The Influence of Holding Time on the Microstructure Evolution of Mg–10Zn–6.8Gd–4Y Alloy during Semi-Solid Isothermal Heat Treatment
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
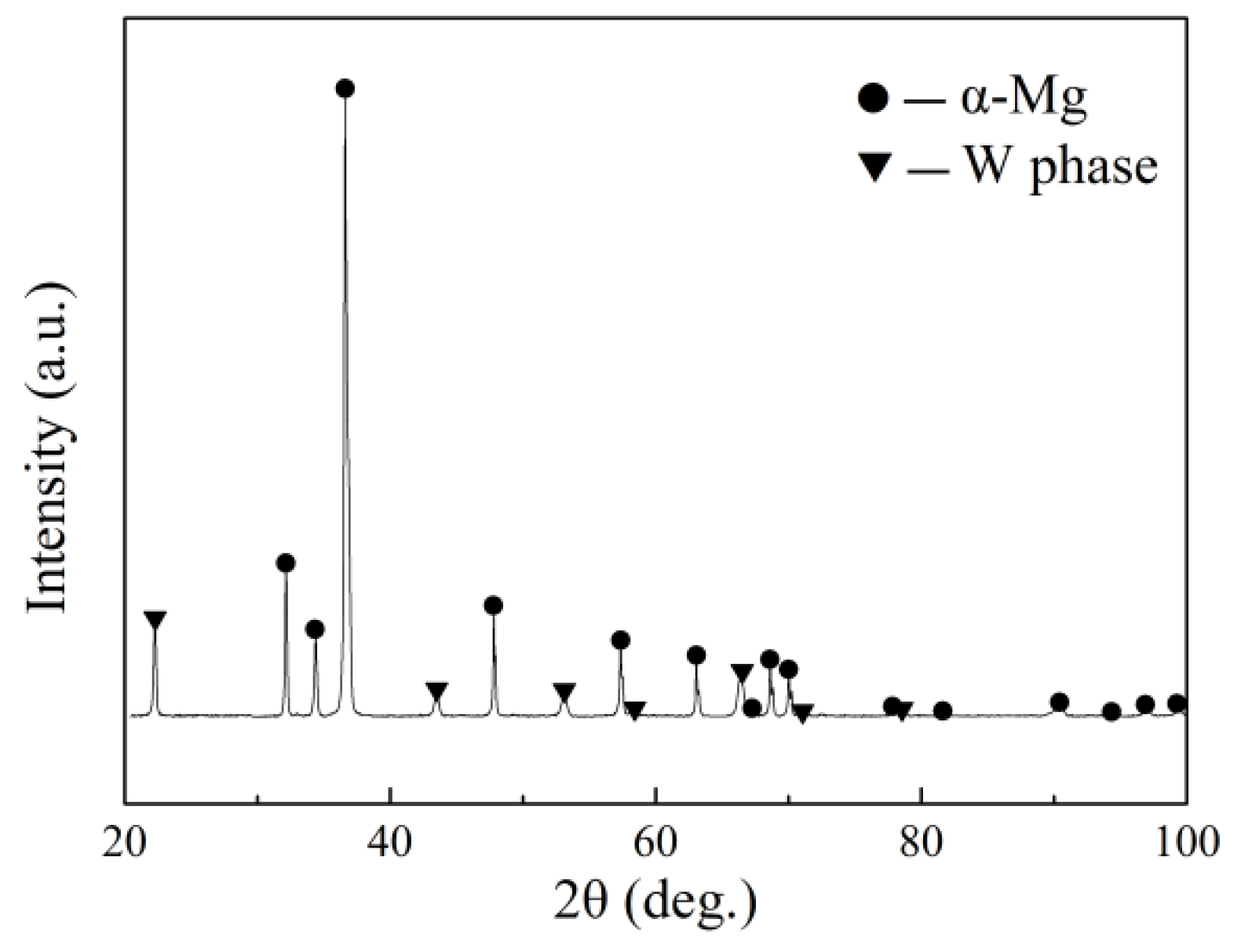
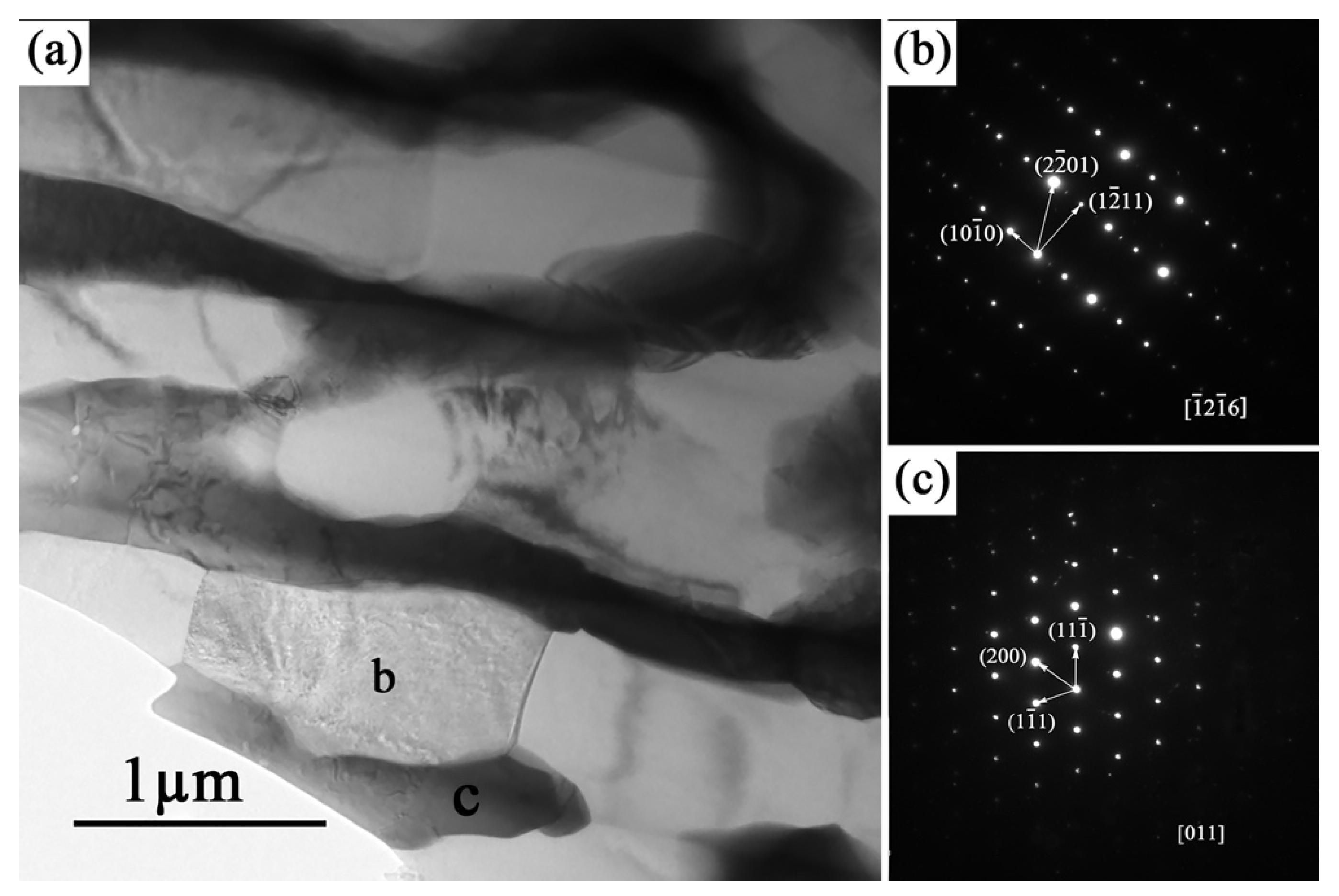
3.1. The Microstructure of As-Cast Mg–10Zn–6.8Gd–4Y Alloy
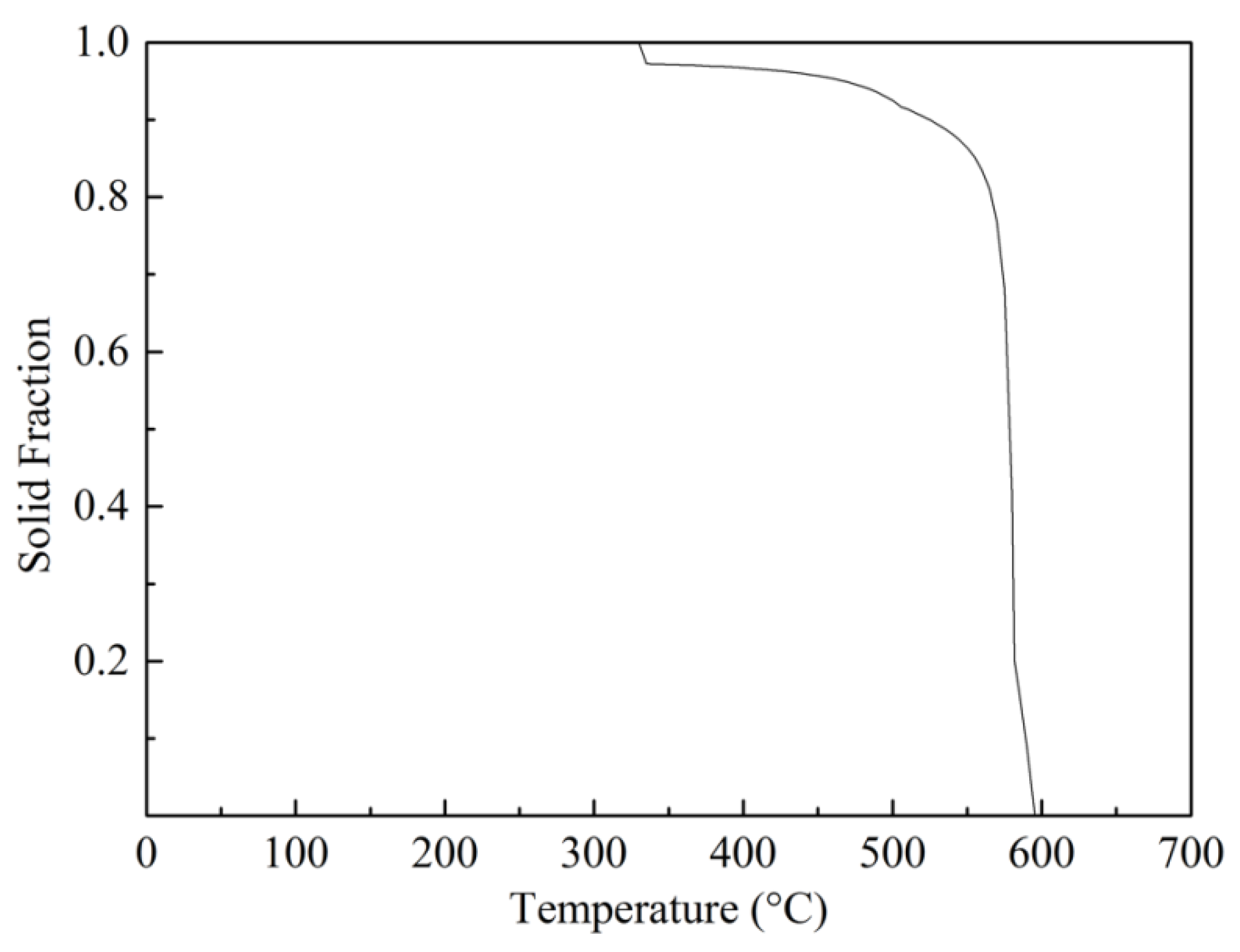
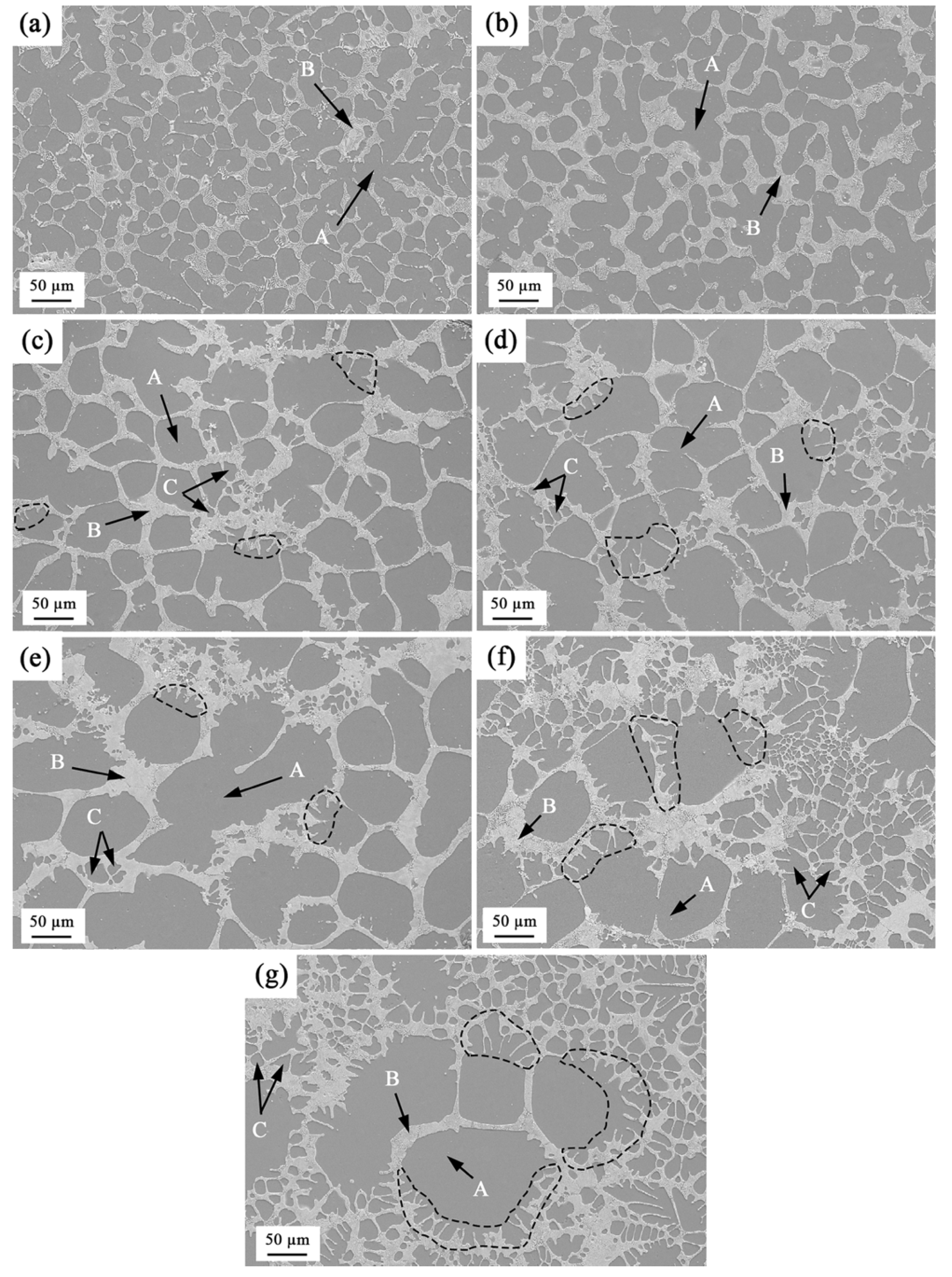
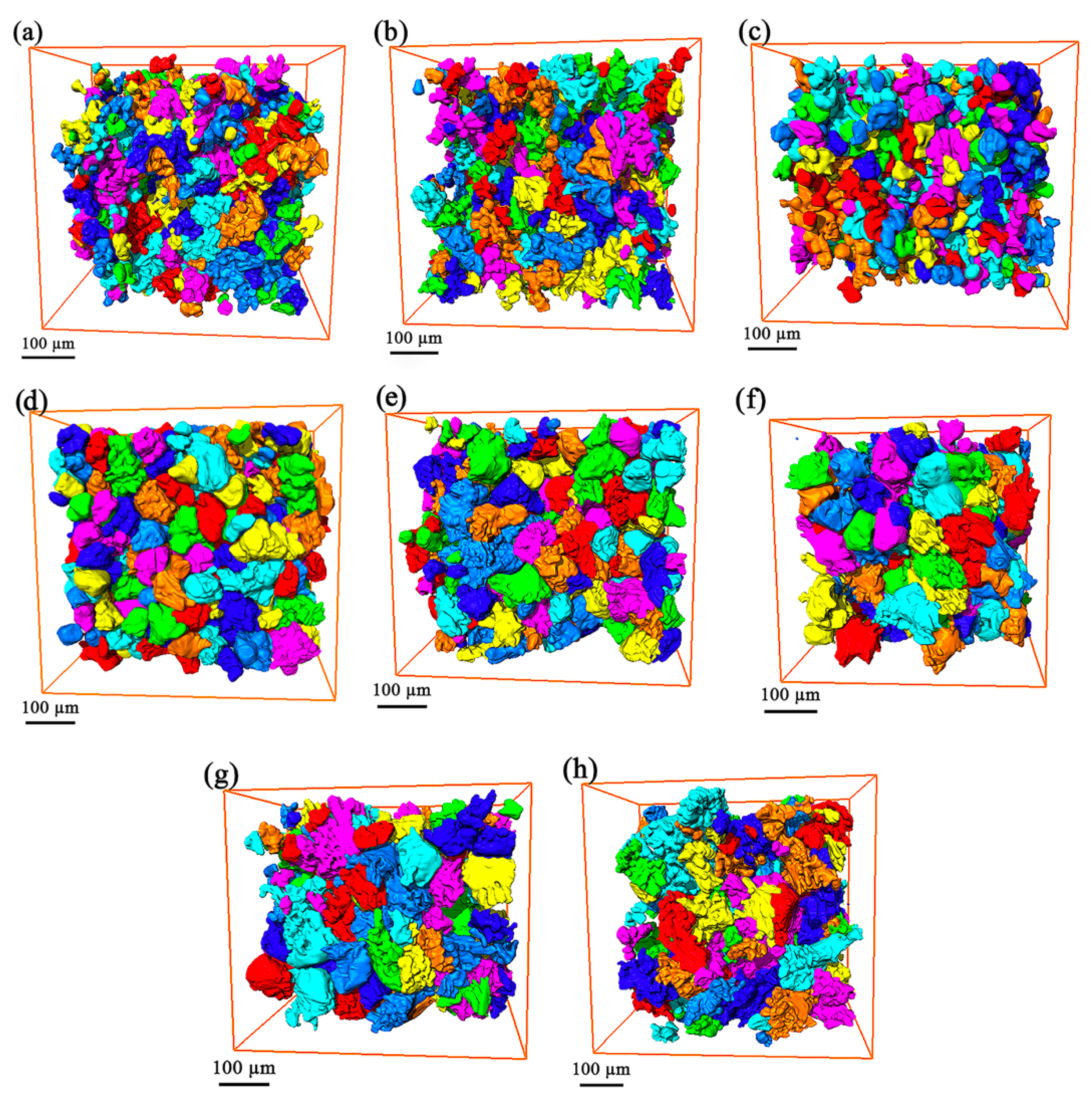
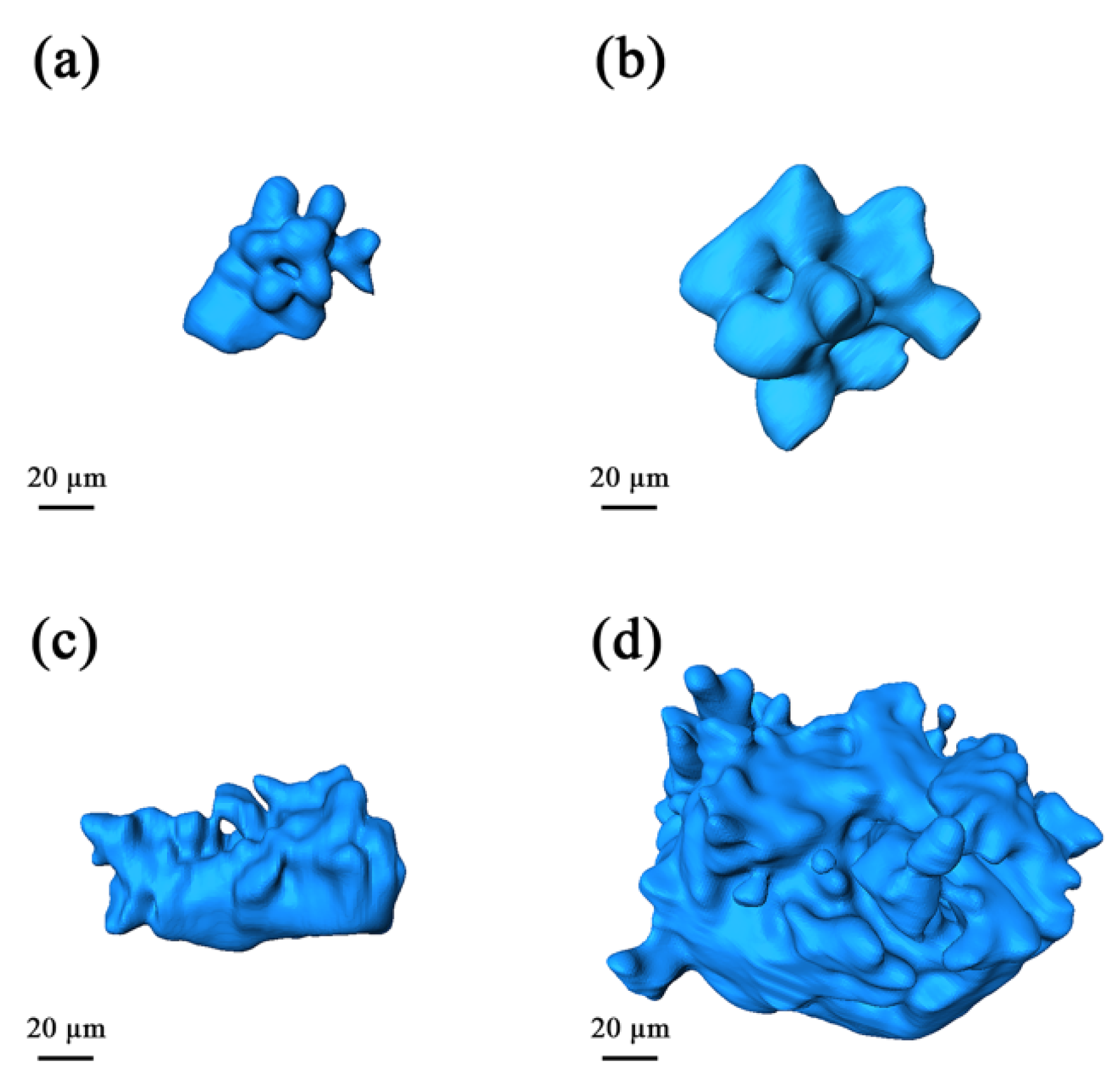
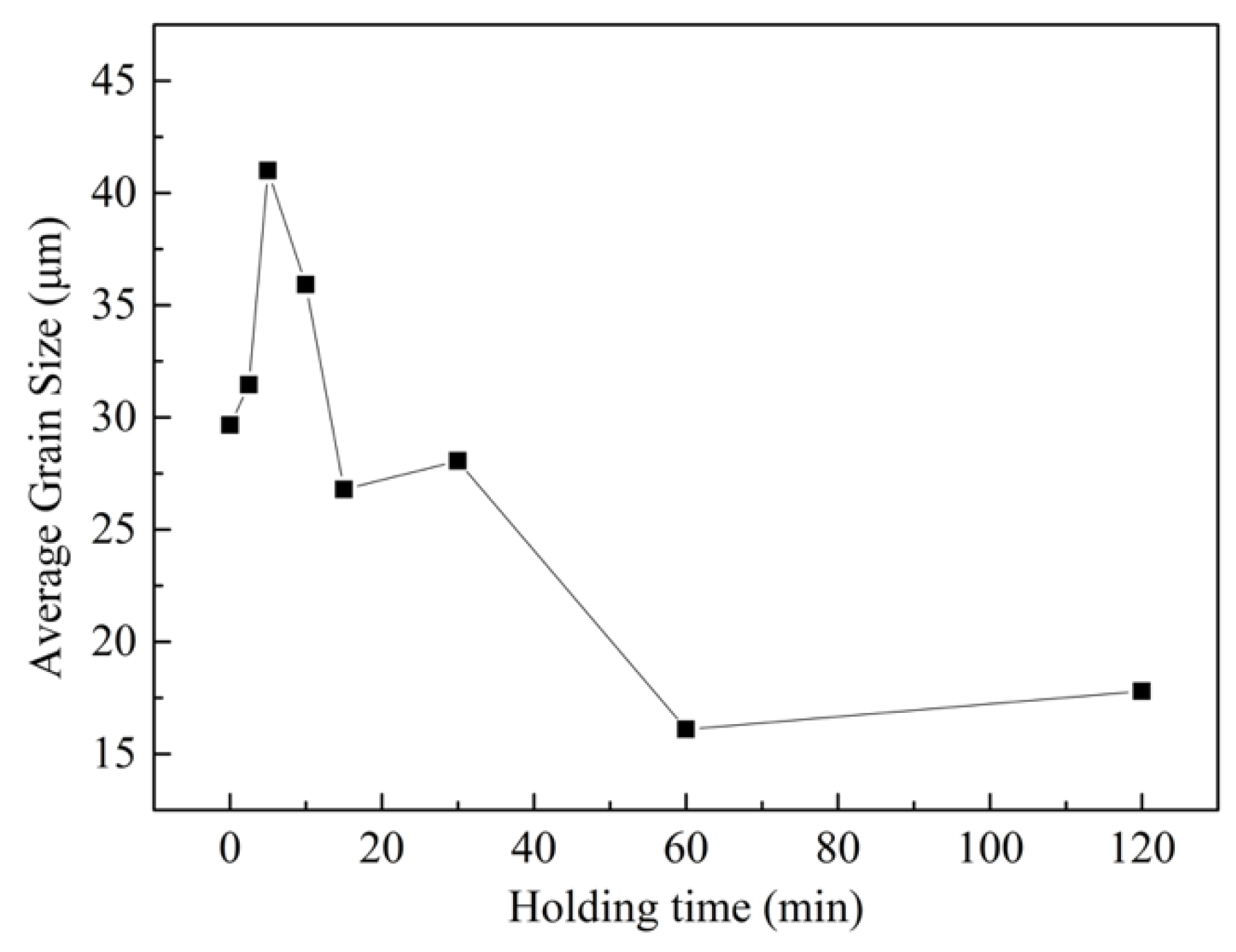
3.2. The Microstructures of Semi-Solid Mg–10Zn–6.8Gd–4Y Alloys
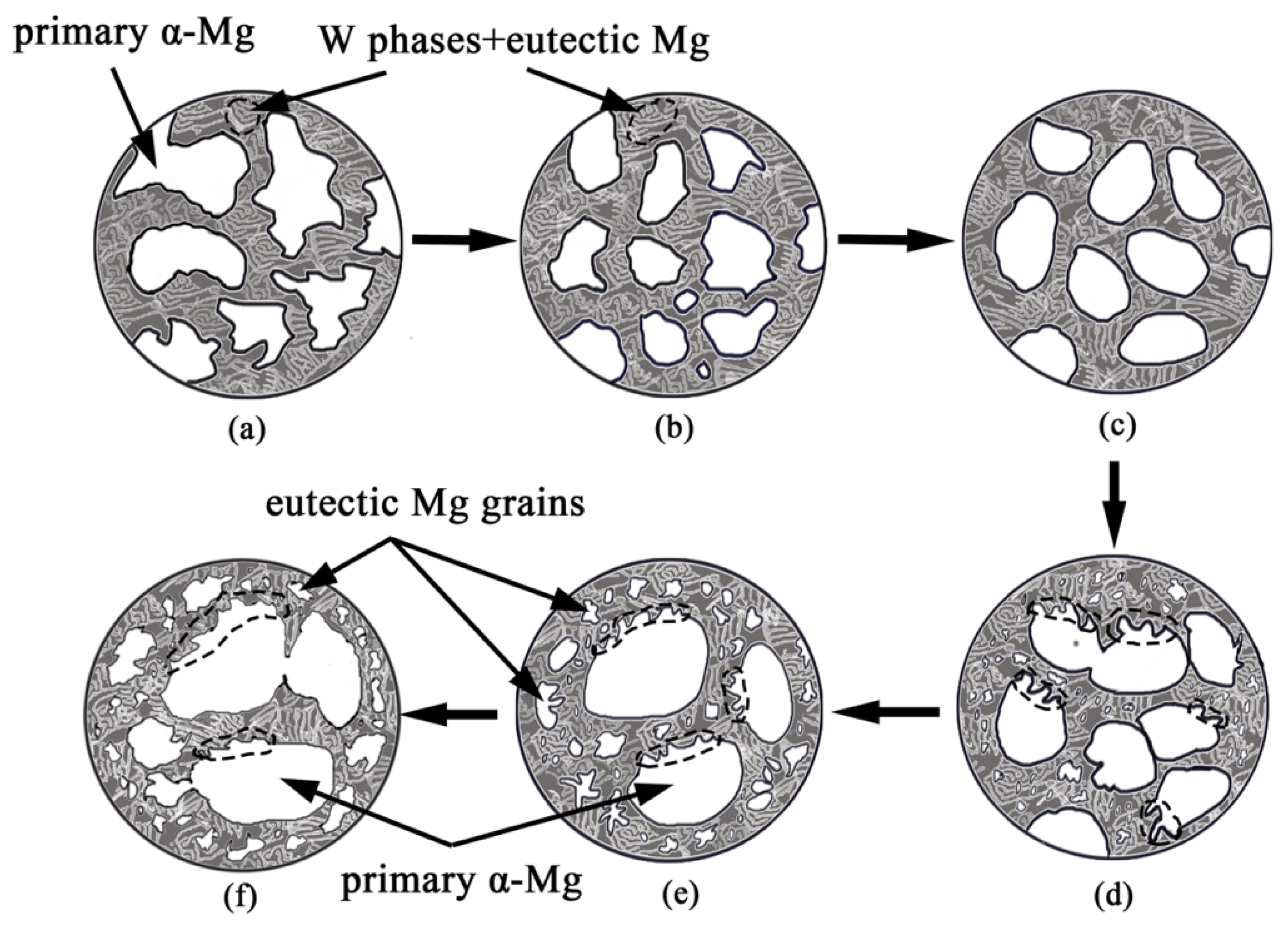
3.3. Coarsening Mechanism during Isothermal Heat Treatment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Diem, W. Magnesium in different applications. Auto Technol. 2001, 1, 40–41. [Google Scholar]
- Tekumalla, S.; Seetharaman, S.; Almajid, A.; Gupta, M. Mechanical properties of magnesium-rare earth alloy systems: a review. Metals 2015, 5, 1–39. [Google Scholar] [CrossRef]
- Liu, H.; Ju, J.; Bai, J.; Sun, J.; Song, D.; Yan, J.; Jiang, J.; Ma, A. Preparation, microstructure evolutions and mechanical property of an ultra-fine grained Mg-10Gd-4Y-1.5Zn-0.5Zr alloy. Metals 2017, 7, 398. [Google Scholar] [CrossRef]
