Bioleaching for Copper Extraction of Marginal Ores from the Brazilian Amazon Region
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Chemical, Mineralogical and Morphological Characterization
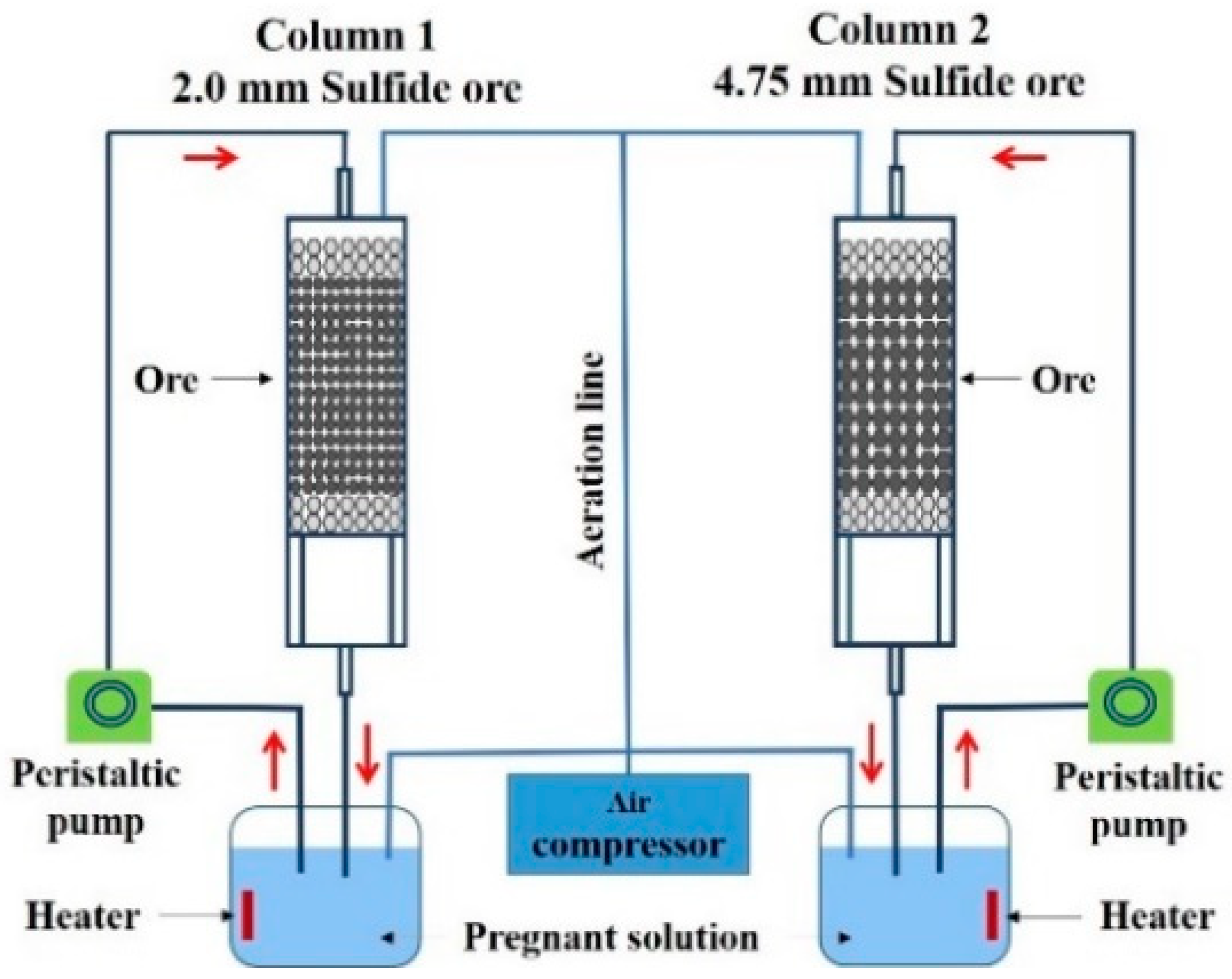
2.3. Assembling of the Bioleaching Columns
