Abstract
To analyze the mechanical properties and reaction characteristics of Al-ZrH2-PTFE (aluminum-zirconium hydride-polytetrafluoroethylene) composites under quasi-static compression, five types of specimens with different ZrH2 contents (0%, 5%, 10%, 20% and 30%) were prepared by molding-vacuum sintering. The true stress-strain curves and reaction rates of the different specimens were measured using quasi-static compression. The specific reaction processes were recorded by a high-speed camera. The corresponding reaction products were characterized by the XRD phase analysis, the calorific value was tested by a Calorimeter, and the reaction mechanism was analyzed. According to the results, the strength of the composites increased first and then decreased with the increase in the content of ZrH2. It reached a maximum of 101.01 MPa at 5%. Violent reaction occurred, and special flames were observed during the reaction of the specimens with 5% ZrH2. With the increase in the content of ZrH2, the chemical reaction was hard to induce due to the reduction in strength and toughness of composites. The reaction mechanism of Al/ZrH2/PTFE reveals that high temperatures at crack tip induced the reaction of Al and PTFE. Subsequently, ZrH2 decomposed to release hydrogen and generate ZrC. Calorimetric experiment shows that the calorific value of Al/ZrH2/PTFE with 20% ZrH2 is higher than that of Al/PTFE. The findings verify the potential of ZrH2 as an energetic additive for the enhancement of strength and release of the energy of the composites.
1. Introduction
Reactive materials generally refer to the metastable energetic composites composed of two or more non-explosive solid materials, e.g., polymers-metals and metals-metals, which can have a violent reaction under impact loading. The materials are largely insensitive under quasi-static or static loading, and the conventional initiation techniques, e.g., explosive bridge wires or flames, cannot trigger their reactions [1,2,3,4]. Among these materials, aluminum-polytetrafluoroethylene (Al-PTFE) is a typical advanced reactive material that has aroused huge attention recently. Compared with the conventional insensitive explosives, Al-PTFE exhibits a higher level of energy release and better mechanical properties [5,6,7]. Hence, it has high application implication in the field of military damage, e.g., the unit functionalized to cause secondary damage, and large application potential in other fields of air defense, anti-missile, barrier breaking, rocket propulsion system, petroleum exploitation, etc. [8,9] For instance, the advantage of reactive materials applied in warheads is that when high-speed projectiles or energetic fragments affect the target, the reactive materials will react quickly and release considerable chemical energy, thereby causing the damage of chemical and mechanical energy to the target [3].
Numerous experimental research on the mechanical properties and reaction characteristics of Al-PTFE composites have been conducted by scholars over the past decades. Osborne and Pantoya studied the effect of micron and nano-sized Al on the thermal decomposition process of Al/PTFE powders [10]. Cai and Qiao investigated the mechanical properties and the effect of particle size grading on the strength of Al-W-PTFE materials, respectively [11,12]. Xu performed a series of experiments to explore the effects of Al contents and strain rate on the mechanical properties and reaction characteristics of Al-PTFE, respectively [13]. Feng reported that Al-PTFE specimens could have a violent chemical reaction under quasi-static compression and originally developed a crack-induced initiation mechanism in accordance with the hot spot theory [14,15]. Al-PTFE has become an emerging research hotspot in the field of energetic materials.