- Tang, C.; Wu, K.; Liu, W.; Feng, D.; Wang, X.; Miao, G.; Yang, M.; Liu, X.; Li, Q. Effects of Gd, Y content on the microstructure and mechanical properties of Mg-Gd-Y-Nd-Zr alloy. Metals 2018, 8, 790. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, D.; Zhang, H.; Wang, L.; Wang, J.; Meng, J. Effect and application of rare earth element in magnesium alloys. Chin. J. Rare Met. 2008, 32, 659–667. [Google Scholar]
- Rokhlin, L.L. Magnesium Alloys Containing Rare Earth Metals: Structure and Properties; Taylor & Francis: London, UK, 2003; pp. 32–45. [Google Scholar]
- Su, G.; Cao, Z.; Liu, Y.; Wang, Y.; Zhang, L.; Cheng, L. Effects of semi-solid isothermal process parameters on microstructure of Mg-Gd alloy. Trans. Nonferrous Met. Soc. Chin. 2010, 20, s402–s406. [Google Scholar] [CrossRef]
- Zheng, W.; Li, S.; Tang, B.; Zeng, D.; Guo, X. Effect of rare earths on hot cracking resistant property of Mg-Al alloys. J. Rare Earths 2006, 24, 346–351. [Google Scholar] [CrossRef]
- Flemings, M.C. Behavior of metal-alloys in the semisolid state. Metall. Trans. A 1991, 22, 957–981. [Google Scholar] [CrossRef]
