Abstract
In this paper, the theoretical and industrial definitions of metallic calcium production by the metallothermic process in a vacuum atmosphere were investigated. In the experiments, Al is the only reductant used for metallothermic calcium production. The effects of Al stoichiometry, time variances, and temperature changes were investigated. The experiments were carried out at 1200 °C, 1250 °C, and 1300 °C, and with 100% Al, 125% Al, and 150% Al stoichiometry to produce metallic calcium from the residue of metallic magnesium production. Both the raw materials and the residue phases were characterized by atomic absorption spectrometry (AAS), X-ray diffraction (XRD) spectrometry, and chemical analysis techniques. Experimental results were investigated to determine the highest efficiency of reduction conditions. From the results of the experiments, reaction kinetics and activation energy were calculated. According to the experimental results, the highest recovery rate parameters for the reduction of calcium are 150% stoichiometric Al for 480 min at 1300 °C, with 72% recovery.
1. Introduction
Calcium is a shiny, silver-white metal and is quite ductile and soft. Calcium metal oxidizes immediately in the air at temperatures above 300 °C [1,2]. The heat of oxide formation is −304 kcal/mole O2 at 25 °C, and the boiling point of the element and the melting point of calcium oxide are 1482 °C and 2580 °C, respectively [3]. The main calcium-containing minerals are given in Table 1.

Table 1.
Main calcium-containing minerals [2,3].
Calcium metal is never found in a natural state due to its chemical reactivity with oxygen. The main components of sedimentary rock samples such as limestone, chalk, marble, and dolomite, as well as eggshells and pearls, are calcite formations. In addition, limestone is the most common source of metallic calcium production [2,3]. Calcium interacts with almost any form of metal oxide at high temperatures and is an excellent reducing agent. Calcium is used in lead refining (separation of bismuth), steel purification (desulfurization, deoxidation, and dephosphorization), and as an alloying agent for silicon and lead. Also, calcium can be used to recover refractory metals from their oxide form (e.g., chromium, rare earths, and thorium), for the reduction of uranium dioxide, and as an agent for the reduction of boron oxide [4,5,6].
The major industrial calcium producers are Russia, China, the USA, and France. Russian calcium production facilities produced 4000 tons of metallic calcium in 2011. China, as a main producer, increased its amount of calcium production between 2006 and 2011. The common reason given for this increase is that only two companies produced calcium by the electrolytic method in the 1960s, but later, the aluminothermic method of production started and a large number of companies started to produce metallic calcium. In 2011, China produced 30,000–35,000 tons of calcium by the aluminothermic method while also producing 4000–6000 tons of calcium using the electrolytic process. The USA produces approximately 1000–2000 tons of calcium annually using the aluminothermic method. Also, France has facilities for the production of calcium metal, but has not been producing it recently. The reserves of calcium are almost unlimited due to the presence of living organisms [7,8,9].
The first production method was the electrolysis of anhydrous molten calcium chloride in 1808 [10]. Then, the aluminothermic process was developed by Goldschmidt in 1898, with the reduction of metal oxides by a metal exchange reaction. This process enhances the significance of the extraction technology of refractory metals. After that, electrolytic calcium production was replaced by the metallothermic reduction process [11].
Nowadays, the use of high-purity raw dolomite materials (99.5% CaCO3·MgCO3) makes it possible to produce 99.95% or better magnesium in commercial quantities, mostly using the silicothermic reduction method under a vacuum atmosphere. In China, the consumption quantities per ton of magnesium in the reactors are as follows: 0.9 MWh of electricity, 10.5 tons of coal, 11 tons of dolomite, 82% magnesium efficiency, and 6.2 tons of residue with a high CaO content [12].
Pure calcium metal is obtained by the industrial-scale metallothermic reduction of calcium oxide with aluminum [13,14]. A mixture of calcium oxide and aluminum powder is heated to 1200 °C under a vacuum atmosphere and the reduction begins to produce calcium metal [15]. The thermite reaction is described as the exothermic reduction of metallic oxides. This process releases a very large amount of heat and is a sufficient way to heat the product phases above their melting points. Recently, the thermite reaction has been described as a wide class of reactions. It is defined as a metal reacting with a metallic or a nonmetallic oxide to produce a more stable metal or a corresponding metal. The form of the general reduction reaction can be written in a general form, as shown by Equation (1) [6,16]:
where M is a metal or alloy, A is a metal or nonmetal, MO and AO are their corresponding oxides, and enthalpy (ΔH) is generated by the reaction. In the aluminothermic Ca production process, aluminum or aluminum-based materials are used as the reductant of CaO-based raw materials. Reduction of Ca is carried out at high temperatures under a vacuum atmosphere. Ca is in the gas phase at reduction temperatures. The general reaction is shown by Equation (2) [17] and the standard enthalpy value of the reaction was calculated by using FactSage Thermfact/CRCT (Montreal, QC, Canada) and GTT-Technologies (Aachen, Germany) thermochemical simulation software, version 6.4.
