Chemical Dealloying Synthesis of CuS Nanowire-on-Nanoplate Network as Anode Materials for Li-Ion Batteries
Abstract
:1. Introduction
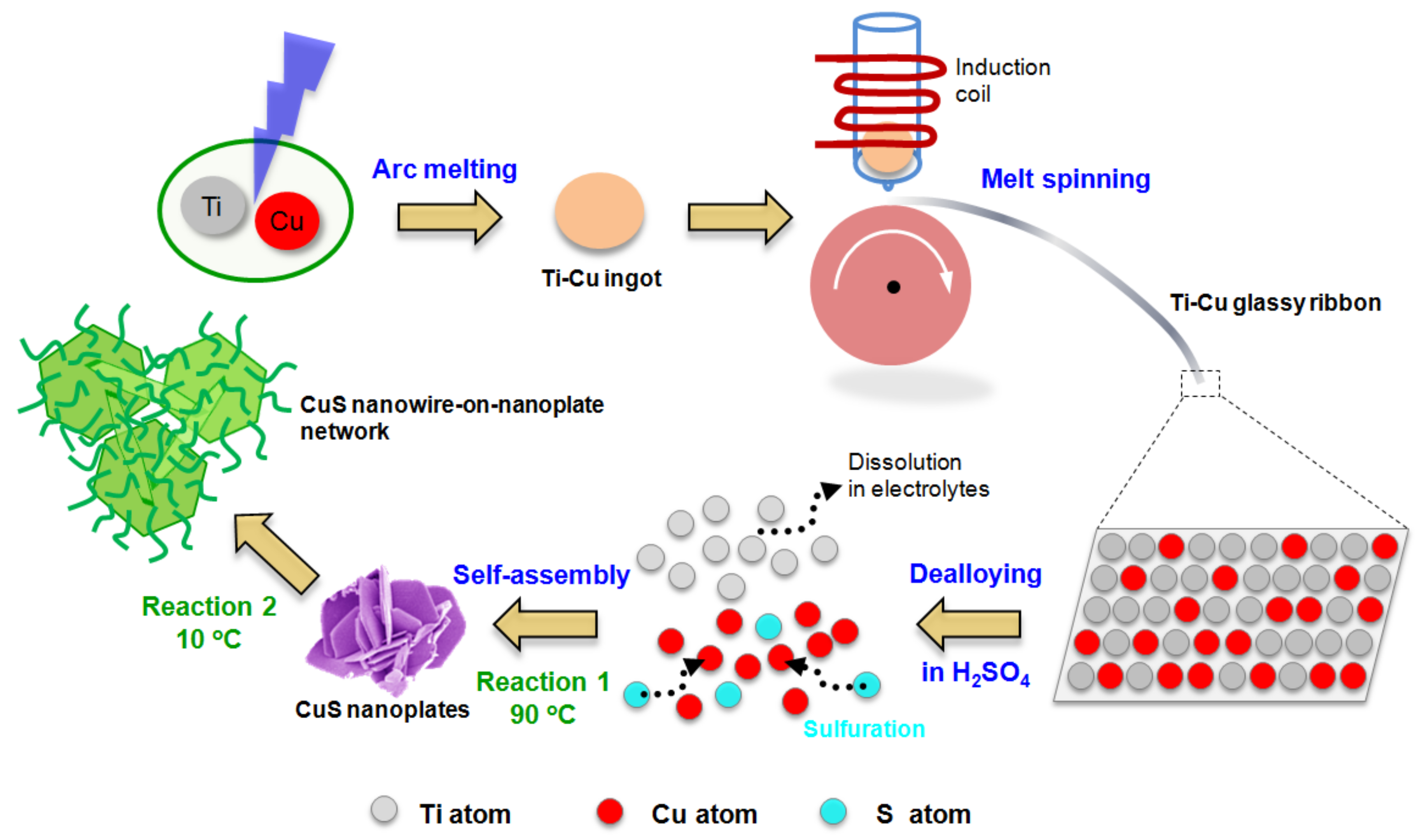
2. Materials and Methods
2.1. Preparation of Materials
2.2. Materials Characterization
2.3. Electrochemical Measurements
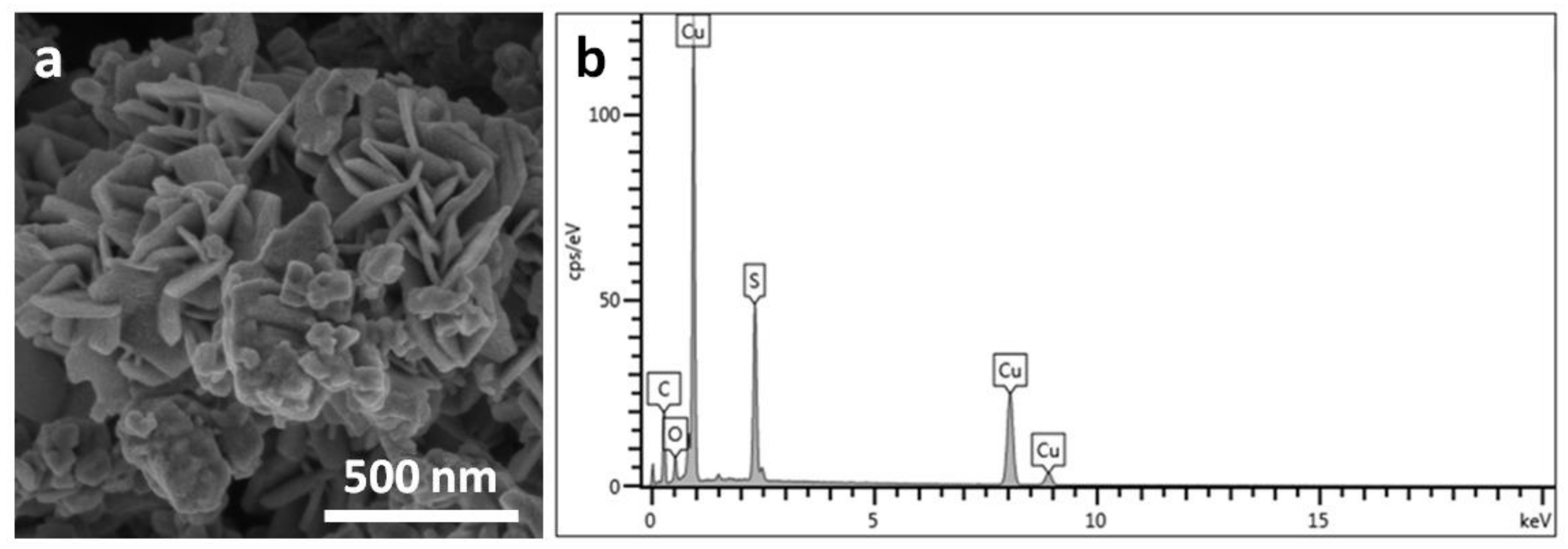
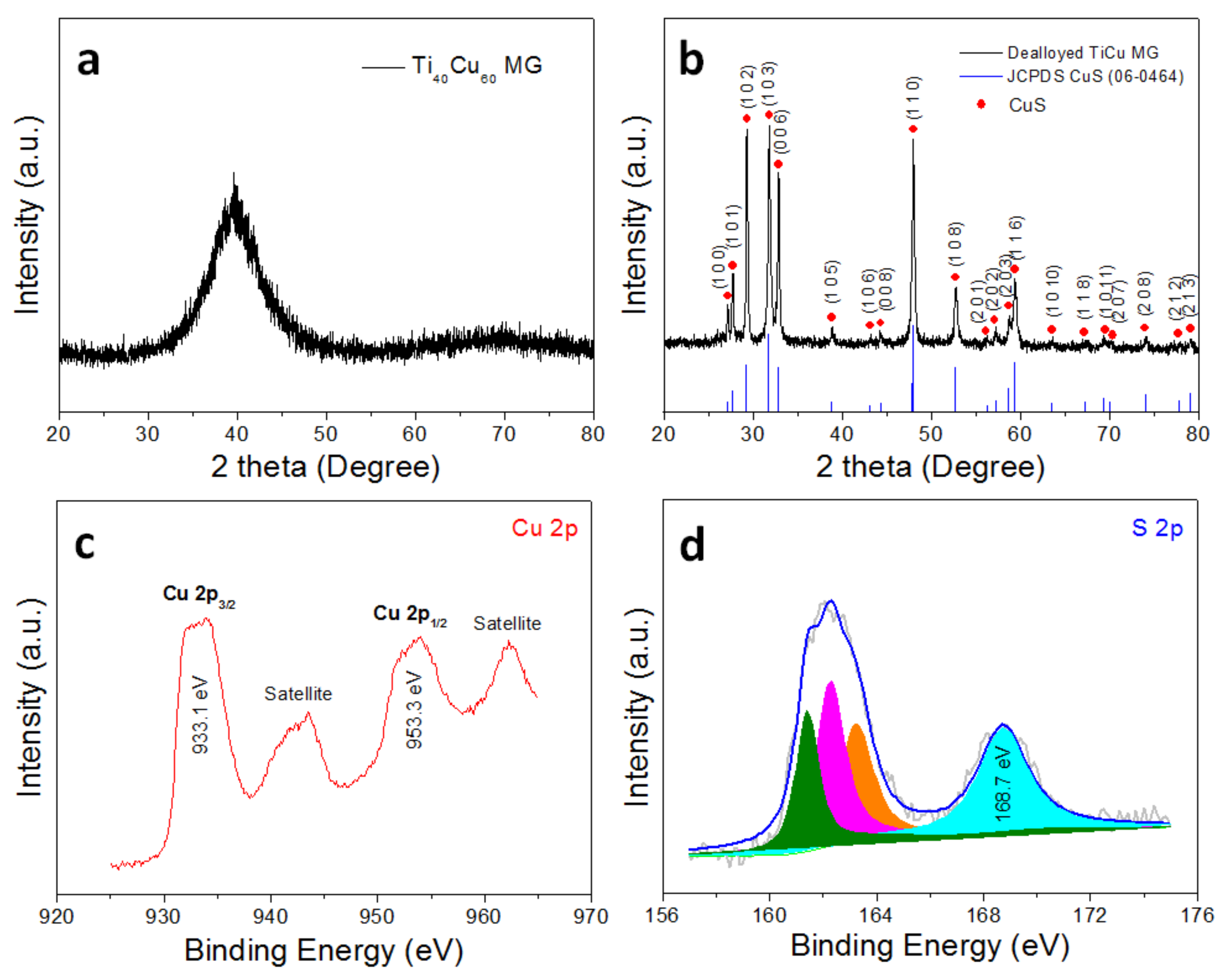
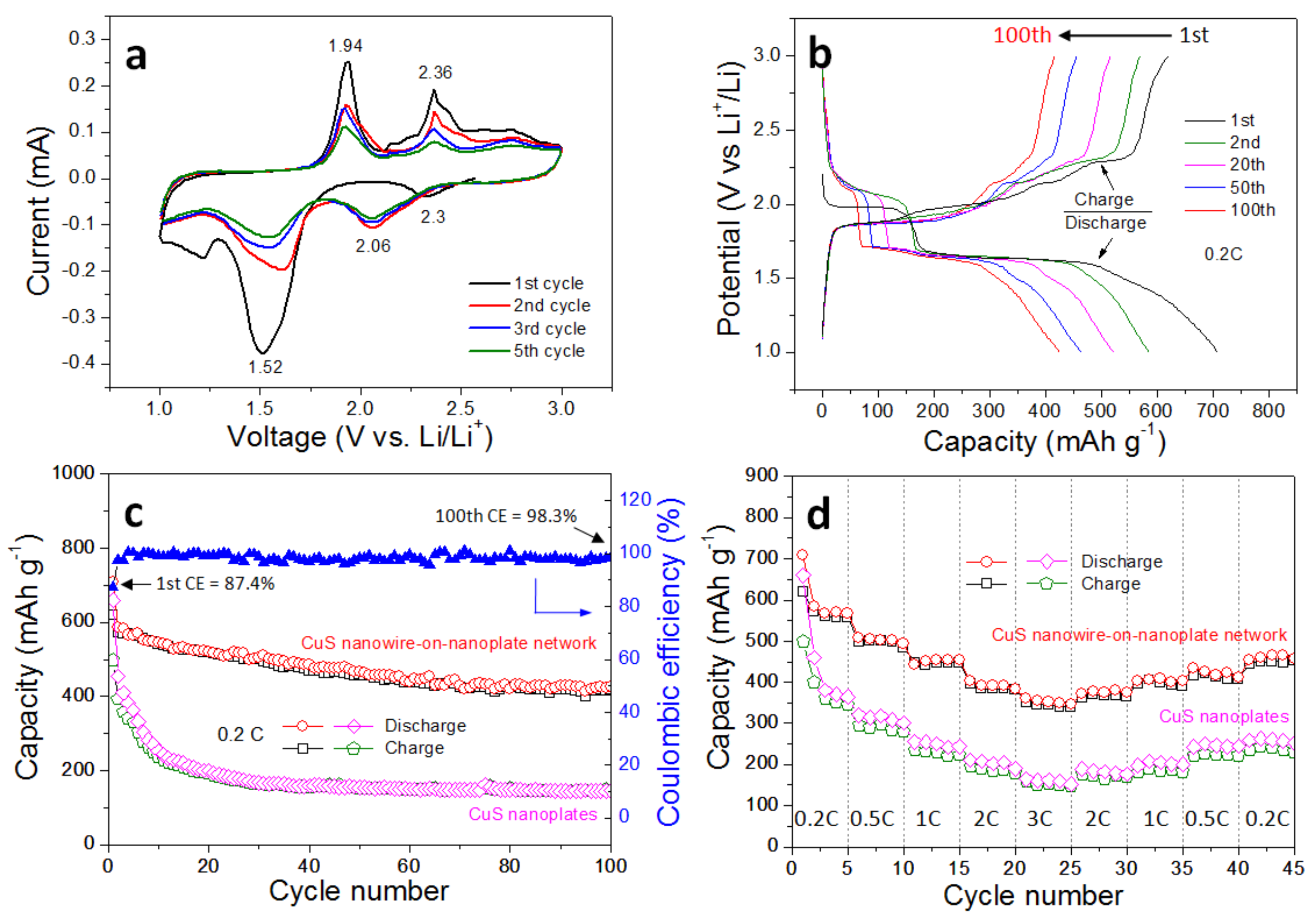
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kennedy, T.; Brandon, M.; Ryan, K.M. Advances in the application of silicon and germanium nanowires for high-performance lithium-ion batteries. Adv. Mater. 2016, 28, 5696–5704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.G.; Li, Y.; Li, H.P.; Yin, F.X.; Zhao, Y.; Bakenov, Z. Synthesis of hierarchical MoS2 microspheres composed of nanosheets assembled via facile hydrothermal method as anode material for lithium-ion batteries. J. Nanopart. Res. 2016, 63, 1–9. [Google Scholar] [CrossRef]
- McNulty, D.; Geaney, H.; Buckley, D.; O’Dwyer, C. High capacity binder-free nanocrystalline GeO2 inverse opal anodes for Li-ion batteries with long cycle life and stable cell voltage. Nano Energy 2018, 43, 11–21. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Li, Y.; Li, H.P.; Yin, F.X.; Bakenov, Z. Electrochemical performance of carbon-encapsulated Fe3O4 nanoparticles in lithium-ion batteries: Morphology and particle size effects. Electrochim. Acta 2016, 216, 475–483. