On Long-Term Stability of Metallic Glasses
Abstract
1. Introduction
2. Experimental Procedure
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Greer, A.L. New horizons for glass formation and stability. Nat. Mater. 2015, 14, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayana, C.; Inoue, A. Bulk Metallic Glasses; CRC Press: Boca Raton, FL, USA, 2011; p. 543. [Google Scholar]
- Louzguine-Luzgin, D.V. Metallic Glasses and Those Composites; Materials Research Forum LLC: Millersville, PA, USA, 2018; p. 336. [Google Scholar]
- Kaloshkin, S.D.; Tomilin, I.A. The crystallization kinetics of amorphous alloys. Thermochim. Acta 1996, 280–281, 303–317. [Google Scholar] [CrossRef]
- Liu, F.; Sommer, F.; Bos, C.; Mittemeijer, E.J. Analysis of solid state phase transformation kinetics: Models and recipes. Int. Mater. Rev. 2007, 52, 193–212. [Google Scholar] [CrossRef]
- Perepezko, J.H. Nucleation-controlled reactions and metastable structures. Prog. Mater Sci. 2004, 49, 263–284. [Google Scholar] [CrossRef]
- Louzguine, D.V.; Inoue, A. Strong influence of supercooled liquid on crystallization of the Al85Ni5Y4Nd4Co2 metallic glass. Appl. Phys. Lett. 2001, 78, 3061–3063. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, D.Q.; Pan, M.X.; Wang, W.H.; Greer, A.L. Amorphous metallic plastic. Phys. Rev. Lett. 2005, 94, 205502. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Li, J.F.; Zhao, D.Q.; Pan, M.X.; Wang, W.H. Degradable Sr-based bulk metallic glasses. Scr. Mater. 2009, 61, 1091–1094. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Polkin, V.I. Properties of bulk metallic glasses. Russ. J. Non-Ferrous Met. 2017, 58, 80–92. [Google Scholar] [CrossRef]
- Madge, S.V. Toughness of bulk metallic glasses. Metals 2015, 5, 1279–1305. [Google Scholar] [CrossRef]
- Peker, A.; Johnson, W.L. Time-temperature-transformation diagram of a highly processable metallic glass. Mater. Sci. Eng. A 1994, 179–180, 173–175. [Google Scholar] [CrossRef]
- Bai, F.X.; Yao, J.H.; Wang, Y.X.; Pan, J.; Li, Y.; Bai, F.X. Crystallization kinetics of an Au-based metallic glass upon ultrafast heating and cooling. Scr. Mater. 2017, 132, 58–62. [Google Scholar] [CrossRef]
