1. Introduction
Powders with metastable structures capable of releasing significant energy/heat during the heating process could be produced by melt spinning, with subsequent milling. The melt-spinning processing technology provides significantly high cooling rates during solidification.
Generally, rapid solidification leads to a substantial improvement in mechanical properties, compared to conventional alloy processing from the same composition, due to the extension of solid solution limits, microstructure refining, and second-phase dispersion [
1,
2,
3]. Rapid solidification results in a refinement of the microstructure (including grains and secondary phase particles), a reduction in macro and micro segregation, a significant increase in solid state solubility, as well as the formation of unbalanced or metastable phases [
4,
5].
Due to their good casting capacity, high mechanical strength, low weight, excellent corrosion resistance, and low thermal expansion coefficient, aluminum–silicon (Al–Si) alloys are used in high-end areas such as aerospace, automotive, and electronics industries. Significant efforts have been done to study the microstructure, mechanical properties, and thermal stability of this family of aluminum alloys [
6]. In the automotive industry, the importance of Al–Si and Al–Si–
X (
X = Me) alloys is paramount, especially for certain components of the engine [
7].
Because high-rate solidification is a practical way of significantly changing the structure, several methods and techniques have been developed in this field. Among the fast solidification techniques, melt-spinning is an approach with unique advantages in refining the microstructure. The single-roll melt-spinning process has one of the highest melt cooling rates throughout the continuous casting process, the solidification occurring over a time-scale of milliseconds [
8]. The major advantage of melt-spinning is the possibility of continuous production of fast-solidified materials in the form of thin ribbons, even on a large industrial scale [
3]. The characteristics of the aluminum alloys thus obtained gives them physical, chemical, and mechanical properties different to those obtained by conventional casting [
9]. The extended solid solution as well as dispersed particle formation can improve the mechanical strength, wear resistance, and thermal stability of melt-spun alloys [
10].
Generally, the metallic powders that are used for additive manufacturing (AM) are obtained by atomization, where the rate of cooling of the metal granules (and hence solidification) is generally in the order of 10
4–10
5 °C/s, which includes this process in the category of fast alloy solidification. Research has been carried out on the influence of cooling during atomization on the structure of aluminum–nickel (Al–Ni) alloys [
11]. It was found that the development of the phases and the lattice parameters of the different phases vary with the size and composition of the droplets, hence with the cooling rate during the atomization process.
Gas atomization is one of the most common methods of obtaining fine metallic powders, with spherical shapes and homogeneous structures [
12]. In a gas atomization process, a stream of hot molten metal performs a heat exchange with high-pressure gas jets, which give rise to high cooling and sub-cooling speeds for the atomized metal droplets. The produced powder particles exhibit a low segregation and a very fine microstructure, which improves the properties of the material, such as mechanical strength, durability, hardness, and corrosion resistance [
13].
Materials with metastable structures based on high-performance alloys have been studied, by combining new technologies and new chemical compositions. It was observed that the mechanical properties of aluminum alloys can be significantly improved by rapid solidification (RS) during the atomization process in gas, as well as applying powder metallurgy (PM) techniques [
14].
Laser-based AM technologies involve selective melting of cross-sections onto a powder bed that is lowered incrementally to build the part layer upon layer. A laser beam is focused onto the powder bed and used as a melting tool, driven along a toolpath by mirrors or lenses. One of the key factors affecting dimensional accuracy of laser-based AM processes is the minimum spot diameter. The obvious consequence is an increasing adoption by system manufacturers of high-energy beams, to be focused into always smaller spots, while preserving the capability to process high-melting alloys. Consequently, in order to obtain a fully melt pool, higher energy input is generally needed. Developing new materials which can be melted/fused with lower input power is of high importance.
In order to reduce the amount of heat required for the laser processing of the alloy and thus to reduce the required laser power, it is necessary that a heat-generating phenomenon occurs when the alloy is heated—in this case, this heat should be subtracted from the heat required for fusion. In order for the heating of the alloy to produce exothermal effects, it is necessary that: (i) The structure is a non-equilibrium, metastable, or amorphous structure, or (ii) the alloy has in its structure a supersaturated solid solution, which, upon heating, decomposes, followed by the formation of intermetallic compounds, which should produce positive energy effects.
In the present paper, the authors highlight the influence of the processing technologies on Al–Si–Ni alloy structures. To date, the number of reports concerning this system (Al–Si–Ni) is significantly low. Moreover, only a limited number of compositions was studied. The aim of the research was to establish correlations between the obtained metastable structures (amorphous/nanostructured) and the thermal effects that arise from the heating process of these materials, with certain potential applications in the AM technology.
2. Materials and Methods
Al–Si–Ni alloys with different elemental concentrations were prepared in an electric furnace, heated with silicon carbide resistors, in graphite crucibles, under an initially powdered protective flux layer. The protective flux was added on the melt pool in powder form, in order to deoxidize, refine, and decontaminate the molten alloy. Aluminum, Al-Si22, and Ni80 pre-alloys (nickel blended with flux) were used to obtain the bulk alloys. The chemical composition of the produced alloys is shown in
Table 1, along with the sample variants obtained for each alloy.
Thin alloy ribbons were obtained by melt-spinning. The alloys were melted inside a perforated Al2O3 crucible, using a copper induction coil. The diameter of the molten metal outlet was 1 mm, positioned at 1 mm distance from the copper wheel. The copper disk rotation speed was 2600 rpm (resulting in a peripheral speed of about 28 m/s). In order to avoid contamination/oxidation of the melt, the crucible was continuously purged with argon (Pargon = 1 bar). Once the alloy was melted, the argon pressure was increased to 8 bars, thus causing the expulsion of the molten alloy on the spinning copper disk. The dimensions of the melt-spun ribbons were: Al79Si11Ni10 alloy, thickness 49 ± 6 µm, width 1.8 ± 0.4 mm; Al67Si18Ni15 thickness 52 ± 8 µm, width 2.4 ± 0.6 mm.
In order to observe the influence of the cooling speed on the structure, for the preparation of Al79Si11Ni10 powder granules by atomization, the same copper induction coil, containing the alumina crucible, was used, but this time the molten alloy was cooled/solidified using a gas atomizer. The atomization was done in water at a pressure of 4 bar using an atomizer with the following characteristics: 16 equidistant discharge outlets of water, 1 mm in diameter, fluid angle α = 45°, water temperature of 20 °C, 2 mm molten metal jet diameter. The average granule dimension obtained by atomization was 360 ± 80 µm.
The samples, which were later-on analyzed, are separated as follows: Al79Si11Ni10 as-cast (referred to as “bulk”), melt-spun (referred to as “ribbons”), and atomized (referred to as “granules”); Al67Si18Ni15 as-cast (referred to as “bulk”), and melt-spun (referred to as “ribbons”).
The structural analysis of the bulk, melt-spun, and atomized samples was performed on an Omnimet-Buehler structural analysis system (Buehler, Esslingen am Neckar, Germany), equipped with a Nikon metallographic microscope (up to 1000× magnification, Nikon Eclipse MA100, Nikon Co, Tokyo, Japan) and a software suitable for quantitative structural analysis (NIS Element, version S4.30.01, Nikon Co., Tokyo, Japan).
The structural properties of the bulk and melt-spun samples (1 cm long strips) were investigated using X-Ray diffraction (Shimadzu LabX XRD-600 diffractometer, Shimadzu, Tokyo, Japan), with Cu Kα radiation (λ = 1.54059 Å). The samples were analyzed in ambient atmosphere, within 2°–90° 2θ range, with a scanning angle step of 0.02° and a 2 deg/min count time.
The samples were observed with a scanning electron microscope (SEM, Quanta FEI, Eindhoven, The Netherland) operated in high vacuum mode, equipped with an X-ray energy dispersion spectroscopy system (X-EDS, INCA Oxford Instruments, Abingdon, UK) for chemical micro-analysis.
The structural stability of the samples was studied by differential scanning calorimetry (DSC) analysis on a MAIA DSC 200F3 machine (Netzsch, Selb, Germany) produced by Netzsch, with a heating/cooling rate of 10 °C/min in nitrogen atmosphere, up to the maximum temperature of 600 °C, and data processing was done using the Netzsch Proteus Analysis software (5.2.1/2011, Netzsch, Selb, Germany). For comparison purposes, a commercially available industrial powder (F357-type (AlSi10Mg equivalent), EOS Gmbh) was also analyzed.
The crystallization temperatures of the samples were studied on a F3 Jupiter STA/TG/DTA (Netzsch, Selb, Germany), with a heating/cooling rate of 10 °C/min in argon atmosphere, up to the maximum temperature of 1050 °C, and data processing was done using the Netzsch Proteus Analysis software (5.2.1/2011, Netzsch, Selb, Germany). The calibration of the instrument was done before each measuring session.