M + AO → MO + A + ΔH
3CaO(s) + 2Al(s) → 3Ca(s) + Al2O3(s), ΔH°298 = 229.5 kJ
Pidgeon et al. performed the first calcium production via the Pidgeon process, which uses aluminum as a reducing agent. The reduction was done at 1170 °C in a steel retort under a vacuum atmosphere. The stoichiometry of aluminum had been taken as 100%, 110%, and 120%. The reaction was realized at 45 kg with charge in one retort, and recovery levels of 85.8%, 92.0%, and 96.0% of calcium were observed. Heating cycles of the experiments were 12, 17, and 24 h [18]. Pidgeon et al. used CaO as a raw material during their experiments.
In Turkey, magnesium metal was not produced until 2015. However, Turkey’s dolomite reserves amount to 16 billion tons (identified and probable). A new Turkish plant started with a production capacity of 15,000 tons/year using the silicothermic process. Since 2002, Yücel et al., using Turkish minerals, have investigated the parameters for the silicothermic reduction of calcined dolomite [13]. In 2017, Turkey produced 5000 tons of magnesium. The production residue includes high amounts of CaO [3,14].
In this study, magnesium production residues were used as a raw material to obtain calcium metal instead of CaO. Starting from a CaO-containing waste (residue), we aimed to reduce CO2 emission (440 kg CO2 for per metric ton of CaCO3) and eliminated energy loss (178 kJ/mol) in the calcination process, which is needed for the decomposition of CaCO3 to CaO [19]. Al was used as a reductant. In order to evaluate magnesium residues, the effects of time, temperature, and reductant stoichiometry on the results were investigated. Experimental studies were made under a vacuum atmosphere within steel retort in a horizontal furnace. The effects of variable process parameters on Ca recovery were investigated and kinetic model studies were performed with the obtained data.
2. Thermodynamic Background
The thermodynamic parameters were calculated by using the FactSage 6.4 database. Figure 1 was plotted to understand the change of the reduction conditions of MgO and CaO using the Al reductant under 1 mbar vacuum atmosphere and 1 bar pressure at standard atmosphere. For 1 bar atmosphere, it can be seen that the reaction temperature needs to be 2332 °C for CaO reduction with an Al reductant. Metallic calcium vapor pressure is important for condensing the calcium as a metal in the process.
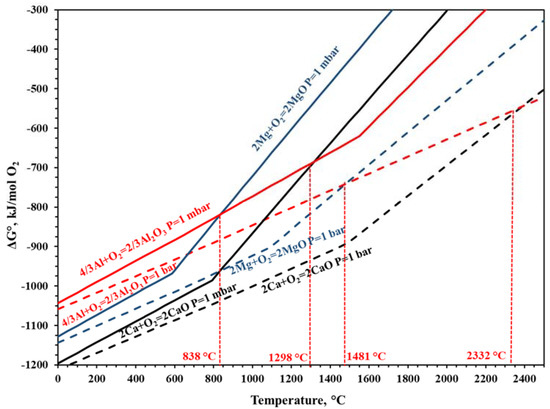
Figure 1.
Change of Gibbs free energy for the reduction of CaO and MgO using Al reductant at 1 mbar and 1 bar.
Figure 1 and Table 2 give reduction temperatures of MgO and CaO with the Al reductant, and it can be clearly seen that CaO reduction starts at 1298 °C. Figure 2 presents probable phases of the CaO reduction process. After 1150 °C, the Ca(g) phase increased dramatically. At 1300 °C, 2.25 mole of Ca(g) occurred. According to the thermodynamic investigations, Ca(g) occurred between 1150 °C and 1200 °C.

Table 2.
Minimum reduction temperatures of CaO and MgO for Al reductant at 1 bar and 1 mbar.
