Study on Intensification Behavior of Bismuth Ions on Gold Cyanide Leaching
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Gold Dissolution
2.2.2. Electrochemistry
2.2.3. Microstructure and Surface Product Analysis
3. Results
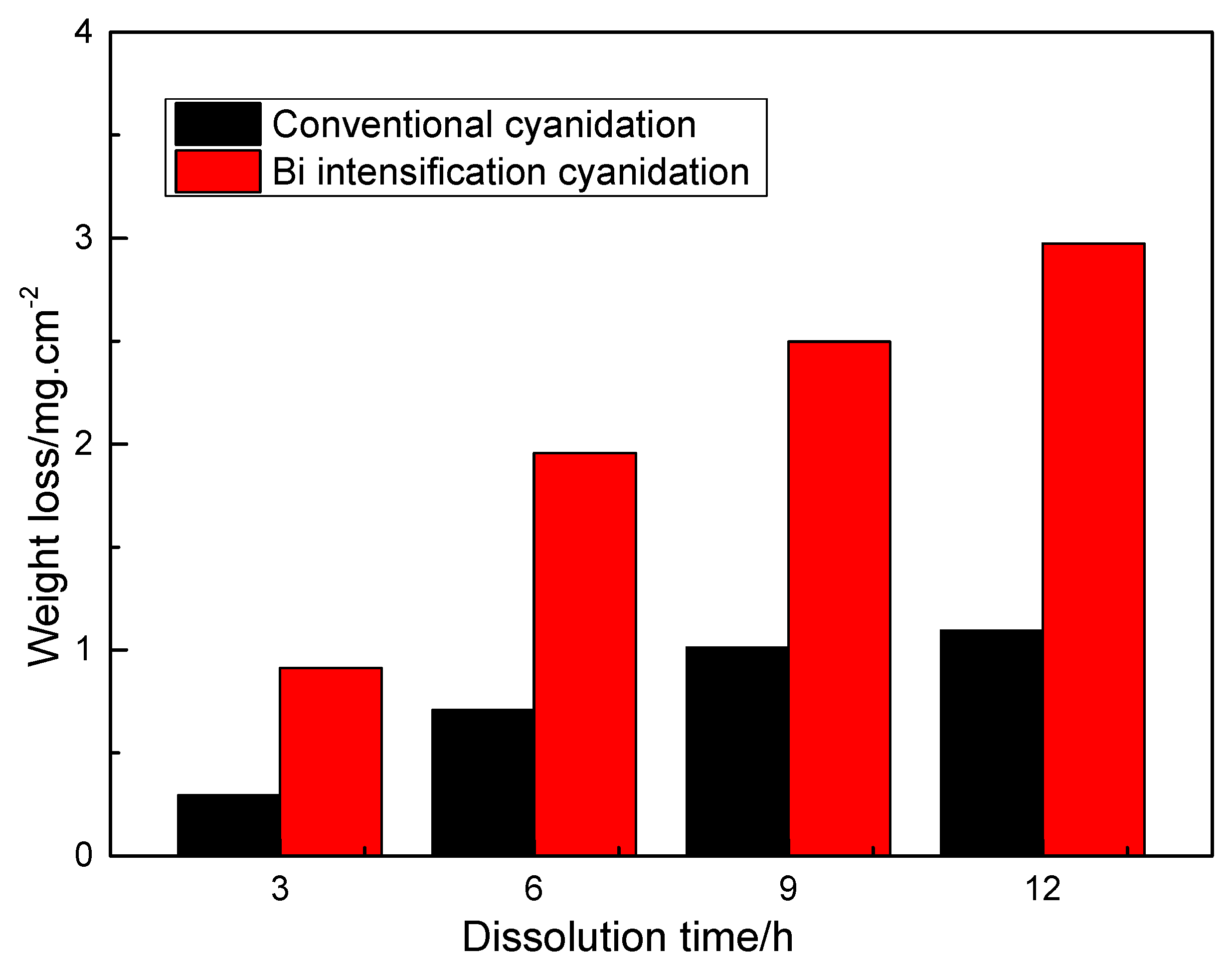
3.1. Effect of Bismuth Ions on Gold Dissolution
3.2. Electrochemical Behavior
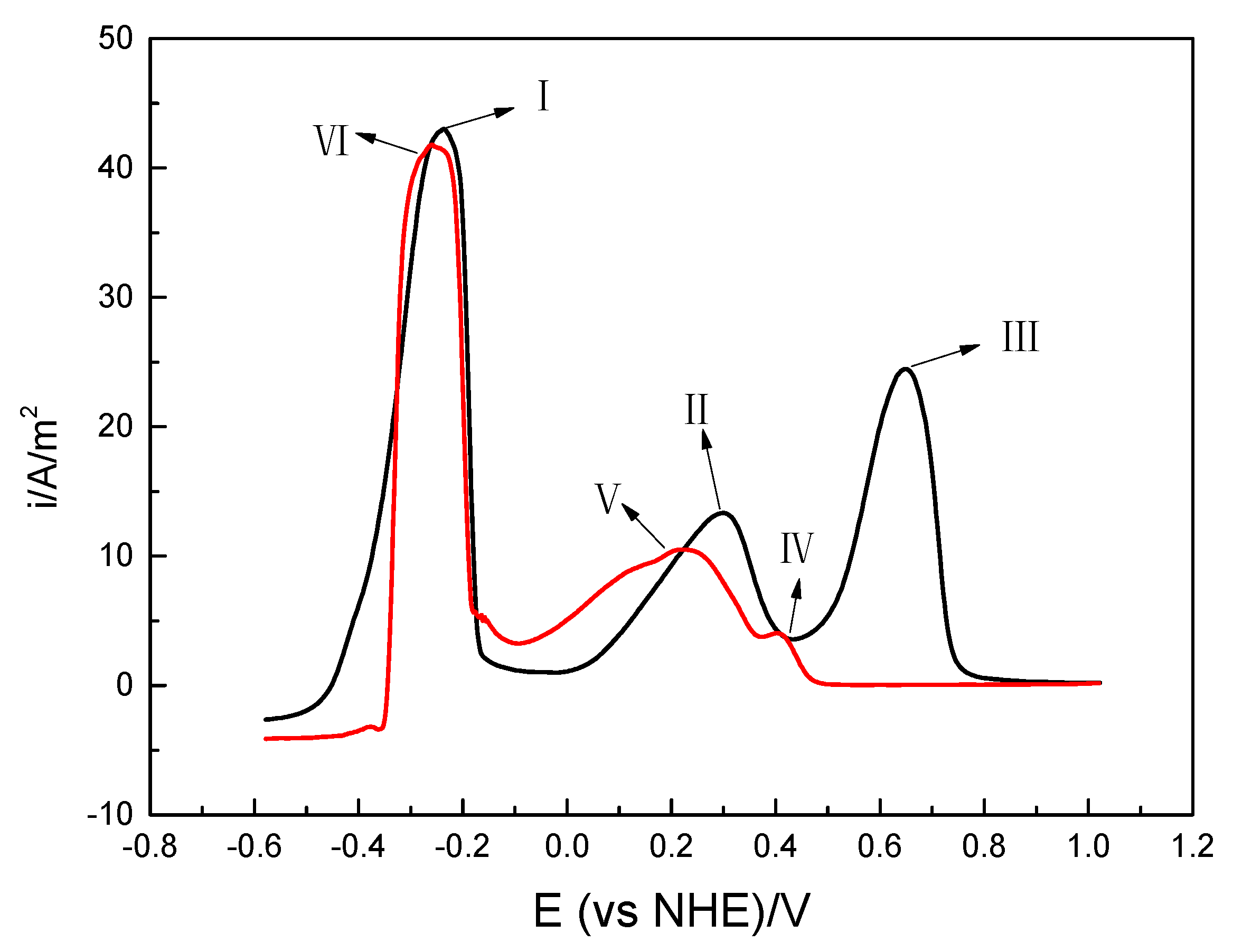
3.2.1. Gold Dissolution Behavior by Cyclic Voltammetry
3.2.2. Gold Dissolution Behavior by Tafel Curves
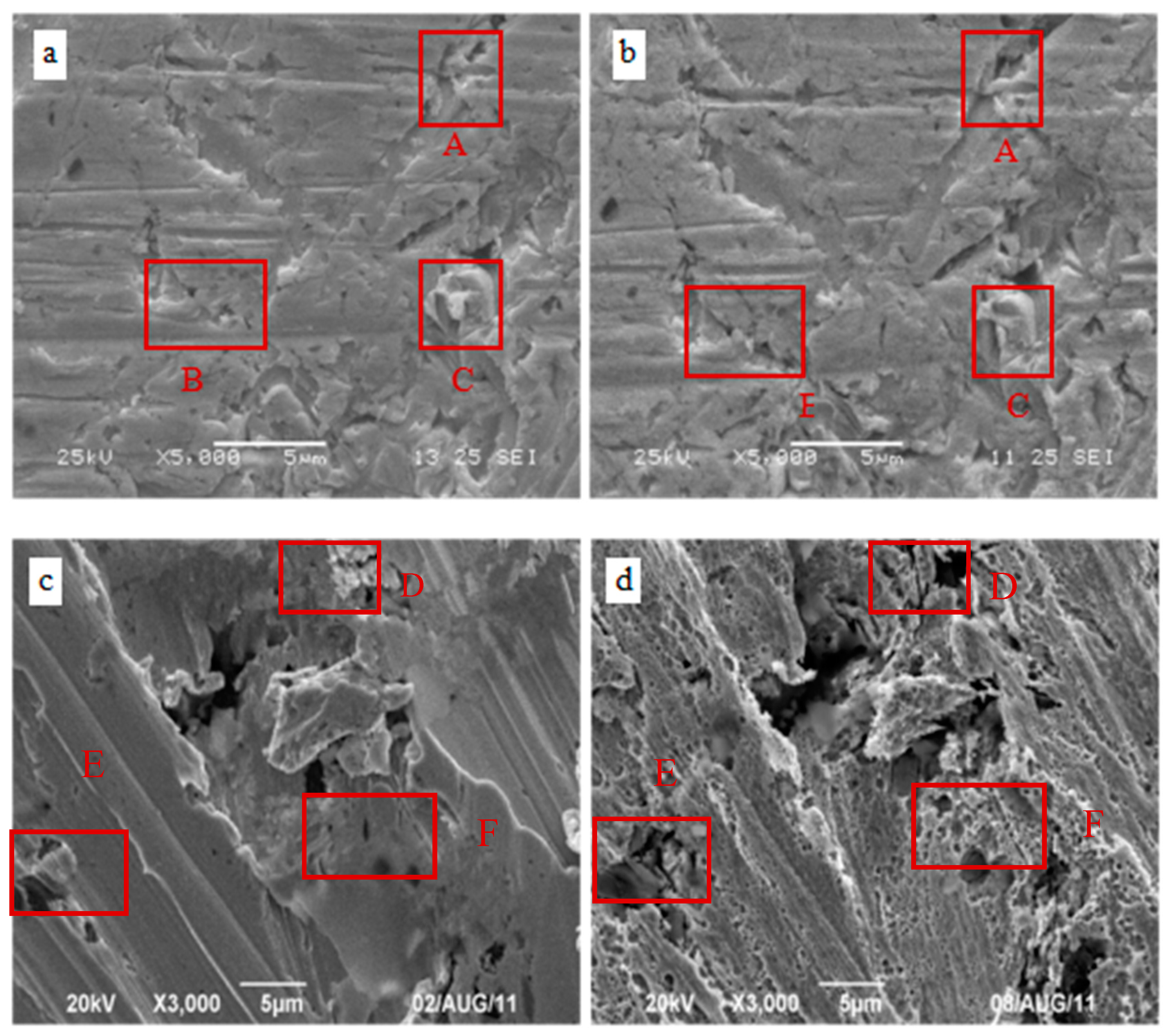
3.3. Microstructure Analysis
3.4. Composition Analysis of Surface Products
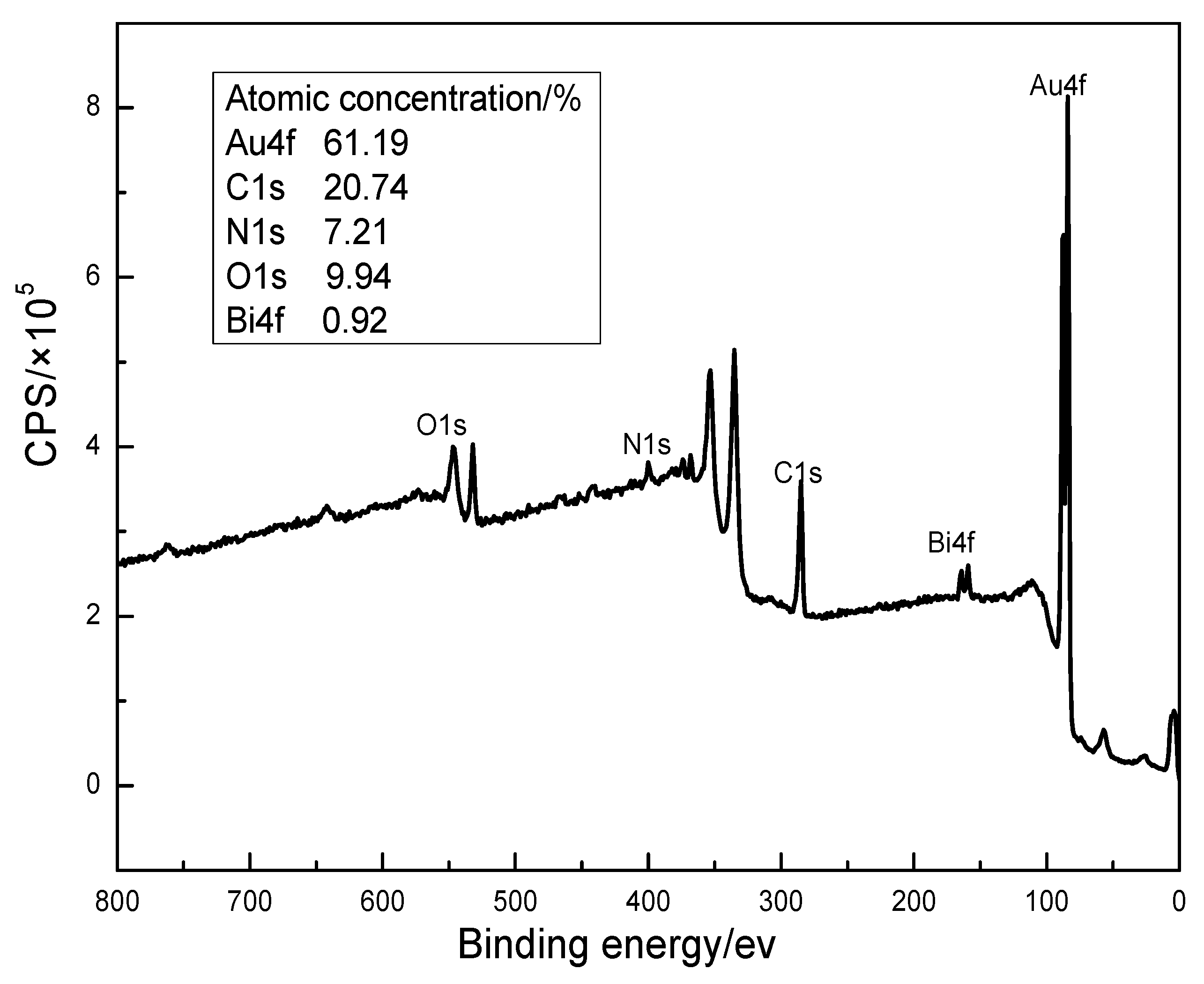
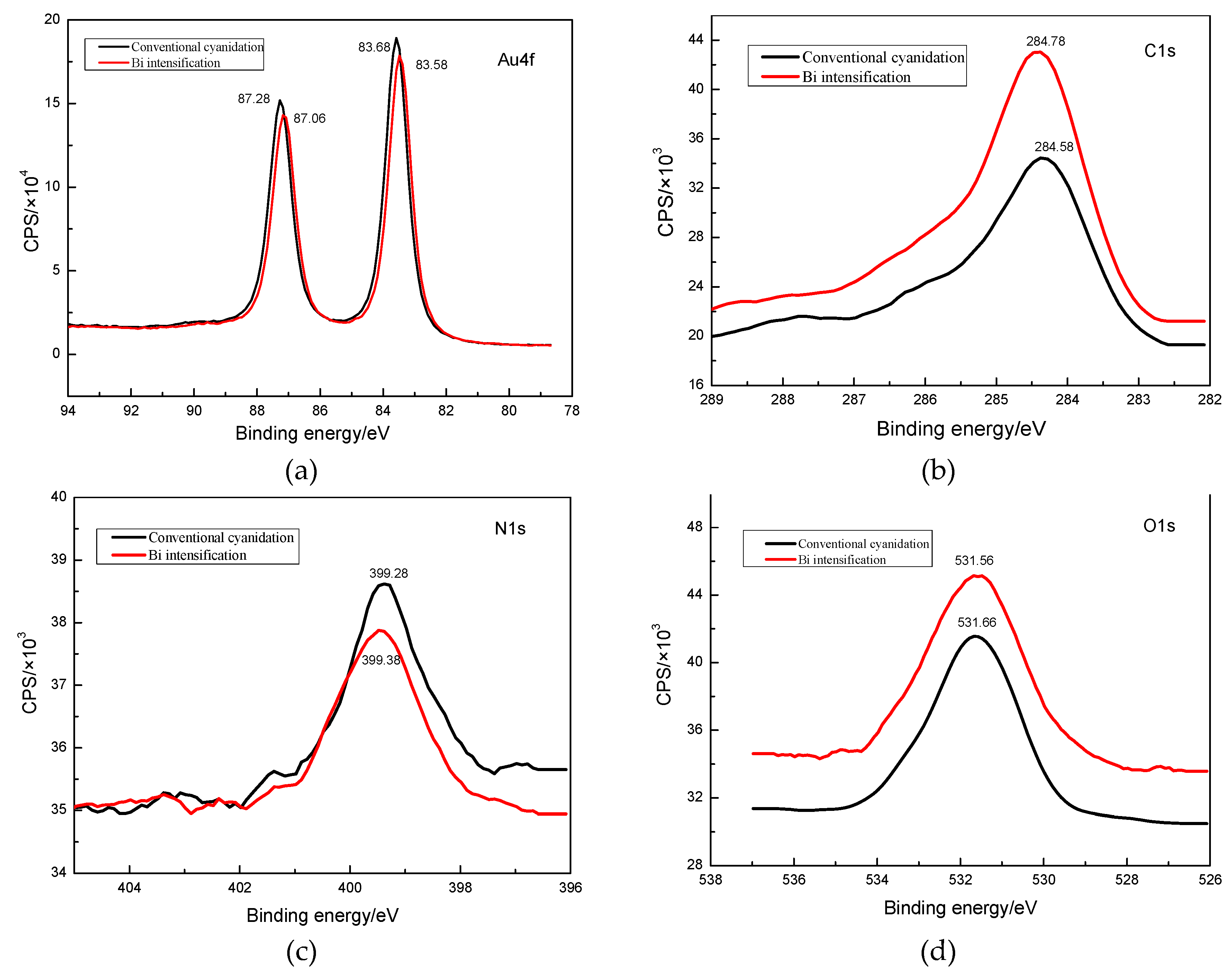
3.4.1. Surface Product Information Analysis by XPS
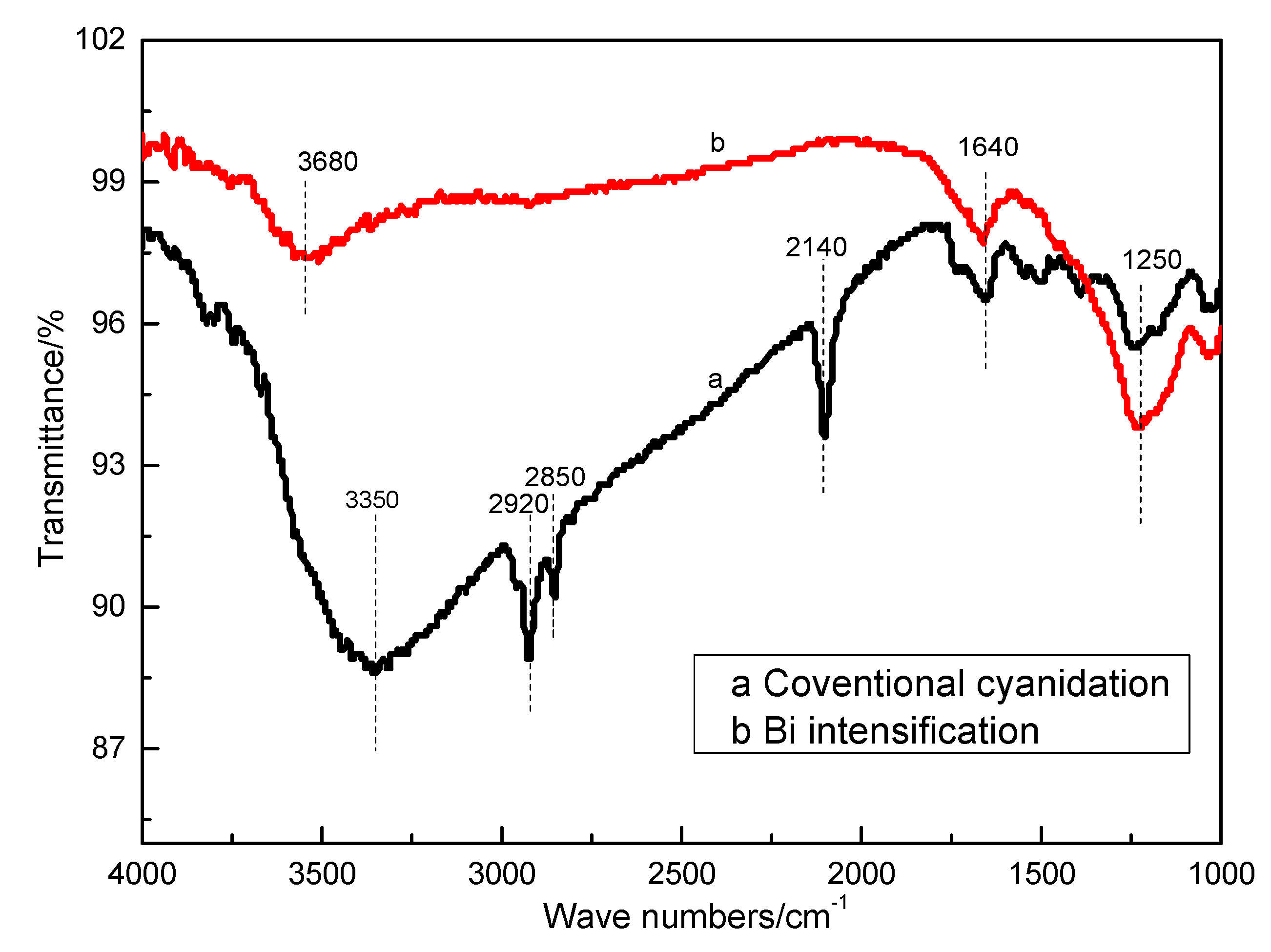
3.4.2. Surface Product Information Analysis by FT-IR
3.4.3. Surface Product Information Analysis by Raman
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hu, C.R.; Yang, F. Review for improvement about the dressing, metallurgy of gold ores. Gold 2000, 21, 29–37. [Google Scholar]