In order to assess some mechanical properties (hardness–H and elastic modulus–E) of the Al–Si–Ni alloys, both in bulk form and fast-cooled samples, instrumented indentation measurements were performed, using a CSM Instruments/Anton Paar NHT2 module (CSM Instruments/Anton Paar, Pesseux, Switzerland), equipped with a diamond Berkovich indenter. The instrumented indentation parameters were: maximum load 20 mN, loading rate 40 mN/min, unloading rate 40 mN/min, dwell time 10 s. At least 20 measurements were performed on each sample, and the results were averaged. The samples (as-cast bulk fragments, melt-spun ribbons, and atomized granules) were embedded in low-contraction resin, in order to minimize the effect on the final results, followed by mechanical polishing to a mirror-like finish. Moreover, in order to assess the microhardness of the bulk samples, a Vickers microhardness tester (Future-Tech FM700, Kawasaki, Japan) was used (FM 700), with the following conditions: 100 gf applied load, 15 s dwell time. At least 10 indentations were performed on the bulk alloys, and the results were averaged. In order to be able to compare the results with the data from the literature, the Vickers values were converted to Brinell units.
Author Contributions
Conceptualization, T.B., B.V., and D.C.; data curation, T.B., B.V., D.C., I.G., M.A.P., and C.G.; formal analysis, T.B., B.V., D.C., A.N., A.G., E.B., G.B., I-L.V., M.A.P., C.G., and M.C.; funding acquisition: A.G.; investigation: C.G.; methodology: D.C., and M.A.P. project administration: E.B.; resources, S.M., and D.M.; writing—original draft preparation, T.B., B.V., D.C., I-L.V., and L.P.; writing—review and editing, D.C., I.G., B.V., and T.B.
Funding
This project has received funding from the European Union′s Horizon 2020 research and innovation programme under grant agreement No. 723699. We hereby acknowledge the structural founds project PRO-DD (POS-CCE, O.2.2.1., ID 123, SMIS 2637, ctr. No. 11/2009) for providing some of the infrastructure used in this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xu, C.L.; Wang, H.Y.; Qiu, F.; Yang, Y.F.; Jiang, Q.C. Cooling rate and microstructure of rapidly solidified Al–20 wt.% Si alloy. Mater. Sci. Eng. A 2006, 417, 275–280. [Google Scholar] [CrossRef]
- Chen, Z.; Lei, Y.; Zhang, H. Structure and properties of nanostructured A357 alloy produced by meltspinning compared with direct chill ingot. J. Alloys Compd. 2011, 509, 7473–7477. [Google Scholar] [CrossRef]
- Jassim, A.K.; Hammood, A.S. Single Roll Melt Spinning Technique Applied as a Sustainable Forming Process to Produce Very Thin Ribbons of 5052 and 5083 Al-Mg Alloys Directly from Liquid State. Procedia CIRP 2016, 40, 133–137. [Google Scholar] [CrossRef][Green Version]
- Lin, Y.; Wu, B.; Li, S.; Mao, S.; Liu, X.; Zhang, Y.; Wang, L. The quantitative relationship between microstructure and mechanical property of a melt spun Al–Mg alloy. Mater. Sci. Eng. A 2015, 621, 212–217. [Google Scholar] [CrossRef]
- Lin, Y.; Mao, S.; Yan, Z.; Zhang, Y.; Wang, L. The enhanced microhardness in a rapidly solidified Al alloy. Mater. Sci. Eng. A 2017, 692, 182–191. [Google Scholar] [CrossRef]
- Dong, X.; He, L.; Li, P. Gradient microstructure and multiple mechanical properties of AlSi9Cu alloy ribbon produced by melt spinning. J. Alloys Compd. 2014, 612, 20–25. [Google Scholar] [CrossRef]
- Karakose, E.; Karaaslan, T.; Keskin, M.; Uzun, O. Microstructural evolution and microhardness of a melt-spunAl–6Ni–2Cu–1Si (in wt.%) alloy. J. Mater. Process. Technol. 2008, 195, 58–62. [Google Scholar] [CrossRef]
- Izadinia, M.; Dehghani, K. Structure and properties of nanostructured Cu-13.2Al-5.1Ni shape memory alloy produced by melt spinning. Trans. Nonferrous Met. Soc. China 2011, 21, 2037–2043. [Google Scholar] [CrossRef]