Metal hydrides, a new type of energetic materials, have also aroused huge attention from scholars in recent years. They have been extensively used in both the military and civil fields for their high hydrogen storage density, high chemical energy and excellent activity [16]. Many scholars added metal hydrides (MgH2, TiH2, AlH3, LiH2, Mg (BH4)2, etc.) to conventional explosives and rocket propellants such as high-energy additives to enhance energy density of raw materials. Research has shown that dehydrogenation takes places, when metal hydrides are involved in the reaction. Besides, the decomposition products, metal and H2, can react with other substances, thereby generating a lot of energy [17]. Zirconium hydride, which possesses a high density of 5.61 g/cm3 and a hydrogen content of approximately 3.8% [18], is thus considered a potential high-density hydrogen-supplying fuel as an energetic additive. Some scholars have made a series of studies on its combustion mechanisms and energy characteristics. Liu studied the effect of ZrH2 on the energy characteristics of propellants and found that the density and specific impulse of propellants containing ZrH2 were significantly higher than those of the conventional propellants [19]. Yang et al. investigated the reaction mechanisms of ZrH2 in the combustion of a double-base (DB) propellant, and selected Al for comparison. During the combustion of DB propellants, ZrH2 showed entirely different behavior compared with the conventional fuel Al. For the formulation containing ZrH2, the combustion of the fuel occurs on the burning surface, which obviously contributes to the heat feedback to the propellant and combustion efficiency of the fuel. Moreover, it was proved that ZrH2 is not oxidized directly by the propellant during combustion but starts with its dehydrogenation to release hydrogen and generate zirconium, which can decrease the relative molecular mass of gases and improve the energy output of the propellant [17]. Stanisław Cudziło, et al. studied the effect of TiH2 and ZrH2 on the detonation heat of the RDX-based explosives. It was found that the explosives with TiH2 and ZrH2 release more energy than the RDX-based explosives [20]. However, there have been no related research on the addition of ZrH2 into the Al/PTFE reactive materials so far.
In this study, ZrH2 was introduced into Al/PTFE composites, and five types of specimens with different ZrH2 contents were prepared. The mechanical properties and reaction characteristics were explored by the quasi-static compression experiments and under the scanning electron microscope (SEM), adiabatic bomb calorimeter and X-ray diffraction (XRD), and the reaction mechanism of the material was analyzed.
2. Experimental Section
2.1. Materials and Specimen Preparation
The initial powders that were adopted to prepare the specimens had the following average sizes: PTFE: 25 μm (from 3F, Shanghai, China); Al: 1–2 μm (from JT-4, Hunan, China); and ZrH2: 10 μm (from Haixin, Shenyang, China).
Five types of Al/ZrH2/PTFE specimens were prepared with different ZrH2 contents. According to the chemical equilibrium ratio (26.5 wt.%/73.5 wt.%), the mass fractions of the Al and PTFE in each type of specimens were proportioned. The mass ratios of the five types of Al/ZrH2/PTFE granular composites and the corresponding theoretical maximum density (ρTMD) are listed in Table 1.

Table 1.
Component ratios and theoretical density of the Al/ZrH2/PTFE granular composites.
The following describes the preparation process of the specimens: the three granular powders (namely Al, ZrH2 and PTFE) were weighed and mixed by the formula in Table 1, suspended in and anhydrous ethanol solution and then stirred using a motor-driven blender for 20 min. The suspension was put into a vacuum oven for 48 h at 60 °C until it was completely dried. Subsequently, the mixtures were sieved (60 meshes) to produce uniform powders, which were cold pressed into cylinders with a size of Φ10 mm × 10 mm using a molding die and a hydraulic press (compressive pressure and holding time were set as 240 MPa and 20 s, respectively). The pressed specimens were kept at ambient pressure and temperature for 2 h to remove the entrapped air and residual stress. Next, they were sintered at 360 °C for 4 h in a vacuum sintering oven, and the heating and cooling rate were set as 90 °C·h−1 and 50 °C·h−1, respectively.
2.2. Experimental Procedures
The initial microscopic morphology and element distributions of the mixtures were observed by the scanning electron microscope. The types of instruments applied are S-3400N II (Hitachi, Tokyo, Japan) and FEI Versa 3D (Hillsboro, OR, USA). Moreover, the calorific value of the powers (1#, 4#) was quantitatively measured by a C2000 Calorimeter (IKA, Staufen, Germany) at a pure oxygen atmosphere.
