Effect of Saliva and Mucin-Based Saliva Substitutes on Fretting Processes of 316 Austenitic Stainless Steel
Abstract
1. Introduction
2. Materials and Methods
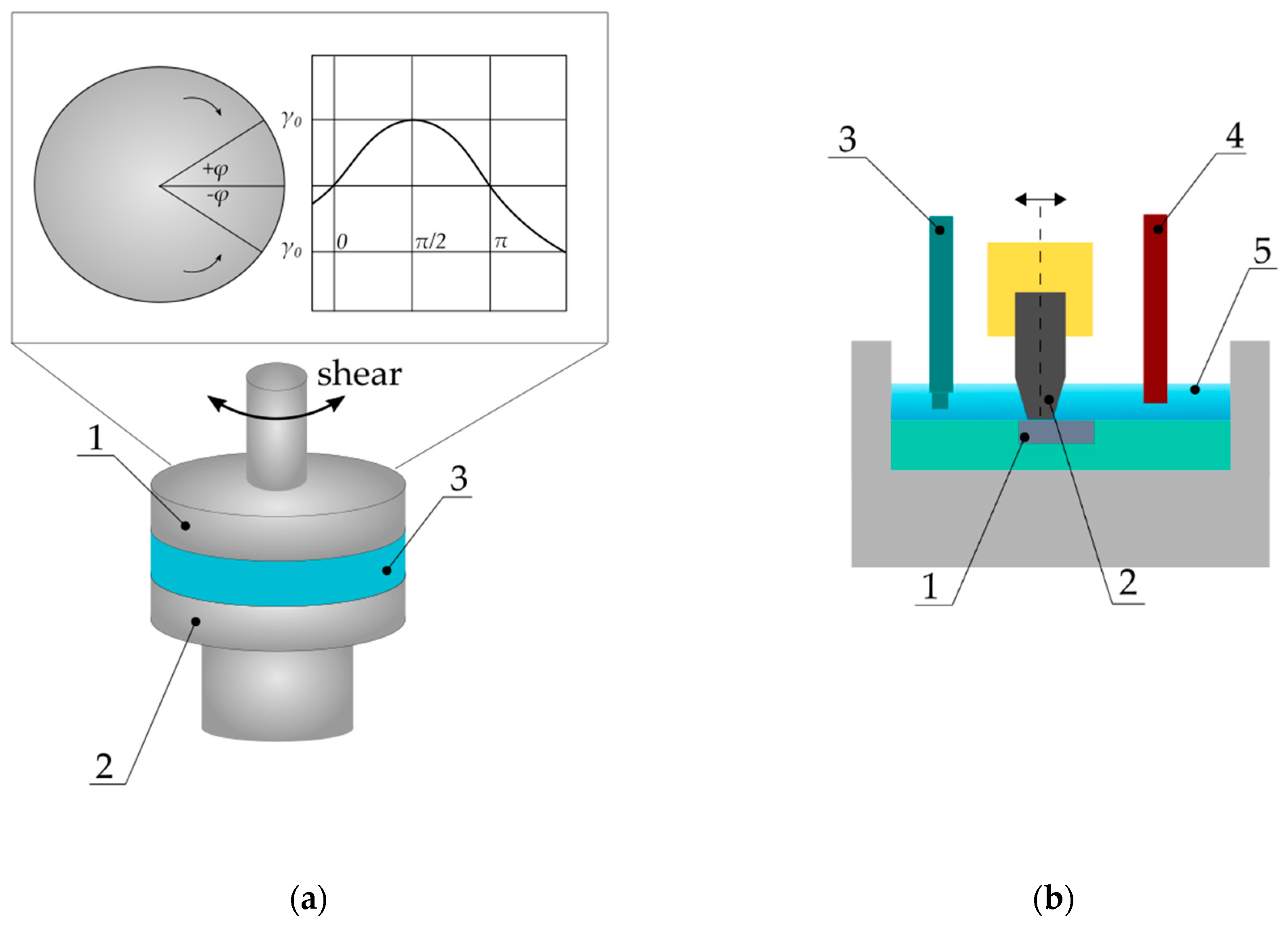
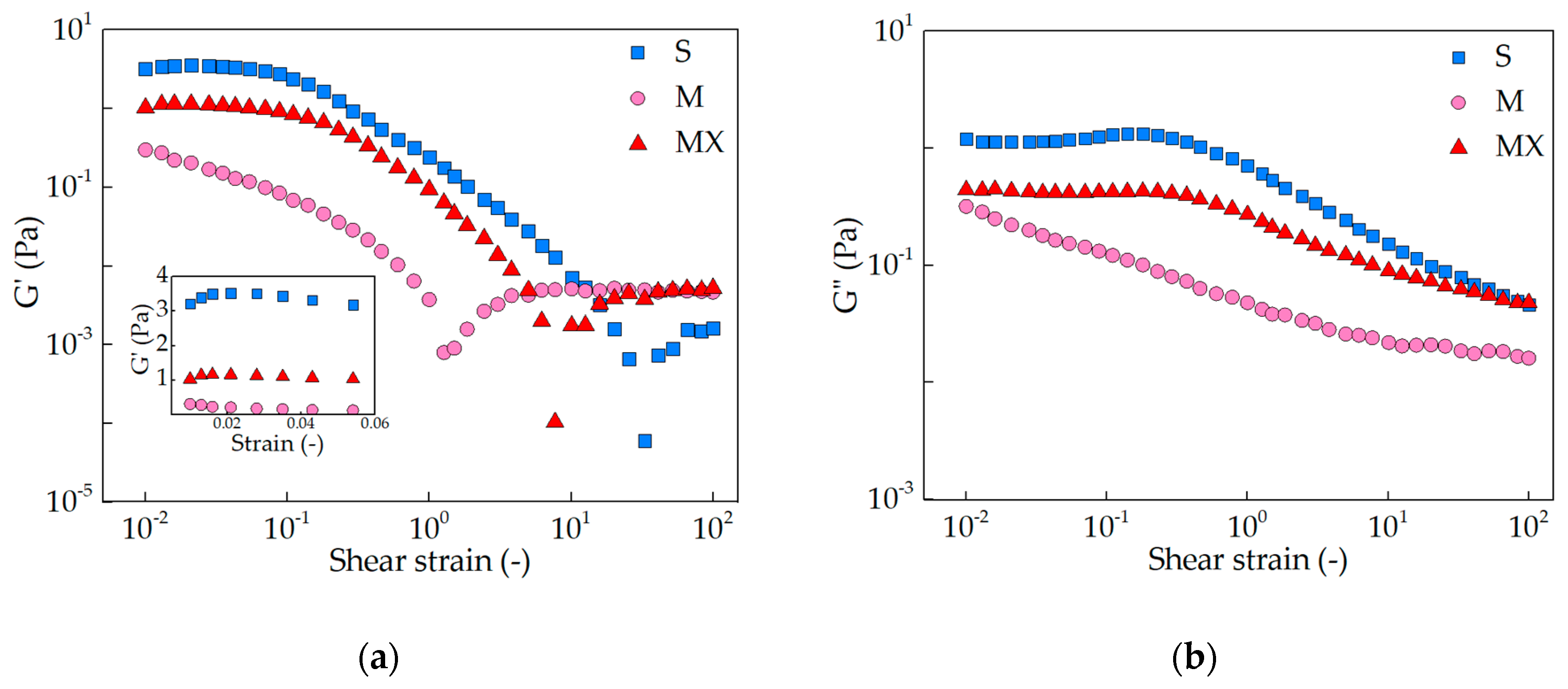
2.1. Viscoelasticity-Oscillatory Rheology Tests