- Ma, J.; Ren, F.; Wang, G.; Yi, X.; Li, Y.; Wen, J. Electrochemical performance of melt-spinning Al-Mg-Sn based anode alloys. Int. J. Hydrogen Energy 2017, 42, 11654–11661. [Google Scholar] [CrossRef]
- Rajabi, M.; Simchi, A.; Davami, P. Microstructure and mechanical properties of Al–20Si–5Fe–2X (X = Cu, Ni, Cr) alloys produced by melt-spinning. Mater. Sci. Eng. A 2008, 492, 443–449. [Google Scholar] [CrossRef]
- Tourret, D.; Reinhart, G.; Gandin, C.-A.; Iles, G.N.; Dahlborg, U.; Calvo-Dahlborg, M.; Bao, C.M. Gas atomization of Al–Ni powders: Solidification modeling and neutron diffraction analysis. Acta Mater. 2011, 59, 6658–6669. [Google Scholar] [CrossRef]
- Ding, P.; Mao, A.; Zhang, X.; Jin, X.; Wang, B.; Liu, M.; Gu, X. Preparation, characterization and properties of multicomponentAlCoCrFeNi2.1 powder by gas atomization method. J. Alloys Compd. 2017, 721, 609–614. [Google Scholar] [CrossRef]
- Li, X.; Fritsching, U. Process modeling pressure-swirl-gas-atomization for metal powder production. J. Mater. Process. Technol. 2017, 239, 1–17. [Google Scholar] [CrossRef]
- Wang, E.R.; Hui, X.D.; Wang, S.S.; Zhao, Y.F.; Chen, G.L. Microstructure and mechanical properties of Al–Si–Ni–Ce alloys prepared by gas-atomization spark plasma sintering and hot-extrusion. Mater. Sci. Eng. A 2011, 528, 5764–5771. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, W.T.; Kim, D.H. Formation and crystallization of Al–Ni–Ti amorphous alloys. Mater. Sci. Eng. A 2004, 385, 44–53. [Google Scholar] [CrossRef]
- Shi, J.; Zheng, A.; Lin, Z.; Chen, R.; Zheng, J.; Cao, Z. Effect of process control agent on alloying andmechanical behavior of L21 phase Ni–Ti–Al alloys. Mater. Sci. Eng. A 2019, 740–741, 130–136. [Google Scholar] [CrossRef]
- Chen, H.; Kong, D. Effects of laser remelting speeds on microstructure, immersion corrosion, and electrochemical corrosion of arc–sprayed amorphous Al–Ti–Ni coatings. J. Alloys Compd. 2019, 771, 584–594. [Google Scholar] [CrossRef]
- Fabrichnaya, O.; Beuers, G.; Bätzner, C.; Lukas, H.L. Al-Ni-Si (Aluminium-Nickel-Silicon). In Light Metal Systems. Part 3. Landolt-Börnstein-Group IV Physical Chemistry (Numerical Data and Functional Relationships in Science and Technology); Effenberg, G., Ilyenko, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 11A3. [Google Scholar]
- Cohen, M. Advancing Materials Research; Psaras, P.A., Langford, H.D., Eds.; National Academy Press: Washington, DC, USA, 1987; p. 57. [Google Scholar]
- Spear, R.E.; Gardner, G.R. Dendrite Cell Size. AFS Trans. 1963, 71, 209–215. [Google Scholar]
- Granger, D.A. Ingot Casting in the Aluminum Industry. Treatise Mater. Sci. Technol. 1989, 31, 109–135. [Google Scholar] [CrossRef]
- Shivkumar, S.; Wang, L.; Apelian, D. Molten Metal Processing of Advanced Cast Aluminum Alloys. JOM 1991, 43, 26–32. [Google Scholar] [CrossRef]
- Şmakov Iu, V.; Zenina, M.V.; Riabov, I.V. Sovremennoje sostoianie i dalnejichee razvitie porchnevah splavov na Al-osnove. Liteino Prod. 2000, 11, 3. [Google Scholar]
- He, S.; Liu, Y.; Guo, S. Cooling rate calculation of non-equilibrium aluminum alloy powders prepared by gas atomization. Rare Metal Mat. Eng. 2009, 38, 353–356. [Google Scholar]
- Scherrer, P. Bestimmung der Grosse und der Inneren Struktur von Kolloidteilchen Mittels Rontgenstrahlen, Nachrichten von der Gesellschaft der Wissenschaften, Göttinger. Math. Phys. 1918, 2, 98–100. [Google Scholar]
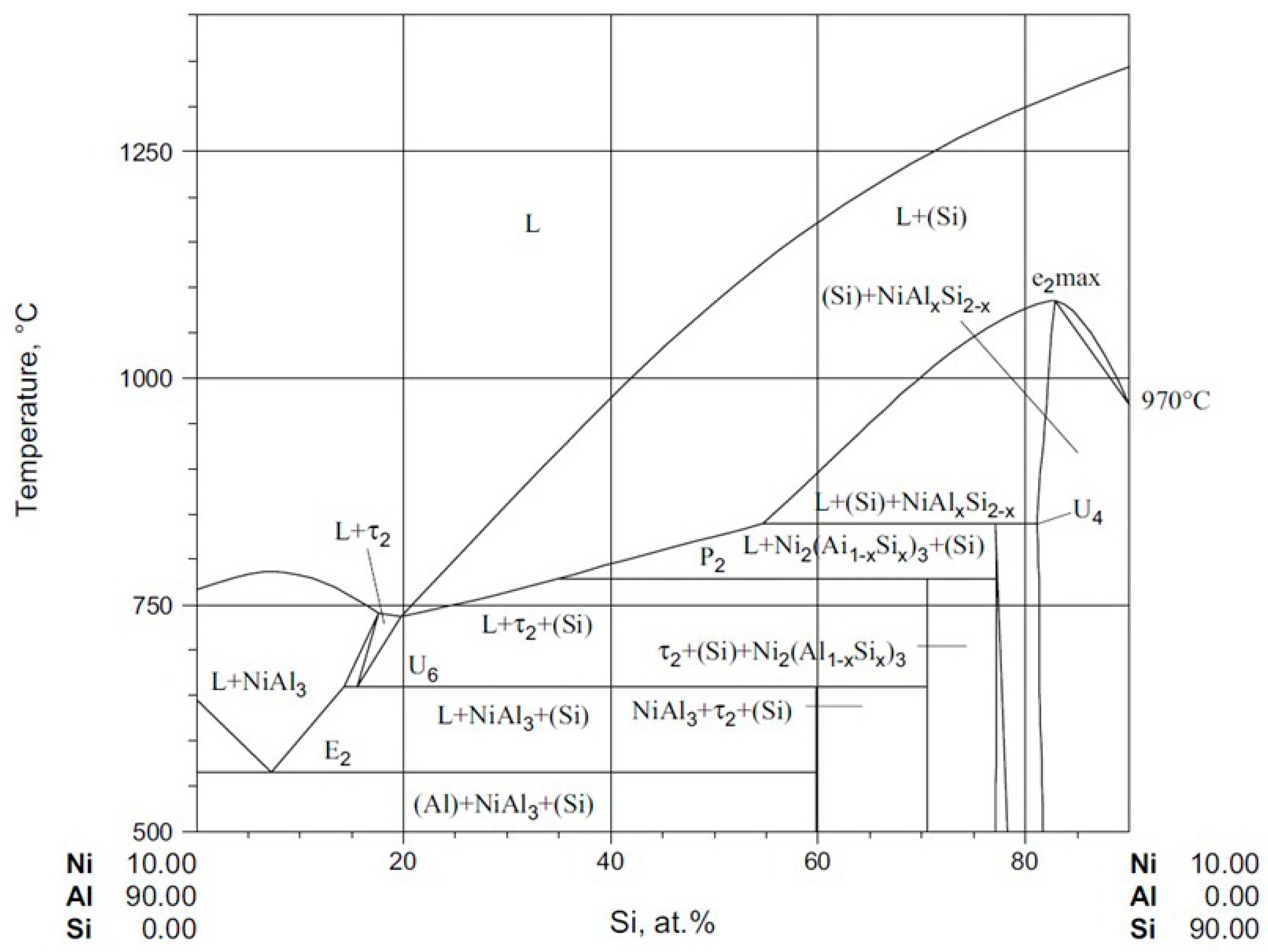
Figure 1.
The position of Al
79Si
11Ni
10 alloy in vertical projection of ternary diagram for 10% nickel [
18]. The deep eutectic, noticed for the 11% Si position, should benefit in the glass-formation capacity of the alloy.
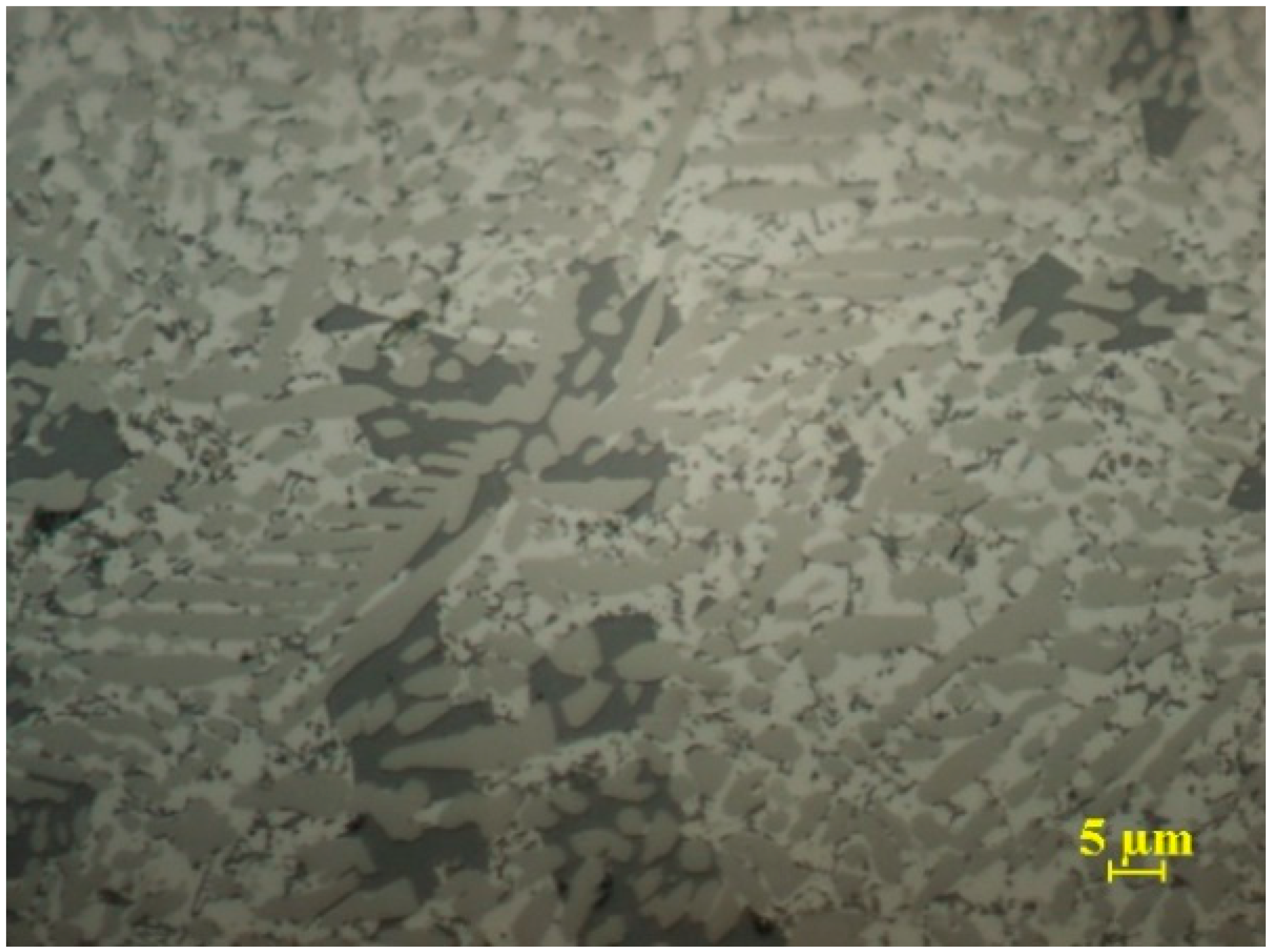
Figure 2.
Metallographic structure of a powder particle obtained by atomization in argon, from the Al79Si11Ni10 alloy.