A microcomputer-controlled electronic universal testing machine with a maximum loading capacity of 100 KN (CMT5105, MTS, Eden Prairie, MN, USA) was employed to perform the quasi-static compression experiments. According to a nominal strain rate of 0.01 s−1, the Compression speed of the indenter was set as 6 mm·min−1. To alleviate the effect of the friction, both ends of specimens were lubricated with an appropriate amount of petroleum jelly before loading. In the meantime, a high-speed camera (FASTCAM SA-Z, Photron, Tokyo, Japan) with a frame rate up to 10,000 frames/s was used to observe the deformation and reaction process of the five types of specimens under quasi-static compression. Specimens of five formulations were tested ten times to ensure the consistency and reliability of the experimental results. The experiment was performed at an ambient temperature of 26 °C.
After quasi-static compression, the residues of the reaction specimens were characterized by the X-ray diffraction (Bruker D8 ADVANCE, Bruker, Berlin, Germany) under Cu Kα radiation (λ = 0.15406 nm) at 40 kV and 40 mA, respectively. The data were collected from 10° to 90° (2θ) at a scanning speed of 5 °·min−1.
3. Results and Discussion
3.1. Mesoscale Characteristics
Figure 1 shows the original microstructures and element distributions of the materials by SEM to assess the homogeneity before cold pressing. Figure 1a–f were obtained by S-3400N II and FEI Versa 3D, respectively. The microstructure of pure ZrH2 power is illustrated in Figure 1a, showing the irregular shape of ZrH2 particles different from that of spherical Al particles. The overall interior microstructure of mixture 2# with 5% content of ZrH2 is illustrated in Figure 1b. There were many voids existing between the three particles. Al and ZrH2 distributed discretely and evenly in the PTFE matrix, respectively, and these could be discriminated facilely due to their spherical geometry. Figure 1c–f illustrate the distributions of the C, F, Al and Zr element. It was further observed that the Al, ZrH2, and PTFE powders were homogeneously mixed by the preparation process.
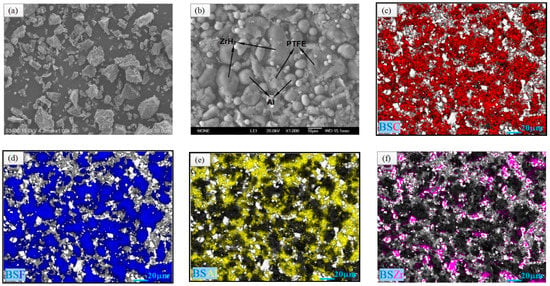
Figure 1.
Internal microstructures and element distributions of the materials. (a) SEM (scanning electron microscope) image of ZrH2 powder, (b) SEM image of mixture 2#, (c) C element, (d) F element, (e) Al element, and (f) Zr element.
3.2. Mechanical Properties of Reactive Materials under Quasi-Static Compression
Figure 2 shows the true stress-strain curves of the five Al/ZrH2/PTFE composites; the corresponding mechanical properties are tabulated in Table 2. It can be obtained that the five types of specimens are all elastoplastic materials, which first underwent a short elastic deformation, reaching the yield point and then experienced the plastic deformation and showed the strain hardening phenomenon under quasi-static compression. The compressive strength of Al/ZrH2/PTFE increased first and then decreased as the content of ZrH2 increased from 0% to 30%. It reached the maximum of 101.01 MPa at 5% content of ZrH2, which increased by 8.78% compared with Al/PTFE specimens. However, the materials strength was lower than that of Al/PTFE when the mass fraction of ZrH2 was higher than 10%.
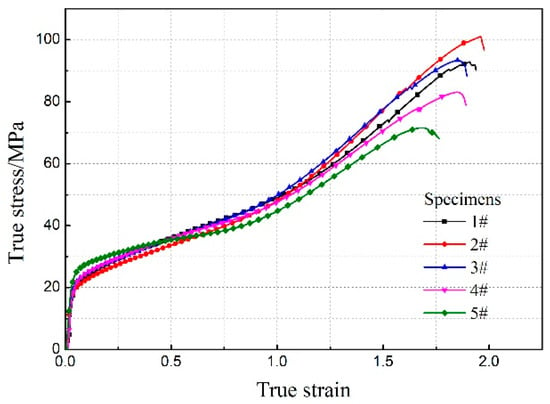
Figure 2.