- Nishiyama, N.; Inoue, A. Supercooling investigation and critical cooling rate for glass formation in Pd-Cu-Ni-P alloy. Acta Mater. 1999, 47, 1487–1495. [Google Scholar] [CrossRef]
- Loeffler, J.; Schroers, J.; Johnson, W.L. Time-temperature-transformation diagram and microstructures of bulk glass forming Pd40Cu30Ni10P20. Appl. Phys. Lett. 2000, 77, 681–683. [Google Scholar] [CrossRef]
- Louzguine, D.V.; Inoue, A. Comparison of the long-term thermal stability of various metallic glasses under continuous heating. Scr. Mater. 2002, 47, 887–891. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Inoue, A. Relation between time-temperature transformation and continuous heating transformation diagrams of metallic glassy alloys. Physica B 2005, 358, 174–180. [Google Scholar] [CrossRef]
- Grange, R.A.; Kiefer, J.M. Transformation of austenite on continuous cooling and its relation to transformation at constant temperature. Trans. ASM 1941, 29, 85–144. [Google Scholar]
- Louzguine-Luzgin, D.V.; Inoue, A. The outline of glass transition phenomenon derived from the viewpoint of devitrification process. Phys. Chem. Glasses—Eur. J. Glass Sci. Technol., Part B 2009, 50, 27–30. [Google Scholar]
- Louzguine, V.; Inoue, A. Electronegativity of the constituent rare-earth metals as a factor stabilizing the supercooled liquid region in Al-based metallic glasses. Appl. Phys. Lett. 2001, 79, 3410–3412. [Google Scholar] [CrossRef]
- Louzguine, D.V.; Inoue, A. Comparative analysis of crystallization of Al85RE8Ni5Co2 (RE-rare earth metals), metallic glasses with and without supercooled liquid region. J. Metastable Nanocryst. Mater. 2002, 386–388, 117–122. [Google Scholar]
- Louzguine, D.V.; Louzguina, L.V.; Inoue, A. Multistage devitrification of Mg-Ni-Mm and Mg-Ni-Y-Mm metallic glasses (Mm = mischmetal). Philos. Mag. A 2003, 83, 203–216. [Google Scholar] [CrossRef]
- Louzguine, D.V.; Inoue, A. Gold as an alloying element promoting formation of a nanoicosahedral phase in a Cu-based alloy. J. Alloys Compd. 2003, 361, 153–156. [Google Scholar] [CrossRef]