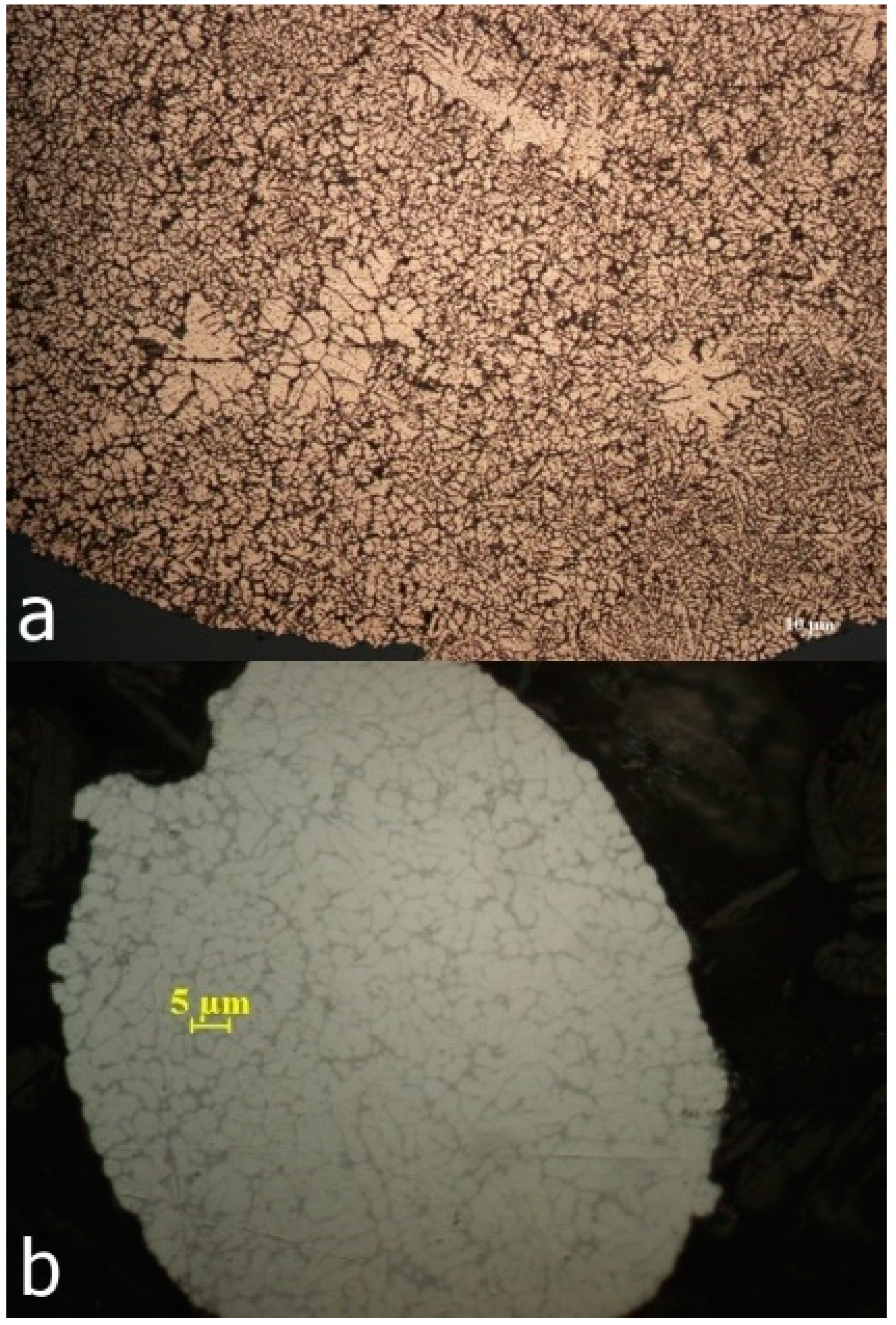
Figure 3.
Powder structure obtained by atomization of Al79Si11Ni10 alloy with high pressure, in water (a); the structure of commercially available powder (b).
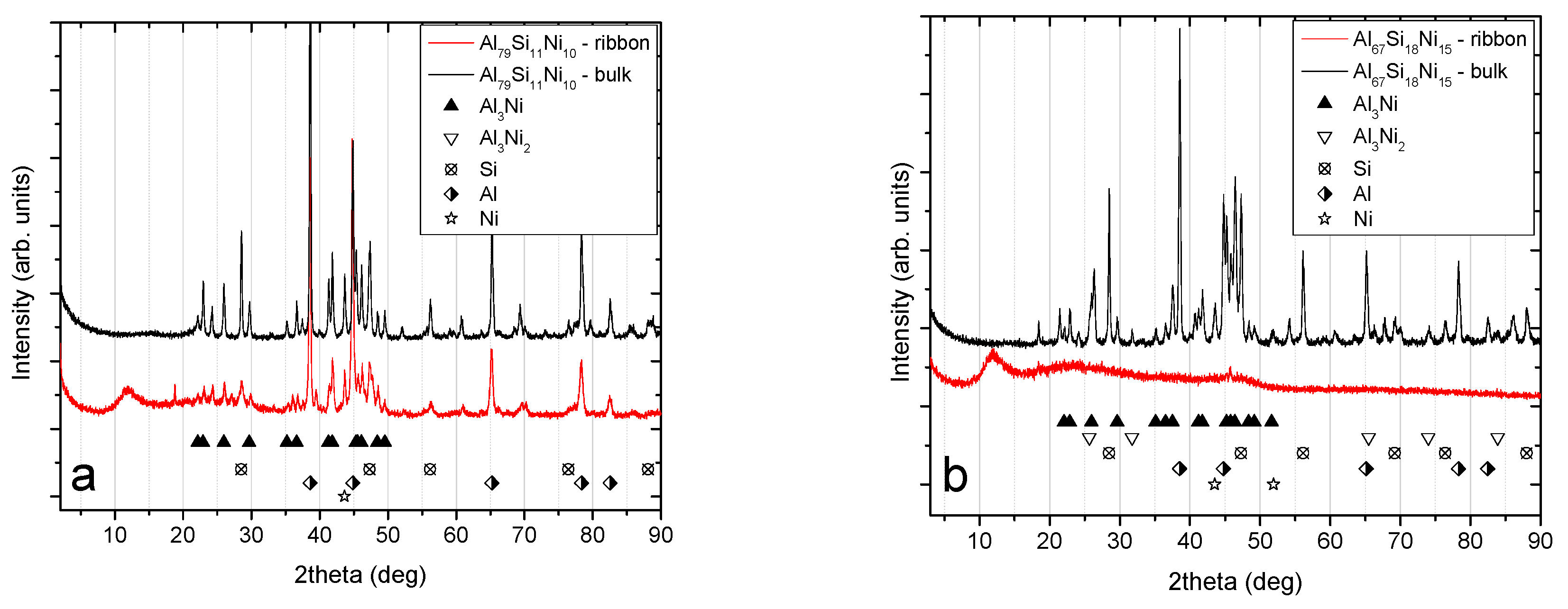
Figure 4.
X-ray diffraction patterns for the bulk and melt-spun alloys: (a) Patterns for the Al79Si11Ni10 bulk and melt-spun ribbons; (b) patterns for the Al67Si18Ni15 bulk and melt-spun ribbons.
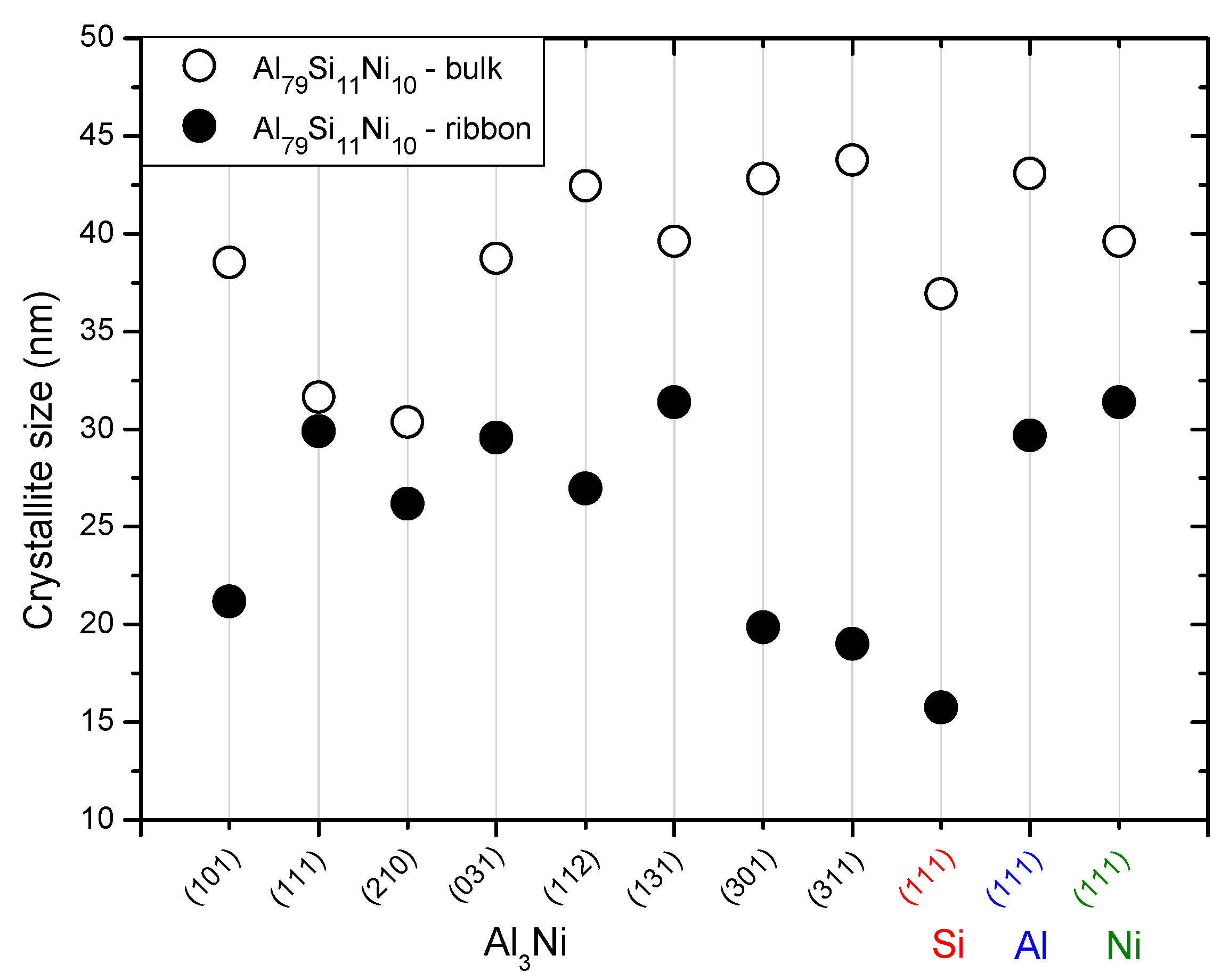
Figure 5.
