MgO Refractory Doped with ZrO2 Nanoparticles: Influence of Cold Isostatic and Uniaxial Pressing and Sintering Temperature in the Physical and Chemical Properties
Abstract
1. Introduction
2. Experimental Procedure
3. Results and Discussion
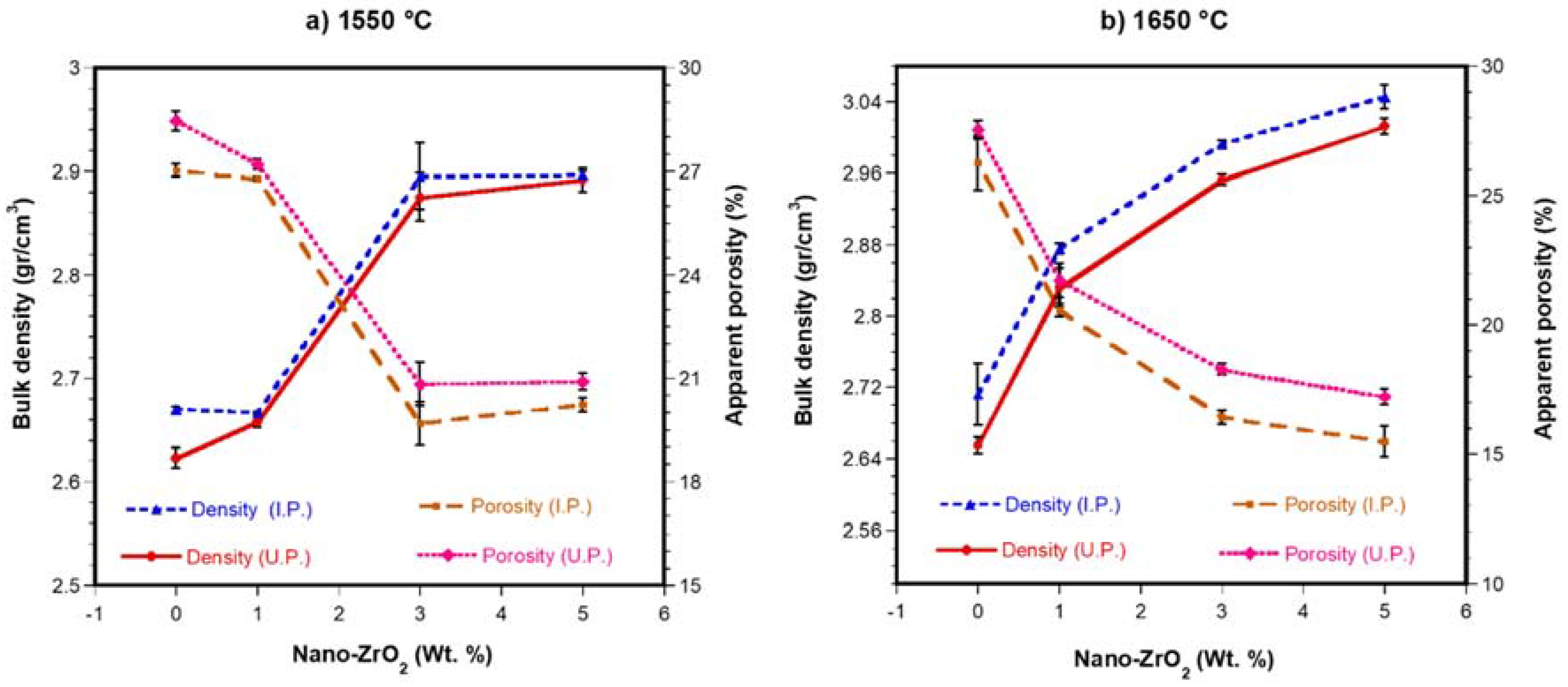
3.1. Densification
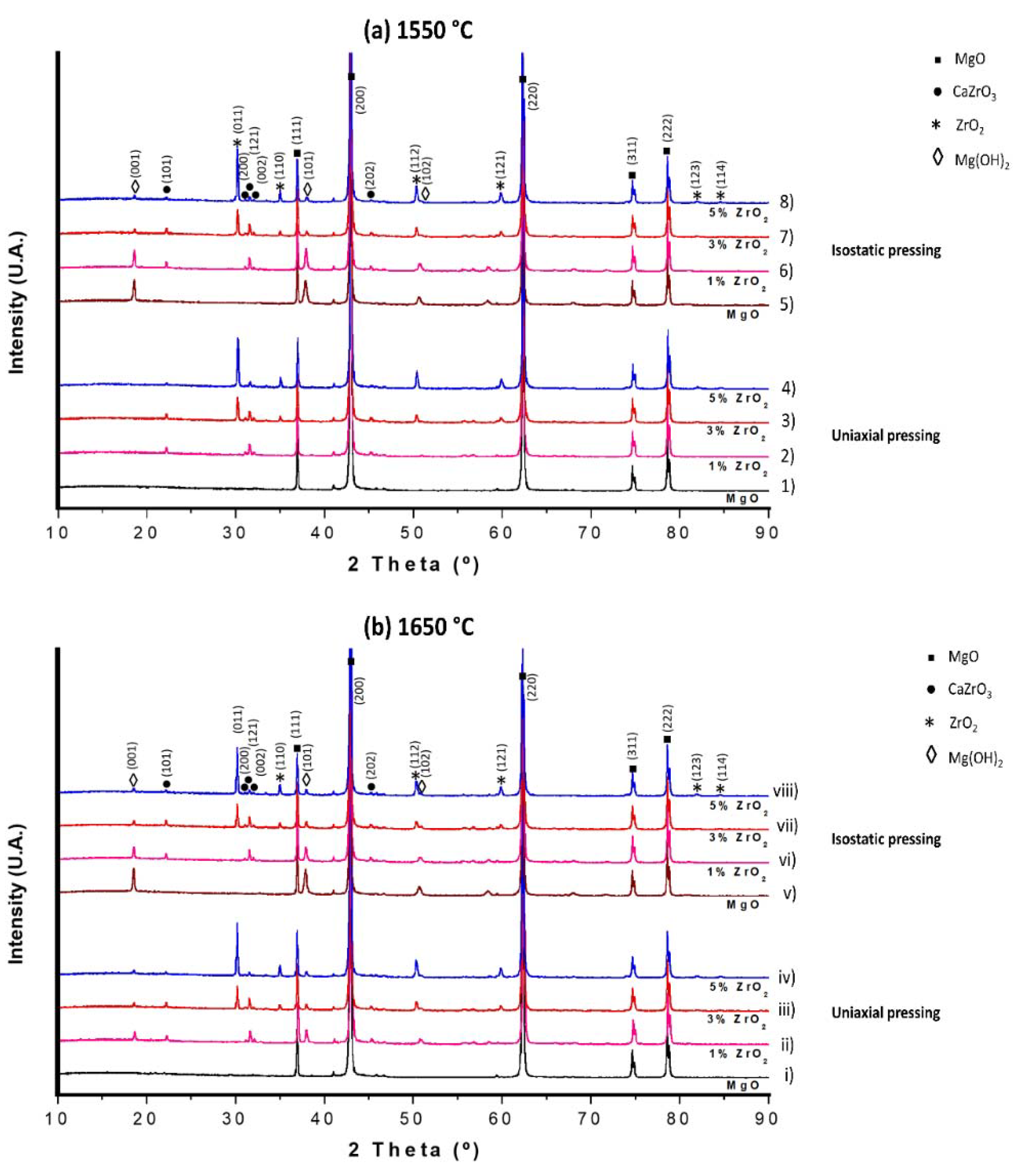
3.2. Phase Analysis (DRX)
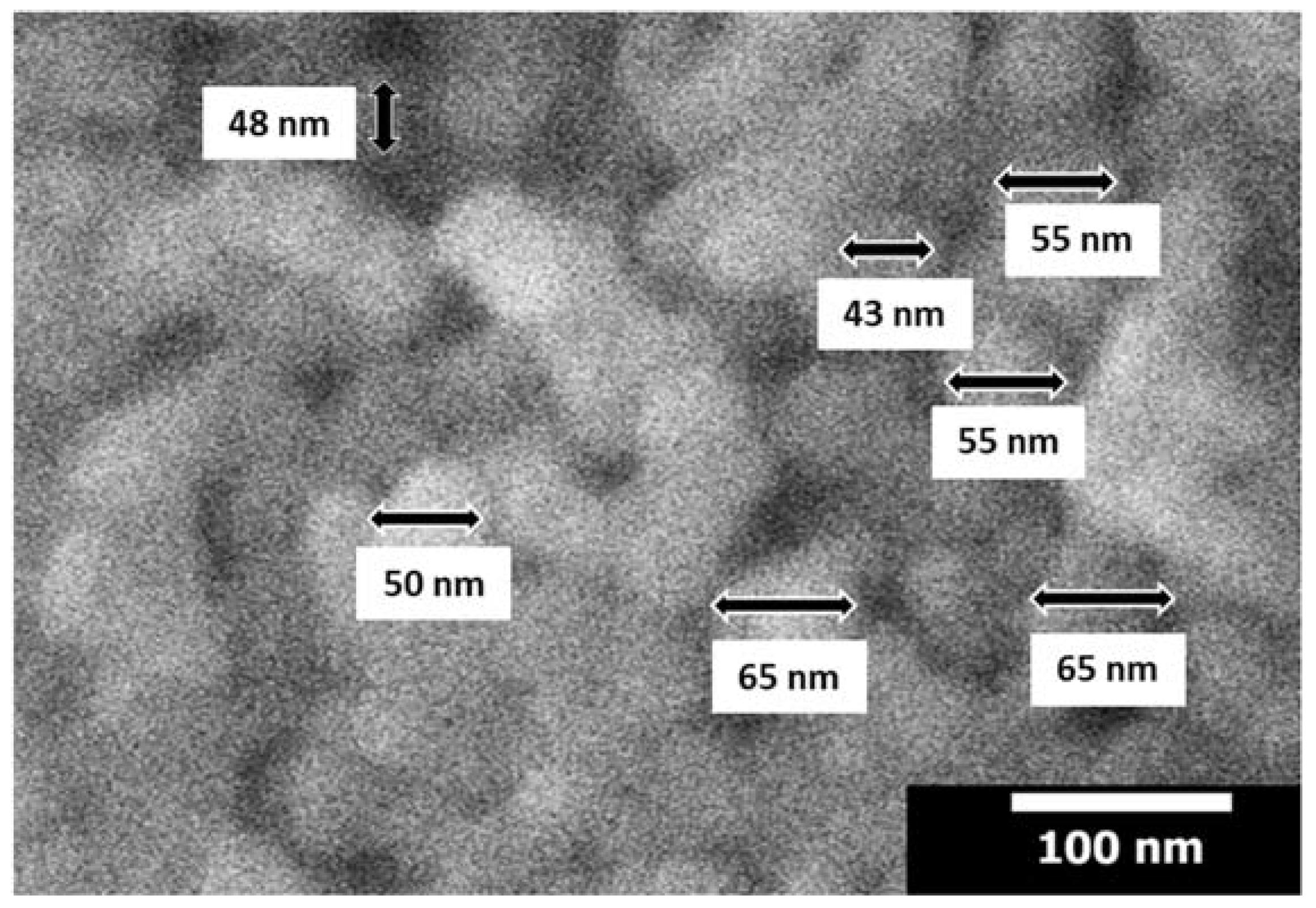
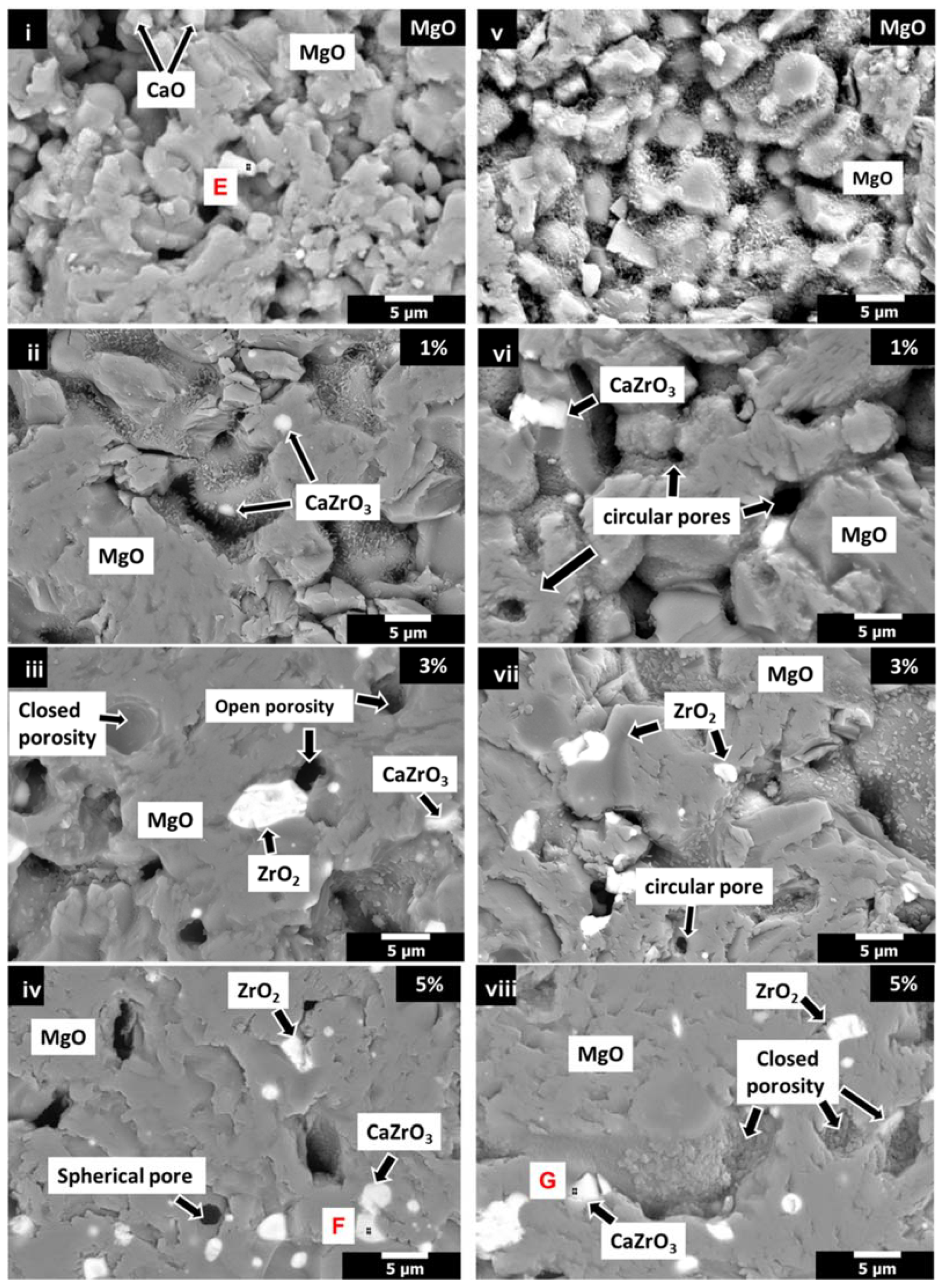
3.3. Microstructure Analysis (SEM-EDX)
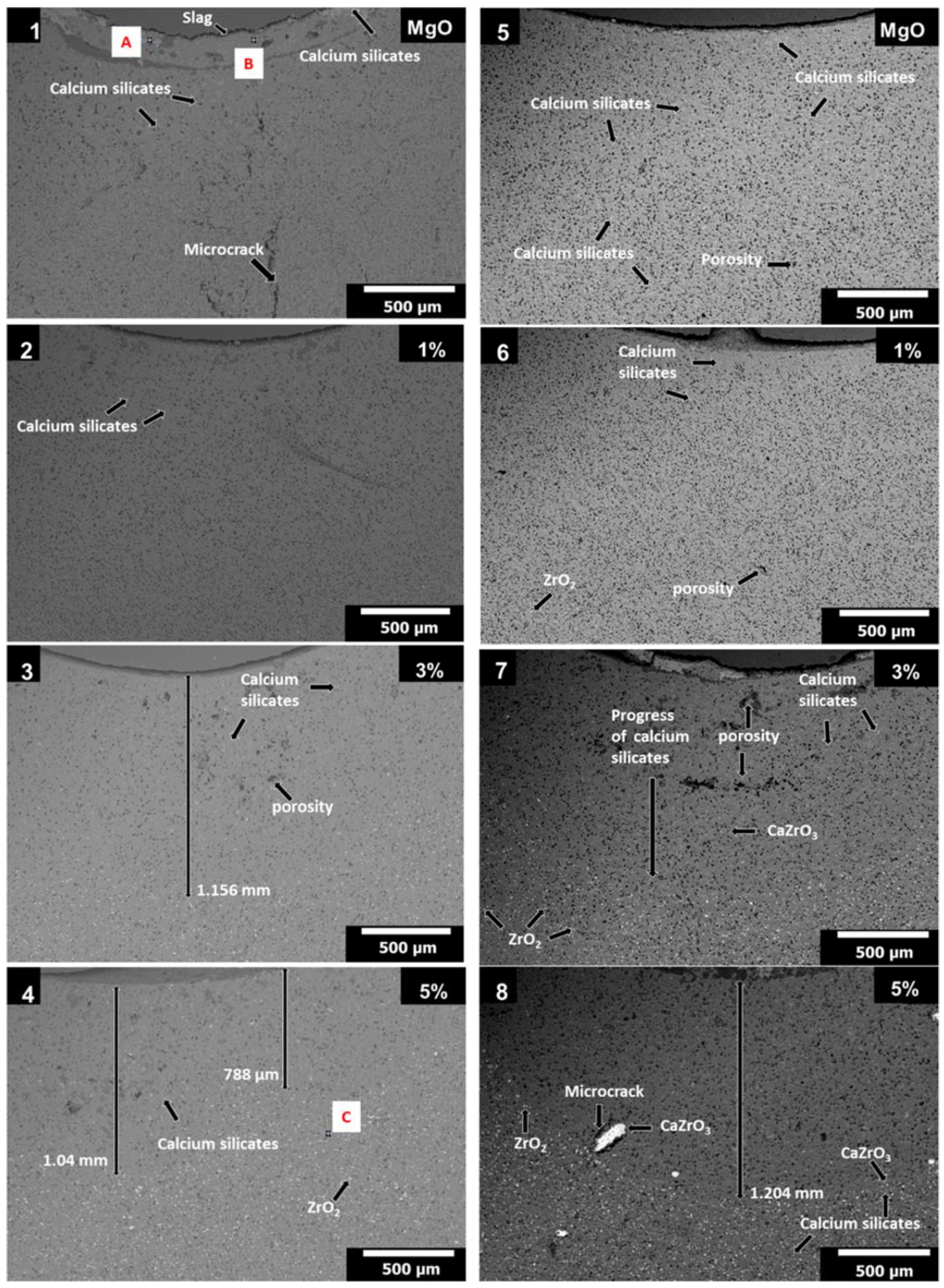
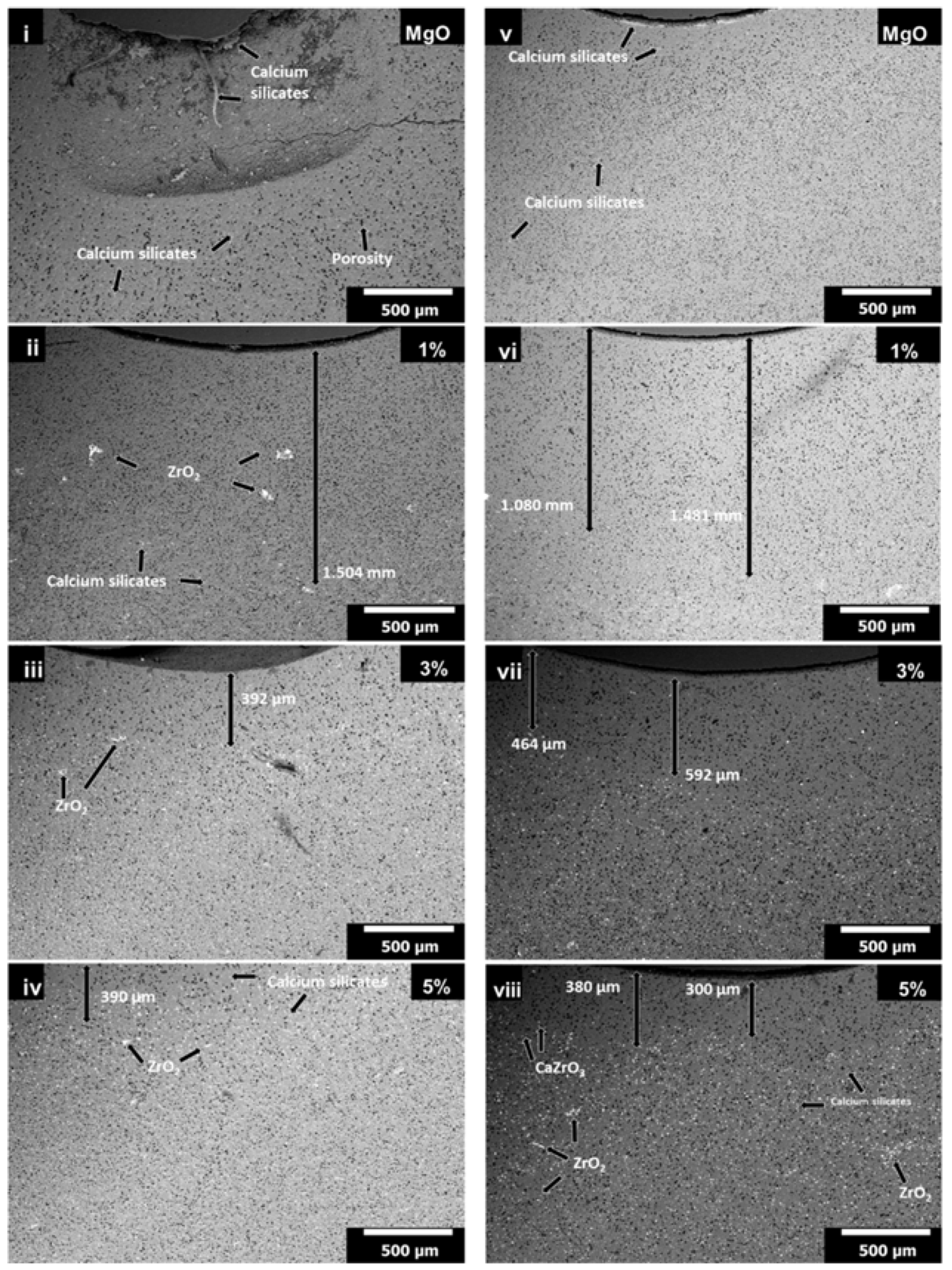
3.4. Slag Corrosion Test
- -
