Microstructural Evaluation and Corrosion Resistance of Semisolid Cast A356 Alloy Processed by Equal Channel Angular Pressing
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. The Effect of Cooling Slope
3.2. The Heat Treatment Solution
3.3. Process of ECAP
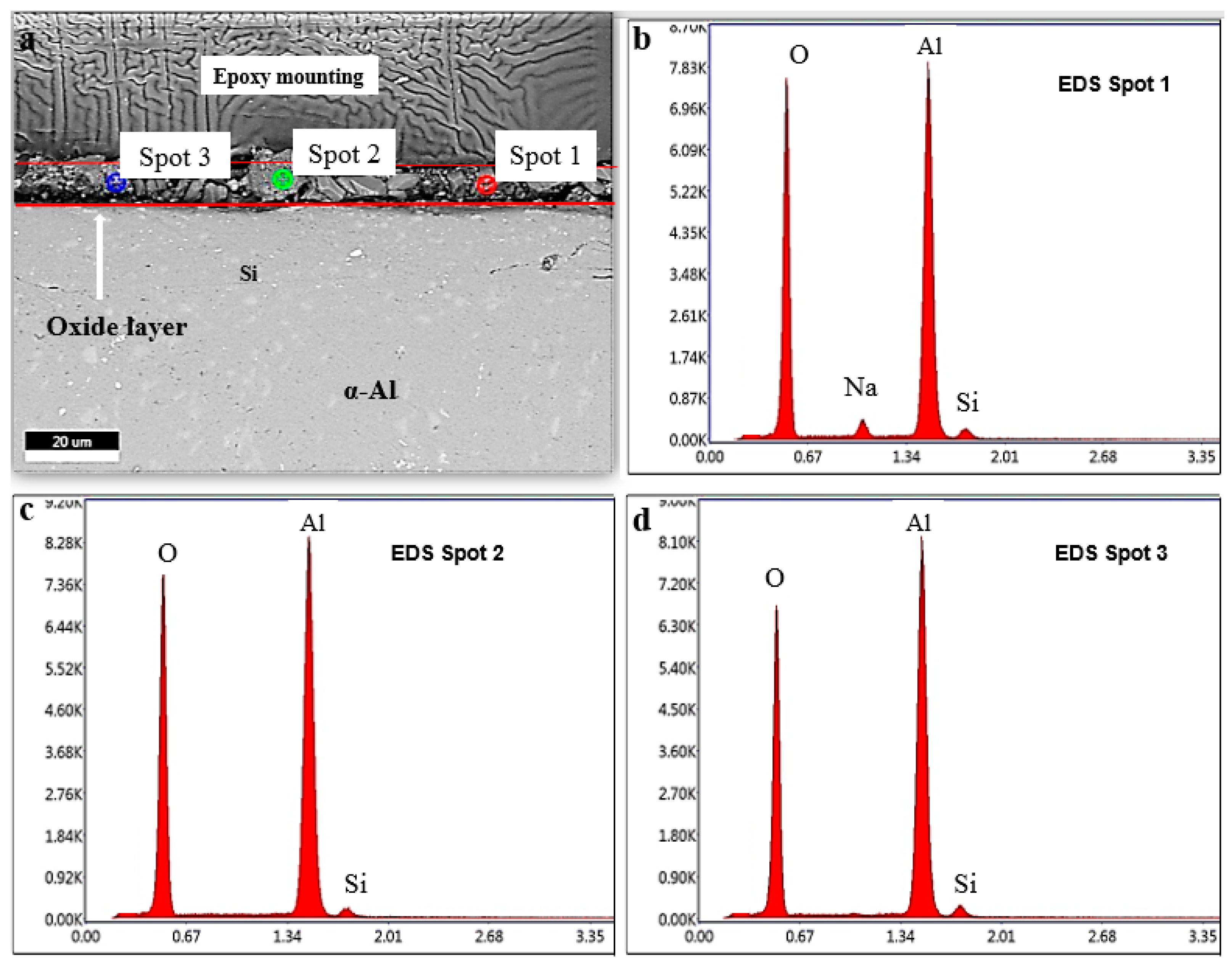
