Characterization of Porous Phosphate Coatings Enriched with Magnesium or Zinc on CP Titanium Grade 2 under DC Plasma Electrolytic Oxidation
Abstract
:1. Introduction
2. Method
3. Results and Discussion
4. Conclusions
- (1)
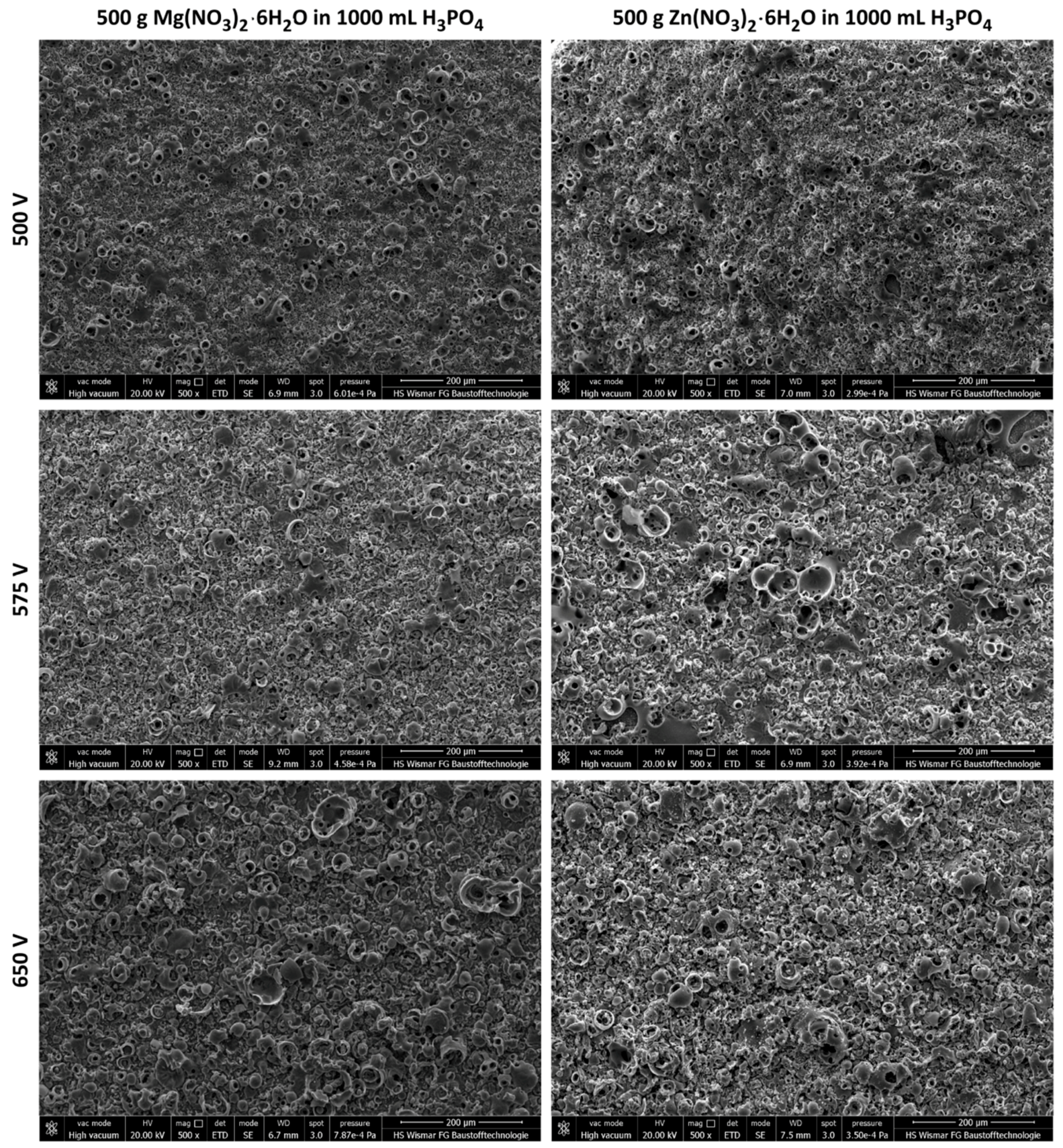
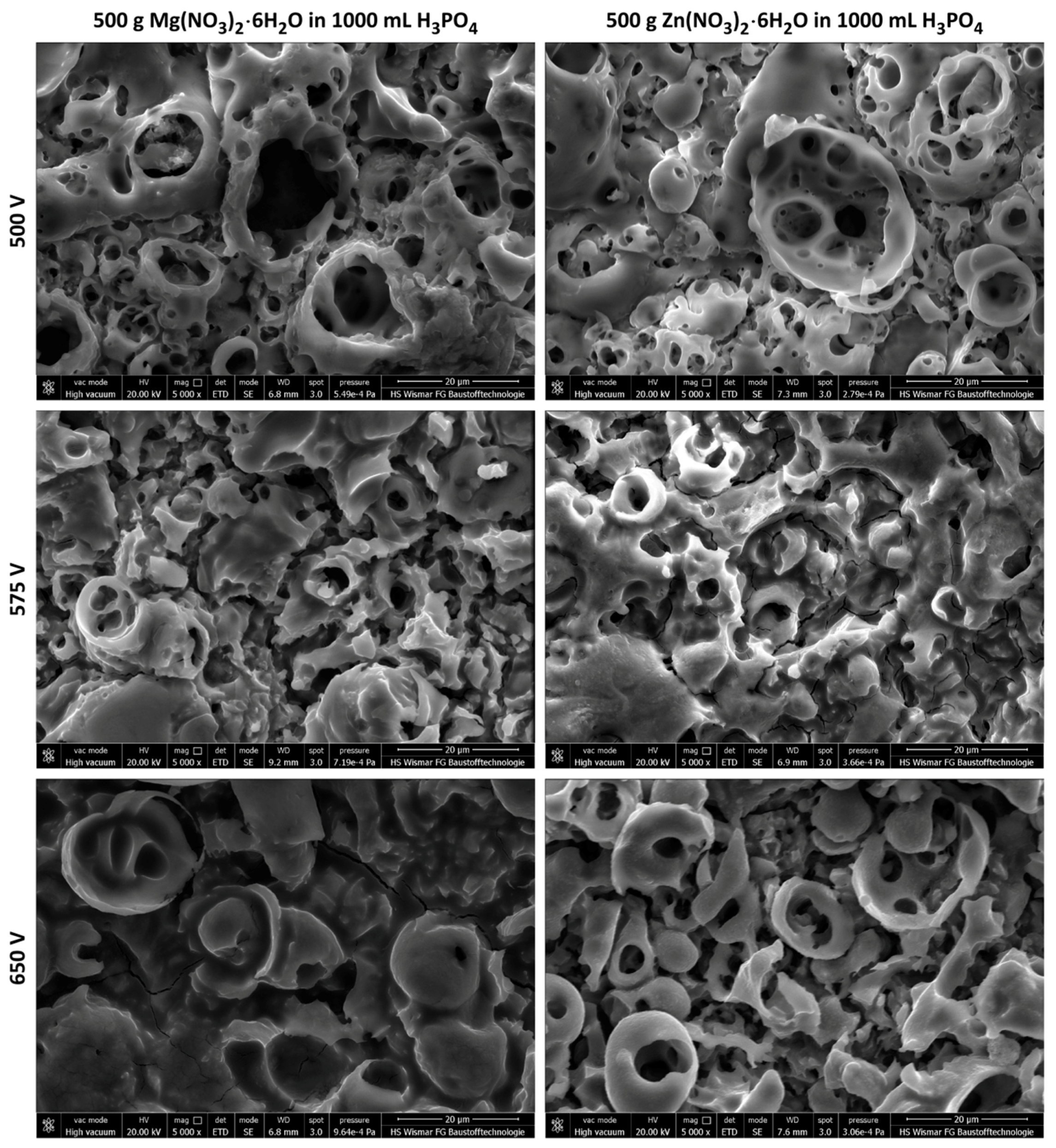