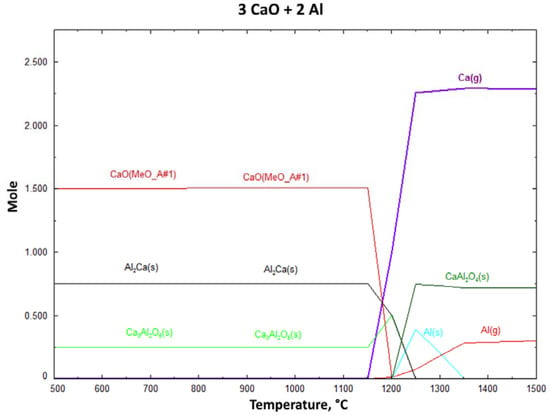
Figure 2.
Change of reaction products with increasing process temperature for Al reductant (1 mbar).
The diagrams were plotted for 100% stoichiometry of the reductant Al at 1 mbar. In accordance with the Pidgeon process, reactions occurred at lower temperatures around 1 mbar than 1 bar [15]. According to the FactSage 6.4 database 3CaO·Al2O3(s) and Al2Ca(s) are stable phases after 500 °C at 1 mbar vacuum atmosphere. In addition, the amount of AlC2(s) phase decreased above 1100 °C, and Al(s) appeared. The Al formed to promote the formation of Ca(g) phase up to 1300 °C and above this temperature, Al(s) dramatically turns into Al(g) phase.
In Figure 3, in the same circumstances, Al(s) cannot be formed, so AlF(g) is observed. So the decreased amount of Ca(g) and the CaF2(s) addition effect are not investigated in experimental studies. Figure 3 shows the effect of CaF2 addition and the decreased Ca(g) products above 1200 °C. At around 1400 °C, the AlF amount slightly decreases, whilst the reduced Ca(g) amount increases simultaneously. Figure 4 shows the effect of FeSi as a reductant and Ca(g) starts to occur above 1400 °C. Neither of these additions had a positive effect on CaO reduction.
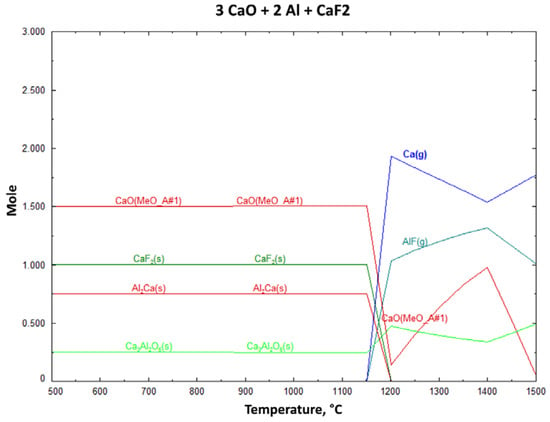
Figure 3.
Change of reaction products with increasing process temperature for Al and CaF2 reductant (1 mbar).
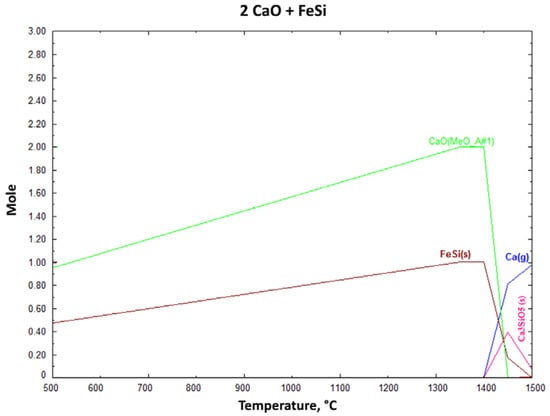
Figure 4.
Change of reaction products with increasing process temperature for FeSi reductant (1 mbar).
Figure 5 plotted the results to understand the cooling behavior of Ca(g). According to Figure 5, Ca(g) converts to solid phase at 794 °C and 1 mbar conditions and it does not present any reduction condition. It was only simulated to represent maximum cooling temperatures of the condensation zone.
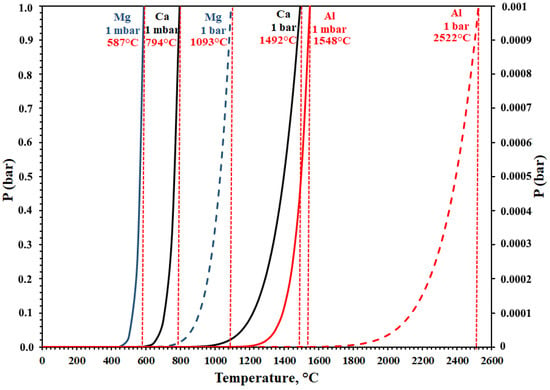
Figure 5.