- Ji, S.; Fan, Z.; Bevis, M.J. Semi-solid processing of engineering alloys by a twin-screw rheomoulding process. Mater. Sci. Eng. A 2001, 299, 210–217. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, B.; Yuan, S.; Jie, W.; Yang, G. Microstructure evolution during solidification of AZ91D magnesium alloy in semisolid. J. Mater. Sci. Technol. 2006, 22, 311–314. [Google Scholar]
- Koren, Z.; Rosenson, H.; Gutman, E.M.; Unigovski, Y.B.; Eliezer, A. Development of semisolid casting for AZ91 and AM50 magnesium alloys. J. Light Met. 2002, 2, 81–87. [Google Scholar] [CrossRef]
- Czerwinski, F. The generation of Mg–Al–Zn alloys by semisolid state mixing of particulate precursors. Acta Mater. 2004, 52, 5057–5069. [Google Scholar] [CrossRef]
- Loué, W.R.; Suéry, M. Microstructural evolution during partial remelting of Al-Si7Mg alloys. Mater. Sci. Eng. A 1995, 203, 1–13. [Google Scholar] [CrossRef]
- Ma, G.R.; Li, X.L.; Xiao, L.; Li, Q.F. Effect of holding temperature on microstructure of an AS91 alloy during semisolid isothermal heat treatment. J. Alloys Compd. 2010, 496, 577–581. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, G.; Cao, Z.; Ding, W. Effects of Ca content on the microstructure of semisolid Mg–13Al alloy produced via isothermal heat treatment. J. Alloys Compd. 2012, 534, 52–58. [Google Scholar] [CrossRef]
