Role of Substrates in the Corrosion Behaviors of Micro-Arc Oxidation Coatings on Magnesium Alloys
Abstract
1. Introduction
2. Materials and Methods
2.1. MAO Process
2.2. Microstructure Characterization and Corrosion Tests
3. Results
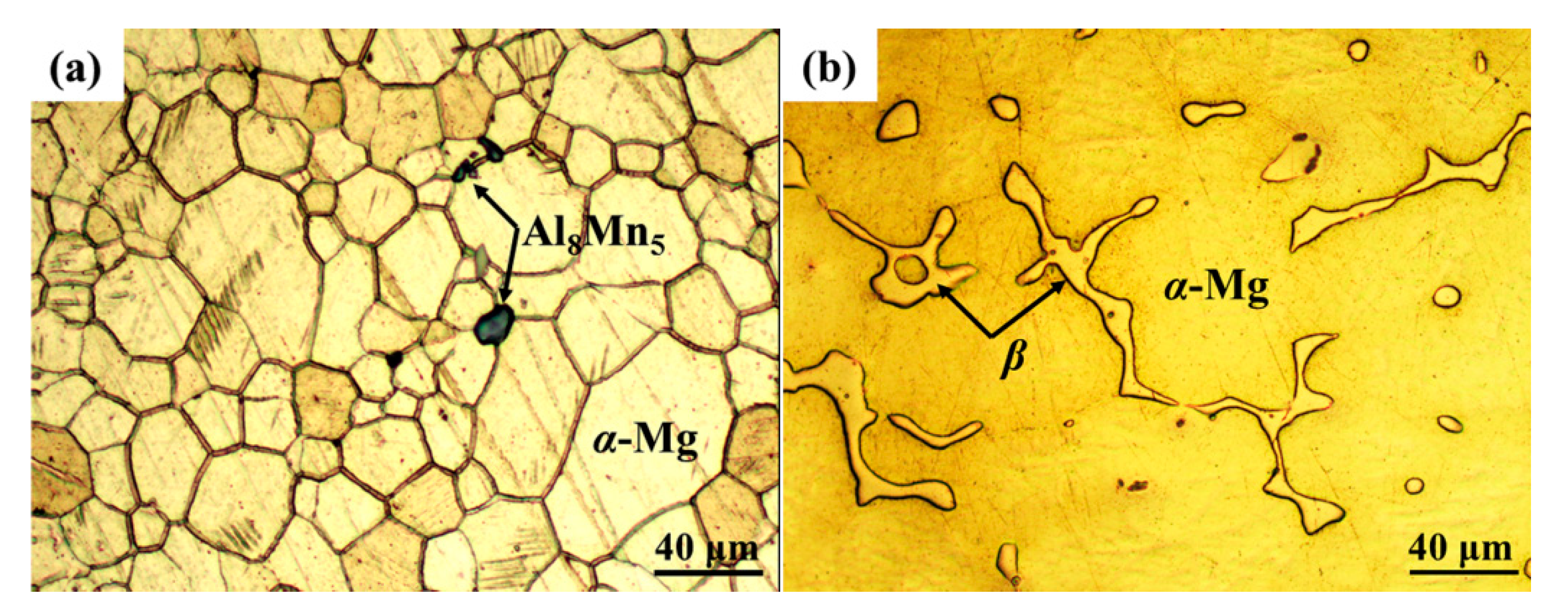
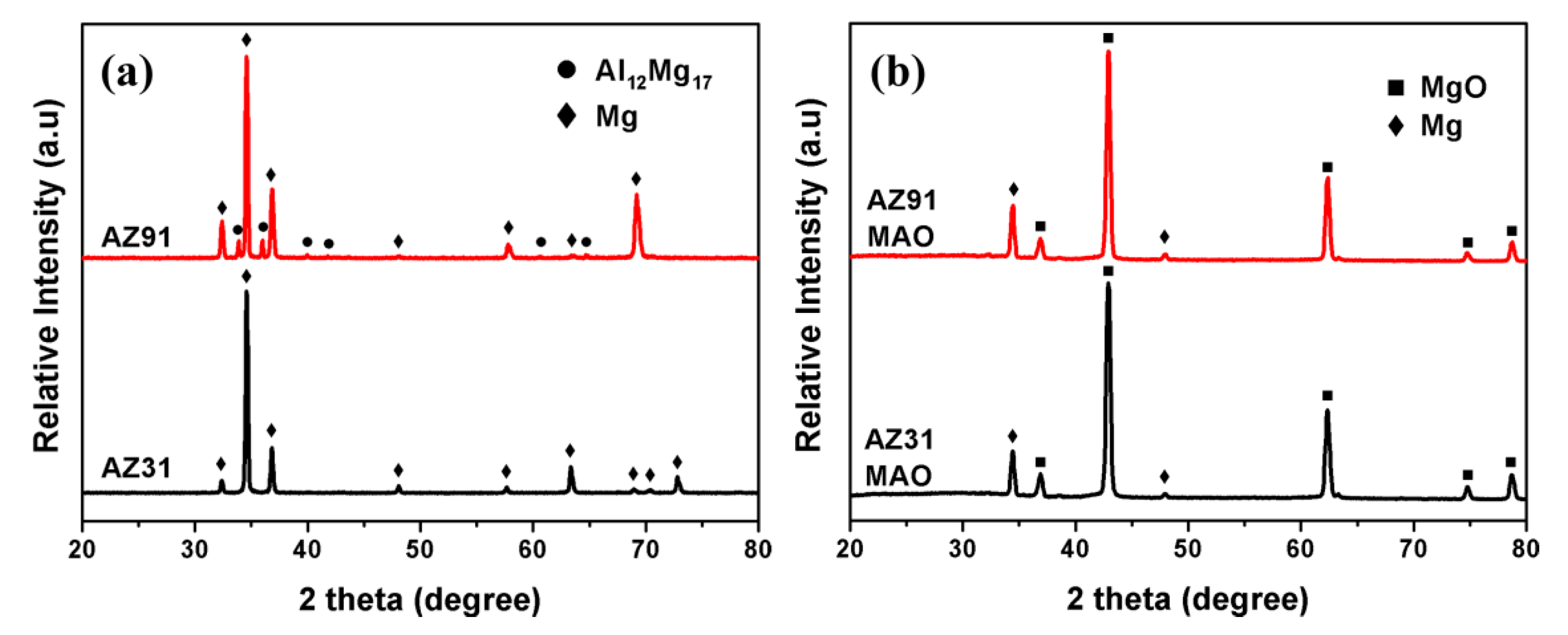
3.1. Microstructure and Composition
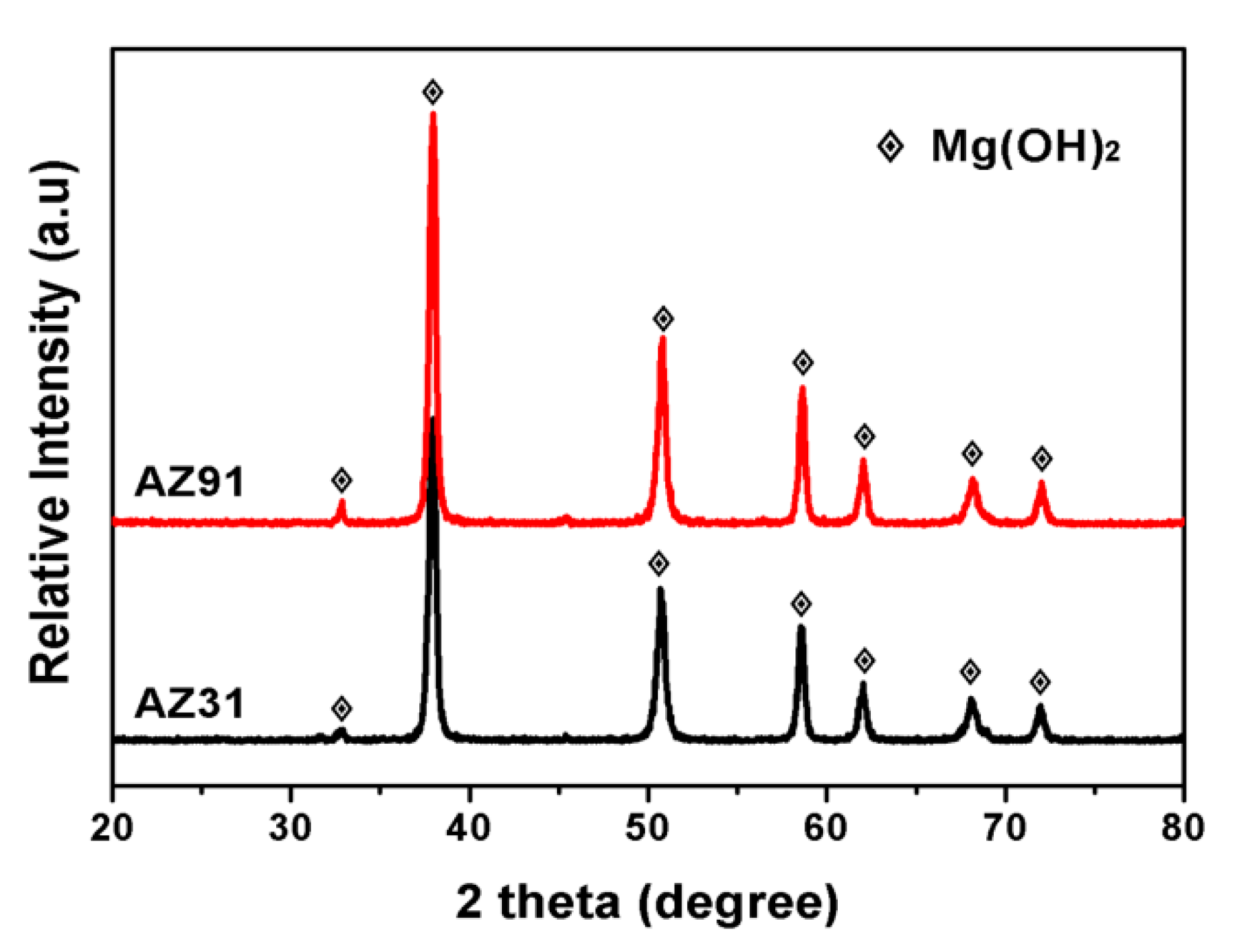
3.2. Corrosion Behaviors of AZ31 and AZ91 Mg Alloys
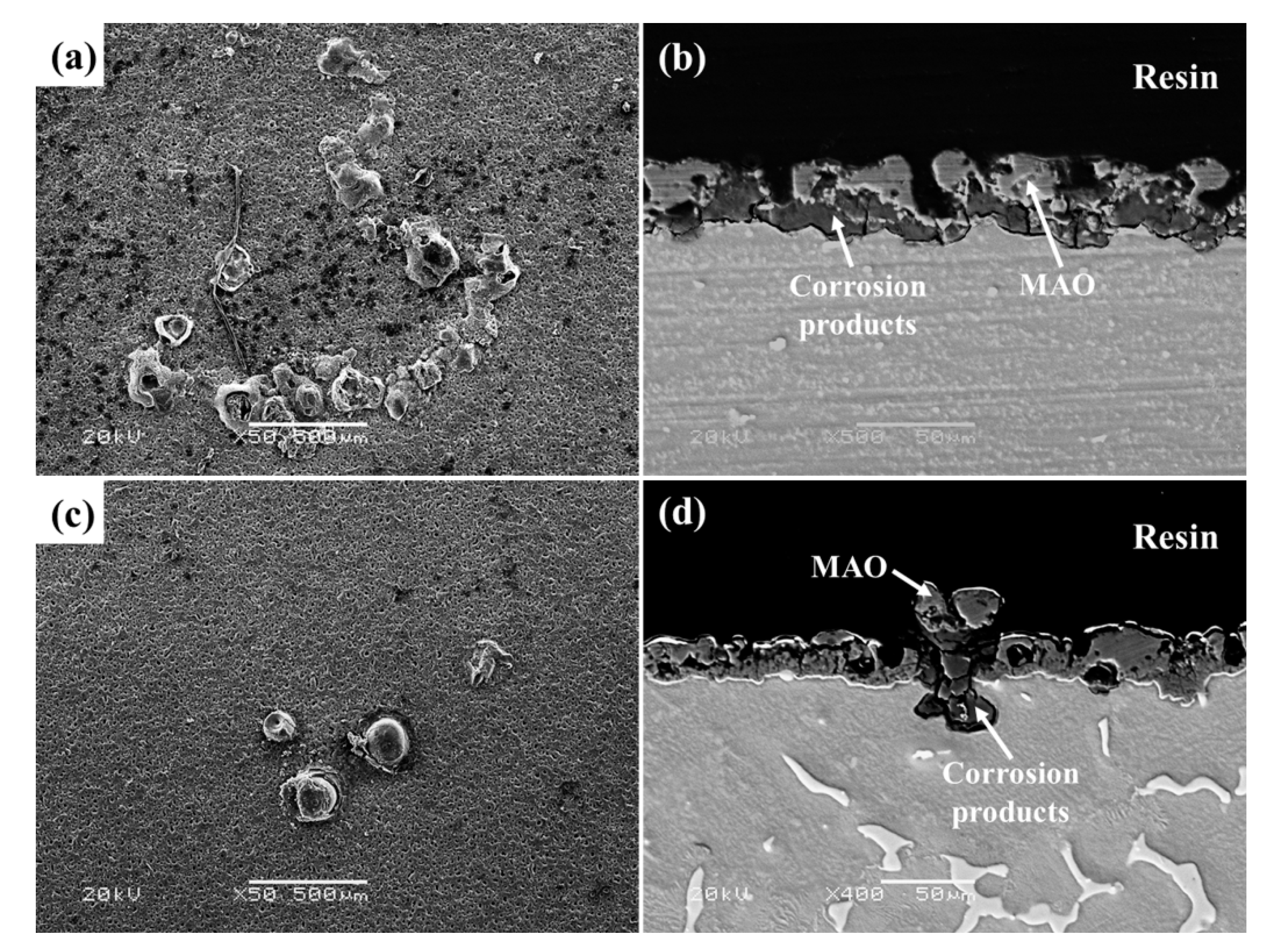
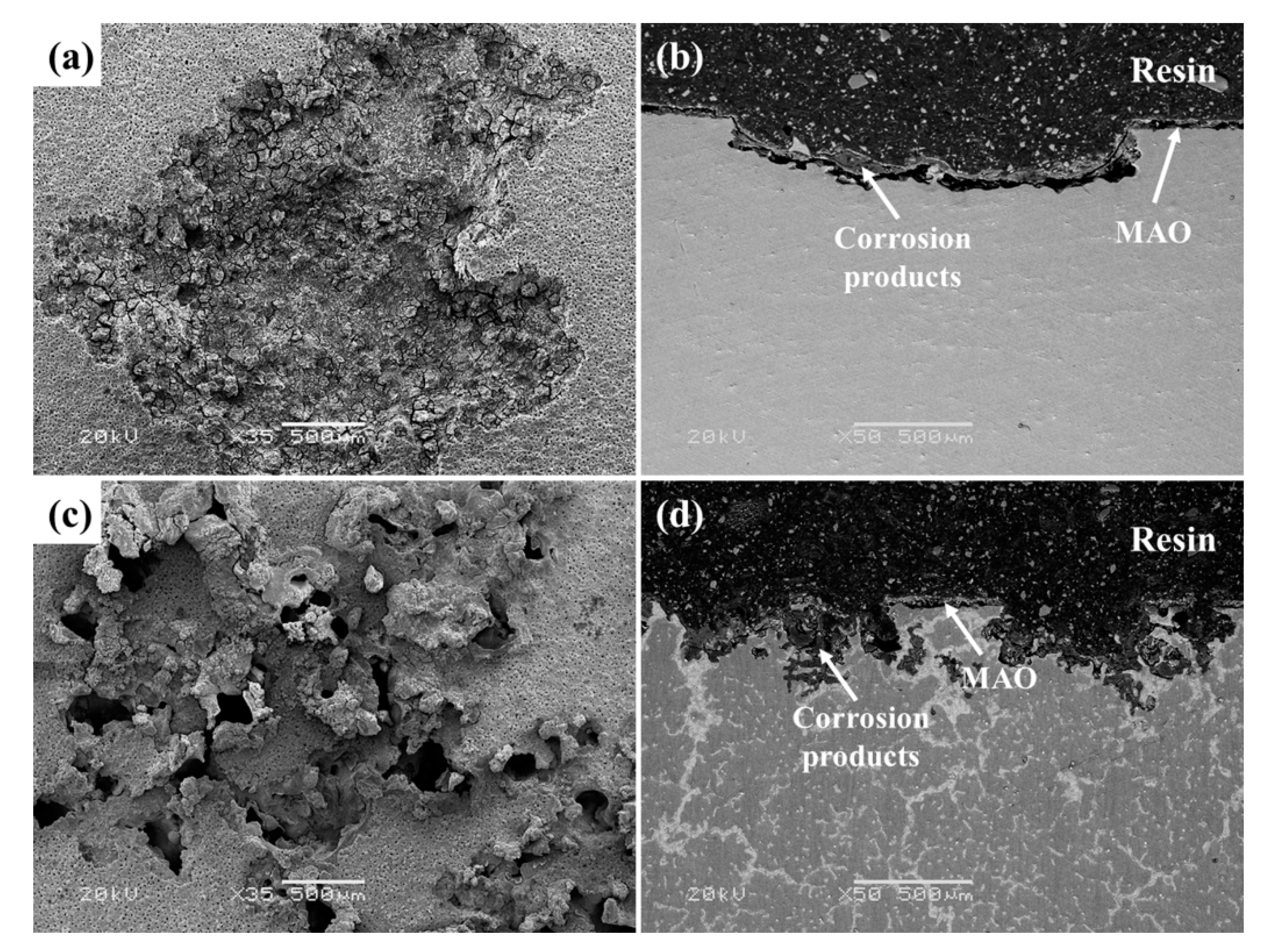
3.3. Corrosion Behaviors of MAO-Coated AZ31 and AZ91 Mg Alloys
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mordike, B.L.; Ebert, T. Magnesium properties-applications-potential. Mater. Sci. Eng. A 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Kulekci, M.K. Magnesium and its alloys applications in automotive industry. J. Adv. Manuf. Technol. 2008, 39, 851–865. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Understanding magnesium corrosion-a frame work for improved alloy performance. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Gray, J.E.; Luan, B. Protective coatings on magnesium and its alloys-a critical review. J. Alloy. Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Zhang, D.F.; Qi, Z.B.; Wei, B.B.; Shen, H.; Wang, Z.C. Microstructure and corrosion behaviors of conductive Hf/HfN multilayer coatings on magnesium alloys. Ceram. Int. 2018, 44, 9958–9966. [Google Scholar] [CrossRef]