Crystallite size of different phases of Al79Si11Ni10 in bulk and melt-spun ribbon form. The refinement of the structure is evident for the melt-spun ribbons, extrapolated from the lower crystallite size for all phases.
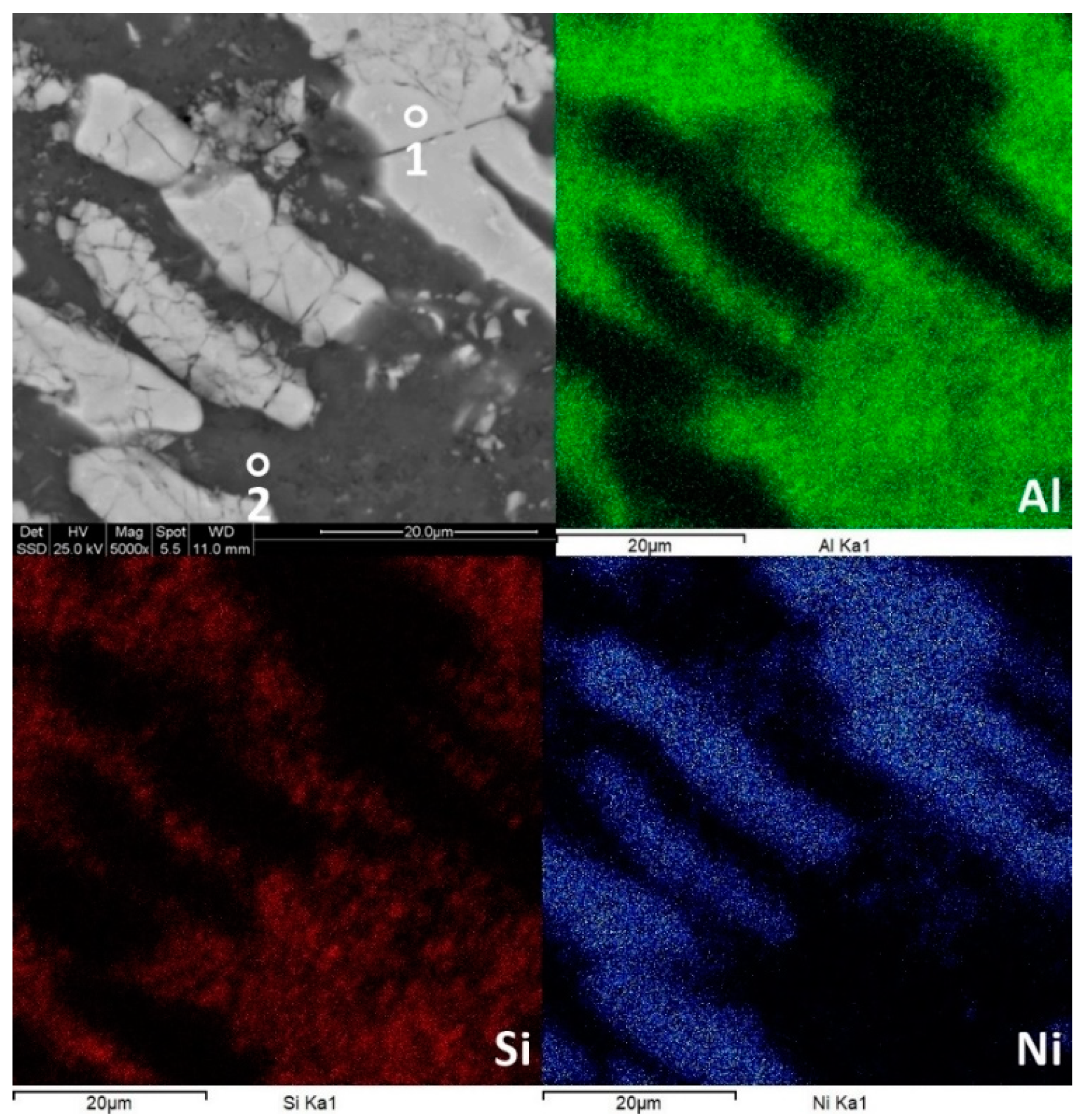
Figure 6.
Surface morphology and chemical composition of Al79Si11Ni10 in bulk form. The segregation of the elements is noticeable, and it is linked to the structural development of the alloy.
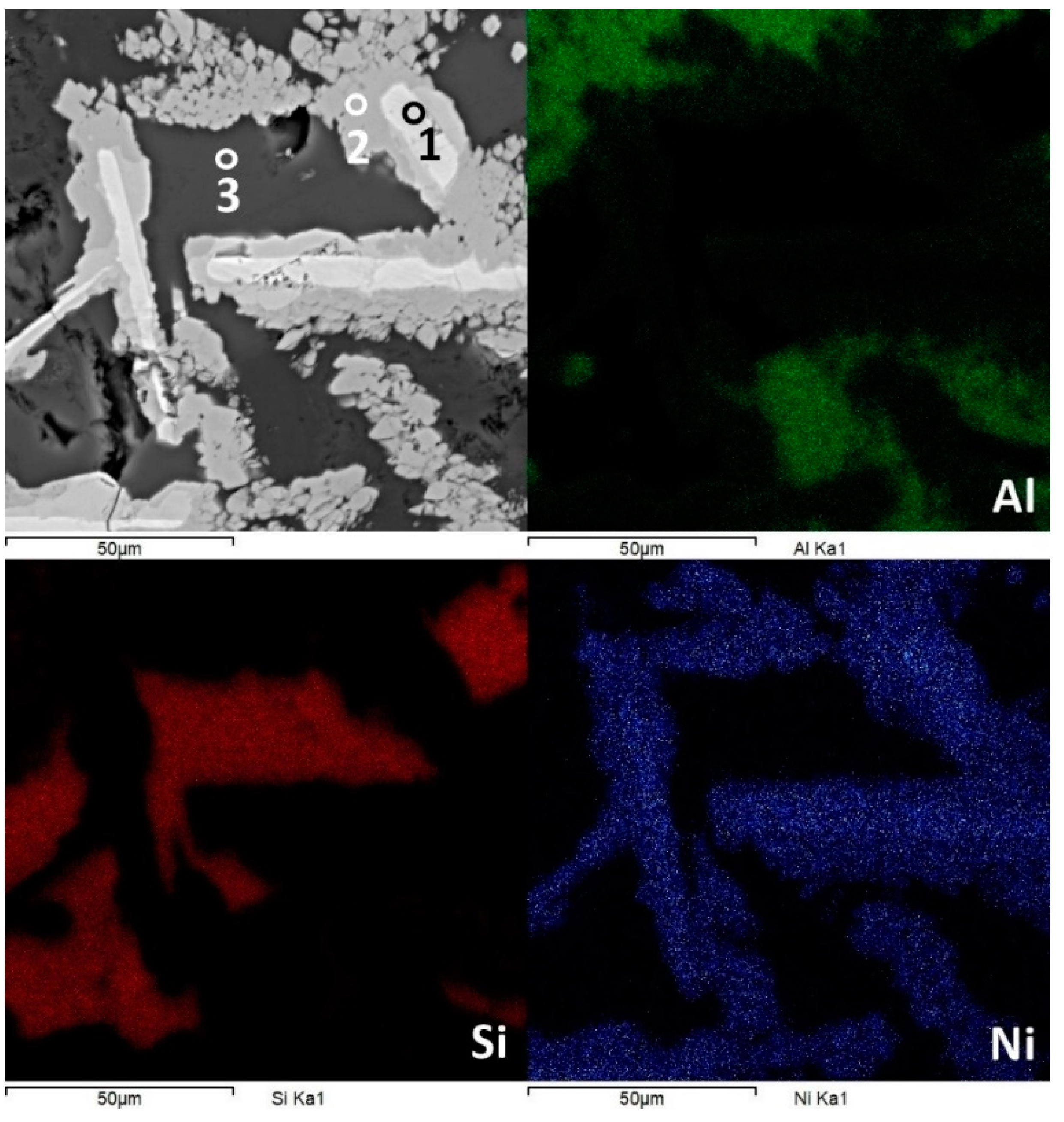
Figure 7.