- Bas, A.D.; Ghali, E.; Choi, Y. A review on electrochemical dissolution and passivation of gold during cyanidation in presence of sulphides and oxides. Hydrometallurgy 2017, 172, 30–44. [Google Scholar] [CrossRef]
- Acar, S. Process development metallurgical studies for gold cyanidation process. Miner. Metall. Proc. 2016, 33, 161–171. [Google Scholar] [CrossRef]
- Cathro, K.J.; Koch, D. The anodic dissolution of gold in cyanide solutions. J. Electrochem. Soc. 1964, 111, 1416–1420. [Google Scholar] [CrossRef]
- Jiang, T. Chemistry of Extractive Metallurgy of Gold; Xu, W., Ed.; Hunan Science and Technology Press: Changsha, China, 1998; pp. 92–103. ISBN 7-5357-1759-4. [Google Scholar]
- Korolev, L.; Altinkaya, P.; Halli, P.; Hannula, P.M.; Yliniemi, K.; Lundstrom, M. Electrochemical recovery of minor concentrations of gold from cyanide-free cupric chloride leaching solutions. J. Clean. Prod. 2018, 186, 840–850. [Google Scholar] [CrossRef]
- Nunan, T.O.; Peixoto, G.C.; Ernesto, H. Improvements in gold ore cyanidation by pre-oxidation with hydrogen peroxide. Miner. Eng. 2017, 108, 67–70. [Google Scholar] [CrossRef]
- Xu, H.F.; Sun, J.F.; Liao, H.; Li, H.L.; Zi, J.X. The leaching of peroxides in cyanide leaching process. Hydrometallurgy 2003, 22, 133–135. [Google Scholar]
