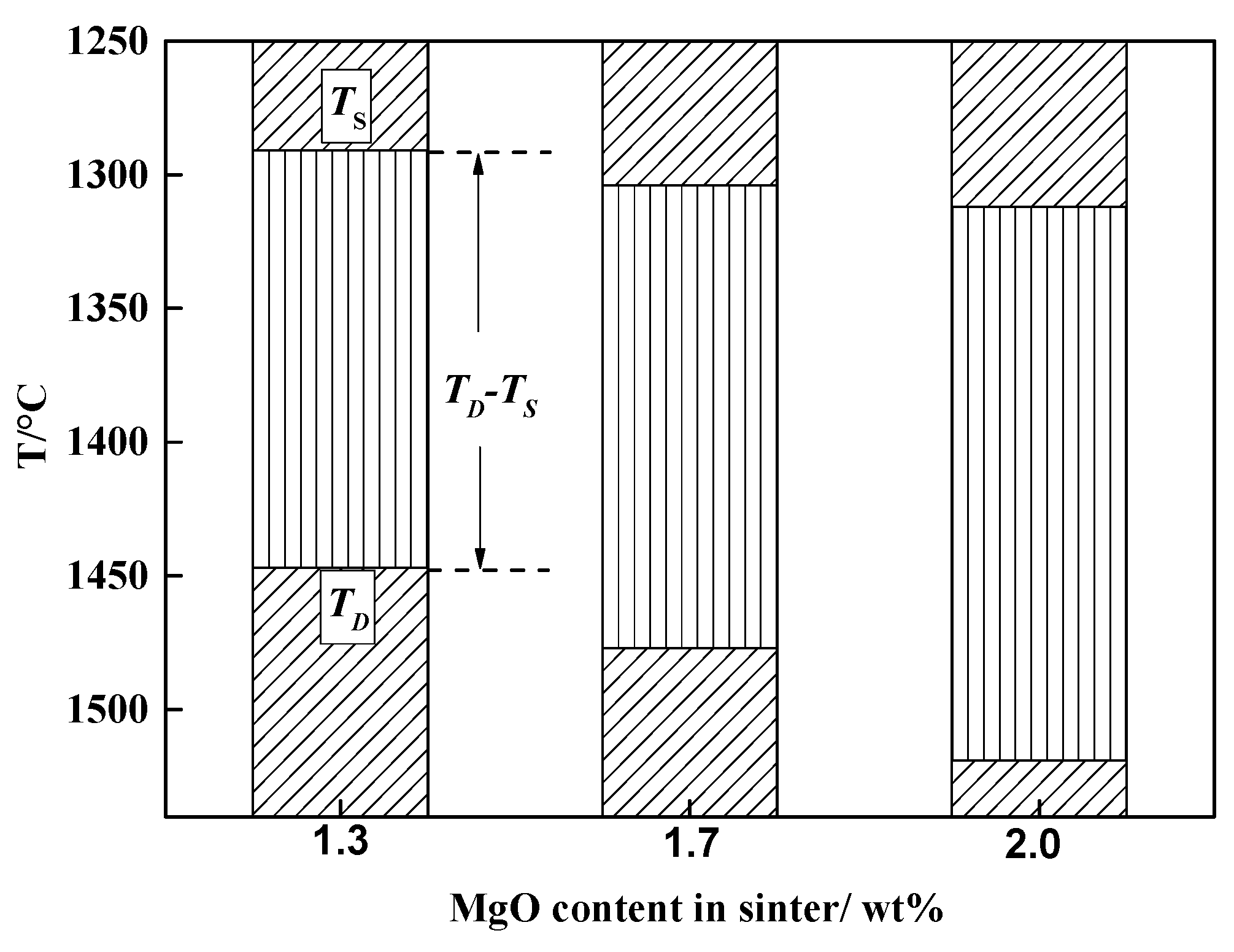
3.2. Softening-Melting Behavior
3.3. Permeability of MgO Sinter
4. Discussion
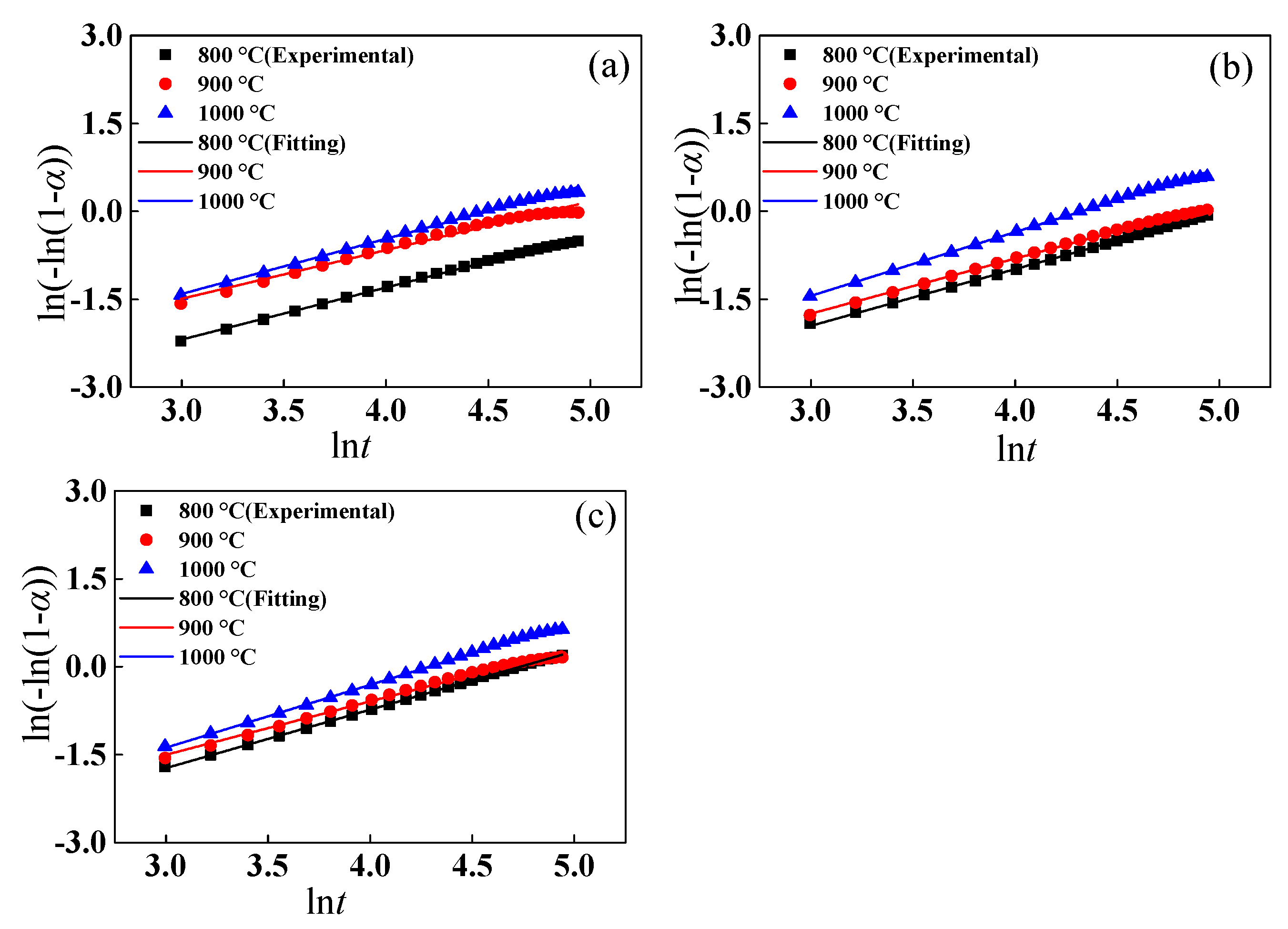
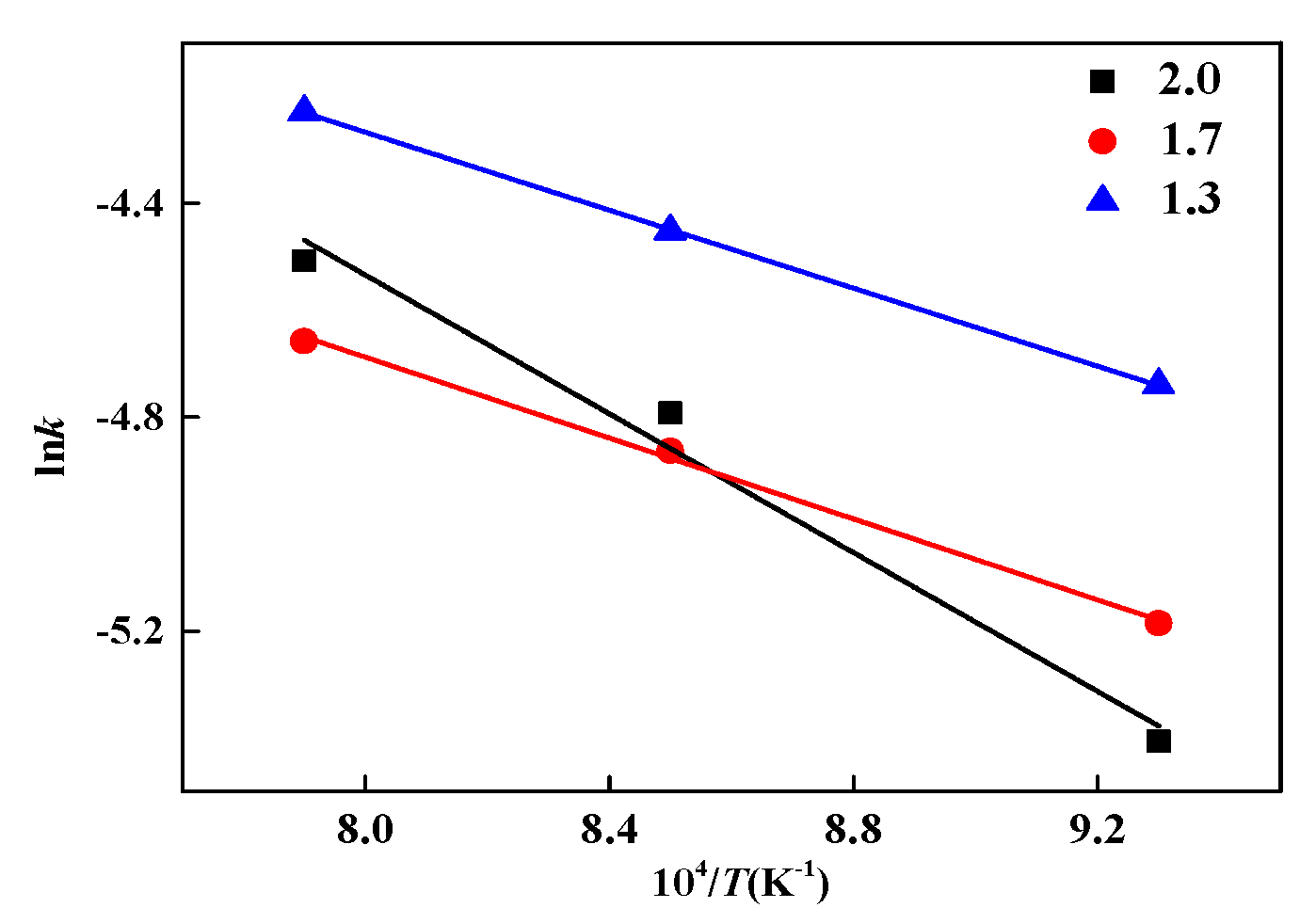
4.1. Reduction Kinetic Analysis
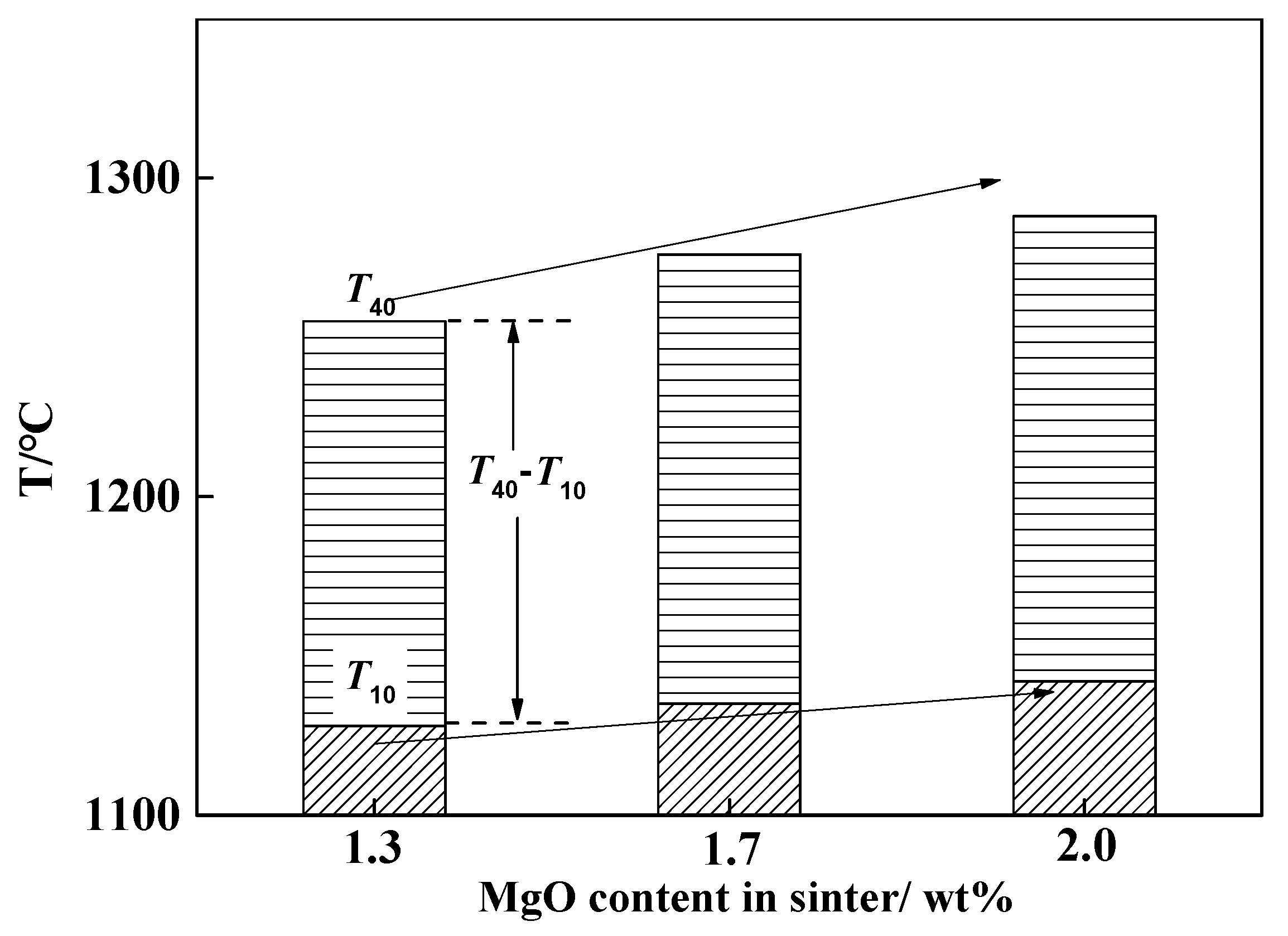
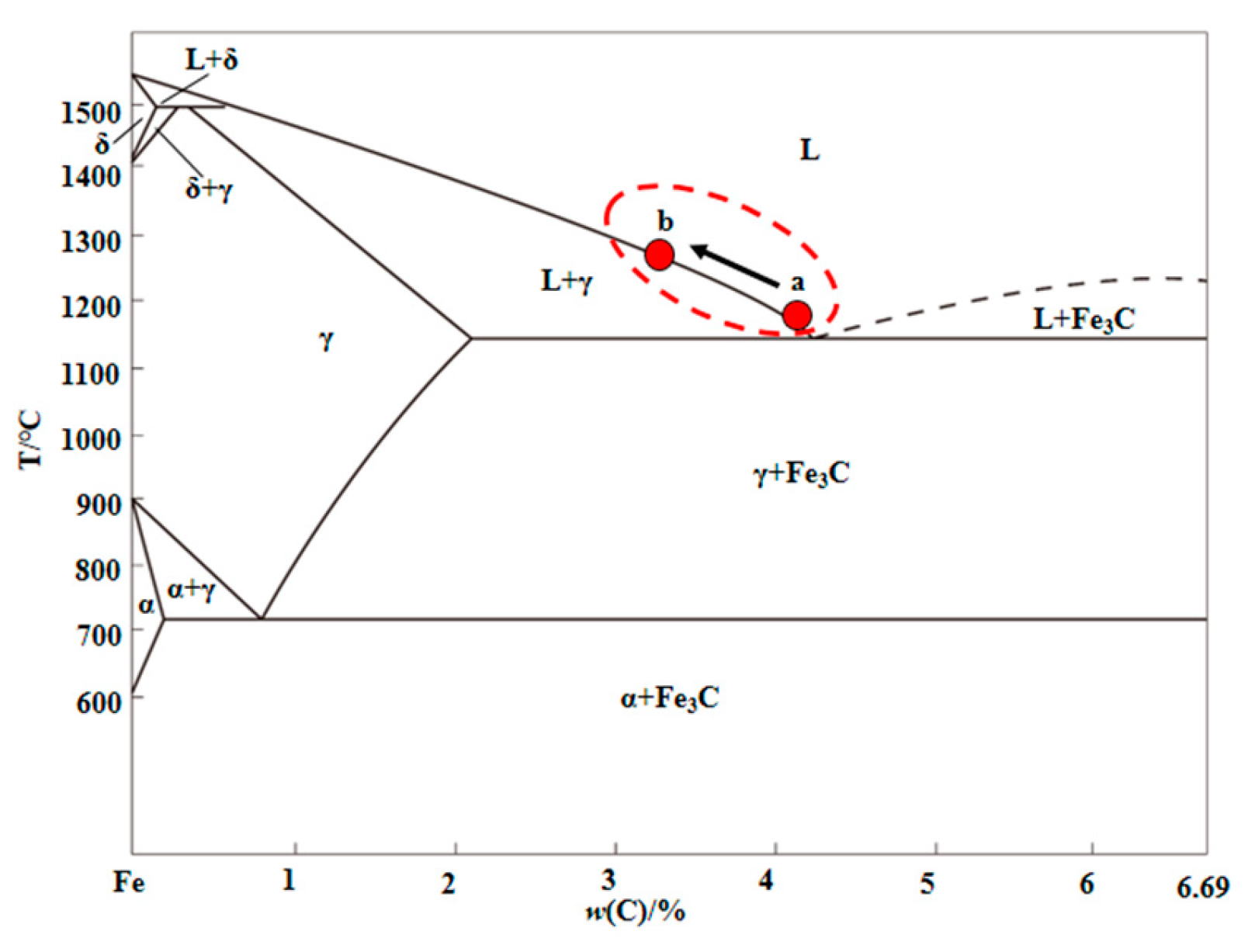
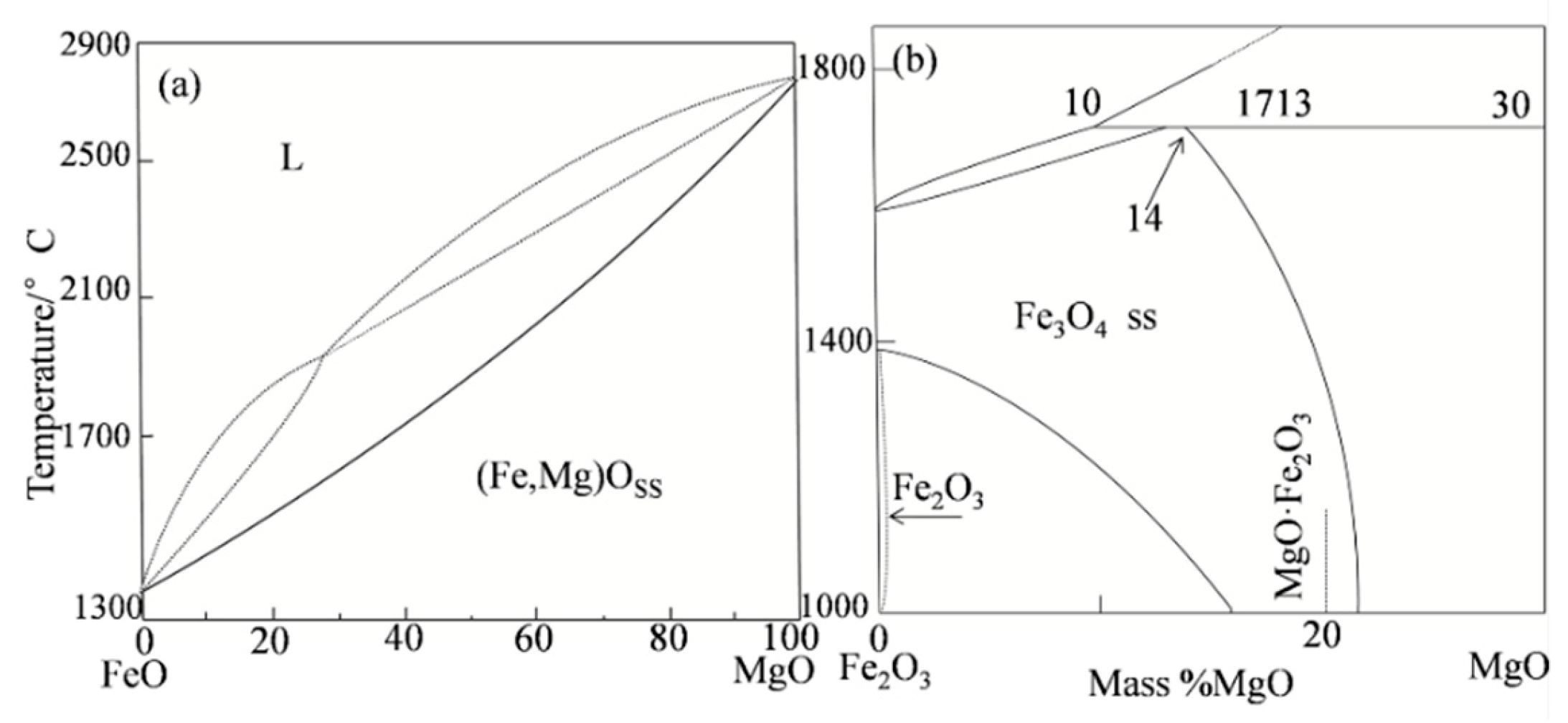
4.2. Factors Determining Melting Temperature
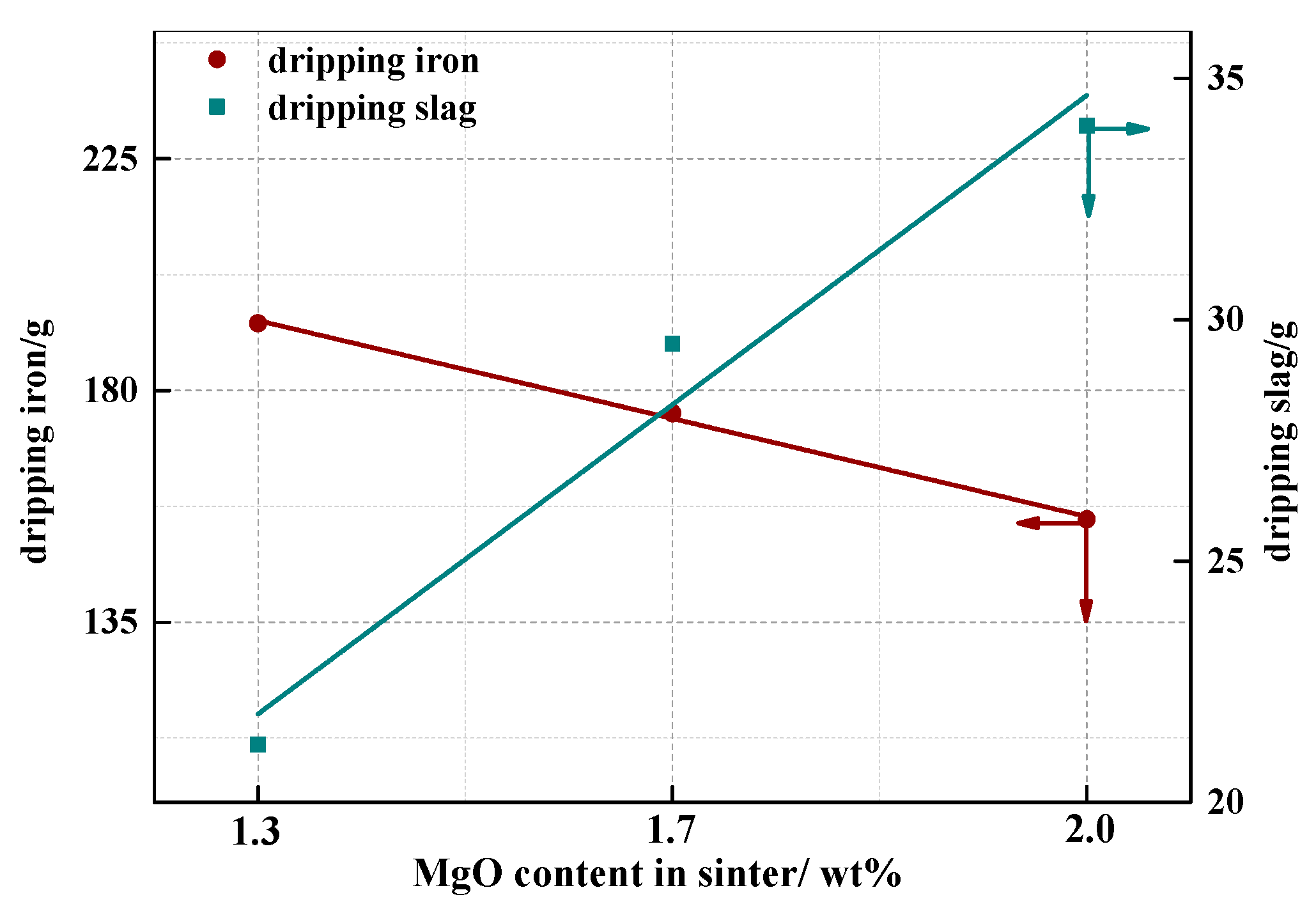
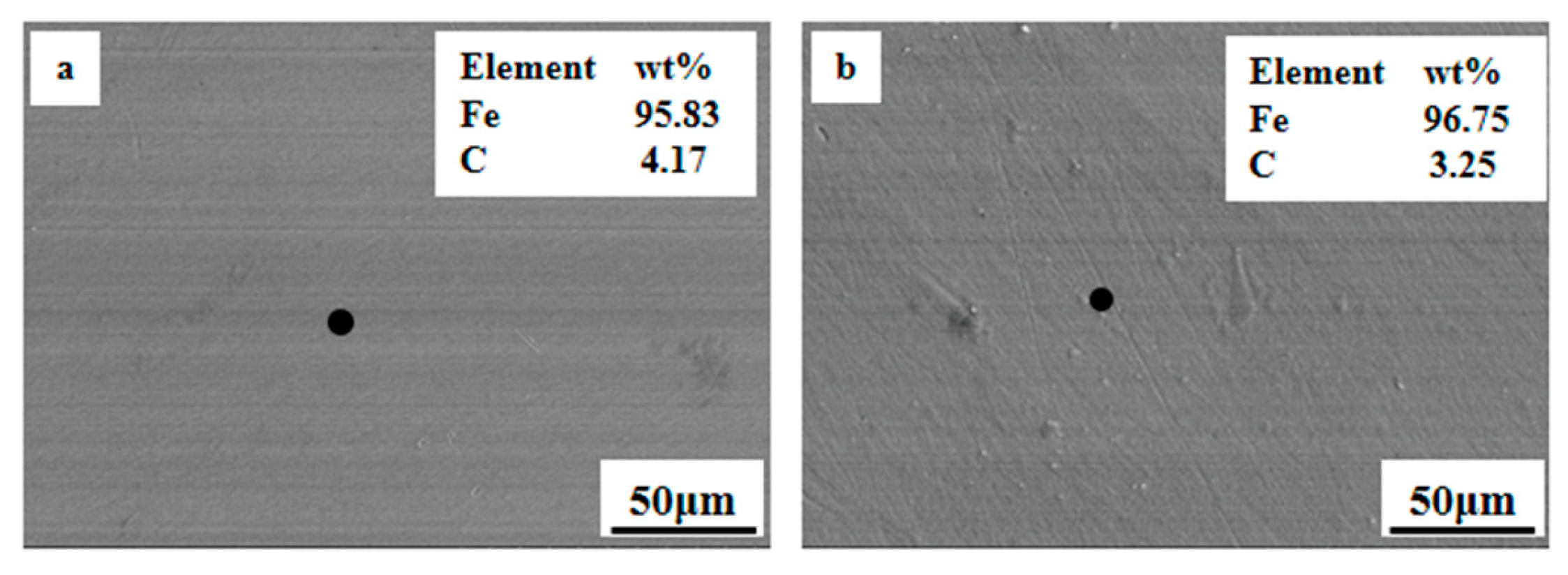
4.3. Effect of MgO on Dripping Behavior of Sinter
4.4. Comprehensive Assessment Index of Melting Properties
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hino, M.; Nagasaka, T.; Katsumata, A.; Higuchi, K.; Yamaguchi, K.; Kon-no, N. Simulation of primary-slag melting behavior in the cohesive zone of a blast furnace, considering the effect of Al2O3, FetO, and basicity in the sinter ore. Metall. Mater. Trans. B 1999, 30, 671–683. [Google Scholar] [CrossRef]
- Liu, Z.G.; Chun, M.S.; Wang, H.T.; Zhao, W.; Xue, X.X. Effect of MgO content in sinter on the softening-melting behavior of mixed burden made from chromium-bearing vanadium-titanium magnetite. Int. J. Miner. Metall. Mater. 2016, 23, 25–32. [Google Scholar] [CrossRef]
- Sugiyama, T.; Shirouchi, S.; Tsuchiya, O.; Ondda, M.; Fujita, I. High temperature reduction and softening properties of pellets with magnesite. ISIJ Int. 1983, 23, 153–160. [Google Scholar] [CrossRef]