Surface morphology and chemical composition of Al67Si18Ni15 in bulk form. The segregation of the elements is noticeable, and it is linked to the structural development of the alloy.
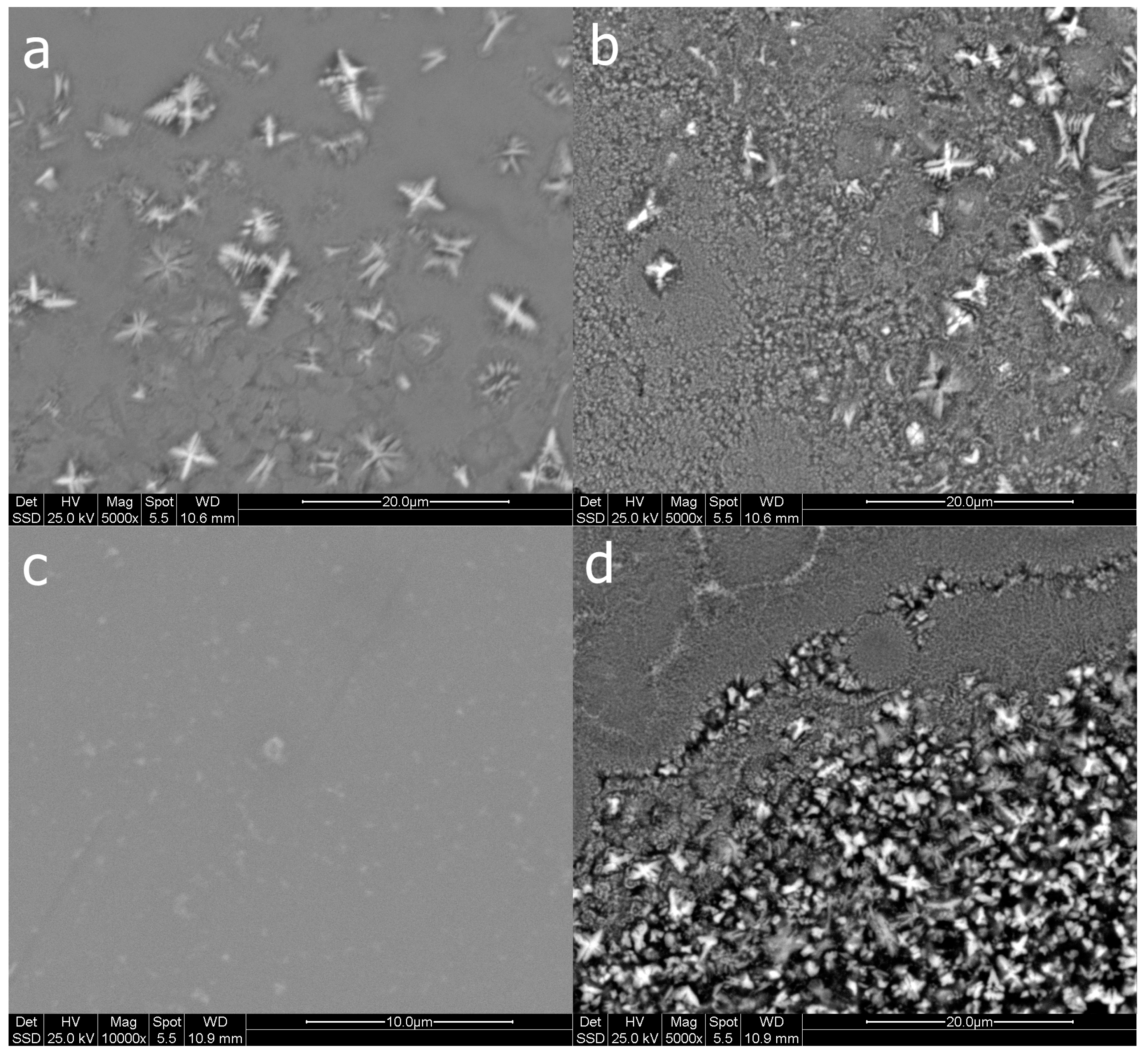
Figure 8.
Surface morphology of melt-spun ribbons from Al79Si11Ni10 ((a) as-cast; (b) after heat treatment) and Al67Si18Ni15 ((c) as-cast; (d) after heat treatment).
Figure 9.
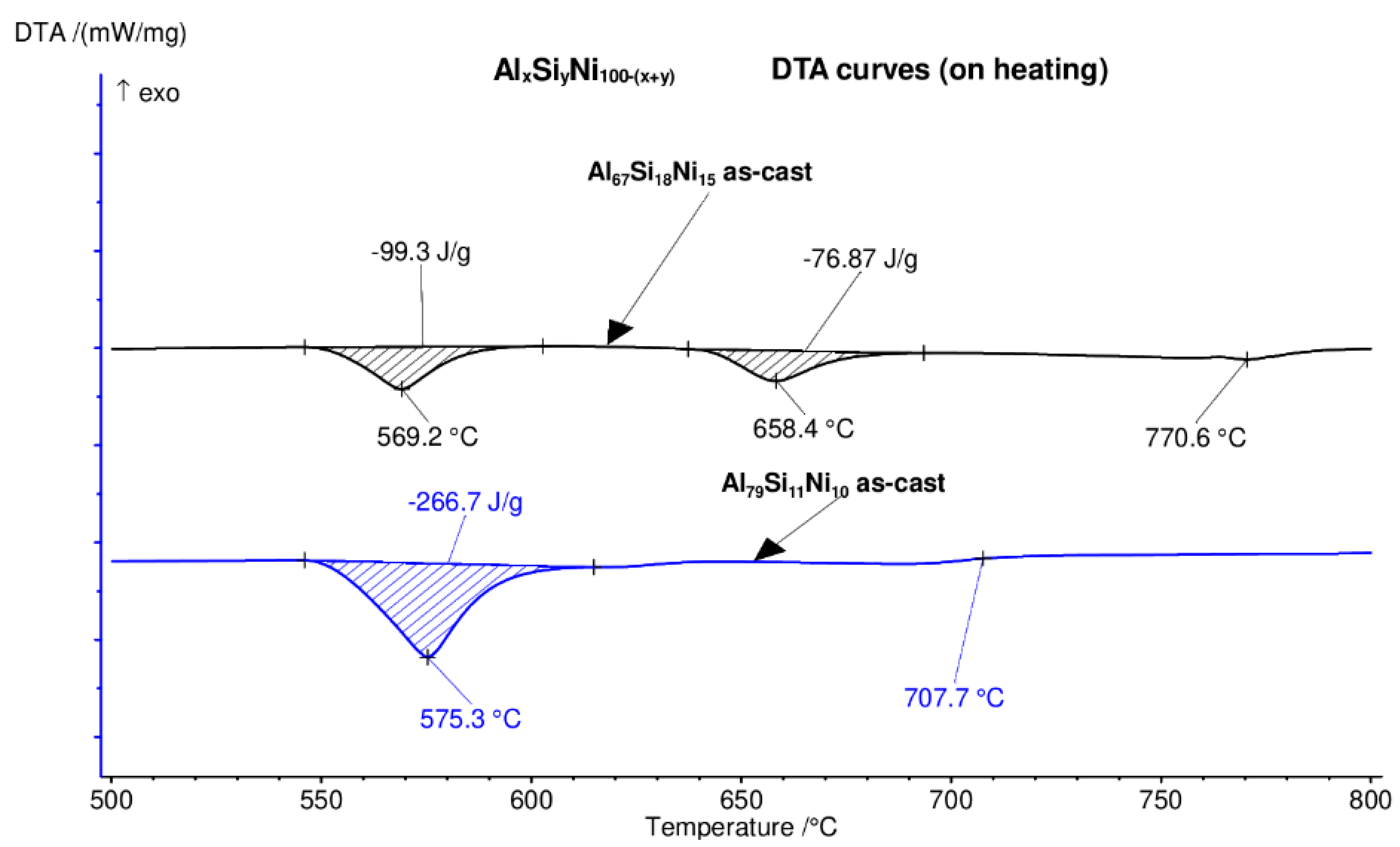
Differential thermal analysis (DTA) heating curves for the Al79Si11Ni10 and Al67Si18Ni15 alloys, in bulk form. The endothermic peaks signify melting events, firstly of the eutectic, followed by the intermetallic compounds.