- Nami, B.; Shabestari, S.G.; Miresmaeili, S.M.; Razavi, H.; Mirdamadi, S. The effect of rare earth elements on the kinetics of the isothermal coarsening of the globular solid phase in semisolid AZ91 alloy produced via SIMA process. J. Alloys Compd. 2010, 489, 570–575. [Google Scholar] [CrossRef]
- Voorhees, P.W.; Glicksman, M.E. Ostwald ripening during liquid phase sintering—Effect of volume fraction on coarsening kinetics. Metall. Trans. A 1984, 15, 1081–1088. [Google Scholar] [CrossRef]
- Masteghin, M.G.; Bertinotti, R.C.; Orlandi, M.O. Coalescence growth mechanism of inserted tin dioxide belts in polycrystalline SnO2-based ceramics. Mater. Charact. 2018, 142, 289–294. [Google Scholar] [CrossRef]
- German, R.M. Coarsening in sintering: grain shape distribution, grain size distribution, and grain growth kinetics in solid-pore systems. Crit. Rev. Solid State Mater. Sci. 2010, 35, 263–305. [Google Scholar] [CrossRef]
- Chen, M.; Kattamis, T.Z. Dendrite coarsening during directional solidification of Al–Cu–Mn alloys. Mater. Sci. Eng. A 1998, 247, 239–247. [Google Scholar] [CrossRef]
- Fife, J.L.; Voorhees, P.W. Self-similar microstructural evolution of dendritic solid–liquid mixtures during coarsening. Scr. Mater. 2009, 60, 839–842. [Google Scholar] [CrossRef]
- Pompe, O.; Rettenmayr, M. Microstructural changes during quenching. J. Cryst. Growth 1998, 192, 300–306. [Google Scholar] [CrossRef]