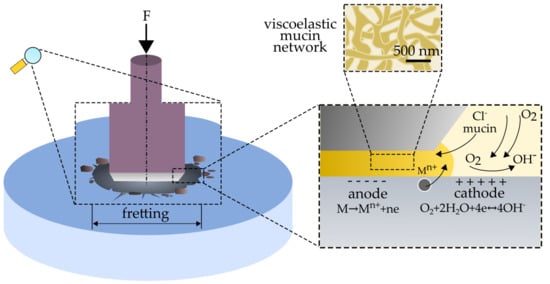
2.2. Fretting and Fretting-Corrosion Tests
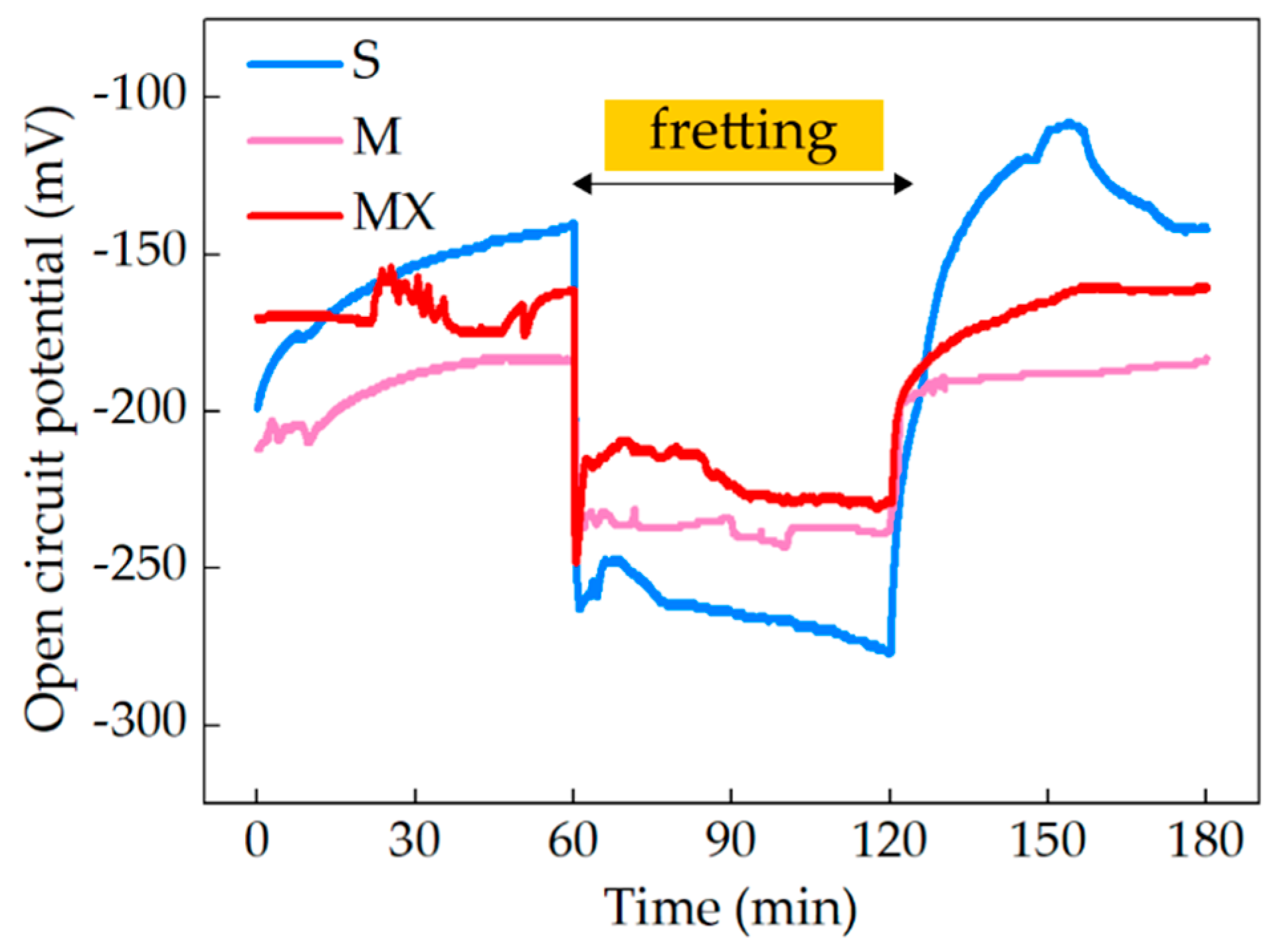
2.2.1. Open Circuit Potential (EOCP)
2.2.2. Potentiodynamic Test
3. Results and Discussion
3.1. Viscoelasticity
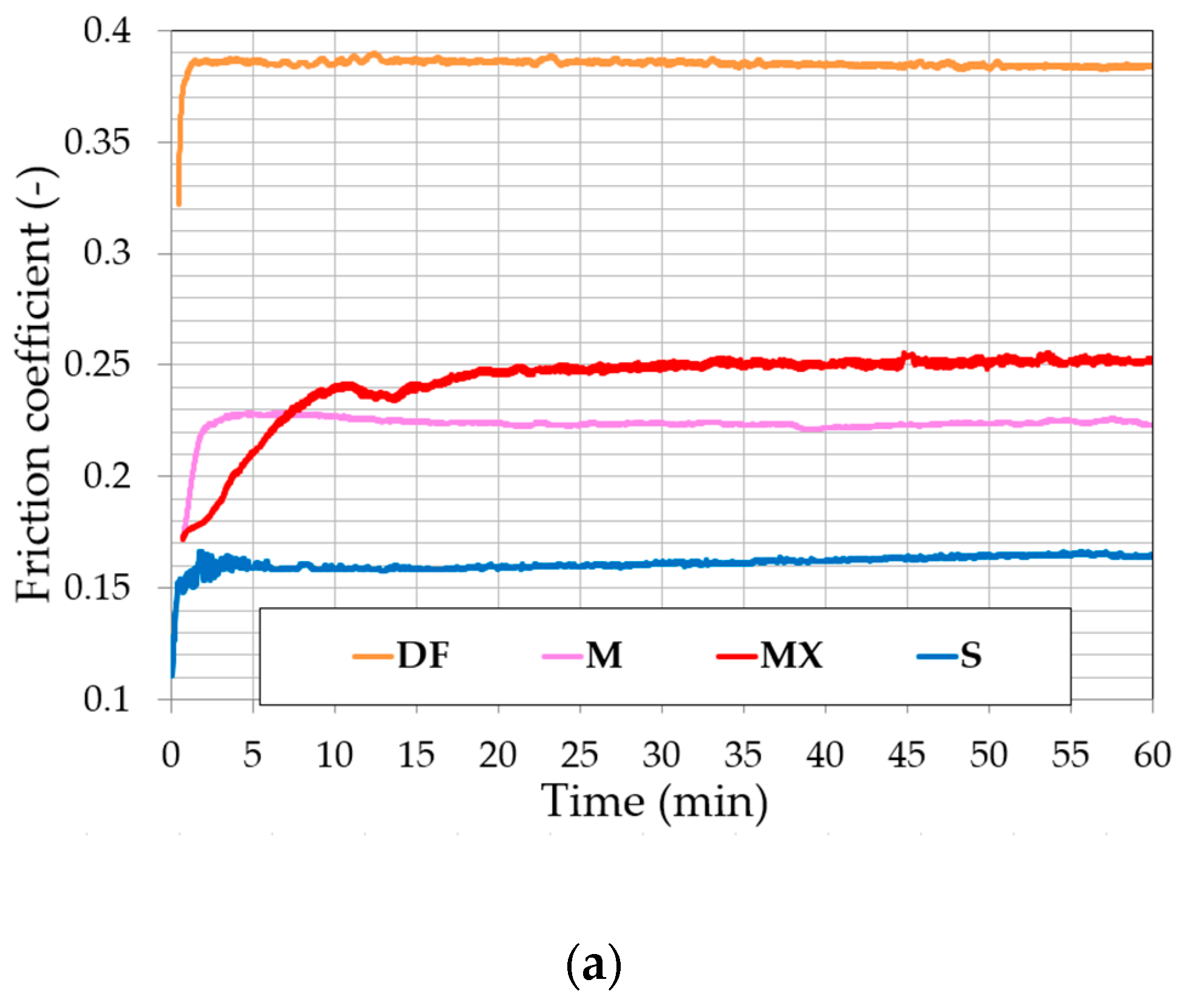
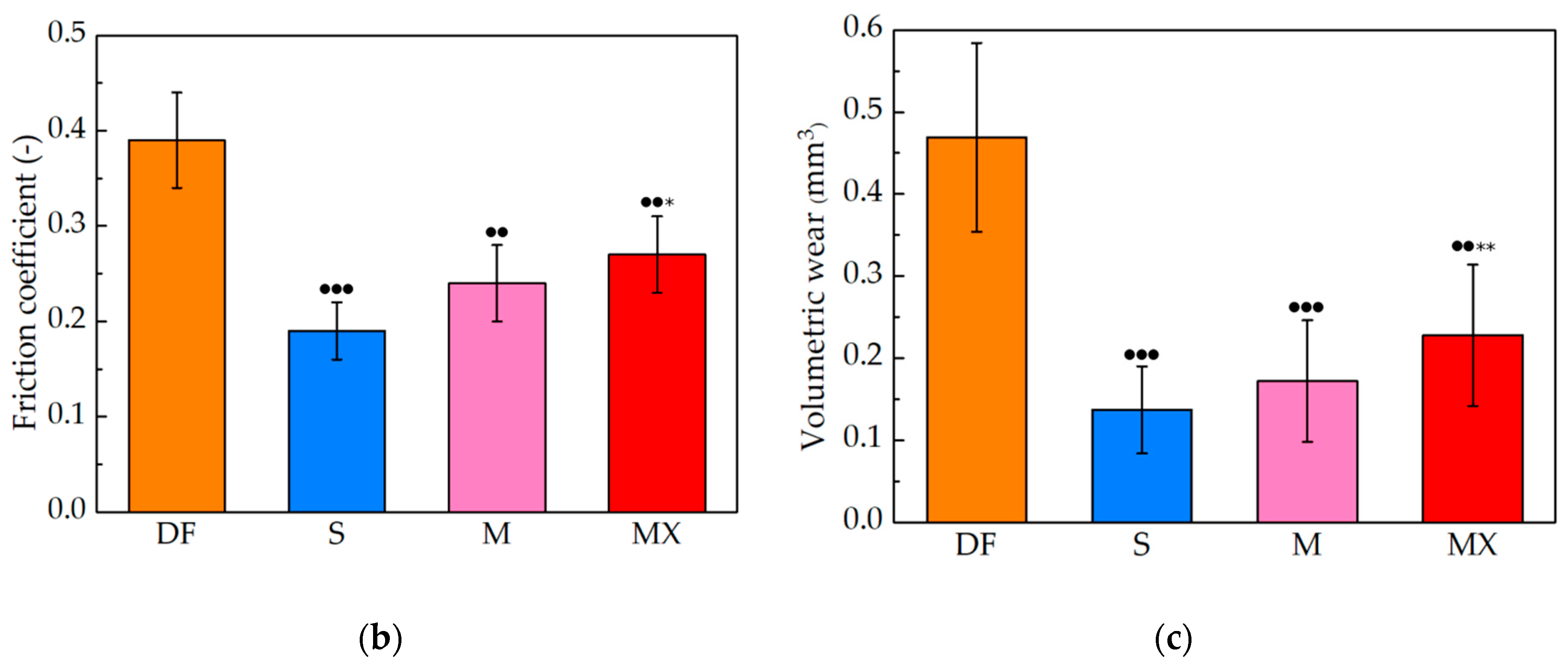