- Senanayake, G. A review of effects of silver, lead, sulfide and carbonaceous matter on gold cyanidation and mechanistic interpretation. Hydrometallurgy 2008, 90, 46–73. [Google Scholar] [CrossRef]
- Breuer, P.L.; Jeffrey, M.I. Mechanisms of sulfide ion oxidation during cyanidation. Part I: The effect of lead (II) ions. Miner. Eng. 2008, 21, 579–586. [Google Scholar] [CrossRef]
- Chimenos, J.M.; Guzman, M.; Hewitt, D.M.; Karagueorguieva, A.; Espiell, F. Kinetics of the reaction of gold cyanidation in the presence of a thallium(I) salt. Hydrometallurgy 1997, 44, 269–286. [Google Scholar] [CrossRef]
- Jeffrey, M.I.; Ritchie, I.M. The leaching of gold in cyanide solutions in the presence of impurities II. The effect of silver. J. Electrochem. Soc. 2000, 147, 3272–3276. [Google Scholar] [CrossRef]
- Yang, Y.B.; Li, Q.; Li, G.H.; Guo, Y.F.; Huang, Z.C.; Jiang, T. An electrochemical investigation on intensification of gold cyanidation by heavy metal ions. In EPD Congress 2005; Schlesinger, M.E., Ed.; TMS (The Minerals, Metals & Materials Society): Pittsburgh, PA, USA, 2005; pp. 977–984. [Google Scholar]