- Van Boggelen, J.W.K.; Eskin, D.G.; Katgerman, L. First stages of grain coarsening in semi-solid Al–Cu alloys. Scr. Mater. 2003, 49, 717–722. [Google Scholar] [CrossRef]
- Chen, F.; Mao, F.; Chen, Z.; Han, J.; Yan, G.; Wang, T.; Cao, Z. Application of synchrotron radiation X-ray computed tomography to investigate the agglomerating behavior of TiB2 particles in aluminum. J. Alloys Compd. 2015, 622, 831–836. [Google Scholar] [CrossRef]
- Wang, M.Y.; Xu, Y.J.; Jing, T.; Peng, G.Y.; Fu, Y.N.; Chawla, N. Growth orientations and morphologies of α-Mg dendrites in Mg–Zn alloys. Scr. Mater. 2012, 67, 629–632. [Google Scholar] [CrossRef]
- Maire, E.; Grenier, J.C.; Daniel, D.; Baldacci, A.; Klöcker, H.; Bigot, A. Quantitative 3D characterization of intermetallic phases in an Al–Mg industrial alloy by X-ray microtomography. Scr. Mater. 2006, 55, 123–126. [Google Scholar] [CrossRef]
- Salvo, L.; Cloetens, P.; Maire, E.; Zabler, S.; Blandin, J.J.; Buffière, J.Y.; Ludwig, W.; Boller, E.; Bellet, D.; Josserond, C. X-ray micro-tomography an attractive characterisation technique in materials science. Nucl. Instrum. Methods Phys. Res. Sect. B 2003, 200, 273–286. [Google Scholar] [CrossRef]