- Louzguine, D.V.; Inoue, A. Nanoparticles with icosahedral symmetry in Cu-based bulk glass former induced by Pd addition. Scr. Mater. 2003, 48, 1325–1329. [Google Scholar] [CrossRef]
- Louzguine, D.V.; Inoue, A. Influence of Ni and Co additions on supercooled liquid region, devitrification behaviour and mechanical properties of Cu-Zr-Ti bulk metallic glass. In Proceedings of the 9th International Symposium on Metastable, Mechanically Alloyed and Nanocrystalline Materials, Seoul, Korea, 8–12 September 2002. [Google Scholar]
- Louzguine, D.V.; Inoue, A. Devitrification of Ni-based glassy alloys containing noble metals in relation with the supercooled liquid region. J. Non-Cryst. Solids 2004, 337, 161–165. [Google Scholar] [CrossRef]
- Louzguine, D.V.; Inoue, A. Microstructure and properties of melt-spun Si-Al-Ge-Transition Metal amorphous alloys containing nanocrystalline Ge particles. Mater. Trans., JIM 1998, 39, 245–251. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Inoue, A. Structure and transformation behaviour of a rapidly solidified Al-Y-Ni-Co-Pd alloy. J. Alloys Compd. 2005, 399, 78–85. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Xie, G.; Zhang, W.; Inoue, A. Influence of Al and Ag on the devitrification behavior of a Cu-Zr glassy alloy. Mater. Trans. 2007, 48, 2128–2132. [Google Scholar] [CrossRef]
- Battezzati, L. Interplay of process kinetics in the undercooled melt in the proximity of the glass transition. Mater. Sci. Eng., A 2004, 375–377, 60–65. [Google Scholar] [CrossRef]
- Debnath, M.R.; Kim, D.-H.; Fleury, E. Dependency of the corrosion properties of in-situ Ti-based BMG matrix composites with the volume fraction of crystalline phase. Intermetallics 2012, 22, 255–259. [Google Scholar] [CrossRef]
- Lee, P.Y.; Cheng, Y.M.; Chen, J.Y.; Hu, C.J. Formation and corrosion behavior of mechanically-alloyed Cu-Zr-Ti bulk metallic glasses. Metals 2017, 7, 148. [Google Scholar] [CrossRef]
- Koster, U. Surface crystallization of metallic glasses. Mater. Sci. Eng. 1988, 97, 233–239. [Google Scholar] [CrossRef]