- It is possible to form porous coatings by using of DC PEO oxidation in electrolytes containing concentrated phosphoric acid and magnesium or zinc nitrate.
- (2)
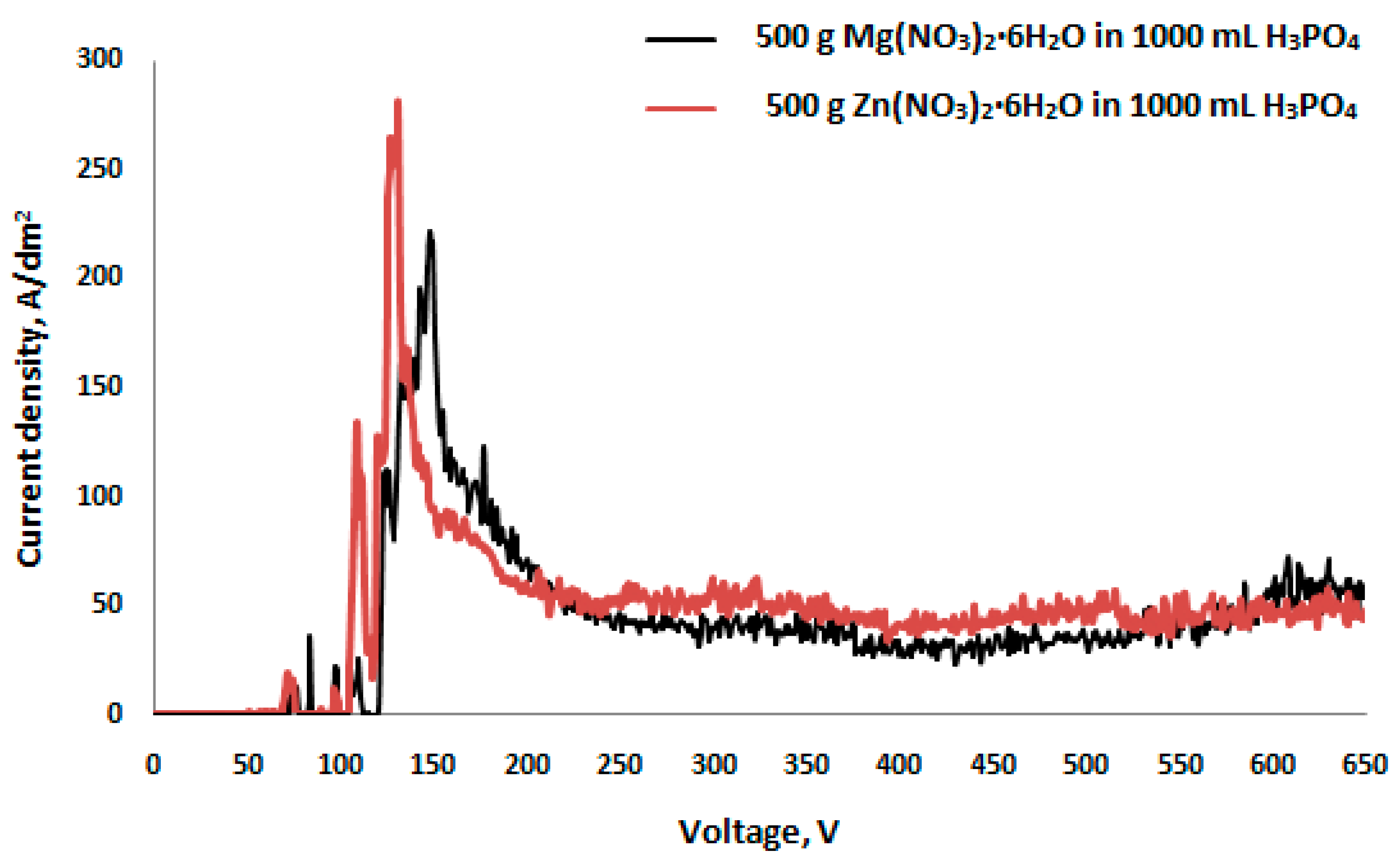
- The voltage of PEO process has an influence on the chemical composition and thickness of the obtained porous coatings as well as on their electrochemical behavior.