- Zone 1 corresponds to chemically attacked layer without CaZrO3 and ZrO2, with a distance of 347 µm. The slag (classified slightly basic) attacked and dissolved the particles of ZrO2 and CaZrO3 (chemically classified as acid and neutral, respectively), so in this area only infiltration path of the slag (in microns) was observed. Likewise, when the CaO of the slag attacked the nano-ZrO2, it helped to release the SiO2, from the same silicate crystalline structure. Both phases are more stable, so ZrO2 nanoparticles dissolved in the first micron’s depth, when the slag advanced.
- -
- Zone 2 corresponds to a less permeable layer where the advance of the blast furnace slag is blocked by the presence of CaZrO3 and ZrO2, with a thickness of 1.153 mm; the above is explained because, when CaO and SiO2 phases (in free form) found a great amount of ZrO2 particles enter into solid solution to form silicates of ZrO2 and MgO (i.e., these compounds enter inside the tetrahedric crystal structure formed by Si-O, since its anion has four negative charges and is compensated by the presence of alkaline earth metal ions mainly [46]). Likewise, the formation of CaZrO3, or simply ZrO2 and CaZrO3 particles surrounded by CaO and SiO2 (white dots within this zone) was also observed, making a refractory material with less permeability to infiltration of blast furnace slag.
- -
- Zone 3: which corresponds to the original refractory with particles of ZrO2, CaZrO3, and MgO. This zone started from an average distance of 1.5 mm with respect to the surface of contact with the slag. The points marked as A, B, and C correspond to the EDX analysis of each zone, whose results are found at the bottom of the same figure.
4. Conclusions
- -
- 4 to 7% reduction in volume was obtained when the specimen was obtained by IP, compared by UP When the samples were sintered (at 1550 °C and 1650 °C), density values were almost similar using UP and IP technique, being the values of the density slightly greater when using IP.
- -
- 5 wt. % ZrO2 nanoparticles are the optimal content of the dopant phase. The optimal process conditions require the obtaining of green compacts by cold isostatic pressing and, later, sintering the green compact at 1650 °C. This leads to well-sintered samples with low porosity and the highest density (3.05 g/cm3) of all the studied specimens. The chemical resistance of the specimens to the slag attack is also the best in this type of samples.