- Paljević, M.; Tudja, M. Unusual oxidation behaviour of Zr50Cu50 alloy at high temperatures. Corros. Sci. 2008, 50, 818–822. [Google Scholar] [CrossRef]
- Lim, K.; Kim, W.; Lee, E.; Jee, S.; Kim, S.; Kim, D.; Eckert, J. Oxidation resistance of the supercooled liquid in Zr50Cu50 and Cu46Zr46Al8 metallic glasses. J. Mater. Res. 2012, 27, 1178–1186. [Google Scholar] [CrossRef]
- Lim, K.R.; Kim, C.E.; Yun, Y.S.; Kim, W.T.; Soon, A.; Kim, D.H. Remarkably stable amorphous metal oxide grown on Zr-Cu-Be metallic glass. Sci. Rep. 2015, 5, 18196. [Google Scholar] [CrossRef]
- Altounian, Z.; Guo-hua, T.; Strom-Olsen, J.O. Crystallization characteristics of Cu-Zr metallic glasses from Cu70Zr30 to Cu25Zr75. J. Appl. Phys. 1982, 53, 4755. [Google Scholar] [CrossRef]
- Freed, R.L.; Vander Sande, J.B. A study of the crystallization of two non-crystalline Cu-Zr alloys. J. Non-Cryst. Solids 1978, 27, 9–28. [Google Scholar] [CrossRef]
- Naka, M.; Hashimoto, K.; Masumoto, T. Corrosion behavior of amorphous and crystalline Cu50Ti50 and Cu50Zr50 alloys. J. Non-Cryst. Solids 1978, 30, 29–36. [Google Scholar] [CrossRef]
- Turn, J.C.; Latanision, R.M. The influence of structure on the corrosion of glassy copper-zirconium alloys. Natl. Assoc. Corros. Eng. 1983, 39, 271–279. [Google Scholar] [CrossRef]
- Cullinan, T.; Kalay, I.; Kalay, Y.E.; Kramer, M.; Napolitano, R. Kinetics and mechanisms of isothermal devitrification in amorphous Cu50Zr50. Metall. Mater. Trans. A 2015, 46A, 600. [Google Scholar] [CrossRef]
- Bala, H.; Szymura, S. Acid corrosion of amorphous and crystalline Cu-Zr alloys. Appl. Surf. Sci. 1988, 35, 41–51. [Google Scholar] [CrossRef]
- Tang, J.; Zhu, Q.H.; Wang, Y.Y.; Apreutesei, M.; Wang, H.; Steyer, P.; Chamas, M.; Billard, A. Insights on the role of copper addition in the corrosion and mechanical properties of binary Zr-Cu metallic glass coatings. Coatings 2017, 7, 223. [Google Scholar] [CrossRef]
- Ellingham, H.J.T. Reducibilty of oxides and sulphides in metallurgical processes. J. Soc. Chem. Ind. Lond. 1944, 63, 125. [Google Scholar]