Change of vapor pressure of Ca, Mg, and Al at 1 mbar and 1 bar.
In Figure 5, it can be seen that Ca vapor pressure started and increased dramatically at 650 °C. In the experimental studies, an average process pressure of 0.5 mbar was used and the process temperatures were 1200 °C, 1250 °C, and 1300 °C. Thus, it is clear that formation of the gas phase of calcium is possible with Al used as a reductant and 1 mbar under vacuum conditions.
3. Experimental Studies
Raw material was provided by domestic sources of Mg reduction residue for use as a calcium source. Residues were analyzed by chemical analysis and AAS (PerkinElmer AAnalyst 800, PerkinElmer Inc., Boston, MA, USA) techniques. Chemical analysis of the residue is given in Table 3. Al was employed as a reductant, with 99.02% Al, 0.84% Fe, and 0.01% Mg. The XRD pattern of the residue was obtained by PANalytical PW3040/60 XRD (Panalytical B.V., Almelo, The Netherland) (Cu Kα, λ = 0.154 nm). The XRD pattern and the reference codes of the patterns are given in Figure 6.

Table 3.
Chemical analysis of the Mg production residue.
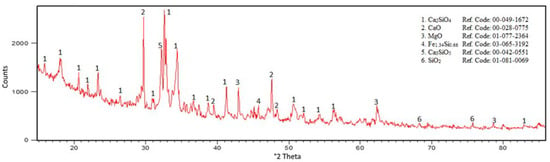
Figure 6.
X-ray diffraction (XRD) pattern of raw materials of residue.
Stainless steel in the shape of a cylindrical retort was used as a pot in the experiments. The calcium metal was vaporized and the residue was placed in the retort inside a ceramic boat [20]. To achieve the vacuum atmosphere inside the retort, a dual-stage Edwards 8 E2M8 rotary vane mechanical vacuum pump was used, which can hold a final pressure of 0.1 mbar (Edwards High Vacuum, Crawley, UK). The furnace had SiC resistance and the retort was externally heated to a maximum temperature of 1350 °C. A schematic sketch of the experimental setup is shown in Figure 7.
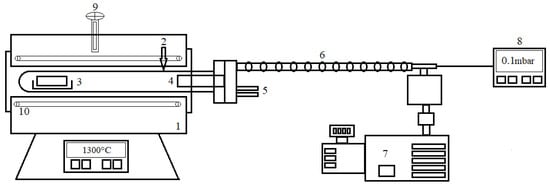
Figure 7.
Schematic sketch of experimental setup: (1) furnace; (2) stainless steel retort; (3) charge; (4) condensation section; (5) cooling water in and out; (6) vacuum connection; (7) vacuum pump; (8) digital pressure gauge; (9) PtRh30/PtRh6 thermocoupler; (10) SiC resistance.
In the present study, experimental sets were developed to understand the effects of reductants on the vacuum aluminothermic process. In the experiments, the effects of Al stoichiometry were examined. The amount of Al reductant that can reduce calcium oxide was defined as 100% stoichiometry. Stoichiometry of 100% reductant was determined for the Al amount to reduce the Mg production residue. It was changed from 100% stoichiometric Al to 150% stoichiometric Al in 25% increments. In these experiments, the effects of time and temperature changes were also investigated for 60, 120, 180, 240, 360, and 480 min and 1200 °C, 1250 °C, and 1300 °C under 1 mbar.
In the final stage of the reduction, the steel retort was left to cool in the furnace at the exact same vacuum values and until it reached room temperature. After that, the cover was opened and the residues were left in the ceramic base. Then, these residues were weighed and analyzed. The level of Ca metal recovery was calculated from the residue by using Equation (3):
where W0 is the weight of raw materials, Ca0% is the weight percentage of calcium in the raw materials, W1 is the weight of residue, and Ca1% is the weight percentage of calcium in the residue after reduction. Experimental procedures were set up to investigate the effects of reductants on the Pidgeon process with changing Al stoichiometry, time, and temperature. The crown calcium recoveries could not be calculated because there was an insufficient quantity of product to analyze.