- Liu, X.L.; Wu, S.L.; Huang, W.; Zhang, K.F.; Du, K.P. Influence of High Temperature Interaction between Sinter and Lump Ores on the Formation Behavior of Primary-slags in Blast Furnace. ISIJ Int. 2014, 9, 2089–2096. [Google Scholar] [CrossRef]
- Iljana, M.; Kemppainen, A.; Paananen, T.; Mattila, O.; Heikkinen, E.P.; Fabritius, T. Evaluating the reduction-softening behaviour of blast furnace burden with an advanced test. ISIJ Int. 2016, 56, 1705–1714. [Google Scholar] [CrossRef]
- Cheng, G.J.; Xue, X.X.; Gao, Z.X.; Jiang, T.; Yang, H.; Duan, P.N. Effect of Cr2O3 on the reduction and smelting mechanism of high-chromium vanadium-titanium magnetite pellets. ISIJ Int. 2016, 56, 1938–1947. [Google Scholar] [CrossRef]
- Hsieh, L. Effect of raw material composition on the sintering properties. ISIJ Int. 2005, 45, 551–559. [Google Scholar] [CrossRef]
- Li, T.L.; Sun, C.Y.; Liu, X.Y.; Song, S.; Wang, Q. The effects of MgO and Al2O3 behaviours on softening-melting properties of high basicity sinter. Ironmak. Steelmak. 2018, 8, 755–763. [Google Scholar] [CrossRef]
- Higuchi, K.; Naito, M.; Nakano, M.; Takamoto, Y. Optimization of chemical composition and microstructure of iron ore sinter for low-temperature drip of molten iron with high permeability. ISIJ Int. 2004, 44, 2057–2066. [Google Scholar] [CrossRef]
- Loo, C.E.; Heikkinen, J. Structural transformation of beds during iron ore sintering. ISIJ Int. 2012, 52, 2158–2167. [Google Scholar] [CrossRef]
