Recovery of Platinum from Spent Petroleum Catalysts: Optimization Using Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
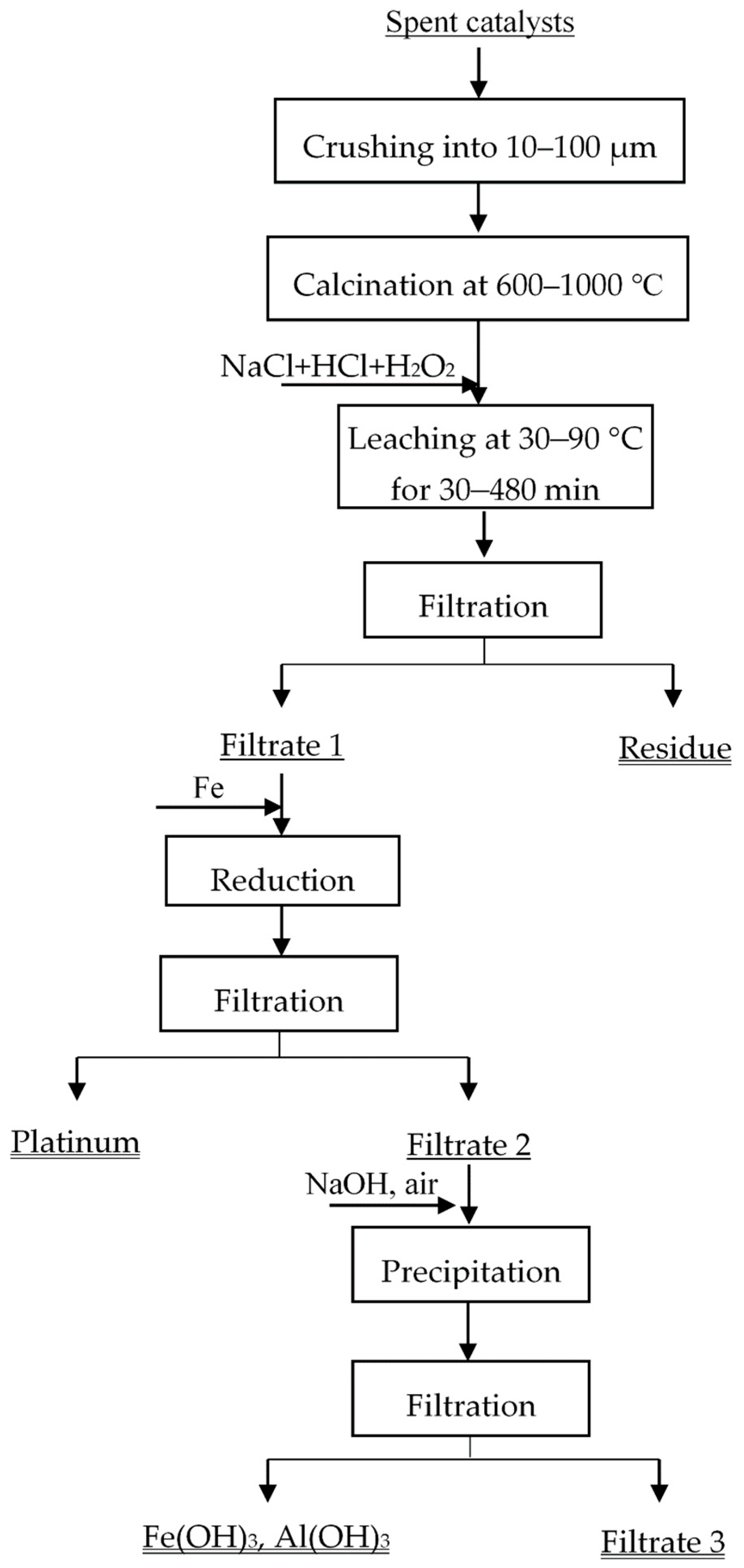
2.2. Experimental Procedures
2.3. Analytical Methods
3. Results and Discussion