True stress-strain curves of Al/ZrH2/PTFE (aluminum-zirconium hydride-polytetrafluoroethylene) specimens in quasi-static compression tests.

Table 2.
Average mechanical parameters of Al/ZrH2/PTFE composites.
According to the results, ZrH2 and Al particles could enhance the strength of the ternary system with the content of ZrH2 less than or equal to 10%. In the elastic stage, the deformed part of specimens was concentrated on the softer amorphous PTFE matrix. Since the ZrH2 and Al particles could slide relative to the deformation of matrices in this stage, their reinforcing effect on the matrix was negligible [21]. With the specimen yielding and exhibiting strain hardening, ZrH2 and Al particles primarily provided the support and reinforcement until the materials failed under the condition of low ZrH2 content. However, excessive ZrH2 could break the integrity and continuity of PTFE, and the lack of bonding between particles led to a reduction of the mixture’s ultimate strength.
3.3. Reaction Behaviors of Reactive Materials under Quasi-Static Compression
The reaction rates of the five types of specimens are listed in Table 3. According to the results, the Al/PTFE composites without ZrH2 reacted completely, 80% of the Al/ZrH2/PTFE samples with the 5% content of ZrH2 were completely reacted, and the other 20% were partially reacted. However, the specimens with the content of ZrH2 at 10%, 20% and 30% were not reacted. The integrity of the materials was broken with further increases in ZrH2, leading to a reduction of the strength and toughness of PTFE matrix so that the material could not absorb sufficient energy [14]. The different reaction rates of the Al/ZrH2/PTFE composites with 0% to 30% ZrH2 suggest that the addition of ZrH2 affects the reaction behaviors of Al/PTFE significantly.

Table 3.
Reaction rates of five types of specimens in quasi-static compression.
The reaction process of 1# and 2# under quasi-static compression are shown in Figure 3a. This figure suggests that the specimens first deformed and then suddenly reacted when the pressure reached the fracture strength of the specimens; then the specimens were deflagrated, releasing a considerable amount of black smog within 2–3 s. Feng et al. also found this reaction phenomenon in the study of Al/PTFE composites under quasi-static compression. The specimens, by a special treatment, underwent deformation, cracking, and violent chemical reactions, generating black smog and solid residues [22]. Besides, a special flame similar to gas combustion was observed in 2#, which contained 5% ZrH2. The partially reacted specimens are shown in Figure 3b. Under the similar compression conditions, the specimen cracked at the edge, with an initial reaction occurring at one of the cracks. More circumferential cracks with weak flares, and immediately the reaction extinguished. The specimens (3#, 4#, 5#) in Figure 3c, which failed due to the formation of developmental cracks inside [23], were not reacted at all.
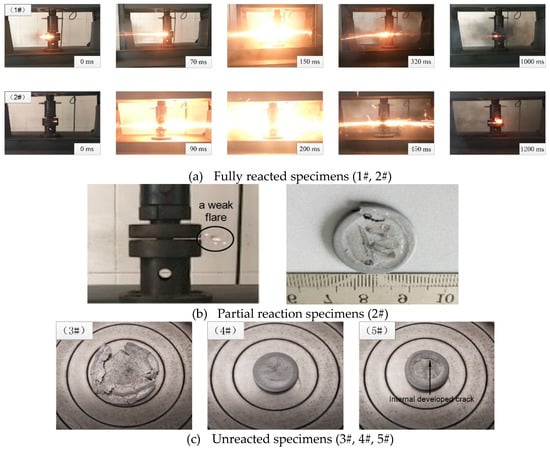
Figure 3.
Reaction phenomenon of different specimens in quasi-static compression. (a) Fully reacted specimens, (b) Partial reaction specimens, (c) Unreacted specimens.