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhang, Y.Y.; Li, H.; Peng, Y.Y.; Li, J.Y.; Wang, J.; Hwang, B.J.; Zhao, J.B. Realizing high reversible capacity: 3D intertwined CNTs inherently conductive network for CuS as an anode for lithium ion batteries. Chem. Eng. J. 2018, 332, 49–56. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.H.; Huang, J.X.; Zhang, Y.Y.; Zhao, J.B. Microwave-assisted synthesis of CuS/graphene composite for enhanced lithium storage properties. Electrochim. Acta 2017, 225, 443–451. [Google Scholar] [CrossRef]
- Ding, C.H.; Su, D.Z.; Ma, W.X.; Zhao, Y.J.; Yan, D.; Li, J.B.; Jin, H.B. Design of hierarchical CuS/graphene architectures with enhanced lithium storage capability. Appl. Surf. Sci. 2017, 403, 1–8. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.H.; Jiang, J.L.; Zhang, Y.Y.; Peng, Y.Y.; Zhao, J.B. CuS microspheres as high-performance anode material for Na-ion batteries. Electrochim. Acta 2017, 247, 851–859. [Google Scholar] [CrossRef]
- Chen, H.; Yeh, Y.M.; Chen, Y.T.; Jiang, Y.L. Influence of growth conditions on hair-like CuS nanowires fabricated by electro-deposition and sulfurization. Ceram. Int. 2014, 40, 9757–9761. [Google Scholar] [CrossRef]
- Zhou, M.J.; Peng, N.; Liu, Z.; Xi, Y.; He, H.Q.; Xia, Y.G.; Liu, Z.P.; Okada, S. Synthesis of sub-10 nm copper sulphide rods as high-performance anode for long-cycle life Li-ion batteries. J. Power Sources 2016, 306, 408–412. [Google Scholar] [CrossRef]