- Chen, D.; Wang, R.Q.; Huang, Z.Q.; Wu, Y.K.; Zhang, Y.; Wu, G.R.; Li, D.L.; Guo, C.H.; Jiang, G.R.; Yu, S.X.; et al. Evolution processes of the corrosion behavior and structural characteristics of plasma electrolytic oxidation coatings on AZ31 magnesium alloy. Appl. Surf. Sci. 2018, 434, 326–335. [Google Scholar] [CrossRef]
- Dey, A.; Rani, R.U.; Thota, H.K.; Sharma, A.K.; Bandyopadhyay, P.; Mukhopadhyay, A.K. Microstructural, corrosion and nanomechanical behaviour of ceramic coatings developed on magnesium AZ31 alloy by micro arc oxidation. Ceram. Int. 2013, 39, 3313–3320. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. An investigation of ceramic coating growth mechanisms in plasma electrolytic oxidation (PEO) processing. Electrochim. Acta 2013, 112, 111–119. [Google Scholar] [CrossRef]
- Curran, J.A.; Clyne, T.W. Porosity in plasma electrolytic oxide coatings. Acta Mater. 2006, 54, 1985–1993. [Google Scholar] [CrossRef]
- Pezzato, L.; Brunelli, K.; Napolitani, E.; Magrini, M.; Dabalà, M. Surface properties of AZ91 magnesium alloy after PEO treatment using molybdate salts and low current densities. Appl. Surf. Sci. 2015, 357, 1031–1039. [Google Scholar] [CrossRef]