3.4. Analysis of Reaction Mechanism
As shown in Figure 4, specimens 1# and completely reacted specimens 2# produced the black solid residues. The reaction residues were detected by XRD to explore the chemical reaction mechanism of Al/ZrH2/PTFE. The XRD patterns of residues are shown in Figure 5, suggesting that AlF3 was formed in the reaction residues of specimens (1#, 2#), and ZrC was generated in the reaction products of specimens 2#. Furthermore, there was no diffraction peak of ZrH2 in the XRD patterns, suggesting that–ZrH2 was fully involved in the reaction.
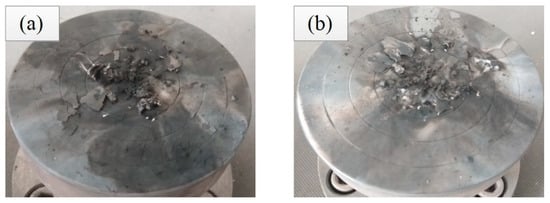
Figure 4.
Reaction residues of completely reacted specimens under quasi-static compression (a) specimen 1# and (b) specimen 2#.
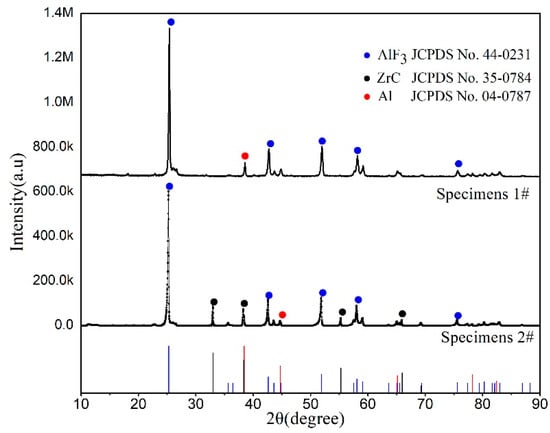
Figure 5.
XRD (X-ray diffraction) results of reaction residues.
Yang et al. found that ZrH2 exhibits a good resistance to the oxidation by a DB propellant. Its combustion starts with the dehydrogenation to generate H2 and metallic Zr, which are further involved in the combustion process [17]. Furthermore, Feng et al. proposed a crack-induced mechanism of Al/PTFE composites under quasi-static compression [15]. Given these two studies, the reaction mechanism of Al/ZrH2/PTFE can be inferred, i.e., when the compressive stress reaches the breaking strength under quasi-static compression, the specimen instantaneously fails and breaks, thereby forming circumferential open cracks. The temperature rises sharply at the crack tip, which causes the formation of a local hot spot along the direction of crack’s extension. Subsequently, the PTFE component reacts with Al particles, leading to the release a considerable amount of heat. The ZrH2 is activated and decomposes to generate H2 and metallic Zr. Next, hydrogen gas burns, and Zr is combined with C (carbon black) to generate ZrC at high temperature. The exothermic reaction further contributes to the releasement of heat. Based on the above analysis results, the possible reaction processes of Al/ZrH2/PTFE ternary system are as follows:
(-C2F4-) → C2F4 (g)
Al + C2F4 → AlF3 + C
ZrH2 → Zr + H2 (g)
Zr + C → ZrC
H2 + O2 → H2O (g)
In addition, the calorimetric result indicates that the calorific value of Al/ZrH2/PTFE powder (about 14.744 MJ/kg) is higher than that of Al/PTFE (about 12.476 MJ/kg). The addition of ZrH2 is favorable for improving the energy release of the reactive materials.
4. Conclusions
The mechanical properties and reaction characteristics of Al/ZrH2/PTFE specimens with different contents of ZrH2 were investigated using the quasi-static compression experiments and XRD phase detection. The main conclusions are drawn as follows:
(1) The five groups of Al/ZrH2/PTFE are all elastoplastic materials. The compressive strength of the specimens increased first and then decreased with the increase in the content of ZrH2. When the mass fraction of ZrH2 was 5%, the strength reached a maximum of 101.01 MPa, 8.78% higher than that of the Al/PTFE specimens. Its strength was lower than that of Al/PTFE with more than 10% ZrH2. An appropriate amount of ZrH2 particles (less than 10%) could enhance the strength of the materials. Excessive ZrH2 could break the integrity and continuity of PTFE, thereby resulting in a reduction of the mixture’s ultimate strength.