- Samoylova, O.V.; Mikhailov, G.G.; Makrovets, L.A.; Trofimov, E.A. Thermodynamic analysis of interaction processes in the Cu-Zr-O system realized in liquid copper melt. Bull. South Ural State Univ. Ser. Metall. 2014, 14, 17–22. [Google Scholar]
- Li, Y.; Guo, Q.; Kalb, J.A.; Thompson, C.V. Matching glass-forming ability with the density of the amorphous phase. Science 2008, 322, 1816–1819. [Google Scholar] [CrossRef] [PubMed]
- Han, X.L.; Qin, Y.S.; Qin, K.; Li, X.L.; Wang, S.H.; Mi, J.; Song, K.K.; Wang, L. Glass-forming ability and early crystallization kinetics of novel Cu-Zr-Al-Co bulk metallic glasses. Metals 2016, 6, 225. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Chen, C.L.; Lin, L.Y.; Wang, Z.C.; Ketov, S.V.; Miyama, M.J.; Trifonov, A.S.; Lubenchenko, A.V.; Ikuhara, Y. Bulk metallic glassy surface native oxide: Its atomic structure, growth rate and electrical properties. Acta Mater. 2015, 97, 282–290. [Google Scholar] [CrossRef]
- Ketov, S.V.; Ivanov, Y.P.; Şopu, D.; Louzguine-Luzgin, D.V.; Suryanarayana, C.; Rodin, A.O.; Schöber, T.; Greer, A.L.; Eckert, J. High-resolution transmission electron microscopy investigation of diffusion in metallic glass multilayer films. Mater. Today Adv. 2019, 1, 100004. [Google Scholar] [CrossRef]
- Ketov, S.V.; Trifonov, A.S.; Ivanov, Y.P.; Churyumov, A.Y.; Lubenchenko, A.V.; Batrakov, A.A.; Jiang, J.; Louzguine-Luzgin, D.V.; Eckert, J.; Orava, J.; et al. On cryothermal cycling as a method for inducing structural changes in metallic glasses. NPG Asia Mater. 2018, 10, 137–145. [Google Scholar] [CrossRef]
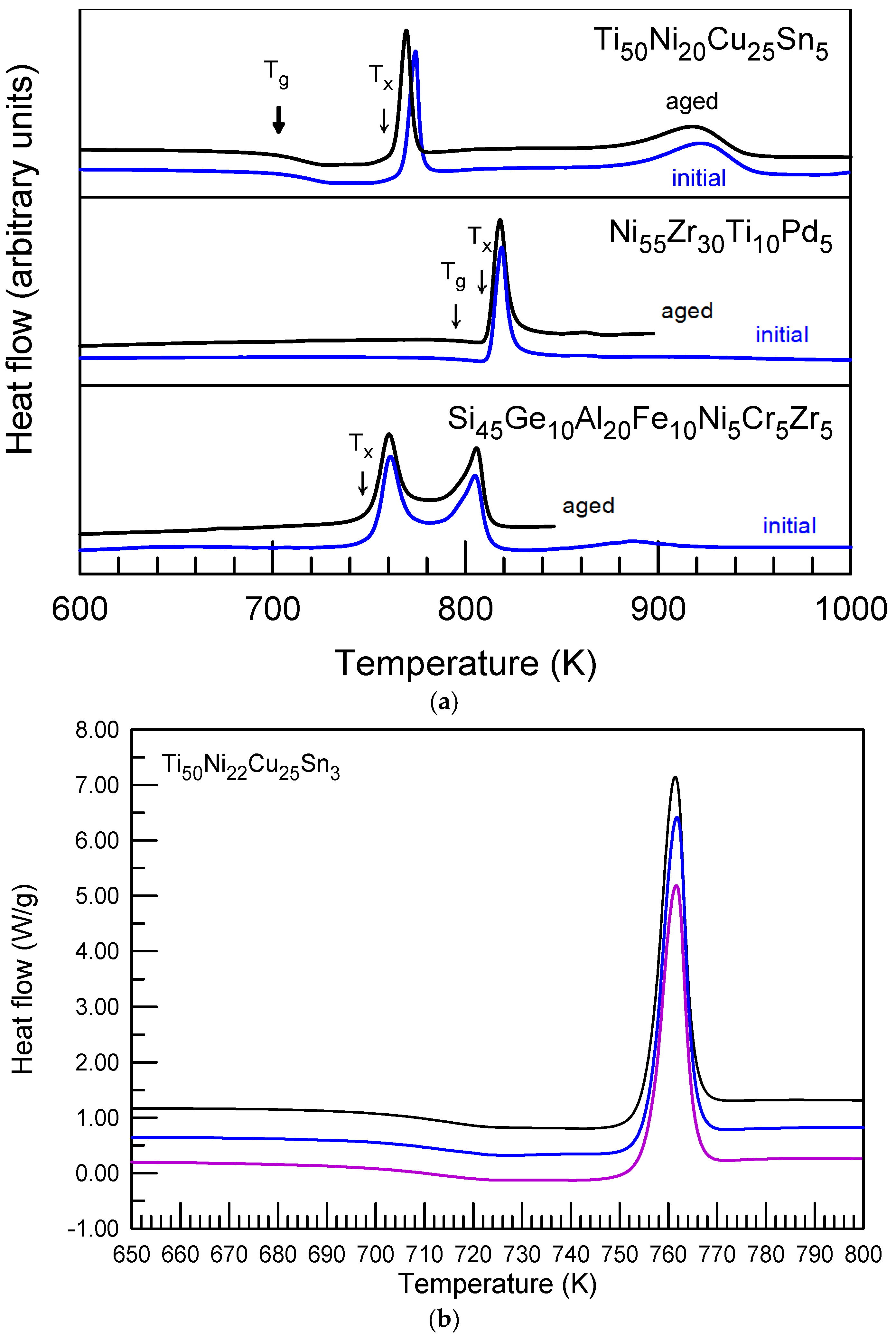
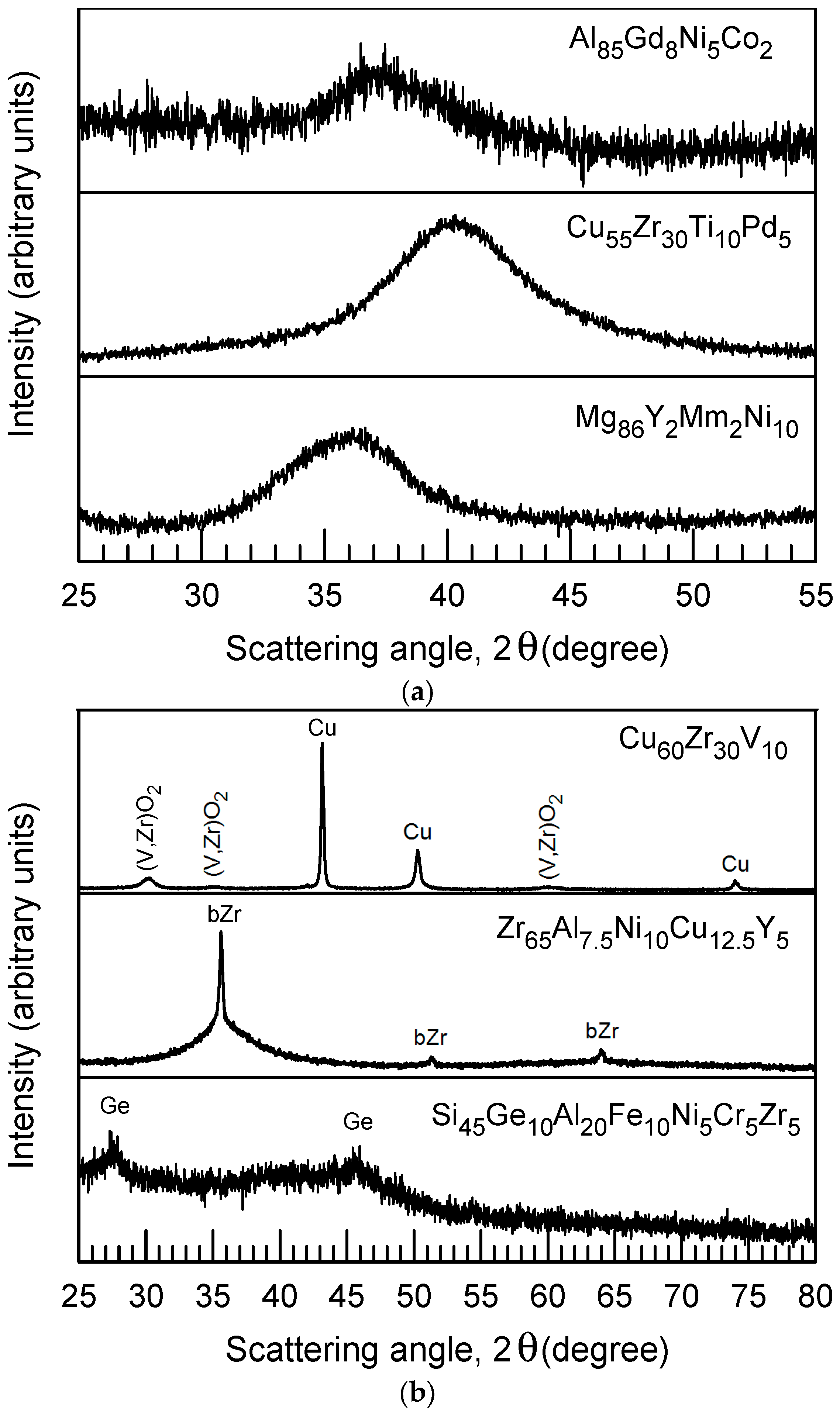




| Alloy | Ref | NY | ΔTg | ΔTx | ΔTrx × 100 | Initial | Aged | ||
|---|---|---|---|---|---|---|---|---|---|
| Tgi (K) | Txi (K) | Tga (K) | Txa (K) | ||||||
| Al85Gd8Ni5Co2 | 20 | 19 | 4 | 3 | 0.52 | 554 | 575 | 550 | 572 |
| Al85Sm8Ni5Co2 | 21 | 18 | 1 | 2 | 0.35 | 557 | 575 | 556 | 573 |
| Mg86Mm4Ni10 | 22 | 17 | - | 3 | 0.66 | - | 456 | - | 453 |
| Mg86Y2Mm2Ni10 | 22 | 17 | - | 1 | 0.22 | - | 451 | - | 450 |