- Mori, Y.; Koshi, A.; Liao, J.S.; Asoh, H.; Ono, S. Characteristics and corrosion resistance of plasma electrolytic oxidation coatings on AZ31B Mg alloy formed in phosphate-silicate mixture electrolytes. Corros. Sci. 2014, 88, 254–262. [Google Scholar] [CrossRef]
- Rendon, M.E.; Duque, V.; Quintero, D.; Harmsen, M.C.; Echeverria, F. Novel coatings obtained by plasma electrolytic oxidation to improve the corrosion resistance of magnesium-based biodegradable implants. Surf. Coat. Technol. 2018, 354, 28–37. [Google Scholar] [CrossRef]
- Zhuang, J.J.; Guo, Y.Q.; Xiang, N.; Xiong, Y.; Hu, Q.; Song, R.G. A study on microstructure and corrosion resistance of ZrO2-containing PEO coatings formed on AZ31 Mg alloy in phosphate-based electrolyte. Appl. Surf. Sci. 2015, 357, 1463–1471. [Google Scholar] [CrossRef]
- Lin, X.; Tan, L.L.; Zhang, Q.; Yang, K.; Hu, Z.Q.; Qiu, J.H.; Cai, Y. The in vitro degradation process and biocompatibility of a ZK60 magnesium alloy with a forsterite-containing micro-arc oxidation coating. Acta Biomater. 2013, 9, 8631–8642. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Bala Srinivasan, P.; Blawert, C.; Dietzel, W. Influence of chloride ion concentration on the electrochemical corrosion behaviour of plasma electrolytic oxidation coated AM50 magnesium alloy. Electrochim. Acta 2010, 55, 6802–6811. [Google Scholar] [CrossRef]