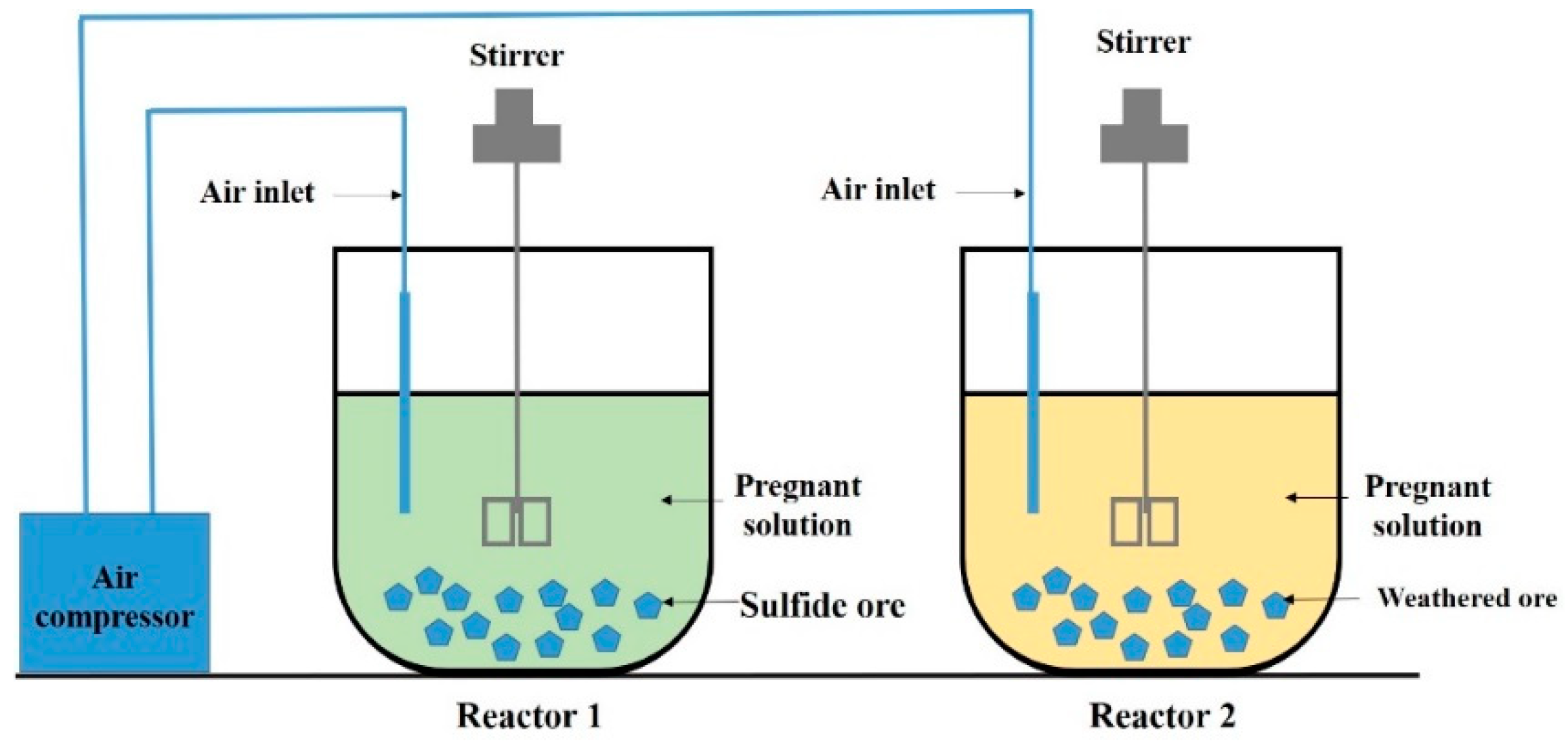
2.4. Assembling of the Bioleaching Stirred Reactors
2.5. Bioleaching Assessment
3. Results and Discussion
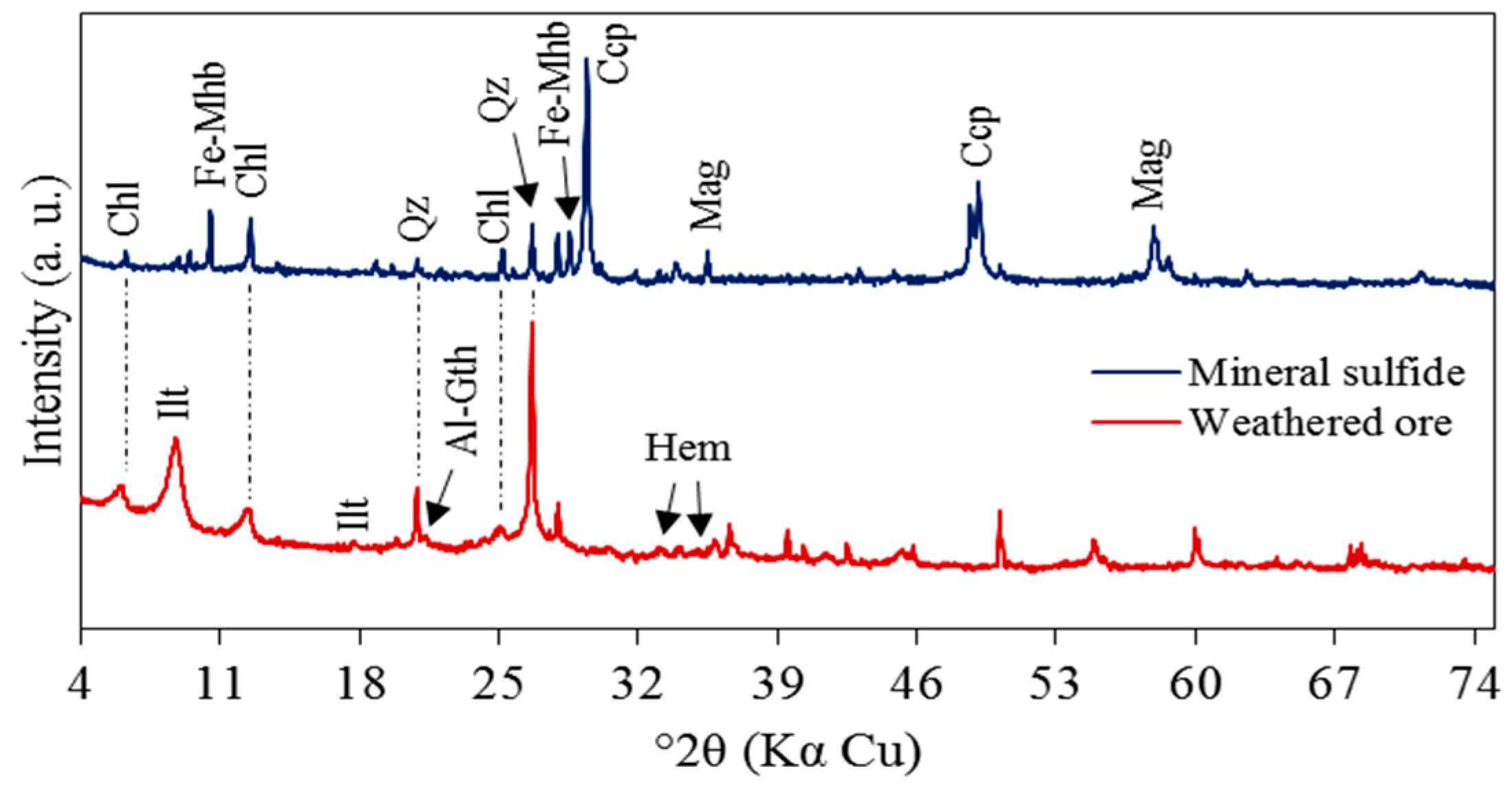
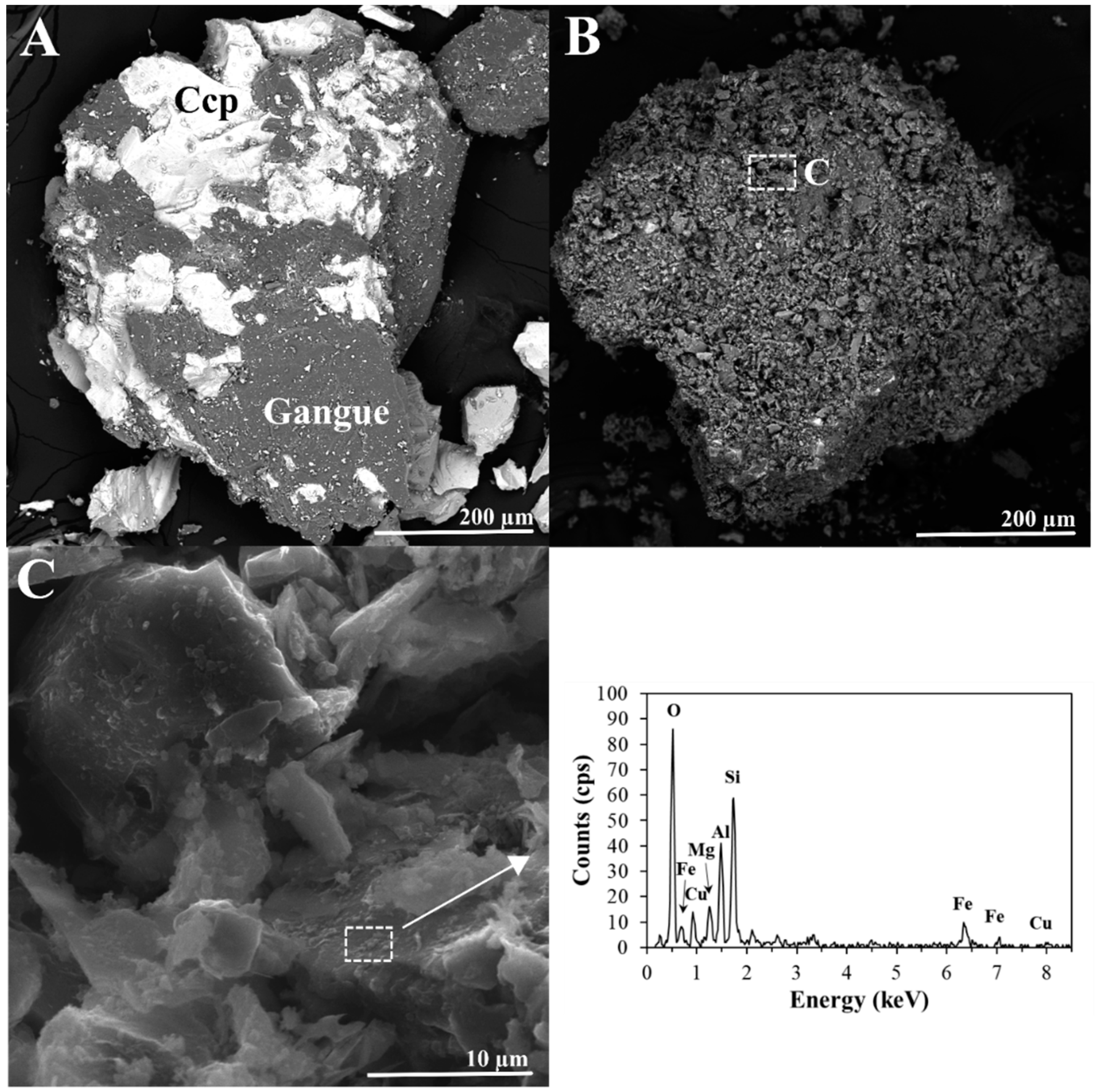
3.1. Mineralogical, Chemical and Morphological Composition