Ca recovery % (from residue) = {[(Ca0% × W0) − (Ca1% × W1)]/(Ca0% × W0)} × 100
4. Results and Discussion
Experimental trials began with the theoretical requirement of 100% stoichiometric Al reductant and the time effect was investigated for calcium reduction at 1200 °C. Raw materials as metallic magnesium production residue and 100% stoichiometric Al powder mixture were prepared and experiments were conducted with the same amount of mixture, 9 g. The recovery efficiency of calcium was calculated from the residue. The XRD patterns of postreduction residual materials are displayed in Figure 8. When the process time is increased from 60 min to 240 min, the (CaO)12(Al2O3)7 phase turns to Ca(AlO2)2. The reason for the lack of Al peaks in the XRD patterns of the residues from low recovery ratios may be the reduction of Si from SiO2-containing phases because of the relatively easy reduction of Si by Al. Thus, metallic Al converts into an aluminium oxide form [21].
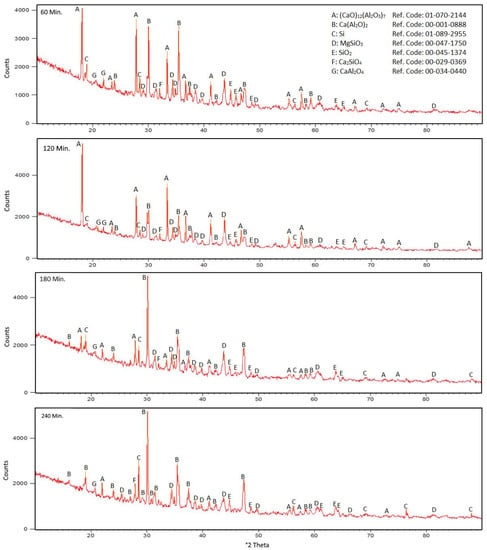
Figure 8.
XRD patterns for 1300 °C, 150% stoichiometry, 60, 120, 180, and 240 min after residual reduction.
It was obvious that the 100% Al reductant and the extended time did not affect the recovery rate of calcium. The recovery rates were 3% to 19% in the first experiments. Then, Al stoichiometry was increased to 125%, and the recovery rates were 5–21%. After 150% Al stoichiometry was tried, the recovery rates were 5–20%. This showed us that the amount of reductant was not enough to obtain Ca reduction with high recovery rates, as shown in Table 4 and Figure 9.

Table 4.
Parameters and efficiency of the experimental studies (pellet wt., 9 g).
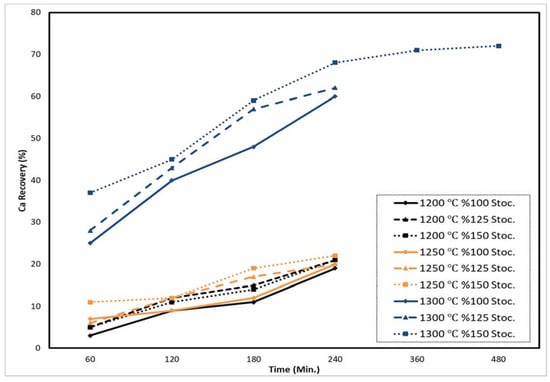
Figure 9.
Ca recovery rates for Al reductant with increasing stoichiometry and process duration at 1200 °C, 1250 °C, and 1300 °C for 60–480 min under 1 mbar.
In the second set of experiments, the reaction temperature was fixed at 1250 °C. This time, the highest recovery detected for the total charge amount of 9 g, 150% Al stoichiometry, 1250 °C, and 240 min was 22%.
Figure 9 shows the Ca amounts in residues and the Ca recovery rates for Al reductant with increasing stoichiometry and process duration at 1250 °C. The difference between Ca recovery rates increased with time and Al stoichiometry.
The recovery results increased from 7% to 20% at 100% Al stoichiometry. When Al stoichiometry was increased to 125%, the recovery rates were 6–20%. After the experimental set was repeated at 150% Al stoichiometry, the recovery rates were 11–22%. This showed us that the amount of reductant was not enough to produce Ca reductions with high recovery rates.
In the third experimental set, the reduction temperature was set at 1300 °C, and the highest CaO amount in residue was obtained in the experiment conducted with 150% Al and 240 min, with a 68% recovery rate. The recovery rates were 25–60% at 100% Al stoichiometry. When Al stoichiometry was changed to 125%, the recovery rates were 36–62%.