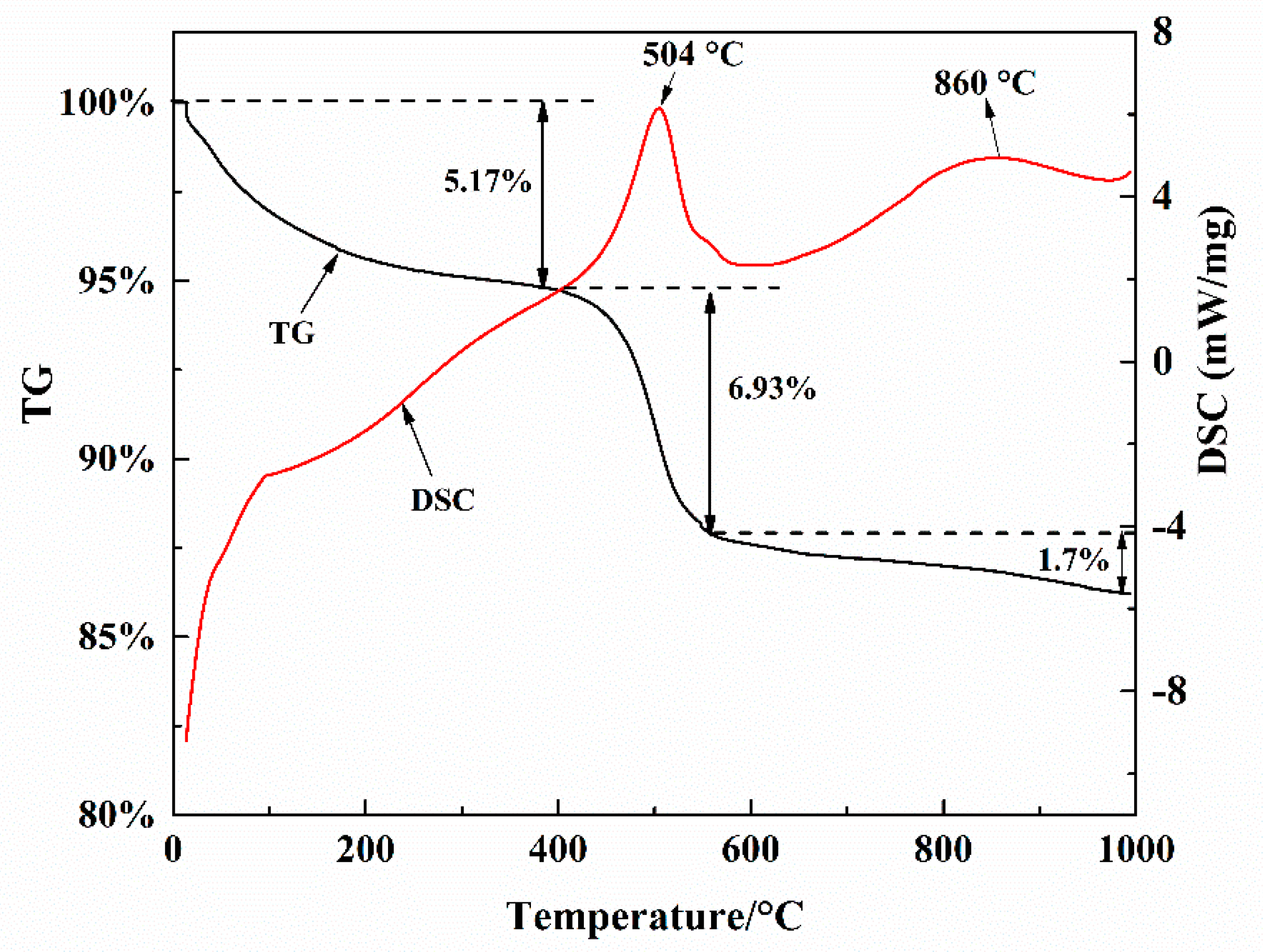
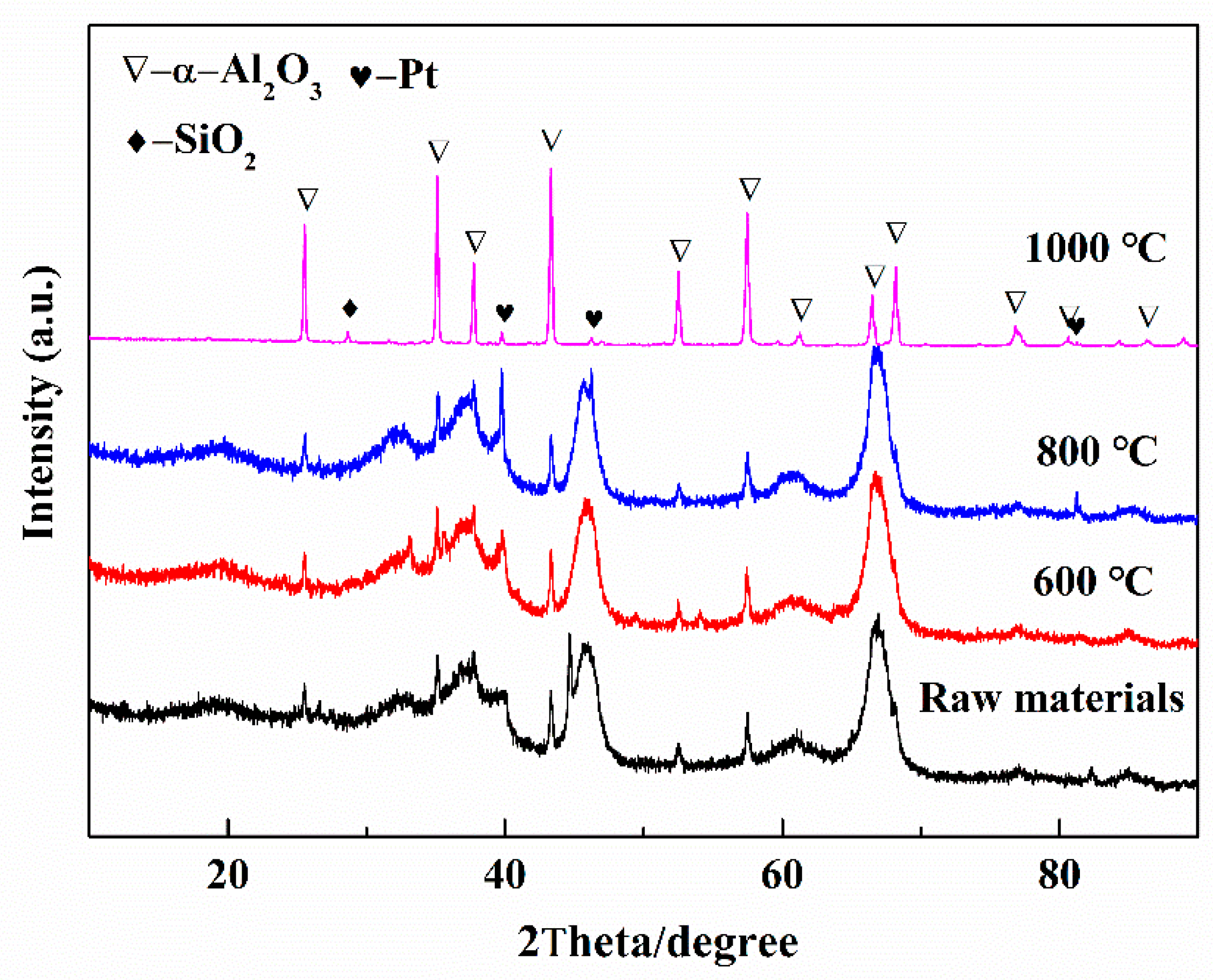
3.1. Characterization of Spent Catalysts
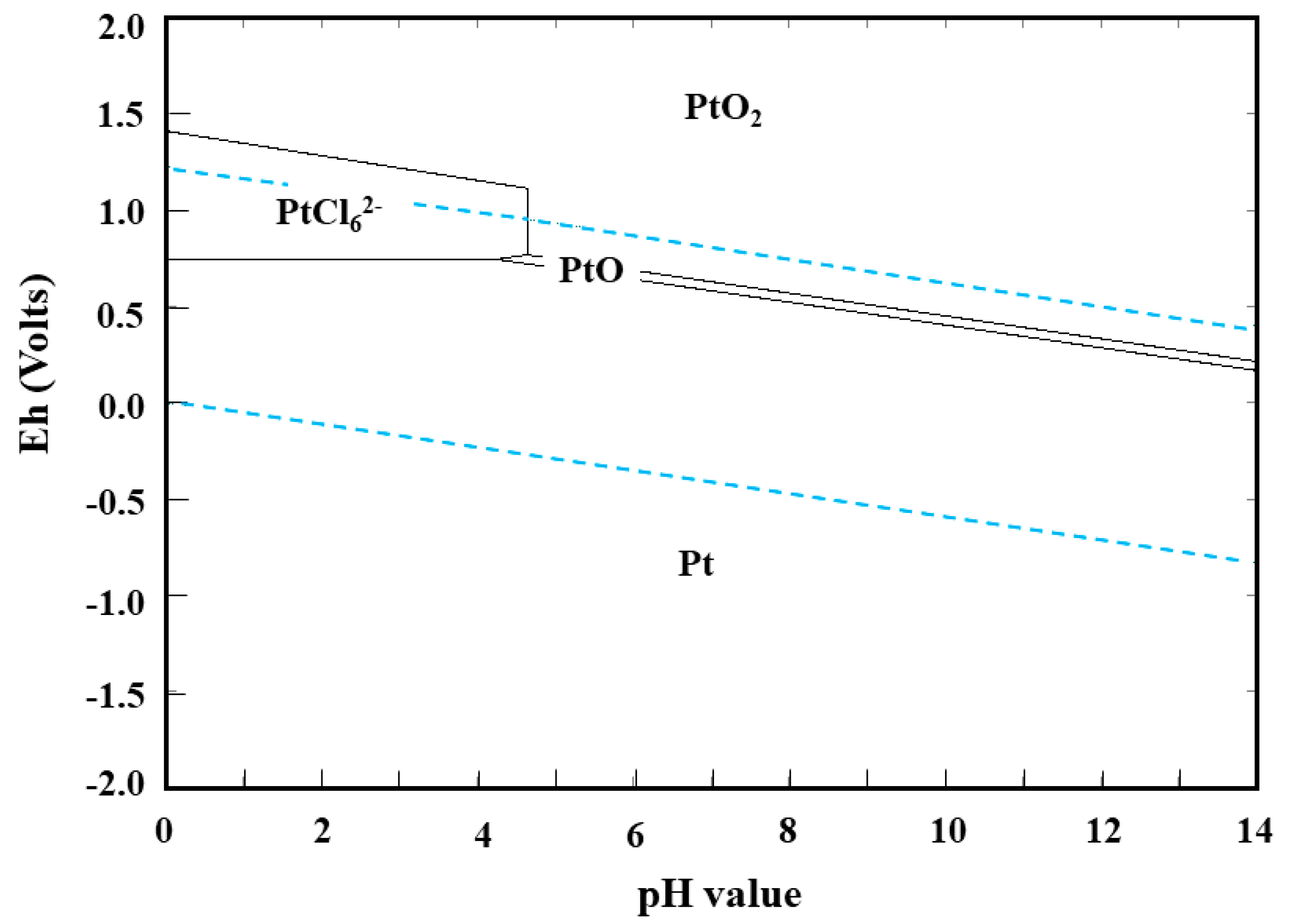
3.2. Platinum Leaching
3.2.1. Effect of Calcination Temperature
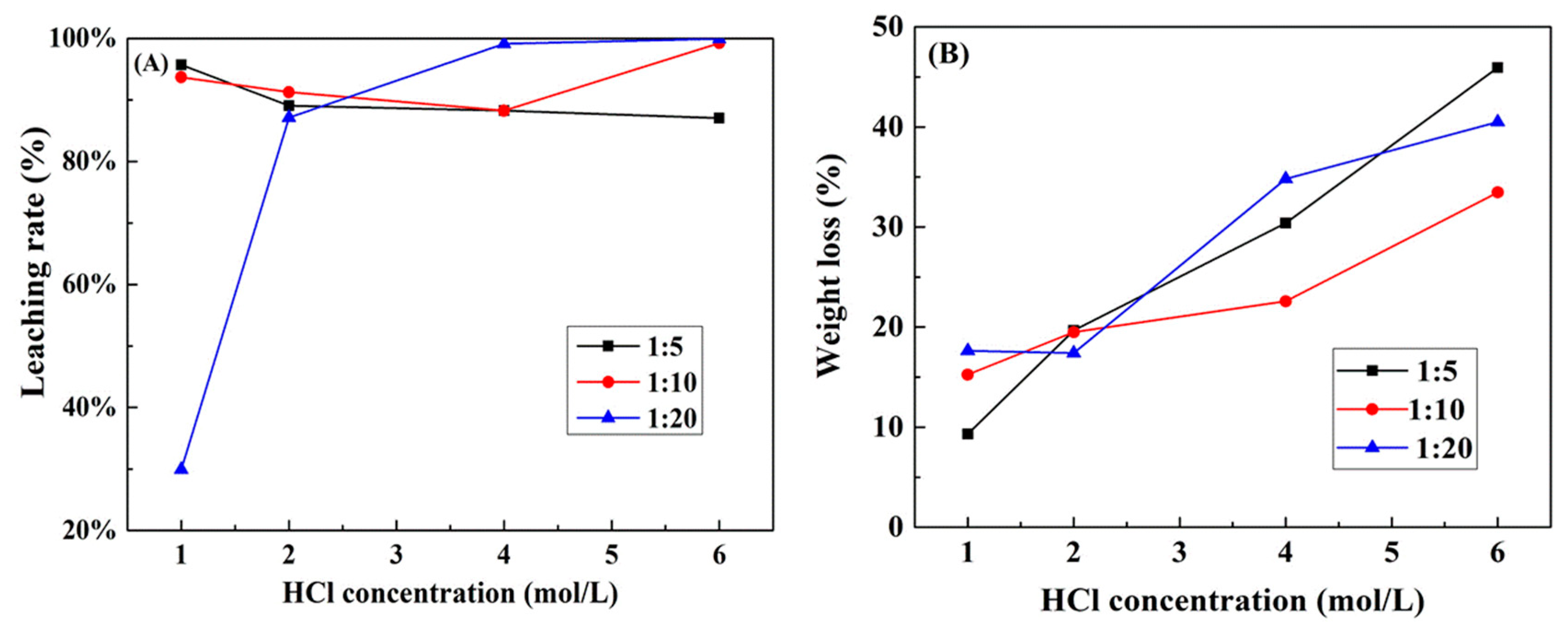