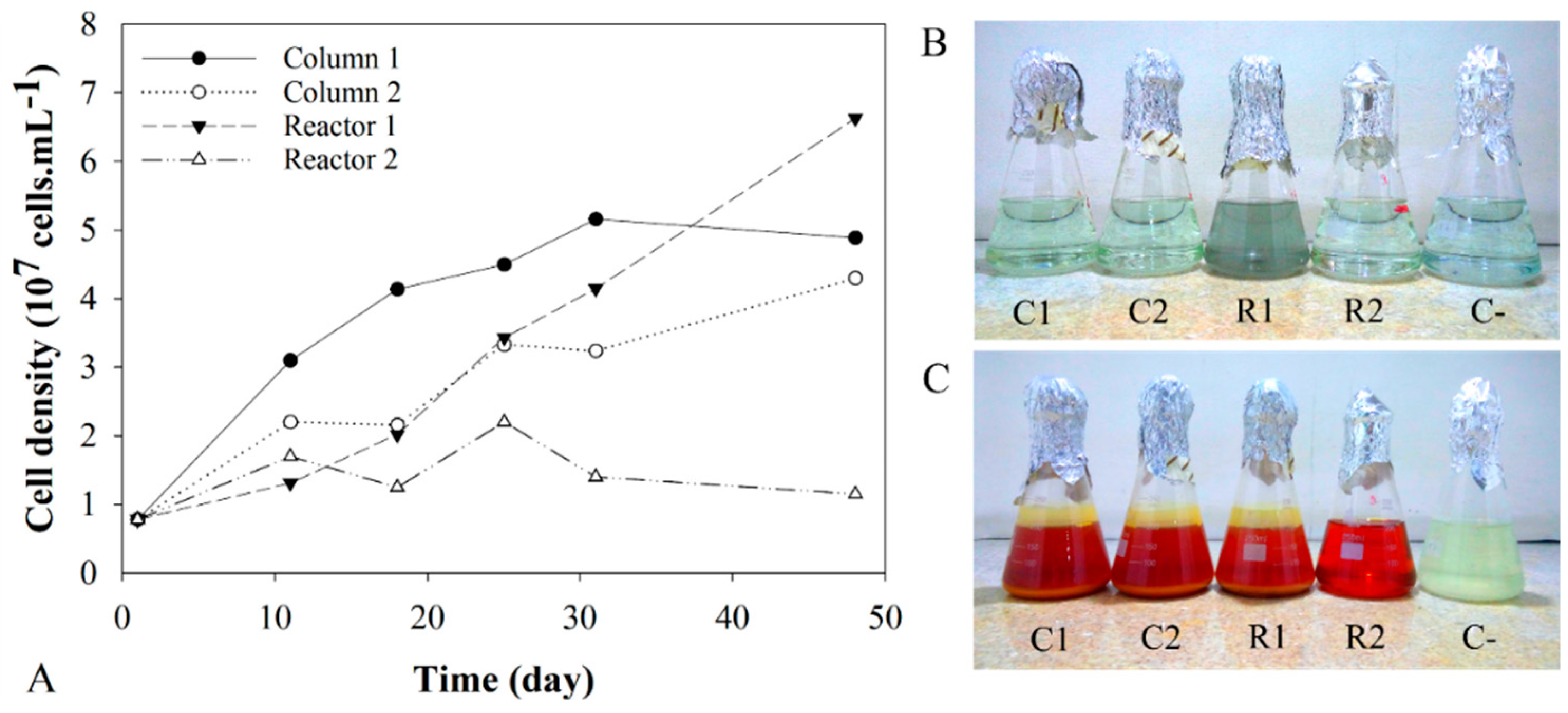
3.2. Shifts in A. ferrooxidans Population
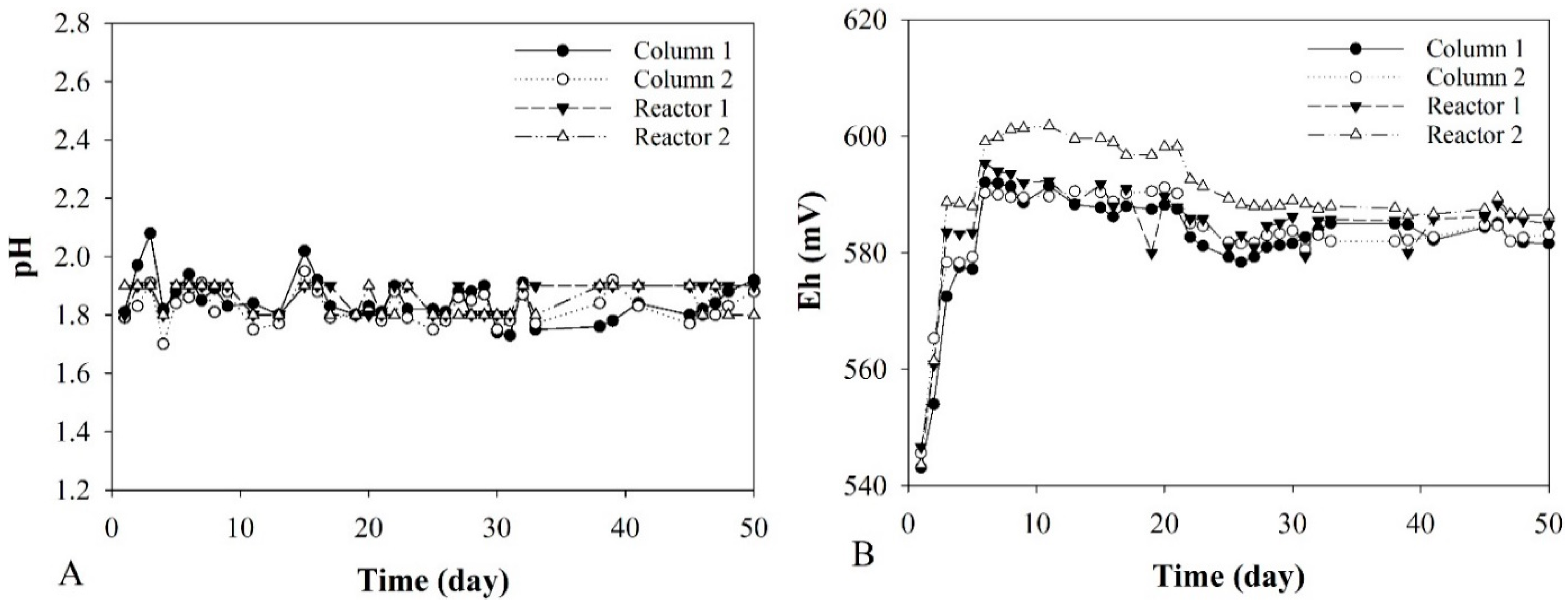
3.3. pH and Eh Monitoring
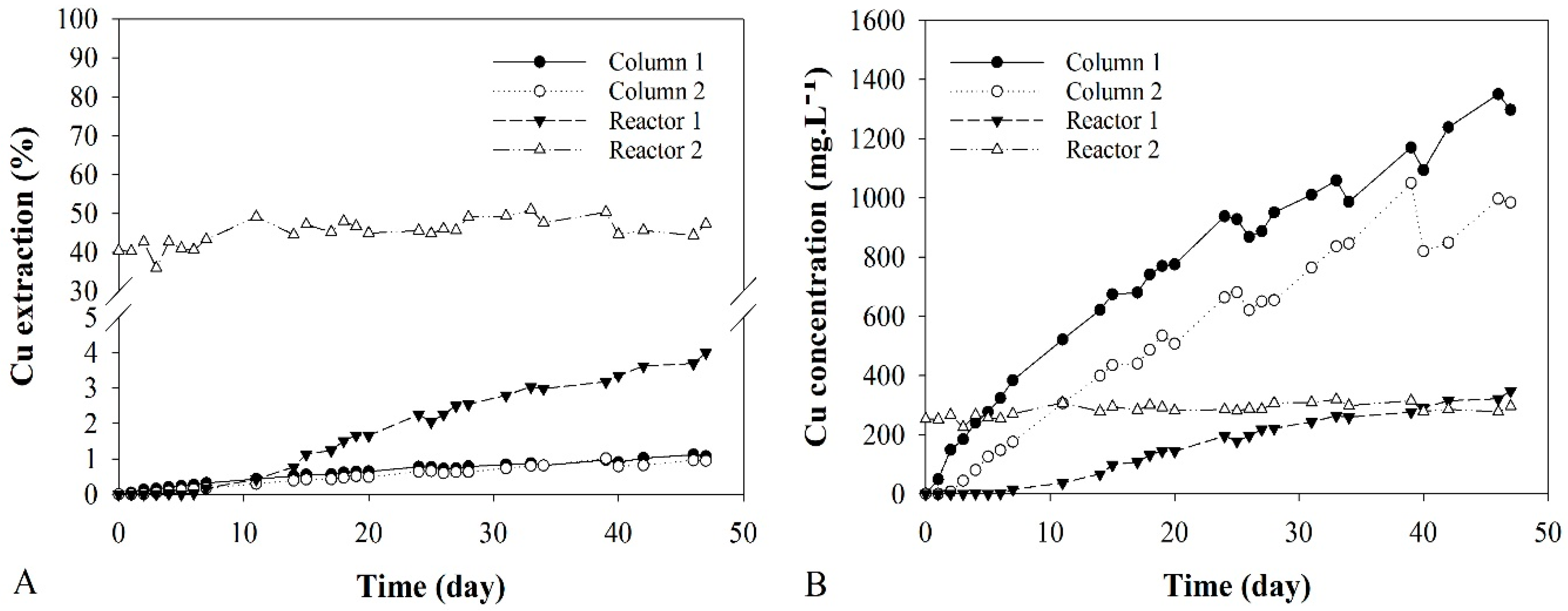
3.4. Copper Extraction
3.4.1. Columns Bioleaching
3.4.2. Stirred Reactors Bioleaching
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brown, T.J.; Idoine, N.E.; Raycraft, E.R.; Shaw, R.A.; Hobbs, S.F.; Everett, P.; Deady, E.A.; Bide, T. World Mineral Production 2012–2016; British Geologycal Survey: Nottingham, UK, 2018; p. 87. [Google Scholar]
- Departamento Nacional de Produção Mineral (DNPM). Anuário Mineral Brasileiro: Principais Substâncias Metálicas; DNPM: Brasília, Brazil, 2018. Available online: http://www.anm.gov.br/dnpm/publicacoes/serie-estatisticas-e-economia-mineral/anuario-mineral/anuario-mineral-brasileiro/amb_metalicos2017 (accessed on 12 March 2018).