- Wang, L.Q.; Zhou, J.S.; Liang, J.; Chen, J.M. Corrosion mechanism of plasma electrolytic oxidation coated magnesium alloy with laser surface melting pretreatment. J. Electrochem. Soc. 2014, 161, C20–C24. [Google Scholar] [CrossRef]
- Lin, X.; Wang, X.; Tan, L.L.; Wan, P.; Yu, X.M.; Li, Q.; Yang, K. Effect of preparation parameters on the properties of hydroxyapatite containing micro-arc oxidation coating on biodegradable ZK60 magnesium alloy. Ceram. Int. 2014, 40, 10043–10051. [Google Scholar] [CrossRef]
- Sankara Narayanan, T.S.N.; Park, I.S.; Lee, M.H. Strategies to improve the corrosion resistance of microarc oxidation (MAO) coated magnesium alloys for degradable implants: Prospects and challenges. Prog. Mater. Sci. 2014, 60, 1–71. [Google Scholar] [CrossRef]
- Ono, S.; Moronuki, S.; Mori, Y.; Koshi, A.; Liao, J.S.; Asoh, H. Effect of electrolyte concentration on the structure and corrosion resistance of anodic films formed on Magnesium through plasma electrolytic oxidation. Electrochim. Acta 2017, 240, 415–423. [Google Scholar] [CrossRef]
- Tjiang, F.; Ye, L.W.; Huang, Y.J.; Chou, C.C.; Tsai, D.S. Effect of processing parameters on soft regime behavior of plasma electrolytic oxidation of magnesium. Ceram. Int. 2017, 43, S567–S572. [Google Scholar] [CrossRef]