Author Contributions
Funding
Conflicts of Interest
References
- Stevens, R. An Introduction to Zirconia, 2nd ed.; Magnesium Elektron Ltd.: Clifton, UK, 1986. [Google Scholar]
- Wolten, G.M. Diffusionless phase transformations in zirconia and hafnia. J. Am. Ceram. Soc. 1963, 46, 418–422. [Google Scholar] [CrossRef]
- Lampman, S.R.; Mara, W.; Theodore, Z. Engineered Materials Handbook: Volume 4: Ceramics and Glasses, 1st ed.; CRS Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Schacht, C. Refractories Handbook, 1st ed.; Marcel Dekker Inc.: Pittsburg, PA, USA, 2004. [Google Scholar]
- Perez, I.; Moreno-Ventas, I.; Parra, R.; Ríos, G. Post-mortem study of magnesia–chromite refractory used in a submerged arc furnace in the copper-making process. J. Miner. Met. Mater. Soc. 2018, 70, 2435–2442. [Google Scholar] [CrossRef]
- García, L.V.; Mendivil, M.I.; Roy, T.K.D.; Castillo, G.A.; Shaji, S. Laser sintering of magnesia with nanoparticles of iron oxide and aluminum oxide. Appl. Surf. Sci. 2015, 336, 59–66. [Google Scholar] [CrossRef]
- Fernández-González, D.; Prazuch, J.; Ruiz-Bustinza, I.; González-Gasca, C.; Piñuela-Noval, J.; Verdeja, L.F. Solar synthesis of calcium aluminates. Sol. Energy 2018, 171, 658–666. [Google Scholar] [CrossRef]
- Fernández-González, D.; Ruiz-Bustinza, I.; González-Gasca, C.; Piñuela Noval, J.; Mochón-Castaños, J.; Sancho-Gorostiaga, J.; Verdeja, L.F. Concentrated solar energy applications in materials science and metallurgy. Sol. Energy 2018, 170, 520–540. [Google Scholar] [CrossRef]
- Martinac, V. Effect of TiO2 addition on the sintering process of magnesium oxide from seawater. In Sintering of Ceramics-New Emerging Techniques, 1st ed.; Lakshmanan, A., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 309–322. [Google Scholar]
- Oghbaei, M.; Mirzaee, O. Microwave versus conventional sintering: A review of fundamentals, advantages and applications. J. Alloy. Compd. 2010, 494, 175–189. [Google Scholar] [CrossRef]
- Qian, B.; Shen, Z. Laser sintering of ceramics. J. Asian Ceram. Soc. 2013, 1, 315–321. [Google Scholar] [CrossRef]
- Zhu, T.; Li, Y.; Sang, S.; Xie, Z. A new approach to fabricate MgO-C refractories with high thermal shock resistance by adding artificial graphite. J. Eur. Ceram. Soc. 2018, 38, 2179–2185. [Google Scholar] [CrossRef]
- Ganesh, I.; Bhattacharjee, S.; Saha, B.P.; Johnson, R.; Rajeshwari, K.; Sengupta, R.; Ramana Rao, M.V.; Mahajan, Y.R. An efficient MgAl2O4 spinel additive for improved slag erosion and penetration resistance of high-Al2O3 and MgO–C refractories. Ceram. Int. 2002, 28, 245–253. [Google Scholar] [CrossRef]
- Calvo, W.A.; Ortega, P.; Velasco, M.J.; Muñoz, V.; Pena, P.; Martinez, A.G.T. Characterization of alumina-magnesia-carbon refractory bricks containing aluminium and silicon. Ceram. Int. 2018, 44, 8842–8855. [Google Scholar] [CrossRef]
- Behera, S.; Sarkar, R. Effect of different metal powder anti-oxidants on N220 nano carbon containing low carbon MgO-C refractory: An in-depth Investigation. Ceram. Int. 2016, 42, 18484–18494. [Google Scholar] [CrossRef]
- Aneziris, C.G.; Hubálková, J.; Barabás, R. Microstructure evaluation of MgO–C refractories with TiO2 and Al-additions. J. Eur. Ceram. Soc. 2007, 27, 73–78. [Google Scholar] [CrossRef]
- Zhang, S.; Yamaguchi, A. Effects of CaO and Al2O3 added to MgO-C refractories on MgO-C reaction. J. Ceram. Soc. Jpn. 1996, 104, 84–88. [Google Scholar] [CrossRef][Green Version]
- Gómez Rodríguez, C.; Das Roy, T.K.; Shaji, S.; Castillo Rodríguez, G.A.; García Quiñonez, L.; Rodríguez, E.; González, J.O.; Aguilar-Martínez, J.A. Effect of addition of Al2O3 and Fe2O3 nanoparticles on the microstructural and physico-chemical evolution of dense magnesia composite. Ceram. Int. 2015, 41, 7751–7758. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, S.; Sedeh, M.B.; Dehsheikh, H.G.; Shahraki, A.; Farooghi, M. Densification and properties of ZrO2 nanoparticles added magnesia-doloma refractories. Ceram. Int. 2016, 42, 15658–15663. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, S.; Nemati, A.; Shahraki, A.; Farooghi, M. Densification and properties of Fe2O3 nanoparticles added CaO refractories. Ceram. Int. 2016, 42, 12270–12275. [Google Scholar] [CrossRef]
- Dehsheikh, H.G.; Ghasemi-Kahrizsangi, S. Performance improvement of MgO-C refractory bricks by the addition of Nano-ZrSiO4. Mater. Chem. Phys. 2017, 202, 369–376. [Google Scholar] [CrossRef]
- Shahraki, A.; Ghasemi-Kahrizsangi, S.; Nemati, A. Performance improvement of MgO-CaO refractories by the addition of nano-sized Al2O3. Mater. Chem. Phys. 2017, 198, 354–359. [Google Scholar] [CrossRef]