| Cu55Zr30Ti10Au5 | 23 | 17 | 3 | 3 | 0.38 | 744 | 787 | 741 | 784 |
| Cu55Zr30Ti10Pd5 | 24 | 17 | −6 | 2 | 0.26 | 735 | 784 | 741 | 782 |
| Cu57.5Ni2.5Zr30Ti10 | 25 | 18 | 3 | 1 | 0.13 | 705 | 749 | 702 | 748 |
| Ni55Zr30Ti10Pd5 | 26 | 16 | −4 | 0 | 0.00 | 787 | 811 | 791 | 811 |
| Ni55Zr30Ti10Pt5 | 26 | 16 | 5 | 3 | 0.36 | 816 | 845 | 811 | 842 |
| Ni60Zr30V10 * | - | 18 | −2 | 4 | 0.47 | 812 | 852 | 814 | 848 |
| Ti50Ni22Cu25Sn3 * | - | 20 | 0 | 4 | 0.53 | 697 | 758 | 697 | 754 |
| Ti50Ni20Cu25Sn5 * | - | 20 | 6 | 4 | 0.52 | 702 | 767 | 696 | 763 |
| Ti50Ni20Cu20Sn10 * | - | 20 | 4 | 3 | 0.39 | 731 | 774 | 727 | 771 |
| Ti50Ni22Cu22V6 * | - | 19 | - | 3 | 0.41 | - | 733 | - | 730 |
| Zr65Al7.5Ni10Cu12.5Y5 ** | - | 19 | - | 1 | 0.15 | - | 665 | - | 664 |
| Si45Al20Fe10Ge10Ni5Cr5Zr5 *** | 27 | 22 | - | 2 | 0.27 | - | 752 | 750 | |
| Al85Ni5Co2Y6Pd2 **** | 28 | 19 | - | 0 | 0.00 | - | 523 | - | 523 |
| Cu60Zr30V10 ***** | - | 18 | - | 12 | 1.52 | - | 790 | - | 778 |
| Zr50Cu50 ***** | 29 | 17 | - | 54 | 8 | 675 | 742 | - | 688 |
| Area | Side | Cu | Zr |
|---|---|---|---|
| Smooth area | silver | 52.6 | 47.4 |
| Smooth area | silver | 52.6 | 47.4 |
| Smooth area | silver | 51.1 | 48.9 |
| Average | - | 52.1 | 47.9 |
| C.I. * | - | ±1.2 | ±1.2 |
| Smooth area | brown | 57.1 | 42.9 |
| Smooth area | brown | 58.4 | 41.6 |
| Average | - | 57.75 | 42.25 |
| Particle area | brown | 67.4 | 32.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Louzguine-Luzgin, D.V.; Jiang, J. On Long-Term Stability of Metallic Glasses. Metals 2019, 9, 1076. https://doi.org/10.3390/met9101076
Louzguine-Luzgin DV, Jiang J. On Long-Term Stability of Metallic Glasses. Metals. 2019; 9(10):1076. https://doi.org/10.3390/met9101076
Chicago/Turabian StyleLouzguine-Luzgin, Dmitri V., and Jing Jiang. 2019. "On Long-Term Stability of Metallic Glasses" Metals 9, no. 10: 1076. https://doi.org/10.3390/met9101076
APA StyleLouzguine-Luzgin, D. V., & Jiang, J. (2019). On Long-Term Stability of Metallic Glasses. Metals, 9(10), 1076. https://doi.org/10.3390/met9101076