- Loo, C.E.; Matthews, L.T.; O'Dea, D.P. Lump ore and sinter behaviour during softening and melting. ISIJ Int. 2011, 51, 930–938. [Google Scholar] [CrossRef]
- Kemppainen, A.; Ohno, K.; Iljana, M.; Panananen, T.; Heikkinen, E.P.; Maeda, T.; Kunitomo, K.; Fabritius, T. Softening behaviors of acid and olivine fluxed iron ore pellets in the cohesive zone of a blast furnace. ISIJ Int. 2015, 55, 2039–2046. [Google Scholar] [CrossRef]
- Guo, W.T.; Xue, Q.G.; Liu, Y.L.; Shen, X.F.; Wang, J.S. Microstructure evolution during softening and melting process in different reduction degrees. Ironmak. Steelmak. 2016, 43, 22–30. [Google Scholar] [CrossRef]
- Tang, W.D.; Yang, S.T.; Cheng, G.J.; Gao, Z.X.; Xue, X.X. Effect of MgO in sinter and primary-slag on smelting mechanism of chromium-bearing vanadium titanomagnetite. Steel Res. Int. 2018, 89, 1800226. [Google Scholar] [CrossRef]
- Zhao, W.; Chu, M.S.; Wang, H.T.; Liu, Z.G.; Tang, J.; Ying, Z.W. Effect of introducing coke into ore layer on softening-meltingdropping characteristics of vanadium-titanium mixed burden under simulating BF conditions. ISIJ Int. 2018, 58, 1989–1998. [Google Scholar] [CrossRef]
- Wu, S.L.; Tuo, B.Y.; Zhang, L.H.; Du, K.P.; Sum, Y. New evaluation methods discussion of softening–melting and dropping characteristic of bf iron bearing burden. Steel Res. Int. 2014, 85, 233–242. [Google Scholar] [CrossRef]