- (3)
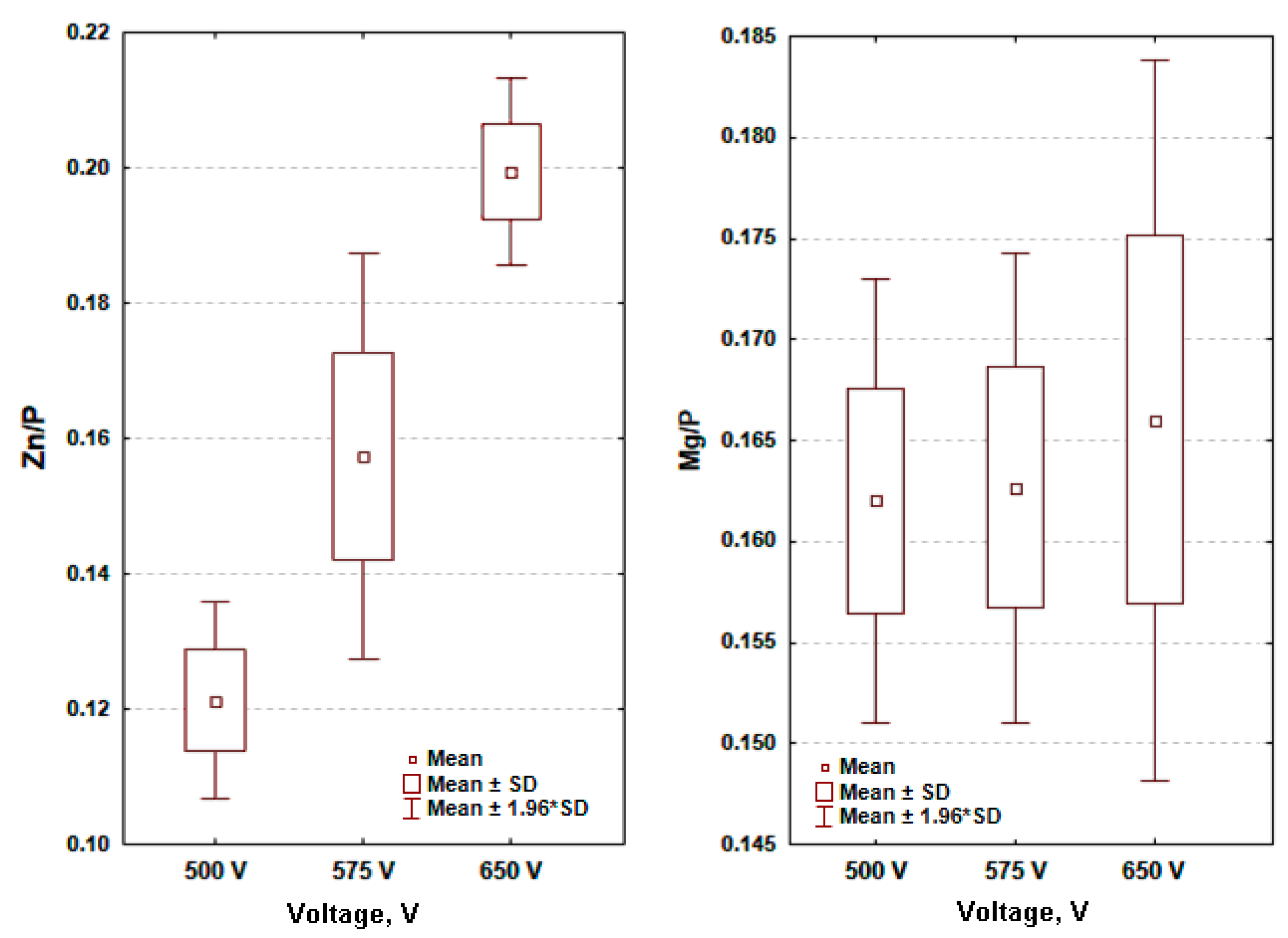
- The higher the voltage of PEO treatment, the higher is zinc-to-phosphorus ratio for zinc enriched coatings; there is a trend for growing as a function of PEO voltage.
- (4)
- The higher the voltage of PEO treatment, the higher the range (max–min) of magnesium-to-phosphorus ratio with very similar averages for magnesium enriched coatings; the trend may be described as a constant function of PEO voltage.
- (5)
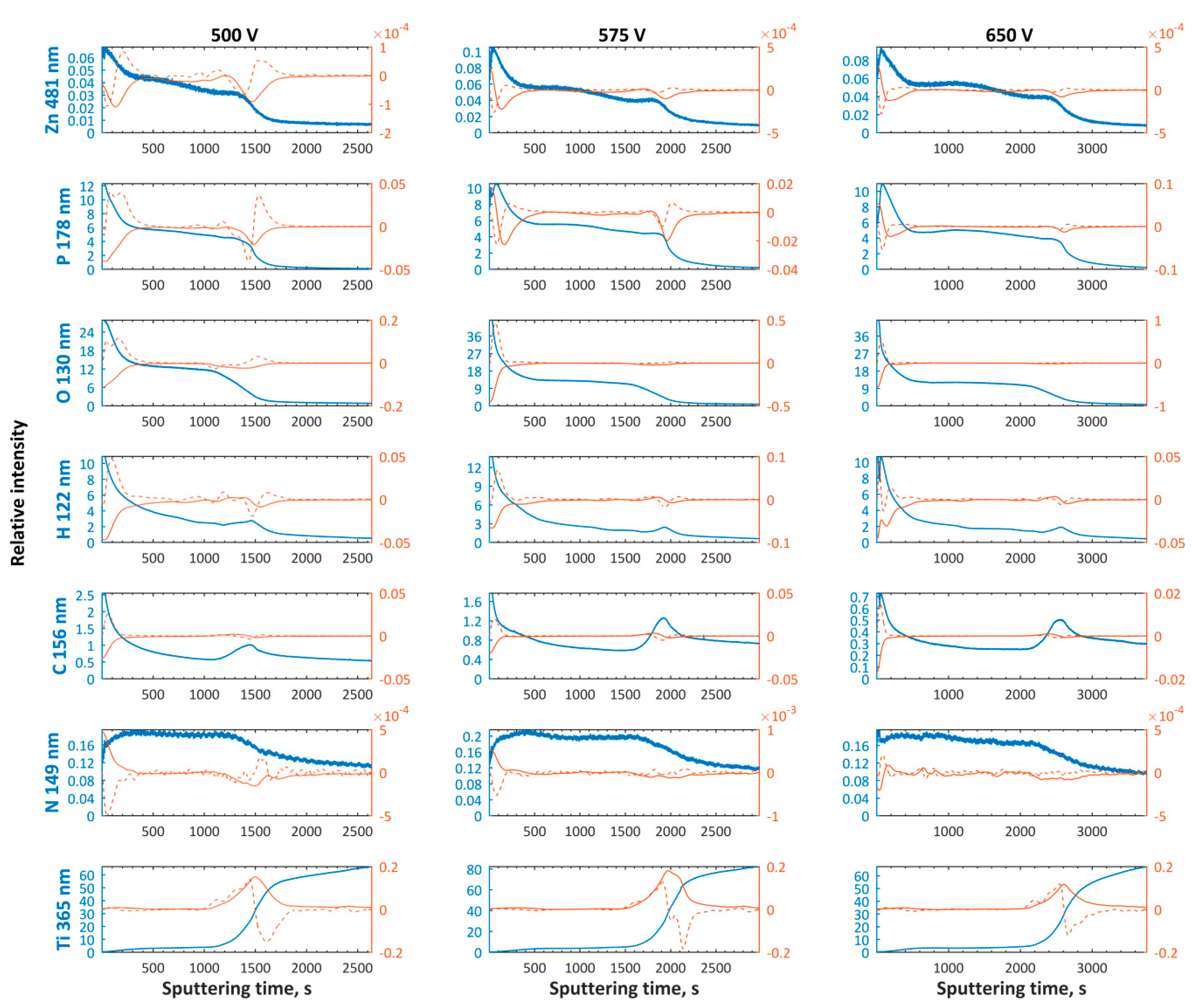
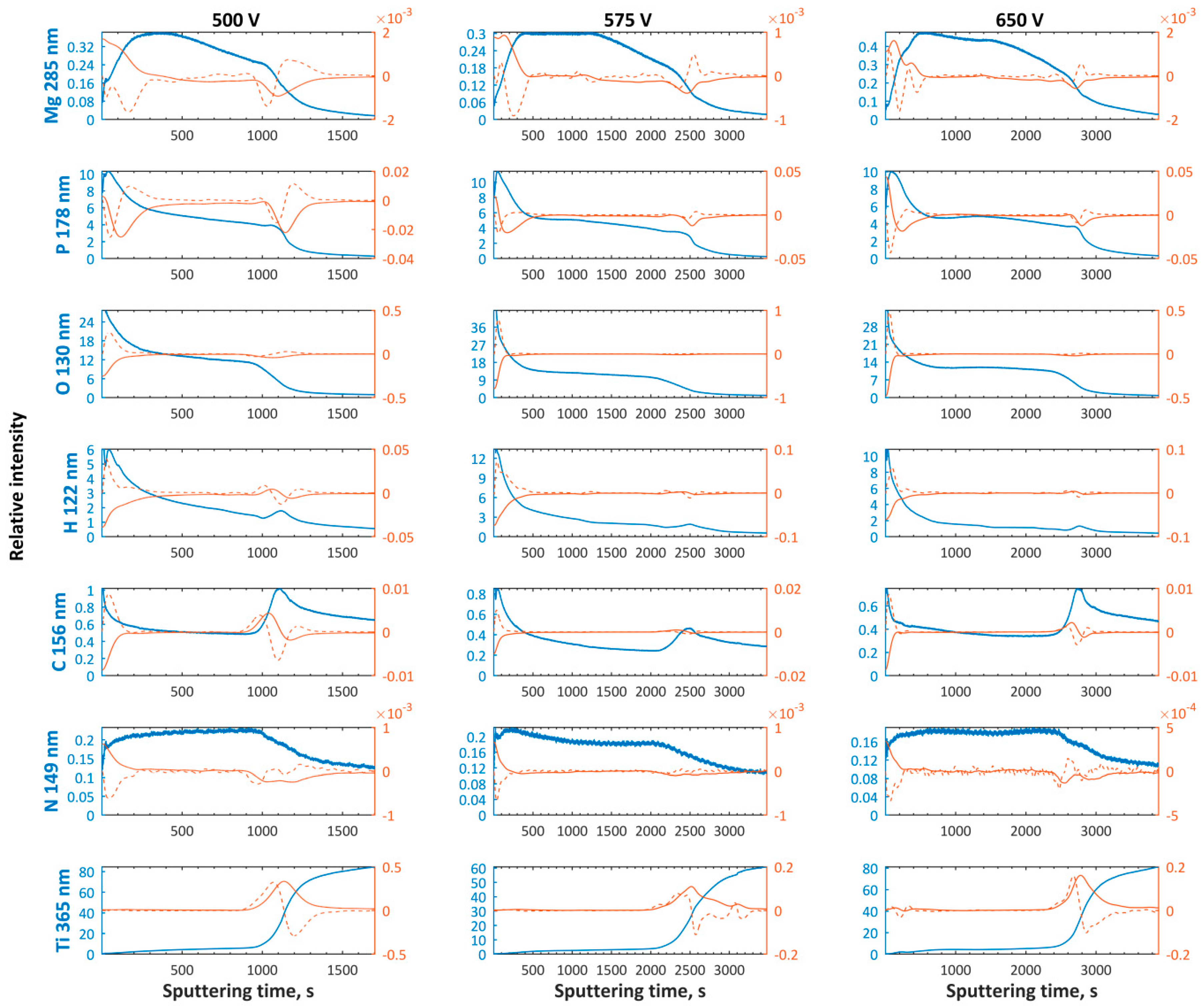
- The four-sub-layer model may be assumed to describe the obtained PEO coatings:
- (a)
- The first and top sub-layers having a thickness corresponding with the sputtering time equaling to 200–600 s and 350–450 s for magnesium and zinc enriched PEO coatings, respectively;
- (b)
- The top sub-layer enriched with magnesium is depleted in magnesium compounds, while the other one, containing zinc in the top, is clearly enriched with zinc;
- (c)
- The thickness of semi-porous sub-layers depends on the PEO treatment voltage; the thickest second and third sub-layers appear to set up at high voltages and they decrease with PEO voltage decreasing;
- (d)
- The transition sub-layers have thickness corresponding to a sputtering time of about 500–900 s.