- Panda, S.; Akcil, A.; Pradhan, N.; Deveci, H. Current scenario of chalcopyrite bioleaching: A review on the recent advances to its heap-leach technology. Bioresour. Technol. 2015, 196, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Borja, D.; Nguyen, K.; Silva, R.; Park, J.; Gupta, V.; Han, Y.; Lee, Y.; Kim, H. Experiences and future challenges of bioleaching research in South Korea. Minerals 2016, 6, 128. [Google Scholar] [CrossRef]
- Johnson, D.B. Biomining—Biotechnologies for extracting and recovering metals from ores and waste materials. Curr. Opin. Biotechnol. 2014, 30, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Nancucheo, I.; Bitencourt, J.A.P.; Sahoo, P.K.; Alves, J.O.; Siqueira, J.O.; Oliveira, G. Recent developments for remediating acidic mine waters using sulfidogenic bacteria. BioMed Res. Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Watling, H.R. The bioleaching of sulphide minerals with emphasis on copper sulphides—A review. Hydrometallurgy 2006, 84, 81–108. [Google Scholar] [CrossRef]
- Tuovinen, O.H.; Kelly, D.P. Studies on the growth of Thiobacillus ferrooxidans. Archiv Mikrobiol. 1973, 88, 285–298. [Google Scholar] [CrossRef]
- Rawlings, D.E. Characteristics and adaptability of iron- and sulfur-oxidizing microorganisms used for the recovery of metals from minerals and their concentrates. Microb. Cell Fact. 2005, 4, 13. [Google Scholar] [CrossRef]
- Natarajan, K.A. Methods in Biohydrometallurgy and Developments. In Biotechnology of Metals Principles, Recovery Methods and Environmental Concerns; Natarajan, K.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; p. 502. [Google Scholar]
- Borja, D.; Lee, E.; Silva, R.A.; Kim, H.; Park, J.H.; Kim, H. Column bioleaching of arsenic from mine tailings using a mixed acidophilic culture: A technical feasibility assessment. J. Korean Inst. Resour. Recycl. 2015, 24, 69–77. [Google Scholar]
- Ghorbani, Y.; Franzidis, J.-P.; Petersen, J. Heap leaching technology—Current state, innovations and future directions: A review. Min. Process. Extr. Metall. Rev. 2016, 37, 73–119. [Google Scholar] [CrossRef]
- Rankin, J. Energy Use in Metal Production. In Proceedings of the High Temperature Processing Symposium 2012, Swinburne University of Technology, Melbourne, Australia, 6–7 February 2012; p. 3. [Google Scholar]
- Tao, H.; Li, D. Presentation on mechanisms and applications of chalcopyrite and pyrite bioleaching in biohydrometallurgy—A presentation. Biotechnol. Rep. 2014, 4, 107–119. [Google Scholar] [CrossRef]
- Wang, J.; Hu, M.; Zhao, H.; Tao, L.; Gan, X.; Qin, W.; Qiu, G. Well-controlled column bioleaching of a low-grade copper ore by a novel equipment. J. Cent. South Univ. 2015, 22, 3318–3325. [Google Scholar] [CrossRef]
- Huang, M.Q.; Wang, Y.M.; Yin, S.H.; Wu, A.X. Enhanced column bioleaching of copper sulfides by forced aeration. Adv. Mater. Res. 2015, 1130, 400–405. [Google Scholar] [CrossRef]
- Dunbar, W.S. Biotechnology and the mine of tomorrow. Trends Biotechnol. 2017, 35, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, F. The use of reactors in biomining processes. Electron. J. Biotechnol. 2000, 3, 10–11. [Google Scholar] [CrossRef]
- Park, J.; Han, Y.; Lee, E.; Choi, U.; Yoo, K.; Song, Y.; Kim, H. Bioleaching of highly concentrated arsenic mine tailings by Acidithiobacillus ferrooxidans. Sep. Purif. Technol. 2014, 133, 291–296. [Google Scholar] [CrossRef]
- Zhou, H.-B.; Zeng, W.-M.; Yang, Z.-F.; Xie, Y.-J.; Qiu, G.-Z. Bioleaching of chalcopyrite concentrate by a moderately thermophilic culture in a stirred tank reactor. Bioresour. Technol. 2009, 100, 515–520. [Google Scholar] [CrossRef]