3.4. Scanning Electron Microscope (FESEM)
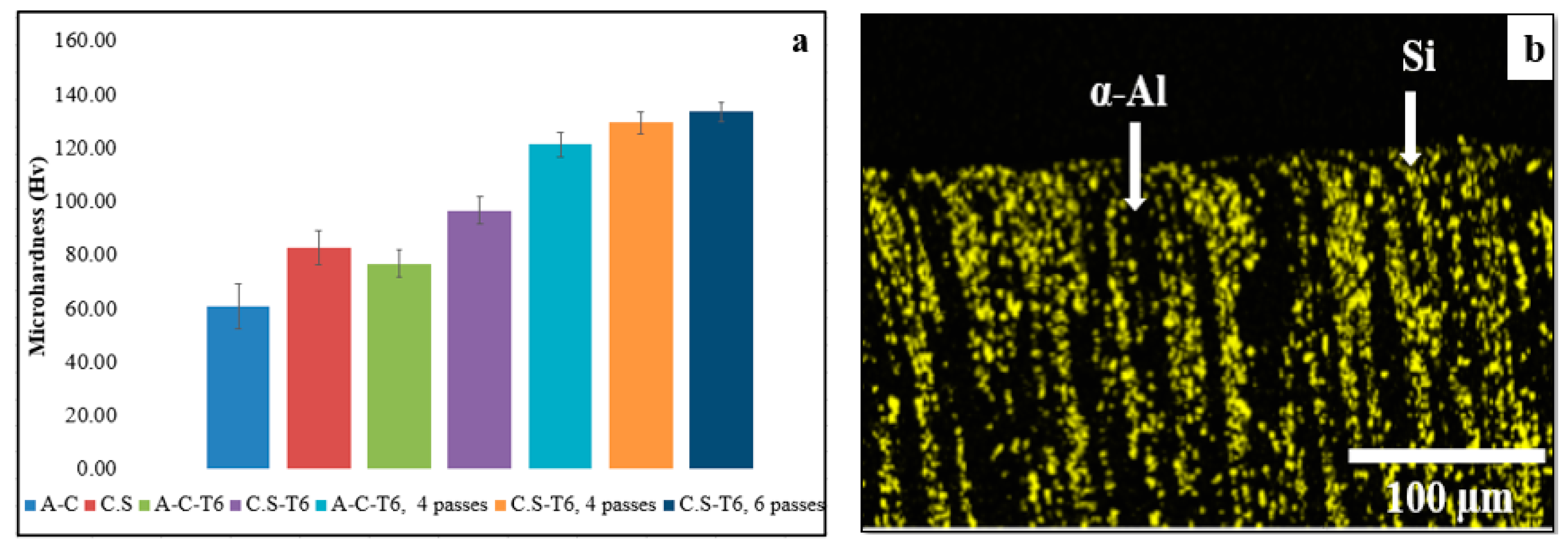
3.5. Hardness
3.6. Corrosion Resistance
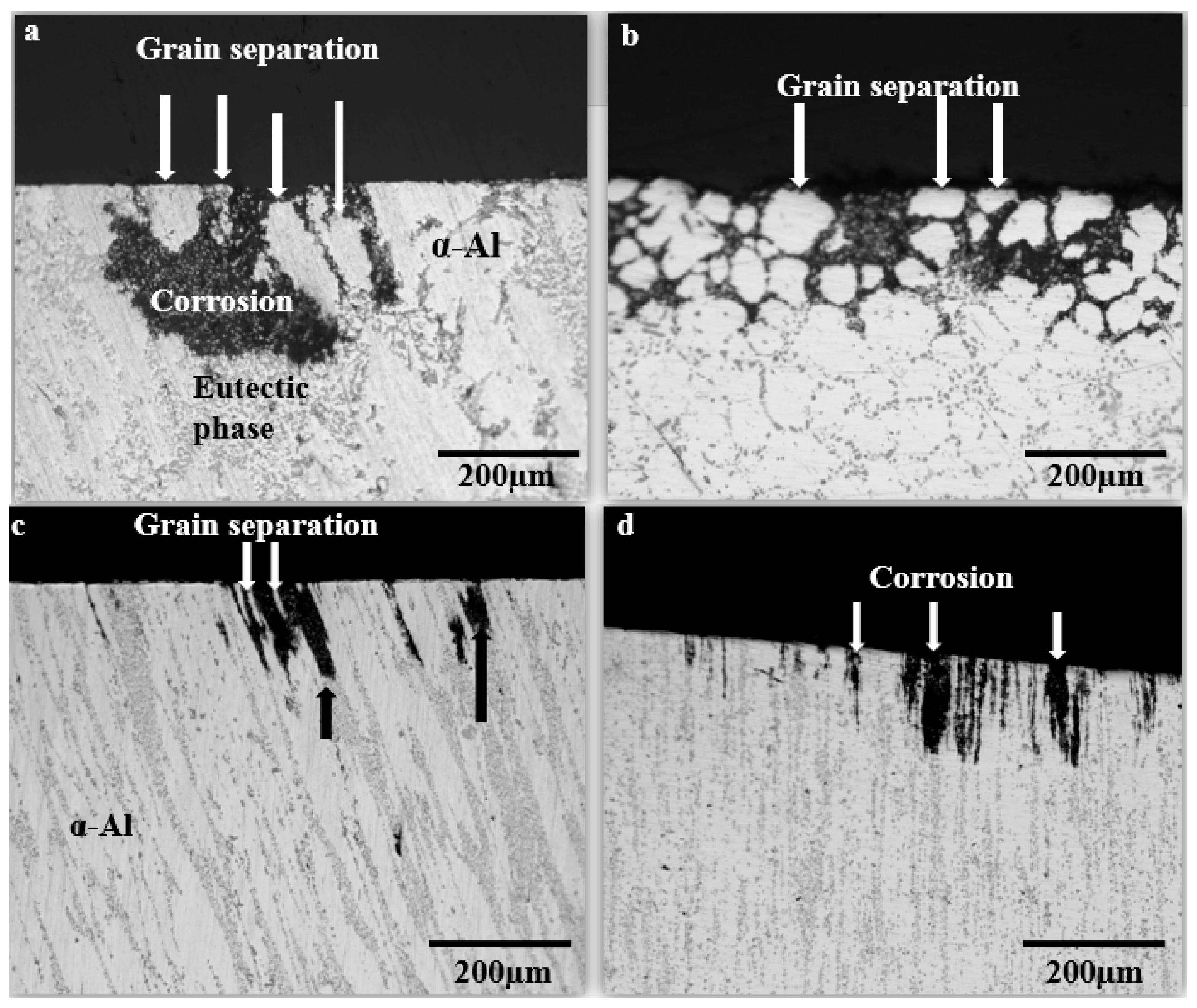
3.6.1. Surface Morphology
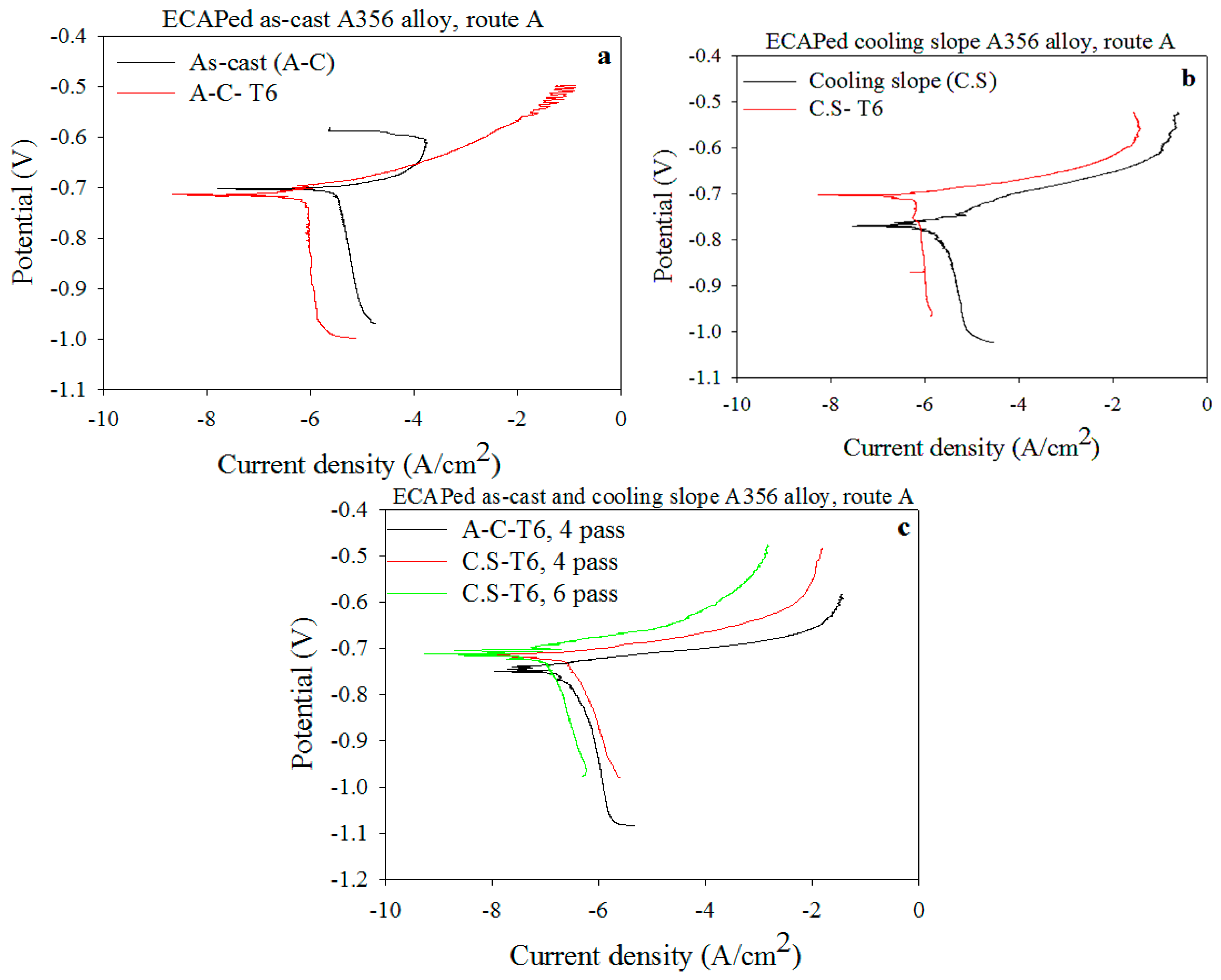
3.6.2. Potentiodynamic Test
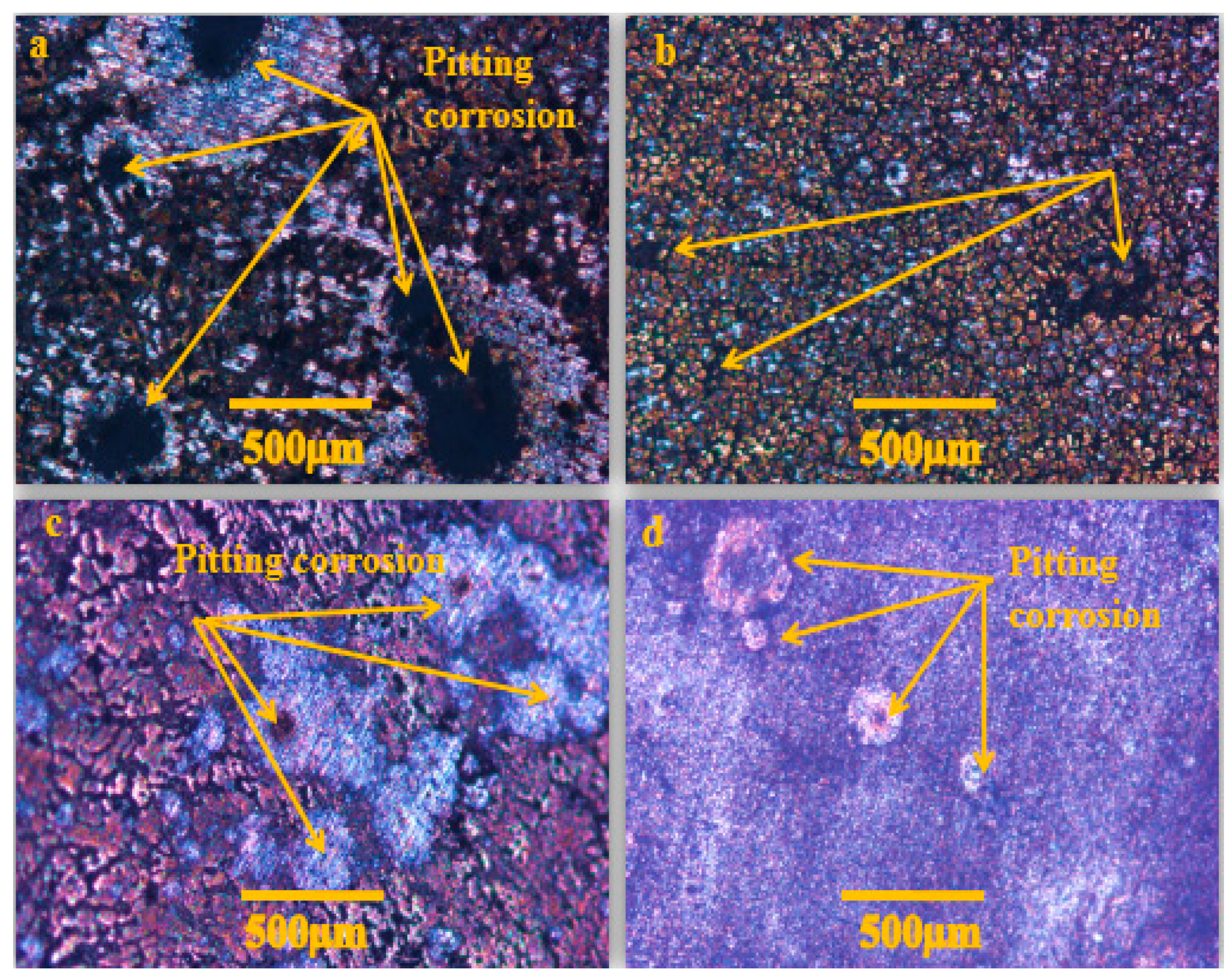
3.6.3. Pitting Corrosion Appearance
4. Conclusions
- The as-cast samples were successfully subjected to ECAP for up to four passes, while the samples from cooling slope casting, six passes.
- The latter also showed finer and more homogeneous distribution of α-Al grains and Si particles. As a result, their hardness values were also higher.