- Lorenzen, L.; Deventer, J.S.J. Electrochemical interactions between gold and its associated minerals during cyanidation. Hydrometallurgy 1992, 30, 177–193. [Google Scholar] [CrossRef]
- Yang, Y.B.; Li, Q.; Jiang, T.; Guo, Y.F.; Li, G.H.; Xu, B. Co-intensification of gold leaching with heavy metals and hydrogen peroxide. Trans. Nonferr. Met. Soc. 2010, 20, 903–909. [Google Scholar] [CrossRef]
- Jeffery, M.I.; Linda, L.; Breuer, P.L.; Chu, C.K. A kinetic and electrochemical study of the ammonia cyanide process for leaching gold in solutions containing copper. Miner. Eng. 2002, 15, 1173–1180. [Google Scholar] [CrossRef]
- Kudryk, V.; Kellogg, H.H. Mechanism and rate controlling factors in the dissolution of gold in cyanide solution. J. Metals 1954, 6, 541–548. [Google Scholar] [CrossRef]
- Pan, T.P.; Wan, C.C. Anodic behavior of gold in cyanide solution. J. Appl. Electrochem. 1979, 9, 653–655. [Google Scholar] [CrossRef]
- Li, N.H.; Sun, S.G.; Chen, S.P. Studies on the role of oxidation states of the platinum surface in electrocatalytic oxidation of small primary alcohols. J. Electroanal. Chem. 1997, 430, 57–67. [Google Scholar] [CrossRef]