- Hedrich, S.; Joulian, C.; Graupner, T.; Schippers, A.; Guézennec, A.-G. Enhanced chalcopyrite dissolution in stirred tank reactors by temperature increase during bioleaching. Hydrometallurgy 2018, 179, 125–131. [Google Scholar] [CrossRef]
- Guezennec, A.-G.; Hedrich, S.; Schippers, A.; Kamradt, A.; Matys, S.; Michels, R.; Joulian, C.; Bodénan, F. Bioleaching in stirred tank reactors to process Kupferschiefer-type ore: Current status and perspectives. In Proceedings of the 16ème Congrès de la Société Française de Génie des Procédés, Nancy, France, 11–13 July 2017; pp. 1–2. [Google Scholar]
- Wang, J.; Zhu, S.; Zhang, Y.; Zhao, H.; Hu, M.; Yang, C.; Qin, W.; Qiu, G. Bioleaching of low-grade copper sulfide ores by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans. J. Cent. South Univ. 2014, 21, 728–734. [Google Scholar] [CrossRef]
- Zeng, W.; Tan, S.; Chen, M.; Qiu, G. Detection and analysis of attached microorganisms on the mineral surface during bioleaching of pure chalcopyrite with moderate thermophiles. Hydrometallurgy 2011, 106, 46–50. [Google Scholar] [CrossRef]
- do Nascimento, D.d.N.O.; Palmieri, M.C.; do Carmo, A.L.V.; Nogueira, E.P.; Lucheta, A.R.; de Ferreira, R.V.P.; Ferreira Filho, H.R.; Alves, J.O. Copper ores bioleaching in reactors with Acidithiobacillus ferrooxidans. Tecnol. Metal. Mater. Min. 2018, 15, 81–85. [Google Scholar]
- Júnior, O.G. Isolation and purification of Thiobacillus ferrooxidans and Thiobacillus thiooxidans from some coal and uranium mines of Brazil. Rev. Microbiol. 1991, 22, 1–6. [Google Scholar]
- Liu, G.; Yin, J.; Cong, W. Effect of fluid shear and particles collision on the oxidation of ferrous iron by Acidithiobacillus ferrooxidans. Min. Eng. 2007, 20, 1227–1231. [Google Scholar] [CrossRef]
- Morin, D.H.R. Bioleaching of sulfide minerals in continuous stirred tanks. In Microbial Processing of Metal Sulfides; Springer: Dordrecht, The Netherlands, 2007; pp. 133–150. [Google Scholar]
- Kim, H.N.; Bradford, S.A.; Walker, S.L. Escherichia coli O157:H7 Transport in saturated porous media: Role of solution chemistry and surface macromolecules. Environ. Sci. Technol. 2009, 43, 4340–4347. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Walker, S.L.; Bradford, S.A. Macromolecule mediated transport and retention of Escherichia coli O157:H7 in saturated porous media. Water Res. 2010, 44, 1082–1093. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.A.; Borja, D.; Hwang, G.; Hong, G.; Gupta, V.; Bradford, S.A.; Zhang, Y.; Kim, H. Analysis of the effects of natural organic matter in zinc beneficiation. J. Clean. Prod. 2017, 168, 814–822. [Google Scholar] [CrossRef]
- Vera, M.; Schippers, A.; Sand, W. Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation—Part A. Appl. Microbiol. Biotechnol. 2013, 97, 7529–7541. [Google Scholar] [CrossRef]
- Pan, H.; Yang, H.; Tong, L.; Zhong, C.; Zhao, Y. Control method of chalcopyrite passivation in bioleaching. Trans. Nonferrous Met. Soc. China 2012, 22, 2255–2260. [Google Scholar] [CrossRef]
- Bevilaqua, D.; Leite, A.L.L.; Garcia, O.; Tuovinen, O. Oxidation of chalcopyrite by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans in shake flasks. Process Biochem. 2002, 38, 587–592. [Google Scholar] [CrossRef]
- Santos, A.L.A.; Arena, F.A.; Benedetti, A.V.; Bevilaqua, D. Effect of redox potential on chalcopyrite dissolution imposed by addition of ferrous ions. Eclética Quím. J. 2017, 42, 40. [Google Scholar] [CrossRef]
- Li, Y.; Kawashima, N.; Li, J.; Chandra, A.P.; Gerson, A.R. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv. Colloid Interface Sci. 2013, 197, 1–32. [Google Scholar] [CrossRef]