- The combination of cooling slope casting and ECAP had given the lowest current density to the A356 alloy, at 1.145 × 10−7 A/cm2 when experimental using 3.5 wt.% NaCl solution. The favorable corrosion resistance was attributed to the refined Si particles that impeded the occurrences of microgalvanic cells on the protective layer of the alloy surface.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haghdadi, N.; Zarei-Hanzaki, A.; Abedi, H.R.; Sabokpa, O. The effect of thermomechanical parameters on the eutectic silicon characteristics in a non-modified cast A356 aluminum alloy. Mater. Sci. Eng. A 2012, 549, 93–99. [Google Scholar] [CrossRef]
- Davis, J.R. Aluminum and Aluminum Alloys. Light Met. Alloy 2001, 66, 351–416. [Google Scholar] [CrossRef]
- Musa, A.Y.; Mohamad, A.B.; Kadhum, A.A.H.; Chee, E.P. Galvanic corrosion of aluminum alloy (Al2024) and copper in 1.0 M nitric acid. Int. J. Electrochem. Sci. 2011, 6, 5052–5065. [Google Scholar]
- Galvin, E.; O’Brien, D.; Cummins, C.; Mac Donald, B.J.; Lally, C. A strain-mediated corrosion model for bioabsorbable metallic stents. Acta Biomater. 2017, 55, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Tahamtan, S.; Fadavi Boostani, A. Evaluation of pitting corrosion of thixoformed A356 alloy using a simulation model. Trans. Nonferrous Met. Soc. China English Ed. 2010, 20, 1702–1706. [Google Scholar] [CrossRef]
- Boostani, A.F.; Tahamtan, S. Fracture behavior of thixoformed A356 alloy produced by SIMA process. J. Alloys Compd. 2009, 481, 220–227. [Google Scholar] [CrossRef]
- Tahamtan, S.; Boostani, A.F. Quantitative analysis of pitting corrosion behavior of thixoformed A356 alloy in chloride medium using electrochemical techniques. Mater. Des. 2009, 30, 2483–2489. [Google Scholar] [CrossRef]
- Barbucci, A.; Bruzzone, G.; Delucchi, M.; Panizza, M.; Cerisola, G. Breakdown of passivity of aluminium alloys by intermetallic phases in neutral chloride solution. Intermetallics 2000, 8, 305–312. [Google Scholar] [CrossRef]
- Wang, Q.G. Microstructural effects on the tensile and fracture behavior of aluminum casting alloys A356/357. Metall. Mater. Trans. A 2003, 34, 2887–2899. [Google Scholar] [CrossRef]
- Arrabal, R.; Mingo, B.; Pardo, A.; Mohedano, M.; Matykina, E.; Rodríguez, I. Pitting corrosion of rheocast A356 aluminium alloy in 3.5 wt.% NaCl solution. Corros. Sci. 2013, 73, 342–355. [Google Scholar] [CrossRef]
- Akhter, R.; Ivanchev, L.; Burger, H.P. Effect of pre/post T6 heat treatment on the mechanical properties of laser welded SSM cast A356 aluminium alloy. Mater. Sci. Eng. A 2007, 447, 192–196. [Google Scholar] [CrossRef]
- Liao, B.; Park, Y.; Ding, H. Effects of rheocasting and heat treatment on microstructure and mechanical properties of A356 alloy. Mater. Sci. Eng. A 2011, 528, 986–995. [Google Scholar] [CrossRef]
- Syarif, J.; Detak, Y.P.; Ramli, R. Modeling of Correlation between Heat Treatment and Mechanical Properties of Ti--6Al--4V Alloy Using Feed Forward Back Propagation Neural Network. ISIJ Int. 2010, 50, 1689–1694. [Google Scholar] [CrossRef]