- Guo, E.; Phillion, A.B.; Cai, B.; Shuai, S.; Kazantsev, D.; Jing, T.; Lee, P.D. Dendritic evolution during coarsening of Mg-Zn alloys via 4D synchrotron tomography. Acta Mater. 2017, 123, 373–382. [Google Scholar] [CrossRef]
- Shuai, S.; Guo, E.; Zheng, Q.; Wang, M.; Jing, T. Characterisation of three-dimensional dendritic morphology and orientation selection of α-Mg in Mg–Ca alloy using synchrotron X-ray tomography. Mater. Charact. 2016, 111, 170–176. [Google Scholar] [CrossRef]
- Tahreen, N.; Zhang, D.F.; Pan, F.S.; Jiang, X.Q.; Li, D.Y.; Chen, D.L. Strengthening mechanisms in magnesium alloys containing ternary I, W and LPSO phases. J. Mater. Sci. Technol. 2018, 34, 1110–1118. [Google Scholar] [CrossRef]
- Luo, S.; Pan, F.; Tang, A. Microstructure and mechanical properties at elevated temperature of Mg-Zn-Zr-Y alloy with W phase. Hot Working Technol. 2012, 41, 41–44. [Google Scholar] [CrossRef]
- Jiang, H.S.; Qiao, X.G.; Zheng, M.Y.; Wu, K.; Xu, C.; Kamado, S. The partial substitution of Y with Gd on microstructures and mechanical properties of as-cast and as-extruded Mg-10Zn-6Y-0.5Zr alloy. Mater. Charact. 2018, 135, 96–103. [Google Scholar] [CrossRef]
- Shi, F.; Wang, C.; Zhang, Z. Microstructures, corrosion and mechanical properties of as-cast Mg–Zn–Y–(Gd) alloys. Trans. Nonferrous Met. Soc. China 2015, 25, 2172–2180. [Google Scholar] [CrossRef]