- Du, Y.P.; Yin, Z.Y.; Zhu, J.X.; Huang, X.; Wu, X.J.; Zeng, Z.Y.; Yan, Q.Y.; Zhang, H. A general method for the large-scale synthesis of uniform ultrathin metal sulphide nanocrystals. Nat. Commun. 2012, 3, 1177. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Tao, F.Q.; Quan, Z.; Zhou, X.L.; Yuan, Y.H.; Hu, J.C. Bubble template synthesis of copper sulfide hollow spheres and their applications in lithium ion battery. Mater. Lett. 2012, 68, 28–31. [Google Scholar] [CrossRef]
- Sabah, F.A.; Ahmedad, N.M.; Hassan, Z.; Almessiere, M.A. A novel CuS thin film deposition method by laser-assisted spray photolysis deposition and its application to EGFET. Sens. Actuators B Chem. 2017, 247, 197–215. [Google Scholar] [CrossRef]
- Hu, X.S.; Shen, Y.; Xu, L.H.; Wang, L.M.; Xing, Y.J. Preparation of flower-like CuS by solvothermal method and its photodegradation and UV protection. J. Alloys Compd. 2016, 674, 289–294. [Google Scholar] [CrossRef]
- Lu, Z.; Li, C.; Han, J.H.; Zhang, F.; Liu, P.; Wang, H.; Wang, Z.L.; Cheng, C.; Chen, L.H.; Hirata, A.; Fujita, T.; Erlebacher, J.; Chen, M.W. Three-dimensional bicontinuous nanoporous materials by vapor phase dealloying. Nat. Commun. 2018, 9, 276. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Higuchi, K.; Yamamoto, Y.; Tokunaga, T.; Arai, S.; Abe, H. In-situ TEM study of a nanoporous Ni–Co catalyst used for the dry reforming of methane. Metals 2017, 7, 406. [Google Scholar] [CrossRef]