- Atkinson, H. Current status of semi-solid processing of metallic materials. Adv. Mater. Form. 2007, 81–98. [Google Scholar] [CrossRef]
- Mohammed, M.N.; Omar, M.Z.; Salleh, M.S.; Alhawari, K.S.; Kapranos, P. Semisolid metal processing techniques for nondendritic feedstock production. Sci. World J. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Salleh, M.S.; Omar, M.Z.; Syarif, J.; Mohammed, M.N. An Overview of Semisolid Processing of Aluminium Alloys. ISRN Mater. Sci. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Spencer, D.B.; Merhrabian, R.; Flemings, M. Rheological behaviour of Sn-15pct Pb in the crystallization range. Metall. Trans. 1972, 3, 1925–1932. [Google Scholar] [CrossRef]
- Haga, T.; Kapranos, P. Billetless simple thixoforming process. J. Mater. Process. Technol. 2002, 130–131, 581–586. [Google Scholar] [CrossRef]
- Birol, Y. A357 thixoforming feedstock produced by cooling slope casting. J. Mater. Process. Technol. 2007, 186, 94–101. [Google Scholar] [CrossRef]
- Mohamed, I.F.; Masuda, T.; Lee, S.; Edalati, K.; Horita, Z.; Hirosawa, S.; Matsuda, K.; Terada, D.; Omar, M.Z. Strengthening of A2024 alloy by high-pressure torsion and subsequent aging. Mater. Sci. Eng. A 2017, 704, 112–118. [Google Scholar] [CrossRef]
- Fritsch, S.; Wagner, M.F.-X. On the effect of natural aging prior to low temperature ECAP of a high-strength aluminum alloy. Metals 2018, 8, 63. [Google Scholar] [CrossRef]
- Wei, J.; Huang, G.; Yin, D.; Li, K.; Wang, Q.; Zhou, H. Effects of ECAP and Annealing Treatment on the Microstructure and Mechanical Properties of Mg-1Y (wt.%) Binary Alloy. Metals 2017, 7, 119. [Google Scholar] [CrossRef]
- Shin, D.H.; Kim, B.C.; Park, K.T.; Choo, W.Y. Microstructural changes in equal channel angular pressed low carbon steel by static annealing. Acta Mater. 2000, 48, 3245–3252. [Google Scholar] [CrossRef]
- Abd El Aal, M.I.; Sadawy, M.M. Influence of ECAP as grain refinement technique on microstructure evolution, mechanical properties and corrosion behavior of pure aluminum. Trans. Nonferr. Met. Soc. China Engl. Ed. 2015, 25, 3865–3876. [Google Scholar] [CrossRef]
- Moradi, M.; Nili-Ahmadabadi, M.; Heidarian, B. Improvement of mechanical properties of AL (A356) cast alloy processed by ecap with different heat treatments. Int. J. Mater. Form. 2009, 2, 85–88. [Google Scholar] [CrossRef]
- Jiang, J.-H.; Ma, A.-B.; Lu, F.-M.; Saito, N.; Watazu, A.; Song, D.; Zhang, P.; Nishida, Y. Improving corrosion resistance of Al-11mass% Si alloy through a large number of ECAP passes. Mater. Corros. 2011, 62, 848–852. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, A.; Song, D.; Yang, D.; Shi, J.; Wang, K.; Zhang, L.; Chen, J. Anticorrosion behavior of ultrafine-grained Al-26 wt% Si alloy fabricated by ECAP. J. Mater. Sci. 2012, 47, 7744–7750. [Google Scholar] [CrossRef]
- Wang, X.; Nie, M.; Wang, C.T.; Wang, S.C.; Gao, N. Microhardness and corrosion properties of hypoeutectic Al-7Si alloy processed by high-pressure torsion. Mater. Des. 2015, 83, 193–202. [Google Scholar] [CrossRef]