- Wei, F.F.; Zhang, W.; Zhang, T.; Wang, F.H. Effect of variations of Al content on microstructure and corrosion resistance of PEO coatings on Mg-Al alloys. J. Alloys Compd. 2017, 690, 195–205. [Google Scholar] [CrossRef]
- Tekin, K.C.; Malayoğlu, U.; Shrestha, S. Electrochemical behavior of plasma electrolytic oxide coatings on rare earth element containing Mg alloys. Surf. Coat. Technol. 2013, 236, 540–549. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.; Zhang, W.; Zhang, T.; Wang, F.H. Influence of second phase on corrosion performance and formation mechanism of PEO coating on AZ91 Mg alloy. J. Alloys Compd. 2017, 718, 92–103. [Google Scholar] [CrossRef]
- Muhaffel, F.; Mert, F.; Cimenoglu, H.; Höche, D.; Zheludkevich, M.L.; Blawer, C. Characterisation and corrosion behaviour of plasma electrolytic oxidation coatings on high pressure die cast Mg-5Al-0.4Mn-xCe (x=0, 0.5, 1) alloys. Surf. Coat. Technol. 2015, 269, 200–211. [Google Scholar] [CrossRef]
- Liu, C.C.; Liang, J.; Zhou, J.S.; Li, Q.B.; Wang, L.Q. Characterization of AZ31 magnesium alloy by duplex process combining laser surface melting and plasma electrolytic oxidation. Appl. Surf. Sci. 2016, 382, 47–55. [Google Scholar] [CrossRef]
- Jia, R.L.; Zhang, M.; Zhang, L.; Zhang, W.; Guo, F. Correlative change of corrosion behavior with the microstructure of AZ91 Mg alloy modified with Y additions. J. Alloys Compd. 2015, 634, 263–271. [Google Scholar] [CrossRef]