- Nguyen, K.A.; Borja, D.; You, J.; Hong, G.; Jung, H.; Kim, H. Chalcopyrite bioleaching using adapted mesophilic microorganisms: Effects of temperature, pulp density, and initial ferrous concentrations. Mater. Trans. 2018, 59, 1860–1866. [Google Scholar] [CrossRef]
- Ngoma, E.; Borja, D.; Smart, M.; Shaik, K.; Kim, H.; Petersen, J.; Harrison, S.T. Bioleaching of arsenopyrite from Janggun mine tailings (South Korea) using an adapted mixed mesophilic culture. Hydrometallurgy 2018, 181, 21–28. [Google Scholar] [CrossRef]
- Hong, J.; Silva, R.A.; Park, J.; Lee, E.; Park, J.; Kim, H. Adaptation of a mixed culture of acidophiles for a tank biooxidation of refractory gold concentrates containing a high concentration of arsenic. J. Biosci. Bioeng. 2016, 121, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Turan, N.G.; Elevli, S.; Mesci, B. Adsorption of copper and zinc ions on illite: Determination of the optimal conditions by the statistical design of experiments. Appl. Clay Sci. 2011, 52, 392–399. [Google Scholar] [CrossRef]
- Alvarez-Puebla, R.A.; dos Santos, D.S.; Blanco, C.; Echeverria, J.C.; Garrido, J.J. Particle and surface characterization of a natural illite and study of its copper retention. J. Colloid Interface Sci. 2005, 285, 41–49. [Google Scholar] [CrossRef]
- Oliveira, D.M.; Sobral, L.; Padrão, D.O. Leaching of copper oxide ore by in situ biological generation of sulphuric acid. In Proceedings of the 5th International Seminar on Process Hydrometallurgy, Santiago, Chile, 10–12 July 2013; pp. 347–353. [Google Scholar]
- Lee, E.; Han, Y.; Park, J.; Hong, J.; Silva, R.A.; Kim, S.; Kim, H. Bioleaching of arsenic from highly contaminated mine tailings using Acidithiobacillus thiooxidans. J. Environ. Manag. 2015, 147, 124–131. [Google Scholar] [CrossRef]

| Sample | SiO2 | TiO2 | Al2O3 | Fe2O3 | CaO | CuO | MgO | NiO | K2O | P2O5 | SO3 | Na2O | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mineral sulfide 4.75 mm | 20.3 | 0.2 | 4.2 | 25.2 | 2.1 | 13.9 | 2.3 | 0.1 | 0.3 | 0.3 | 31.1 | - | - |
| Mineral sulfide 2 mm | 14.1 | 0.1 | 2.9 | 26.8 | 2.0 | 16.1 | 1.9 | 0.2 | 0.2 | 0.4 | 35.2 | - | 0.1 |
| Mineral sulfide <1.68 | 11.4 | 0.1 | 2.5 | 26.1 | 1.6 | 17.4 | 1.5 | 0.1 | 0.2 | 0.4 | 38.6 | - | 0.1 |
| Weathered ore <1.68 | 47.4 | 1.0 | 16.8 | 24.6 | 1.2 | 1.3 | 2.8 | - | 3.6 | 0.7 | - | 0.3 | 0.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, D.N.O.d.; Lucheta, A.R.; Palmieri, M.C.; Carmo, A.L.V.d.; Silva, P.M.P.; Ferreira, R.V.d.P.; Junca, E.; Grillo, F.F.; Alves, J.O. Bioleaching for Copper Extraction of Marginal Ores from the Brazilian Amazon Region. Metals 2019, 9, 81. https://doi.org/10.3390/met9010081
Nascimento DNOd, Lucheta AR, Palmieri MC, Carmo ALVd, Silva PMP, Ferreira RVdP, Junca E, Grillo FF, Alves JO. Bioleaching for Copper Extraction of Marginal Ores from the Brazilian Amazon Region. Metals. 2019; 9(1):81. https://doi.org/10.3390/met9010081
Chicago/Turabian StyleNascimento, Dryelle Nazaré Oliveira do, Adriano Reis Lucheta, Maurício César Palmieri, Andre Luiz Vilaça do Carmo, Patricia Magalhães Pereira Silva, Rafael Vicente de Pádua Ferreira, Eduardo Junca, Felipe Fardin Grillo, and Joner Oliveira Alves. 2019. "Bioleaching for Copper Extraction of Marginal Ores from the Brazilian Amazon Region" Metals 9, no. 1: 81. https://doi.org/10.3390/met9010081
APA StyleNascimento, D. N. O. d., Lucheta, A. R., Palmieri, M. C., Carmo, A. L. V. d., Silva, P. M. P., Ferreira, R. V. d. P., Junca, E., Grillo, F. F., & Alves, J. O. (2019). Bioleaching for Copper Extraction of Marginal Ores from the Brazilian Amazon Region. Metals, 9(1), 81. https://doi.org/10.3390/met9010081