- (6)
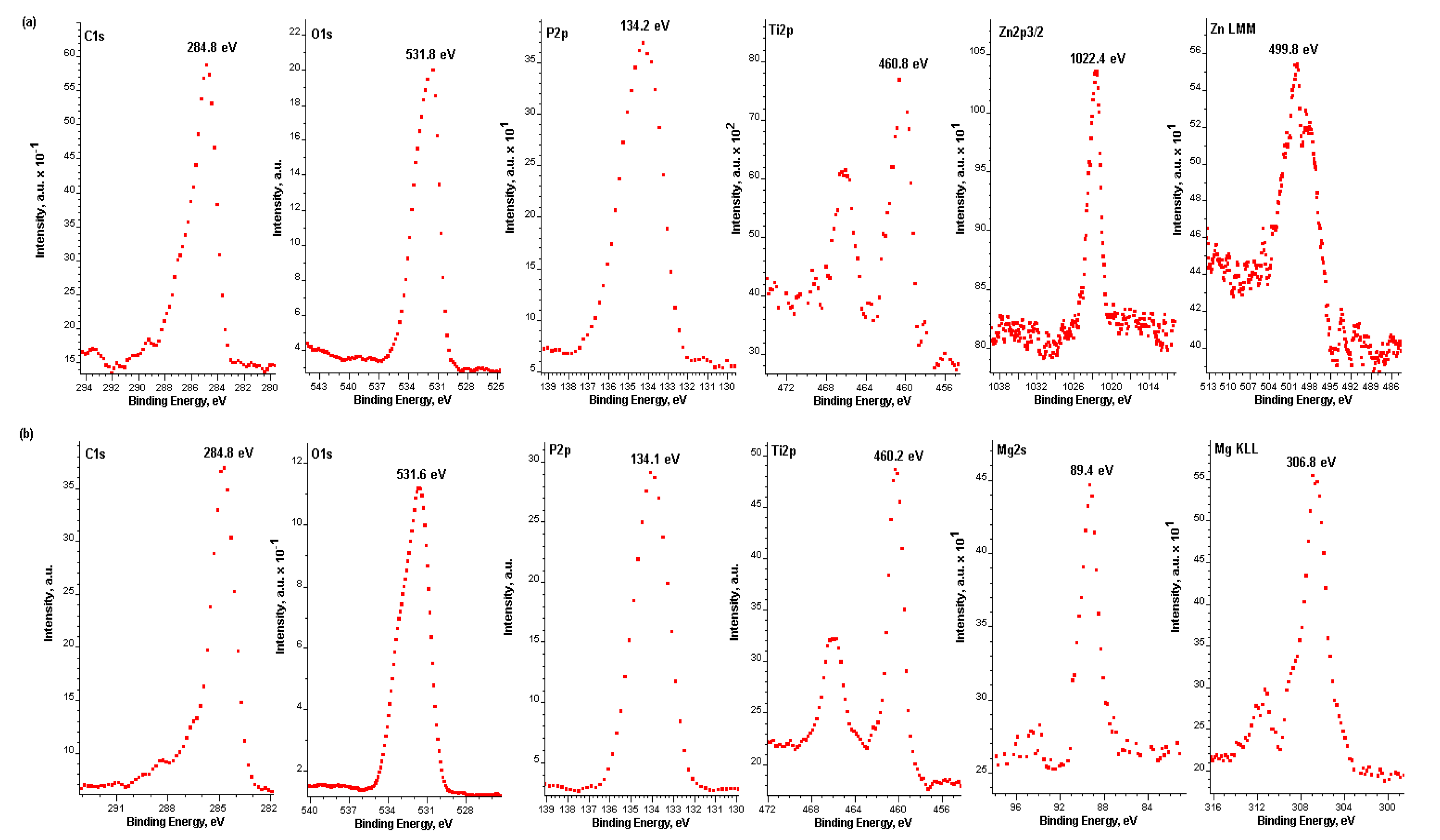
- Most likely the top 10 nm of porous coatings is constituted of titanium (Ti4+), magnesium (Mg2+), zinc (Zn2+), and phosphates PO43−, and/or HPO42−, and/or H2PO4−, and/or P2O74−.
- (7)
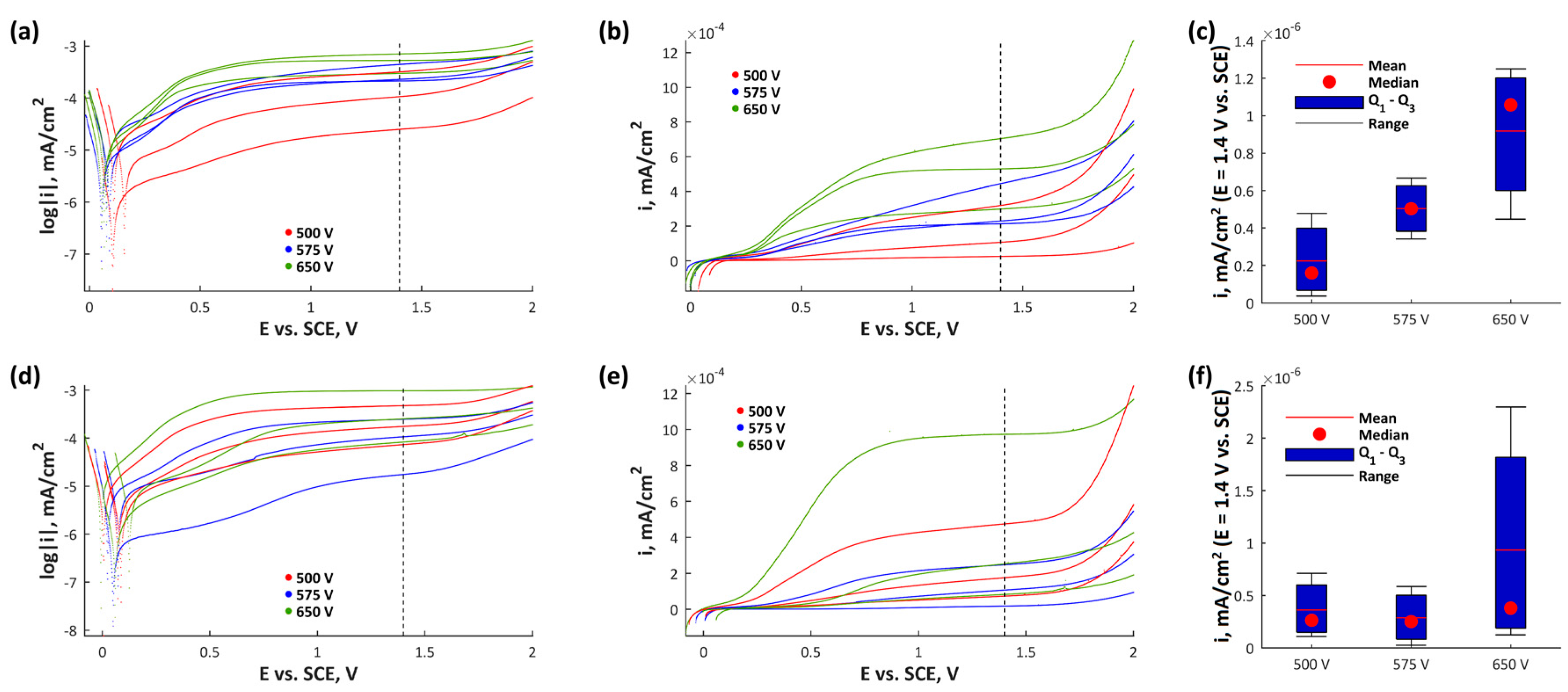
- Based on the potentiodynamic corrosion measurements one may conclude that the best electrochemical repeatability was noted for magnesium and zinc enriched coatings obtained at 575 VDC.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hryniewicz, T.; Hryniewicz, Z. On the solution of equation of diffusion in electropolishing. J. Electrochem. Soc. 1989, 136, 3767–3769. [Google Scholar] [CrossRef]
- Hryniewicz, T. Concept of microsmoothing in the electropolishing process. Surf. Coat. Technol. 1994, 64, 75–80. [Google Scholar] [CrossRef]
- Rokicki, R.; Hryniewicz, T. Enhanced oxidation-dissolution theory of electropolishing. Trans. Inst. Met. Finish. 2012, 90, 188–196. [Google Scholar] [CrossRef]
- Aliasghari, S. Plasma Electrolytic Oxidation of Titanium. Ph.D. Thesis, Faculty of Engineering and Physical Sciences, School of Materials, The University of Manchester, Manchester, UK, 2014. [Google Scholar]
- Han, Y.; Hong, S.H.; Xu, K.W. Synthesis of nanocrystalline titania films by micro-arc oxidation. Mater. Lett. 2002, 56, 744–747. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Sharkeev, Y.P.; Sinebryukhov, S.L.; Khrisanfova, O.A.; Legostaeva, E.V.; Zavidnaya, A.G.; Puz’, A.V.; Khlusov, I.A.; Opra, D.P. Functional coatings formed on the titanium and magnesium alloys as implant materials by plasma electrolytic oxidation technology: Fundamental principles and synthesis conditions. Corros. Rev. 2016, 34, 65–83. [Google Scholar] [CrossRef]
- Hryniewicz, T.; Rokosz, K.; Sandim, H.R.Z. SEM/EDX and XPS studies of niobium after electropolishing. Appl. Surf. Sci. 2012, 263, 357–361. [Google Scholar] [CrossRef]
- Hryniewicz, T.; Konarski, P.; Rokicki, R.; Valiček, J. SIMS analysis of hydrogen content in near surface layers of AISI 316L SS after electrolytic polishing under different conditions. Surf. Coat. Technol. 2011, 205, 4228–4236. [Google Scholar] [CrossRef]
- Rokosz, K. Electrochemical Polishing in Magnetic Field; Koszalin University of Technology Publishing House: Koszalin, Poland, 2012; Monograph No 219; ISSN 0239-7129. (In Polish) [Google Scholar]
- Hryniewicz, T.; Rokicki, R.; Rokosz, K. Co-Cr alloy corrosion behaviour after electropolishing and “magnetoelectropolishing” treatments. Surf. Coat. Technol. 2008, 62, 3073–3076. [Google Scholar] [CrossRef]
- Hryniewicz, T.; Rokicki, R.; Rokosz, K. Magnetoelectropolishing for metal surface modification. Trans. Inst. Met. Finish. 2007, 85, 325–332. [Google Scholar] [CrossRef]
- Hryniewicz, T.; Rokosz, K. Polarization characteristics of magnetoelectropolishing stainless steels. Mater. Chem. Phys. 2010, 122, 169–174. [Google Scholar] [CrossRef]
- Hryniewicz, T.; Rokicki, R.; Rokosz, K. Corrosion and surface characterization of titanium biomaterial after magnetoelectropolishing. Surf. Coat. Technol. 2008, 203, 1508–1515. [Google Scholar] [CrossRef]