(2) A violent chemical reaction occurred on specimens 1# and part of specimens 2#. The difference was that special burning flames was observed in the reaction of 2# but not in 1#. The specimens with 10%, 20% and 30% ZrH2 were not reacted. The reason is that excessive ZrH2 destroy the integrity of the entire material causing a decrease in strength and toughness of the PTFE matrix. Because of the insufficient energy absorption when the material was compressed, it was difficult to trigger a chemical reaction.
(3) According to the analysis of the reaction mechanism, the specimens failed and ruptured instantaneously under quasi-static compression. Temperature sharply rose at the crack tip, thereby triggering the reaction of Al and PTFE with the release of a considerable amount of heat. Then, ZrH2 was activated and decomposes owing to the heat, causing combustion of released hydrogen and combination of Zirconium and C (carbon black). The reaction further promoted the release of heat. There were no diffraction peaks corresponding to ZrH2, suggesting that ZrH2 participated completely in the reaction. Meanwhile, the calorific value of Al/ZrH2/PTFE powder is about 14.744 MJ/kg, higher than that of Al/PTFE (about 12.476 MJ/kg). As an energetic additive, an appropriate amount of ZrH2 could enhance the mechanical strength of the reactive materials and promote the release of energy.
Author Contributions
Y.L. and J.H. conceived and designed the experiments; J.Z., Z.Y. and J.W. performed the experiments; Z.G., J.S., S.W., X.F. and J.K. analyzed the data; and J.Z. wrote the paper.
Funding
This research was funded by the National Natural Science Foundation of China (No. 51673213).
Acknowledgments
The financial support from the National Natural Science Foundation of China (General Program. Grant No. 51673213) is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kubiatowicz, J.; Bindel, D.; Chen, Y.; Czerwinski, S.; Eaton, P.; Geels, D.; Gummadi, R.; Rhea, S.; Weatherspoon, H.; Weimer, W.; et al. Oceanstore: An Architecture for Global-scale Persistent Storage. In Proceedings of the ASPLOS, Cambridge, MA, USA, 12–15 November 2000. [Google Scholar]
- Bhagwan, R.; Tati, K.; Cheng, Y.; Savage, S.; Voelker, G.M. Total Recall: System Support for Automated Availability Management. In Proceedings of the NSDI, San Francisco, CA, USA, 29–31 March 2004. [Google Scholar]
- Zhao, P.D.; Lu, F.Y.; Li, J.L.; Chen, R.; Xu, S.L.; Yang, S.Q. Dynamic Compression Properties of Reactive Material PTFE/Al. Energ. Mater. 2009, 17, 459–462. [Google Scholar]
- Yang, S.Q.; Xu, S.L.; Zhang, T. Preparation and Performance of PTFE/Al Reactive Materials. J. Natl. Univ. Def. Technol. 2008, 30, 39–42. [Google Scholar]
- Daniel, B.N.; Richard, M.T.; Benjamin, N.A. Reactive Material Enhanced Projectiles and Related Methods. U.S. Patent 20080035007[P], 14 February 2008. [Google Scholar]
- Mock, W.; Holt, W.H. Impact Initiation of Rods of Pressed Polytetrafluoroethylene (PTFE) and Aluminum Powders. AIP Conf. Proc. 2006, 845, 1097–1100. [Google Scholar]
- Wang, H.; Zheng, Y.; Yu, Q.; Liu, Z.; Yu, W. Impact-induced initiation and energy release behavior of reactive materials. J. Appl. Phys. 2011, 110, 074904. [Google Scholar]