- Liu, C.C.; Zheng, H.; Gu, X.; Jiang, B.L.; Liang, J. Effect of severe shot peening on corrosion behavior of AZ31 and AZ91 magnesium alloys. J. Alloys Compd. 2019, 770, 500–506. [Google Scholar] [CrossRef]
- Joni, M.S.; Fattah-alhosseini, A. Effect of KOH concentration on the electrochemical behavior of coatings formed by pulsed DC micro-arc oxidation (MAO) on AZ31B Mg alloy. J. Alloys Compd. 2016, 661, 237–244. [Google Scholar] [CrossRef]
- Cui, X.J.; Liu, C.H.; Yang, R.S.; Li, M.T.; Lin, X.Z. Self-sealing micro-arc oxidation coating on AZ91D Mg alloy and its formation mechanism. Surf. Coat. Technol. 2015, 269, 228–237. [Google Scholar] [CrossRef]
- Arrabal, R.; Pardo, A.; Merino, M.C.; Mohedano, M.; Casajús, P.; Matykina, E.; Skeldon, P.; Thompson, G.E. Corrosion behaviour of a magnesium matrix composite with a silicate plasma electrolytic oxidation coating. Corros. Sci. 2010, 52, 3738–3749. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, C.Y. Corrosion protection behavior of AZ31 magnesium alloy with cathodic electrophoretic coating pretreated by silane. Prog. Org. Coat. 2009, 66, 387–392. [Google Scholar] [CrossRef]