- Rokicki, R.; Hryniewicz, T.; Rokosz, K. Modifying metallic implants by magnetoelectropolishing. Med. Device Diagn. Ind. 2008, 30, 102–111. [Google Scholar]
- Rokicki, R.; Hryniewicz, T. Nitinol surface finishing by magnetoelectropolishing. Trans. Inst. Met. Finish. 2008, 86, 280–285. [Google Scholar] [CrossRef]
- Rokicki, R.; Haider, W.; Hryniewicz, T. Influence of sodium hypochlorite treatment of electropolished and magnetoelectropolished nitinol surfaces on adhesion and proliferation of MC3T3 preosteoblast cells. J. Mater. Sci. Mater. Med. 2012, 23, 2127–2139. [Google Scholar] [CrossRef] [PubMed]
- Rokosz, K.; Hryniewicz, T.; Simon, F.; Rzadkiewicz, S. Comparative XPS analysis of passive layers composition formed on AISI 304 L SS after standard and high-current density electropolishing. Surf. Interface Anal. 2015, 47, 87–92. [Google Scholar] [CrossRef]
- Rokosz, K.; Lahtinen, J.; Hryniewicz, T.; Rzadkiewicz, S. XPS depth profiling analysis of passive surface layers formed on austenitic AISI 304L and AISI 316L SS after high-current-density electropolishing. Surf. Coat. Technol. 2015, 276, 516–520. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Simon, F.; Rzadkiewicz, S. Comparative XPS analyses of passive layers composition formed on duplex 2205 SS after standard and high-current-density electropolishing. Teh. Vjesn. Tech. Gaz. 2016, 23, 731–735. [Google Scholar]
- Hryniewicz, T.; Rokosz, K. Analysis of XPS results of AISI 316L SS electropolished and magnetoelectropolished at varying conditions. Surf. Coat. Technol. 2010, 204, 2583–2592. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Raaen, S. Cr/Fe ratio by XPS spectra of magnetoelectropolished AISI 316L SS fitted by Gaussian-Lorentzian shape lines. Teh. Vjesn. Tech. Gaz. 2014, 21, 533–538. [Google Scholar]
- Rokosz, K.; Hryniewicz, T. XPS measurements of LDX 2101 duplex steel surface after magnetoelectropolishing. Int. J. Mater. Res. 2013, 104, 1223–1232. [Google Scholar] [CrossRef]
- Hryniewicz, T.; Rokosz, K. Investigation of selected surface properties of AISI 316L SS after magnetoelectropolishing. Mater. Chem. Phys. 2010, 123, 47–55. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T. XPS Analysis of nanolayers obtained on AISI 316L SS after Magnetoelectropolishing. World Sci. News 2016, 37, 232–248. [Google Scholar]
- Rokosz, K.; Hryniewicz, T.; Raaen, S. Characterization of passive film formed on AISI 316L stainless steel after magnetoelectropolishing in a broad range of polarization parameters. Steel Res. Int. 2012, 83, 910–918. [Google Scholar] [CrossRef]
- Hryniewicz, T.; Rokicki, R.; Rokosz, K. Chapter 11. Magnetoelectropolished Titanium Biomaterial. In Biomaterials Science and Engineering; InTech: London, UK, 2011; pp. 227–248. ISBN 978-953-308-118-2. Available online: http://www.intechopen.com/articles/show/title/magnetoelectropolished-titanium-biomaterial (accessed on 3 February 2018).