- Xu, C.X.; Wang, R.Y.; Zhang, Y.; Ding, Y. A general corrosion route to nanostructured metal oxides. Nanoscale 2010, 2, 906–909. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.F.; Fei, P.Y.; Xiong, H.Q.; Qin, C.L.; Zhao, W.M.; Liu, X.Z. CoFe2O4 nanoplates synthesized by dealloying method as high performance Li-ion battery anodes. Electrochim. Acta 2017, 252, 295–305. [Google Scholar] [CrossRef]
- Xu, W.C.; Liang, Y.Q.; Su, Y.G.; Zhu, S.L.; Cui, Z.D.; Yang, X.J.; Inoue, A.; Wei, Q.; Liang, C.Y. Synthesis and properties of morphology controllable copper sulphide nanosheets for supercapacitor application. Electrochim. Acta 2016, 211, 891–899. [Google Scholar] [CrossRef]
- Xu, W.C.; Zhu, S.L.; Liang, Y.Q.; Li, Z.Y.; Cui, Z.D.; Yang, X.J.; Inoue, A. Nanoporous CuS with excellent photocatalytic property. Sci. Rep. 2015, 5, 15125. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.F.; Liu, J.Y.; Qin, C.L.; Liu, L.; Zhao, W.M.; Inoue, A. Fabrication and new electrochemical properties of nanoporous Cu by dealloying amorphous Cu–Hf–Al alloys. Intermetallics 2015, 56, 48–55. [Google Scholar] [CrossRef]
- Zhao, W.M.; Fei, P.Y.; Zhang, X.M.; Zhang, Y.G.; Qin, C.L.; Wang, Z.F. Porous TiO2/Fe2O3 nanoplate composites prepared by de-alloying method for Li-ion batteries. Mater. Lett. 2018, 211, 254–257. [Google Scholar] [CrossRef]
- Venkadesh, A.; Radhakrishnan, S.; Mathiyarasu, J. Eco-friendly synthesis and morphology-dependent superior electrocatalytic properties of CuS nanostructures. Electrochim. Acta 2017, 246, 544–552. [Google Scholar] [CrossRef]
- Qin, C.L.; Zhang, Y.S.; Wang, Z.F.; Xiong, H.Q.; Yu, H.; Zhao, W.M. One-step synthesis of CuO@brass foil by dealloying method for low-cost flexible supercapacitor electrodes. J. Mater. Sci. Mater. Electron. 2016, 27, 9206–9215. [Google Scholar] [CrossRef]