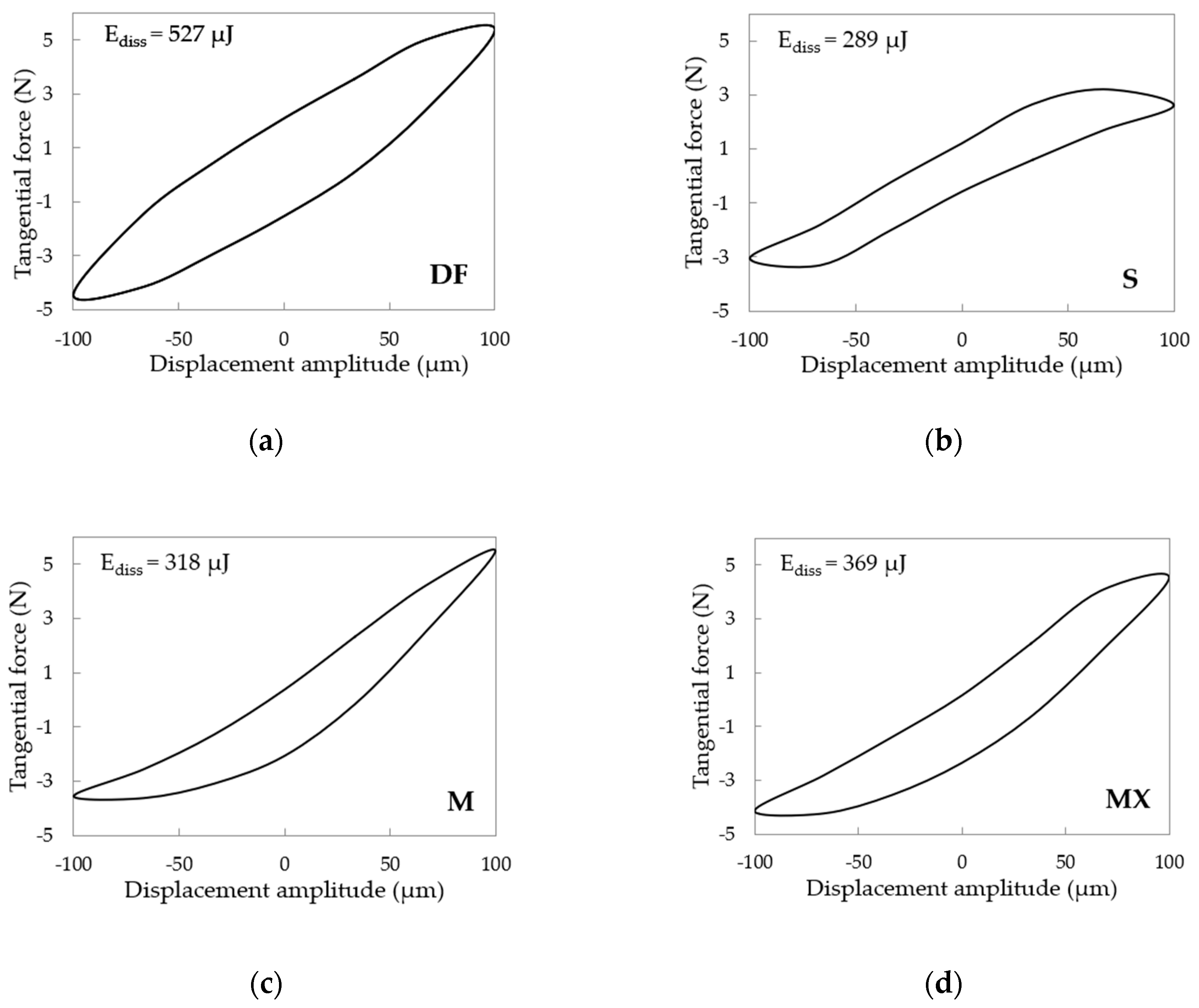
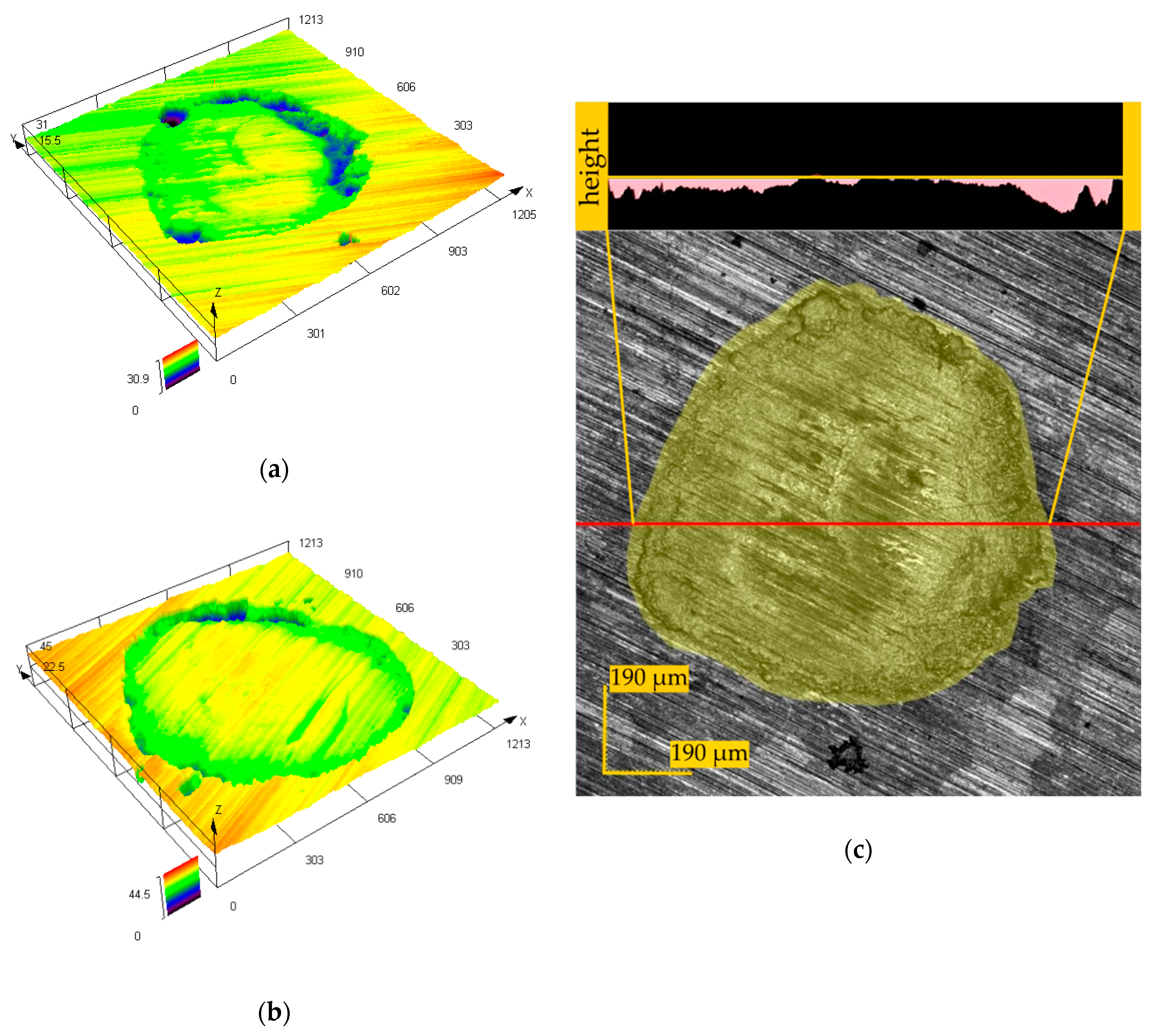
3.2. Fretting
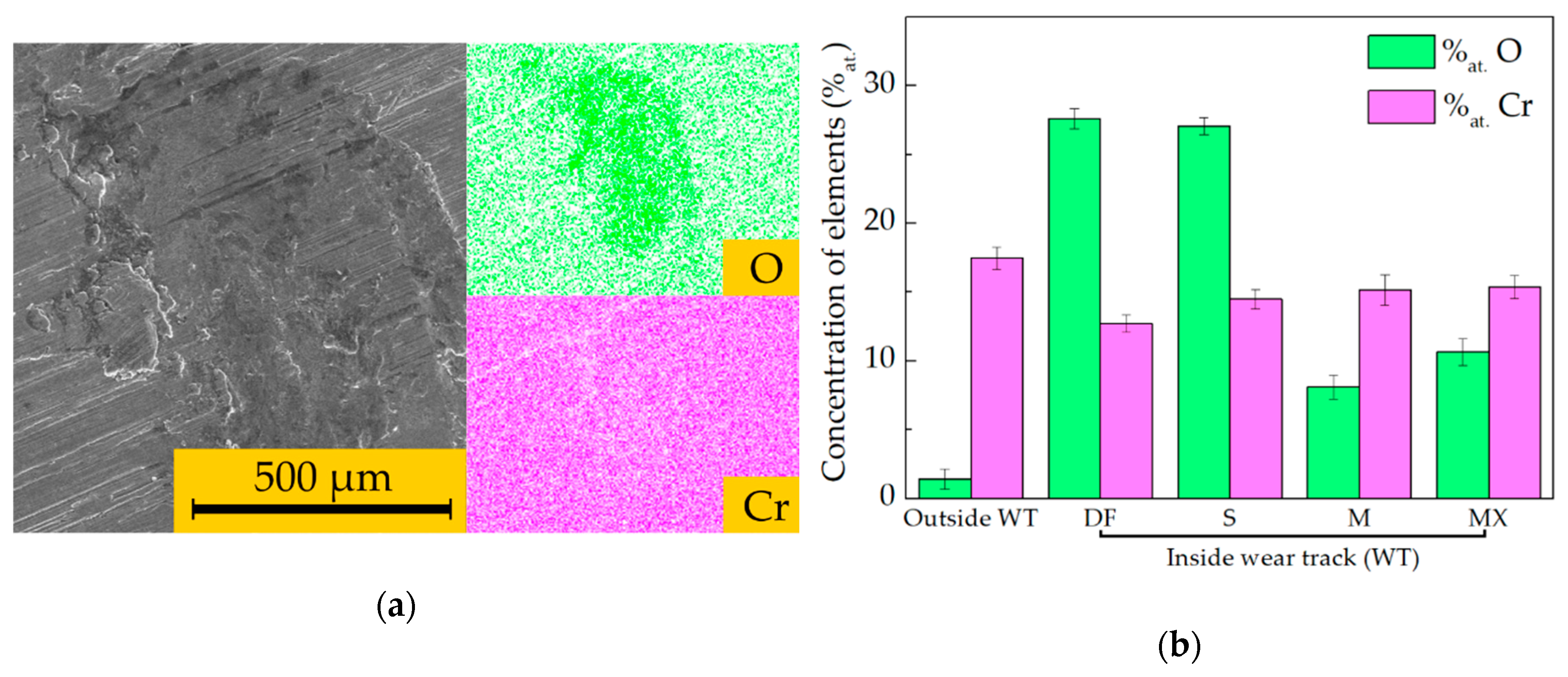
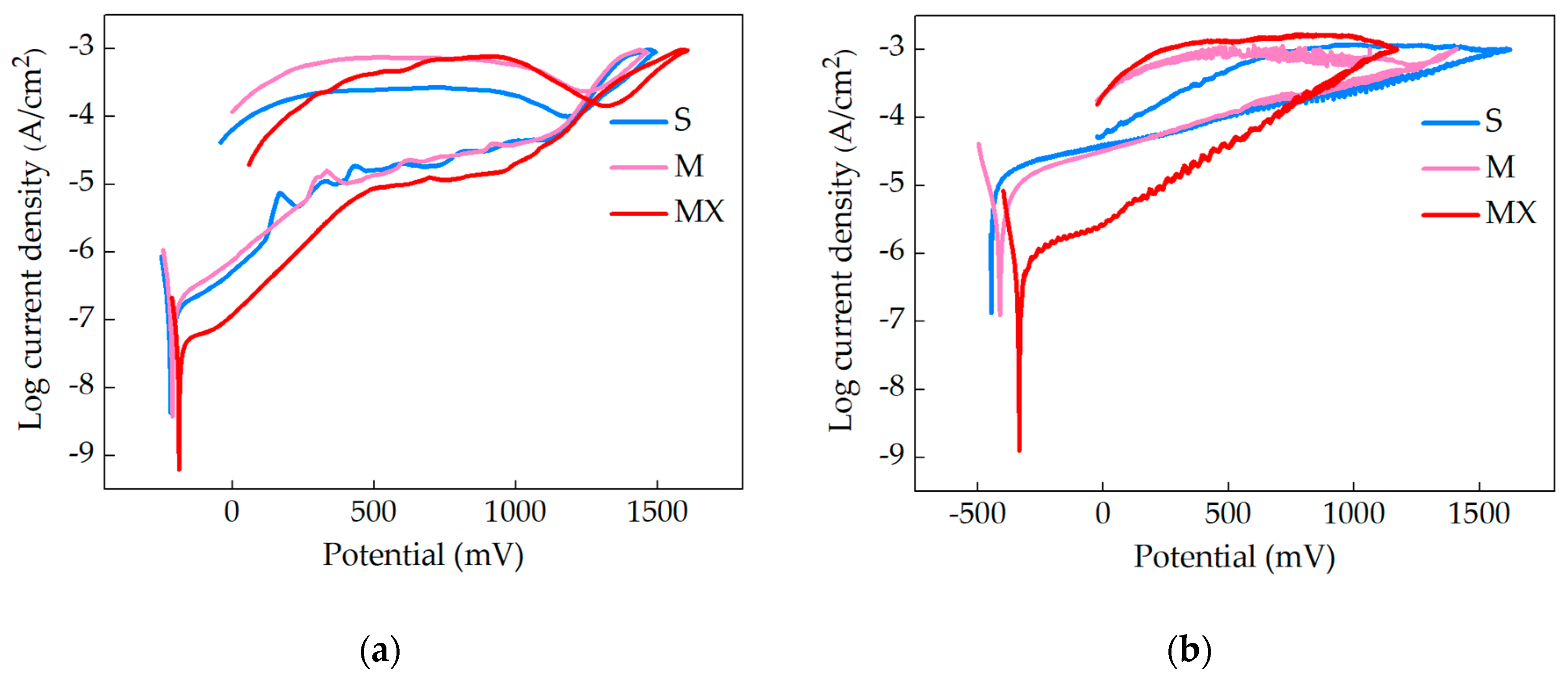
3.3. Fretting-Corrosion
4. Conclusions
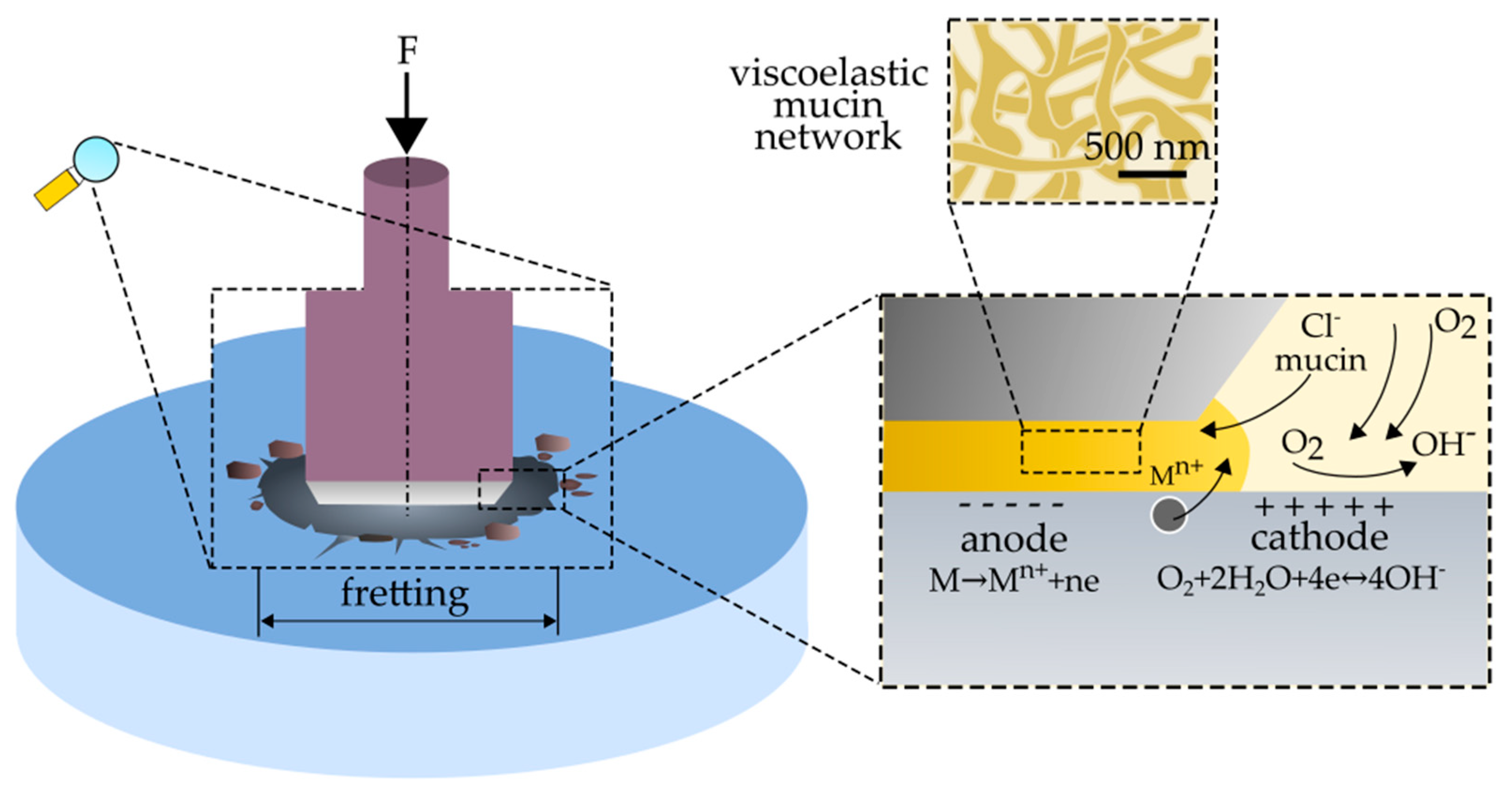
- fretting processes lead to an intensification of the corrosive wear of austenitic steel. The mechanism of corrosion failure in fretting conditions is similar to crevice corrosion and is based on gradients of oxygen concentration at the friction location.
- the presence of organic components (mucin, xanthan gum) in the preparation of artificial saliva accelerates and improves the quality of the adsorptive layer formed on the steel surface. The formed layer, in static conditions, protects the material against corrosion.
- the xanthan gum in the saliva preparation can lead to an intensification of the secondary wear of steel, which is related to the difficult removal of wear products from the friction area.
- the proposed compositions of artificial saliva have favorable rheological, tribological, and electrochemical properties, and can be used by people that have problems with the secretion of natural saliva.