- Hryniewicz, T.; Konarski, P.; Rokicki, R.; Valiček, J. SIMS studies of titanium biomaterial hydrogenation after magnetoelectropolishing. Surf. Coat. Technol. 2012, 206, 4027–4031. [Google Scholar] [CrossRef]
- Hryniewicz, T.; Konarski, P.; Rokicki, R. Hydrogen reduction in MEP niobium studied by secondary ion mass spectrometry (SIMS). Metals 2017, 7, 442. [Google Scholar] [CrossRef]
- Rokicki, R.; Hryniewicz, T.; Konarski, P.; Rokosz, K. The alternative, novel technology for improvement of surface finish of SRF niobium cavities. World Sci. News 2017, 74, 152–163. [Google Scholar]
- Rokosz, K.; Hryniewicz, T. Effect of magnetic field on the pitting corrosion of austenitic steel type AISI 304. Ochr. Przed Koroz. 2011, 54, 487–491. [Google Scholar]
- Hryniewicz, T.; Rokosz, K. Corrosion resistance of magnetoelectropolished AISI 316L SS biomaterial. Anti-Corros. Methods Mater. 2014, 61, 57–64. [Google Scholar] [CrossRef]
- Hryniewicz, T.; Rokosz, K.; Rokicki, R.; Prima, F. Nanoindentation and XPS studies of titanium TNZ alloy after electrochemical polishing in a magnetic field. Materials 2015, 8, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Hryniewicz, T.; Rokosz, K.; Valiček, J.; Rokicki, R. Effect of magnetoelectropolishing on nanohardness and Young’s modulus of titanium biomaterial. Mater. Lett. 2012, 83, 69–72. [Google Scholar] [CrossRef]
- Han, Y.; Hong, S.H.; Xu, K.W. Structure and in vitro bioactivity of titania-based films by micro-arc oxidation. Surf. Coat. Technol. 2003, 168, 249–258. [Google Scholar] [CrossRef]
- The, T.H.; Berkani, A.; Mato, S.; Skeldon, P.; Thompson, G.E.; Habazaki, H.; Shimizu, K. Initial stages of plasma electrolytic oxidation of titanium. Corros. Sci. 2003, 45, 2757–2768. [Google Scholar]
- Rokosz, K.; Hryniewicz, T.; Dudek, Ł.; Matysek, D.; Valiček, J.; Harničarova, M. SEM and EDS analysis of surface layer formed on titanium after plasma electrolytic oxidation in H3PO4 with the addition of Cu(NO3)2. J. Nanosci. Nanotechnol. 2016, 16, 7814–7817. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Dalibor, M.; Raaen, S.; Valiček, J.; Dudek, Ł.; Harničarova, M. SEM, EDS and XPS analysis of the coatings obtained on titanium after plasma electrolytic oxidation in electrolytes containing copper nitrate. Materials 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Krząkala, A.; Młyński, J.; Dercz, G.; Michalska, J.; Maciej, A.; Nieużyła, L.; Simka, W. Modification of Ti-6Al-4V alloy surface by EPD-PEO process in ZrSiO4 suspension. Arch. Metall. Mater. 2014, 59, 199–204. [Google Scholar] [CrossRef]
- Simka, W.; Nawrat, G.; Chlode, J.; Maciej, A.; Winiarski, A.; Szade, J.; Radwanski, K.; Gazdowicz, J. Electropolishing and anodic passivation of Ti6Al7Nb alloy. Przem. Chem. 2011, 90, 84–90. [Google Scholar]
- Wang, Y.; Jiang, B.; Lei, T.; Guo, L. Dependence of growth features of microarc oxidation coatings of titanium alloy on control modes of alternate pulse. Mater. Lett. 2004, 58, 1907–1911. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Raaen, S. Development of Plasma Electrolytic Oxidation for improved Ti6Al4V biomaterial surface properties. Int. J. Adv. Manuf. Technol. 2016, 85, 2425–2437. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Raaen, S.; Chapon, P. Investigation of porous coatings obtained on Ti-Nb-Zr-Sn alloy biomaterial by Plasma Electrolytic Oxidation: Characterisation and Modelling. Int. J. Adv. Manuf. Technol. 2016, 87, 3497–3512. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Raaen, S.; Chapon, P. Development of copper-enriched porous coatings on ternary Ti-Nb-Zr alloy by Plasma Electrolytic Oxidation. Int. J. Adv. Manuf. Technol. 2017, 89, 2953–2965. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T. Characteristics of porous and biocompatible coatings obtained on Niobium and Titanium-Niobium-Zirconium (TNZ) alloy by Plasma Electrolytic Oxidation. Mechanik 2015, 12, 15–18. [Google Scholar] [CrossRef]
- Simka, W.; Sadowski, A.; Warczak, M.; Iwaniak, A.; Dercz, G.; Michalska, J.; Maciej, A. Modification of titanium oxide layer by calcium and phosphorus. Electrochim. Acta 2011, 56, 8962–8968. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Gaiaschi, S.; Chapon, P.; Raaen, S.; Pietrzak, K.; Malorny, W. Characterisation of calcium- and phosphorus-enriched porous coatings on CP Titanium Grade 2 fabricated by plasma electrolytic oxidation. Metals 2017, 7. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Chapon, P.; Raaen, S.; Sandim, H.R.Z. XPS and GDOES characterisation of porous coating enriched with copper and calcium obtained on Tantalum via Plasma Electrolytic Oxidation. J. Spectrosc. 2016, 2016, 7093071. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Raaen, S.; Chapon, P.; Dudek, Ł. GDOES, XPS and SEM with EDS analysis of porous coatings obtained on Titanium after Plasma Electrolytic Oxidation. Surf. Interface Anal. 2016, 49, 303–315. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Chapon, P.; Dudek, Ł. A new approach to porous PEO coating sub-layers determination on the basis of GDOES signals. World Sci. News 2016, 57, 289–299. [Google Scholar]