- Hunt, E.M.; Pantoya, M.L. Impact sensitivity of intermetallic nanocomposites: A study on compositional and bulk density. Intermetallics 2010, 18, 1612–1616. [Google Scholar] [CrossRef]
- Leslie, R.B.; Brian, B. Oil Well Perforators. U.S. Patent 20070056462[P], 15 March 2007. [Google Scholar]
- Osborne, D.T.; Pantoya, M.L. Effect of Al particle size on the thermal degradation of Al/Teflon mixtures. Combust. Sci. Technol. 2007, 179, 1467–1480. [Google Scholar] [CrossRef]
- Cai, J.; Walley, S.M.; Hunt, R.J.; Proud, W.G.; Nesterenko, V.F.; Meyers, M.A. High-strain, high-strain-rate flow and failure in PTFE/Al/W granular composites. Mater. Sci. Eng. A 2008, 472, 308–315. [Google Scholar] [CrossRef]
- Qiao, L.; Tu, J.; Zhao, L.J. Influence of Particle Size Grading on Strength of Al/W/PTFE Composite. Ordnance Mater. Sci. Eng. 2014, 37, 17–21. [Google Scholar]
- Xu, S.L.; Yang, S.Q.; Zhao, P.D.; Li, J.; Lu, F. The Study on the Compressive Behavior of PTFE/Al Energetic Composite. Chin. J. Theor. Appl. Mech. 2009, 41, 708–712. [Google Scholar]
- Feng, B.; Fang, X.; Li, Y.C.; Wang, H.X.; Mao, Y.M.; Wu, S.Z. An Initiation Phenomenon of Al-PTFE under Quasi-Static Compression. Chem. Phys. Lett. 2015, 637, 38–41. [Google Scholar] [CrossRef]
- Feng, B.; Li, Y.C.; Wu, S.Z.; Wang, H.X.; Tao, Z.M.; Fang, X. A Crack-Induced Initiation Mechanism of Al-PTFE under Quasi-Static Compression and the investigation of Influencing Factors. Mater. Des. 2016, 108, 411–417. [Google Scholar] [CrossRef]
- Dreizin, E.L. Metal-based reactive nanomaterials. Prog. Energy Combust. Sci. 2009, 35, 141–167. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, F.; Yuan, Z.; Wang, Y.; An, T.; Chen, X.; Xuan, C.; Zhang, J. On the combustion mechanisms of ZrH2 in the double-base propellant. Phys. Chem. Chem. Phys. PCCP 2017, 19, 32597–32604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shen, H.; Che, X.K.; Wang, L. Zirconium Powder Production through Hydrogenation and Dehydrogenation Process. Chin. J. Rare Met. 2011, 35, 417–421. [Google Scholar]
- Liu, Q.; Chen, L.Q.; Wang, J.R.; Zheng, K.B. Analysis of Energy Characteristics of the Propellants Containing Zirconium or its hydride. J. Solid Rocket Technol. 2019, 42, 54–59. (In Chinese) [Google Scholar]
- Cudziło, S.; Trzciński, W.A.; Paszula, J.; Szala, M.; Chyłek, Z. Effect of Titanium and Zirconium Hydrides on the Detonation Heat of RDX-based Explosives—A Comparison to Aluminium. Propellants Explos. Pyrotech. 2018, 43, 280–285. [Google Scholar] [CrossRef]
- Jordan, J.L.; Siviour, C.R.; Foley, J.R.; Brown, E.N. Compressive Properties of Extruded Polytetrafluoroethylene. Polymer 2007, 48, 4184–4195. [Google Scholar] [CrossRef]
- Feng, B.; Fang, X.; Li, Y.C.; Wang, H.X.; Mao, Y.M. Reaction of Al/Teflon under compression strain rate. Chin. J. Energ. Mater. (Hanneng Cailiao) 2016, 24, 599–603. [Google Scholar]
- Wu, J.X.; Li, Y.C.; Fang, X.; Wang, H.X.; Feng, B.; Wu, S.Z. Effect of Al Particle Size on Quasi-Static Pressure Reaction and Drop Weight Impact Sensitivity of Al-PTFE. Chin. J. Energ. Mater. 2018, 26, 524–529. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).