- Osório, W.R.; Garcia, L.R.; Goulart, P.R.; Garcia, A. Effects of eutectic modification and T4 heat treatment on mechanical properties and corrosion resistance of an Al-9 wt% Si casting alloy. Mater. Chem. Phys. 2007, 106, 343–349. [Google Scholar] [CrossRef]
- Tahamtan, S.; Fadavi Boostani, A. Microstructural characteristics of thixoforged A356 alloy in mushy state. Trans. Nonferr. Met. Soc. China Engl. Ed. 2010, 20, s781–s787. [Google Scholar] [CrossRef]
- Samsudin, M.; Omar, M.Z.; Abdullah, S. Effects of rheocasting and thixoforming on the microstructure and mechanical properties of A356 aluminium alloy. J. Teknol. 2016, 78, 107–113. [Google Scholar]
- Rooy, E.L.; Linden, J.H.L. Van ASM Metals Handbook, Vol 02 Properties and Selection: Nonferrous Alloys and Special-Purpose Materials; ASM International: Geauga, OH, USA, 1990; pp. 3330–3345. [Google Scholar]
- Kurz, W.; Fisher, D. Fundamentals of solidification; Trans Tech Publication Ltd.: Aedermannsdorf, Switzerland, 1986; ISBN 0878495223. [Google Scholar]
- Flemings, M.C. Behavior of metal alloys in the semisolid state. Metall. Trans. B 1991, 22, 269–293. [Google Scholar] [CrossRef]
- Ogris, E.; Wahlen, A.; Lüchinger, H.; Uggowitzer, P.J. On the silicon spheroidization in Al-Si alloys. J. Light Met. 2002, 2, 263–269. [Google Scholar] [CrossRef]
- Tiryakioǧlu, M. Si particle size and aspect ratio distributions in an Al-7%Si-0.6%Mg alloy during solution treatment. Mater. Sci. Eng. A 2008, 473, 1–6. [Google Scholar] [CrossRef]
- Li, B.; Wang, H.; Jie, J.; Wei, Z. Effects of yttrium and heat treatment on the microstructure and tensile properties of Al-7.5Si-0.5Mg alloy. Mater. Des. 2011, 32, 1617–1622. [Google Scholar] [CrossRef]
- Ishak, N.N.M.; Salleh, M.S.; Yahaya, S.H.; Mohamad, E.; Sulaiman, M.A. The Effect of Equal Channel Angular Pressing (ECAP) on the Microstructure and Hardness of A356 Aluminium Alloy. J. Adv. Manuf. Technol. 2017, 11, 47–58. [Google Scholar]
- Cepeda-Jiménez, C.M.; García-Infanta, J.M.; Zhilyaev, A.P.; Ruano, O.A.; Carreño, F. Influence of the supersaturated silicon solid solution concentration on the effectiveness of severe plastic deformation processing in Al–7 wt.% Si casting alloy. Mater. Sci. Eng. A 2011, 528, 7938–7947. [Google Scholar] [CrossRef]
- Ritwik, R.; Rao, A.K.P.; Dhindaw, B.K. Low-convection-cooling slope cast AlSi7Mg alloy: A rheological perspective. J. Mater. Eng. Perform. 2013, 22, 2487–2492. [Google Scholar] [CrossRef]
- Natori, K.; Utsunomiya, H.; Tanaka, T. Improvement in formability of semi-solid cast hypoeutectic Al-Si alloys by equal-channel angular pressing. J. Mater. Process. Technol. 2017, 240, 240–248. [Google Scholar] [CrossRef]
- Mill, E.B.; Sherif, E.M.; Mohammed, J.A.; Abdo, H.S.; Almajid, A.A. Corrosion Behavior in Highly Concentrated Sodium Chloride Solutions of Nanocrystalline Aluminum Processed by High. Int. J. Electrochem. Sci. 2016, 11, 1355–1369. [Google Scholar]
- Nguyen, V.T.; Hussain, Z.; Anasyida, A.S.; Huy, T.D.; Almanar, I.P. Influence of Semi-Solid Casting and Equal Channel Pressing on Microstructure of a Hypoeutectic Al-Si Alloy. Mater. Sci. Forum 2015, 819, 9–14. [Google Scholar] [CrossRef]