Figure 10.
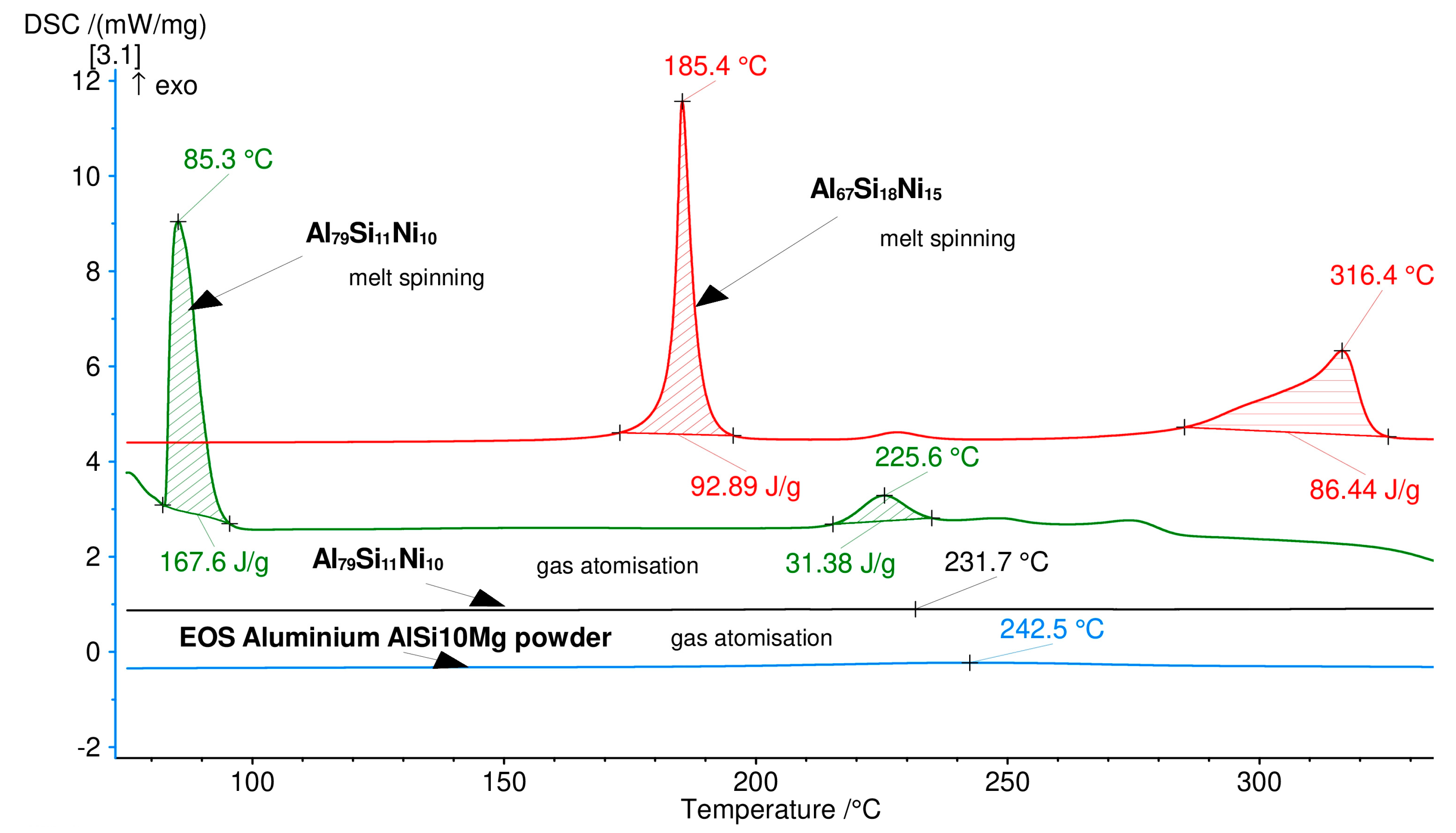
Differential scanning calorimetry (DSC) heating curves for melt-spinning; Al79Si11Ni10–gas atomization, compared to commercially available atomized powder (F357 EOS). The exothermal peaks signify phase transformations, which occur during heating.
Figure 11.
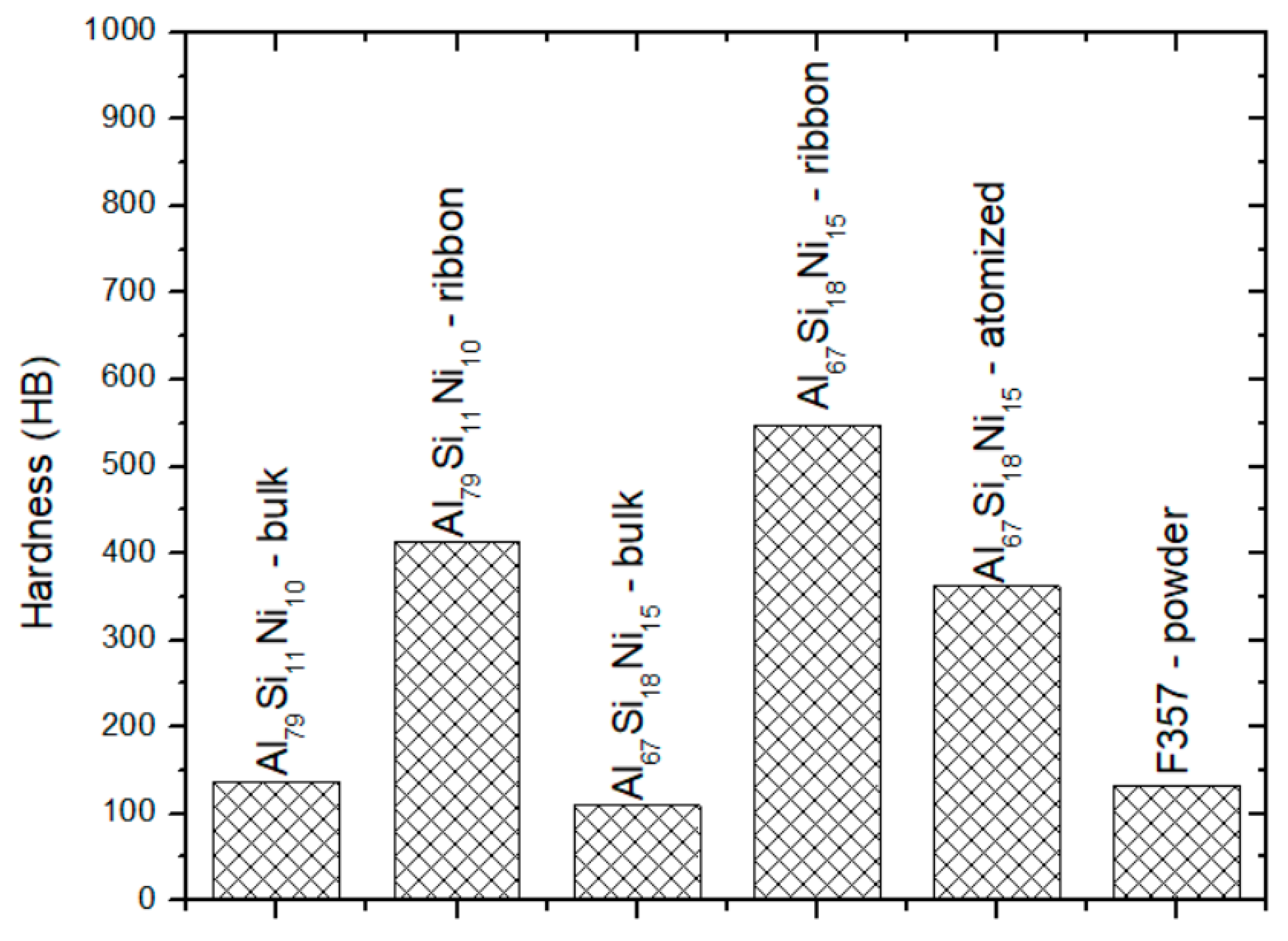
Brinell hardness (converted) for the bulk, melt-spun, and atomized Al79Si11Ni10 and Al67Si18Ni15 alloys, and the F357 (AlSi10Mg) alloy. A hardening effect due to the fast cooling techniques is noticed.