- Karikalan, N.; Karthik, R.; Chen, S.M.; Karuppiah, C.; Elangovan, A. Sonochemical synthesis of sulfur doped reduced graphene oxide supported CuS nanoparticles for the non-enzymatic glucose sensor applications. Sci. Rep. 2017, 7, 2494. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.G.; Du, J.W.; Ma, T.L. Cysteine-assisted synthesis of CuS-TiO2 composites with enhanced photocatalytic activity. Ceram. Int. 2017, 43, 9559–9563. [Google Scholar] [CrossRef]
- Hu, X.S.; Shen, Y.; Zhang, Y.T.; Zhang, H.F.; Xu, L.H.; Xing, Y.J. Synthesis of flower-like CuS/reduced graphene oxide (RGO) composites with significantly enhanced photocatalytic performance. J. Alloys Compd. 2017, 695, 1778–1785. [Google Scholar] [CrossRef]
- Song, C.D.; Wang, X.; Zhang, J.; Chen, X.B.; Li, C. Enhanced performance of direct Z-scheme CuS-WO3 system towards photocatalytic decomposition of organic pollutants under visible light. Appl. Surf. Sci. 2017, 425, 788–795. [Google Scholar] [CrossRef]
- Hosseinpour, Z.; Scarpellini, A.; Najafishirtari, S.; Marras, S.; Colombo, M.; Alemi, A.; De Volder, M.; George, C.; Lesnyak, V. Morphology-dependent electrochemical properties of CuS hierarchical superstructures. ChemPhysChem 2015, 16, 3418–3424. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Ni, S.B.; Chen, Q.C.; Zhang, J.C.; Yang, X.L. CuS@Cu freestanding electrode via electrochemical corrosion for high performance Li-ion batteries. Mater. Lett. 2017, 201, 13–17. [Google Scholar] [CrossRef]