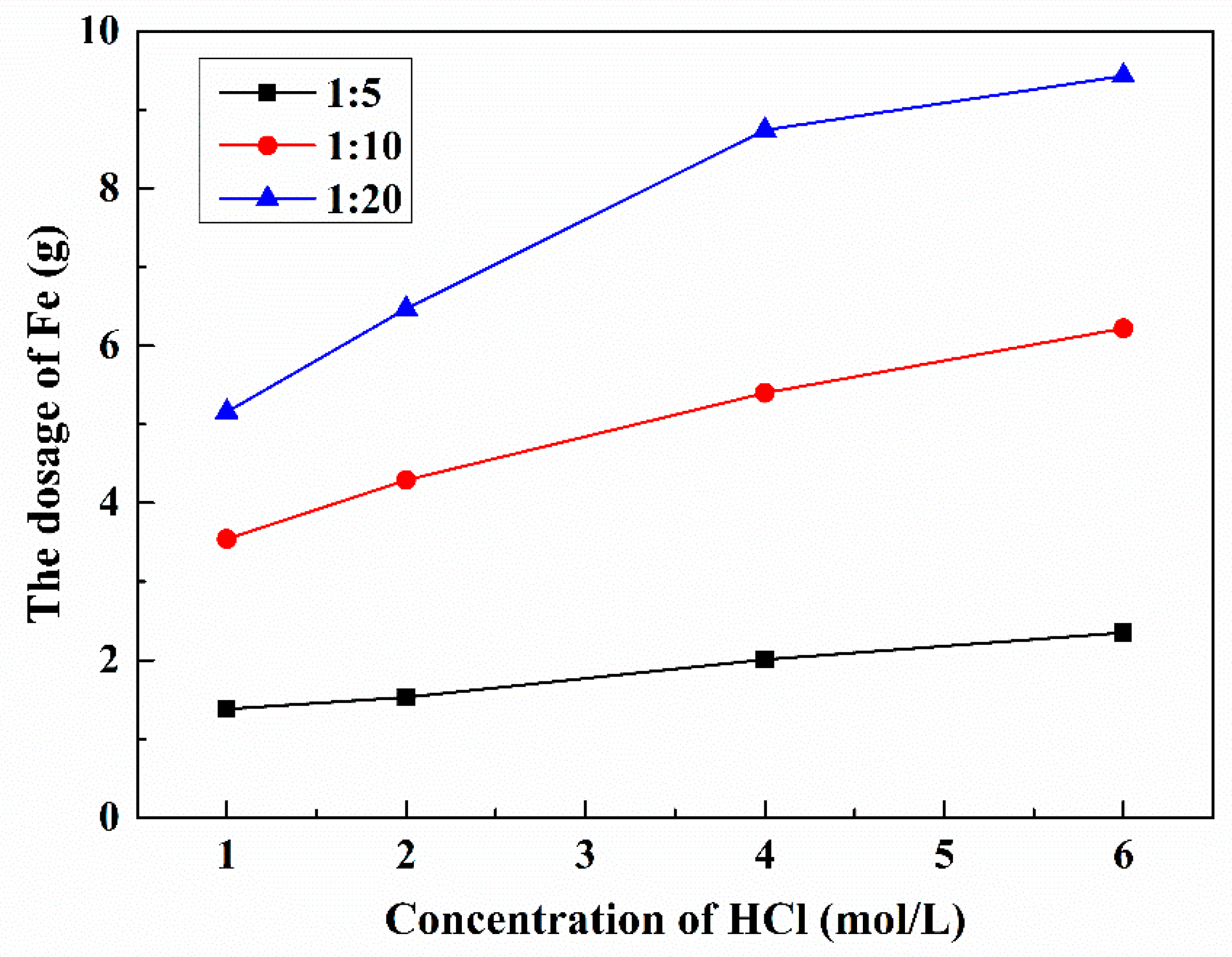
3.2.2. Effect of S/L and HCl Concentration
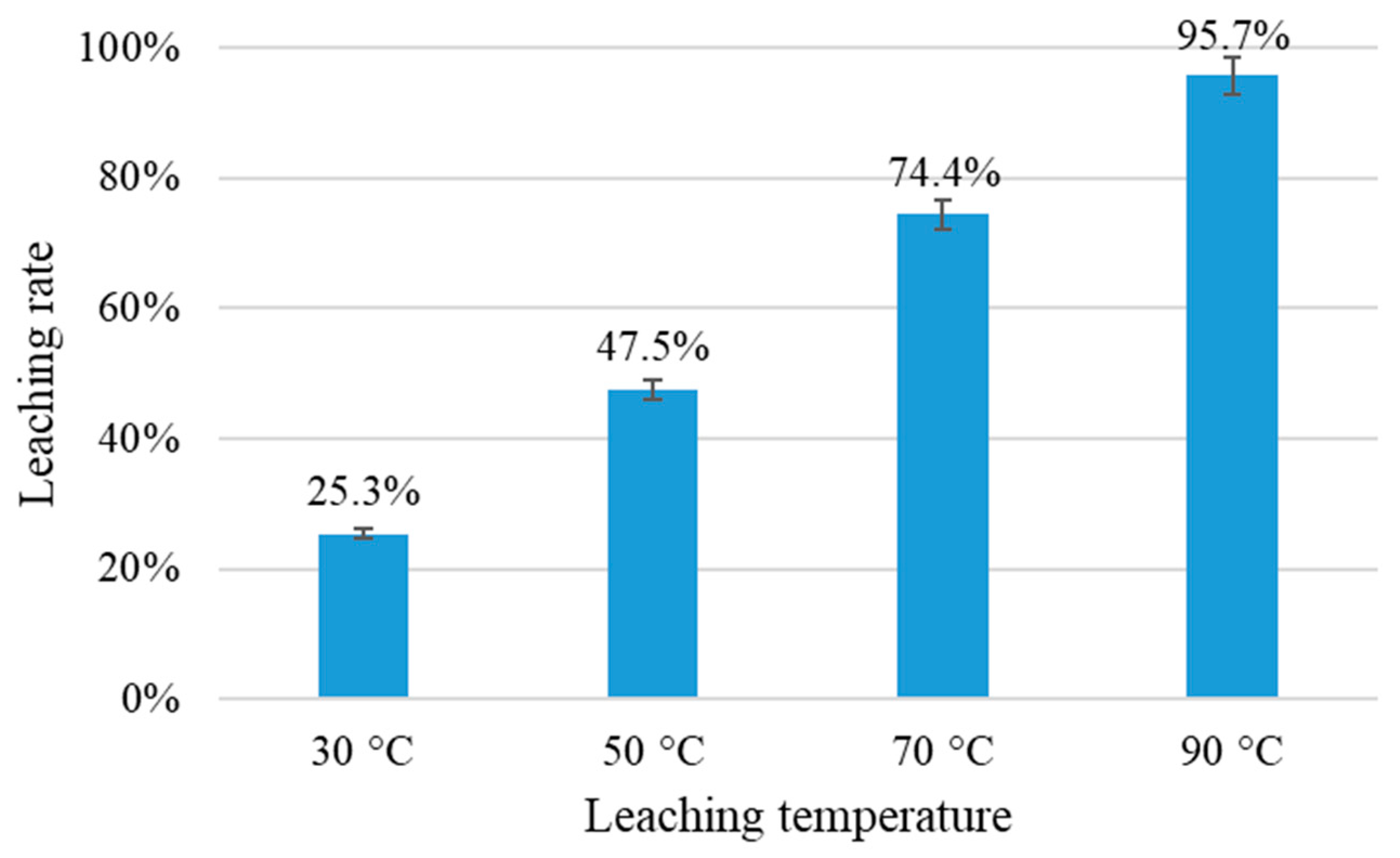
3.2.3. Effect of Leaching Temperature
3.2.4. Effect of Hydrogen Peroxide Dosage
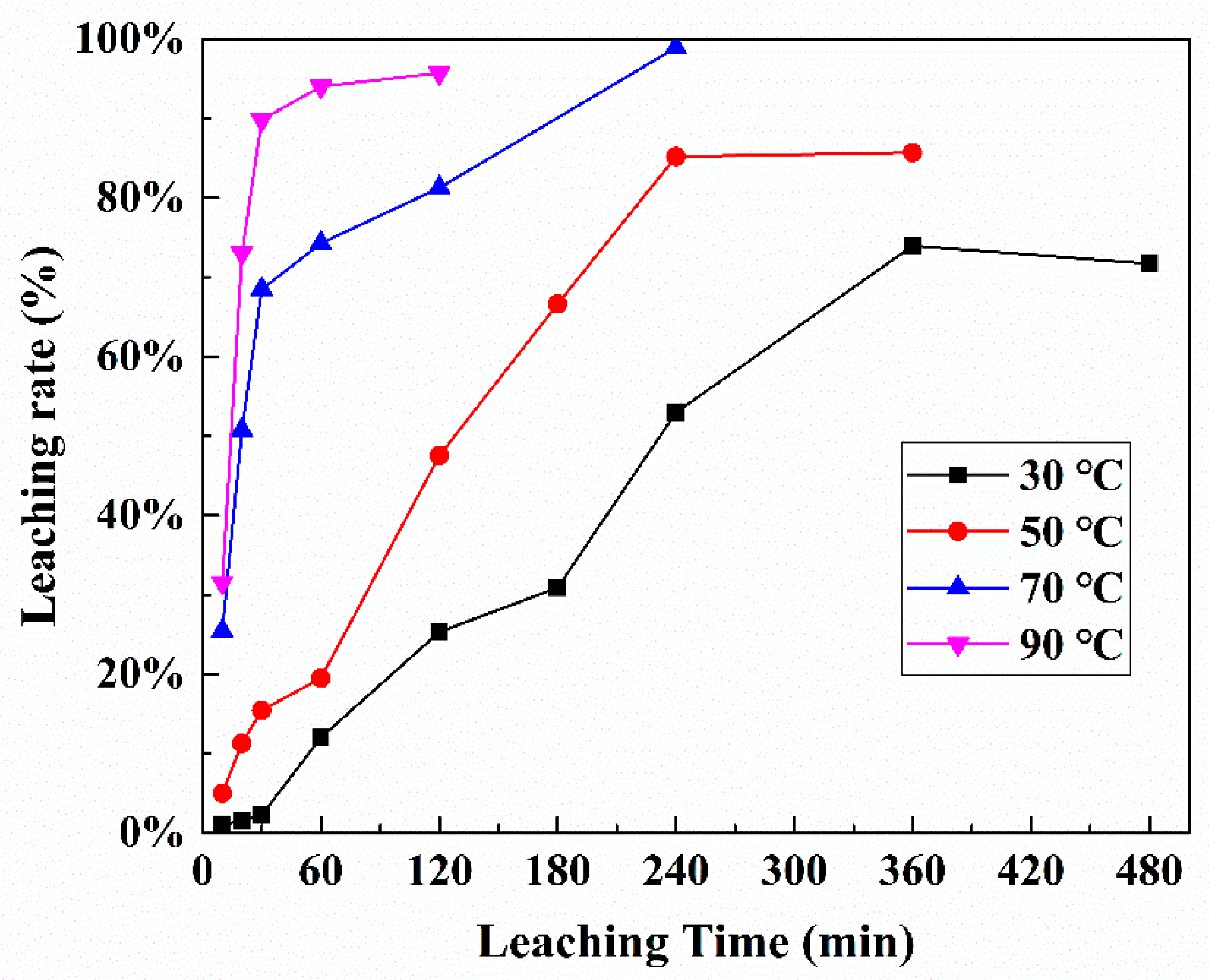
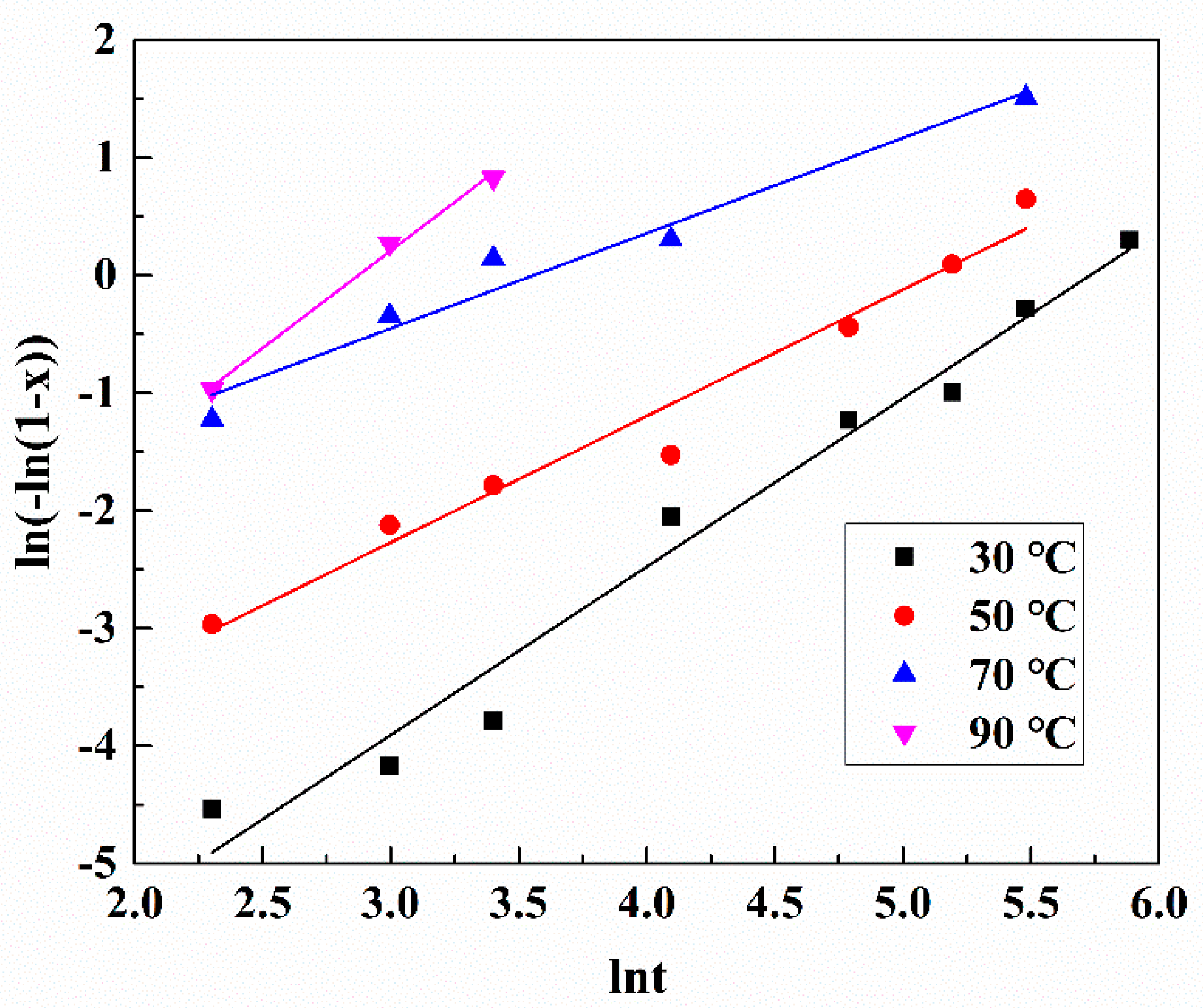