- Pan, L.; Zhou, Z.; Chen, D.; Sun, S. Electrochemical and in situ FTIR studies of adsorption and oxidation of dimethyl ether on Platinum electrode. Acta Phys. Chim. Sin. 2008, 24, 1739–1744. [Google Scholar] [CrossRef]
- Nicol, M.; Fleming, C.; Paul, R. The chemistry of the extraction of gold. In The Extractive Metallurgy of Gold, 2nd ed.; Stanley, G.G., Ed.; South African Institute of Mining and Metallurgy: Johannesburg, South Africa, 1987; pp. 831–905. [Google Scholar]
- Nicol, M.J. Anodic behavior of gold part II-oxidation in alkaline solutions. Gold Bull. 1980, 13, 105–111. [Google Scholar] [CrossRef]
- Lee, S.J.; Kwon, H.S.; Kim, J.S. Analysis of microstructure and corrosion behavior of laser surface alloyed zircaloy-4 with niobium. Met. Mater. Int. 2000, 6, 145–149. [Google Scholar] [CrossRef]
- Lu, H.; Gao, K.W.; Chu, W.Y. Determination of tensile stress corrosion induced by dezincification layer during corrosion for brass. Corros. Sci. 1998, 40, 1663–1670. [Google Scholar] [CrossRef]
- Jeffrey, M.I.; Ritchie, I.M. The leaching of gold in cyanide solutions in the presence of impurities I. The effect of lead. J. Electrochem. Soc. 2000, 147, 3257–3262. [Google Scholar] [CrossRef]