- Wang, J.X.; Lyu, X.J.; Wang, L.Y.; Yu, S.; Zhu, W.X.; Han, C.; Cao, X.Q. Preparation and electrochemical performance of hierarchical CuS-rGO composite. J. Alloys Compd. 2017, 694, 1067–1072. [Google Scholar] [CrossRef]
- Tao, H.C.; Yang, X.L.; Zhang, L.L.; Ni, S.B. One-pot facile synthesis of CuS/graphene composite as anode materials for lithium ion batteries. J. Phys. Chem. Solids 2014, 75, 1205–1209. [Google Scholar] [CrossRef]
- Ren, Y.R.; Wei, H.M.; Yang, B.; Wang, J.W.; Ding, J.N. “Double-sandwich-like” CuS@reduced graphene oxide as an anode in lithium ion batteries with enhanced electrochemical performance. Electrochim. Acta 2014, 145, 193–200. [Google Scholar] [CrossRef]
- Kalimuldina, G.; Taniguchi, I. Electrochemical properties of stoichiometric CuS coated on carbon fiber paper and Cu foil current collectors as cathode material for lithium batteries. J. Mater. Chem. A 2017, 5, 6937–6946. [Google Scholar] [CrossRef]
- Cheng, J.J.; Pan, Y.; Zhu, J.T.; Li, Z.Z.; Pan, J.N.; Ma, Z.S. Hybrid network CuS monolith cathode materials synthesized via facile in situ melt-diffusion for Li-ion batteries. J. Power Sources 2014, 257, 192–197. [Google Scholar] [CrossRef]


| Anode Material | Preparation Method | Current Density | Cycle Number | Final Discharge Capacity (mAh·g−1) | Reference |
|---|---|---|---|---|---|
| Hierarchical CuS/graphene | Hydrothermal method | 0.09 C | 100 | 568 | [7] |
| CuS-rGO | Hydrothermal process | 0.18 C | 50 | 451 | [31] |