- Bag, M.; Adak, S.; Sarkar, R. Study on low carbon containing MgO-C refractory: Use of nano carbon. Ceram. Int. 2012, 38, 2339–2346. [Google Scholar] [CrossRef]
- Dehsheikh, H.G.; Ghasemi-Kahrizsangi, S.; Karamian, E. Addition impact of nano-carbon black on the performance of MgO.CaO compounds. Ceram. Int. 2018, 44, 5524–5527. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, S.; Dehsheikh, H.G.; Karamian, E.; Boroujerdnia, M.; Payandeh, K. Effect of MgAl2O4 nanoparticles addition on the densification and properties of MgO-CaO refractories. Ceram. Int. 2017, 43, 5014–5019. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, S.; Dehsheikh, H.G.; Karamian, E. Impact of titania nanoparticles addition on the microstructure and properties of MgO-C refractories. Ceram. Int. 2017, 43, 15472–15477. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, S.; Shahraki, A.; Farooghi, M. Effect of nano-TiO2 additions on the densification and properties of magnesite-dolomite ceramic composites. Iran. J. Sci. Technol. 2018, 42, 567–575. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, S.; Dehsheikh, H.G.; Boroujerdnia, M. MgO-CaO-Cr2O3 composition as a novel refractory brick: Use of Cr2O3 nanoparticles. Boletín Soc. Española Cerámica Vidr. 2017, 56, 83–89. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, S.; Karamian, E.; Ghasemi-Kahrizsangi, A.; Desheikh, H.G.; Soheily, A. The impact of trivalent oxide nanoparticles on the microstructure and performance of magnesite-dolomite refractory bricks. Mater. Chem. Phys. 2017, 193, 413–420. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, S.; Karamian, E.; Dehsheikh, H.G. The impact of ZrSiO4 nanoparticles addition on the microstructure and properties of dolomite based refractories. Ceram. Int. 2017, 43, 13932–13937. [Google Scholar] [CrossRef]
- Dehsheikh, H.G.; Ghasemi-Kahrizsangi, S. The influence of silica nanoparticles addition on the physical, mechanical, thermo-mechanical as well as microstructure of Mag-Dol refractory composites. Ceram. Int. 2017, 43, 16780–16786. [Google Scholar] [CrossRef]
- Kahrizsangi, S.G.; Nemati, A.; Shahraki, A.; Farooghi, M. Effect of nano-sized Fe2O3 on microstructure and hydration resistance of MgO-CaO refractories. Int. J. Nanosci. Nanotechnol. 2016, 12, 19–26. [Google Scholar]
- Zargar, H.R.; Oprea, C.; Oprea, G.; Troczynski, T. The effect of nano-Cr2O3 on solid-solution assisted sintering of MgO refractories. Ceram. Int. 2012, 38, 6235–6241. [Google Scholar] [CrossRef]
- Dudczig, S.; Veres, O.; Aneziris, C.G.; Skiera, E.; Steinbrech, R.W. Nano- and micrometre additions of SiO2, ZrO2 and TiO2 in fine grained alumina refractory ceramics for improved thermal shock performance. Ceram. Int. 2012, 38, 2011–2019. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, S.; Dehsheikh, H.G.; Boroujerdnia, M. Effect of micro and nano-Al2O3 addition on the microstructure and properties of MgO-C refractory ceramic composite. Mater. Chem. Phys. 2017, 189, 230–236. [Google Scholar] [CrossRef]
- Ballester, A.; Verdeja, L.F.; Sancho, J.P. Metalurgia extractiva: Fundamentos (Volumen I), 1st ed.; Sintesis: Madrid, Spain, 2000. [Google Scholar]
- Puertas, F. Cementos de escorias activadas alcalinamente: Situación actual y perspectivas de futuro. Mater. Construcc. 1995, 45, 53–64. [Google Scholar] [CrossRef]
- Tiryakioğlu, M.; Dispinar, D.; Uludağ, M.; Yazman, Ş.; Gemi, L. The effect of 0.5 wt % additions of carbon nanotubes and ceramic nanoparticles on tensile properties of epoxy-matrix composites: A comparative study. Mater. Sci. Nanotechnol. 2017, 1, 15–22. [Google Scholar] [CrossRef]
- Assaedi, H.; Shaikh, F.U.A.; Low, I.M. Effect of nano-clay on mechanical and thermal properties of geopolymer. J. Asian Ceram. Soc. 2016, 4, 19–28. [Google Scholar] [CrossRef]
- Szczerba, J.; Pdzich, Z.; Madej, D. Synthesis of spinel-calcium zirconate materials. Ceram. Mater. 2011, 63, 27–33. [Google Scholar]
- Chen, M.; Lu, C.; Yu, J. Improvement in performance of MgO-CaO refractories by addition of nano-sized ZrO2. J. Eur. Ceram. Soc. 2007, 27, 4633–4638. [Google Scholar] [CrossRef]
- Kehl, G.L. The Principles of Metallographic Laboratory Practice, 3rd ed.; McGraw Hill Publishing Co., Ltd.: London, UK, 1949. [Google Scholar]
- Wang, J.A.; Novaro, O.; Bokhimi, X.; López, T.; Gomez, R.; Navarrete, J.; Llanos, M.E.; López-Salinas, E. Characterization of the thermal decomposition of brucite prepared by sol-gel technique for synthesis of nanocrystalline MgO. Mater. Lett. 1998, 35, 317–323. [Google Scholar] [CrossRef]