- Liu, J.X.; Cheng, G.J.; Liu, Z.G.; Chu, M.S.; Xue, X.X. Softening and melting properties of different burden structures containing high chromic vanadium titano-magnetite. Int. J. Miner. Process. 2015, 142, 113–118. [Google Scholar] [CrossRef]
- Matsumura, M.; Hoshi, M.; Kawaguchi, T. Improvement of sinter softening property and reducibility by controlling chemical compositions. ISIJ Int. 2005, 45, 594–602. [Google Scholar] [CrossRef]
- Nishimura, T.; Higuchi, K.; Nation, M.; Kunitomo, K. Evaluation of softening, shrinking and melting reduction behaviour of raw materials for blast furnace. ISIJ Int. 2011, 51, 1316–1321. [Google Scholar] [CrossRef]
- Kaushik, P.; Fruehan, R.J. Mixed burden softening and melting phenomena in the blast furnace operation Part 2-mechanism of softening and melting and impact on the cohesive zone. Ironmak. Steelmak. 2006, 33, 520–528. [Google Scholar] [CrossRef]
- Guha, M.; Sinha, M. Tracking softening-melting behaviour of blast furnace burden. ISIJ Int. 2015, 55, 2033–2035. [Google Scholar] [CrossRef]
- Sterneland, J.; Lahiri, A.K. Contraction and meltdown behaviour of olivine iron ore pellets under simulated blast furnace conditions. Ironmak. Steelmak. 1999, 26, 339–348. [Google Scholar] [CrossRef]
- Sterneland, J.; Andersson, M.; Jonsson, P. Comparison of iron ore reduction in the experiment blast furnace and laboratory scale simulation of blast furnace process. Ironmak. Steelmak. 2003, 30, 313–327. [Google Scholar] [CrossRef]