Finally, Al stoichiometry was fixed at 150% and the recovery rates were 37–72%. This showed us that the amount of reductant was enough to obtain Ca reductions with high recovery rates. In brief, increasing the time and the reductant stoichiometry increased the recovery of Ca. All experimental study parameters and efficiencies are given in Table 4. Pressures were from 0.2 mbar to 0.9 mbar.
Ca recovery is given in Figure 9, and the amount of Ca in the residual after the experiments is shown in Figure 10. In the experiment with the longest time and highest temperature (8 h, 1300 °C), mass balance was calculated and it was observed that 3.86% of Al turned into vapor phase. Details on the results and discussions of the Al turning into vapor phase and the Mg reduction from raw materials which have a higher MgO content will be published in another research article.
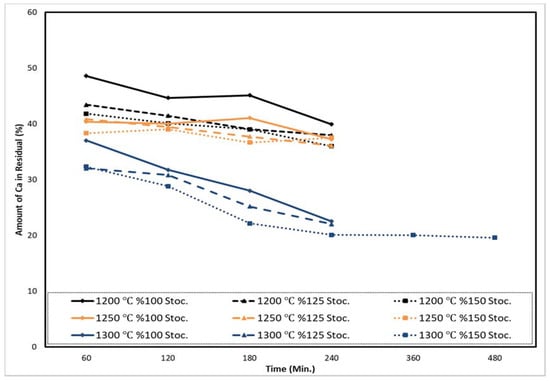
Figure 10.
Ca amounts in residues of Al reductant with increasing stoichiometry and process duration at 1200 °C, 1250 °C, and 1300 °C for 60–480 min under 1 mbar.
It can be clearly seen that temperature directly affects residual Ca recovery rates. The highest Ca recovery rate at 1200 °C for 240 min was 21%, at 1250 °C for 240 min it was 22%, and at 1300 °C for 480 min it was 72%. Figure 9 and Figure 10 show experimental data after chemical analysis to determine the Ca recovery plot, supported by the amount of Ca in the residue.
Figure 9 shows that when the reduction time increased from 60 to 480 min, 150% Al reductant stoichiometry increased Ca recovery from 37% to 72%. Up to a 240 min recovery rate, it increased from 37% to 68%. From 240 min to 480 min of reduction, the recovery rate increased 68–72%. Temperature from 1200–1250 °C to 1300 °C affected Ca recovery positively. Figure 10 shows that after reduction, the amount of Ca in the residual decreased, depending on the Ca recovery efficiency during the reduction. Ca in the residual decreased when the temperature increased from 1200–1250 °C to 1300 °C. This shows that the results of Ca recovery and amount of Ca residual were in agreement.
To determine the rate constant (k) of the Pidgeon process in the temperature range of 1200–1300 °C, Equation (4), the Jander kinetic model, was plotted over time, as shown in Figure 11 and Figure 12:
where k is the rate constant, t is the time (hour), and α is the calcium recovery fraction [22,23].
kt = [1 − (1 − α)1/3]2
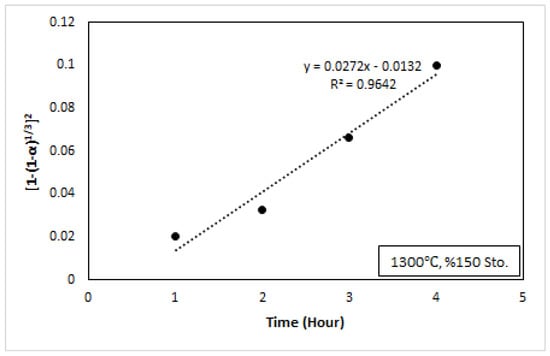
Figure 11.
Ca reduction recovery at 1300 °C and 150% stoichiometry, with the plot of kt = [1 − (1 − α)1/3]2 vs. time.
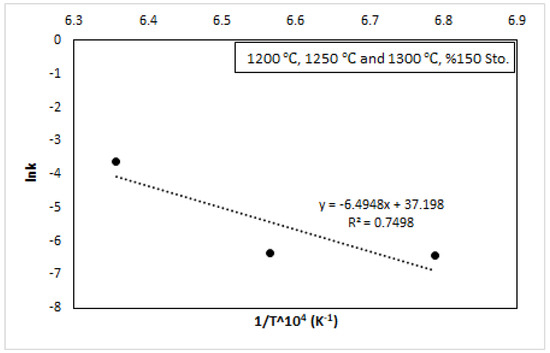
Figure 12.