| CuS/graphene composite | Hydrothermal method | 0.09 C | 25 | 296 | [32] |
| CuS nanospheres | Microwave irradiation method | 0.36 C | 100 | 379 | [6] |
| CuS particles | Hydrothermal process | 0.2 C | 100 | 159.7 | [33] |
| CuS coated on Cu foil | Spray pyrolysis | 0.1 C | 30 | 450 | [34] |
| CuS nanosheet network | In situ melt-diffusion strategy | 0.2 C | 100 | 468.3 | [35] |
| CuS nanowire-on-nanoplate network | Dealloying | 0.2 C | 100 | 425 (395/expected at 0.36 C) | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhang, X.; Zhang, Y.; Li, M.; Qin, C.; Bakenov, Z. Chemical Dealloying Synthesis of CuS Nanowire-on-Nanoplate Network as Anode Materials for Li-Ion Batteries. Metals 2018, 8, 252. https://doi.org/10.3390/met8040252
Wang Z, Zhang X, Zhang Y, Li M, Qin C, Bakenov Z. Chemical Dealloying Synthesis of CuS Nanowire-on-Nanoplate Network as Anode Materials for Li-Ion Batteries. Metals. 2018; 8(4):252. https://doi.org/10.3390/met8040252
Chicago/Turabian StyleWang, Zhifeng, Xiaomin Zhang, Yongguang Zhang, Man Li, Chunling Qin, and Zhumabay Bakenov. 2018. "Chemical Dealloying Synthesis of CuS Nanowire-on-Nanoplate Network as Anode Materials for Li-Ion Batteries" Metals 8, no. 4: 252. https://doi.org/10.3390/met8040252
APA StyleWang, Z., Zhang, X., Zhang, Y., Li, M., Qin, C., & Bakenov, Z. (2018). Chemical Dealloying Synthesis of CuS Nanowire-on-Nanoplate Network as Anode Materials for Li-Ion Batteries. Metals, 8(4), 252. https://doi.org/10.3390/met8040252