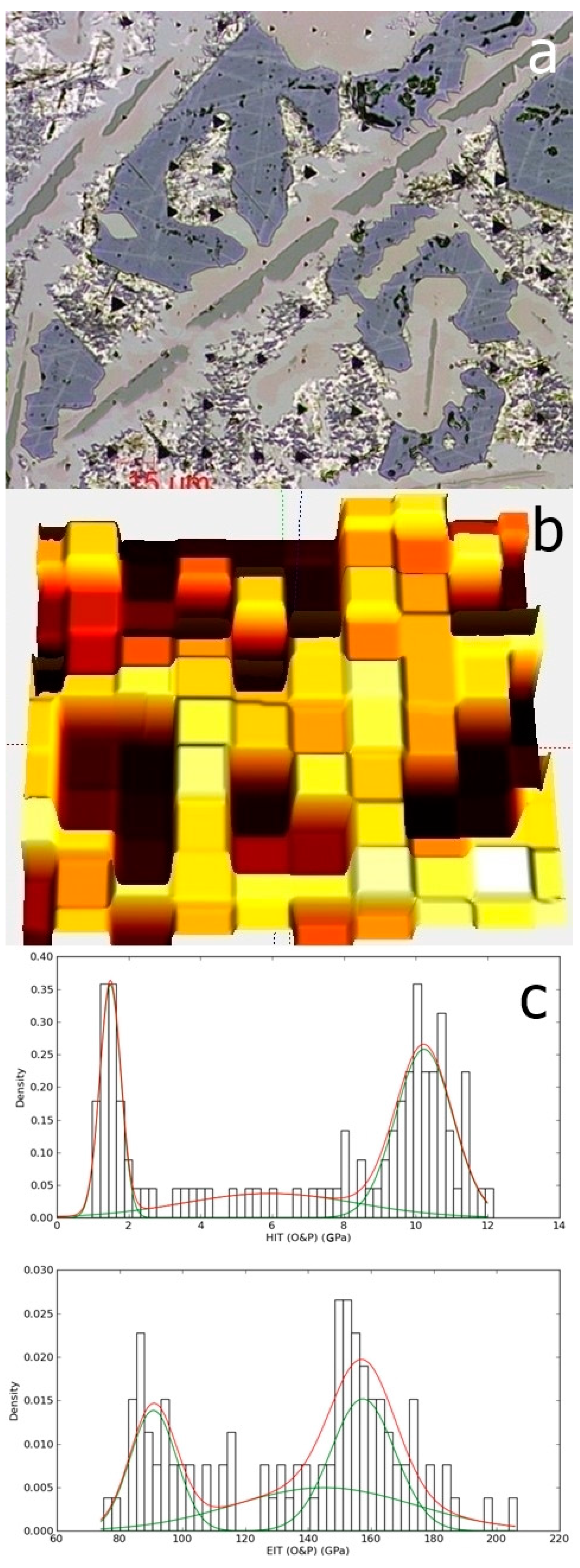
Figure 12.
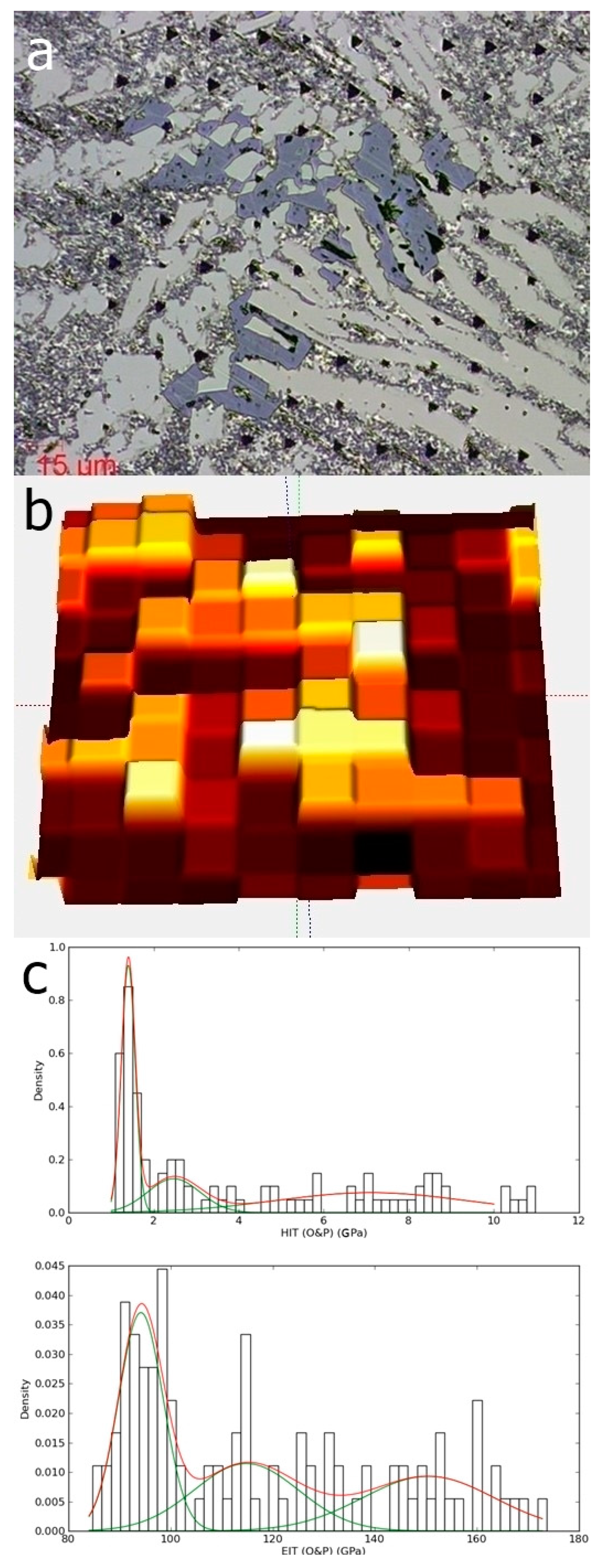
The indentation matrix (a) and the surface mapping as function of hardness (b); the hardness and elastic modulus distribution (c), extracted from the surface mapping, for the Al79Si11Ni10 alloy.
Figure 13.
The indentation matrix (a) and the surface mapping as function of hardness (b); the hardness and elastic modulus distribution (c), extracted from the surface mapping, for the Al67Si18Ni15 alloy.
Table 1.
Chemical composition of the Al–Si–Ni alloys.
| Alloy | Chemical Composition | Al | Si | Ni | Bulk | Melt-Spun | Atomized |
|---|
| Al79Si11Ni10 | (at. %) | 79 | 11 | 10 | - | - | - |
| (wt. %) | 70.41 | 10.2 | 19.39 |
| Al67Si18Ni15 | (at. %) | 67 | 18 | 15 | - | - | - |
| (wt. %) | 56.6 | 15.83 | 27.57 |
Table 2.
The surface chemical composition, obtained by energy dispersion spectroscopy system.
| Alloy | Acquisition Site | Chemical Composition (at. %) |
|---|
| Al | Si | Ni |
|---|
| Al79Si11Ni10 in bulk form | spectrum 1 | 74.51 | - | 25.49 |
| spectrum 2 | 80.83 | 18.66 | 0.51 |
| Al67Si18Ni15 in bulk form | spectrum 1 | 48.95 | 10.69 | 40.36 |
| spectrum 2 | 73.01 | 1.26 | 25.73 |
| spectrum 3 | 0.42 | 99.10 | 0.48 |
| Al79Si11Ni10 melt-spun ribbons, as cast | sum spectrum | 78.23 | 12.92 | 8.85 |
| Al79Si11Ni10 melt-spun ribbons, heat treated | sum spectrum | 77.64 | 14.01 | 8.35 |
| Al67Si18Ni15 melt-spun ribbons, as cast | sum spectrum | 64.78 | 22.04 | 13.18 |
| Al67Si18Ni15 melt-spun ribbons, heat treated | sum spectrum | 76.01 | 14.76 | 9.23 |
Table 3.
Mechanical characteristics of the fast solidified Al79Si11Ni10 and Al67Si18Ni15 alloys.
| Mechanical | Al79Si11Ni10 | Al67Si18Ni15 | Al67Si18Ni15 |
|---|
| Properties | Melt Spun | Melt Spun | Atomized |
|---|
| H (GPa) | 4.74 ± 0.46 | 6.29 ± 0.27 | 4.13 ± 0.31 |
| E (GPa) | 94.03 ± 6.70 | 70.29 ± 1.02 | 87.85 ± 4.55 |
| H/E | 0.050 | 0.090 | 0.047 |
| H3/E2 | 0.012 | 0.051 | 0.009 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).