Author Contributions
Funding
Conflicts of Interest
References
- Park, J.; Lakes, R.S. Biomaterials: An introduction, 3rd ed.; Springer: New York, NY, USA, 2007. [Google Scholar]
- Chen, Q.Z.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Dabrowski, J.R.; Klekotka, M.; Sidun, J. Fretting and fretting corrosion of 316L implantation steel in oral cavity environment. Maint. Reliab. 2014, 16, 441–446. [Google Scholar]
- Mystkowska, J.; Ferreira, J.A.; Leszczyńska, K.; Chmielewska, S.; Dąbrowski, J.R.; Wieciński, P.; Kurzydłowski, K.J. Biocorrosion of 316LV steel used in oral cavity due to Desulfotomaculum nigrificans bacteria. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Mystkowska, J.; Niemirowicz-Laskowska, K.; Łysik, D.; Tokajuk, G.; Dąbrowski, J.R.; Bucki, R. The Role of Oral Cavity Biofilm on Metallic Biomaterial Surface Destruction–Corrosion and Friction Aspects. Int. J. Mol. Sci. 2018, 19, 743. [Google Scholar] [CrossRef] [PubMed]
- Andrysewicz, E.; Mystkowska, J.; Dąbrowski, J.R.; Olchowik, R. Influence of self-made saliva substitutes on tribological characteristics of human enamel. Acta Bioeng. Biomech. 2014, 16, 67–74. [Google Scholar] [PubMed]
- Duisabeau, L.; Combrade, P.; Forest, B. Environmental effect on fretting of metallic materials for orthopaedic implants. Wear 2004, 256, 805–816. [Google Scholar] [CrossRef]
- Geringer, J.; Forest, B.; Combrade, B. Fretting-corrosion of materials used as orthopedic implants. Wear 2005, 259, 943–951. [Google Scholar] [CrossRef]
- Vadiraj, A.; Kamaraj, M. Effect of surface treatments on fretting fatigue damage of biomedical titanium alloys. Tribol. Int. 2007, 40, 82–88. [Google Scholar] [CrossRef]
- Sivakumar, B.; Kumar, S.; Sankara Narayanan, T.S.N. Fretting corrosion behaviour of Ti–6Al–4V alloy in artificial saliva containing varying concentrations of fluoride ions. Wear 2011, 270, 317–324. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Barbosa, S.L.; Ariza, E.; Celis, J.P.; Rocha, L.A. Simultaneous degradation by corrosion and wear of titanium in artificial saliva containing fluorides. Wear 2012, 292–293, 82–88. [Google Scholar] [CrossRef]
- Močnik, P.; Kosec, T.; Kovač, J.; Bizjak, M. The effect of pH, fluoride and tribocorrosion on the surface properties of dental wires. Mater. Sci. Eng. C 2017, 78, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Andrysewicz, E.; Mystkowska, J.; Kolmas, J.; Jalbrzykowski, M.; Olchowik, R.; Dabrowski, J.R. Influence of artificial saliva compositions on tribological characteristics of Ti-6Al-4V implant alloy. Acta Bioeng. Biomech. 2012, 14, 71–79. [Google Scholar] [PubMed]
- Ranjitkar, S.; Kaidonis, J.A.; Towsend, G.C.; Richards, L.C. An in vitro assessment of the effect of load and pH on wear between opposing enamel and dentin surfaces. Arch. Oral Biol. 2008, 53, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Mystkowska, J.; Car, H.; Dąbrowski, J.R.; Romanowska, J.; Klekotka, M.; Milewska, A.J. Artificial mucin-based saliva preparations—Physicochemical and tribological properties. Oral Health Prev. Dent. 2018, 16, 183–193. [Google Scholar]
- Yakubov, G.E.; Macakova, L.; Wilson, S.; Windust, J.H.C.; Stokes, J.R. Aqueous lubrication by fractionated salivary proteins: Synergistic interaction of mucin polymer brush with low molecular weight macromolecules. Tribol. Int. 2015, 89, 34–45. [Google Scholar] [CrossRef]
- Glantz, P.O. Interfacial phenomena in the oral cavity. Colloids Surf. A Physicochem. Eng. Asp. 1997, 123–124, 657–670. [Google Scholar] [CrossRef]
- Bansil, R.; Turner, B.S. Mucin structure, aggregation, physiological functions and biomedical applications. Curr. Opin. Colloid Interface Sci. 2006, 11, 164–170. [Google Scholar] [CrossRef]
- Hannig, C.; Hannig, M.; Kensche, A.; Carpenter, G. The mucosal pellicle? An underestimated factor in oral physiology. Arch. Oral Biol. 2017, 80, 144–152. [Google Scholar] [CrossRef]
- Mystkowska, J.; Karalus, W.; Sidorenko, J.; Dąbrowski, J.R.; Kalska-Szostko, B. Biotribological properties of dentures lubricated with artificial saliva. J. Frict. Wear 2016, 37, 544–551. [Google Scholar] [CrossRef]
- Saleh, J.; Figueiredo, M.A.; Cherubini, K.; Salum, F.G. Salivary hypofunction: An update on aetiology, diagnosis and therapeutics. Arch. Oral Biol. 2015, 60, 242–255. [Google Scholar] [CrossRef]
- Hahnel, S.; Rosentritt, M.; Handel, G.; Burgers, R. Influence of saliva substitute films on initial Streptococcus mutans adhesion to enamel and dental plaque. J. Dent. 2008, 36, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Mitchell, J.R.; Gaddipati, S.R.; Hill, S.E.; Wolf, B. Shear rheology and filament stretching behavior of xanthan gum and carboxymethyl cellulose solution in presence of saliva. Food Hydrocoll. 2014, 40, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.H.; Salehi, M.; Saatchi, A.; Mortazavi, V.; Moosavi, S.B. In vitro corrosion behavior of bioceramic, metallic, and bioceramic-metallic coated stainless steel dental implants. Dent. Mater. 2003, 19, 188–198. [Google Scholar] [CrossRef]
- Sharifnabi, A.; Fathi, M.H.; Eftekhari Yekta, B.; Hossainalipour, M. The structural and bio-corrosion barrier performance of Mg-substituted fluorapatite coating on 316l stainless steel human body implant. Appl. Surf. Sci. 2014, 288, 331–340. [Google Scholar] [CrossRef]
- Harvey, N.M.; Yakubov, G.E.; Stokes, J.R.; Klein, J. Lubrication and load-bearing properties of human salivary pellicles adsorbed ex vivo on molecularly smooth substrata. Biofouling 2012, 28, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Celli, J.; Gregor, B.; Turner, B.; Afdhal, N.H.; Bansil, R.; Erramilli, S. Viscoelastic properties and dynamics of porcine gastric mucin. Biomacromolecules 2005, 6, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Heintze, S.D. How to qualify and validate wear simulation devices and methods. Dent. Mater. 2006, 22, 712–734. [Google Scholar] [CrossRef]