- Samaniego, A.; Llorente, I.; Feliu, S., Jr. Combined effect of composition and surface condition on corrosion behavior of magnesium alloys AZ31 and AZ61. Corros. Sci. 2013, 68, 66–71. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A.; Dargusch, M. Influence of microstructure on the corrosion of diecast AZ91D. Corros. Sci. 1999, 41, 249–273. [Google Scholar] [CrossRef]
- Liang, J.; Bala Srinivasan, P.; Blawert, C.; Störmer, M.; Dietzel, W. Electrochemical corrosion behaviour of plasma electrolytic oxidation coatings on AM50 magnesium alloy formed in silicate and phosphate based electrolytes. Electrochim. Acta 2009, 54, 3842–3850. [Google Scholar] [CrossRef]




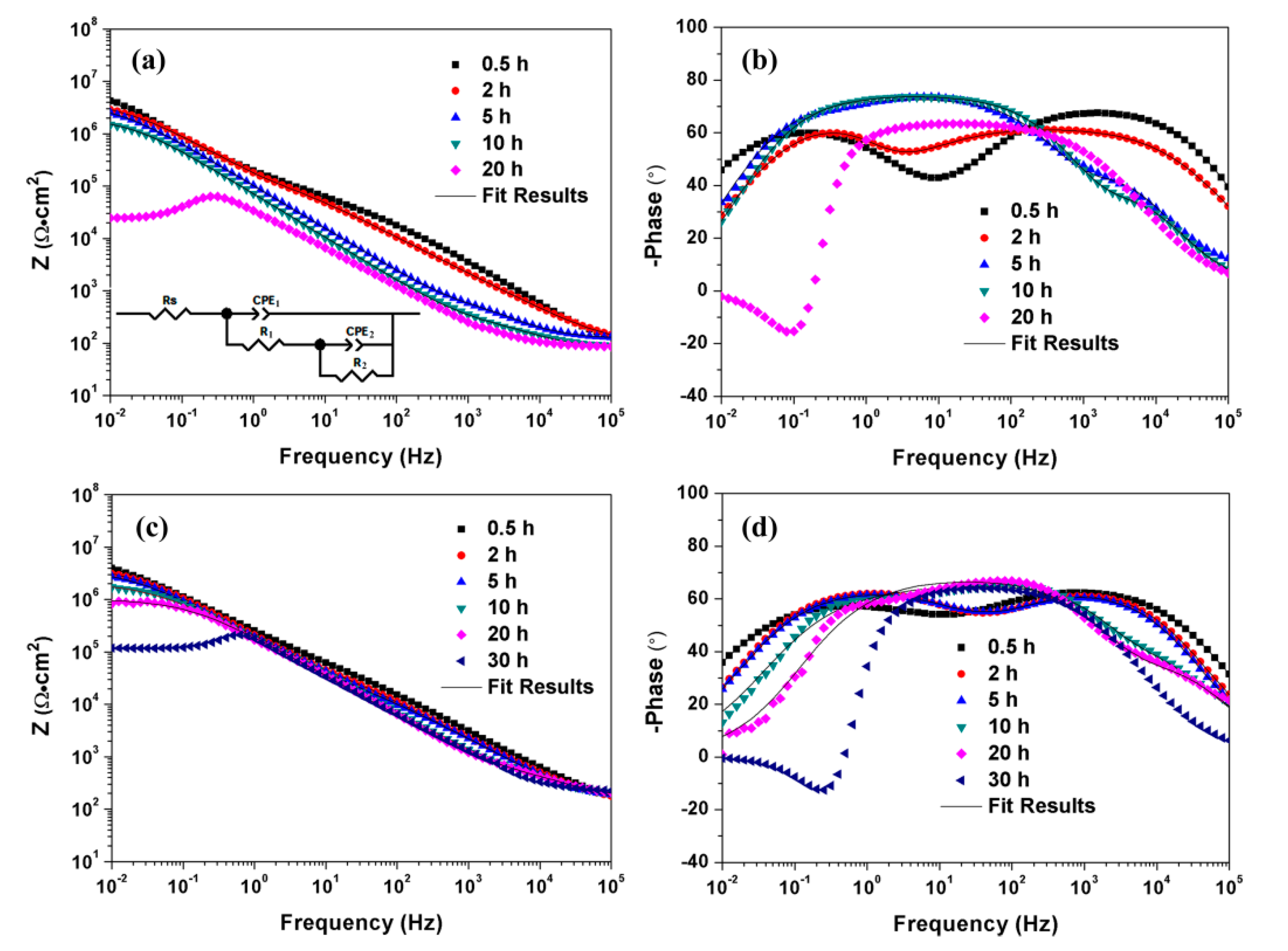


| Sample | CPE-T | CPE-P | Rct (Ω·cm2) | L (H) | RL (Ω·cm2) |
|---|---|---|---|---|---|
| AZ31 | 5.32 × 10−6 | 0.93 | 1.92 × 103 | 1.06 × 103 | 1.24 × 103 |
| AZ91 | 4.74 × 10−6 | 0.91 | 6.98 × 103 | 1.84 × 104 | 3.72 × 104 |
| Time (h) | (CPE-T)1 | (CPE-P)1 | R1 (Ω·cm2) | (CPE-T)2 | (CPE-P)2 | R2 (Ω·cm2) |
|---|---|---|---|---|---|---|
| 0.5 | 4.13 × 10−7 | 0.75 | 9.86 × 104 | 9.82 × 10−7 | 0.76 | 8.62 × 106 |
| 2 | 1.02 × 10−6 | 0.70 | 2.64 × 105 | 4.86 × 10−7 | 0.89 | 4.08 × 106 |
| 5 | 1.34 × 10−6 | 0.79 | 9.57 × 102 | 8.32 × 10−7 | 0.86 | 3.75 × 106 |
| 10 | 1.14 × 10−6 | 0.83 | 2.84 × 102 | 1.88 × 10−6 | 0.83 | 1.96 × 106 |
| Time (h) | (CPE-T)1 | (CPE-P)1 | R1 (Ω·cm2) | (CPE-T)2 | (CPE-P)2 | R2 (Ω·cm2) |