- Ding, C.Y.; Lv, X.W.; Xuan, S.W.; Tang, K.; Bai, C.G. Isothermal reduction kinetics of powdered hematite and calcium ferrite with CO–N2 gas mixtures. ISIJ Int. 2016, 56, 2118–2125. [Google Scholar] [CrossRef]
- Panigrahy, S.C.; Verstraeten, P.; Dilewijns, J. Influence of MgO addition on mineralogy of iron ore sinter. Metall. Trans. B 1984, 15B, 23–32. [Google Scholar] [CrossRef]
- Mccune, R.C.; Wynblatt, P. Calcium segregation to a magnesium oxide (100) surface. J. Am. Ceram. Soc. 1983, 66, 111–117. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. I General theory. J. Chem. Phys. 1939, 12, 1103–1112. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. II Transformation-time relations for random distribution of nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Avrami, M. Granulation, phase change, and microstructure kinetics of phase change. III. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Hancock, J.D.; Sharp, J.H. Method of comparing solid-state kinetic data and its application to the decomposition of kaolinite, brucite, and BaCO3. J. Am. Ceram. Soc. 1972, 55, 74–77. [Google Scholar] [CrossRef]
- Gao, Q.J.; Shen, F.M.; Wei, G.; Jiang, X.; Zheng, H.Y. Effects of MgO containing additive on low-Temperature metallurgical properties of oxidized pellet. J. Iron Steel Res. Int. 2013, 20, 25–28. [Google Scholar] [CrossRef]
- Eisenhüttenleute, V.D.; Allibert, M. Slag Atlas, 2nd ed.; Verlag Stahleisen GmbH: Düsseldorf, Germany, 1995. [Google Scholar]