- Moulder, J.F.; Chastain, J.; King, R.C.J. Handbook of X-ray photoelectron spectroscopy: A reference book of standard spectra for identification and interpretation of XPS data. Chem. Phys. Lett. 1995, 220, 7–10. [Google Scholar]
- Bas, A.D.; Safizadeh, F.; Zhang, W.; Ghali, E.; Choi, Y. Active and passive behaviors of gold in cyanide solutions. Trans. Nonferr. Met. Soc. 2015, 25, 3442–3453. [Google Scholar] [CrossRef]
- Goff, A.L.; Artero, V.; Metay, R.; Moggia, F.; Jousselme, B. Immobilization of Fe hydrogenase mimics onto carbon and gold electrodes by controlled aryldiazonium salt reduction: An electrochemical, XPS and ATR-IR study. Int. J. Hydrog. Energy 2010, 35, 10790–10796. [Google Scholar] [CrossRef]
- Cao, L.L.; Shi, F.X.; Song, W.J.; Zhu, Y.F. Study of the electromigration behavior of Au-Ag thin films deposited on SiO2 substrate using AES, XPS and AFM techniques. Surf. Interface Anal. 2001, 28, 258–263. [Google Scholar] [CrossRef]
- Barroso, B.A.; Alexandre, F.M.; Fernández, G.C.; Gomez, S.V. FT-IR analysis of pyrone and chromene structures in activated carbon. Energy Fuel 2014, 28, 4096–4103. [Google Scholar] [CrossRef]