- Rollett, A.; Humphreys, F.; Rohrer, G.; Hatherly, M. Recrystallization and Related Annealing Phenomena; Elsevier Science Ltd.: Oxford, UK, 2004. [Google Scholar]
- Mckenzie, P.W.J.; Lapovok, R.; Estrin, Y. The influence of back pressure on ECAP processed AA 6016: Modeling and experiment. Acta Mater. 2007, 55, 2985–2993. [Google Scholar] [CrossRef]
- Meyers, M.A.; Mishra, A.; Benson, D.J. Mechanical properties of nanocrystalline materials. Prog. Mater. Sci. 2006, 51, 427–556. [Google Scholar] [CrossRef]
- Zhu, M. Effects of T6 heat treatment on the microstructure, tensile properties, and fracture behavior of the modified A356 alloys. J. Mater. 2017, 36, 243–249. [Google Scholar] [CrossRef]
- Möller, H.; Govender, G.; Stumpf, W. Factors influencing tensile mechanical properties of Al-7Si-Mg casting alloys A356/7. In Light Alloys 2012; Springer: Berlin, Germany, 2012; pp. 467–471. [Google Scholar]
- Goodarzy, M.H.; Arabi, H.; Boutorabi, M.A.; Seyedein, S.H.; Najafabadi, S.H.H. The effects of room temperature ECAP and subsequent aging on mechanical properties of 2024 Al alloy. J. Alloys Compd. 2014, 585, 753–759. [Google Scholar] [CrossRef]
- Kumar, S.R.; Gudimetla, K.; Venkatachalam, P.; Ravisankar, B.; Jayasankar, K. Microstructural and mechanical properties of Al 7075 alloy processed by Equal Channel Angular Pressing. Mater. Sci. Eng. A 2012, 533, 50–54. [Google Scholar] [CrossRef]
- Thuong, N.; Zuhailawati, H.; Seman, A.A.; Huy, T.D.; Dhindaw, B.K. Microstructural evolution and wear characteristics of equal channel angular pressing processed semi-solid-cast hypoeutectic aluminum alloys. Mater. Des. 2015, 67, 448–456. [Google Scholar] [CrossRef]
- Hughes, A.E.; Birbilis, N.; Mol, J.M.C.; Garcia, S.J.; Zhou, X.; Thompson, G.E. Corrosion and principles of protection. In Recent Trends in Processing and Degradation of Aluminum Alloys; Intech: Rijeka, Croatia, 1968. [Google Scholar]
- Birbilis, N.; Buchheit, R.G. Electrochemical Characteristics of Intermetallic Phases in Aluminum Alloys: An Experimental Survey and Discussion. J. Electrochem. Soc. 2005, 152, 140–151. [Google Scholar] [CrossRef]
- Boag, A.; Hughes, A.E.; Glenn, A.M.; Muster, T.H.; McCulloch, D. Corrosion of AA2024-T3 Part I: Localised corrosion of isolated IM particles. Corros. Sci. 2011, 53, 17–26. [Google Scholar] [CrossRef]
- Balyanov, A.; Kutnyakova, J.; Amirkhanova, N.A.; Stolyarov, V.V.; Valiev, R.Z.; Liao, X.Z.; Zhao, Y.H.; Jiang, Y.B.; Xu, H.F.; Lowe, T.C.; et al. Corrosion resistance of ultra fine-grained Ti. Scr. Mater. 2004, 51, 225–229. [Google Scholar] [CrossRef]
- Di Schino, A.; Kenny, J.M. Effects of the grain size on the corrosion behavior of refined AISI 304 austenitic stainless steels. J. Mater. Sci. Lett. 2002, 21, 1631–1634. [Google Scholar] [CrossRef]
- Yahya, S.; Rahim, A.A. Inhibitive Behaviour of Corrosion of Aluminium Alloy in NaCl by Mangrove Tannin. Sains Malaysiana 2011, 40, 953–957. [Google Scholar]
- Inturi, R.B. Localized Corrosion of Nanocrystalline 304 Type Stainless Steel Films. Corrosion 1992, 48, 398–403. [Google Scholar] [CrossRef]