Ca reduction recovery at 1200 °C, 1250 °C, and 1300 °C, 150% stoichiometry, with the Arrhenius plot of ln (k) vs. 104/T.
The slope of the graph gives the value of the activation energy, which was calculated from Arrhenius Equation (5):
K = A × e−Ea/RT
Taking the logarithms of both sides of the equation and separating the exponential and pre-exponential terms yields the equation of a straight line, and the slope gives −Ea/R. The k values are calculated from the slope of the curves and are given in Table 5. These mathematical calculations are the easiest way to determine the activation energy from the values of k observed at different temperatures and plotting lnk for a function of 1/T, as seen in Equation (6):
lnk = lnA − Ea/RT

Table 5.
Kinetic results of the experimental studies.
Figure 11 shows 150% reductant stoichiometry and 1300 °C conditions, time, and the value of kt = [1 − (1 − α)1/3]2. From the slope of this figure, the value of k was calculated. In the experimental studies, 1200 °C, 1250 °C, and 1300 °C, and 100% Al, 125% Al, and 150% Al reductant stoichiometry were applied. The kinetic results are given in Table 5. It was observed that the value of k increased as the temperature increased. The result of the rate constant and Arrhenius equation was related to Figure 11. As seen in Figure 11, the rate constant increased with increasing temperature. The result was in accordance with the fact that the reaction rate increases with increasing temperature.
5. Conclusions
This study had three main objectives. First, we wanted to recycle the Mg production residue to use it as a raw material for Ca production. Starting from the residue, our experiments reduced CO2 emission and eliminated energy loss in the calcination process, which is needed for the decomposition of CaCO3 to CaO and for eliminating energy consumption for the calcination process.
The reduction to obtain metallic calcium was investigated by using reductant stoichiometry of 100% Al, 125% Al, and 150% Al at 1200 °C, 1250 °C, and 1300 °C, while varying the process duration from 60 to 240 min. The results show that the highest Ca recovery from the residue was obtained in the experiment with a 9 g charge weight, 150% stoichiometric Al at 1300 °C, and a 480 min reduction time. The highest recovery rate obtained was 72%.
Al is used as a reductant for the metallothermic reduction of calcium. The results show that when the time increased from 60 min to 240 min, the recovery rate of Ca increased. For instance, 150% Al stoichiometry for 60 min had a recovery ratio of about 37%. Meanwhile, with 150% Al stoichiometry at 1300 °C for 240 min, the Ca recovery ratio increased to 68%. The exponentially increasing slope of the line was broken after 240 min at 1300 °C and then no significant increment was identified, as seen in Figure 9. Therefore, the optimum parameters for the reduction of calcium are 150% stoichiometric Al at 1300 °C for 240 min, with 68% recovery.
Kinetic studies showed that Mg production residues need a higher activation energy than the raw CaO reduction process. The reduction of CaO with Al required ΔH°298 = 229.5 kJ at room temperature. From our experiments with residues, it can clearly be seen that reducing Ca needs 540.01 kJ/mol with 150% reductant stoichiometry. Our model fits the Jander kinetic model, a diffusion-controlled reaction model.
From the results of these experiments, it was understood that the use of Al with increasing stoichiometry and a longer reduction time raises the efficiency of metallic calcium production.
Author Contributions
K.C.T. and M.B. conceived and designed the experiments; O.Y. is the advisor of the experiments and the project.