- Ratke, L.; Voorhees, P.W. Growth and Coarsening: Ostwald Ripening in Material Processing; Springer: Berlin, Germany, 2002; pp. 31–41. [Google Scholar]
- Marsh, S.P.; Glicksman, M.E. Overview of Geometric Effects on Coarsening of Mushy Zones. Metall. Mater. Trans. A 1996, 27, 557–567. [Google Scholar] [CrossRef]
- Nishioka, K.; Maksimov, I.L. Reconsideration of the concept of critical nucleus and the Gibbs—Thomson equation. J. Cryst. Growth 1996, 163, 1–7. [Google Scholar] [CrossRef]
- Das, S.K.; Kang, Y.B.; Ha, T.; Jung, I.H. Thermodynamic modeling and diffusion kinetic experiments of binary Mg–Gd and Mg–Y systems. Acta Mater. 2014, 71, 164–175. [Google Scholar] [CrossRef]
- Das, S.K.; Kim, Y.M.; Ha, T.K.; Jung, I.H. Investigation of anisotropic diffusion behavior of Zn in hcp Mg and interdiffusion coefficients of intermediate phases in the Mg–Zn system. Calphad 2013, 42, 51–58. [Google Scholar] [CrossRef]
- Bermudez, K.; Brennan, S.; Sohn, Y.H. Intermetallic phase formation and growth in the Mg-Y System. In Magnesium Technology 2012; Mathaudhu, S.N., Sillekens, W.H., Neelameggham, N.R., Hort, N., Eds.; Springer International Publishing: Berlin, Germany, 2016; pp. 145–148. [Google Scholar]