- Geng, W.; Nakajima, T.; Takanashi, H.; Ohki, A. Analysis of carboxyl group in coal and coal aromaticity by Fourier transform infrared (FT-IR) spectrometry. Fuel 2009, 88, 139–144. [Google Scholar] [CrossRef]
- Zhong, Q.; Yang, Y.B.; Jiang, T.; Li, Q.; Xu, B. Xylene activation of coal tar pitch binding characteristics for production of metallurgical quality briquettes from coke breeze. Fuel Process. Technol. 2016, 148, 12–18. [Google Scholar] [CrossRef]
- Patrick, H.; Jonas, O.; Willian, A. Ligand field strengths of carbon monoxide and cyanide in octahedral coordination. J. Coord. Chem. 2005, 58, 41–45. [Google Scholar]

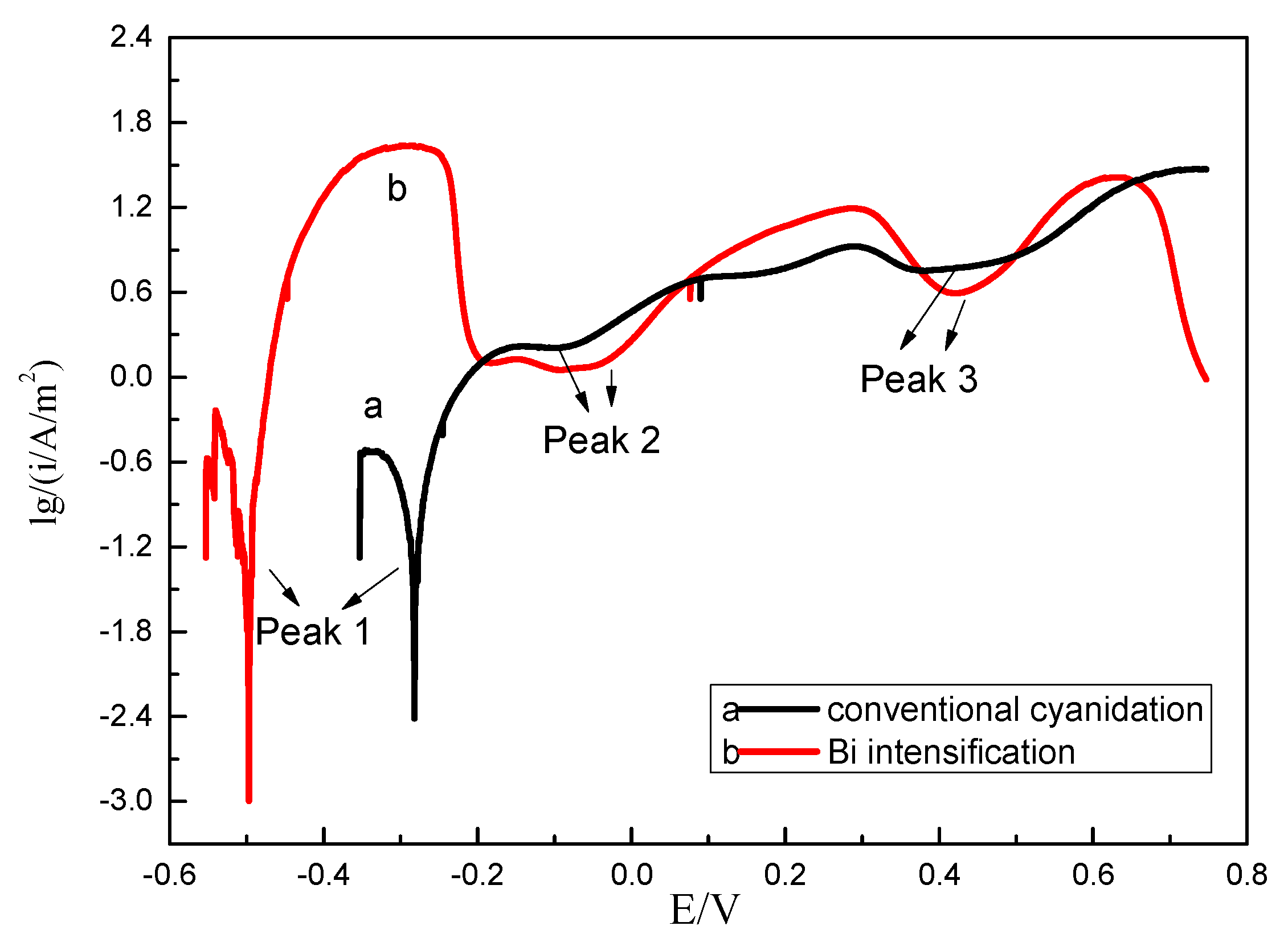


| Cyanide Method | Corrosion 1 | Corrosion 2 | Corrosion 3 | |||
|---|---|---|---|---|---|---|
| E/V | i/(mA/m2) | E/V | i/(A/m2) | E/V | i/(A/m2) | |
| Convention | −0.28 | 3.87 | −0.10 | 1.62 | 0.38 | 5.75 |
| Bi Intensification | −0.50 | 9.41 | −0.11 | 1.13 | 0.43 | 3.98 |
| Cyanide Method | Au | C | N | O | Bi |
|---|---|---|---|---|---|
| Conventional/% | 75.77 | 13.57 | 5.22 | 5.44 | 0 |