Acknowledgments
The authors gratefully acknowledge the Istanbul Technical University BAP department, which made this research possible (ITU BAP Project ID: 40654).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cardarelli, F. Materials Handbook: A Concise Desktop Reference, 3rd ed.; Springer: London, UK, 2008; pp. 620–626. [Google Scholar]
- Mantell, C.L. The alkaline earth metals: Calcium, barium, and strontium. In Rare Metals Handbook, 2nd ed.; Hampel, C.A., Ed.; Reinhold: New York, NY, USA, 1973; pp. 15–25. [Google Scholar]
- Belitskus, D. Aluminothermic production of metals and alloys. JOM 1972, 24, 30–34. [Google Scholar] [CrossRef]
- Sokic, M.; Matković, V.; Marković, B.; Gulišija, Z.; Patarić, A.; Mihailović, M.; Janjušević, Z. The possibilities of obtaining metallic calcium from Serbian carbonate mineral raw materials. Chem. Ind. Chem. Eng. Q. 2014, 20, 397–405. [Google Scholar] [CrossRef]
- Liu, L.; Chen, M.; Xu, L.; Yin, X.; Sun, W. Effect of BaO Addition on Densification and Mechanical Properties of Al2O3-MgO-CaO Refractories. Metals 2016, 6, 84. [Google Scholar] [CrossRef]
- Giorgi, F.T.; Alexander, S. Production of Advanced Materials by Methods of Self-Propagating High-Temperature Synthesis; Springer: New York, NY, USA, 2013. [Google Scholar]
- Theodore, G. The Elements, a Visual Exploration of Every Known Atom in the Universe; Black Dog & Leventhal: New York, NY, USA, 2009. [Google Scholar]
- Albert, S. A Guide to the Elements, 3rd ed.; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- John, E. Nature’s Building Blocks: An A-Z Guide to the Elements; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Mantell, C.L.; Hardy, C. Calcium Metallurgy and Technology; Reinhold: New York, NY, USA, 1945; p. 358. [Google Scholar]
- Mehra, O.K.; Bose, D.K.; Gupta, C.K. Molybdenum Metal by the Aluminothermic Reduction of Calcium Molybdate. Metall. Trans. 1973, 4, 691–694. [Google Scholar] [CrossRef]
- Xiaoming, M. Magnesium technology 2001. In Proceedings of the TMS Annual Meeting, New Orleans, LA, USA, 11–15 February 2001; pp. 13–15. [Google Scholar]
- Buğdaycı, M.; Turan, A.; Alkan, M.; Yücel, O. Effect of Reductant Type on the Metallothermic Magnesium Production Process. High Temp. Mater. Process. 2017. [CrossRef]
- Rodriguez-Navarro, C.; Ruiz-Agudo, E.; Luque, A.; Rodriguez-Navarro, A.B.; Ortega-Huertas, M. Thermal decomposition of calcite: Mechanisms of formation and textural evolution of CaO nanocrystals. Am. Mineral. 2009, 94, 578–593. [Google Scholar] [CrossRef]
- Beruto, D.; Searcy, A.W.; Kim, M.G. Microstructure, kinetic, structure, thermo- dynamic analysis for calcite decomposition: Free-surface and powder bed experiments. Thermochim. Acta 2004, 424, 99–109. [Google Scholar] [CrossRef]
- Wang, L.L.; Munir, Z.A.; Maximov, Y.M. Review Thermite reactions: Their utilization in the synthesis and processing of materials. J. Mater. Sci. 1993, 28, 3693–3708. [Google Scholar] [CrossRef]
- Kulifeev, V.K.; Kropachev, A.N.; Tarasov, V.P. Thermodynamic Investigations and Substantiation of the Aluminothermic Fabrication Method of Calcium. Russ. J. Non-Ferr. Met. 2016, 57, 15–22. [Google Scholar] [CrossRef]
- Pidgeon, L.M.; MacCatty, S.A. Production of Calcium. U.S. Patent Office Patent Number 2,464,767, 15 October 1949. [Google Scholar]
- Senthoorselvan, S.; Gleis, S.; Hartmut, S.; Yrjas, P.; Hupa, M. Cyclic Carbonation Calcination Studies of Limestone and Dolomite for CO2 Separation from Combustion Flue Gases. J. Eng. Gas Turbines Power 2009. [CrossRef]
- Yücel, O.; Yiğit, S.; Derin, B. Production of Magnesium Metal from Turkish Calcined Dolomite using Vacuum Silicothermic Reduction Method. Mater. Sci. Forum 2005, 488–489, 39–42. [Google Scholar]
- Hasegawa, M. Ellingham Diagram. In Treatise on Process Metallurgy, Volume 3: Industrial Processes, 1st ed.; Elsevier: New York, NY, USA, 2013. [Google Scholar]
- Zhang, C.; Wang, C.; Zhang, S.; Guo, L. The effects of hydration activity of calcined dolomite (HCD) on the silicothermic reduction process. Int. J. Miner. Process. 2015, 142, 154–160. [Google Scholar] [CrossRef]
- Wulandari, W.; Brooks, G.A.; Rhamdhani, M.A.; Monaghan, B.J. Kinetic analysis of silicothermic process under flowing argon atmosphere. Can. Metall. Q. 2014, 53, 17–25. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).