- Martinze, A.M.; Kak, A.C. PCA versus LDA. IEEE Trans. Pattern Anal. Mach. Intell. 2001, 23, 228–233. [Google Scholar] [CrossRef]
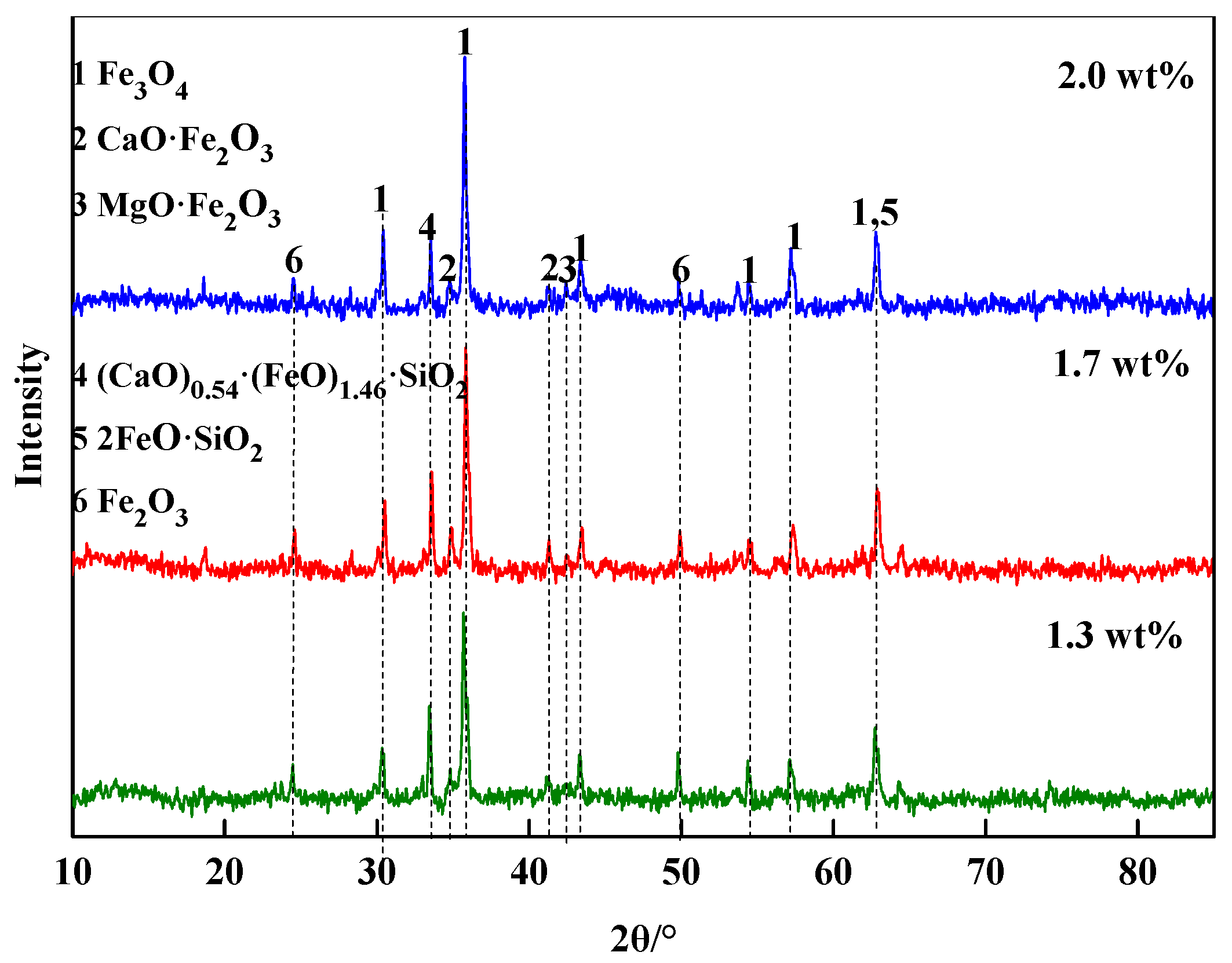
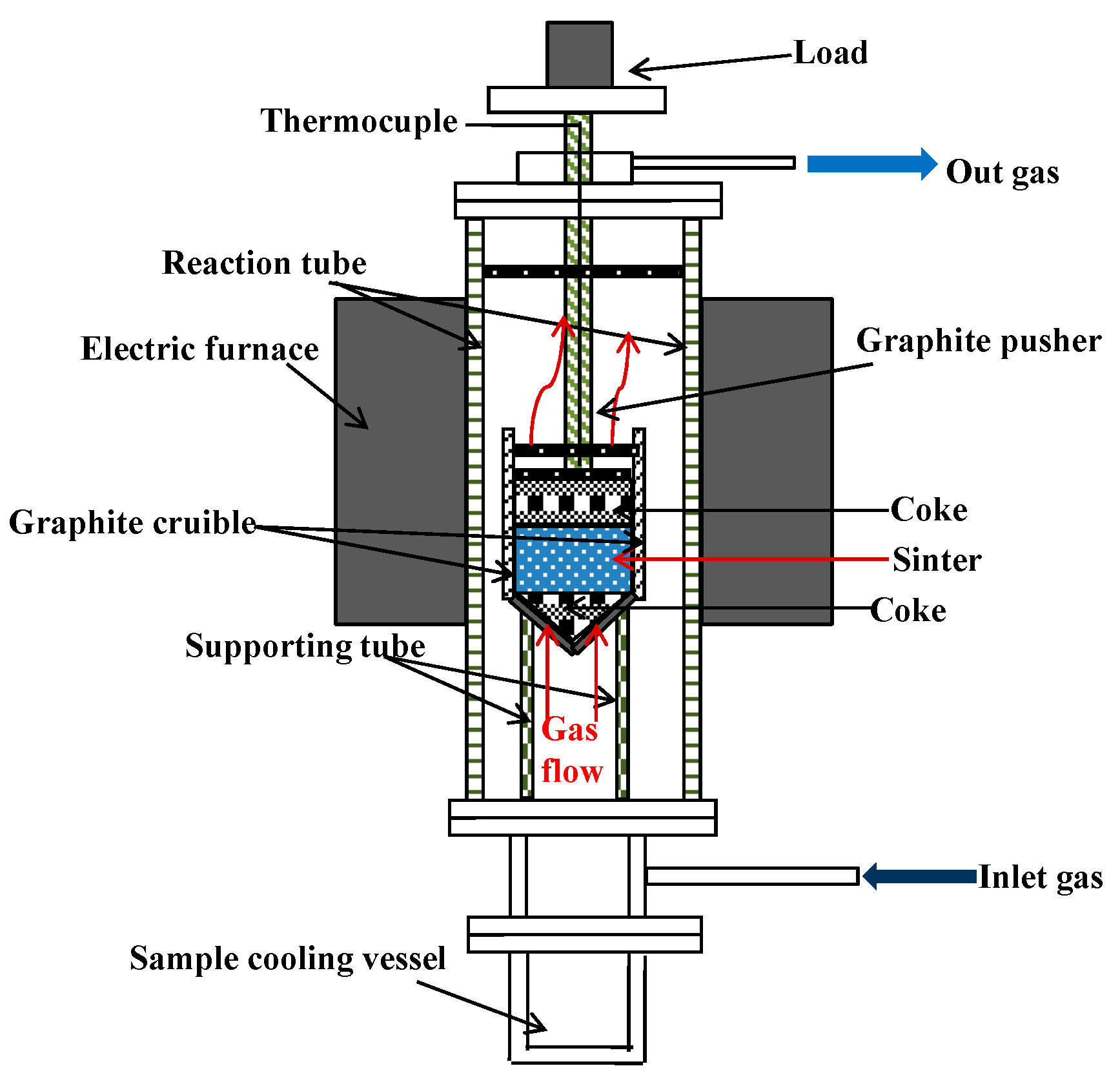
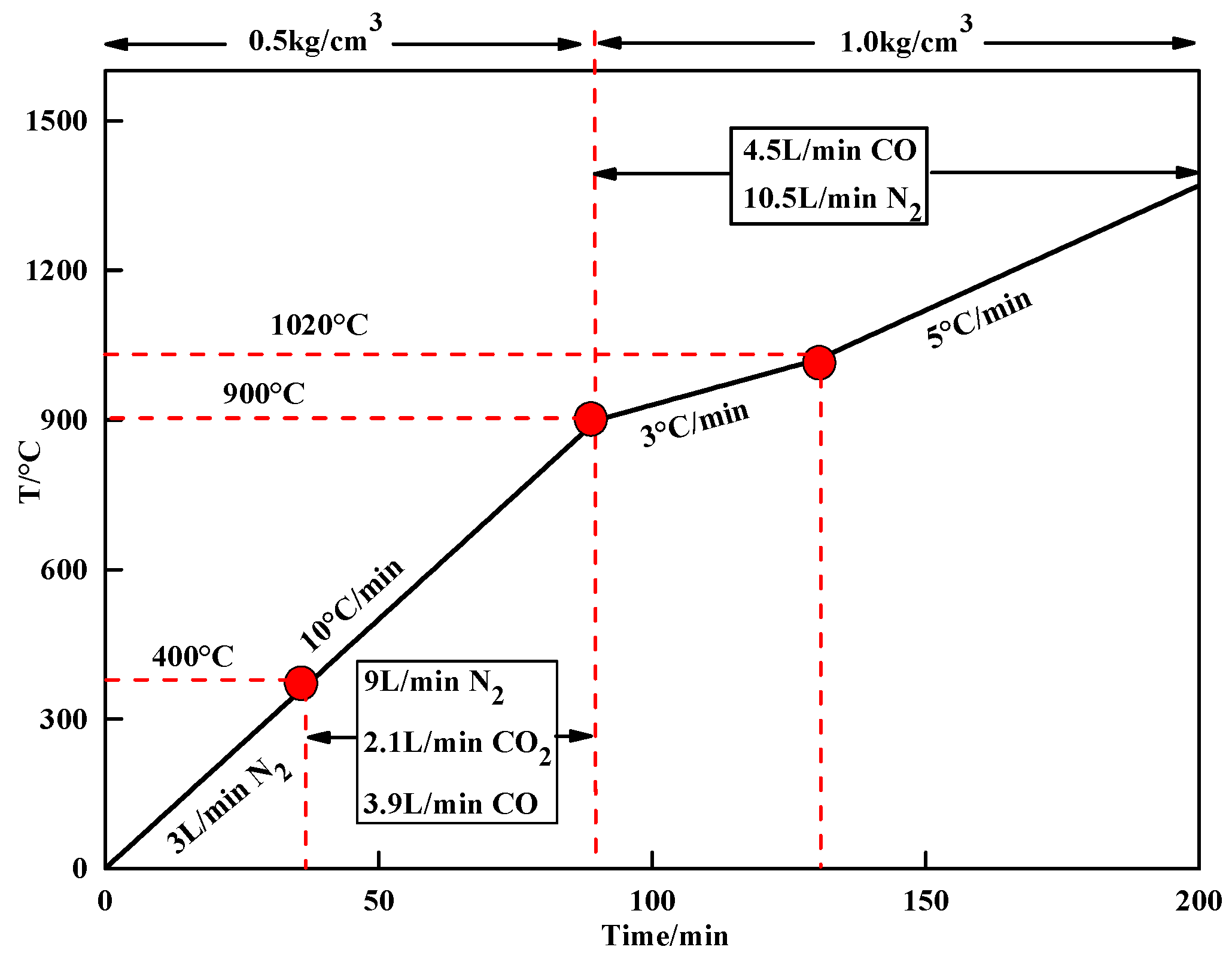
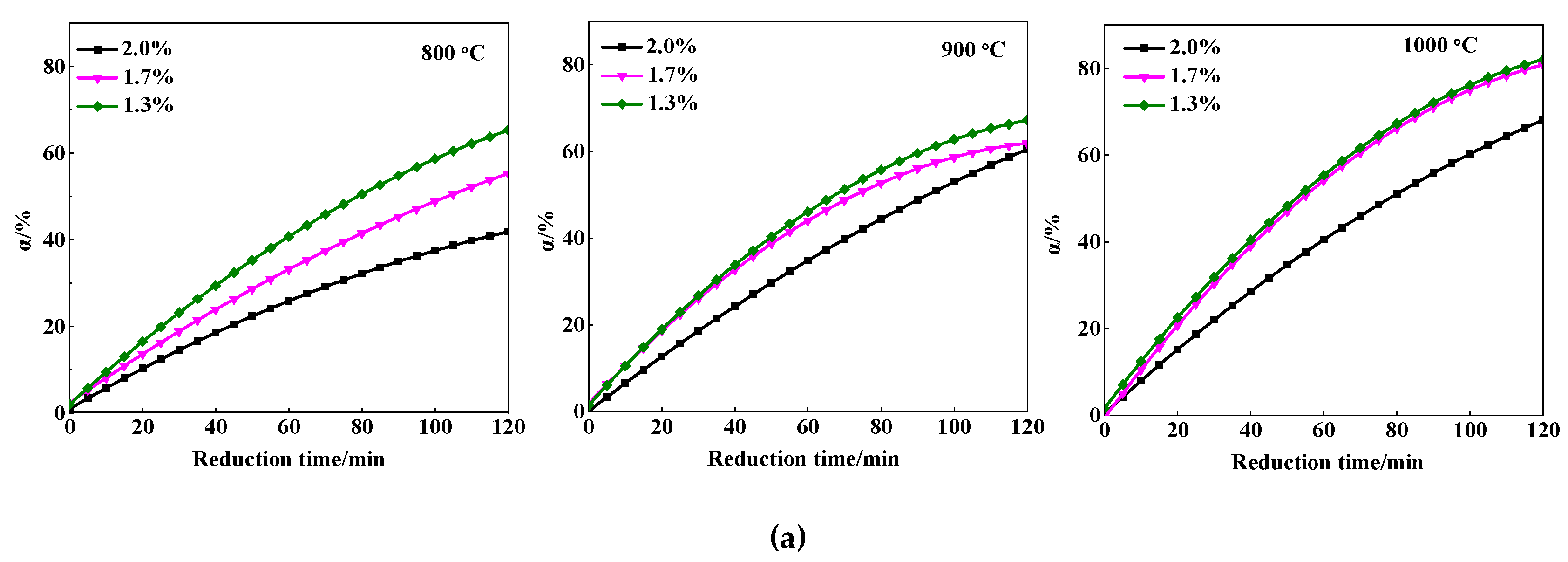





| MgO Contents | TFe | FeO | CaO | SiO2 | MgO | Al2O3 | R |
|---|---|---|---|---|---|---|---|
| 1.3 | 57.63 | 9.02 | 8.76 | 5.15 | 1.31 | 1.91 | 1.70 |
| 1.7 | 57.65 | 9.01 | 8.63 | 5.05 | 1.69 | 1.89 | 1.71 |
| 2.0 | 57.55 | 8.89 | 8.65 | 5.09 | 2.02 | 1.93 | 1.69 |
| Reference | Crucible Diameter | Bed Height | Sample Amount | Degree of Pre-Reduction | Temperature | Gas Atmosphe-re | load |
|---|---|---|---|---|---|---|---|
| Nandy et al. [19] | 48 mm | 90 mm | Variable (constant bed height) | No pre-reduction | <230 °C: 3.8 °C/min 230–900 °C: 8.4 °C/min 900–1100 °C: 2.2 °C/min 1100–1650 °C: 5 °C/min | 30CO-70N2 | 10 kPa |
| Nishimura et al. [20] | 85 mm | 70 mm | Hundreds of grams | No pre-reduction | <1000 °C: 10 °C/min 1000–1550 °C: 5 °C/min | 29.4CO-3.5H2-67.1N2[vol-%] | 100 kPa at above 800 °C |
| Kaushik and Fruehan [21] | 80 mm | 80 mm | ~500 g | No pre-reduction | Mainly 5 °C/min | Changing CO-CO2-N2 gas | 50 kPa |
| Guha and Sinha [22] | 48 mm | 90 mm | Variable (constant bed height) | No pre-reduction | Dynamic temperature program | Changing CO-CO2-N2 gas | 10 kPa |