| Bi Intensification/% | 61.19 | 20.74 | 7.21 | 9.94 | 0.92 |
| Position (cm−1) | Assignment | Group |
|---|---|---|
| 1250 | O-H | Alcohol compound |
| 1640 | NO2 | Nitro compound |
| 2140 | C≡N | Cyanide compound |
| 2850, 2920 | C-H | Hydrocarbon compound |
| 3350, 3680 | O-H | Alkaline compound |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Lai, M.; Zhong, Q.; Li, Q.; Xu, B.; Jiang, T. Study on Intensification Behavior of Bismuth Ions on Gold Cyanide Leaching. Metals 2019, 9, 362. https://doi.org/10.3390/met9030362
Yang Y, Lai M, Zhong Q, Li Q, Xu B, Jiang T. Study on Intensification Behavior of Bismuth Ions on Gold Cyanide Leaching. Metals. 2019; 9(3):362. https://doi.org/10.3390/met9030362
Chicago/Turabian StyleYang, Yongbin, Meixiang Lai, Qiang Zhong, Qian Li, Bin Xu, and Tao Jiang. 2019. "Study on Intensification Behavior of Bismuth Ions on Gold Cyanide Leaching" Metals 9, no. 3: 362. https://doi.org/10.3390/met9030362
APA StyleYang, Y., Lai, M., Zhong, Q., Li, Q., Xu, B., & Jiang, T. (2019). Study on Intensification Behavior of Bismuth Ions on Gold Cyanide Leaching. Metals, 9(3), 362. https://doi.org/10.3390/met9030362