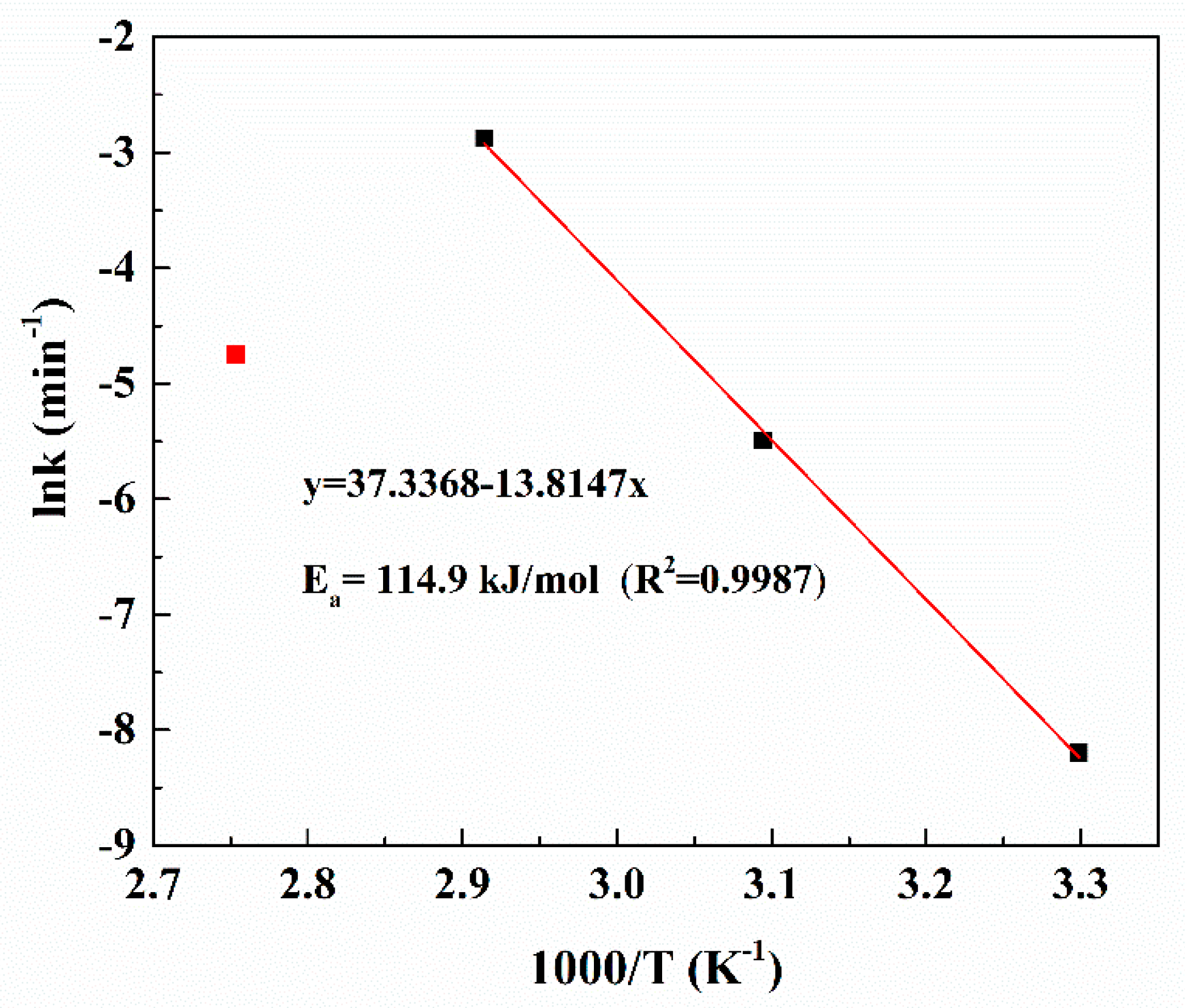
3.3. Kinetics Analysis of Platinum Leaching
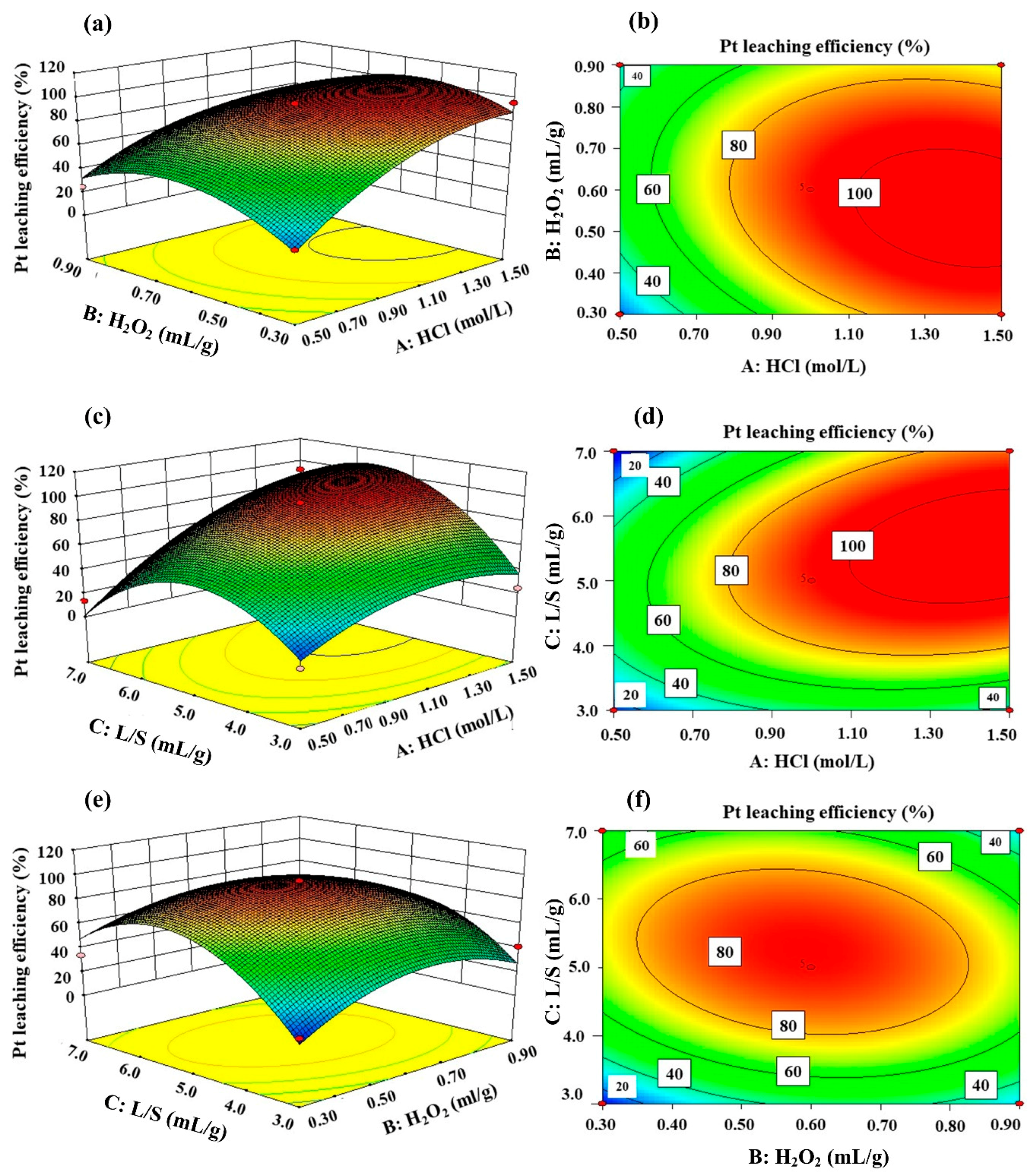
3.4. Optimization Design by Using Response Surface Methodology
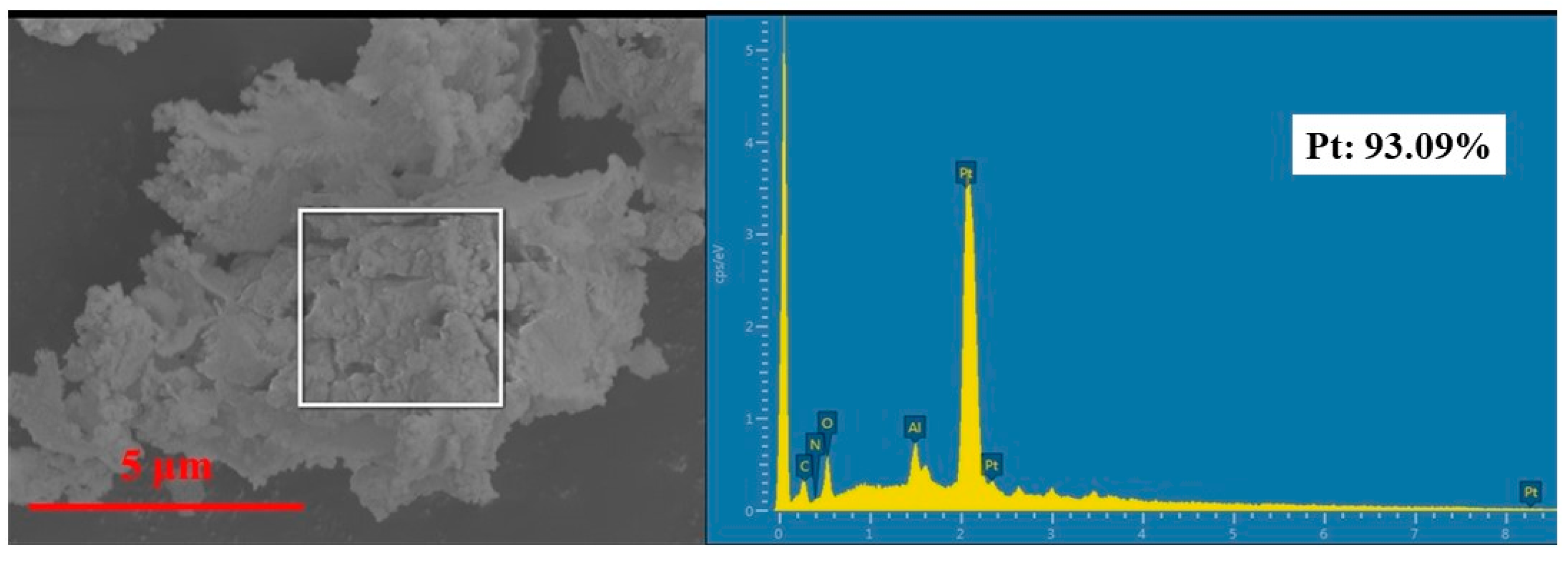
3.5. Recovery of Platinum from Leaching Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hennebel, T.; Boon, N.; Maes, S.; Lenz, M. Biotechnologies for critical raw material recovery from primary and secondary sources: R&D priorities and future perspectives. New Biotechnol. 2015, 32, 121–127. [Google Scholar]
- US Geological Survey (USGS). Platinum-Group Metals Statistics and Information. In Mineral Commodity Summaries; 2018. Available online: https://minerals.usgs.gov/minerals/pubs/commodity/platinum/mcs-2018-plati.pdf (accessed on 2 February 2019).