- Rokosz, K.; Hryniewicz, T.; Malorny, W. Characterisation of porous coatings obtained on materials by Plasma Electrolytc Oxidation. Mater. Sci. Forum 2016, 862, 86–95. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Raaen, S. SEM, EDS and XPS analysis of nanostructured coating obtained on NiTi biomaterial alloy by Plasma Electrolytic Oxidation (PEO). Teh. Vjesn. Tech. Gaz. 2017, 24, 193–198. [Google Scholar]
- Rokosz, K.; Hryniewicz, T.; Pietrzak, K.; Sadlak, P.; Valíček, J. Fabrication and characterisation of porous, calcium enriched coatings on titanium after Plasma Electrolytic Oxidation under DC regime. Adv. Mater. Sci. 2017, 17, 55–67. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T. Comparative SEM and EDX analysis of surface coatings created on niobium and titanium alloys after Plasma Electrolytic Oxidation (PEO). Teh. Vjesn. Tech. Gaz. 2017, 24, 465–472. [Google Scholar]
- Rokosz, K.; Hryniewicz, T.; Raaen, S.; Malorny, W. Fabrication and characterization of porous coatings obtained by Plasma Electrolytic Oxidation. J. Mech. Energy Eng. 2017, 1, 23–30. [Google Scholar]
- Valiček, J.; Drzik, M.; Hryniewicz, T.; Harničarova, M.; Rokosz, K.; Kusnerova, M.; Barcova, K.; Brazina, D. Noncontact method for surface roughness measurement after machining. Meas. Sci. Rev. 2012, 12, 184–188. [Google Scholar] [CrossRef]
- Kusnerova, M.; Valiček, J.; Harničarova, M.; Hryniewicz, T.; Rokosz, K.; Palkova, Z.; Vaclavik, V.; Repka, M.; Bendova, M. A proposal for simplifying the method of evaluation of uncertainties in measurement results. Meas. Sci. Rev. 2013, 13, 1–6. [Google Scholar] [CrossRef]
- Casa Software Ltd. CasaXPS: Processing Software for XPS, AES, SIMS and More. 2009. Available online: http://www.casaxps.com (accessed on 12 December 2017).
- Wagner, C.D.; Naumkin, A.V.; Kraut-Vass, A.; Allison, J.W.; Powell, C.J.; Rumble, J.R. NIST Standard Reference Database 20, Version 4.1. 2003. Available online: http://srdata.nist.gov/xps (accessed on 3 February 2018).
- Nelis, T.; Payling, R. Practical Guide to Glow Discharge Optical Emission Spectroscopy; RSC Analytical Spectroscopy Monographs; Barnett, N.W., Ed.; Royal Society of Chemistry: Cambridge, UK, 2002. [Google Scholar]
- Pulsed RF Glow Discharge Optical Emission Spectrometry; Ultra Fast Elemental Depth Profiling; HORIBA Jobin Yvon: Paris, France, 2014; Available online: http://www.horiba.com/scientific/products/atomic-emission-spectroscopy/glow-discharge/ (accessed on 12 December 2017).
- DiP: Differential Interferometry Profiling; HORIBA Jobin Yvon: Paris, France, 2015; Available online: http://www.horiba.com/fileadmin/uploads/Scientific/Documents/GDS/HJY_BRO_GDOES_DiP.pdf (accessed on 12 December 2017).
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rokosz, K.; Hryniewicz, T.; Gaiaschi, S.; Chapon, P.; Raaen, S.; Pietrzak, K.; Malorny, W.; Salvador Fernandes, J. Characterization of Porous Phosphate Coatings Enriched with Magnesium or Zinc on CP Titanium Grade 2 under DC Plasma Electrolytic Oxidation. Metals 2018, 8, 112. https://doi.org/10.3390/met8020112
Rokosz K, Hryniewicz T, Gaiaschi S, Chapon P, Raaen S, Pietrzak K, Malorny W, Salvador Fernandes J. Characterization of Porous Phosphate Coatings Enriched with Magnesium or Zinc on CP Titanium Grade 2 under DC Plasma Electrolytic Oxidation. Metals. 2018; 8(2):112. https://doi.org/10.3390/met8020112
Chicago/Turabian StyleRokosz, Krzysztof, Tadeusz Hryniewicz, Sofia Gaiaschi, Patrick Chapon, Steinar Raaen, Kornel Pietrzak, Winfried Malorny, and João Salvador Fernandes. 2018. "Characterization of Porous Phosphate Coatings Enriched with Magnesium or Zinc on CP Titanium Grade 2 under DC Plasma Electrolytic Oxidation" Metals 8, no. 2: 112. https://doi.org/10.3390/met8020112
APA StyleRokosz, K., Hryniewicz, T., Gaiaschi, S., Chapon, P., Raaen, S., Pietrzak, K., Malorny, W., & Salvador Fernandes, J. (2018). Characterization of Porous Phosphate Coatings Enriched with Magnesium or Zinc on CP Titanium Grade 2 under DC Plasma Electrolytic Oxidation. Metals, 8(2), 112. https://doi.org/10.3390/met8020112