- Sidun, J.; Dąbrowski, J.R. The method of fretting wear assessment with the application of 3D laser measuring microscope. In Advances in Intelligent Systems and Computing; Gzik, M., Tkacz, E., Paszenda, Z., Pietka, E., Eds.; Springer: Switzerland, Poland, 2018; pp. 369–377, 2194–5357. [Google Scholar]
- Nosonovsky, M.; Bhushan, B. Multiscale friction mechanisms and hierarchical surfaces in nano- and bio-tribology. Mater. Sci. Eng. R Rep. 2007, 58, 162–193. [Google Scholar] [CrossRef]
- Bansil, R.; Stanley, E.; LaMont, J.T. Mucin biophysics. Annu. Rev. Physiol. 1995, 57, 635–657. [Google Scholar] [CrossRef]
- Chen, D.T.N.; Wen, Q.; Janmey, P.A.; Crocker, J.; Yodh, A.G. Rheology of Soft Materials. Annu. Rev. Condens. Matter Phys. 2010, 1, 301–322. [Google Scholar] [CrossRef]
- Wagner, N.J.; Brady, J.F. Shear thickening in colloidal dispersions. Phys. Today 2009, 62, 27–32. [Google Scholar] [CrossRef]
- Fouvry, S.; Kapsa, P.; Vincent, L. Quantification of fretting damage. Wear 1996, 200, 186–205. [Google Scholar] [CrossRef]
- Fouvry, S.; Kapsa, P. An energy description of hard coating wear mechanisms. Surf. Coat. Technol. 2001, 138, 141–148. [Google Scholar] [CrossRef]
- Mystkowska, J.; Jałbrzykowski, M.; Dąbrowski, J.R. Tribological properties of selected self-made solutions of synthetic saliva. Solid State Phenom. 2013, 199, 567–572. [Google Scholar] [CrossRef]
- Zhu, M.H.; Zhou, Z.R. On the mechanisms of various fretting wear modes. Tribol. Int. 2011, 44, 1378–1388. [Google Scholar] [CrossRef]
- Zhang, B.; Cai, Z.; Gan, X.; Zhu, M.; Yu, H. Dual motion fretting wear behaviors of titanium and its alloy in artificial saliva. Trans. Nonferrous Met. Soc. China 2014, 24, 100–107. [Google Scholar] [CrossRef]
- Licausi, M.P.; Munoz, A.I.; Borras, V.A. Influence of the fabrication process and fluoride content on the tribocorrosion behaviour of Ti6Al4V biomedical alloy in artificial saliva. J. Mech. Behav. Biomed. Mater. 2013, 20, 137–148. [Google Scholar] [CrossRef]
- Revathi, A.; Borrás, A.D.; Muñoz, A.I.; Richard, C.; Manivasagam, G. Degradation mechanisms and future challenges of titanium and its alloys for dental implant applications in oral environment. Mater. Sci. Eng. C 2017, 76, 1354–1368. [Google Scholar] [CrossRef]
- Vieira, A.C.; Ribeiro, A.R.; Rocha, L.A.; Celis, J.P. Influence of pH and corrosion inhibitors on the tribocorrosion of titanium in artificial saliva. Wear 2006, 261, 994–1001. [Google Scholar] [CrossRef]
- Mroczkowski, M.L.; Hertzler, J.S.; Humphrey, S.M.; Johnson, T.; Blanchard, C.R. Effect of impact assembly on the fretting corrosion of modular hip tapers. J. Orthop. Res. 2006, 24, 271–279. [Google Scholar] [CrossRef]
- Khan, M.A.; Williams, R.L.; Williams, D.F. The corrosion behavior of Ti-6Al-4V, Ti-6Al-7Nb and Ti-13Nb-13Zr in protein solutions. Biomaterials 1999, 20, 631–637. [Google Scholar] [CrossRef]
- Buciumeanu, M.; Bagheri, A.; Souza, J.C.M.; Silva, F.S.; Henriques, B. Tribocorrosion behavior of hot pressed CoCrMo alloys in artificial saliva. Tribol. Int. 2016, 97, 423–430. [Google Scholar] [CrossRef]
- Holmes, D.; Sharifi, S.; Stack, M.M. Tribo-corrosion of steel in artificial saliva. Tribol. Int. 2014, 75, 80–86. [Google Scholar] [CrossRef]
- Schipper, R.G.; Silletti, E.; Vingerhoeds, M.H. Saliva as research material: Biochemical, physicochemical and practical aspects. Arch. Oral Biol. 2007, 52, 1114–1135. [Google Scholar] [CrossRef] [PubMed]
- Chiappin, S.; Antonelli, G.; Gatti, R.; De Palo, E.F. Saliva specimen: A new laboratory tool for diagnostic and basic investigation. Clin. Chim. Acta 2007, 383, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Lata, S.; Sharma, C.H.; Singh, A.K. Microbial corrosion by thermophilic bacteria. Cent. Eur. J. Eng. 2012, 2, 113–122. [Google Scholar]
- Pawlak, Z.; Urbaniak, W.; Oloyede, A. The relationship between friction and wettability in aqueous environment. Wear 2011, 271, 1745–1749. [Google Scholar] [CrossRef]
- Liu, S.Y.; Han, Y.; Yin, L.P.; Long, L.; Liu, R. Toxicology studies of a superparamagnetic iron oxide nanoparticles in vivo. Adv. Mat. Res. 2008, 47, 1097–1100. [Google Scholar] [CrossRef]


| Fe | Cr | Ni | Mo | Mn | Other |
|---|---|---|---|---|---|
| 60.3 | 17.7 | 13.6 | 2.4 | 1.9 | 4.1 |
| Preparation | ΔEOCP (mV) |
|---|---|
| S | 125 ± 21 |
| M | 57 ± 7 |
| MX | 63 ± 11 |
| Preparation | Corrosion | Fretting-Corrosion | ||
|---|---|---|---|---|
| Ecor (mV) | Rp (kΩ·cm2) | Ecor (mV) | Rp (kΩ·cm2) | |
| S | −214 | 146.02 | −446 | 6.24 |
| M | −209 | 104.75 | −418 | 5.78 |
| MX | −189 | 345.93 | −340 | 35.94 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mystkowska, J.; Łysik, D.; Klekotka, M. Effect of Saliva and Mucin-Based Saliva Substitutes on Fretting Processes of 316 Austenitic Stainless Steel. Metals 2019, 9, 178. https://doi.org/10.3390/met9020178
Mystkowska J, Łysik D, Klekotka M. Effect of Saliva and Mucin-Based Saliva Substitutes on Fretting Processes of 316 Austenitic Stainless Steel. Metals. 2019; 9(2):178. https://doi.org/10.3390/met9020178
Chicago/Turabian StyleMystkowska, Joanna, Dawid Łysik, and Marcin Klekotka. 2019. "Effect of Saliva and Mucin-Based Saliva Substitutes on Fretting Processes of 316 Austenitic Stainless Steel" Metals 9, no. 2: 178. https://doi.org/10.3390/met9020178
APA StyleMystkowska, J., Łysik, D., & Klekotka, M. (2019). Effect of Saliva and Mucin-Based Saliva Substitutes on Fretting Processes of 316 Austenitic Stainless Steel. Metals, 9(2), 178. https://doi.org/10.3390/met9020178