- Sverdrup, H.U.; Ragnarsdottir, K.V. A system dynamics model for platinum group metal supply, market price, depletion of extractable amounts, ore grade, recycling and stocks-in-use. Resour. Conserv. Recycl. 2016, 114, 130–152. [Google Scholar] [CrossRef]
- Graedel, T.E.; Harper, E.M.; Nassar, N.T.; Nuss, P.; Reck, B.K. Criticality of metals and metalloids. Proc. Natl. Acad. Sci. USA 2015, 112, 4257–4262. [Google Scholar] [CrossRef] [PubMed]
- Westerhoff, P.; Lee, S.; Yang, Y.; Gordon, G.W.; Hristovski, K.; Halden, R.U.; Herckes, P. Characterization, Recovery Opportunities, and Valuation of Metals in Municipal Sludges from U.S. Wastewater Treatment Plants Nationwide. Environ. Sci. Technol. 2015, 49, 9479–9488. [Google Scholar] [CrossRef] [PubMed]
- Ferella, F.; Innocenzi, V.; Maggiore, F. Oil refining spent catalysts: A review of possible recycling technologies. Resour. Conserv. Recycl. 2016, 108, 10–20. [Google Scholar] [CrossRef]
- US Geological Survey (USGS). Platinum-Group Metals Statistics and Information. In Minerals Yearbook of Platinum-Group Metals; 2015. Available online: https://minerals.usgs.gov/minerals/pubs/commodity/platinum/myb1-2015-plati.pdf (accessed on 2 February 2019).
- Marafi, M.; Stanislaus, A. Spent catalyst waste management: A review: Part I—Developments in hydro-processing catalyst waste reduction and use. Resour. Conserv. Recycl. 2008, 52, 859–873. [Google Scholar] [CrossRef]
- Akcil, A.; Vegliò, F.; Ferella, F.; Okudan, M.D.; Tuncuk, A. A review of metal recovery from spent petroleum catalysts and ash. Waste Manag. 2015, 45, 420–433. [Google Scholar] [CrossRef]
- Mahmoud, M.H.H. Leaching platinum-group metals in a sulfuric acid/chloride solution. JOM 2003, 55, 37–40. [Google Scholar] [CrossRef]
- He, X.; Li, Y.; Wu, X.; Zhao, Y.; Wang, H.; Liu, W. Study on the Process of Enrichment Platinum Group Metals by Plasma Melting Technology. Precious Met. 2016, 37, 1–5. [Google Scholar]
- Rumpold, R.; Antrekowitsch, J. Recycling of platinum group metals from automotive catalysts by an acidic leaching process. S. Afr. Inst. Min. Metall. Platinum 2012, 1, 695–714. [Google Scholar]
- Jha, M.K.; Lee, J.C.; Kim, M.S.; Jeong, J.; Kim, B.S.; Kumar, V. Hydrometallurgical Recovery/Recycling of Platinum by the Leaching of Spent Catalysts: A review. Hydrometallurgy 2013, 133, 23–32. [Google Scholar] [CrossRef]
- Sun, P.P.; Lee, M.S. Separation of Pt (IV) and Pd (II) from the loaded Alamine 336 by stripping. Hydrometallurgy 2011, 109, 181–184. [Google Scholar] [CrossRef]
- Hubicki, Z.; Wójcik, G. Studies of removal of platinum (IV) ion microquantities from the model solutions of aluminium, copper, iron, nickel and zinc chloride macroquantities on the anion exchanger Duolite S 37. J. Hazard. Mater. 2006, 136, 770–775. [Google Scholar] [CrossRef]
- Marinho, R.S.; Afonso, J.C.; da Cunha, J.W.S.D. Recovery of platinum from spent catalysts by liquid–liquid extraction in chloride medium. J. Hazard. Mater. 2010, 179, 488–494. [Google Scholar] [CrossRef]
- Paiva, A.P.; Ortet, O.; Carvalho, G.I.; Nogueira, C.A. Recovery of palladium from a spent industrial catalyst through leaching and solvent extraction. Hydrometallurgy 2017, 171, 394–401. [Google Scholar] [CrossRef]
- Marinho, R.S.; Da, S.C.; Afonso, J.C.; Da, C.J. Recovery of platinum, tin and indium from spent catalysts in chloride medium using strong basic anion exchange resins. J. Hazard. Mater. 2011, 192, 1155–1160. [Google Scholar] [CrossRef]
- Hammadi, M.Q.; Yassen, R.S.; Abid, K.N. Recovery of Platinum and Palladium from Scrap Automotive Catalytic Converters. Al-Khwarizmi Eng. J. 2017, 13, 131–141. [Google Scholar]
- Britton, L.A.; Markarian, G.Z. Method for platinum recovery from materials containing rhenium and platinum metals. US9702021B2, 11 July 2017. [Google Scholar]
- Nogueira, C.A.; Paiva, A.P.; Oliveira, P.C.; Costa, M.C.; Da, C.A. Oxidative leaching process with cupric ion in hydrochloric acid media for recovery of Pd and Rh from spent catalytic converters. J. Hazard. Mater. 2014, 278, 82–90. [Google Scholar] [CrossRef]
- Aberasturi, D.J.; Pinedo, R.; Larramendi, I.R.; Larramendi, J.I.; Rojo, T. Recovery by hydrometallurgical extraction of the platinum-group metals from car catalytic converters. Miner. Eng. 2011, 24, 505–513. [Google Scholar] [CrossRef]
- Kizilaslan, E.; Aktaş, S.; Şeşen, M.K. Towards environmentally safe recovery of platinum from scrap automotive catalytic converters. Tur. J. Eng. Environ. Sci. 2010, 33, 83–90. [Google Scholar]