| Sternelandand Labiri, [23,24] | 80 mm | ~55 mm | 500 g | No pre-reduction | 3 dynamic temperature program | Changing CO-CO2-H2-N2 gas 3 dynamic load programs | 3 Differ-ent dyna-mic load prog-rm |
| Function | Kinetic Model | Equation | n |
|---|---|---|---|
| D1(α) | One-dimensional diffusion | α2 = kt | 0.62 |
| D2(α) | Two-dimensional diffusion | (1 − α)ln(1 − α)+ α = kt | 0.57 |
| D3(α) | Three-dimensional diffusion | [1 − (1 − α)1/3]2 = kt | 0.54 |
| A1(α) | Avrami-Erofeev, n = 1 | [−ln(1 − α)]1/2 = kt | 2 |
| A2(α) | Avrami-Erofeev, n = 2 | [−ln(1 − α)]1/3 = kt | 3 |
| F1(α) | First-order reaction | −ln(1 − α) = kt | 1 |
| Item | T/°C | Slope | R2 |
|---|---|---|---|
| 1.3 wt% | 800 | 0.99628 | 0.99971 |
| 900 | 0.97316 | 0.99975 | |
| 1000 | 1.08597 | 0.99951 | |
| 1.7 wt% | 800 | 0.95547 | 0.9995 |
| 900 | 0.97211 | 0.99994 | |
| 1000 | 1.11765 | 0.99989 | |
| 2.0 wt% | 800 | 0.91508 | 0.99988 |
| 900 | 0.91755 | 0.99902 | |
| 1000 | 0.98516 | 0.99988 |
| MgO Content | T/°C | k/T | R2 |
|---|---|---|---|
| 1.3 wt% | 800 | 0.00874 | 0.99968 |
| 900 | 0.00955 | 0.99702 | |
| 1000 | 0.01457 | 0.99809 | |
| 1.7 wt% | 800 | 0.00654 | 0.99976 |
| 900 | 0.00781 | 0.99909 | |
| 1000 | 0.01426 | 0.99866 | |
| 2.0 wt% | 800 | 0.00449 | 0.99785 |
| 900 | 0.0083 | 0.99042 | |
| 1000 | 0.01103 | 0.99888 |
| MgO Content | 1.3 | 1.7 | 2.0 |
|---|---|---|---|
| E/(kJ·mol−1) | 3.03 | 3.14 | 5.39 |