- Rashida, S.; Islamia, N.; Ariffina, A.K.; Ridhab, M.; Fonnab, S. The effect of immersion time on the corrosion behavior of SUS304 in brine using half-cell potential measurement. Polarization 2016, 5, 7. [Google Scholar]
- Krishna, K.G.; Sivaprasad, K.; Narayanan, T.S.N.S.; Kumar, K.C.H. Localized corrosion of an ultrafine grained Al–4Zn–2Mg alloy produced by cryorolling. Corros. Sci. 2012, 60, 82–89. [Google Scholar] [CrossRef]
- Akiyama, E.; Zhang, Z.; Watanabe, Y. Effects of severe plastic deformation on the corrosion behavior of aluminum alloys. J. Solid State Electrochem. 2009, 13, 277–282. [Google Scholar] [CrossRef]
- Song, D.; Ma, A.; Jiang, J.; Lin, P.; Yang, D. Corrosion behavior of ultra-fine grained industrial pure Al fabricated by ECAP. Trans. Nonferr. Met. Soc. China Engl. Ed. 2009, 19, 1065–1070. [Google Scholar] [CrossRef]

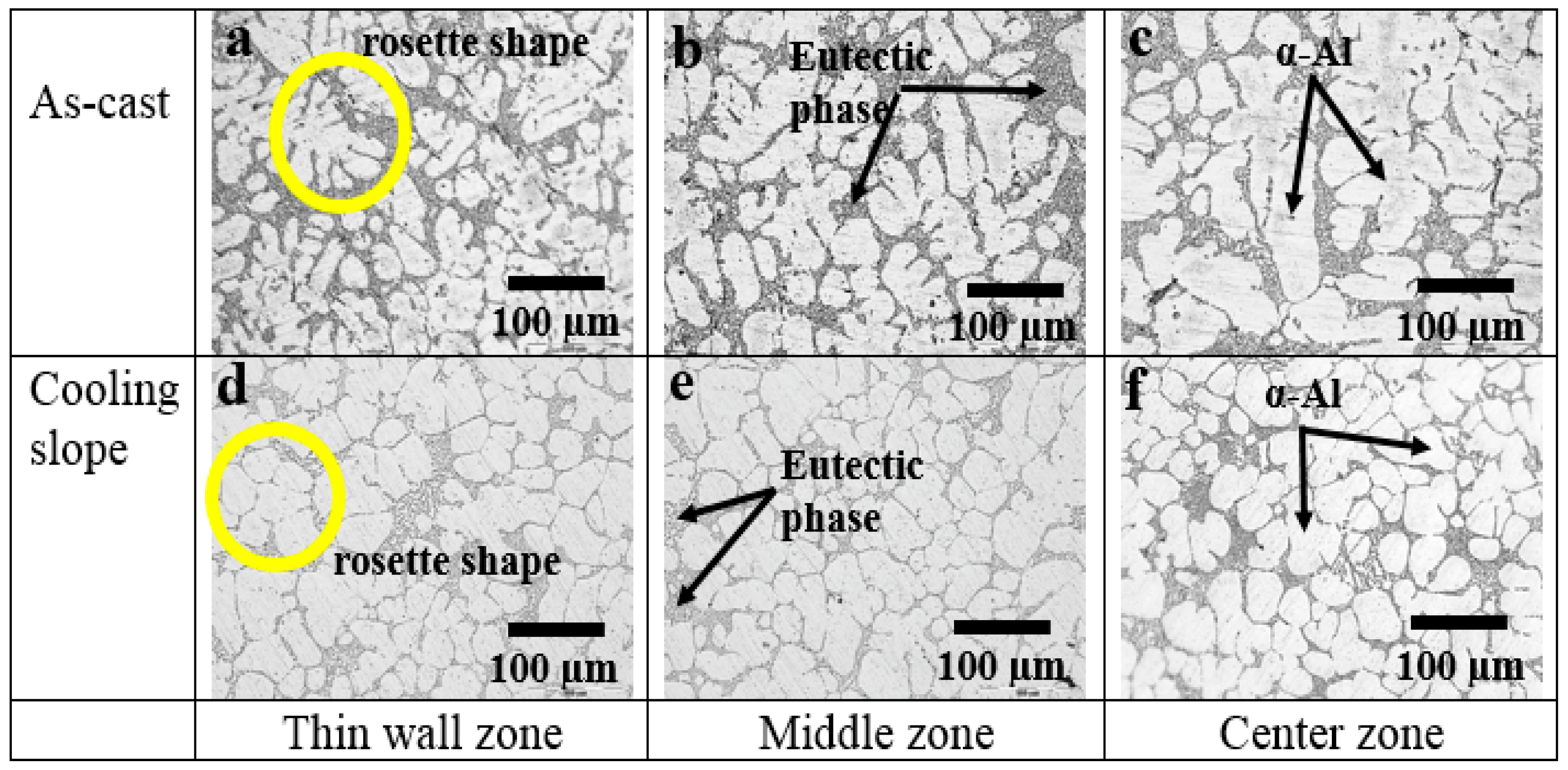
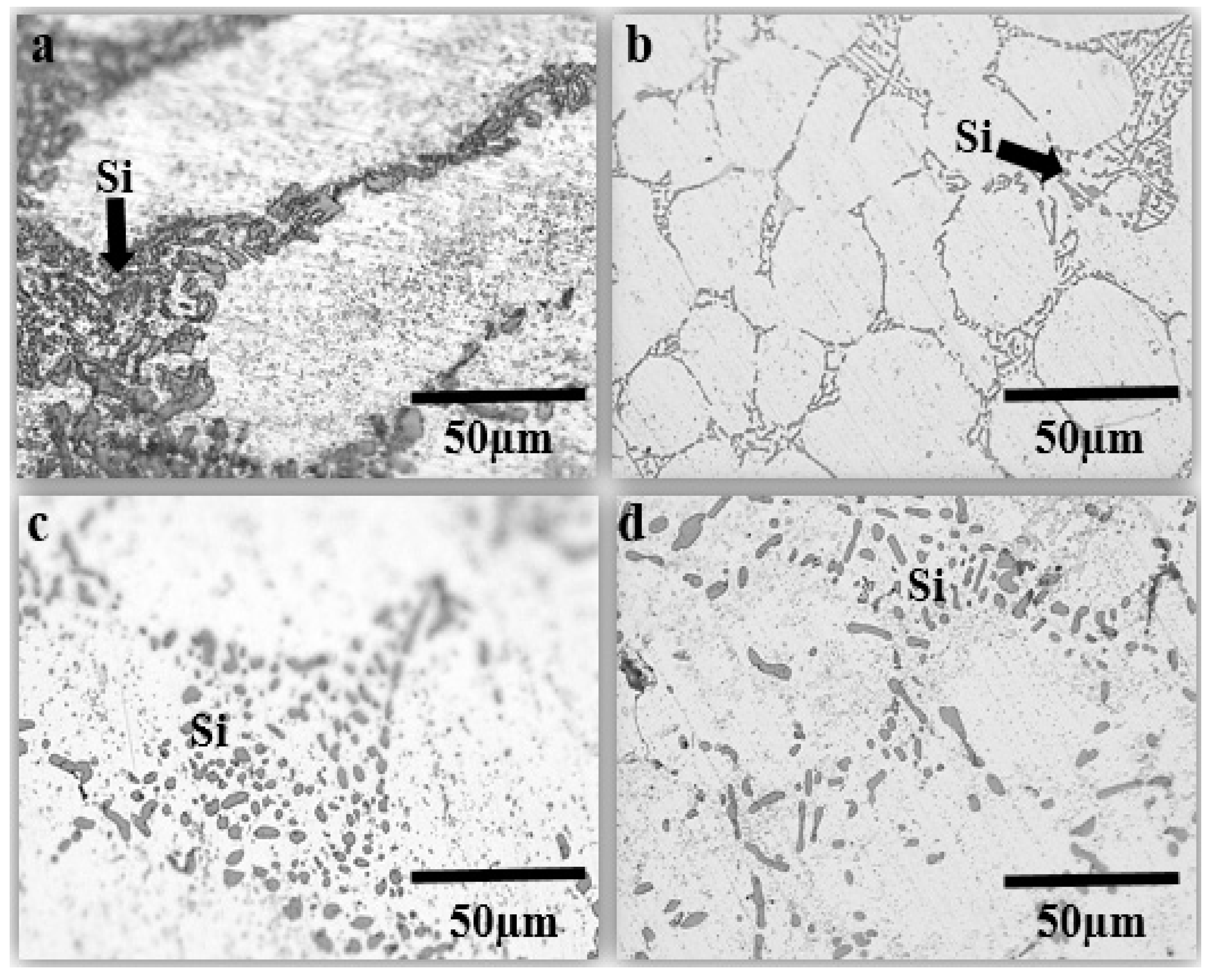

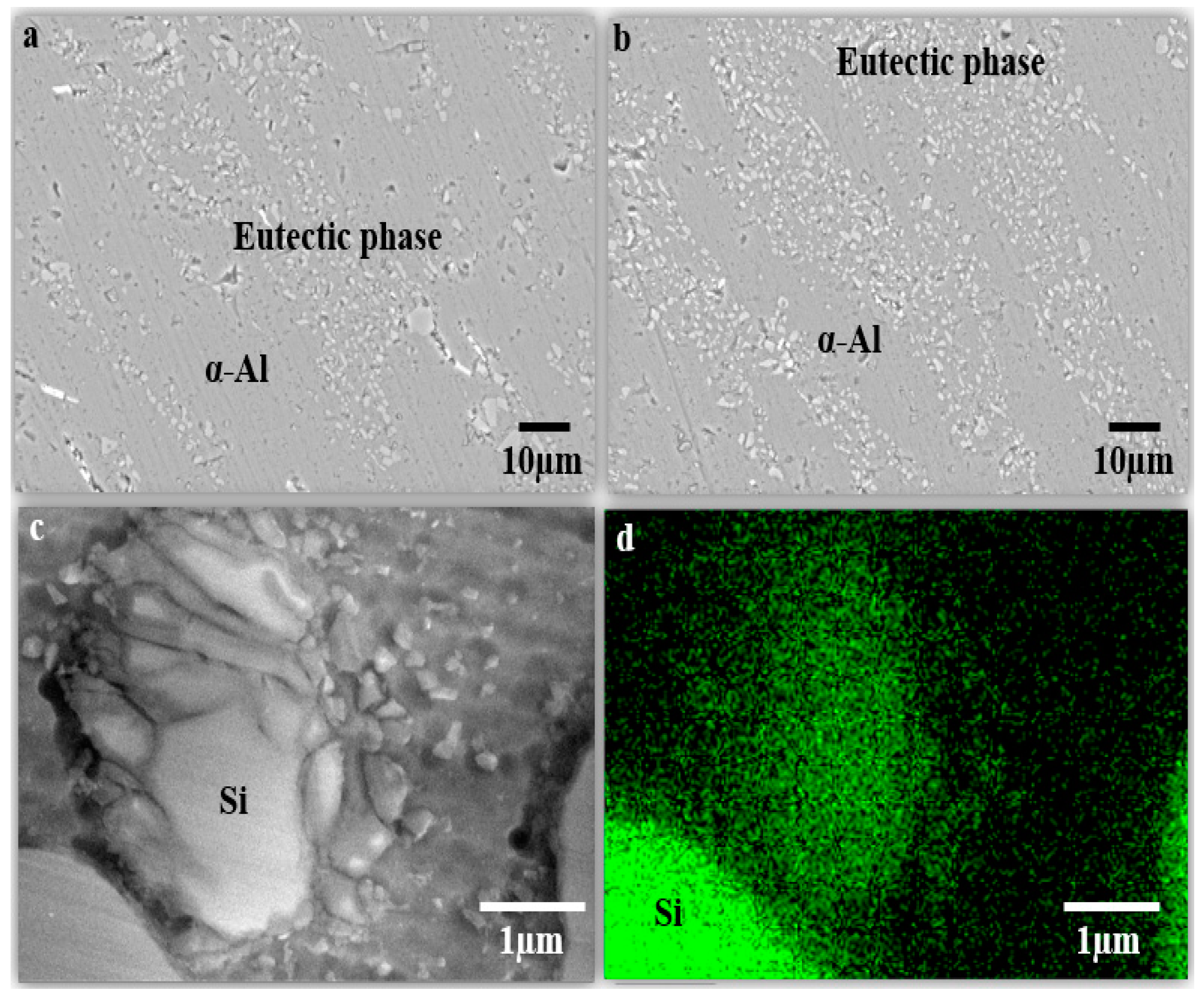
| Samples | Si Size (µm) | Grain Size (µm) |
|---|---|---|
| As-cast | 4.22 | 170.51 |
| ECAPed as-cast-T6, 4 passes | 0.761 | 40.40 |
| Cooling slope | 3.01 | 53.55 |
| ECAPed cooling slope-T6, 4 passes | 0.74 | 29.34 |
| ECAPed cooling slope-T6, 6 passes | 0.71 | 23.12 |
| Samples | Ecorr (V) | Icorr (A/cm2) | Rp (Ω·m2) | βc (V·dec−1) | βa (V·dec−1) | CR (mmy−1) |
|---|---|---|---|---|---|---|
| As-cast (A-C) | −0.698 | 3.894 × 10−6 | 5.212 × 103 | 0.642 | 0.0504 | 0.0424 |
| A-C-T6 | −0.713 | 8.432 × 10−7 | 2.035 × 104 | 0.443 | 0.0499 | 0.0160 |
| A-C-T6, 4 passes | −0.751 | 1.369 × 10−7 | 9.147 × 104 | 0.268 | 0.0286 | 0.0015 |
| Cooling slope (C.S) | −0.769 | 1.790 × 10−6 | 9.690 × 103 | 0.333 | 0.0480 | 0.0195 |
| C.S-T6 | −0.702 | 7.258 × 10−7 | 2.180 × 104 | 0.321 | 0.0474 | 0.0079 |
| C.S-T6, 4 passes | −0.717 | 1.285 × 10−7 | 8.516 × 104 | 0.280 | 0.047 | 0.0014 |
| C.S-T6, 6 passes | −0.709 | 1.145 × 10−7 | 9.525 × 104 | 0.259 | 0.0466 | 0.00124 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gebril, M.A.; Omar, M.Z.; Mohamed, I.F.; Othman, N.K. Microstructural Evaluation and Corrosion Resistance of Semisolid Cast A356 Alloy Processed by Equal Channel Angular Pressing. Metals 2019, 9, 303. https://doi.org/10.3390/met9030303
Gebril MA, Omar MZ, Mohamed IF, Othman NK. Microstructural Evaluation and Corrosion Resistance of Semisolid Cast A356 Alloy Processed by Equal Channel Angular Pressing. Metals. 2019; 9(3):303. https://doi.org/10.3390/met9030303
Chicago/Turabian StyleGebril, Mohamed Abdelgawad, Mohd Zaidi Omar, Intan Fadhlina Mohamed, and Norinsan Kamil Othman. 2019. "Microstructural Evaluation and Corrosion Resistance of Semisolid Cast A356 Alloy Processed by Equal Channel Angular Pressing" Metals 9, no. 3: 303. https://doi.org/10.3390/met9030303
APA StyleGebril, M. A., Omar, M. Z., Mohamed, I. F., & Othman, N. K. (2019). Microstructural Evaluation and Corrosion Resistance of Semisolid Cast A356 Alloy Processed by Equal Channel Angular Pressing. Metals, 9(3), 303. https://doi.org/10.3390/met9030303