- Haynes, W.M. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Li, L.; Fan, E.; Guan, Y.; Zhang, X.X.; Xue, Q.; Wei, L.; Wu, F.; Chen, R.J. Sustainable recovery of cathode materials from spent lithium-ion batteries using lactic acid leaching system. ACS Sustain. Chem. Eng. 2017, 5, 5224–5233. [Google Scholar] [CrossRef]
- Li, G.H.; Rao, M.J.; Jiang, T.; Huang, Q.Q.; Peng, Z.W. Leaching of limonitic laterite ore by acidic thiosulfate solution. Miner. Eng. 2011, 24, 859–863. [Google Scholar] [CrossRef]
- Bahrami, H.; Eslami, A.; Nabizadeh, R.; Mohseni-Bandpi, A.; Asadi, A.; Sillanpää, M. Degradation of trichloroethylene by sonophotolytic-activated persulfate processes: Optimization using response surface methodology. J. Clean. Prod. 2018, 198, 1210–1218. [Google Scholar] [CrossRef]

| Materials | Leaching Conditions | Recovery Rate | References | |||
|---|---|---|---|---|---|---|
| Reagents | Temp. (°C) | Time | S/L (g/mL) | |||
| Spent catalysts | Aqua regia | 75 | 20 min | 1:4 | Pt 97% | [18] |
| Spent auto-catalysts | aqua regia | 100 | 2 h | 1:20 | Pt 97.58% | [19] |
| Super alloys | H2SO4 800 g/L, 35% H2O2 | 80–90 | 35 h | 1:20 | Pt 99.98% | [20] |
| Spent catalytic converters | HCl 6 mol/L, Cu2+ 0.3 mol/L | 80 | 4 h | 1:20 | Pd 95%, Rh 86% | [21] |
| Industrial catalyst | H2SO4 60%, NaCl 0.1 mol/L | 125 | 10 h | 1:100 | Pt 99% | [10] |
| Car catalytic converters | HCl 12 mol/L, H2O2 7% and H2SO4 2.0 mol/L | 90 | 6 h | 1:3 | Pt >95% | [22] |
| Scrap automotive catalytic converters | 10 HCl (37%): 1 H2O2 (36%) | 80 | 2 h | 1:20 | Pt 95% | [23] |
| Spent petroleum catalysts | HCl 1.45 mol/L, NaCl 4.55 mol/L, 10% H2O2/spent catalysts of 0.66 mL/g | 90 | 2 h | 1:4.85 | Pt 98.1% | This study |
| Temperature | 600 °C | 800 °C | 1000 °C |
| Leaching efficiency | 77.4% | 99.9% | 82.5% |
| Pt in leaching residue | 0.2% | 0.03% | 17.4% |
| T/°C | n | lnk | R2 |
|---|---|---|---|
| 30 | 1.43027 | −8.19704 | 0.97262 |
| 50 | 1.07385 | −5.49141 | 0.96562 |
| 70 | 0.80941 | −2.88015 | 0.95073 |
| 90 | 1.65294 | −4.75054 | 0.99106 |
| Factors | Coded Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A: HCl concentration (mol/L) | 0.5 | 1 | 1.5 |
| B: 10% H2O2/spent catalysts (mL/g) | 0.3 | 0.6 | 0.9 |
| C: Liquid/solid ratio | 3 | 5 | 7 |
| Run | Factor 1 HCl Concentration (mol/L) | Factor 2 H2O2 Dosage (mL/g) | Factor 3 Liquid/Solid (L/S) | Response Pt Leaching Rate (%) |
|---|---|---|---|---|
| 1 | 1.0 | 0.3 | 3 | 13.9% |
| 2 | 0.5 | 0.3 | 5 | 19.8% |
| 3 | 1.0 | 0.3 | 7 | 33.9% |
| 4 | 1.5 | 0.3 | 5 | 95.7% |
| 5 | 1.0 | 0.6 | 5 | 93.9% |
| 6 | 1.0 | 0.9 | 3 | 41.1% |
| 7 | 1.0 | 0.6 | 5 | 94.4% |
| 8 | 1.0 | 0.6 | 5 | 95.1% |
| 9 | 1.0 | 0.9 | 7 | 21.7% |
| 10 | 0.5 | 0.9 | 5 | 24.7% |
| 11 | 1.0 | 0.6 | 5 | 94.8% |
| 12 | 1.5 | 0.9 | 5 | 68.5% |
| 13 | 1.5 | 0.6 | 7 | 92.3% |
| 14 | 0.5 | 0.6 | 3 | 7.4% |
| 15 | 1.0 | 0.6 | 5 | 95.2% |
| 16 | 0.5 | 0.6 | 7 | 13.4% |
| 17 | 1.5 | 0.6 | 3 | 24.4% |
| Source | Sun of Squares | df | Mean Square | F Value | p Value |
|---|---|---|---|---|---|
| Model | 20710.34 | 9 | 695.56 | 47.52 | <0.0001 |
| A | 5805.03 | 1 | 3892.27 | 265.93 | <0.0001 |
| B | 6.28 | 1 | 3.39 | 0.23 | 0.6449 |
| C | 695.09 | 1 | 123.32 | 8.43 | 0.0229 |
| AB | 258.73 | 1 | 3.31 | 0.23 | 0.6488 |
| AC | 958.21 | 1 | 58.98 | 4.03 | 0.0847 |
| BC | 388.09 | 1 | 7.05 | 0.48 | 0.5101 |
| A2 | 1350.30 | 1 | 2071.80 | 141.55 | <0.0001 |
| B2 | 2553.33 | 1 | 13.57 | 0.93 | 0.3677 |
| C2 | 7571.48 | 1 | 57.17 | 3.91 | 0.0887 |
| Residual | 920.57 | 7 | 14.64 | ||
| Lack of fit | 919.49 | 3 | 34.05 | 453.29 | <0.0001 |
| Pure error | 1.08 | 4 | 0.075 | ||
| Cor total | 21630.92 | 16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Zheng, H.; Li, J.; Zhang, S.; Liu, B.; Ekberg, C.; Jian, Z. Recovery of Platinum from Spent Petroleum Catalysts: Optimization Using Response Surface Methodology. Metals 2019, 9, 354. https://doi.org/10.3390/met9030354
Ding Y, Zheng H, Li J, Zhang S, Liu B, Ekberg C, Jian Z. Recovery of Platinum from Spent Petroleum Catalysts: Optimization Using Response Surface Methodology. Metals. 2019; 9(3):354. https://doi.org/10.3390/met9030354
Chicago/Turabian StyleDing, Yunji, Huandong Zheng, Jiayi Li, Shengen Zhang, Bo Liu, Christian Ekberg, and Zhuming Jian. 2019. "Recovery of Platinum from Spent Petroleum Catalysts: Optimization Using Response Surface Methodology" Metals 9, no. 3: 354. https://doi.org/10.3390/met9030354
APA StyleDing, Y., Zheng, H., Li, J., Zhang, S., Liu, B., Ekberg, C., & Jian, Z. (2019). Recovery of Platinum from Spent Petroleum Catalysts: Optimization Using Response Surface Methodology. Metals, 9(3), 354. https://doi.org/10.3390/met9030354