| A/min−1 | 0.26 | 0.19 | 1.92 |
| MgO Content | Al2O3 | CaO | MgO | TFe | SiO2 | MFe | FeO |
|---|---|---|---|---|---|---|---|
| 1.3 wt% | 2.43 | 11.07 | 1.53 | 68.29 | 6.05 | 25.32 | 62.32 |
| 2.0 wt% | 2.16 | 10.95 | 2.68 | 69.26 | 5.36 | 26.48 | 61.01 |
| Item | CaO | SiO2 | Al2O3 | MgO | FeO | |
|---|---|---|---|---|---|---|
| 2.0 wt% MgO | residual | 40.15 | 25.32 | 10.10 | 11.89 | 14.58 |
| dripping | 43.93 | 27.42 | 11.31 | 12.58 | 5.32 | |
| 1.3 wt% MgO | residual | 44.96 | 28.14 | 11.26 | 7.07 | 8.66 |
| dripping | 45.99 | 32.14 | 11.84 | 7.90 | 2.00 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Shen, F.; Zhang, H.; Gao, Q.; Jiang, X. High-Temperature Reduction and Melting Mechanism of Sinter with Different MgO Content. Metals 2019, 9, 510. https://doi.org/10.3390/met9050510
Guo H, Shen F, Zhang H, Gao Q, Jiang X. High-Temperature Reduction and Melting Mechanism of Sinter with Different MgO Content. Metals. 2019; 9(5):510. https://doi.org/10.3390/met9050510
Chicago/Turabian StyleGuo, He, Fengman Shen, Huaiyu Zhang, Qiangjian Gao, and Xin Jiang. 2019. "High-Temperature Reduction and Melting Mechanism of Sinter with Different MgO Content" Metals 9, no. 5: 510. https://doi.org/10.3390/met9050510
APA StyleGuo, H., Shen, F., Zhang, H., Gao, Q., & Jiang, X. (2019). High-Temperature Reduction and Melting Mechanism of Sinter with Different MgO Content. Metals, 9(5), 510. https://doi.org/10.3390/met9050510