- Liu, W.; Yang, D.D.; Quan, G.F.; Zhang, Y.B.; Yao, D.D. Microstructure evolution of semisolid Mg-2Zn-0.5Y alloy during isothermal heat treatment. Rare Met. Mater. Eng. 2016, 45, 1967–1972. [Google Scholar]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, S.; Gao, B.; Wang, X.; Cao, Z.; Guo, E.; Wang, T. The Influence of Holding Time on the Microstructure Evolution of Mg–10Zn–6.8Gd–4Y Alloy during Semi-Solid Isothermal Heat Treatment. Metals 2019, 9, 420. https://doi.org/10.3390/met9040420
Nie S, Gao B, Wang X, Cao Z, Guo E, Wang T. The Influence of Holding Time on the Microstructure Evolution of Mg–10Zn–6.8Gd–4Y Alloy during Semi-Solid Isothermal Heat Treatment. Metals. 2019; 9(4):420. https://doi.org/10.3390/met9040420
Chicago/Turabian StyleNie, Shuang, Bingyang Gao, Xuejian Wang, Zhiqiang Cao, Enyu Guo, and Tongmin Wang. 2019. "The Influence of Holding Time on the Microstructure Evolution of Mg–10Zn–6.8Gd–4Y Alloy during Semi-Solid Isothermal Heat Treatment" Metals 9, no. 4: 420. https://doi.org/10.3390/met9040420
APA StyleNie, S., Gao, B., Wang, X., Cao, Z., Guo, E., & Wang, T. (2019). The Influence of Holding Time on the Microstructure Evolution of Mg–10Zn–6.8Gd–4Y Alloy during Semi-Solid Isothermal Heat Treatment. Metals, 9(4), 420. https://doi.org/10.3390/met9040420