- Rodríguez, E.; Castillo, G.; Contreras, J.E.; Aguilar Martínez, J.A.; González, Y.; Guzman, A.; García Ortiz, L.I. Desarrollo de un refractario MgO-CaZrO3 dopado con MgAl2O4 para la industria cementera. CIENCIA-UANL 2011, 14, 31–38. [Google Scholar]
- Schafföner, S.; Aneziris, C.G.; Berek, H.; Hubálková, J.; Priese, A. Fused calcium zirconate for refractory applications. J. Eur. Ceram. Soc. 2013, 33, 3411–3418. [Google Scholar] [CrossRef]
- Verdeja, L.F.; Sancho, J.P.; Ballester, A.; González, R. Refractory and Ceramic Materials, 1st ed.; Sintesis: Madrid, Spain, 2008. [Google Scholar]


| Raw Material and Slag | Chemical Analysis (wt. %) | |||||||||||
| SiO2 | B2O3 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | TiO2 | P2O5 | L.O.I. | |
| Magnesia | 0.20 | 0.01 | 0.15 | 0.12 | - | 98.50 | 1.00 | - | - | - | - | - |
| Slag | 34.30 | - | 11.10 | 0.60 | 0.30 | 7.62 | 40.00 | 0.25 | 0.40 | 0.50 | 0.13 | 4.80 |
| Physical and chemical properties of the magnesia (MgO) | ||||||||||||
| Bulk density | 3.48–3.52 g/cm3 | |||||||||||
| Melting point | 2800 °C | |||||||||||
| Boiling point | 3600 °C | |||||||||||
| pH | 10.5 | |||||||||||
| Powder | Size (nm) | Purity (%) | Relative Density (g/cm3) | Specific Surface Area (m2/g) | Color |
|---|---|---|---|---|---|
| ZrO2 nanoparticles | <100 | >99 | 5.89 | ≥25 | White |
| Chemical Composition (wt. %)) | Temperature and Pressing Methods | ||||
|---|---|---|---|---|---|
| MgO | ZrO2 Nanoparticles | IP 1550 °C | UP 1550 °C | UP 1650 °C | IP 1650 °C |
| 99 | 1 | CGR1–6 | CGR19–24 | CGR37–42 | CGR55–60 |
| 97 | 3 | CGR7–12 | CGR25–30 | CGR43–48 | CGR61–66 |
| 95 | 5 | CGR13–18 | CGR31–36 | CGR49–54 | CGR67–72 |
| 100 | 0 | CGRi–vi | CGRvii–xii | CGRxiii–xviii | CGRxix–xxiv |
| Figure | Figure 4 (1) | Figure 4 (1) | Figure 4 (4) | Figure 4 (8) | Figure 5 (i) | Figure 5 (iv) | Figure 5 (viii) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Element | Point A | Point B | Point C | Point D | Point E | Point F | Point G | |||||||
| wt. % | at. % | wt. % | at. % | wt. % | at. % | wt. % | at. % | wt. % | at. % | wt. % | at. % | wt. % | at. % | |
| O | 36.5 | 46.6 | 57 | 73.61 | 29.25 | 63.71 | 36.12 | 69.9 | 60.18 | 76.72 | 35.7 | 67.7 | 41.7 | 71.65 |
| Mg | 63.5 | 53.4 | 12.66 | 10.75 | 7.81 | 11.19 | 8.18 | 10.41 | 9.15 | 7.67 | 4.58 | 5.71 | 6.58 | 7.44 |
| Ca | - | - | 30.34 | 15.64 | 2.17 | 1.89 | 1.83 | 1.41 | 30.67 | 15.61 | 15.87 | 12.01 | 13.87 | 9.51 |
| Zr | - | - | - | - | 60.77 | 23.21 | 53.87 | 18.28 | - | - | 43.85 | 14.58 | 37.85 | 11.4 |
| Figure | Figure 6 (1) | Figure 6 (1) | Figure 6 (4) | |||
|---|---|---|---|---|---|---|
| Element | Point A | Point B | Point C | |||
| wt. % | at. % | wt. % | at. % | wt. % | at. % | |
| O | 40.83 | 59.59 | 44.25 | 54.69 | 41.11 | 61.23 |
| Mg | 4.26 | 4.09 | 55.71 | 45.31 | 24.77 | 24.28 |
| Si | 17.36 | 14.43 | - | - | 5.91 | 5.02 |
| Ca | 37.56 | 21.89 | - | - | 6.31 | 3.75 |
| Zr | - | - | - | - | 21.9 | 5.72 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Rodríguez, C.; Fernández-González, D.; García-Quiñonez, L.V.; Castillo-Rodríguez, G.A.; Aguilar-Martínez, J.A.; Verdeja, L.F. MgO Refractory Doped with ZrO2 Nanoparticles: Influence of Cold Isostatic and Uniaxial Pressing and Sintering Temperature in the Physical and Chemical Properties. Metals 2019, 9, 1297. https://doi.org/10.3390/met9121297
Gómez-Rodríguez C, Fernández-González D, García-Quiñonez LV, Castillo-Rodríguez GA, Aguilar-Martínez JA, Verdeja LF. MgO Refractory Doped with ZrO2 Nanoparticles: Influence of Cold Isostatic and Uniaxial Pressing and Sintering Temperature in the Physical and Chemical Properties. Metals. 2019; 9(12):1297. https://doi.org/10.3390/met9121297
Chicago/Turabian StyleGómez-Rodríguez, Cristian, Daniel Fernández-González, Linda Viviana García-Quiñonez, Guadalupe Alan Castillo-Rodríguez, Josué Amilcar Aguilar-Martínez, and Luis Felipe Verdeja. 2019. "MgO Refractory Doped with ZrO2 Nanoparticles: Influence of Cold Isostatic and Uniaxial Pressing and Sintering Temperature in the Physical and Chemical Properties" Metals 9, no. 12: 1297. https://doi.org/10.3390/met9121297
APA StyleGómez-Rodríguez, C., Fernández-González, D., García-Quiñonez, L. V., Castillo-Rodríguez, G. A., Aguilar-Martínez, J. A., & Verdeja, L. F. (2019). MgO Refractory Doped with ZrO2 Nanoparticles: Influence of Cold Isostatic and Uniaxial Pressing and Sintering Temperature in the Physical and Chemical Properties. Metals, 9(12), 1297. https://doi.org/10.3390/met9121297