|---|---|---|---|---|---|---|
| 0.5 | 5.38 × 10−7 | 0.73 | 9.58 × 104 | 6.16 × 10−7 | 0.66 | 7.96 × 106 |
| 2 | 8.47 × 10−7 | 0.71 | 1.01 × 105 | 3.75 × 10−7 | 0.82 | 4.30 × 106 |
| 5 | 6.98 × 10−7 | 0.74 | 3.55 × 104 | 6.30 × 10−7 | 0.72 | 3.86 × 106 |
| 10 | 1.21 × 10−6 | 0.70 | 1.41 × 103 | 1.36 × 10−7 | 0.86 | 2.16 × 106 |
| 20 | 7.31 × 10−6 | 0.73 | 7.34 × 102 | 5.01 × 10−7 | 0.79 | 1.02 × 106 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Yuan, J.; Li, H.; Jiang, B. Role of Substrates in the Corrosion Behaviors of Micro-Arc Oxidation Coatings on Magnesium Alloys. Metals 2019, 9, 1100. https://doi.org/10.3390/met9101100
Liu C, Yuan J, Li H, Jiang B. Role of Substrates in the Corrosion Behaviors of Micro-Arc Oxidation Coatings on Magnesium Alloys. Metals. 2019; 9(10):1100. https://doi.org/10.3390/met9101100
Chicago/Turabian StyleLiu, Cancan, Junwen Yuan, Hongtao Li, and Bailing Jiang. 2019. "Role of Substrates in the Corrosion Behaviors of Micro-Arc Oxidation Coatings on Magnesium Alloys" Metals 9, no. 10: 1100. https://doi.org/10.3390/met9101100
APA StyleLiu, C., Yuan, J., Li, H., & Jiang, B. (2019). Role of Substrates in the Corrosion Behaviors of Micro-Arc Oxidation Coatings on Magnesium Alloys. Metals, 9(10), 1100. https://doi.org/10.3390/met9101100




